Abstract
A novel flavonoid glucoside, ruthenicunoid A (1), together with eight known substances, were isolated from the fruits of Lycium ruthenicun Murr. Their structures were elucidated by extensive spectroscopic data and chemical methods. Especially, the absolute configuration of glucose residue in 1 was assigned by acid hydrolysis followed by derivatization and GC analysis. Biological evaluation towards Sirtuin 1 (SIRT1) found that compounds 1 and 2 exhibit inhibitory activity against SIRT1 in a concentration-dependent manner, indicating its potential on SIRT1-associated disorders.
1. Introduction
Lycium ruthenicun Murr. is found in the northwest regions of China. Its fruit is edible and has been used as a remedy for the treatment of hypertension, ureteral stones, tinea and furuncle, and gingvial bleeding [1,2,3]. The fruits of L. ruthenicun contains a variety of bioactive ingredients, in particular, polyphenols such as anthocyanins, which have antioxidant effects and are beneficial for the prevention and treatment of cardiovascular diseases are rich in the fruits [4,5]. A literature search found that the major research in the past focused on the extraction methods and measurement of the total anthocyanins [6,7,8]; no comprehensive study has been conducted to explore the chemical constituents of L. ruthenicun. This attracted our attention. In the course of continuous study, a new flavonoid glucoside, ruthenicunoid A, and eight known compounds were isolated and identified. All the compounds were tested for their biological activity on SIRT1, a nicotinamide adenosine dinucleotide (NAD)-dependent deacetylase. Our efforts will be described below.
2. Results and Discussion
2.1. Structure Elucidation of the Compounds
The EtOH extract of L. ruthenicun was suspended in water and partitioned with EtOAc. The EtOAc soluble part was submitted to a combination of chromatography to afford compounds 1–9 (Figure 1).
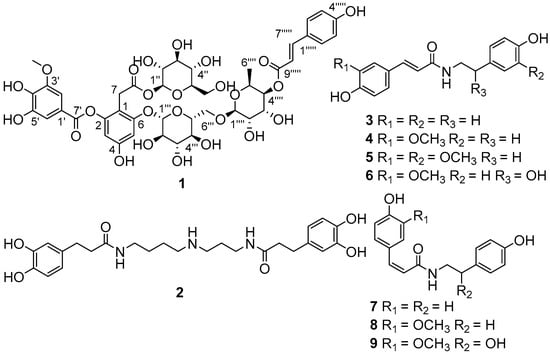
Figure 1.
Chemical structures of compounds 1–9.
Compound 1, obtained as a brownish auburn gum, has the molecular formula C43H50O25 (19 degrees of unsaturation) based on analysis of its HRESIMS at m/z 989.2546 [M + Na]+ (calcd. for C43H50O25Na, 989.2539). The 1H NMR spectrum of 1 (Table 1) shows an AABB coupling system characteristic of a group of protons at δH 7.48 (2H, d, J = 8.5 Hz, H-2′′′′′, 6′′′′′) and 6.81 (2H, d, J = 8.5 Hz, H-3′′′′′, 5′′′′′), four aromatic protons at δH 6.42 (1H, d, J = 1.8 Hz, H-3), δH 6.67 (1H, d, J = 1.8 Hz, H-5), δH 7.30 (1H, d, J = 1.8 Hz, H-2′), and δH 7.35 (1H, d, J = 1.8 Hz, H-6′), suggesting the presence of two 1,2,3,5-tetrasubstituted benzene rings. In addition, one methoxy group at δH 3.88 (3H, s, 3′-OCH3) and two olefinic protons respectively at δH 7.63 (1H, d, J = 15.9 Hz, H-7′′′′′) and δH 6.37 (1H, d, J = 16.0 Hz, H-8′′′′′) were observed. The 13C NMR and DEPT spectra of 1 (Table 1) show 43 carbon signals attributed to two methyl (one oxygenated), three sp3 methylene, twenty-five methine (ten olefinic and fifteen aliphatic), and thirteen quaternary carbons (three carbonyls, ten sp2 including seven oxygenated). Inspection of these NMR data found that the partial signals resemble those of malvone [9,10], differing in that 5′-OMe in malvone was replaced by 5′-OH in 1. The HMBC correlation (Figure 2) of OCH3/C-3′ and ROESY correlation of OCH3/H-2′ (Figure 2), in consideration of the chemical shifts of C-4′ (δC 141.6), C-5′ (δC 146.5), secured the presence of 3-methoxy,4,5-dihydroxyl substituted pattern. Further HMBC correlations of H-1′′/C-8, H-1′′′/C-6, H-7/C-1, C-2, C-6, in consideration of chemical shifts of C-2, C-4, and C-6 indicated the position of two glucose residues. HMBC correlations of H-2′, H-6′/C-7′ and the significant upfield shift of C-2 (δC 152.1) secured an ester carbonyl attached to C-2 instead of C-4. Apart from the red part, the remaining signals (blue part) are in accordance with those of 4-p-cumaroyl-α-rhamnosyl-(1 → 6)-β-glucose [11]. The observation of the above-mentioned AABB coupling system, a transformed double bond (JH-7′′′′′,H-8′′′′′ = 15.9 Hz), and two sugar moieties in the middle field supported our conclusion. Additional HMBC cross peaks of H-1′′′′/C-6′′′, H-4′′′′/C-9′′′′′ further indicated the linkage pattern in the blue part of 1. The red and blue parts were connected via C-6-O-C-1′′′ supported by the HMBC correlation of H-1′′′/C-6 and the ROESY correlation of H-5/H-1′′′. Thus, the planar structure of 1 was deduced. For the configuration of the sugar moieties, acid hydrolysis of 1 followed by TLC comparison and GC analysis allowed the assignment of d-glucose and l-rhamnose. In detail, the L-cysteine methyl ester hydrochloride derivatives of the hydrolysis product of 1, d-, l-glucose and L-rhamnose were prepared and subjected to GC analysis. The retention time for that of 1 is 17.698 min and 21.290 min, close to that of L-rhamnose (17.847 min) and d-glucose (21.276 min) rather than l-glucose (21.768 min), clarifying the type of sugar and its configuration. It should be noted that d-rhamnose or d,l-rhamnose in this study was not readily available, so that the derivative of d-rhamnose couldn’t be prepared and analyzed by GC. However, it is possible to differentiate l- from d-form of rhamnose by comparing the consistency of retention time between the derivative of l-rhamnose and that of the mixture of l-rhamnose with 1. In this way, we found that the retention time for l-cysteine methyl ester hydrochloride derivative of l-rhamnose is identical with that of co-injection of the mixture (16.827 min for the latter) by GC/MS analysis, securing the type of rhamnose and its configuration accordingly. Taken together, the structure of 1 was identified and named as ruthenicunoid A.

Table 1.
1H (600 MHz) and 13C NMR (150 MHz) data of 1 (δ in ppm, J in Hz, methanol-d4).
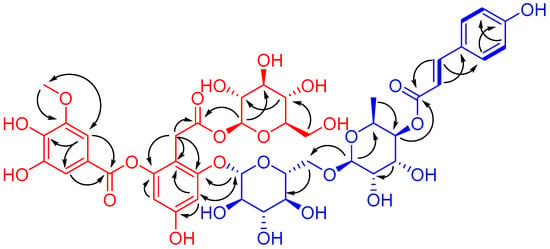
Figure 2.
1H-1H COSY (  ) and key HMBC (
) and key HMBC (  ) and ROESY (
) and ROESY (  ) correlations of 1.
) correlations of 1.
 ) and key HMBC (
) and key HMBC (  ) and ROESY (
) and ROESY (  ) correlations of 1.
) correlations of 1.
By analysis of the NMR spectroscopic data and comparison with the literature, the known compounds were respectively identified as N1,N10-bis(dihydrocaffeoyl)spermidine (2) [12], N-trans-coumaroyltyramine (3) [13], N-trans-feruloyltyramine (4) [14], N-trans-feruloyl 3′-O-methyldopamine (5) [15], N-trans-feruloyloctopamine (6) [14], N-cis-coumaroyltyramine (7) [16], N-cis-feruloyltyramine (8) [14], and N-cis-feruloyloctopamine (9) [14].
2.2. Biological Evaluation
SIRT1 is a nicotinamide adenosine dinucleotide (NAD)-dependent deacetylase which regulates a wide range of cellular functions and is implicated in many diseases such as aging, cancer and so on [17,18,19,20]. So far, several SIRT1 activators and inhibitors such as nicotinamide (IC50 value less than 50 μM), salermide (IC50 value = 76.2 μM), and cambinol (IC50 value = 56 μM) were documented [21]. With this assay in hand and considering the title species is used for aging, compounds 1–9 were thus tested for their inhibitory activity against SIRT1. The results showed that compounds 1 and 2 are active towards SIRT1 (Figure 3) with 2 to be more potent than 1, comparable to that of nicotinamide at the concentration of 100 μM, whereas compounds 3–9 are not active (data not shown). The finding of 2 as a SIRT1 inhibitory substance indicated that such type of amide or aliphatic amine might be of important structure class for antiaging drug design.
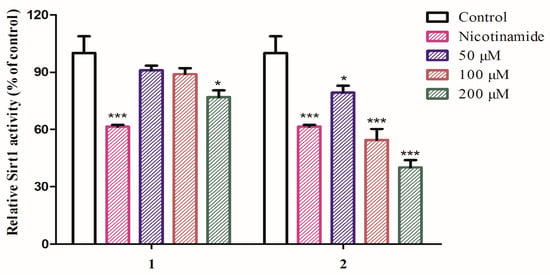
Figure 3.
SIRT1 activation of compounds 1 and 2. SIRT1 enzyme activity was measured using the SIRT1 Fluorometric Drug Discovery Kit. Statistical analysis was performed using one-way analysis of the variance (ANOVA) followed by Bonferroni’s multiple comparison tests. All error bars are S.E.M. * p < 0.05, *** p < 0.001 versus control (n = 3).
3. Experimental Section
3.1. General Procedures
Optical rotations were recorded on a Horiba SEPA-300 polarimeter. UV spectrum was recorded on a Shimadzu UV-2401PC spectrometer (Shimadzu Corporation, Tokyo, Japan). GC analysis was performed using an Agilent 6890N gas chromatography instrument (Agilent Technologies, Santa Clara, CA, USA). GC/MS analysis was performed using an Agilent 7890B GC System (Agilent Technologies, Santa Clara, CA, USA) and a Asilent 5977 MSD inrun (Agilent Technologies, Santa Clara, CA, USA). NMR spectra were recorded on a Bruker AV-400 (Bruker, Karlsruhe, Germany) or an AV-600 spectrometer (Bruker, Karlsruhe, Germany), with TMS as an internal standard. ESIMS, and HRESIMS were measured on an Agilent G6230TOF MS spectrometer (Agilent Technologies, Santa Clara, CA, USA). C-18 silica gel (40–60 μm; Daiso Co., Tokyo, Japan), MCI gel CHP 20P (75–150 μm, Mitsubishi Chemical Industries, Tokyo, Japan) and Sephadex LH-20 (Amersham Pharmacia, Uppsala, Sweden) were used for column chromatography. Semi-preparative HPLC was carried out using an Agilent 1200 liquid chromatograph with a YMC-Pack ODS-A column (250 mm × 10 mm, i.d., 5 μm) and Thermo Hypersil GOLD-C18 column (250 mm × 21.2 mm, i.d., 5 μm).
3.2. Plant Material
The fruits of L. ruthenicum were collected from the market of herbal medicine in Yunnan province, People’s Republic of China, in September 2016. The material was identified by Mr. Bin Qiu at Yunnan Institute of Materia Medica, and a voucher specimen (CHYX-0605) is deposited at the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, People’s Republic of China.
3.3. Extraction and Isolation
The fruits of L. ruthenicum (5 kg) were powdered and soaked by 80% aqueous EtOH (3 × 25 L × 24 h) to give a crude extract, which was suspended in water followed by extraction with EtOAc to afford an EtOAc soluble extract (85 g). The EtOAc extract was divided into six parts (Fr.1–Fr.6) by using a MCI gel CHP 20P column eluted with gradient aqueous MeOH (20–100%). Fr.2 (3.5 g) was purified by Sephadex LH-20 (MeOH) followed by semipreparative HPLC (MeOH/H2O, 27:73, containing 0.05% formic acid) to afford compound 2 (78.4 mg, tR = 9.8 min). Fr.4 (10.1 g) was separated by Sephadex LH-20 (MeOH) to yield six fractions (Fr.4.1–Fr.4.6). Fr.4.3 (2.1 g) was separated by RP-18 column (MeOH/H2O, 30–100%) to get three fractions (Fr.4.3.1–Fr.4.3.3). Fr.4.3.3 (490 mg) was separated by Sephadex LH-20 (MeOH) to yield four fractions (Fr.4.3.3.1–Fr.4.3.3.4). Among these, Fr.4.3.3.4 (48 mg) was purified by semi-preparative HPLC (MeCN/H2O, 28:72) to yield compounds 4 (2.1 mg, tR = 16.1 min) and 5 (2.3 mg, tR = 21.3 min). Fr.4.4 (1.0 g) was separated by RP-18 column (MeOH/H2O, 35–100%) to get five fractions (Fr.4.4.1–Fr.4.4.5). Fr.4.4.2 (180 mg) was separated by preparative HPLC (MeOH/H2O, 10–100%) to get three fractions (Fr.4.4.2.1–Fr.4.4.2.3). Fr.4.4.2.1 (23 mg) was purified by semi-preparative HPLC (MeCN/H2O, 21:79) to afford compound 1 (4.9 mg, tR = 15.4 min). Fr.4.4.3 (380 mg) was separated by preparative HPLC (MeOH/H2O, 10–100%) to get nine fractions (Fr.4.4.3.1–Fr.4.4.3.9). Of which, Fr.4.4.3.3 (56.3 mg) was purified by semipreparative HPLC (MeCN/H2O, 18:82) to afford compounds 6 (5.4 mg, tR = 27.9 min) and 9 (1.0 mg, tR = 30.3 min). Fr.4.4.3.7 (23 mg) was purified by semipreparative HPLC (MeCN/H2O, 27:73) to yield compound 7 (2.3 mg, tR = 22.8 min). Fr.4.4.3.8 (44 mg) was purified by semi-preparative HPLC (MeCN/H2O, 23:77) to afford compounds 3 (7.1 mg, tR = 27.0 min) and 8 (2.1 mg, tR = 29.6 min).
3.4. Compound Characterization Data
Ruthenicunoid A (1): Brownish auburn gum; : −23.5 (c 0.49, MeOH). UV (MeOH) λmax (log ε): 203 (4.66), 313 (4.47) nm. ESIMS m/z: 989 [M + Na]+. HRESIMS m/z: 989.2546 [M + Na]+ (calcd. for C43H50O25Na, 989.2539); 1H- and 13C-NMR, see Table 1.
3.5. Acid Hydrolysis and Sugar Analysis
A solution of 1 (1.0 mg) in 1 N HCl was stirred at 70 °C for 5 h. After cooling, the mixtures were extracted with EtOAc. The aqueous layer was neutralized with 1 N NaOH and concentrated in vacuo, which was subsequently dissolved in anhydrous pyridine (2 mL). To these solutions L-cysteine methyl ester hydrochloride (2.0 mg) was added, and the mixtures were stirred at 60 °C for 1 h and concentrated in vacuo at 0 °C. Slow addition of 1-(trimethylsiyl) imidazole to the mixtures was followed by stirring at 60 °C for 2 h. Aliquots (4 µL) of the supernatants were subjected to chiral GC analysis to determine that D-glucose and L-rhamnose unitis are present in 1 [22,23].
3.6. SIRT1 Inhibition
For examination of SIRT1 inhibition of the compounds, each well contained 0.5 U (1 U = 1 pmol/min at 37 °C) of SIRT1 enzyme, 1000 μM of NAD+ (Enzo Life Sciences, Farmingdale, NY, USA), 100 μM of SIRT1 peptide substrate (Enzo Life Sciences) and SIRT1 assay buffer (50 mM Tris-HCl, pH 8.0, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 1 mg/mL BSA) along with the test compounds at a concentration of 50, 100 and 200 μM, respectively. Nicotinamide, a known inhibitor of SIRT1 enzyme was used as a control at a concentration of 100 μM. The plate was incubated at 37 °C for 30 min and the reaction was stopped using Fluor de Lys developer II solution (Enzo Life Sciences) containing 2 mM nicotinamide. The plate was further incubated at 37 °C for another 30 min and the samples were read by a fluorimeter with an excitation wavelength of 360 nm and emission wavelength of 460 nm [24].
4. Conclusions
To conclude, this study led to the isolation of a new flavonoid glucoside and eight known amide derivatives from the edible fruits of L. ruthenicun. Biological evaluation found that both 1 and 2 showed inhibitory activity against SIRT1, indicating their roles in SIRT1-associated disorders and suggesting 2 to be a potent structure template worth for further optimization as SIRT1 inhibitors.
Supplementary Materials
The following data are available online.
Acknowledgments
This study was supported by National Key Research and Development Program of China (2017YFA0503900) and National Natural Science Fund for Distinguished Young Scholars (81525026).
Author Contributions
Y.-X.C. conceived and designed the experiments, J.-J.Q. performed the experiments. Y.-M.Y., L.-Z.C.., F.-Y.Q. and B.-H.L. analyzed the data; Y.-X.C. wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Zhao, J.; Xu, F.; Ji, T.F.; Li, J. A new spermidine from the fruits of Lycium ruthenicum. Chem. Nat. Compd. 2014, 50, 880–883. [Google Scholar] [CrossRef]
- Rao, A.V.; Snyde, D.M. Raspberries and human health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Zilic, S.; Serpen, A.; Akillioglu, G.; Gokmen, V.; Vancetovic, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) Kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, W.J.; Zhang, S.J.; Lv, H.Y. Study on antioxidant activity of pigment of Lycium ruthenicum. Chin. J. Chin. Mater. Med. 2006, 31, 1179–1183. [Google Scholar] [CrossRef]
- Zheng, J.; Ding, C.X.; Wang, L.S.; Li, G.L.; Shi, J.Y.; Li, H.; Wang, H.L.; Suo, Y.R. Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Food Chem. 2011, 126, 859–865. [Google Scholar] [CrossRef]
- Li, J.; Qu, W.J.; Lv, H.Y.; Yuan, H. Study on extracting and refining of the pigments from Lycium ruthenicum Murr. Nat. Prod. Res. Dev. 2006, 18, 650–654. [Google Scholar] [CrossRef]
- Li, S.Z.; Li, J.; Yang, Z.J.; Yuan, H. Study on separation and purifeation of total flavonoids from Lycium ruthenicum Murr. with macroreticular resin. Food Sci. 2009, 30, 19–24. [Google Scholar] [CrossRef]
- Luo, H.; Jin, L.; Gao, S.F.; Chen, H.G. Determination of Anthocyanin in Lycium ruthenicum Murr. from different producing areas in Hei River basin by UV. Chin. Med. J. Res. Pract. 2015, 29, 24–27. [Google Scholar] [CrossRef]
- Lopes, P.; Richard, T.; Saucier, C.; Teissedre, P.; Monti, J.; Glories, Y. Anthocyanone A: A quinone methide derivative resulting from malvidin 3-O-glucoside degradation. J. Agric. Food Chem. 2007, 55, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, H.; Yanase, E.; Nakatsuka, S. Novel oxidation products of cyanidin 3-O-glucoside with 2,2′-azobis-(2,4-dimethyl)valeronitrile and evaluation of anthocyanin content and its oxidation in black rice. Food Chem. 2014, 155, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.J.; Yan, Y.M.; Wang, C.X.; Cheng, Y.X. Compounds from Lycium ruthenicum. Nat. Prod. Res. Dev. 2017. Available online: http://kns.cnki.net/kcms/detail/51.1335.Q.20170904.1418.016.html (accessed on 4 September 2017).
- Yingyongnarongkul, B.; Apiratikul, N.; Aroonrerk, N.; Suksamrarn, A. Synthesis of bis, tris and tetra (dihydrocaffeoyl) polyamine conjugates as antibacterial agents against VRSA. Arch. Pharm. Res. 2008, 31, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.X.; Hui, Y.X.; Rupprecht, J.K.; Mclaughlin, J.L.; Wood, K.V. Additional bioactive compounds and trilobacin, a novel highly cytotoxic acetogenin, from the bark of Asimina triloba. J. Nat. Prod. 1992, 55, 347–356. [Google Scholar] [CrossRef] [PubMed]
- King, R.R.; Calhoun, L.A. Characterization of cross-linked hydroxycinnamic acid amides isolated from potato common scab lesions. Phytochemistry 2005, 66, 2468–2473. [Google Scholar] [CrossRef] [PubMed]
- Cutillo, F.; Dabrosca, B.; Dellagreca, M.; Marino, C.D.; Golino, A.; Previtera, L.; Zarrelli, A. Cinnamic acid amides from Chenopodium album: Effects on seeds germination and plant growth. Phytochemistry 2003, 64, 1381–1387. [Google Scholar] [CrossRef]
- Kim, D.K.; Lim, J.P.; Kim, J.W.; Park, H.W.; Eun, J.S. Antitumor and antiinflammatory constituents from Celtis sinensis. Arch. Pharm. Res. 2005, 28, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, Y. SIRT1: Role in cardiovascular biology. Clin. Chim. Acta 2015, 440, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Y.; Qu, S.L.; Tang, Z.H.; Zhang, Y.; Liu, M.H.; Peng, J.; Tang, H.; Yu, K.L.; Zhang, C.; Ren, Z.; et al. SIRT1 in cardiovascular aging. Clin. Chim. Acta 2014, 437, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Clark-Knowles, K.V.; He, X.H.; Jardine, K.; Coulombe, J.; Dewar-Darch, D.; Caron, A.Z.; Gray, D.A.; McBurney, M.W. Reversible modulation of SIRT1 activity in a mouse strain. PLoS ONE 2017. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.W.; Buer, B.C.; Marshab, E.N.G.; Kennedy, R.T. A label-free Sirtuin 1 assay based on dropletelectrospray ionization mass spectrometry. Anal. Methods 2016, 8, 3458–3465. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chauhan, S. How much successful are the medicinal chemists in modulation of SIRT1: A critical review. Eur. J. Med. Chem. 2016, 119, 45–69. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.N.; Tu, Z.C.; Wang, X.L.; Yan, Y.M.; Fang, P.; Zuo, Z.L.; Hou, B.; Yang, T.H.; Cheng, Y.X. Bioactive compounds from the insect Aspongopus chinensis. Bioorg. Med. Chem. Lett. 2014, 24, 5164–5169. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.S.; Daubenspeck, J.M.; Dybvig, K. Rhamnose biosynthesis in mycoplasmas requires precursor glycans larger than monosaccharide. Mol. Microbiol. 2013, 89, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Karbasforooshan, H.; Karimi, G. The role of SIRT1 in diabetic cardiomyopathy. Biomed. Pharmacother. 2017, 90, 386–392. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the compound 1 is available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).