Antioxidant and Cytoprotective Effects of Tibetan Tea and Its Phenolic Components
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Animals and Chemicals
3.2. Preparation of Lyophilized Aqueous Extract of Tibetan Tea (LATT)
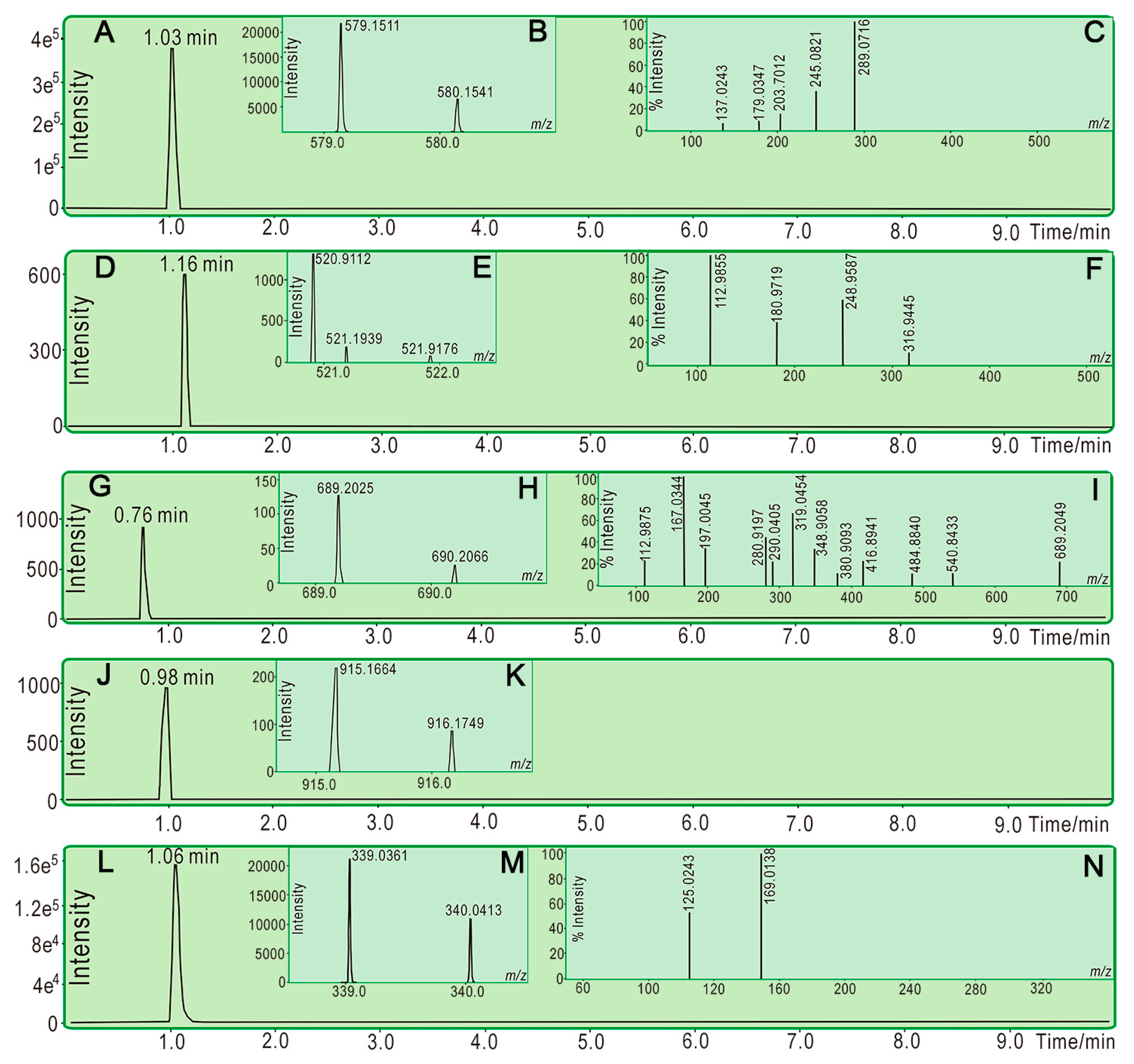
3.3. HPLC Analysis for Phenolic Components in LATT
3.4. Ferric Reducing Antioxidant Power (FRAP) Assay
3.5. Free Radical-Scavenging Assays In Vitro
3.6. UPLC−ESI−Q−TOF−MS/MS Analysis of Reaction Products of PTIO• with Phenolic Components
3.7. Cytoprotective Effect Assay
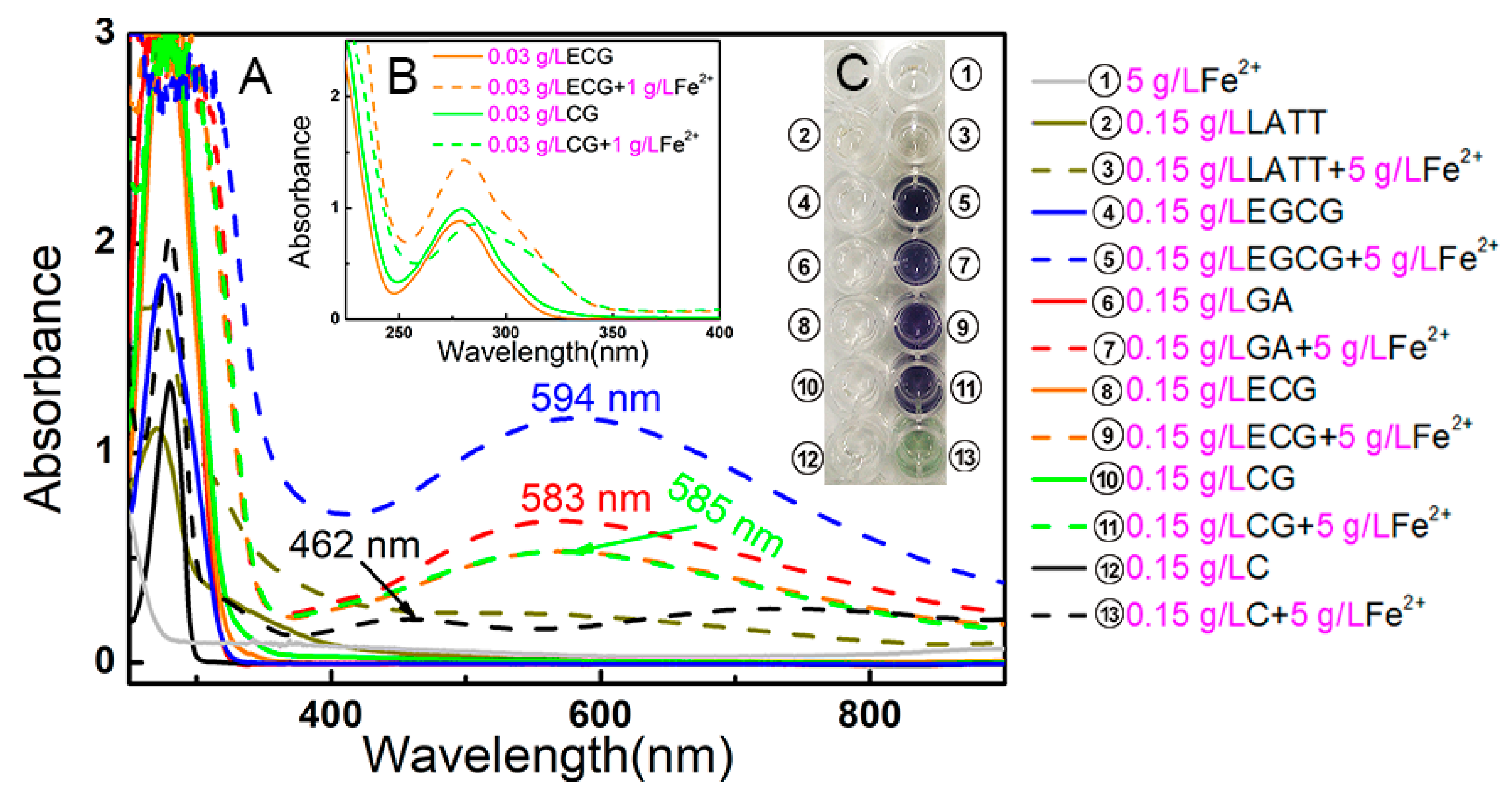
3.8. UV Spectral Determination of Fe2+-Chelation
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABTS | [2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid diammonium salt)] |
| bmMSCs | bone marrow-derived mesenchymal stem cells |
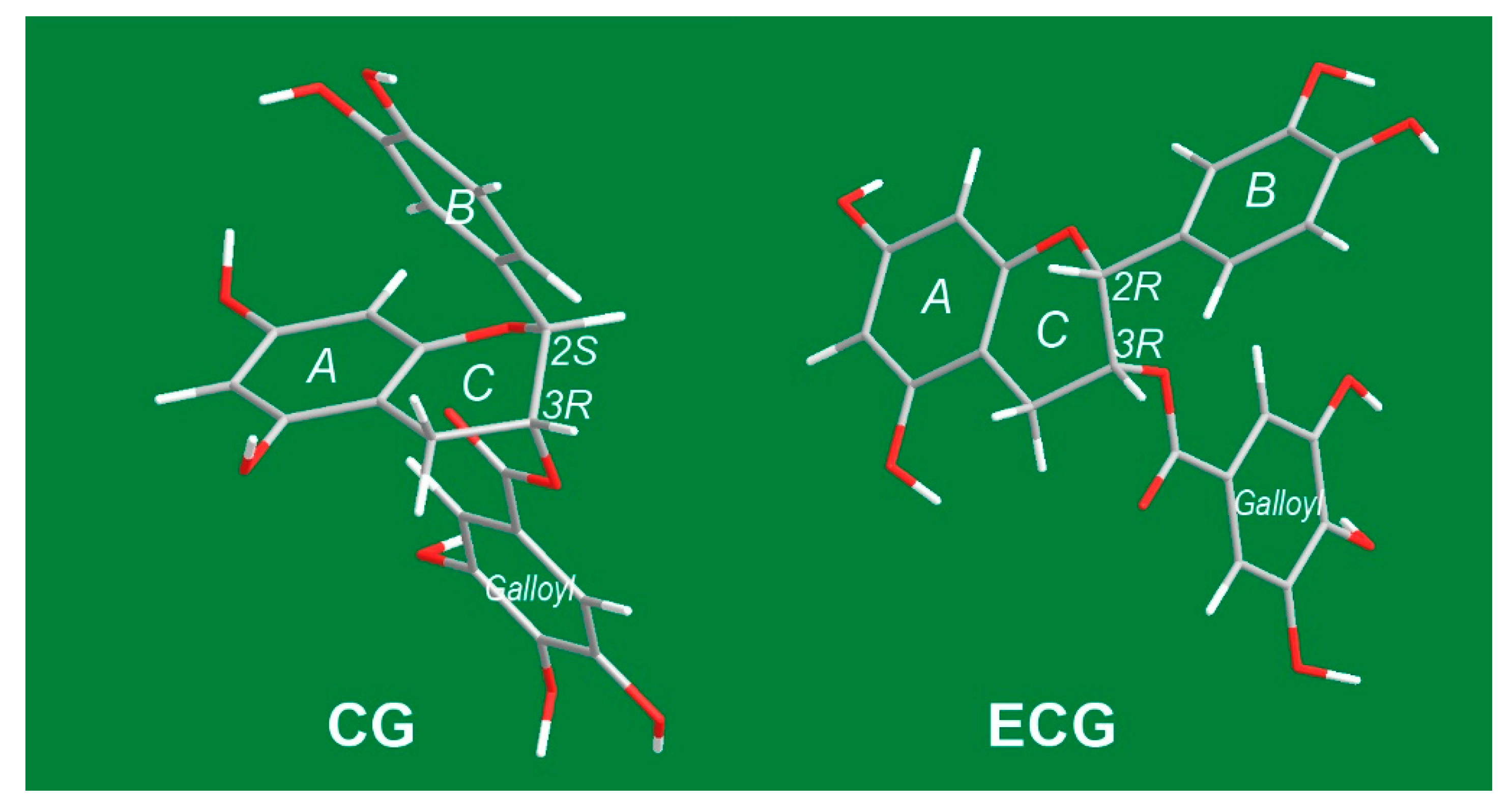
| CG | (−)-catechin gallate |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DPPH• | 1,1-diphenyl-2-picryl-hydrazl |
| ET | electron transfer |
| ECG | (−)-epicatechin gallate |
| EGCG | (−)-epigallocatechin gallate |
| FBS | fetal bovine serum |
| FRAP | ferric reducing antioxidant power |
| GA | gallic acid |
| HAT | hydrogen atom transfer |
| LATT | lyophilized aqueous extract of Tibetan tea |
| PTIO• | 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide radical |
| ROS | reactive oxygen species |
| RAF | radical adduct formation |
| SD | standard deviation |
| TPTZ | 2,4,6-tris(2-pyridyl-s-triazine) |
References
- Jiang, A.L.; He, Y.B.; Hu, W.Z.; Liu, C.H.; Xu, G.K. The inheritance and development of Ya’an Tibetan tea. Sci. Technol. Food Ind. 2012, 33, 391–394. [Google Scholar]
- Lu, X.H.; Xu, J.Y.; Sun, R.; Wang, J.Y. Human trial of health effects of Tibetan tea. Food Res. Dev. 2017, 38, 168–171. [Google Scholar]
- Larrick, J.W. The methyl xanthine hypothesis: Does tea consumption by Tibetan natives blunt the effects of high altitude? Med. Hypotheses 1991, 34, 99–104. [Google Scholar] [CrossRef]
- Lai, X. Ya’an Tibetan tea: The treasures of Chinese intangible cultural heritage. Sichuan Provincial Cond. 2012, 4, 33–35. [Google Scholar]
- Zhong, T.; Qi, G.N.; Xu, W.; Chen, S.X.; Li, J.H. Identification of fungal populations during the storage period of Tibetan tea. Guizhou Agri. Sci. 2010, 38, 101–103. [Google Scholar]
- Wu, J.; Wang, W.; Liu, Y.; Sun, J.; Ye, Y.; Li, B.; Liu, X.; Liu, H.; Sun, Z.; Li, M.; et al. Modifying role of GSTP1 polymorphism on the association between tea fluoride exposure and the brick-tea type fluorosis. PLoS ONE 2015, 10, e128280. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Bai, X.; Zhao, Y.; Liu, J.; Zhou, D.; Fang, S.; Jia, M.; Wu, J. The relationship of fluorosis and brick tea in Chinese Tibetan people. Environ. Health Perspect. 1996, 104, 1340–1343. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.K.; Ham, H.M.; Jeong, M.H.; Kim, D.H.; Kim, H.J. Simultaneous determination of 15 phenolic compounds and caffeine in teas and mate using RP-HPLC/UV detection: Method development and optimization of extraction process. Food Chem. 2015, 172, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Beer, D.; Walters, N.A.; Villiers, A.; Joubert, E.; Walczak, B. Multivariate analysis of variance of designed chromatographic data. A case study involving fermentation of rooibos tea. J. Chromatogr. A 2017, 1489, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; Beelders, T.; Beer, D.; Malherbe, C.J.; Villiers, A.J.; Sigge, G.O. Variation in phenolic content and antioxidant activity of fermented rooibos herbal tea infusions: role of production season and quality grade. J. Agric. Food Chem. 2012, 60, 9171–9179. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Chen, D.F.; Mai, Y.; Wen, B.; Wang, X.Z. Concordance between antioxidant activities in vitro and chemical components of Radix Astragali. Nat. Prod. Res. 2012, 26, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D. Comparison of the antioxidant effects of quercitrin and isoquercitrin: Understanding the role of the 6″-OH group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef] [PubMed]
- Rusak, G.; Komes, D.; Likic, S.; Horzic, D.; Kovac, M. Phenolic content and antioxidative capacity of green and white tea extracts depending on extraction conditions and the solvent used. Food Chem. 2008, 110, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.F.; Li, X.C.; Xu, Z.; Liu, X.; Du, S.H.; Li, H.; Zhou, J.H.; Zeng, H.P.; Hua, Z.C. Hexadecanoic acid from Buzhong Yiqi decoction induces proliferation of bone marrow mesenchymal stem cells. J. Med. Food. 2010, 13, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Wei, G.; Wang, X.Z.; Liu, D.H.; Deng, R.D.; Li, H.; Zhou, J.H.; Li, Y.W.; Zeng, H.P.; Chen, D.F. Targeting of the sonic hedgehog pathway by atractylenolides promotes chondrogenic differentiation of mesenchymal stem cells. Biol. Pharm. Bull. 2012, 35, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Du, G.J.; Zhang, Z.Y.; Wen, X.D.; Yu, C.H.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of tea-phenolics beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin-3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant activity of food constituents: an overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.M.; Rhile, I.J.; Larsen, F.B.; Mader, E.A.; Markle, T.F.; DiPasquale, A.G. Models for proton-coupled electron transfer in Photosystem II. Photosynth. Res. 2006, 87, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Russo, A.; Samuni, A. Reactions of PTIO• and carboxy-PTIO• with •NO, •NO2, and O2. J. Biol. Chem. 2003, 278, 50949–50955. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, T.; Liu, J.; Liu, Y.; Zhang, J.; Lin, J.; Zhao, Z.; Chen, D. Effect and mechanism of wedelolactone as antioxidant-coumestan on •OH-treated mesenchymal stem cells. Arab J. Chem. 2017. [Google Scholar] [CrossRef]
- Li, X.C. 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO•) radical-scavenging: a new and simple antioxidant assay in vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Han, W.; Mai, W.; Wang, L. Antioxidant Activity and mechanism of tetrahydroamentoflavone in vitro. Nat. Prod. Commun. 2013, 8, 787–789. [Google Scholar]
- Lin, J.; Li, X.C.; Chen, L.; Chen, D.F. Protective effect against hydroxylradical-induced DNA damage and antioxidant mechanism of [6]-gingerol: A chemical study. Bull Korean Chem. Soc. 2014, 35, 1633–1638. [Google Scholar] [CrossRef]
- Musialik, M.; Litwinienko, G. Scavenging of DPPH• radicals by Vitamin E is accelerated by its partial ionization: the role of sequential proton loss electron transfer. Org. Lett. 2005, 7, 4951–4954. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Hu, Q.P.; Jiang, S.X.; Li, F.; Lin, J.; Han, L.; Hong, Y.L.; Lu, W.B.; Gao, Y.X.; Chen, D.F. Flos chrysanthemi indici protects against hydroxyl-induced damages to DNA and MSCs via antioxidant mechanism. J. Saudi Chem. Soc. 2015, 19, 454–460. [Google Scholar] [CrossRef]
- Osman, A.M.; Wong, K.K.Y.; Fernyhough, A. ABTS radical-driven oxidation of polyphenols: Isolation and structural elucidation of covalent adducts. Biochem. Biophys. Res. Commun. 2006, 346, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Lópezmartínez, L. M.; Santacruzortega, H.; Navarro, R. E.; Sotelomundo, R.R.; Gonzálezaguilar, G.A. A 1H-NMR investigation of the interaction between phenolic acids found in mango (manguifera indica cv ataulfo) and papaya (carica papaya cv maradol) and 1,1-diphenyl-2-picrylhydrazyl (dpph) free radicals. PLoS ONE 2015, 10, e0140242. [Google Scholar]
- Osman, A.M. Multiple pathways of the reaction of 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) with (+)-catechin: Evidence for the formation of a covalent adduct between DPPH and the oxidized form of the polyphenol. Biochem. Biophys. Res. Commun. 2011, 412, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron chelating. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.Z.; Zheng, R.L. Theory and Application of Free Radical Biology; Science Press: Beijing, China, 2002; pp. 56–78. [Google Scholar]
- Tu, Y.; Yang, X.; Kong, J.; Zhang, S.; Zhu, Y.; Wang, Y. Antioxidant capability of Epi-catechins and theaflavins in vitro by scavenging hydroxyl free radical. Nat. Prod. Res. Dev. 2012, 24, 653–659. [Google Scholar]
- Lee, L.S.; Kim, S.H.; Kim, Y.B.; Kim, Y.C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Liu, J.; Lin, J.; Wang, T.; Huang, J.; Lin, Y.; Chen, D. Protective effects of dihydromyricetin against •OH-induced mesenchymal stem cells damage and mechanistic chemistry. Molecules 2016, 21, 604. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Li, X.C.; Lin, J.; Li, Y.R.; Wang, T.T.; Jiang, Q.; Chen, D.F. Sarcandra glabra (Caoshanhu) protects mesenchymal stem cells from oxidative stress: a bioevaluation and mechanistic chemistry. BMC Complement. Altern. Med. 2016, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Zeng, G.C.; Li, X.C.; Zeng, H.P. In vitro studies on the antioxidant and protective effect of 2-substituted-8-hydroxyquinoline derivatives against H2O2-Induced oxidative stress in BMSCs. Chem. Biol. Drug Des. 2010, 75, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Zeng, H. Synthesis, antioxidation activity of (E)-9-p-Tolyl-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and (E)-9-(p-Anisyl)-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and their induction proliferation of mesenchymal stem cells. Acta Chim. Sin. 2009, 67, 974–982. [Google Scholar]
- Li, X.C.; Gao, Y.X.; Li, F.; Liang, A.F.; Xu, Z.M.; Bai, Y.; Mai, W.Q.; Han, L.; Chen, D.F. Maclurin protects against hydroxyl radical-induced damages to mesenchymal stem cells: Antioxidant evaluation and mechanistic insight. Chem. Biol. Interact. 2014, 219, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Han, L.; Li, Y.R.; Zhang, J.; Chen, J.; Lu, W.B.; Zhao, X.J.; Lai, Y.T.; Chen, D.F.; Wei, G. Protective effect of sinapine against hydroxyl radical-induced damage to mesenchymal stem cells and possible mechanisms. Chem. Pharm. Bull. 2016, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, X.C.; Tian, Y.G.; Lin, Q.; Xie, H.; Lu, W.B.; Chi, Y.G.; Chen, D.F. Lyophilized aqueous extracts of Mori Fructus and Mori Ramulus protect mesenchymal stem cells from •OH–treated damage: Bioassay and antioxidant mechanism. BMC Complement. Altern. Med. 2017, 17, 242. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mai, W.; Chen, D.F. Chemical study on protective effect against hydroxyl-induced DNA damage and antioxidant mechanism of myricitrin. J. Chin. Chem. Soc. 2014, 61, 383–391. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Huang, L. Correlation between antioxidant activities and phenolic contents of radix Angelicae sinensis (Danggui). Molecules 2009, 14, 5349–5361. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, X.; Xie, H.; Wang, X.; Xie, Y.; Chen, C.; Chen, D. Protective mechanism of the antioxidant baicalein toward hydroxyl radical-treated bone marrow-derived mesenchymal stem cells. Molecules 2018, 23, 223. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (+)-catechin and (−)-catechin gallate are available from the authors. |



| FRAP | PTIO•-Scavenging | ABTS+•-Scavenging | DPPH•-Scavenging | |
|---|---|---|---|---|
| LATT μg/mL | 33.2 ± 1.2 | 531.9 ± 26.0 | 15.0 ± 0.4 | 48.7 ± 1.7 |
| ECG μg/mL(μM) | 2.7 ± 0.1 (6.0 ± 0.2 a) | 23.1 ± 0.6 (52.2 ± 1.4 a) | 0.8 ± 0.0 (1.8 ± 0.0 a) | 2.5 ± 0.1 (5.7 ± 0.2 a) |
| EGCG μg/mL(μM) | 4.9 ± 0.2 (10.9 ± 0.5 b) | 23.8 ± 0.23 (53.7 ± 0.5 a) | 1.4 ± 0.1 (3.0 ± 0.1 b) | 2.9 ± 0.1 (6.9 ± 0.3 b) |
| CG μg/mL(μM) | 5.3 ± 0.1 (11.9 ± 0.1 b) | 28.2 ± 3.1 (63.8 ± 7.1 b) | 1.3 ± 0.0 (2.9 ± 0.1 b) | 4.1 ± 0.2 (9.4 ± 0.4 b) |
| (+)-catechin μg/mL(μM) | 10.8 ± 0.5 (37.3 ± 1.9 d) | 71.2 ± 4.0 (161.0 ± 9.1 d) | 1.1 ± 0.0 (3.6 ± 0.1 c) | 5.1 ± 0.1 (18.2 ± 0.3 d) |
| GA μg/mL(μM) | 3.1 ± 0.1 (18.1 ± 0.8 c) | 50.4 ± 0.64 (113.9 ± 1.4 c) | 0.7 ± 0.0 (4.3 ± 0.2 d) | 2.1 ± 0.1 (12.6 ± 0.4 c) |
| Trolox μg/mL(μM) | 11.2 ± 0.2 (44.6 ± 1.0 e) | 23.9 ± 0.7 (54.1 ± 1.5 a) | 5.4 ± 1.2 (21.4 ± 4.9 f) | 9.2 ± 1.1 (36.3 ± 4.5 e) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, H.; Li, X.; Ren, Z.; Qiu, W.; Chen, J.; Jiang, Q.; Chen, B.; Chen, D. Antioxidant and Cytoprotective Effects of Tibetan Tea and Its Phenolic Components. Molecules 2018, 23, 179. https://doi.org/10.3390/molecules23020179
Xie H, Li X, Ren Z, Qiu W, Chen J, Jiang Q, Chen B, Chen D. Antioxidant and Cytoprotective Effects of Tibetan Tea and Its Phenolic Components. Molecules. 2018; 23(2):179. https://doi.org/10.3390/molecules23020179
Chicago/Turabian StyleXie, Hong, Xican Li, Zhenxing Ren, Weimin Qiu, Jianlan Chen, Qian Jiang, Ban Chen, and Dongfeng Chen. 2018. "Antioxidant and Cytoprotective Effects of Tibetan Tea and Its Phenolic Components" Molecules 23, no. 2: 179. https://doi.org/10.3390/molecules23020179
APA StyleXie, H., Li, X., Ren, Z., Qiu, W., Chen, J., Jiang, Q., Chen, B., & Chen, D. (2018). Antioxidant and Cytoprotective Effects of Tibetan Tea and Its Phenolic Components. Molecules, 23(2), 179. https://doi.org/10.3390/molecules23020179





