Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities
Abstract
1. Introduction
2. Methodology
3. Sulphated Flavonoids: General Information and Chemotaxonomic Aspects
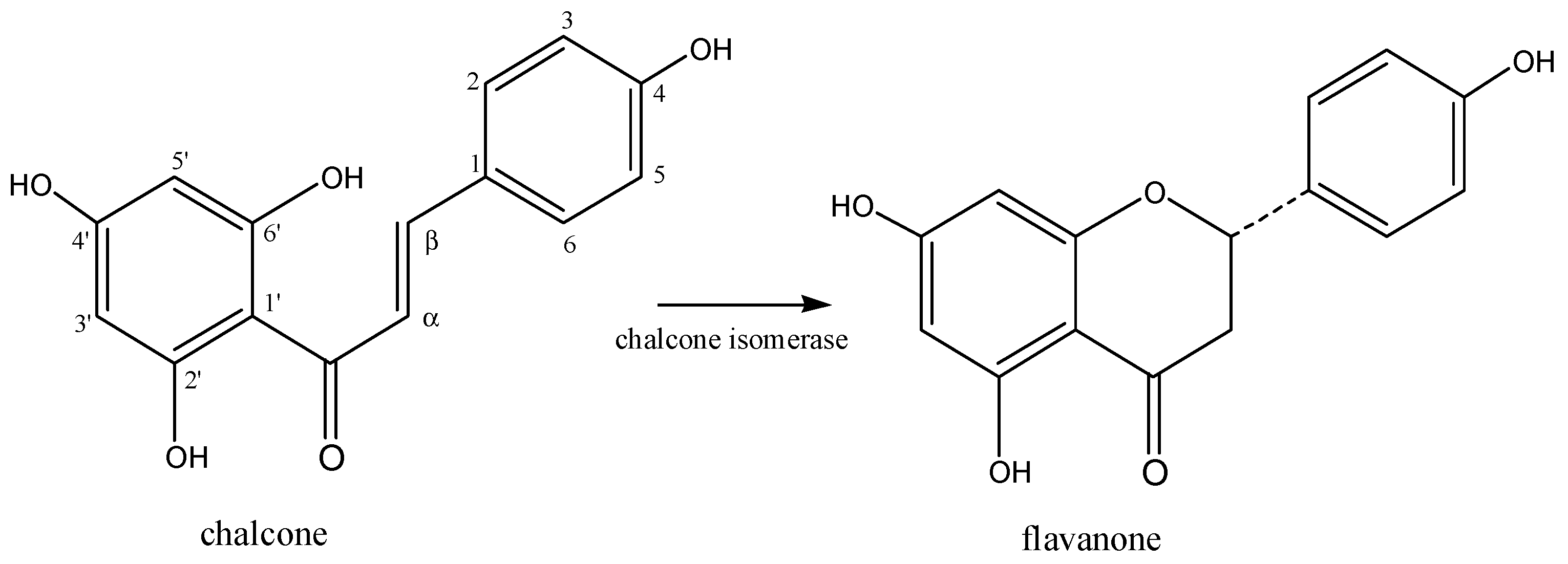
3.1. Biosynthesis
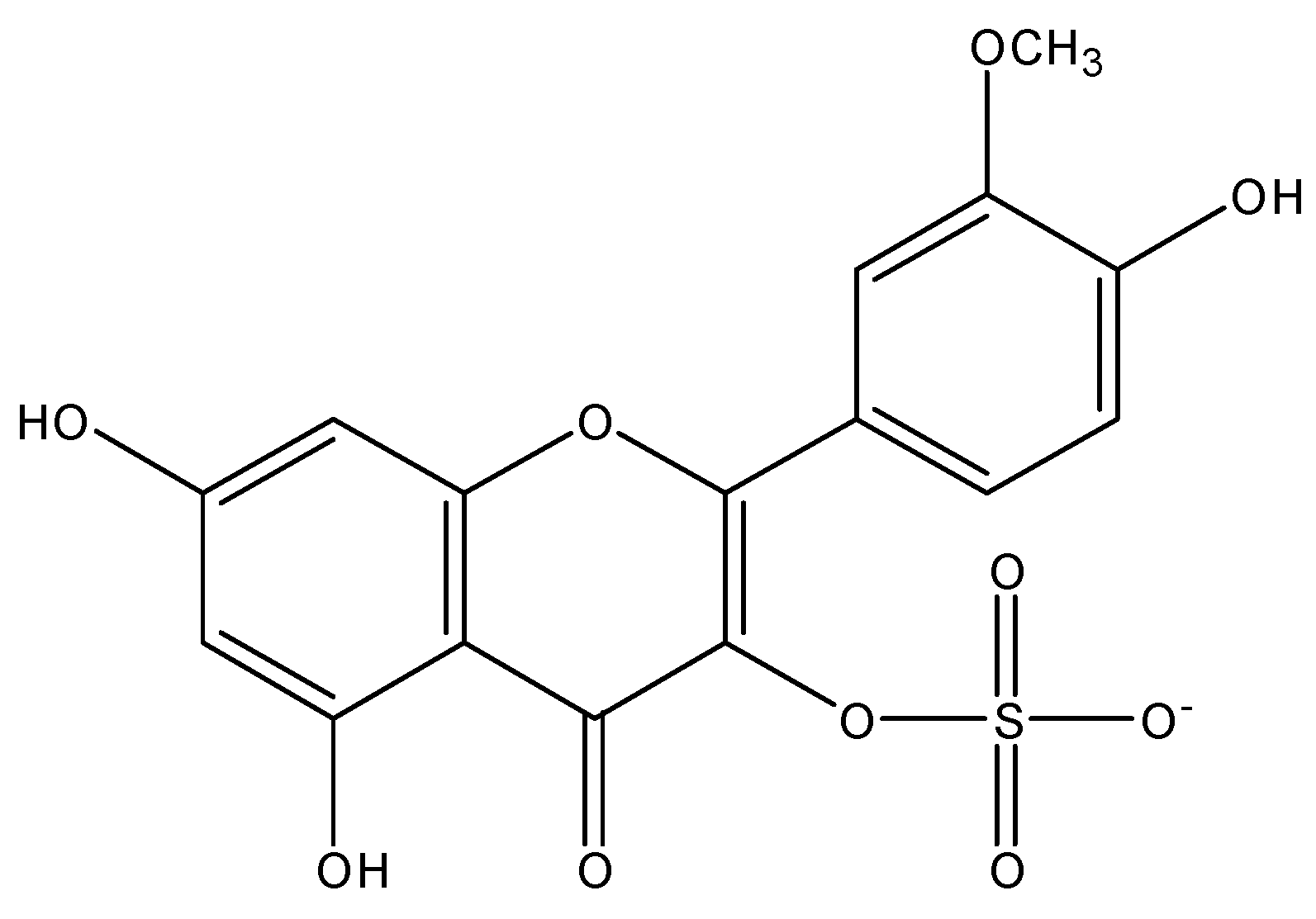
3.2. Chemical Structures
3.3. Biological Activities
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Weng, J.K.; Philippe, R.N.; Noel, J.P. The rise of chemodiversity in plants. Science 2012, 336, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Heiling, S.; Baldwin, I.T.; Gaquerel, E. Illuminating a plant’s tissue-specific metabolic diversity using computational metabolomics and information theory. Proc. Natl. Acad. Sci. USA 2016, 113, E7610–E7618. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.G.; Mahajan, V.; Bedi, Y.S. Changing trends in biotechnology of secondary metabolism in medicinal and aromatic plants. Planta 2015, 241, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Das, G.; Das, S.K. Roles of Flavonoids in Plants. Int. J. Pharm. Sci. Technol. 2011, 6, 12–35. [Google Scholar]
- Gledhill, J.R.; Montgomery, M.G.; Leslie, A.G.; Walker, J.E. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc. Natl. Acad. Sci. USA 2007, 104, 13632–13637. [Google Scholar] [CrossRef] [PubMed]
- Calzia, D.; Oneto, M.; Caicci, F.; Bianchini, P.; Ravera, S.; Bartolucci, M.; Diaspro, A.; Degan, P.; Manni, L.; Traverso, C.E.; et al. Effect of polyphenolic phytochemicals on ectopic oxidative phosphorylation in rod outer segments of bovine retina. Br. J. Pharmacol. 2015, 172, 3890–3903. [Google Scholar] [CrossRef] [PubMed]
- Teles, Y.C.F.; Horta, C.C.R.; Agra, M.F.; Siheri, W.; Boyd, M.; Igoli, J.O.; Gray, A.I.; de Souza, M.F.V. New Sulphated Flavonoids from Wissadula periplocifolia (L.) C. Presl (Malvaceae). Molecules 2015, 20, 20161–20172. [Google Scholar] [CrossRef] [PubMed]
- Barron, D.; Varin, L.; Ibrahim, R.K.; Harborne, J.B.; Williams, C.A. Sulphated Flavonoids—An update. Phytochemistry 1988, 27, 2375–2395. [Google Scholar] [CrossRef]
- Guglielmone, H.A.; Agnese, A.M.; Montoya, S.C.N.; Cabrera, J.L. Inhibitory effects of sulphated flavonoids isolated from Flaveria bidentis on platelet aggregation. Thromb. Res. 2005, 115, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Gurni, A.A.; König, W.A.; Kubitzki, K. Flavonoid glycosides and sulphates from the Dilleniaceae. Phytochemistry 1981, 20, 1057–1059. [Google Scholar] [CrossRef]
- Bohm, B.A.; Stuessy, T.F. Flavonoids of the Sunflower Family (Asteraceae); Springer Science & Business Media: Wien, Austria, 2001; pp. 116–119. ISBN 3-211-83479-6. [Google Scholar]
- Harborne, J.B. Flavonoid Sulphates: A new class of sulphur compounds in higher plants. Phytochemistry 1975, 14, 1147–1155. [Google Scholar] [CrossRef]
- Ananvoranich, S.; Varin, L.; Gulick, P.; Ibrahim, R.K. Cloning and regulation of flavonol 3-sulfotransferase in cell-suspension cultures of Flaveria bidentis. Plant Physiol. 1994, 106, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Gigolashvili, T.; Kopriva, S. Transporters in plant sulfur metabolism. Front. Plant Sci. 2014, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Enerstvedt, K.H.; Jordheim, M.; Andersen, Ø.M. Isolation and Identification of Flavonoids Found in Zostera marina Collected in Norwegian Coastal Waters. Am. J. Plant Sci. 2016, 7, 1163–1172. [Google Scholar] [CrossRef]
- Hell, R. Molecular physiology of plant sulfur metabolism. Planta 1997, 202, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Davidian, J.C.; Kopriva, S. Regulation of Sulphate Uptake and Assimilation—The Same or Not the Same? Mol. Plant 2010, 3, 314–325. [Google Scholar] [CrossRef] [PubMed]
- De Graffenried, C.L.; Bertozzi, C.R. Golgi localization of carbohydrate sulfotransferases is a determinant of l-selectin ligand biosynthesis. J. Biol. Chem. 2003, 278, 40282–40295. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, F.; Krause, F.; Papenbrock, J. The multi-protein family of sulfotransferases in plants: Composition, occurrence, substrate specificity, and functions. Front. Plant Sci. 2014, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Qiu, F.; Zhu, S.; Zhang, T.; Qu, G.; Yao, X. Isolation and identification of ten metabolites of breviscapine in rat urine. Biol. Pharm. Bull. 2007, 30, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Bylka, W.; Stobiecki, M.; Frahski, R. Sulphated flavonoid glycosides from leaves of Atriplex hortensis. Acta Physiol. Plant. 2001, 23, 285–290. [Google Scholar] [CrossRef]
- Dantuluri, M.; Gunnarsson, G.T.; Riaz, M.; Nguyen, H.; Desai, U.R. Capillary electrophoresis of highly sulphated flavanoids and flavonoids. Anal. Biochem. 2005, 336, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Varin, L. Flavonoid Sulfation: Phytochemistry, Enzymology and Molecular Biology. In Phenolic Metabolism in Plants; Stafford, H.A., Ibrahim, R.K., Eds.; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Lima, C.C.; Lemos, R.P.L.; Conserva, L.M. Dilleniaceae family: An overview of its ethnomedicinal uses, biological and phytochemical profile. J. Pharmacogn. Phytochem. 2014, 3, 181–204. [Google Scholar]
- Reinhold, L.; Harborne, J.B.; Swain, T. Progress in Phytochemistry; Pergamon Press: New York, NY, USA, 1977; Volume 4, pp. 189–208. ISBN 9781483144429. [Google Scholar]
- Gadetskaya, A.V.; Tarawneh, A.H.; Zhusupova, G.E.; Gemejiyeva, N.G.; Cantrell, C.L.; Cutler, S.J.; Ross, S.A. Sulfated phenolic compounds from Limonium caspium: Isolation, structural elucidation, and biological evaluation. Fitoterapia 2015, 104, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Pardini, M.; Morelli, I. A flavonoid sulphate and other compounds from the roots of Centaurea bracteata. Phytochemistry 2001, 58, 1229–1233. [Google Scholar] [CrossRef]
- Harborne, J.B.; Mabry, T.J. The Flavonoids: Advances in Research; Springer Science & Business Media: London, UK, 1982; pp. 274–295. ISBN 978-0-412-22480-5. [Google Scholar]
- Nawwar, M.; Buddrus, J. A gossypetin glucuronide sulphate from the leaves of Malva sylvestris. Phytochemistry 1981, 20, 2446–2448. [Google Scholar] [CrossRef]
- Paul, D. A review on biological activities of common Mallow (Malva sylvestris L.). J. Life Sci. 2016, 4, 1–5. [Google Scholar]
- Tomás-Barberán, F.; Harborne, J.B.; Self, R. Twelve 6-Oxygenated-Flavone Sulphates from Lippia nodiflora and L. canescens. Phytochemistry 1987, 26, 2281–2284. [Google Scholar] [CrossRef]
- Buchholz, H.; Wirth, C.; Carola, C.; Alves Fontes, R. Flavonoid Derivative. US Patent 20,070,134,172A1, 14 July 2007. [Google Scholar]
- Habbu, P.V.; Mahadevan, K.M.; Shastry, R.A.; Manjunatha, H. Antimicrobial activity of flavanoid sulphates and other fractions of Argyreia speciosa (Burm.F) Boj. Indian J. Exp. Biol. 2009, 47, 121–128. [Google Scholar] [PubMed]
- El-Sayed, N.H.; Norris, J.A.; Ahmed, A.A.; Mabry, T.J. Flavonoids of Brickellia longifolia. Phytochemistry 1990, 29, 2364–2365. [Google Scholar] [CrossRef]
- Karker, M.; De Tommasi, N.; Smaoui, A.; Abdelly, C.; Ksouri, R.; Braca, A. New Sulphated Flavonoids from Tamarix africana and Biological Activities of Its Polar Extract. Planta Med. 2016, 82, 374–1380. [Google Scholar] [CrossRef] [PubMed]
- Seabra, R.M.; Alves, A.C. Quercetin-3-sulphate from Hypericum elodes. Phytochemistry 1991, 30, 1344–1345. [Google Scholar] [CrossRef]
- Massi, A.; Bortolini, O.; Ragno, D.; Bernardi, T.; Sacchetti, G.; Tacchini, M.; De Risi, C. Research Progress in the Modification of Quercetin Leading to Anticancer Agents. Molecules 2017, 22, 1270–1297. [Google Scholar] [CrossRef] [PubMed]
- Barron, D.; Ibrahim, R.K. Ombuin 3-sulphate from Flaveria chloraefolia. Phytochemistry 1988, 27, 2362–2363. [Google Scholar] [CrossRef]
- Grignon-Dubois, M.; Rezzonico, B. First Phytochemical Evidence of Chemotypes for the Seagrass Zostera noltii. Plants 2012, 1, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Correia-da-Silva, M.; Sousa, E.; Pinto, M.M. Emerging sulfated flavonoids and other polyphenols as drugs: Nature as an inspiration. Med. Res. Rev. 2014, 34, 223–279. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, G.T.; Desai, U.R. Interaction of designed sulfated flavanoids with antithrombin: Lessons on the design of organic activators. J. Med. Chem. 2002, 45, 4460–4470. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Kim, T.H.; Bae, J.S. Anticoagulant activities of persicarin and isorhamnetin. Vasc. Pharmacol. 2013, 58, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liang, N.C. Inhibitory effect of disodium quercetin-7,4′-disulfate on aggregation of pig platelets induced by thrombin and its mechanism. Acta Pharmacol. Sin. 2000, 21, 737–741. [Google Scholar] [PubMed]
- Guglielmone, H.A.; Agnese, A.M.; Nunez Montoya, S.C.; Cabrera, J.L. Anticoagulant effect and action mechanism of sulphated flavonoids from Flaveria bidentis. Thromb. Res. 2002, 105, 183–188. [Google Scholar] [CrossRef]
- Op de Beck, P.; Cartier, G.; David, B.; Dijoux-Franca, M.G.; Mariotte, A.M. Antioxidant flavonoids and phenolic acids from leaves of Leea guineense G Don (Leeaceae). Phytother. Res. 2003, 17, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Yagi, A.; Uemura, T.; Okamura, N.; Hiraguchi, H.; Imoto, T.; Hashimoto, K. Antioxidative sulphated flavonoids in leaves of Polygonum hydropiper. Phytochemistry 1994, 34, 884–887. [Google Scholar] [CrossRef]
- Haraguchi, H.; Ohmi, I.; Sakai, S.; Fukuda, A.; Toihara, Y.; Fujimoto, T.; Okamura, N.; Yagi, A. Effect of Polygonum hydropiper sulfated flavonoids on lens aldose reductase and related enzymes. J. Nat. Prod. 1996, 59, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Teresa, S.; Johnston, K.L.; DuPont, M.S.; O’Leary, K.A.; Needs, P.W.; Morgan, L.M.; Clifford, M.N.; Bao, Y.; Williamson, G. Quercetin metabolites down regulate cyclooxygenase-2 transcription inhuman lymphocytes ex vivo but not in vivo. J. Nutr. 2004, 134, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, V.; Estevez Braun, A.; Gutierrez, R.A.; Murray, A.P. Sulfated flavonoid isolated from Flaveria bidentis and its semisynthetic derivatives as potential drugs for Alzheimer’s disease. In Proceedings of the 17th International Electronic Conference on Synthetic Organic Chemistry, Basel, Switzerland, 1–30 November 2013. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Seok, J.K.; Suh, H.J.; Choi, Y.H.; Hong, S.S.; Kim, D.S.; Boo, Y.C. Antimelanogenic effects of luteolin 7-sulfate isolated from Phyllospadix iwatensis Makino. Br. J. Dermatol. 2016, 175, 501–511. [Google Scholar] [CrossRef] [PubMed]

| Sulphated Flavonoids | Species Name and Family | Reference |
|---|---|---|
| Acacetin 7-sulphate | Wissadula periplocifolia (Malvaceae) | [9] |
| Apigenin 7-sulphate | Tetracera mandagascariensis (Dilleniaceae) Bixa orllana (Bixaceae) | [12,27,28] |
| Ampelopsin (dihydromyricetin)-3′-sulphate | Limonium caspium (Plumbaginaceae) | [29] |
| Axillarin 7-sulphate | Centaurea bracteata (Asteraceae) | [30] |
| Chrysoeriol 7-sulphate | Zostera marina (Zosteraceae) | [31] |
| Eupatin 3-sulphate | Brickellia californica, B. laciniata (Asteraceae) | [31] |
| Gossypetin 3-sulphate | Malva sylvestris (Malvaceae) | [32] |
| Gossypetin 8-o-β-d-glucuronide-3-sulphate | Malva sylvestris (Malvaceae) | [32] |
| Hypoaletin 3′-sulphate | Malva sylvestris (Malvaceae) | [33] |
| Hypoaletin 8-sulphate | Bixa orllana (Bixaceae) | [28] |
| Hypolaetin 3′-methyl ether 8-sulphate | Wissadula periplocifolia (Malvaceae) | [9] |
| Hispidulin 7-sulphate | Lippia nodiflora (Verbenaceae) | [34] |
| Hispidulin 4′-sulphate | Lippia nodiflora (Verbenaceae) | [34] |
| Hispidulin 7,4′-disulphate | Lippia nodiflora (Verbenaceae) | [34] |
| Isoscutellarein 8-sulphate | Wissadula periplocifolia (Malvaceae) | [9] |
| Isorhamnetin 3-sulphate | Senecio galicus (Asteraceae); Polygonum hydropiper (Polygoniaceae) | [13,14] |
| Isorhamnetin 7-sulphate | Frankenia pulverulenta (Frankeniaceae) | [31] |
| Isorhamnetin 3,7-disulphate | Flaveria bidentis (Asteraceae) | [10,31] |
| Isorhamnetin 3-glucoronide 7-sulphate | Frankenia pulverulenta (Frankeniaceae) | [10,31] |
| Isorhamnetin 3,7,4′-trisulphate | Acrotrema uniflorum (Dilleniaceae) | [31] |
| Isoscutellarein 4′-methyl ether 7-sulphate | Wissadula periplocifolia (Malvaceae) | [9] |
| Isoscutellarein 4′-methyl ether-8-sulphate | Wissadula periplocifolia (Malvaceae) | [9] |
| Isoscutellarein 7,4′-dimethyl ether 8-sulphate | Wissadula periplocifolia (L.), Sidastrum micranthum (Malvaceae) | [9,35] |
| Isovitexina 7-sulphate | Phoenix roebelenii (Arecaceae) | [28] |
| Isorientin 7-sulphate | Phoenix roebelenii (Arecaceae) | [28] |
| Jaceosidin 7-sulphate | Lippia nodiflora (Verbenaceae) | [34] |
| Jaceosidin 7,4′-disulphate | Lippia nodiflora (Verbenaceae) | [34] |
| Kaempferol 3-sulphate | Dillenia bracteata, D. triquetra, Schumacheria casteinifolia, Tetracera alnifolia, T. boiviniana, T. breyniana, T. costata, T. oblongata, T. rasiflora, T. rutenbergii, T. volubilis, T. willdenowiana (Dilleniaceae) | [12,31] |
| Kaempferol 7-sulphate | F. pulverulenta (Frankeniaceae) | [31] |
| Kaempferol 3,7-disulphate | Reamuria mucronata, R. vermiculata (Tamaricaceae); Dillenia bracteata, Schumacheria castaneifolia (Dilleniaceae) | [27,31] |
| Kaempferol 3,7,4′-trisulphate | Acrotrema uniflorum (Dilleniaceae) | [27] |
| Kaempferol 7-methyl ether 3-sulphate | Ammi visnaga (Umbeliferaceae); Acrotrema uniflorum, Tetracera alnifolia, T. puggei, T. rosiflora, T. rutenbergii (Dilleniaceae); Argyreia speciosa (Convolvulaceae) | [12,31,36] |
| Kaempferol 7,4′-dimethyl ether 3-sulphate | Tamarix apphyla, T. nilotica (Tamaricaceae) | [30,37] |
| Kaempferol 6,7,4′-trimethyl ether 3-sulphate | Brickellia longifolia (Asteraceae) | [31,36] |
| Kaempferol 3-glucoronide 7-sulphate | Frankenia pulverulenta (Frankeniaceae) | [27] |
| Kaempferol 3-sulphate 7-o-α-arabinopyranoside | Atriplex hortensis (Chenopodiaceae) | [24] |
| Luteolin 7-sulphate | Tetracera stuhimanniana (Dilleniaceae) Bixa orllana (Bixaceae) | [28,31] |
| Luteolin 4′-sulphate | Daucus carota (Umbelliferae) | [28] |
| Luteolin 3′-sulphate | Lachenalia unifolia (Hyacinthaceae) | [28] |
| Luteolin 7,3′-disulphate | Zostera marina (Zosteraceae) | [28] |
| Luteolin 7-sulphate 3′-glucoside | Mascarena verscafeltii (Arecaceae) | [28] |
| Luteolin 7-sulphate 3′-rutinoside | Zostera marina (Zosteraceae); Mascarena verschaffeltii, Opsiandra maya (Arecaceae) | [28,30] |
| Luteolin 4′-methyl ether (diosmetin) 7-sulphate | Zostera marina, Z. nana (Zosteraceae) | [28] |
| Luteolin 4′-methyl ether (diosmetin) 7,3′-disulphate | Lachenalia unifolia (Hyacinthaceae) | [28] |
| Luteolin 6-hydroxy 7-sulphate | Lippia nodiflora (Verbenaceae) | [34] |
| Luteolin 6-hydroxy 6-sulphate | Lippia nodiflora (Verbenaceae) | [34] |
| Luteolin 6-hydroxy 6,7-disulphate | Lippia nodiflora (Verbenaceae) | [34] |
| Luteolin 7-sulphate 8-C-glucoside | Phoenix roebelenii (Arecaceae) | [28] |
| Myricetin-3′-sulphate | Limonium caspium (Plumbaginaceae) | [28] |
| (2S,3S)-5-Methyldihydromyricetin-3′-sulphate β-d-glucopyranoside | Limonium caspium (Plumbaginaceae) | [28] |
| (2S)-Naringenin 4′-o-sulphate | Tamarix africana (Tamaricaceae) | [38] |
| Nepetin 7-sulphate | Lippia nodiflora (Verbenaceae) | [34] |
| Nepetin 3′,4′-sulphate | Lippia nodiflora (Verbenaceae) | [34] |
| Nodifloretin 7-sulphate | Lippia nodiflora (Verbenaceae) | [34] |
| Nodifloretin 6,7-disulphate | Lippia nodiflora (Verbenaceae) | [34] |
| Patuletin 3-sulphate | Brickellia californica (Asteraceae) | [10] |
| Patuletin 7-sulphate | Lasthenia conjugens, L. fremontii (Asteraceae) | [28] |
| Patuletin 7-sulphate 3-Glucoside | Lasthenia conjugens, L. fremontii (Asteraceae) | [28] |
| Quercetin 3-sulphate | Acrotrema uniflorum, Dillenia bracteata, D. triquetra, Schumacheria angustifólia, S. casteinifolia, Tetracera alnifolia, T. boiviniana, T. breyniana, T. costata, T. madagascariensis, T. masuiana, T. oblongata, T. rasiflora, T. rutenbergii, T. sarmentosa, T. sellowiana, T. tigara, T. volubilis, T. willdenowiana (Dilleniaceae); Hypericum elodes (Guttiferae); Oenanthe crocata (Umbelliferae) | [12,28,34,39] |
| Quercetin 3,4′-disulphate | Flaveria bidentis (Asteraceae) | [13] |
| Quercetin 3,7-disulphate | Flaveria bidentis (Asteraceae) | [13] |
| Quercetin 3,7,4′-trisulphate | Flaveria bidentis (Asteraceae) | [13] |
| Quercetin 3,7,3′-trisulphate | Flaveria bidentis (Asteraceae) | [13] |
| Quercetin 3,3′,4′,7-tetrasulphate | Flaveria bidentis (Asteraceae) | [40] |
| Quercetin 7-methyl ether 3-sulphate | Ammi visnaga (Umbeliferaceae) | [31] |
| Quercetin 4′-methyl ether 3,7-disulphate | Reaumuria vermiculata (Tamaricaceae) | [37] |
| Quercetin 7-methyl ether 3,5,4′-trisulphate | Tamarix apphyla (Tamaricaceae) | [37] |
| Quercetin 7,4′-dimethyl ether 3-sulphate | Flaveria chloraefolia (Asteraceae) | [41] |
| Quercetin 7,3′dimethyl ether 3-sulphate | Argyreia speciosa (Convolvulaceae) | [36] |
| Quercetin 7,4′-dimethyl ether 3,3′-disulphate | Acrotrema uniflorum (Dilleniaceae) | [10] |
| Quercetin 3-acetyl-7,3′,4′-trisulphate | Flaveria bidentis (Asteraceae) | [11] |
| Quercetin 3-sulphate 7-o-α-arabinopyranoside | Atriplex hortensis (Chenopodiaceae) | [24] |
| Quercetagetin 3-methyl ether 7-sulphate | Neuroleana oaxacana (Asteraceae) | [13] |
| Quercetagetin 6,7-dimethyl ether 3-sulphate | Brickellia veronikaefolia (Asteraceae) | [13] |
| Quercetagetin 6,7,3′-trimethyl ether 3-sulphate | Brickellia californica (Asteraceae) | [13] |
| Quercetagetin 6,7,4′-trimethyl ether 3-sulphate | Brickellia longifolia (Asteraceae) | [13] |
| Tricetin 3′-sulphate | Lachenalia unifolia (Hyacinthaceae) | [28] |
| Tricetin 7,3′-disulphate | Lachenalia unifolia (Hyacinthaceae) | [28] |
| (2S,4R)-5,7,4′-Trihydroxyflavan-4-ol 5,7-disulphate | Tamarix africana (Tamaricaceae) | [38] |
| (2S)-5,7,4′-Trihydroxyflavan 7-o-sulphate | Tamarix africana (Tamaricaceae) | [38] |
| Vitexina 7-sulphate | Washingtonia robusta (Arecaceae) | [28] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teles, Y.C.F.; Souza, M.S.R.; Souza, M.D.F.V.d. Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities. Molecules 2018, 23, 480. https://doi.org/10.3390/molecules23020480
Teles YCF, Souza MSR, Souza MDFVd. Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities. Molecules. 2018; 23(2):480. https://doi.org/10.3390/molecules23020480
Chicago/Turabian StyleTeles, Yanna C. F., Maria Sallett R. Souza, and Maria De Fátima Vanderlei de Souza. 2018. "Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities" Molecules 23, no. 2: 480. https://doi.org/10.3390/molecules23020480
APA StyleTeles, Y. C. F., Souza, M. S. R., & Souza, M. D. F. V. d. (2018). Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities. Molecules, 23(2), 480. https://doi.org/10.3390/molecules23020480





