Confirmatory Analysis of Nitroimidazoles and Hydroxy Metabolites in Honey by Dispersive-Solid Phase Extraction and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
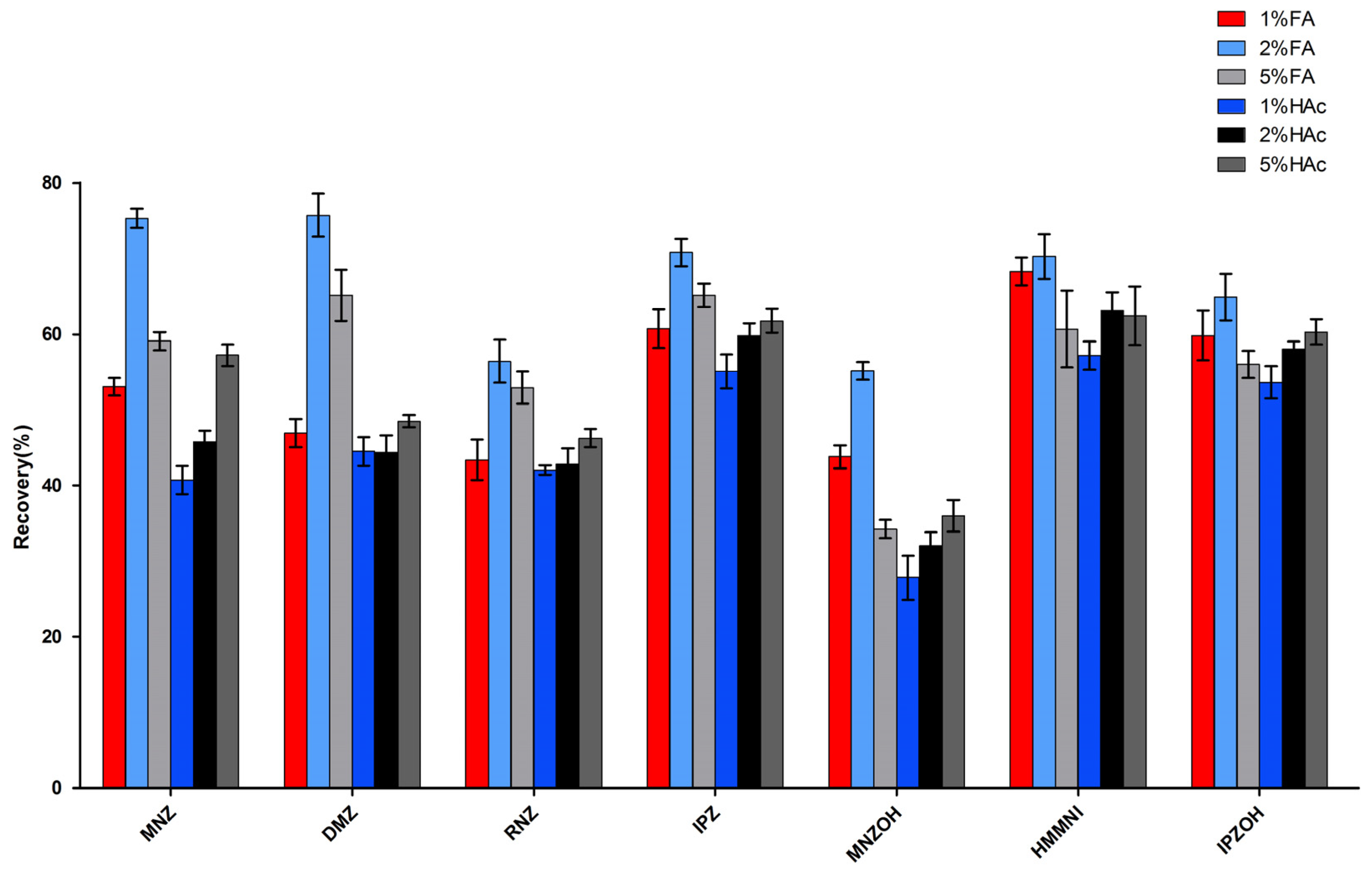
2.1. Optimization of Sample Preparation
2.2. Matrix Effect
2.3. Method Validation
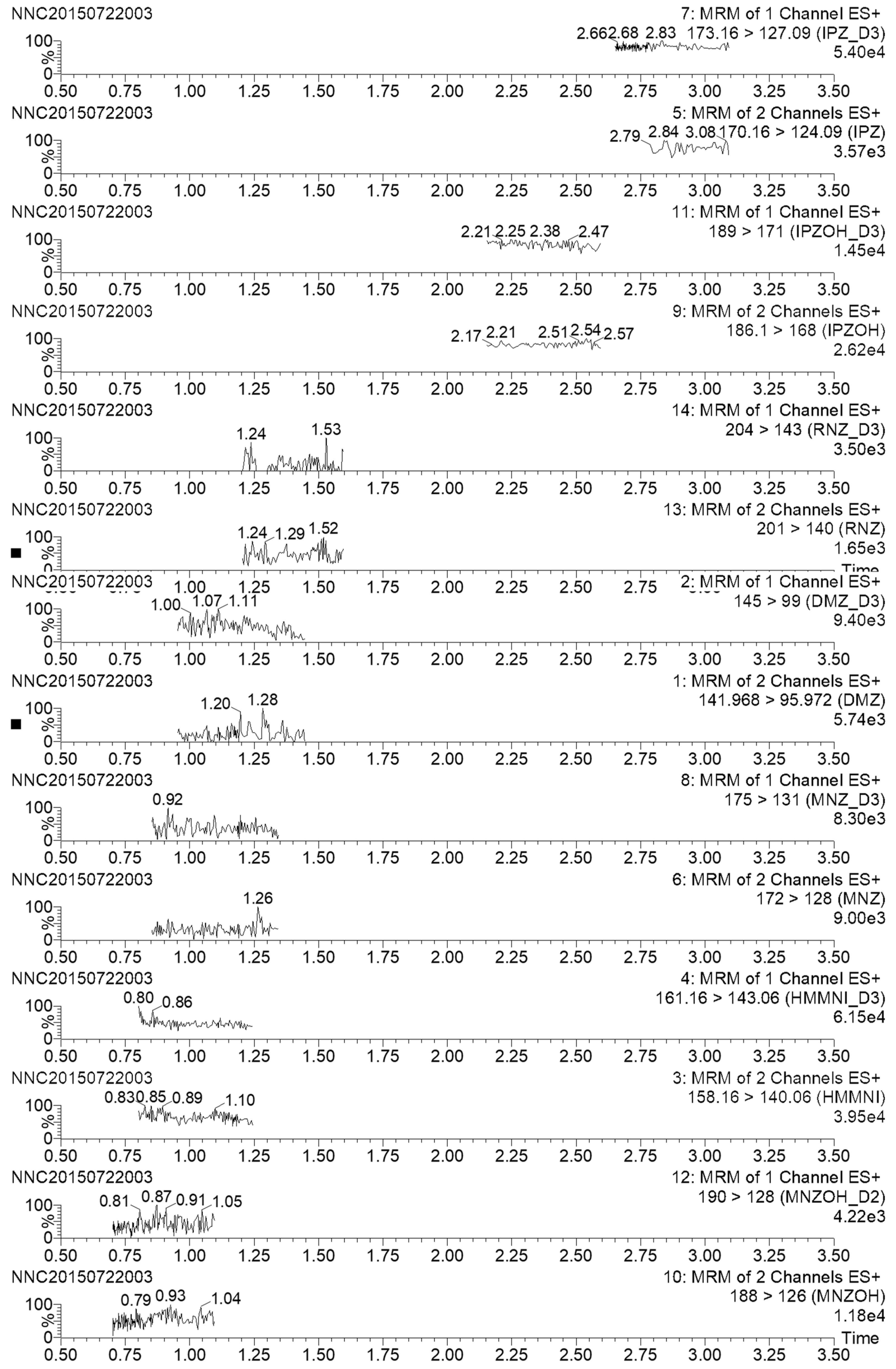
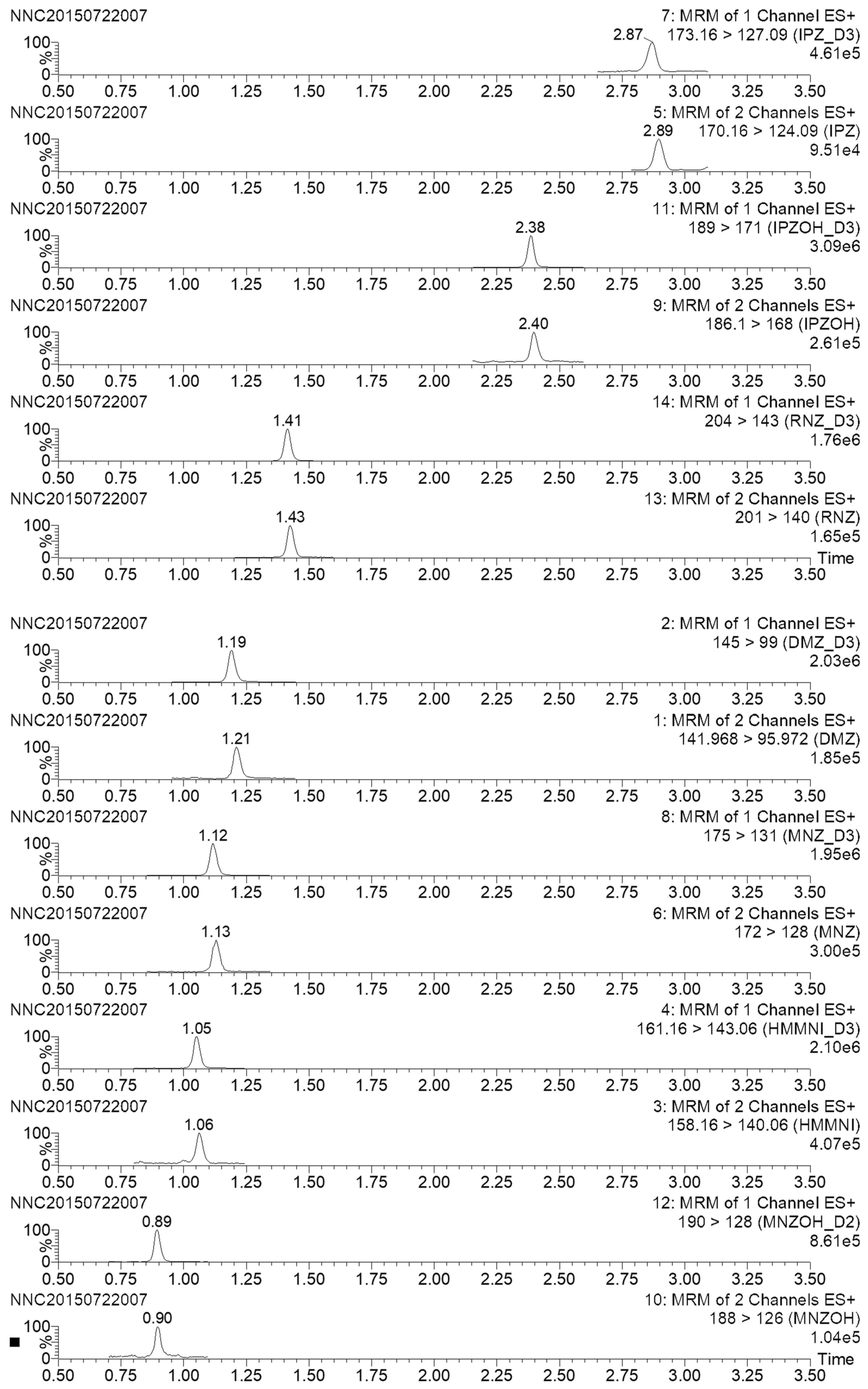
2.4. Analysis of Real Samples
3. Materials and Methods
3.1. Reagents and Materials
3.2. Sample Preparation
3.3. UHPLC-MS/MS Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Regulation No. 235 on Maximum Residue Limits of Veterinary Drugs in Foodstuffs of Animal Origin. 2002; Chinese Ministry of Agriculture. Available online: http://jiuban.moa.gov.cn/zwllm/tzgg/gg/200302/t20030226_59300.htm (accessed on 15 December 2018).
- Commission Regulation (EU) No 37/2010 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. European Commission, 2010. Available online: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-5/reg_2010_37/reg_2010_37_en.pdf (accessed on 15 December 2018).
- Overall Evaluations of Carcinogenicity. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Supplement 7. 1987. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/Suppl7-110.pdf (accessed on 15 December 2018).
- Evaluation of Certain Veterinary drug Residues in Food (Thirty-Fourth Report of the Joint FAO/WHO Expert Committee on Food Additives); WHO Technical Report Series; World Health Organisation: Geneva, Switzerland, 1989.
- Wang, J. Determination of three nitroimidazole residues in poultry meat by gas chromatography with nitrogen-phosphorus detection. J. Chromatogr. A 2001, 918, 435–438. [Google Scholar] [CrossRef]
- Shen, J.; Yhang, Y.; Zhang, S.; Ding, S.; Xiang, X. Determination of nitroimidazoles and their metabolites in swine tissues by liquid chromatography. J. AOAC Int. 2003, 86, 505–509. [Google Scholar]
- Huet, A.C.; Mortier, L.; Daeseleire, E.; Fodey, T.; Elliott, C.; Delahaut, P. Development of an ELISA screening test for nitroimidazoles in egg and chicken muscle. Anal. Chim. Acta 2005, 534, 157–162. [Google Scholar] [CrossRef]
- Polzer, J.; Gowik, P. Validation of a method for the detection and confirmation of nitroimidazoles and corresponding hydroxy metabolites in turkey and swine muscle by means of gas chromatography-negative ion chemical ionization mass spectrometry. J. Chromatogr. B 2001, 761, 47–60. [Google Scholar] [CrossRef]
- Ho, C.; Sin, D.W.M.; Wong, K.M.; Tang, H.P.O. Determination of dimetridazole and metronidazole in poultry and porcine tissues by gas chromatography-electron capture negative ionization mass spectrometry. Anal. Chim. Acta 2005, 530, 23–31. [Google Scholar] [CrossRef]
- Cannavan, A.; Kennedy, D.G. Determination of dimetridazole in poultry tissues and eggs using liquid chromatography thermospray mass spectrometry. Analyst 1997, 122, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Sams, M.J.; Strutt, P.R.; Barnes, K.A.; Damant, A.P.; Rose, M.D. Determination of dimetridazole, ronidazole and their common metabolite in poultry muscle and eggs by high performance liquid chromatography with UV detection and confirmatory analysis by atmospheric pressure chemical ionisation mass spectrometry. Analyst 1998, 123, 2545–2549. [Google Scholar] [CrossRef] [PubMed]
- Hurtaud-Pessel, D.; Delepine, B.; Laurentie, M. Determination of four nitroimidazole residues in poultry meat by liquid chromatography-mass spectrometry. J. Chromatogr. A 2000, 882, 89–98. [Google Scholar] [CrossRef]
- Capitan-Vallvey, L.F.; Ariza, A.; Checa, R.; Navas, N. Determination of five nitroimidazoles in water by liquid chromatography-mass spectrometry. J. Chromatogr. A 2002, 978, 243–248. [Google Scholar] [CrossRef]
- Daeseleire, E.; De Ruyck, K.; Van Renterghem, R. Rapid confirmatory assay for the simultaneous detection of ronidazole, metronidazole and dimetridazole in eggs using liquid chromatography-tandem mass spectrometry. Analyst 2000, 125, 1533–1535. [Google Scholar] [CrossRef]
- Mottier, P.; Huré, I.; Gremaud, E.; Guy, P.A. Analysis of four 5-nitroimidazoles and their corresponding hydroxylated metabolites in egg, processed egg, and chicken meat by isotope dilution liquid chromatography tandem mass spectrometry. J. Agric. Food Chem. 2006, 54, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Fraselle, S.; Derop, V.; Degroodt, J.M.; van Loco, J. Validation of a method for the detection and confirmation of nitroimidazoles and the corresponding hydroxy metabolites in pig plasma by high performance liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2007, 586, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, X.; Zhang, S.; Ding, S.; Jiang, H.; Shen, J. Confirmation of four nitroimidazoles in porcine liver by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2007, 586, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Malone, L.A.; Gatehouse, H.S.; Tregidga, E.L. Effects of time, temperature, and honey on Nosema apis (Microsporidia: Nosematidae), a parasite of the honeybee, Apis mellifera (Hymenoptera: Apidae). J. Invertebr. Pathol. 2001, 77, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Cronly, M.; Behan, P.; Foley, B.; Malone, E.; Martin, S.; Doyle, M.; Regan, L. Rapid multi-class multi-residue method for the confirmation of chloramphenicol and eleven nitroimidazoles in milk and honey by liquid chromatography-tandem mass spectrometry (LC-MS). Food Addit. Contam. 2010, 27, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xia, Z.; Wang, H.; Kang, W.; Lin, L.; Cao, W.; Zhang, H.; Zhou, W. Molecularly imprinted solid phase extraction method for simultaneous determination of seven nitroimidazoles from honey by HPLC-MS/MS. Talanta 2017, 166, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, X.; Ding, S.; Zhang, S.; Jiang, H.; Li, J.; Shen, J. Determination of 5-nitroimidazoles and corresponding hydroxy metabolites in swine kidney by ultra-performance liquid chromatography coupled to electrospray tandem mass spectrometry. Anal. Chim. Acta 2009, 637, 79–86. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Analytes | External Standard (%) | Internal Standard (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Acacia Honey | Jujube Honey | Chaste Honey | Linden Honey | Acacia Honey | Jujube Honey | Chaste Honey | Linden Honey | |
| MNZ | 35 | 23 | 33 | 30 | 96 | 95 | 94 | 96 |
| DMZ | 45 | 41 | 47 | 43 | 96 | 96 | 98 | 99 |
| RNZ | 40 | 47 | 45 | 49 | 98 | 101 | 98 | 96 |
| IPZ | 76 | 80 | 81 | 80 | 97 | 98 | 97 | 98 |
| MNZOH | 33 | 37 | 35 | 35 | 96 | 96 | 95 | 96 |
| HMMNI | 54 | 55 | 52 | 60 | 98 | 97 | 99 | 99 |
| IPZOH | 73 | 70 | 68 | 75 | 98 | 100 | 96 | 96 |
| Analyte | Spiking Level (μg/kg) | Recovery (%) | Intra-Day RSD (n = 6, %) | Inter-Day RSD (n = 18, %) | LOD (μg/kg) | LOQ (μg/kg) |
|---|---|---|---|---|---|---|
| MNZ | 0.2 | 90.8 | 7.8 | 8.2 | 0.02 | 0.05 |
| 0.5 | 105.6 | 5.2 | 8.9 | |||
| 1.0 | 96.3 | 4.3 | 7.3 | |||
| DMZ | 0.2 | 101.8 | 8.8 | 10.1 | 0.05 | 0.1 |
| 0.5 | 97.8 | 6.7 | 8.7 | |||
| 1.0 | 93.4 | 5.7 | 7.8 | |||
| RNZ | 0.2 | 93.8 | 9.0 | 11.2 | 0.02 | 0.05 |
| 0.5 | 98.2 | 8.6 | 10.6 | |||
| 1.0 | 97.7 | 7.9 | 9.4 | |||
| IPZ | 0.2 | 90.2 | 6.4 | 8.0 | 0.05 | 0.1 |
| 0.5 | 93.6 | 6.2 | 9.9 | |||
| 1.0 | 96.9 | 5.8 | 9.3 | |||
| MNZOH | 0.2 | 92.3 | 7.1 | 10.3 | 0.07 | 0.2 |
| 0.5 | 94.6 | 7.0 | 8.7 | |||
| 1.0 | 95.0 | 4.7 | 8.5 | |||
| HMMNI | 0.2 | 92.6 | 8.6 | 9.2 | 0.07 | 0.2 |
| 0.5 | 94.1 | 9.9 | 10.5 | |||
| 1.0 | 97.2 | 7.2 | 9.5 | |||
| IPZOH | 0.2 | 98.3 | 4.8 | 6.4 | 0.07 | 0.2 |
| 0.5 | 104.2 | 6.5 | 7.8 | |||
| 1.0 | 92.7 | 5.1 | 7.9 |
| Sample | Analyte Detected | Product Ions (m/z) | Incurred Sample | Standard | Level (μg/kg) | ||
|---|---|---|---|---|---|---|---|
| RT (min) | Ion Ratio a | RT (min) | Ion Ratio | ||||
| Honey 5 | MNZ | 82/128 | 1.13 | 0.5726 | 1.13 | 0.5812 | 0.6 |
| Honey 17 | MNZ | 82/128 | 1.13 | 0.5711 | 0.3 | ||
| Honey 19 | MNZ | 82/128 | 1.13 | 0.5738 | 1.1 | ||
| Analyte | Precursor Ion (m/z) | Product Ions (m/z) | Cone Voltage (V) | Collision Energy (eV) |
|---|---|---|---|---|
| MNZ | 172 | 82/128 * | 15 | 20/10 |
| MNZ-d3 | 175 | 131 | 15 | 10 |
| DMZ | 142 | 81/96 * | 10 | 18/14 |
| DMZ-d3 | 145 | 99 | 10 | 14 |
| RNZ | 201 | 55/140 * | 20 | 15/10 |
| RNZ-d3 | 204 | 143 | 20 | 10 |
| IPZ | 170 | 109/124 * | 32 | 24/18 |
| IPZ-d3 | 173 | 127 | 32 | 18 |
| MNZOH | 188 | 123/126 * | 20 | 15/10 |
| MNZOH-d2 | 190 | 128 | 20 | 10 |
| HMMNI | 158 | 55/140 * | 18 | 18/10 |
| HMMNI-d3 | 161 | 143 | 18 | 10 |
| IPZOH | 186 | 122/168 * | 20 | 15/10 |
| IPZOH-d3 | 189 | 171 | 20 | 10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Ke, Y.; Wang, Y.; Wang, C.; Ye, D.; Hu, X.; Zhou, L.; Xia, X. Confirmatory Analysis of Nitroimidazoles and Hydroxy Metabolites in Honey by Dispersive-Solid Phase Extraction and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2018, 23, 3350. https://doi.org/10.3390/molecules23123350
Li X, Ke Y, Wang Y, Wang C, Ye D, Hu X, Zhou L, Xia X. Confirmatory Analysis of Nitroimidazoles and Hydroxy Metabolites in Honey by Dispersive-Solid Phase Extraction and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Molecules. 2018; 23(12):3350. https://doi.org/10.3390/molecules23123350
Chicago/Turabian StyleLi, Xiaowei, Yuebin Ke, Yingyu Wang, Chengfei Wang, Dongyang Ye, Xue Hu, Lan Zhou, and Xi Xia. 2018. "Confirmatory Analysis of Nitroimidazoles and Hydroxy Metabolites in Honey by Dispersive-Solid Phase Extraction and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry" Molecules 23, no. 12: 3350. https://doi.org/10.3390/molecules23123350
APA StyleLi, X., Ke, Y., Wang, Y., Wang, C., Ye, D., Hu, X., Zhou, L., & Xia, X. (2018). Confirmatory Analysis of Nitroimidazoles and Hydroxy Metabolites in Honey by Dispersive-Solid Phase Extraction and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Molecules, 23(12), 3350. https://doi.org/10.3390/molecules23123350





