Structures and Bioactive Properties of Myrtucommulones and Related Acylphloroglucinols from Myrtaceae
Abstract
1. Introduction
2. Biological Sources
3. Structures and Chemical Properties
4. Other Biological Sources
5. Biological Activities
5.1. Antibacterial Activity
5.2. Bioactivities against Other Microorganisms and Viruses
5.3. Antioxidant and Anti-Inflammatory Activities
5.4. Cytotoxic and Antiproliferative Activities
5.5. Other Pharmacological Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zilkah, S.; Goldschdmidt, E.E. Myrtle (Myrtus communis L.)—A native Mediterranean and cultured crop species. In Medicinal and Aromatic Plants of the Middle-East. Medicinal and Aromatic Plants of the World; Yaniv, Z., Dudai, N., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 2. [Google Scholar]
- Alipour, G.; Dashti, S.; Hosseinzadeh, H. Review of pharmacological effects of Myrtus communis L. and its active constituents. Phytother. Res. 2014, 28, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microb. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Rosa, A.; Bifulco, E.; Melis, M.P.; Atzeri, A.; Pirisi, F.M.; Dessì, M.A. Chemical composition and antioxidant activities of Myrtus communis L. berries extracts. Food Chem. 2010, 123, 1242–1251. [Google Scholar] [CrossRef]
- Appendino, G.; Bianchi, F.; Minassi, A.; Sterner, O.; Ballero, M.; Gibbons, S. Oligomeric acylphloroglucinols from myrtle (Myrtus communis). J. Nat. Prod. 2002, 65, 334–338. [Google Scholar] [CrossRef]
- Yoshimura, M.; Amakura, Y.; Tokuhara, M.; Yoshida, T. Polyphenolic compounds isolated from the leaves of Myrtus communis. J. Nat. Med. 2008, 62, 366–368. [Google Scholar] [CrossRef]
- Piras, F.M.; Dettori, M.F.; Magnani, A. ToF-SIMS PCA analysis of Myrtus communis L. Appl. Surface Sci. 2009, 255, 7805–7811. [Google Scholar] [CrossRef]
- Barboni, T.; Cannac, M.; Massi, L.; Perez-Ramirez, Y.; Chiaramonti, N. Variability of polyphenol compounds in Myrtus communis L. (Myrtaceae) berries from Corsica. Molecules 2010, 15, 7849–7860. [Google Scholar] [CrossRef] [PubMed]
- Usai, M.; Mulas, M.; Marchetti, M. Chemical composition of essential oils of leaves and flowers from five cultivars of myrtle (Myrtus communis L.). J. Essent. Oil Res. 2015, 27, 465–476. [Google Scholar] [CrossRef]
- Hamdy, A.A.; Kassem, H.A.; Awad, G.E.A.; El-Kady, S.M.; Benito, M.T.; Doyagüez, E.G.; Jimeno, M.L.; Lall, N.; Hussein, A.A. In-vitro evaluation of certain Egyptian traditional medicinal plants against Propionibacterium acnes. S. Afr. J. Bot. 2017, 109, 90–95. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- de Oliveira Bernardes, C.; Tuler, A.C.; Ferreira, A.; Carvalho, M.S.; Nogueira, A.M.; da Silva Ferreira, M.F. Transferability of Psidium microsatellite loci in Myrteae (Myrtaceae): A phylogenetic signal. Euphytica 2018, 214, 105. [Google Scholar] [CrossRef]
- Stefanello, M.E.A.; Pascoal, A.C.; Salvador, M.J. Essential oils from neotropical Myrtaceae: Chemical diversity and biological properties. Chem. Biodivers. 2011, 8, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Moraes Cascaes, M.; Skelding Pinheiro Guilhon, G.M.; de Aguiar Andrade, E.H.; Bichara Zoghbi, M.G.; da Silva Santos, L. Constituents and pharmacological activities of Myrcia (Myrtaceae): A review of an aromatic and medicinal group of plants. Int. J. Mol. Sci. 2015, 16, 23881–23904. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.M.; Freitas de Oliveira, C.; Bednarczuk de Oliveira, V.; Martins Betim, F.C.; Gomes Miguel, O.; Dallarmi Miguel, M. Traditional uses, phytochemistry, and antimicrobial activities of Eugenia species—A review. Planta Med. 2018, 84, 1232–1248. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.A.; Woods, R.R.; Horswell, J.; Robinson, B.H. The potential in-situ antimicrobial ability of Myrtaceae plant species on pathogens in soil. Soil Biol. Biochem. 2016, 96, 1–3. [Google Scholar] [CrossRef]
- Kashman, Y.; Rotstein, A.; Lifshitz, A. The structure determination of two new acylphloroglucinols from Myrtus communis L. Tetrahedron 1974, 30, 991–997. [Google Scholar] [CrossRef]
- Bianchi, A. The Mediterranean aromatic plants and their culinary use. Nat. Prod. Res. 2015, 29, 201–206. [Google Scholar] [CrossRef]
- Montoro, P.; Tuberoso, C.I.; Piacente, S.; Perrone, A.; De Feo, V.; Cabras, P.; Pizza, C. Stability and antioxidant activity of polyphenols in extracts of Myrtus communis L. berries used for the preparation of myrtle liqueur. J. Pharm. Biomed. Anal. 2006, 41, 1614–1619. [Google Scholar] [CrossRef]
- Craven, L.A. New combinations in Melaleuca for Australian species of Callistemon (Myrtaceae). Novon 2006, 16, 468–478. [Google Scholar] [CrossRef]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef]
- Lounasmaa, M.; Puri, H.S.; Widén, C.-J. Phloroglucinol derivatives of Callistemon lanceolatus leaves. Phytochemistry 1977, 16, 1851–1852. [Google Scholar] [CrossRef]
- Carroll, A.R.; Lamb, J.; Moni, R.; Guymer, G.P.; Forster, P.I.; Quinn, R.J. Myrtucommulones F−I, phloroglucinols with thyrotropin-releasing hormone receptor-2 binding affinity from the seeds of Corymbia scabrida. J. Nat. Prod. 2008, 71, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Khanh, P.N.; Duc, H.V.; Huong, T.T.; Son, N.T.; Ha, V.T.; Van, D.T.; Tai, B.H.; Kim, J.E.; Jo, A.R.; Kim, Y.H.; et al. Alkylphloroglucinol derivatives and triterpenoids with soluble epoxide hydrolase inhibitory activity from Callistemon citrinus. Fitoterapia 2016, 109, 39–44. [Google Scholar] [CrossRef]
- Qin, X.J.; Liu, H.; Yu, Q.; Yan, H.; Tang, J.F.; An, L.K.; Khan, A.; Chen, Q.R.; Hao, X.J.; Liu, H.Y. Acylphloroglucinol derivatives from the twigs and leaves of Callistemon salignus. Tetrahedron 2017, 73, 1803–1811. [Google Scholar] [CrossRef]
- Bloor, S.J. Antiviral phloroglucinols from New Zealand Kunzea species. J. Nat. Prod. 1992, 55, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Kaneshima, T.; Myoda, T.; Toeda, K.; Fujimori, T.; Nishizawa, M. Antimicrobial constituents of peel and seeds of camu-camu (Myrciaria dubia). Biosci. Biotechnol. Biochem. 2017, 81, 1461–1465. [Google Scholar] [CrossRef]
- Salni, D.; Sargent, M.V.; Skelton, B.W.; Soediro, I.; Sutisna, M.; White, A.H.; Yulinah, E. Rhodomyrtone, an antibotic from Rhodomyrtus tomentosa. Aus. J. Chem. 2002, 55, 229–232. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R. Eucalyptone G, a new phloroglucinol derivative and other constituents from Eucalyptus globulus Labill. Arkivoc 2007, 15, 281–291. [Google Scholar]
- Senadeera, S.P.; Duffy, S.; Avery, V.M.; Carroll, A.R. Antiplasmodial β-triketones from the flowers of the Australian tree Angophora woodsiana. Bioorg. Med. Chem. Lett. 2017, 27, 2602–2607. [Google Scholar] [CrossRef]
- Larsen, L.; Benn, M.H.; Parvez, M.; Perry, N.B. A cytotoxic triketone–phloroglucinol–bullatenone hybrid from Lophomyrtus bullata. Org. Biomol. Chem. 2005, 3, 3236–3241. [Google Scholar] [CrossRef]
- Woollard, J.M.R.; Perry, N.B.; Weavers, R.T.; van Klink, J.W. Bullatenone, 1, 3-dione and sesquiterpene chemotypes of Lophomyrtus species. Phytochemistry 2008, 69, 1313–1318. [Google Scholar] [CrossRef]
- Shaheen, F.; Ahmad, M.; Khan, S.N.; Hussain, S.S.; Anjum, S.; Tashkhodjaev, B.; Turguniv, K.; Sultankhodzhaev, M.N.; Choudhary, M.I.; Ur-Rahman, A. New α-glucosidase inhibitors and antibacterial compounds from Myrtus communis L. Eur. J. Org. Chem. 2006, 2016, 2371–2377. [Google Scholar] [CrossRef]
- Hiranrat, A.; Mahabusarakam, W. New acylphloroglucinols from the leaves of Rhodomyrtus tomentosa. Tetrahedron 2008, 64, 11193–11197. [Google Scholar] [CrossRef]
- Cottiglia, F.; Casu, L.; Leonti, M.; Caboni, P.; Floris, C.; Busonera, B.; Farci, P.; Ouhtit, A.; Sanna, G. Cytotoxic phloroglucinols from the leaves of Myrtus communis. J. Nat. Prod. 2012, 75, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Hiranrat, A.; Chitbankluoi, W.; Mahabusarakam, W.; Limsuwan, S.; Voravuthikunchai, S.P. A new flavellagic acid derivative and phloroglucinol from Rhodomyrtus tomentosa. Nat. Prod. Res. 2012, 26, 1904–1909. [Google Scholar] [CrossRef]
- Hiranrat, A.; Mahabusarakam, W.; Carroll, A.R.; Duffy, S.; Avery, V.M. Tomentosones A and B, hexacyclic phloroglucinol derivatives from the Thai shrub Rhodomyrtus tomentosa. J. Org. Chem. 2012, 77, 680–683. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Khan, N.; Ahmad, M.; Yousuf, S.; Fun, H.-K.; Soomro, S.; Asif, M.; Mesaik, M.A.; Shaheen, F. New inhibitors of ROS generation and T-cell proliferation from Myrtus communis. Org. Lett. 2013, 15, 1862–1865. [Google Scholar] [CrossRef]
- Rattanaburi, S.; Mahabusarakam, W.; Phongpaichit, S.; Carroll, A.R. Acylphloroglucinols from Callistemon lanceolatus DC. Tetrahedron 2013, 69, 6070–6075. [Google Scholar] [CrossRef]
- Carroll, A.R.; Avery, V.M.; Duffy, S.; Forster, P.I.; Guymer, G.P. Watsonianone A-C, anti-plasmodial β-triketones from the Australian tree, Corymbia watsoniana. Org. Biomol. Chem. 2013, 21, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, J.; Zhao, P.; Zhou, Q.; Mei, Z.; Yang, G.; Yang, X.; Feng, Y. Chemical constituents from Eucalyptus citriodora Hook leaves and their glucose transporter 4 translocation activities. Bioorg. Med. Chem. Lett. 2014, 24, 3096–3099. [Google Scholar] [CrossRef]
- Hans, M.; Charpentier, M.; Huch, V.; Jauch, J.; Bruhn, T.; Bringmann, G.; Quandt, D. Stereoisomeric composition of natural myrtucommulone A. J. Nat. Prod. 2015, 78, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Dalal, S.; Goetz, M.; Cassera, M.B.; Kingston, D.G.I. Antiplasmodial phloroglucinol derivatives from Syncarpia glomulifera. Bioorg. Med. Chem. 2016, 24, 2544–2548. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-X.; Chen, Y.-C.; Liu, Y.; Zhang, W.-M.; Wu, J.-W.; Tan, H.-B.; Qiu, S.-X. Acylphloroglucinols from the leaves of Callistemon viminalis. Fitoterapia 2016, 114, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Q.; Huang, X.J.; Li, Y.T.; Wang, Y.; Wang, L.; Jiang, R.W.; Ye, W.C. Callistrilones A and B, triketone–phloroglucinol–monoterpene hybrids with a new skeleton from Callistemon rigidus. Org. Lett. 2016, 18, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-X.; Tan, H.-B.; Qiu, S.-X. Antimicrobial acylphloroglucinols from the leaves of Rhodomyrtus tomentosa. J. Asian Nat. Prod. Res. 2016, 18, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Hiranrat, W.; Hiranrat, A.; Mahabusarakam, W. Rhodomyrtosones G and H, minor phloroglucinols from the leaves of Rhodomyrtus tomentosa. Phytochem. Lett. 2017, 21, 25–28. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Wang, X.; Liu, R.; Yang, M.; Kong, L.; Luo, J. Acylphloroglucinols from the fruits of Callistemon viminalis. Phytochem. Lett. 2017, 20, 61–65. [Google Scholar] [CrossRef]
- Cao, J.Q.; Wu, Y.; Zhong, Y.L.; Li, N.P.; Chen, M.; Li, M.M.; Ye, W.C.; Wang, L. Antiviral triketone-phloroglucinol-monoterpene adducts from Callistemon rigidus. Chem. Biodiv. 2018, 15, e1800172. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Li, W.; Jiang, L.; Yang, L.; Chen, N.H.; Wu, Z.N.; Li, Y.L.; Wang, G.C. Cytotoxic and anti-inflammatory active phloroglucinol derivatives from Rhodomyrtus tomentosa. Phytochemistry 2018, 153, 111–119. [Google Scholar] [CrossRef]
- Tanaka, N.; Jia, Y.; Niwa, K.; Imabayashi, K.; Tatano, Y.; Yagi, H.; Kashiwada, Y. Phloroglucinol derivatives and a chromone glucoside from the leaves of Myrtus communis. Tetrahedron 2018, 74, 117–123. [Google Scholar] [CrossRef]
- Hou, J.Q.; Wang, B.L.; Han, C.; Xu, J.; Wang, Z.; He, Q.W.; Zhang, P.L.; Zhao, S.M.; Pei, X.; Wang, H. Atropisomeric meroterpenoids with rare triketone-phloroglucinol-terpene hybrids from Baeckea frutescens. Org. Biomol. Chem. 2018, 16, 8513–8524. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2009; ISBN 0471496413. [Google Scholar]
- Appendino, G.; Maxia, L.; Bettoni, P.; Locatelli, M.; Valdivia, C.; Ballero, M.; Stavri, M.; Gibbons, S.; Sterner, O. Antibacterial galloylated alkylphloroglucinol glucosides from myrtle (Myrtus communis). J. Nat. Prod. 2006, 69, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lu, W.J.; Li, N.P.; Liu, J.W.; He, J.; Ye, W.C.; Wang, L. Four new cinnamoyl-phloroglucinols from the leaves of Xanthostemon chrysanthus. Fitoterapia 2018, 128, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Su, J.C.; Wang, S.; Cheng, W.; Huang, X.J.; Li, M.M.; Jiang, R.W.; Li, Y.L.; Wang, L.; Ye, W.C.; Wang, Y. Phloroglucinol derivatives with unusual skeletons from Cleistocalyx operculatus and their in vitro antiviral activity. J. Org. Chem. 2018, 83, 8522–8532. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ang, S.; Huang, X.J.; Tian, H.Y.; Deng, Y.Y.; Zhang, D.M.; Wang, Y.; Ye, W.C.; Wang, L. Meroterpenoids with new skeletons from Myrtus communis and structure revision of myrtucommulone K. Org. Lett. 2016, 18, 4004–4007. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, X.B.; Li, R.J.; Zhang, Y.L.; Yang, M.H.; Luo, J.; Kong, L.Y. Callistiviminenes AO: Diverse adducts of β-triketone and sesqui-or monoterpene from the fruits of Callistemon viminalis. Phytochemistry 2016, 131, 140–149. [Google Scholar] [CrossRef]
- Ito, H.; Iwamori, H.; Kasajima, N.; Kaneda, M.; Yoshida, T. Kunzeanones A, B, and C: Novel alkylated phloroglucinol metabolites from Kunzea ambigua. Tetrahedron 2004, 60, 9971–9976. [Google Scholar] [CrossRef]
- Singh, I.P.; Sidana, J.; Bharate, S.B.; Foley, W.J. Phloroglucinol compounds of natural origin: Synthetic aspects. Nat. Prod. Rep. 2010, 27, 393–416. [Google Scholar] [CrossRef]
- Müller, H.; Paul, M.; Hartmann, D.; Huch, V.; Blaesius, D.; Koeberle, A.; Werz, O.; Jauch, J. Total synthesis of myrtucommulone A. Angewandte Chemie 2010, 49, 2045–2049. [Google Scholar] [CrossRef]
- Charpentier, M.; Hans, M.; Jauch, J. Enantioselective synthesis of myrtucommulone A. Eur. J. Org. Chem. 2013, 19, 4078–4084. [Google Scholar] [CrossRef]
- Charpentier, M.; Jauch, J. Metal catalysed versus organocatalysed stereoselective synthesis: The concrete case of myrtucommulones. Tetrahedron 2017, 73, 6614–6623. [Google Scholar] [CrossRef]
- Morkunas, M.; Dube, L.; Götz, F.; Maier, M.E. Synthesis of the acylphloroglucinols rhodomyrtone and rhodomyrtosone B. Tetrahedron 2013, 69, 8559–8563. [Google Scholar] [CrossRef]
- Morkunas, M.; Maier, M.E. Alternative routes to the acylphloroglucinol rhodomyrtone. Tetrahedron 2015, 71, 9662–9666. [Google Scholar] [CrossRef]
- Gervais, A.; Lazarski, K.E.; Porco, J.A., Jr. Divergent total syntheses of rhodomyrtosones A and B. J. Org. Chem. 2015, 80, 9584–9591. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huo, L.; Yang, B.; Yuan, Y.; Zhang, W.; Xu, Z.; Qiu, S.; Tan, H. Biomimetic-inspired syntheses of myrtucommuacetalone and myrtucommulone J. Org. Lett. 2017, 19, 4786–4789. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.J.; Cao, J.Q.; Yang, X.Y.; Zhong, L.P.; Hu, L.J.; Lu, X.; Hou, B.L.; Hu, Y.J.; Wang, Y.; You, X.F.; et al. Catalytic asymmetric total syntheses of myrtucommuacetalone, myrtucommuacetalone B, and callistrilones A, C, D and E. Chem. Sci. 2018, 9, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Dethe, D.H.; Dherange, B.D.; Das, S. Biomimetic total syntheses of callistrilones A, B, and D. Org. Lett. 2018, 20, 680–683. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Xiao, M.; Xie, Z. Biomimetic syntheses of callistrilones A–E via an oxidative [3 + 2] cycloaddition. Org. Lett. 2018, 20, 2509–2512. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.J.; Cheng, M.J.; Cao, J.Q.; Zhong, L.P.; Hu, Y.J.; Wang, Y.; Wang, L.; Ye, W.C.; Li, C.C. Asymmetric total syntheses of callistrilones B, G and J. Org. Chem. Front. 2018, 5, 1506–1510. [Google Scholar] [CrossRef]
- Nicoletti, R.; Fiorentino, A. Plant bioactive metabolites and drugs produced by endophytic fungi of Spermatophyta. Agriculture 2015, 5, 918–970. [Google Scholar] [CrossRef]
- Nicoletti, R.; Ferranti, P.; Caira, S.; Misso, G.; Castellano, M.; Di Lorenzo, G.; Caraglia, M. Myrtucommulone production by a strain of Neofusicoccum australe endophytic in myrtle (Myrtus communis). World J. Microbiol. Biotechnol. 2014, 30, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Massaro, C.F.; Smyth, T.J.; Smyth, W.F.; Heard, T.; Leonhardt, S.D.; Katouli, M.; Wallace, H.M.; Brooks, P. Phloroglucinols from anti-microbial deposit-resins of Australian stingless bees (Tetragonula carbonaria). Phytother. Res. 2015, 29, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, A.; Lifshitz, A.; Kashman, Y. Isolation and antibacterial activity of acylphloroglucinols from Myrtus communis. Antimicrob. Agents Chemother. 1974, 6, 539–542. [Google Scholar] [CrossRef]
- Limsuwan, S.; Trip, E.N.; Kouwen, T.R.; Piersma, S.; Hiranrat, A.; Mahabusarakam, W.; Voravuthikunchai, S.P.; Van Dijl, J.M.; Kayser, O. Rhodomyrtone: A new candidate as natural antibacterial drug from Rhodomyrtus tomentosa. Phytomedicine 2009, 16, 645–651. [Google Scholar] [CrossRef]
- Saising, J.; Ongsakul, M.; Voravuthikunchai, S.P. Rhodomyrtus tomentosa (Aiton) Hassk. ethanol extract and rhodomyrtone: A potential strategy for the treatment of biofilm-forming staphylococci. J. Med. Microbiol. 2011, 60, 1793–1800. [Google Scholar] [CrossRef]
- Saising, J.; Götz, F.; Dube, L.; Ziebandt, A.K.; Voravuthikunchai, S.P. Inhibition of staphylococcal biofilm-related gene transcription by rhodomyrtone, a new antibacterial agent. Ann. Microbiol. 2015, 65, 659–665. [Google Scholar] [CrossRef]
- Sianglum, W.; Srimanote, P.; Wonglumsom, W.; Kittiniyom, K.; Voravuthikunchai, S.P. Proteome analyses of cellular proteins in methicillin-resistant Staphylococcus aureus treated with rhodomyrtone, a novel antibiotic candidate. PLoS ONE 2011, 6, e16628. [Google Scholar] [CrossRef]
- Visutthi, M.; Srimanote, P.; Voravuthikunchai, S.P. Responses in the expression of extracellular proteins in methicillin-resistant Staphylococcus aureus treated with rhodomyrtone. J. Microbiol. 2011, 49, 956–964. [Google Scholar] [CrossRef]
- Limsuwan, S.; Homlaead, S.; Watcharakul, S.; Chusri, S.; Moosigapong, K.; Saising, J.; Voravuthikunchai, S.P. Inhibition of microbial adhesion to plastic surface and human buccal epithelial cells by Rhodomyrtus tomentosa leaf extract. Arch. Oral Biol. 2014, 59, 1256–1265. [Google Scholar] [CrossRef]
- Voravuthikunchai, S.P.; Dolah, S.; Charernjiratrakul, W. Control of Bacillus cereus in foods by Rhodomyrtus tomentosa (Ait.) hassk. leaf extract and its purified compound. J. Food Prot. 2010, 73, 1907–1912. [Google Scholar] [CrossRef]
- Srisuwan, S.; Tongtawe, P.; Srimanote, P.; Voravuthikunchai, S.P. Rhodomyrtone modulates innate immune responses of THP-1 monocytes to assist in clearing methicillin-resistant Staphylococcus aureus. PLoS ONE 2014, 9, e110321. [Google Scholar] [CrossRef] [PubMed]
- Limsuwan, S.; Hesseling-Meinders, A.; Voravuthikunchai, S.P.; Van Dijl, J.M.; Kayser, O. Potential antibiotic and anti-infective effects of rhodomyrtone from Rhodomyrtus tomentosa (Aiton) Hassk. on Streptococcus pyogenes as revealed by proteomics. Phytomedicine 2011, 18, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Saising, J.; Voravuthikunchai, S.P. Anti Propionibacterium acnes activity of rhodomyrtone, an effective compound from Rhodomyrtus tomentosa (Aiton) Hassk. leaves. Anaerobe 2012, 18, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Wunnoo, S.; Saising, J.; Voravuthikunchai, S.P. Rhodomyrtone inhibits lipase production, biofilm formation, and disorganizes established biofilm in Propionibacterium acnes. Anaerobe 2017, 43, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Leejae, S.; Taylor, P.W.; Voravuthikunchai, S.P. Antibacterial mechanisms of rhodomyrtone against important hospital-acquired antibiotic-resistant pathogenic bacteria. J. Med. Microbiol. 2013, 62, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Mordmuang, A.; Shankar, S.; Chethanond, U.; Voravuthikunchai, S.P. Effects of Rhodomyrtus tomentosa leaf extract on staphylococcal adhesion and invasion in bovine udder epidermal tissue model. Nutrients 2015, 7, 8503–8517. [Google Scholar] [CrossRef]
- Mitsuwan, W.; Olaya-Abril, A.; Calderon-Santiago, M.; Jimenez-Munguia, I.; Gonzalez-Reyes, J.A.; Priego-Capote, F.; Voravuthikunchai, S.P.; Rodriguez-Ortega, M.J. Integrated proteomic and metabolomic analysis reveals that rhodomyrtone reduces the capsule in Streptococcus pneumoniae. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Liu, H.X.; Wang, L.; Xu, Z.F.; Tan, H.B.; Qiu, S.X. Rhodomyrtosone B, a membrane-targeting anti-MRSA natural acylgphloroglucinol from Rhodomyrtus tomentosa. J. Ethnopharmacol. 2019, 228, 50–57. [Google Scholar] [CrossRef]
- Sianglum, W.; Srimanote, P.; Taylor, P.W.; Rosado, H.; Voravuthikunchai, S.P. Transcriptome analysis of responses to rhodomyrtone in methicillin-resistant Staphylococcus aureus. PLoS ONE 2012, 7, e45744. [Google Scholar] [CrossRef]
- Leejae, S.; Hasap, L.; Voravuthikunchai, S.P. Inhibition of staphyloxanthin biosynthesis in Staphylococcus aureus by rhodomyrtone, a novel antibiotic candidate. J. Med. Microbiol. 2013, 62, 421–428. [Google Scholar] [CrossRef]
- Saeloh, D.; Wenzel, M.; Rungrotmongkol, T.; Hamoen, L.W.; Tipmanee, V.; Voravuthikunchai, S.P. Effects of rhodomyrtone on Gram-positive bacterial tubulin homologue FtsZ. PeerJ 2017, e2962. [Google Scholar] [CrossRef]
- Tan, H.; Liu, H.; Zhao, L.; Yuan, Y.; Li, B.; Jiang, Y.; Gong, L.; Qiu, S. Structure-activity relationships and optimization of acyclic acylphloroglucinol analogues as novel antimicrobial agents. Eur. J. Med. Chem. 2017, 125, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, H.; Huo, L.; Wang, M.; Yang, B.; Zhang, W.; Xu, Z.; Tan, H.; Qiu, S.X. Structural optimization and antibacterial evaluation of rhodomyrtosone B analogues against MRSA strains. Med. Chem. Comm. 2018, 9, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Verotta, L. Are acylphloroglucinols lead structures for the treatment of degenerative diseases? Phytochem. Rev. 2003, 1, 389–407. [Google Scholar] [CrossRef]
- Senadeera, S.P.; Lucantoni, L.; Duffy, S.; Avery, V.M.; Carroll, A.R. Antiplasmodial β-triketone-flavanone hybrids from the flowers of the Australian tree Corymbia torelliana. J. Nat. Prod. 2018, 81, 1588–1597. [Google Scholar] [CrossRef]
- Rosa, A.; Deiana, M.; Casu, V.; Corona, G.; Appendino, G.; Bianchi, F.; Ballero, M.; Dessì, M.A. Antioxidant activity of oligomeric acylphloroglucinols from Myrtus communis L. Free Radic. Res. 2003, 37, 1013–1019. [Google Scholar]
- Rosa, A.; Melis, M.P.; Deiana, M.; Atzeri, A.; Appendino, G.; Corona, G.; Incani, A.; Loru, D.; Dessì, M.A. Protective effect of the oligomeric acylphloroglucinols from Myrtus communis on cholesterol and human low density lipoprotein oxidation. Chem. Phys. Lipids 2008, 155, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Feißt, C.; Franke, L.; Appendino, G.; Werz, O. Identification of molecular targets of the oligomeric nonprenylated acylphloroglucinols from Myrtus communis and their implication as anti-inflammatory compounds. J. Pharmacol. Exp. Ther. 2005, 315, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, A.; Pollastro, F.; Northoff, H.; Werz, O. Myrtucommulone, a natural acylphloroglucinol, inhibits microsomal prostaglandin E2 synthase-1. Br. J. Pharmacol. 2009, 156, 952–961. [Google Scholar] [CrossRef]
- Rossi, A.; Di Paola, R.; Mazzon, E.; Genovese, T.; Caminiti, R.; Bramanti, P.; Pergola, C.; Koeberle, A.; Werz, O.; Sautebin, L.; et al. Myrtucommulone from Myrtus communis exhibits potent anti-inflammatory effectiveness in vivo. J. Pharmacol. Exp. Ther. 2009, 329, 76–86. [Google Scholar] [CrossRef]
- Chorachoo, J.; Lambert, S.; Furnholm, T.; Roberts, L.; Reingold, L.; Auepemkiate, S.; Voravuthikunchai, S.P.; Johnston, A. The small molecule rhodomyrtone suppresses TNF-α and IL-17A-induced keratinocyte inflammatory responses: A potential new therapeutic for psoriasis. PLoS ONE 2018, 13, e0205340. [Google Scholar] [CrossRef] [PubMed]
- Chorachoo, J.; Saeloh, D.; Srichana, T.; Amnuaikit, T.; Musthafa, K.S.; Sretrirutchai, S.; Voravuthikunchai, S.P. Rhodomyrtone as a potential anti-proliferative and apoptosis inducing agent in HaCaT keratinocyte cells. Eur. J. Pharmacol. 2016, 772, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Srisuwan, S.; Mackin, K.E.; Hocking, D.; Lyras, D.; Bennett-Wood, V.; Voravuthikunchai, S.P.; Robins-Browne, R.M. Antibacterial activity of rhodomyrtone on Clostridium difficile vegetative cells and spores in vitro. Int. J. Antimicrob. Agents 2018, 52, 724–729. [Google Scholar] [CrossRef]
- Na-Phatthalung, P.; Teles, M.; Voravuthikunchai, S.P.; Tort, L.; Fierro-Castro, C. Immunomodulatory effects of Rhodomyrtus tomentosa leaf extract and its derivative compound, rhodomyrtone, on head kidney macrophages of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2018, 44, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Tretiakova, I.; Blaesius, D.; Maxia, L.; Wesselborg, S.; Schulze-Osthoff, K.; Cinatl, J.; Michaelis, M.; Werz, O. Myrtucommulone from Myrtus communis induces apoptosis in cancer cells via the mitochondrial pathway involving caspase-9. Apoptosis 2008, 13, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Wiechmann, K.; Müller, H.; Fischer, D.; Jauch, J.; Werz, O. The acylphloroglucinols hyperforin and myrtucommulone A cause mitochondrial dysfunctions in leukemic cells by direct interference with mitochondria. Apoptosis 2015, 20, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Wiechmann, K.; Müller, H.; König, S.; Wielsch, N.; Svatoš, A.; Jauch, J.; Werz, O. Mitochondrial chaperonin HSP60 is the apoptosis-related target for myrtucommulone. Cell Chem. Biol. 2017, 24, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Grandjenette, C.; Schnekenburger, M.; Morceau, F.; Mack, F.; Wiechmann, K.; Werz, O.; Dicato, M.; Diederich, M. Dual induction of mitochondrial apoptosis and senescence in chronic myelogenous leukemia by myrtucommulone A. Anti-Cancer Agents Med. Chem. 2015, 15, 363–373. [Google Scholar] [CrossRef]
- Izgi, K.; Iskender, B.; Jauch, J.; Sezen, S.; Cakir, M.; Charpentier, M.; Canatan, H.; Sakalar, C. Myrtucommulone-A induces both extrinsic and intrinsic apoptotic pathways in cancer cells. J. Biochem. Mol. Toxicol. 2015, 29, 432–439. [Google Scholar] [CrossRef]
- Izgi, K.; Iskender, B.; Sakalar, C.; Arslanhan, A.; Yüksek, E.H.; Hizar, E.; Canatan, H. Effects of epirubicin and cisplatin against 4T1 breast cancer cells are enhanced by myrtucommulone-A. Anti-Cancer Agents Med. Chem. 2017, 17, 404–414. [Google Scholar] [CrossRef]
- Izgi, K.; Sonmez, M.F.; Canatan, H.; Iskender, B. Long term exposure to myrtucommulone-A changes CD105 expression and differentiation potential of mesenchymal stem cells. Tissue Engin. Regenener. Med. 2017, 14, 113–121. [Google Scholar] [CrossRef]
- Iskender, B.; Izgi, K.; Sakalar, C.; Canatan, H. Priming hMSCs with a putative anti-cancer compound, myrtucommulone-a: A way to harness hMSC cytokine expression via modulating PI3K/Akt pathway? Tumor Biol. 2016, 37, 1967–1981. [Google Scholar] [CrossRef] [PubMed]
- Iskender, B.; Izgi, K.; Canatan, H. Novel anti-cancer agent myrtucommulone-A and thymoquinone abrogate epithelial–mesenchymal transition in cancer cells mainly through the inhibition of PI3K/AKT signalling axis. Mol. Cell. Biochem. 2016, 416, 71–84. [Google Scholar] [CrossRef]
- Iskender, B.; Izgi, K.; Karaca, H.; Canatan, H. Myrtucommulone-A treatment decreases pluripotency-and multipotency-associated marker expression in bladder cancer cell line HTB-9. J. Nat. Med. 2015, 69, 543–554. [Google Scholar] [CrossRef]
- Gerbeth, K.; Meins, J.; Werz, O.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Determination of myrtucommulone from Myrtus communis in human and rat plasma by liquid chromatography/tandem mass spectrometry. Planta Med. 2011, 77, 450–454. [Google Scholar] [CrossRef]
- Saising, J.; Nguyen, M.-T.; Haertner, T.; Ebner, P.; Al Mamun Bhuyan, A.; Berscheid, A.; Muehlenkamp, M.; Schaekermann, S.; Kumari, N.; Maier, M.E.; et al. Rhodomyrtone (Rom) is a membrane-active compound. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Saeloh, D.; Tipmanee, V.; Jim, K.K.; Dekker, M.P.; Bitter, W.; Voravuthikunchai, S.P.; Wenzel, M.; Hamoen, L.W. The novel antibiotic rhodomyrtone traps membrane proteins in vesicles with increased fluidity. PLoS Pathog. 2018, 14, e1006876. [Google Scholar] [CrossRef]
- Tayeh, M.; Nilwarangoon, S.; Mahabusarakum, W.; Watanapokasin, R. Anti-metastatic effect of rhodomyrtone from Rhodomyrtus tomentosa on human skin cancer cells. Int. J. Oncol. 2017, 50, 1035–1043. [Google Scholar] [CrossRef]
- Romani, A.; Coinu, R.; Carta, S.; Pinelli, P.; Galardi, C.; Vincieri, F.F.; Franconi, F. Evaluation of antioxidant effect of different extracts of Myrtus communis L. Free Radic. Res. 2004, 38, 97–103. [Google Scholar] [CrossRef]
- Geetha, K.M.; Sridhar, C.; Murugan, V. Antioxidant and healing effect of aqueous alcoholic extract of Rhodomyrtus tomentosa (Ait.) Hassk on chronic gastric ulcers in rats. J. Pharm. Res. 2010, 3, 2860–2862. [Google Scholar]
- Jeong, D.; Yang, W.S.; Yang, Y.; Nam, G.; Kim, J.H.; Yoon, D.H.; Noh, H.J.; Lee, S.; Kim, T.W.; Sung, G.H.; et al. In vitro and in vivo anti-inflammatory effect of Rhodomyrtus tomentosa methanol extract. J. Ethnopharmacol. 2013, 146, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, M.; Kopaei, M.R.; Miraj, S. A comparison of the efficacy of metronidazole vaginal gel and Myrtus (Myrtus communis) extract combination and metronidazole vaginal gel alone in the treatment of recurrent bacterial vaginosis. Avicenna J. Phytomed. 2017, 7, 129–136. [Google Scholar] [PubMed]
- Fiorini-Puybaret, C.; Aries, M.F.; Fabre, B.; Mamatas, S.; Luc, J.; Degouy, A.; Ambonati, M.; Mejean, C.; Poli, F. Pharmacological properties of Myrtacine® and its potential value in acne treatment. Planta Med. 2011, 77, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Feuillolay, C.; Pecastaings, S.; Le Gac, C.; Fiorini-Puybaret, C.; Luc, J.; Joulia, P.; Roques, C. A Myrtus communis extract enriched in myrtucummulones and ursolic acid reduces resistance of Propionibacterium acnes biofilms to antibiotics used in acne vulgaris. Phytomedicine 2016, 23, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Chorachoo, J.; Amnuaikit, T.; Voravuthikunchai, S.P. Liposomal encapsulated rhodomyrtone: A novel antiacne drug. Evid.-Based Complement. Altern. Med. 2013, 157635. [Google Scholar] [CrossRef] [PubMed]
- Leejae, S.; Yingyongnarongkul, B.E.; Suksamrarn, A.; Voravuthikunchai, S.P. Synthesis and structure–activity relationship of rhodomyrtone derivatives as antibacterial agent. Chin. Chem. Lett. 2012, 23, 1011–1014. [Google Scholar] [CrossRef]
- Wiechmann, K.; Müller, H.; Huch, V.; Hartmann, D.; Werz, O.; Jauch, J. Synthesis and biological evaluation of novel myrtucommulones and structural analogues that target mPGES-1 and 5-lipoxygenase. Eur. J. Med. Chem. 2015, 101, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Cebola, M.J.; Oliveira, M.C.; Bernardo-Gil, M.G. Supercritical fluid extraction vs conventional extraction of myrtle leaves and berries: Comparison of antioxidant activity and identification of bioactive compounds. J. Supercrit. Fluids 2016, 113, 1–9. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Arráez-Román, D.; Segura-Carretero, A.; Ferranti, P.; Nicoletti, R.; Perrotta, G.M.; Gómez-Caravaca, A.M. Establishment of pressurized-liquid extraction by response surface methodology approach coupled to HPLC-DAD-TOF-MS for the determination of phenolic compounds of myrtle leaves. Anal. Bioanal. Chem. 2018, 410, 3547–3557. [Google Scholar] [CrossRef]
- González de Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Jiménez-Cantizano, A.; Ferreiro-González, M.; Amores-Arrocha, A.; Palma, M.; Barroso, C.G.; Barbero, F.G. Development of new analytical microwave-assisted extraction methods for bioactive compounds from myrtle (Myrtus communis L.). Molecules 2018, 23, 2992. [Google Scholar] [CrossRef]
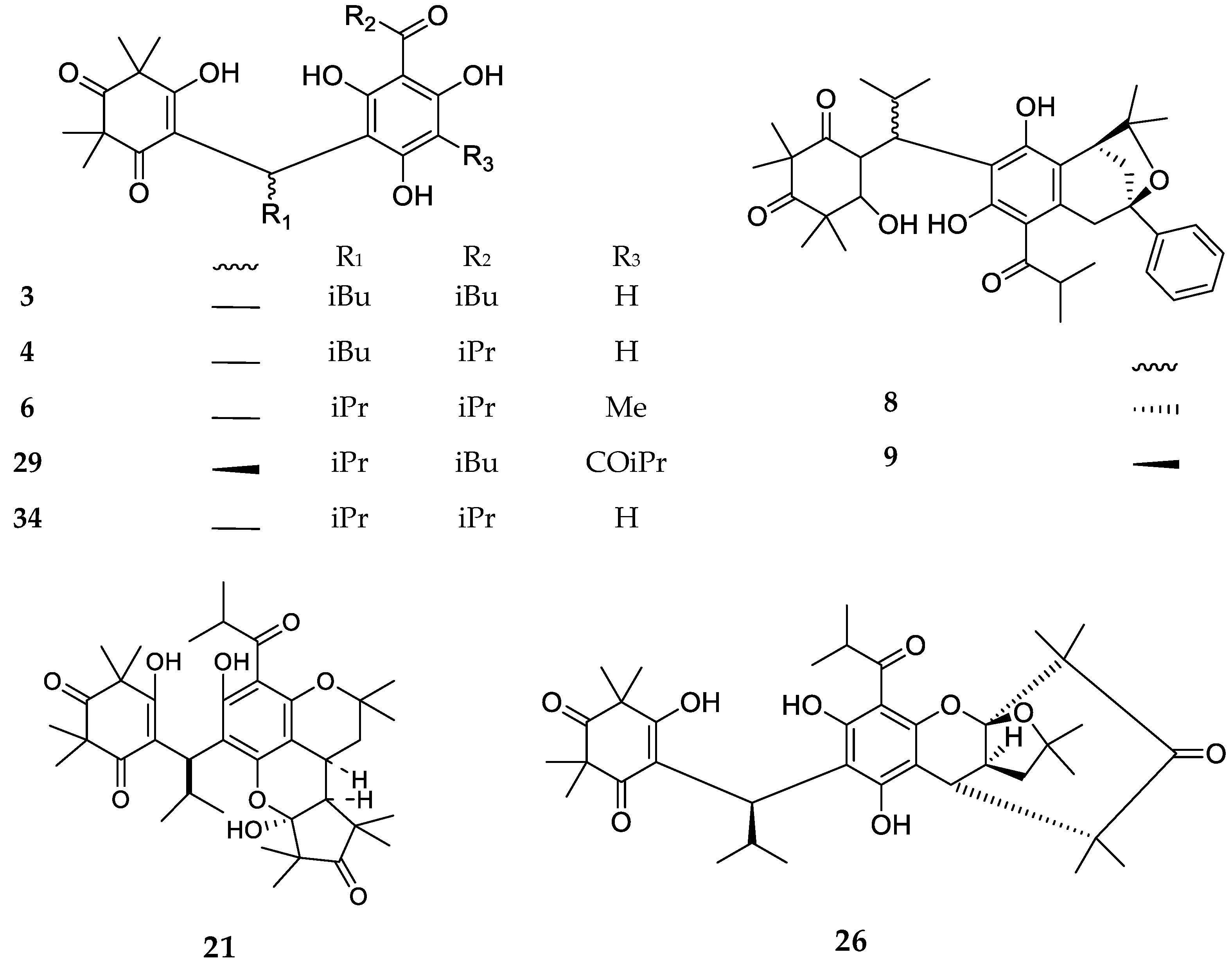
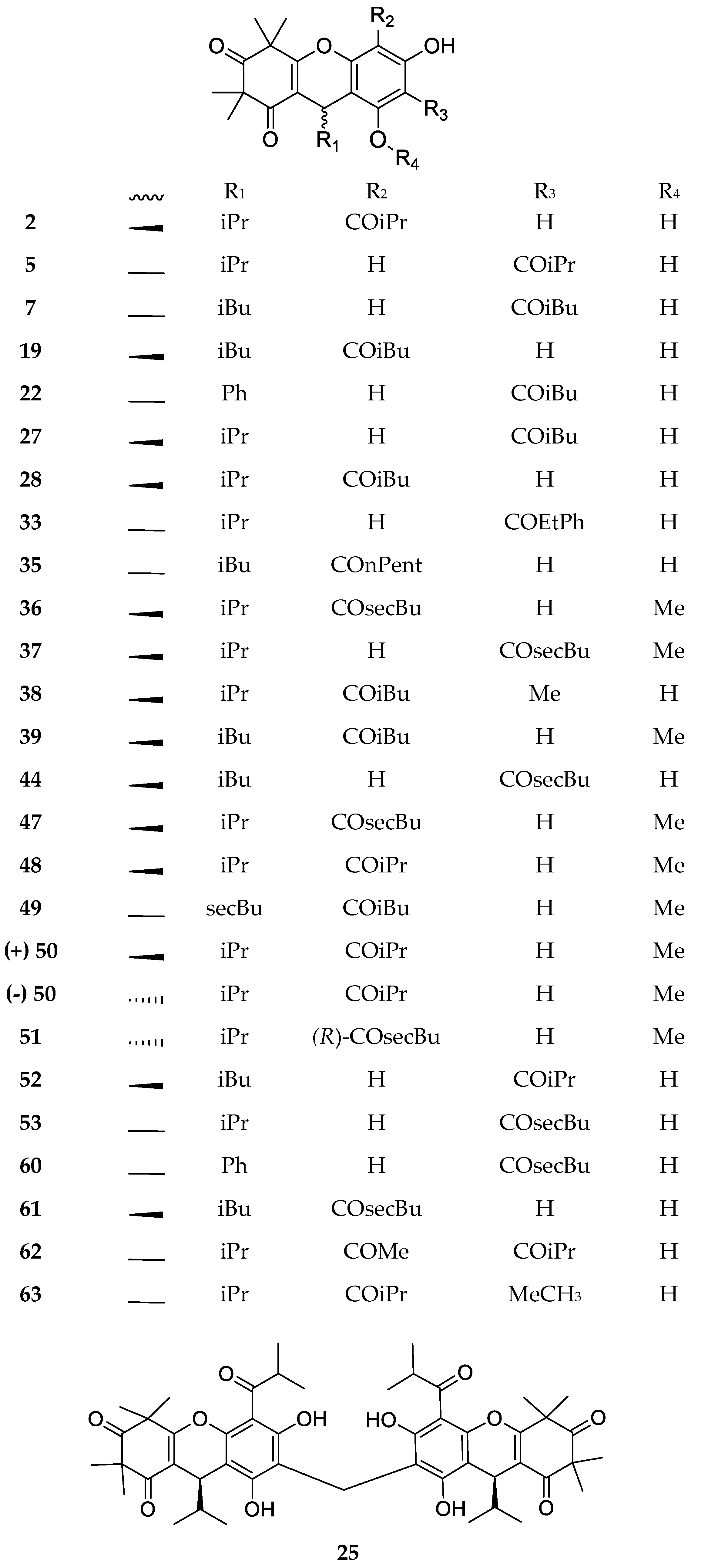
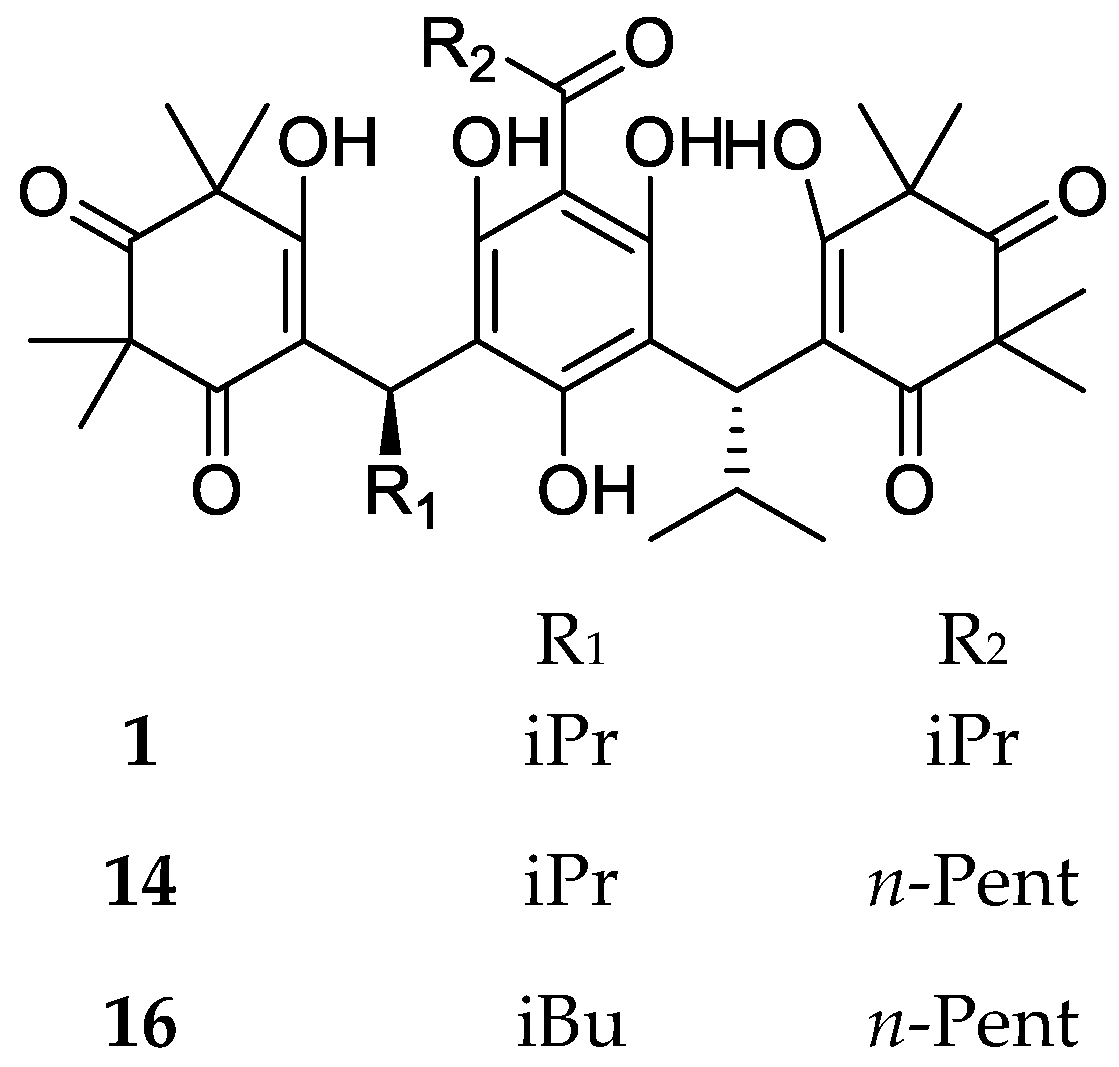
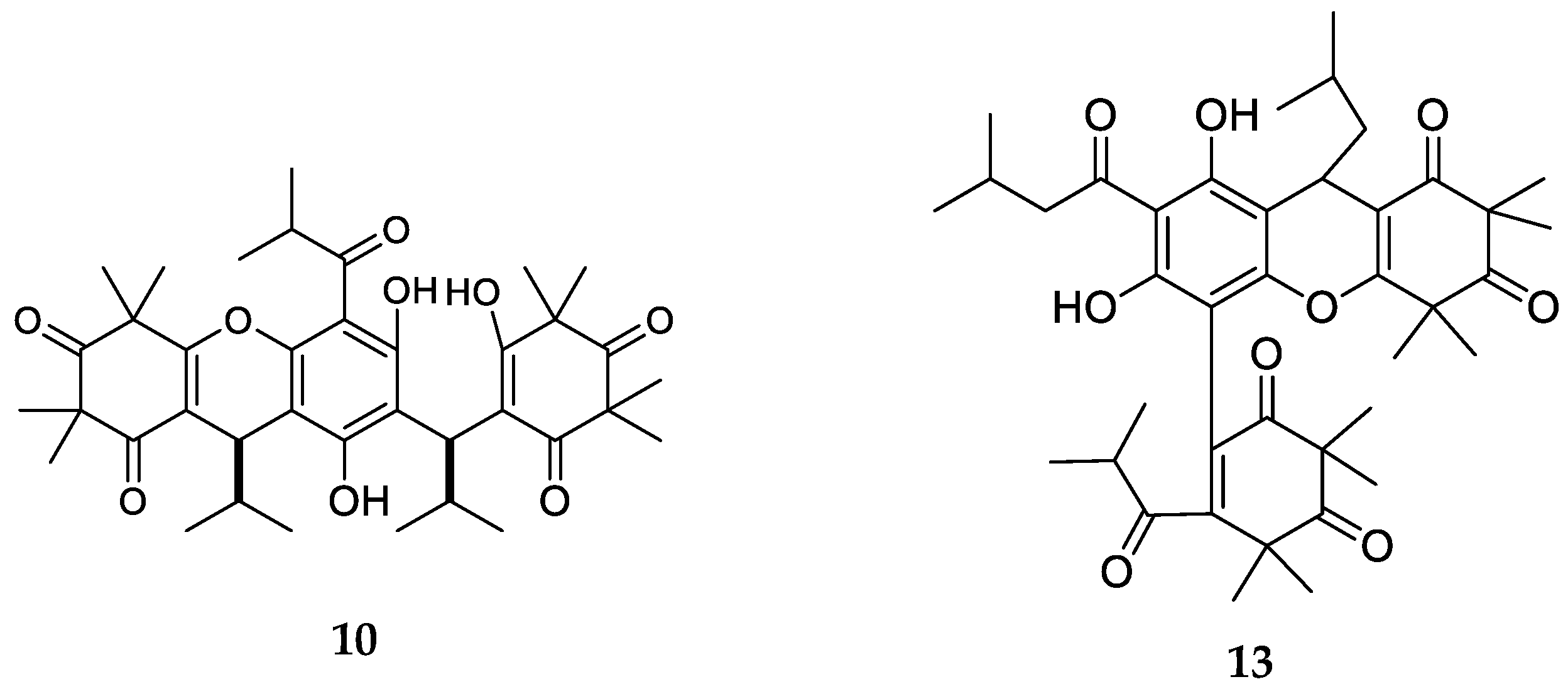
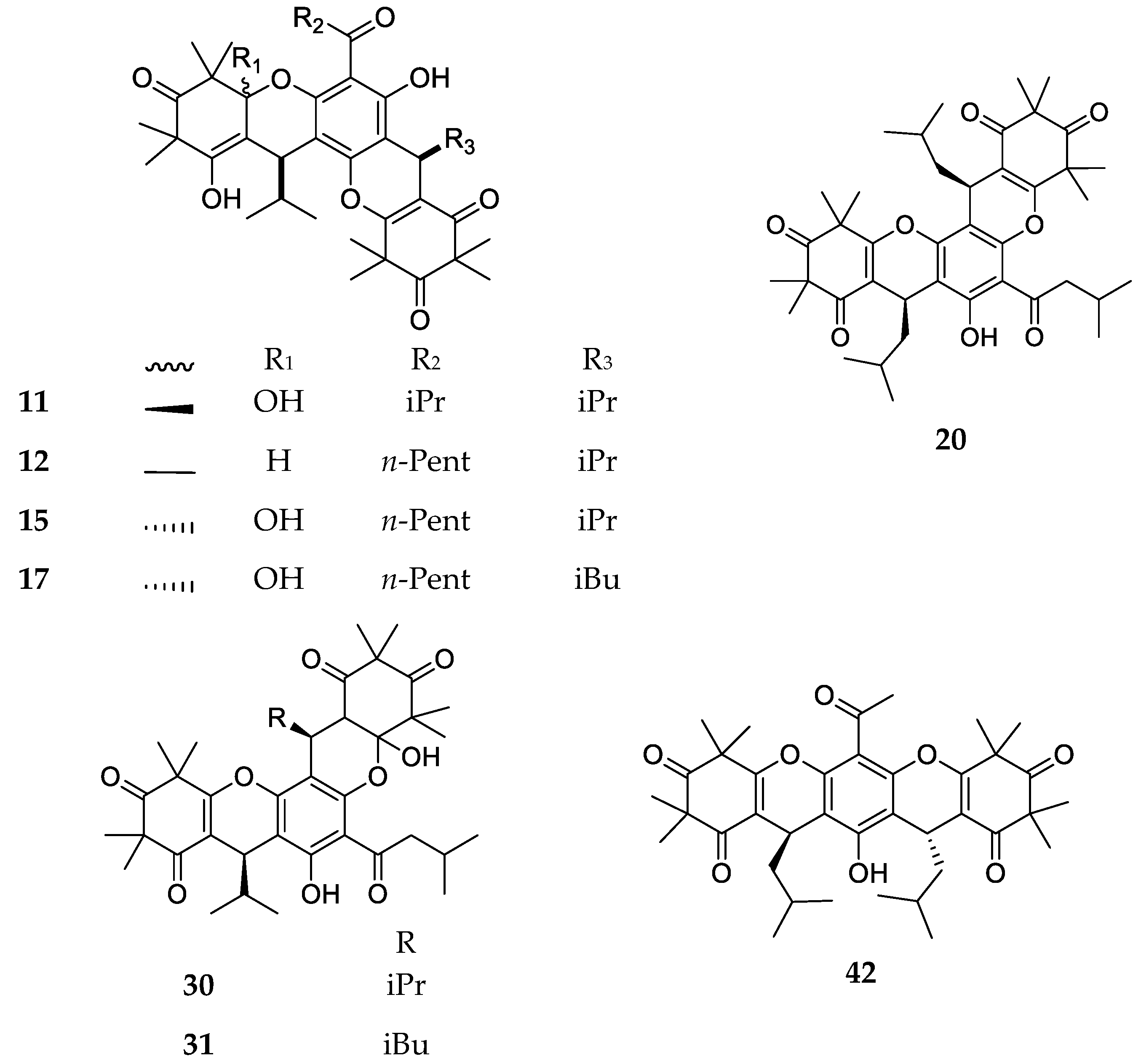
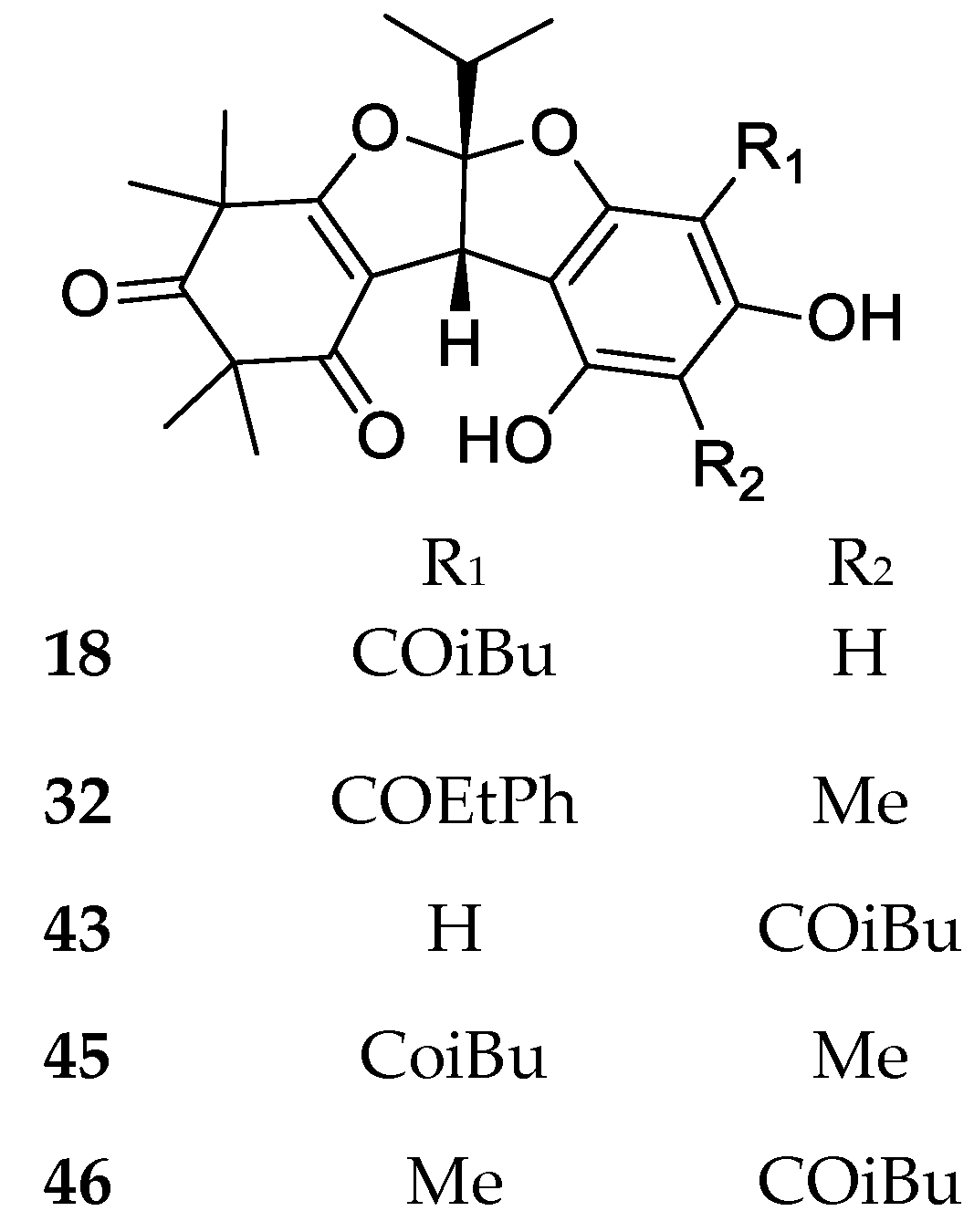
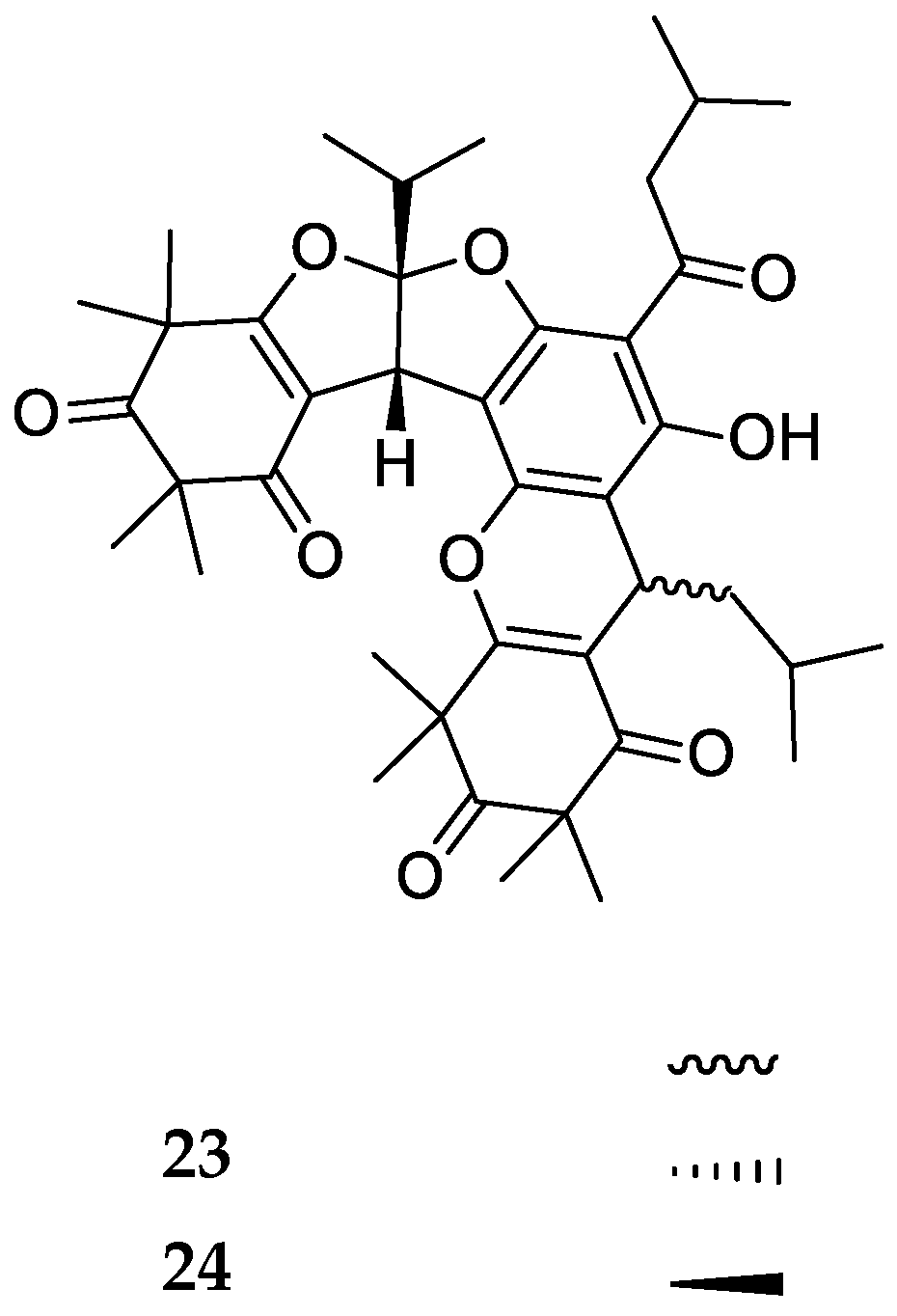

| Code | Compound Name | Formula, Nominal Mass (U) | Source | Ref. |
|---|---|---|---|---|
| 1 | Myrtucommulone A | C38H52O10, 668 | Myrtus communis Melaleuca citrina1 Corymbia scabrida | [17] [22] [23] |
| 2 | Myrtucommulone B | C25H32O5, 412 | Myrtus communis Melaleuca citrina1 Melaleuca salicina1 | [17] [24] [25] |
| 3 | 4-Cyclohexene-1,3-dioxo-5-hydroxy-2,2,6,6-tetramethy1-4-{1-[2,6-dihydroxy-4-methoxy-3-(3-methyl-1-oxo-butyl)phenyl]-3-methylbutyl} | C26H36O7, 460 | Kunzea ericoides Kunzea sinclairii | [26] |
| 4 | 4-Cyclohexene-l,3-dioxo-5-hydroxy-2,2,6,6-tetramethyl-4-{l-[2,6-dihydroxy-4-methoxy-3-(2-methyl-1-oxopropyl)pheny1]-3-methylbutyl} | C25H34O7, 446 | Kunzea ericoides Kunzea sinclairii | [26] |
| 5 | Isomyrtucommulone B | C24H30O6, 414 | Myrtus communis Melaleuca salicina1 Myrciaria dubia | [5] [25] [27] |
| 6 | Semimyrtucommulone | C25H34O7, 446 | Myrtus communis | [5] |
| 7 | Rhodomyrtone A | C26H34O6, 442 | Rhodomyrtus tomentosa Eucalyptus globulus Angophora woodsiana Myrciaria dubia | [28] [29] [30] [27] |
| 8 | Bullataketal A | C37H48O7, 604 | Lophomyrtus bullata Lophomyrtus obcordata | [31] [32] |
| 9 | Bullataketal B | C37H48O7, 604 | ||
| 10 | Myrtucommulone C | C38H50O9, 650 | Myrtus communis | [33] |
| 11 | Myrtucommulone D | C38H50O9, 650 | Myrtus communis Corymbia scabrida Melaleuca salicina1 | [33] [23] [25] |
| 12 | Myrtucommulone E | C38H48O8, 632 | Myrtus communis | [33] |
| 13 | Eucalyptone G | C40H52O9, 676 | Eucalyptus globulus | [29] |
| 14 | Myrtucommulone F | C40H56O10, 696 | Corymbia scabrida | [23] |
| 15 | Myrtucommulone G | C40H54O9, 678 | ||
| 16 | Myrtucommulone H | C41H58O10, 710 | ||
| 17 | Myrtucommulone I | C41H56O9, 692 | ||
| 18 | Rhodomyrtosone A | C26H32O7, 456 | Rhodomyrtus tomentosa Angophora woodsiana | [34] [30] |
| 19 | Rhodomyrtosone B | C26H34O6, 442 | Rhodomyrtus tomentosa | [34] |
| 20 | Rhodomyrtosone C | C41H54O8, 674 | ||
| 21 | Myrtucommulone J | C38H52O8, 636 | Myrtus communis | [35] |
| 22 | Rhodomyrtosone I | C28H30O6, 462 | Rhodomyrtus tomentosa | [36] |
| 23 | Tomentosone A | C41H52O9, 688 | Rhodomyrtus tomentosa | [37] |
| 24 | Tomentosone B | C41H52O9, 688 | ||
| 25 | Myrtucommulone M | C49H60O12, 840 | Myrtus communis | [38] |
| 26 | Myrtucommuacetalone | C38H52O9, 652 | ||
| 27 | Callistenone A | C25H32O6, 428 | Melaleuca citrina1 | [39] |
| 28 | Callistenone B | C25H32O6, 428 | Melaleuca citrina1 Melaleuca salicina1 | [39] [25] |
| 29 | Callistenone C | C29H40O8, 516 | Melaleuca citrina1 | [39] |
| 30 | Callistenone D | C39H52O9, 664 | ||
| 31 | Callistenone E | C40H54O8, 662 | ||
| 32 | Watsonianone B | C31H34O7, 518 | Corymbia watsoniana | [40] |
| 33 | Rhodomyrtosone E | C30H34O6, 490 | Eucalyptus citriodora | [41] |
| 34 | Nor-semimyrtucommulone | C24H32O7, 432 | Myrtus communis | [42] |
| 35 | Rhodomyrtosone F | C27H36O6, 456 | Syncarpia glomulifera | [43] |
| 36 | Callistenone F | C26H34O6, 442 | Melaleuca viminalis1 | [44] |
| 37 | Callistenone G | C26H34O6, 442 | ||
| 38 | Callistenone H | C26H34O6, 442 | Melaleuca viminalis1 Melaleuca salicina1 | [44] [25] |
| 39 | Callistenone I | C27H36O6, 456 | Melaleuca viminalis1 | [44] |
| 40 | Callistrilone A | C33H42O7, 550 | Melaleuca linearis1 | [45] |
| 41 | Callistrilone B | C35H46O6, 562 | ||
| 42 | Tomentosone C | C38H48O8, 632 | Rhodomyrtus tomentosa | [46] |
| 43 | Rhodomyrtosone G | C26H32O7, 456 | Rhodomyrtus tomentosa | [47] |
| 44 | Rhodomyrtosone H | C26H34O6, 442 | ||
| 45 | Callistenone L | C27H34O7, 470 | Melaleuca viminalis1 | [48] |
| 46 | Callistenone M | C27H34O7, 470 | ||
| 47 | Callistenone N | C26H34O6, 442 | ||
| 48 | Callistenone O | C25H32O6, 428 | ||
| 49 | Callistenone P | C27H36O6, 456 | ||
| 50 | Callisalignone B | C25H32O6, 428 | Melaleuca salicina1 | [25] |
| 51 | Callisalignone C | C26H34O6, 442 | ||
| 52 | Myrciarone A | C25H32O6, 428 | Myrciaria dubia | [27] |
| 53 | Myrciarone B | C25H32O6, 428 | ||
| 54 | Callistrilone F | C35H48O8, 596 | Melaleuca linearis1 | [49] |
| 55 | Callistrilone G | C35H48O7, 580 | ||
| 56 | Callistrilone H | C35H52O7, 608 | ||
| 57 | Callistrilone I | C36H50O7, 594 | ||
| 58 | Callistrilone J | C37H52O7, 608 | ||
| 59 | Callistrilone K | C36H48O6, 576 | ||
| 60 | Tomentodione S | C28H32O5, 448 | Rhodomyrtus tomentosa | [50] |
| 61 | Tomentodione T | C28H32O5, 448 | ||
| 62 | 6-Methylisomyrtucommulone B | C25H32O6, 428 | Myrtus communis | [51] |
| 63 | 4-Methylmyrtucommulone B | C25H32O6, 428 | ||
| 64 | Baefrutone A | C35H48O7, 580 | Baeckea frutescens | [52] |
| 65 | Baefrutone B | C35H48O7, 580 | ||
| 66 | Baefrutone C | C35H48O7, 580 | ||
| 67 | Baefrutone D | C35H48O7, 580 | ||
| 68 | Baefrutone E | C40H56O7, 648 | ||
| 69 | Baefrutone F | C40H56O7, 648 |
| Code | Compound Name | Subclass | Ref. |
|---|---|---|---|
| 1 | Myrtucommulone A | Trimeric type | [61,62,63] |
| 7 | Rhodomyrtone A | Dimeric-monopyrane | [64,65] |
| 18 | Rhodomyrtosone A | Dimeric-bisfurane type | [66] |
| 19 | Rhodomyrtosone B | Dimeric-monopyrane type | [64,65,66] |
| 21 | Myrtucommulone J | Dimeric type | [67] |
| 26 | Myrtucommuacetalone | Dimeric type | [67,68] |
| 40 | Callistrilone A | Terpene-adduct type | [68,69,70] |
| 55, 58 | Callistrilone G, J | Terpene-adduct type | [71] |
| Bc | Bs | Cd | Ef | Ml | Pa | Sa | MRSa | Se | Sg | Sm | Spn | Spy | Ss | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1–2 | 0.5 | [5] | ||||||||||||
| 2 | 7.813 | [25] | |||||||||||||
| 8–16 | 16–32 | 16–32 | [51] | ||||||||||||
| 5 | 1.56 | 0.78 | 1.56 | 6.25 | 3.13 | [27] | |||||||||
| 0.8 | [10] | ||||||||||||||
| 0.488 | [25] | ||||||||||||||
| 1–2 | 1–2 | 0.5–1 | [51] | ||||||||||||
| 6 | 32–64 | 32 | [5] | ||||||||||||
| 7 | 0.39 | 0.39 | 1.56 | 0.39 | 0.39–0.78 | 0.39 | 0.19 | 0.19 | 0.39 | 0.39 | 0.39 | [76] | |||
| 0.5–1 | 0.25–1 | [77,78] | |||||||||||||
| 2 | 0.5 | [79] | |||||||||||||
| 0.5 | 0.5 | [80] | |||||||||||||
| 0.78 | 0.39 | [81] | |||||||||||||
| 0.5 | [82] | ||||||||||||||
| 0.62–2.5 | [83] | ||||||||||||||
| 0.78 | [84] | ||||||||||||||
| 0.12–0.5 | [85,86] | ||||||||||||||
| 1.83 | [46] | ||||||||||||||
| 1–32 | 0.5–1 | 0.5–1 | [87] | ||||||||||||
| 0.5 | 0.5 | [80] | |||||||||||||
| 0.78 | 0.78 | 0.78 | 0.78 | 0.78 | 1.56 | [27] | |||||||||
| 0.5–1 | 0.5–1 | [88] | |||||||||||||
| 0.5–1 | [89] | ||||||||||||||
| 11 | 1.953 | 0.975 | [25] | ||||||||||||
| 19 | 2.5 | 0.62–1.25 | 0.62–1.25 | [90] | |||||||||||
| 21 | 0.38 | [35] | |||||||||||||
| 27 | 0.5 | 1 | [39] | ||||||||||||
| 28 | 8 | 8 | [39] | ||||||||||||
| 29 | 8 | 8 | [39] | ||||||||||||
| 38 | 20.3 | [48] | |||||||||||||
| 40 | 32 | 16 | 16 | [45] | |||||||||||
| 41 | 64 | [45] | |||||||||||||
| 42 | 3.66 | [46] | |||||||||||||
| 52 | 1.56 | 1.56 | 1.56 | 1.56 | 3.13 | 3.13 | [27] | ||||||||
| 53 | 1.56 | 1.56 | 1.56 | 3.13 | 1.56 | [27] | |||||||||
| 62 | 8 | 8 | 8–16 | [51] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoletti, R.; Salvatore, M.M.; Ferranti, P.; Andolfi, A. Structures and Bioactive Properties of Myrtucommulones and Related Acylphloroglucinols from Myrtaceae. Molecules 2018, 23, 3370. https://doi.org/10.3390/molecules23123370
Nicoletti R, Salvatore MM, Ferranti P, Andolfi A. Structures and Bioactive Properties of Myrtucommulones and Related Acylphloroglucinols from Myrtaceae. Molecules. 2018; 23(12):3370. https://doi.org/10.3390/molecules23123370
Chicago/Turabian StyleNicoletti, Rosario, Maria Michela Salvatore, Pasquale Ferranti, and Anna Andolfi. 2018. "Structures and Bioactive Properties of Myrtucommulones and Related Acylphloroglucinols from Myrtaceae" Molecules 23, no. 12: 3370. https://doi.org/10.3390/molecules23123370
APA StyleNicoletti, R., Salvatore, M. M., Ferranti, P., & Andolfi, A. (2018). Structures and Bioactive Properties of Myrtucommulones and Related Acylphloroglucinols from Myrtaceae. Molecules, 23(12), 3370. https://doi.org/10.3390/molecules23123370









