Identification and Characterization of Phenylpropanoid Biosynthetic Genes and Their Accumulation in Bitter Melon (Momordica charantia)
Abstract
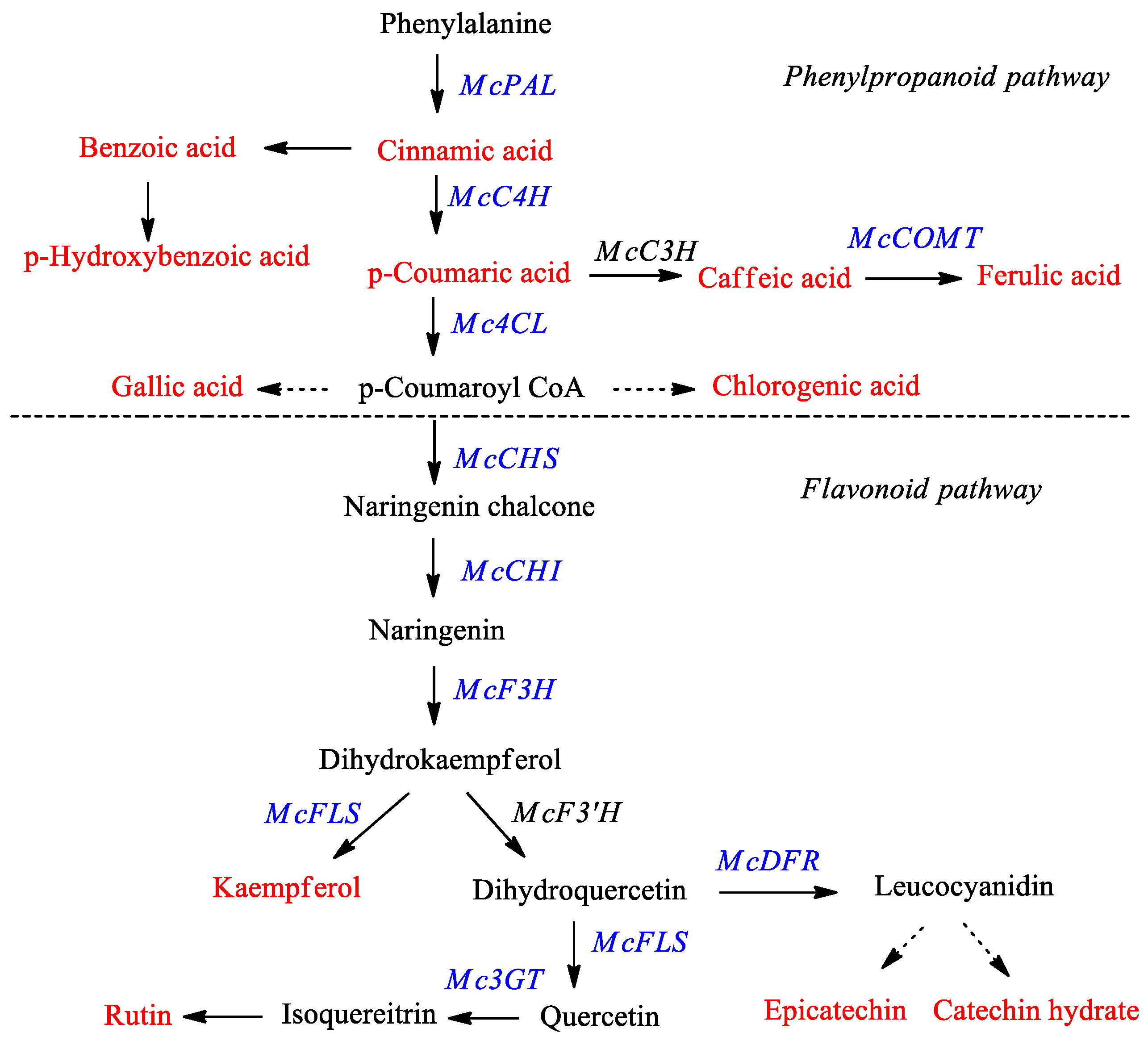
1. Introduction
2. Results and Discussion
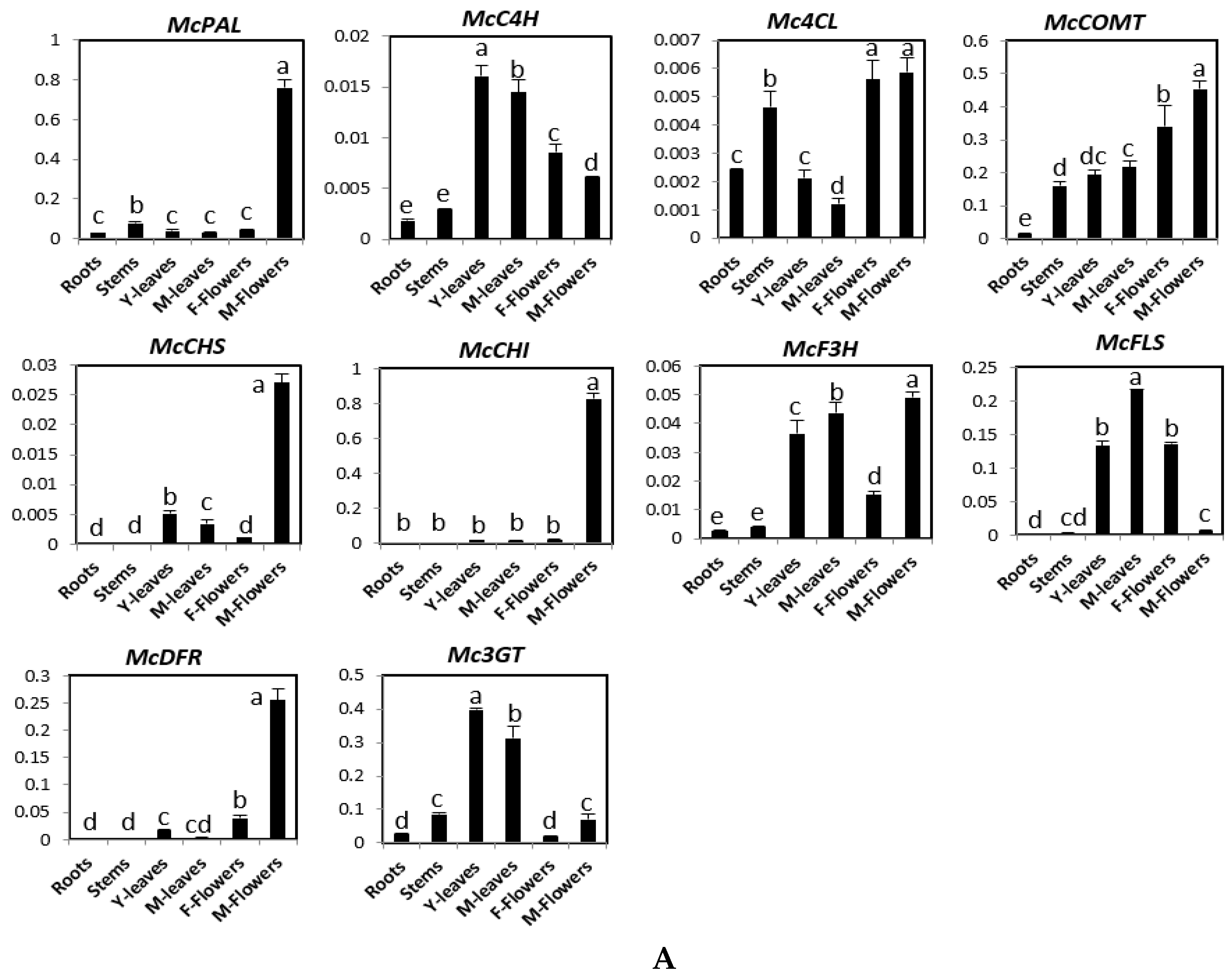
2.1. Expression of Phenylpropanoid and Flavonoid Genes in Different Organs of Bitter Melon
2.2. Phenylpropanoid and Flavonoid Accumulation in Different Organs of Bitter Melon
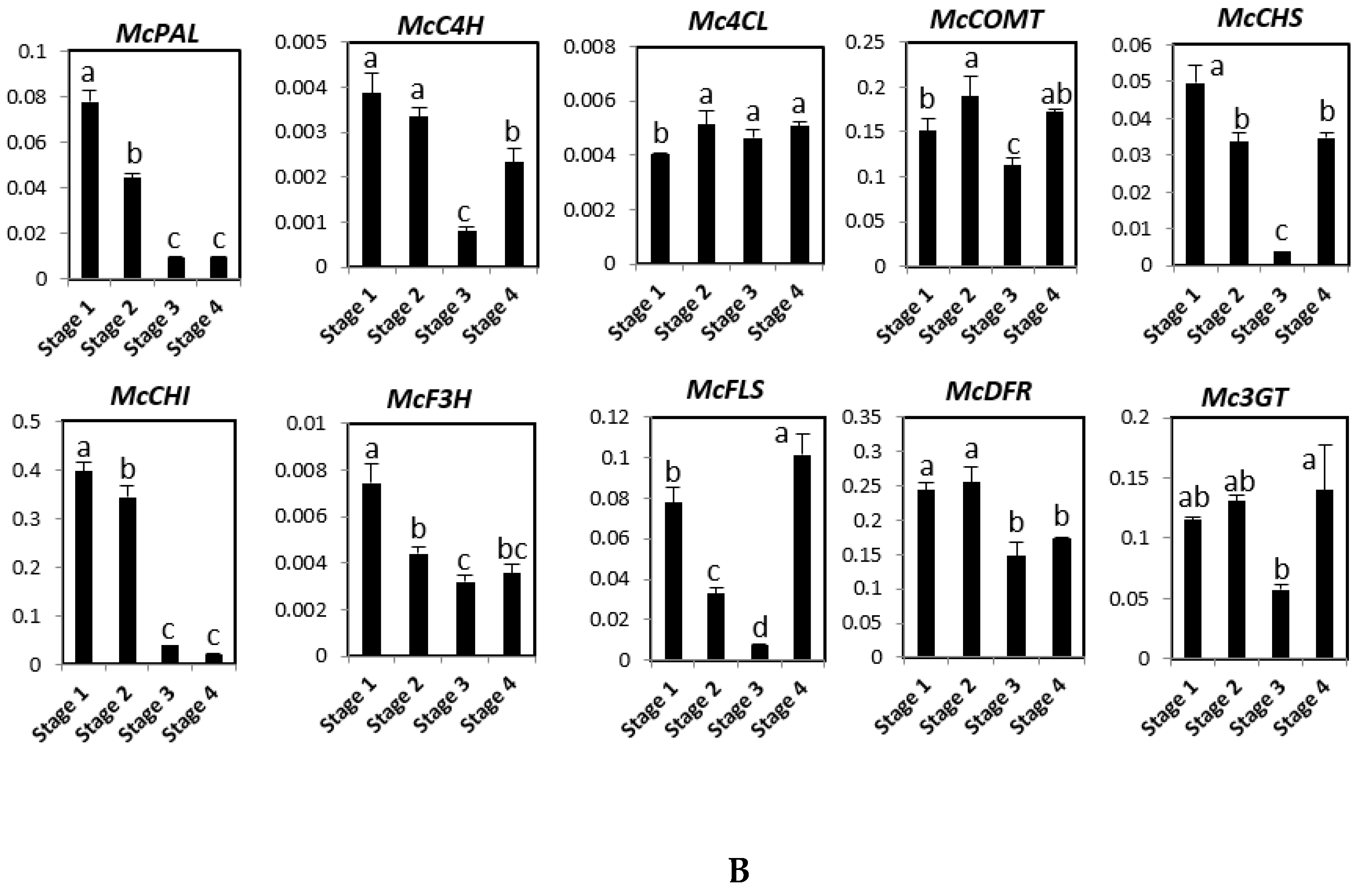
2.3. Expression of Phenylpropanoid and Flavonoid Genes in Different Developmental Stages of Bitter Melon Plantlets
2.4. Phenylpropanoid and Flavonoid Accumulation in Different Developmental Stages of Bitter Melon Plantlets
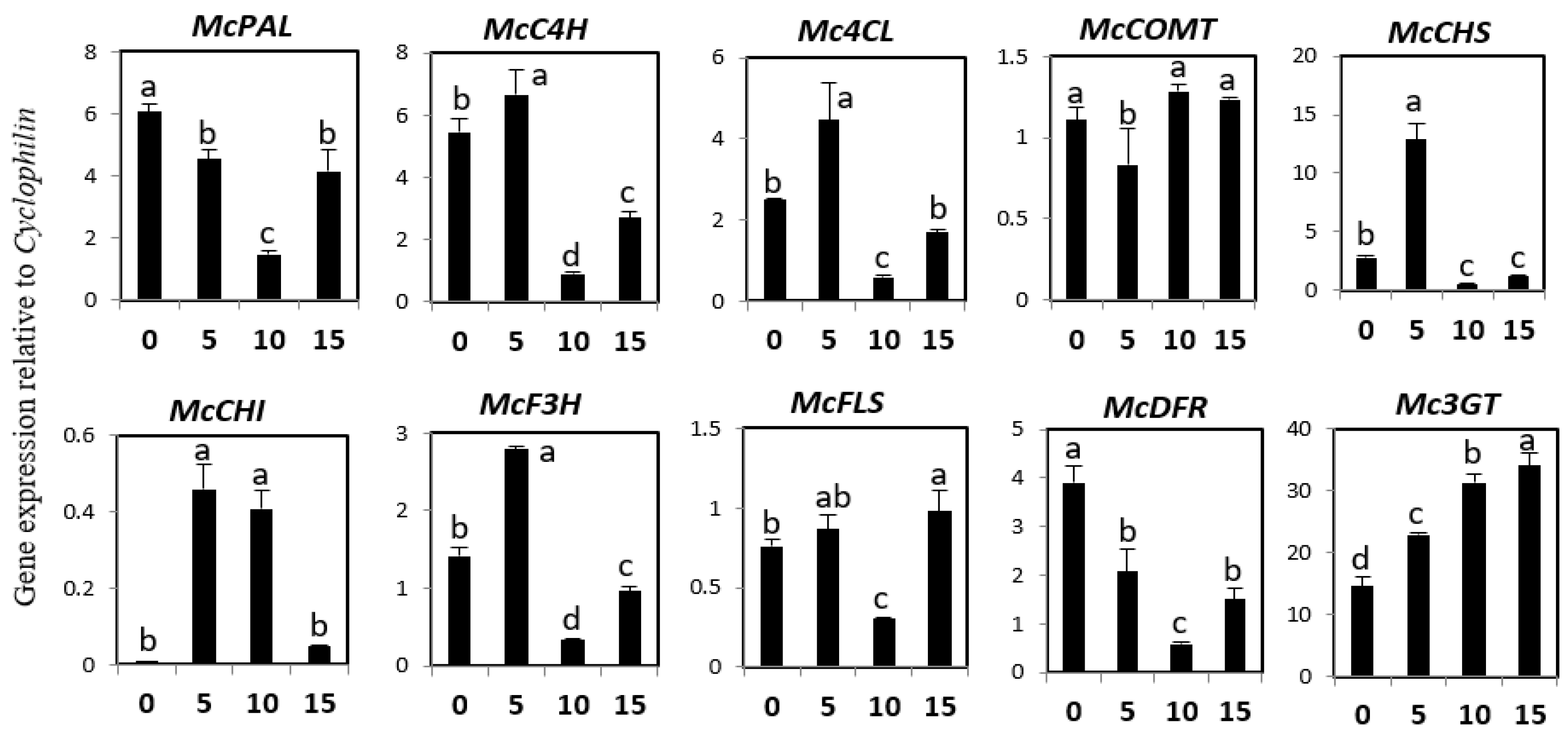
2.5. Effects of White, Blue, and Red Lights on the Expression of Phenylpropanoid and Flavonoid Genes
2.6. Effects of White, Blue, and Red Lights on Phenylpropanoid and Flavonoid Content
3. Material and Methods
3.1. Plant Materials
3.2. RNA Isolation and cDNA Synthesis
3.3. Quantitative Real-Time PCR Analysis
3.4. High Performance Liquid Chromatography (HPLC) Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Meng, Y.; Liu, S.; Li, J.; Meng, Y.; Zhao, X. Preparation of an antitumor and antivirus agent: Chemical modification of alpha-mmc and map30 from Momordica charantia L. with covalent conjugation of polyethyelene glycol. Int. J. Nanomed. 2012, 7, 3133–3142. [Google Scholar] [PubMed]
- Fang, E.F.; Ng, T.B. Bitter gourd (Momordica charantia) is a cornucopia of health: A review of its credited antidiabetic, anti-HIV, and antitumor properties. Curr. Mol. Med. 2011, 11, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Tahira, S.; Hussain, F. Antidiabetic evaluation of Momordica charantia L. fruit extracts. West Indian Med. J. 2014, 63, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Kaur, I.; Kanwar, R.K.; Gupta, R.C.; Chauhan, A.; Kanwar, J.R. Ribosome inactivating proteins (RIPs) from Momordica charantia for antiviral therapy. Curr. Mol. Med. 2009, 9, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.R.; Nabi, M.M.; Nurujjaman, M.; Abu Reza, M.; Alam, A.H.; Uz Zaman, R.; Khalid-Bin-Ferdaus, K.M.; Amin, R.; Khan, M.M.; Hossain, M.A.; et al. Momordica charantia seed lectin: Toxicity, bacterial agglutination and antitumor properties. Appl. Biochem. Biotechnol. 2015, 175, 2616–2628. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, I.; Akyuz, C.; Yesilada, E.; Sener, B. Anti-ulcerogenic effect of Momordica charantia L. fruits on various ulcer models in rats. J. Ethnopharmacol. 2000, 71, 77–82. [Google Scholar] [CrossRef]
- Saeed, M.K.; Shahzadi, I.; Ahmad, I.; Ahma, R.; Shahzad, K.; Ashraf, M.; Viqar-un-Nisa, A. Nutritional analysis and antioxidant activity of bitter gourd (Momordica charantia L.) from Pakistan. Pharmacologyonline 2010, 1, 252–260. [Google Scholar]
- Korkina, L.G. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell. Mol. Biol. 2007, 53, 11. [Google Scholar]
- Castelluccio, C.; Paganga, G.; Melikian, N.; Bolwell, G.P.; Pridham, J.; Sampson, J.; Riceevans, C. Antioxidant potential of intermediates in phenylpropanoid metabolism in higher-plants. FEBS Lett. 1995, 368, 188–192. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant. Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Il Park, N.; Xu, H.; Woo, S.H.; Park, C.H.; Park, S.U. Differential expression of flavonoid biosynthesis genes and accumulation of phenolic compounds in common buckwheat (Fagopyrum esculentum). J. Agric. Food Chem. 2010, 58, 12176–12181. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, J.K.; Park, S.Y.; Zhao, S.; Kim, Y.B.; Lee, S.; Park, S.U. Comparative analysis of flavonoids and polar metabolite profiling of tanno-original and tanno-high rutin buckwheat. J. Agric. Food Chem. 2014, 62, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Ghimeray, A.K.; Sharma, P.; Phoutaxay, P.; Salitxay, T.; Woo, S.H.; Park, S.U.; Park, C.H. Far infrared irradiation alters total polyphenol, total flavonoid, antioxidant property and quercetin production in tartary buckwheat sprout powder. J. Cereal Sci. 2014, 59, 167–172. [Google Scholar] [CrossRef]
- Lee, L.S.; Choi, E.J.; Kim, C.H.; Sung, J.M.; Kim, Y.B.; Seo, D.H.; Choi, H.W.; Choi, Y.S.; Kum, J.S.; Park, J.D. Contribution of flavonoids to the antioxidant properties of common and tartary buckwheat. J. Cereal Sci. 2016, 68, 181–186. [Google Scholar] [CrossRef]
- Thwe, A.A.; Kim, J.K.; Li, X.H.; Kim, Y.B.; Uddin, M.R.; Kim, S.J.; Suzuki, T.; Park, N.I.; Park, S.U. Metabolomic analysis and phenylpropanoid biosynthesis in hairy root culture of tartary buckwheat cultivars. PLoS ONE 2013, 8, e65349. [Google Scholar] [CrossRef]
- Park, D.; Park, Y.; Lee, Y.H.; Choi, I.Y.; Park, K.C.; Park, S.U.; Kim, B.S.; Yeoung, Y.R.; Park, N.I. A comparative study of phenolic antioxidant activity and flavonoid biosynthesis-related gene expression between summer and winter strawberry cultivars. J. Food Sci. 2017, 82, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Park, C.H.; Li, X.; Kim, Y.B.; Yang, J.; Sung, G.B.; Park, N.I.; Kim, S.; Park, S.U. Accumulation of rutin and betulinic acid and expression of phenylpropanoid and triterpenoid biosynthetic genes in mulberry (Morus alba L.). J. Agric. Food Chem. 2015, 63, 8622–8630. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, Y.S.; Kim, Y.; Uddin, M.R.; Kim, Y.B.; Kim, H.H.; Park, S.Y.; Lee, M.Y.; Chung, S.O.; Park, S. Comparative analysis of flavonoids and polar metabolites from hairy roots of Scutellaria baicalensis and Scutellaria lateriflora. World J. Microbiol. Biotechnol. 2014, 30, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, K.K.; Han, Q.B.; Ko, J.K. Astragalus membranaceus: A review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Thwe, A.A.; Li, X.H.; Kim, Y.J.; Kim, J.K.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Triterpene and flavonoid biosynthesis and metabolic profiling of hairy roots, adventitious roots, and seedling roots of Astragalus membranaceus. J. Agric. Food Chem. 2015, 63, 8862–8869. [Google Scholar] [CrossRef] [PubMed]
- Horax, R.; Hettiarachchy, N.; Islam, S. Total phenolic contents and phenolic acid constituents in 4 varieties of bitter melons (Momordica charantia) and antioxidant activities of their extracts. J. Food Sci. 2005, 70, C275–C280. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to led lighting. HortScience 2008, 43, 1951–1956. [Google Scholar]
- Morrow, R.C. Led lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar]
- Hossen, M.Z. Light emitting diodes increase phenolics of buckwheat (Fagopyrum esculentum) sprouts. J. Plant Interact. 2007, 2, 71–78. [Google Scholar] [CrossRef]
- Thwe, A.A.; Kim, Y.B.; Li, X.; Seo, J.M.; Kim, S.J.; Suzuki, T.; Chung, S.O.; Park, S.U. Effects of light-emitting diodes on expression of phenylpropanoid biosynthetic genes and accumulation of phenylpropanoids in Fagopyrum tataricum sprouts. J. Agric. Food Chem. 2014, 62, 4839–4845. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, Y.B.; Li, X.; Choi, S.R.; Park, S.; Park, J.S.; Lim, Y.P.; Park, S.U. Accumulation of phenylpropanoids by white, blue, and red light irradiation and their organ-specific distribution in chinese cabbage (Brassica rapa ssp. Pekinensis). J. Agric. Food Chem. 2015, 63, 6772–6778. [Google Scholar] [CrossRef] [PubMed]
- Cuong, D.M.; Jeon, J.; Morgan, A.M.A.; Kim, C.; Kim, J.K.; Lee, S.Y.; Park, S.U. Accumulation of charantin and expression of triterpenoid biosynthesis genes in bitter melon (Momordica charantia). J. Agric. Food Chem. 2017, 65, 7240–7249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Tuan, P.A.; Li, X.; Kim, Y.B.; Kim, H.; Park, C.G.; Yang, J.; Li, C.H.; Park, S.U. Identification of phenylpropanoid biosynthetic genes and phenylpropanoid accumulation by transcriptome analysis of Lycium chinense. BMC Genomics. 2013, 14, 802. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Park, N.I.; Xu, H.; Woo, S.H.; Park, C.H.; Park, S.U. Differential expression of flavonoid biosynthesis genes and accumulation of phenolic compounds in common buckwheat (Fagopyrum esculentum). J. Agric. Food Chem. 2010, 58, 12176–12181. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–479. [Google Scholar]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the phenolic compounds are available from the authors. |


| Genes | Description | Orthologous Genes | Identity (%) |
|---|---|---|---|
| McPAL (416 aa) | Phenylalanine ammonia-lyase | Cucumis sativus (XP_004145752.1) | 91 |
| Cucumis melo (CAA53733.1) | 88 | ||
| Vitis vinifera (XP_002285277.1) | 87 | ||
| McC4H (502 aa) | Cinnamate 4-hydroxylase | Cucumis sativus (XP_004151467.1) | 89 |
| Cucumis melo (XP_008457287.1) | 89 | ||
| Salvia miltiorrhiza (ABC75596.1) | 83 | ||
| Mc4CL (556 aa) | 4-Coumaroyl CoA ligase | Cucumis sativus (XP_004145532.1) | 88 |
| Cucumis melo (XP_008452936.1) | 87 | ||
| Ziziphus jujuba (XP_015884135.1) | 77 | ||
| McCOMT (369 aa) | Caffeic acid 3-O-methyltransferase | Ricinus communis (XP_002525818.1) | 81 |
| Ziziphus jujuba (XP_015878697.1) | 81 | ||
| Prunus mume (XP_008234634.1) | 82 | ||
| McCHS (402 aa) | Chalcone synthase | Siraitia grosvenorii (ADF57184.1) | 92 |
| Cucumis sativus (XP_004145707.1) | 91 | ||
| Cucumis melo (XP_008449984.1) | 92 | ||
| McCHI (252 aa) | Chalcone isomerase | Cucumis melo (XP_008456952.1) | 86 |
| Cucumis sativus (XP_011655047.1) | 86 | ||
| Ricinus communis (XP_002520870.2) | 73 | ||
| McF3H (370 aa) | Flavanone 3-hydroxylase | Nierembergia sp. NB17 (BAC10996.1) | 81 |
| Hibiscus sabdariffa (ALB35017.1) | 86 | ||
| Litchi chinensis (ADO95201.1) | 81 | ||
| McFLS (335 aa) | Flavonol synthase | Cucumis melo (XP_008445671.1) | 85 |
| Cucumis sativus (XP_004140862.1) | 84 | ||
| Theobroma cacao (XP_007018993.1) | 76 | ||
| McDFR (310 aa) | Dihydroflavonol 4-reductase | Cucumis sativus (XP_011650548.1) | 78 |
| Cucumis melo (XP_008452320.1) | 78 | ||
| Cucumis melo (XP_008452321.1) | 78 | ||
| Mc3GT (462 aa) | Flavonoid 3-O-glucosyltransferase | Cucumis sativus (XP_004140708.1) | 89 |
| Cucumis melo (XP_008456146.1) | 88 |
| Compound | Roots | Stems | Y-leaves | M-leaves | F-flowers | M-flowers |
|---|---|---|---|---|---|---|
| Gallic acid | 0 b | 0 b | 0 b | 0 b | 38.34 ± 9.12 a | 0 b |
| 4-Hydroxybenzoic acid | 0 c | 0 c | 29.49 ± 2.72 a | 10.86 ± 2.93 b | 2.85 ± 1.08 c | 0 c |
| Catechin hydrate | 0 b | 0 b | 0 b | 0 b | 239.06 ± 19.58 a | 0 b |
| Chlorogenic acid | 37.425 ± 0.83 c | 40.62 ± 0.90 c | 0 d | 0 d | 49.39 ± 0.57 b | 58.7 ± 6.39 a |
| Caffeic acid | 14.43 ± 1.02 c | 12.01 ± 0.84 c,d | 33.36 ± 2.19 a | 24.18 ± 3.27 b | 10.17 ± 0.04 d | 9.54 ± 2.26 d |
| Epicatechin | 0 c | 0 c | 0 c | 0 c | 568.67 ± 12.54 b | 660.35 ± 85.84 a |
| p-Coumaric acid | 7.65 ± 0.98 e | 12.54 ± 0.16 d | 54.68 ± 2.66 a | 38.07 ± 1.36 b | 19.47 ± 0.34 c | 10.25 ± 2.94 d,e |
| Ferulic acid | 0 c | 4.76 ± 0.26 b | 4.8 ± 0.26 b | 6.105 ± 1.80 b | 9.49 ± 0.23 a | 10.27 ± 2.25 a |
| Benzoic acid | 7.19 ± 1.51 b | 15.4 ± 4.52 a | 0 c | 2.85 ± 0.08 b,c | 0 c | 1.59 ± 0.92 c |
| Rutin | 37.04 ± 2.48 d | 153.48 ± 3.12 c | 3970.83 ± 27.27 a | 2277.93 ± 71.62 b | 204.19 ± 28.28 c | 7.17 ± 2.76 d |
| trans-Cinnamic acid | 0 b | 0 b | 0 b | 0 b | 0 b | 9.13 ± 2.28 a |
| Kaempferol | 0 d | 0 d | 1.89 ± 0.00 c | 5.49 ± 0.13 a | 4.67 ± 0.52 b | 0 d |
| Compound | Stage 1 | Stage 2 | Stage 3 | Stage 4 |
|---|---|---|---|---|
| Chlorogenic acid | 37.89 ± 0.03 b | 37.74 ± 0.82 b | 40.39 ± 1.31 a | 40.47 ± 1.65 a |
| Caffeic acid | 13.91 ± 1.66 a | 0 b | 0 b | 0 b |
| Epicatechin | 380.71 ± 10.15 a | 386.9 ± 4.75 a | 333.22 ± 2.34 b | 316.22 ± 9.27 c |
| p-Coumaric acid | 0.98 ± 0.43 b | 3.02 ± 0.52 a | 0.94 ± 0.08 b | 1.34 ± 0.44 b |
| Ferulic acid | 4.74 ± 0.00 a | 4.79 ± 0.96 a | 4.69 ± 0.02 a | 4.73 ± 0.06 a |
| Rutin | 12.49 ± 1.09 b,c | 13.66 ± 0.68 b | 20.7 ± 0.29 a | 11.15 ± 0.02 c |
| Kaempferol | 2.66 ± 0.60 a | 0 b | 0 b | 2.72 ± 0.66 a |
| Compound | 0 DAS | 5 DAS | 10 DAS | 15 DAS |
|---|---|---|---|---|
| 4-Hydroxybenzoic acid | 0.375 ± 0.15 c | 0 c | 40.98 ± 1.19 a | 18.82 ± 0.54 b |
| Catechin hydrate | 26.52 ± 2.54 a | 28.875 ± 0.62 a | 13.935 ± 0.40 b | 13.12 ± 1.49 b |
| Chlorogenic acid | 34.455 ± 0.23 a | 0 ± 0 c | 33.225 ± 0.15 b | 33.25 ± 0.08 b |
| Caffeic acid | 6.795 ± 0.06 a | 6.585 ± 0.02 a | 0 b | 6.5 ± 0.21 a |
| p-Coumaric acid | 2.19 ± 0.29 b | 5.145 ± 0.36 a | 0.585 ± 0.15 c | 0.71 ± 0.03 c |
| Ferulic acid | 4.965 ± 0.15 a | 4.905 ± 0.11 a | 5.295 ± 0.28 a | 5.2 ± 0.15 a |
| Benzoic acid | 4.14 ± 2.20 b | 7.92 ± 0.93 a | 4.665 ± 0.91 b | 6.93 ± 0.24 a,b |
| Rutin | 12.27 ± 0.00 d | 27.72 ± 0.85 c | 157.935 ± 0.91 b | 208.97 ± 3.35 a |
| trans-Cinnamic acid | 4.605 ± 0.40 a | 1.74 ± 0.00 b | 0.66 ± 0.08 c | 0.85 ± 0.14 c |
| Light | Days | Caffeic acid | Rutin | Kaempferol | Total |
|---|---|---|---|---|---|
| White | 7 days | 11.36 ± 1.11 e | 433.9 ± 23.3 b | 4.71 ± 1.47 b | 449.97 ± 24.82 b |
| 14 days | 14.75 ± 0.58 d,e | 103.6 ± 6.20 d | 9.93 ± 0.41 a | 128.28 ± 7.05 d | |
| 21 days | 26.71 ± 0.36 b,c | 195.62 ± 1.35 c | 3.1 ± 0.05 b | 225.43 ± 1.58 c | |
| Blue | 7 days | 14.99 ± 0.50 d,e | 457.07 ± 13.17 a,b | 4.89 ± 0.59 b | 476.95 ± 13.17 b |
| 14 days | 31.47 ± 11.67 a,b | 221.39 ± 43.85 c | 4.56 ± 0.94 b | 257.42 ± 56.16 c | |
| 21 days | 36.36 ± 0.89 a | 493.96 ± 9.02 a | 4.3 ± 0.26 b | 534.62 ± 9.13 a | |
| Red | 7 days | 11.1 ± 0.49 e | 87.47 ± 25.18 d | 8.58 ± 0.67 a | 107.15 ± 25.56 d |
| 14 days | 20.94 ± 0.70 c,d,e | 22.34 ± 0.75 e | 3.67 ± 0.21 b | 46.95 ± 0.89 e | |
| 21 days | 21.67 ± 0.43 c,d | 23.15 ± 4.5 e | 4.67 ± 0.32 b | 49.49 ± 4.64 e |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuong, D.M.; Kwon, S.-J.; Jeon, J.; Park, Y.J.; Park, J.S.; Park, S.U. Identification and Characterization of Phenylpropanoid Biosynthetic Genes and Their Accumulation in Bitter Melon (Momordica charantia). Molecules 2018, 23, 469. https://doi.org/10.3390/molecules23020469
Cuong DM, Kwon S-J, Jeon J, Park YJ, Park JS, Park SU. Identification and Characterization of Phenylpropanoid Biosynthetic Genes and Their Accumulation in Bitter Melon (Momordica charantia). Molecules. 2018; 23(2):469. https://doi.org/10.3390/molecules23020469
Chicago/Turabian StyleCuong, Do Manh, Soon-Jae Kwon, Jin Jeon, Yun Ji Park, Jong Seok Park, and Sang Un Park. 2018. "Identification and Characterization of Phenylpropanoid Biosynthetic Genes and Their Accumulation in Bitter Melon (Momordica charantia)" Molecules 23, no. 2: 469. https://doi.org/10.3390/molecules23020469
APA StyleCuong, D. M., Kwon, S.-J., Jeon, J., Park, Y. J., Park, J. S., & Park, S. U. (2018). Identification and Characterization of Phenylpropanoid Biosynthetic Genes and Their Accumulation in Bitter Melon (Momordica charantia). Molecules, 23(2), 469. https://doi.org/10.3390/molecules23020469





