Deuterated Arachidonic Acids Library for Regulation of Inflammation and Controlled Synthesis of Eicosanoids: An In Vitro Study
Abstract
1. Introduction
2. Results
2.1. COX-2 Kinetic Parameters for AA Isotopologues
2.2. 15-LOX-2 Kinetic Parameters for AA Isotopologues
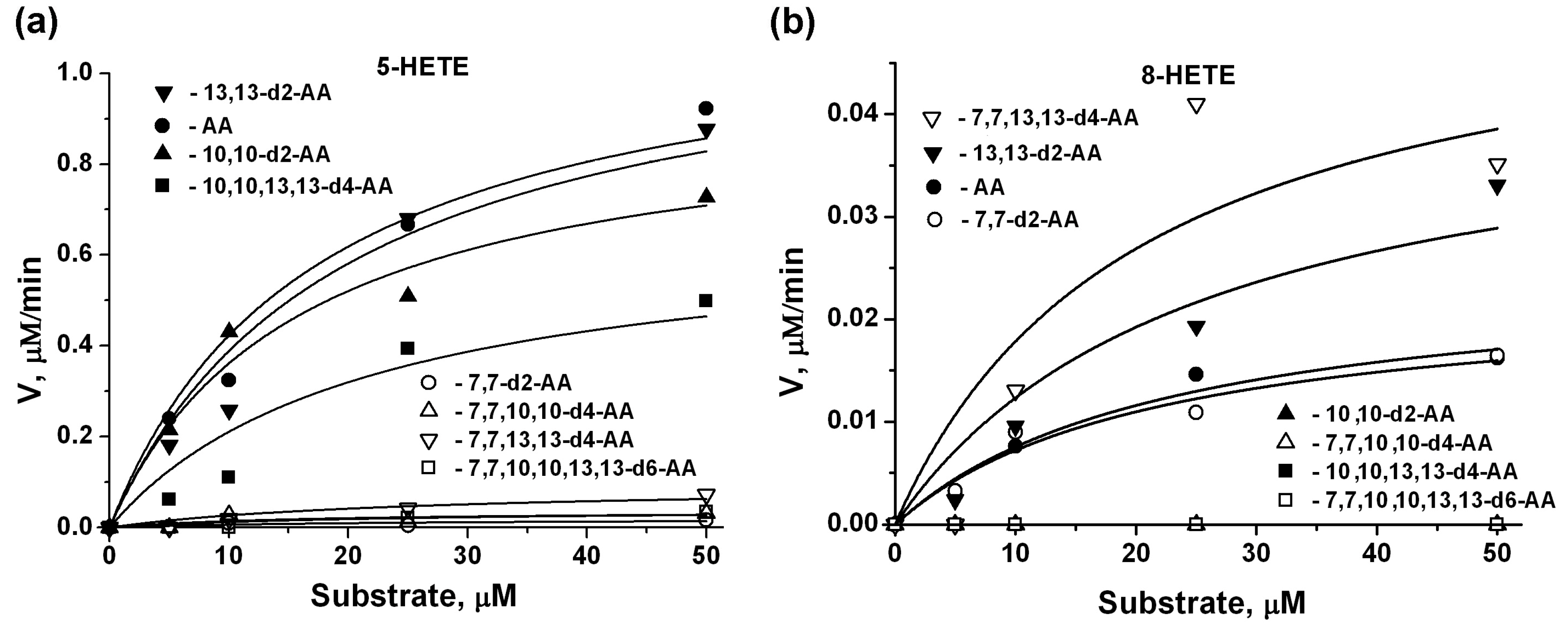
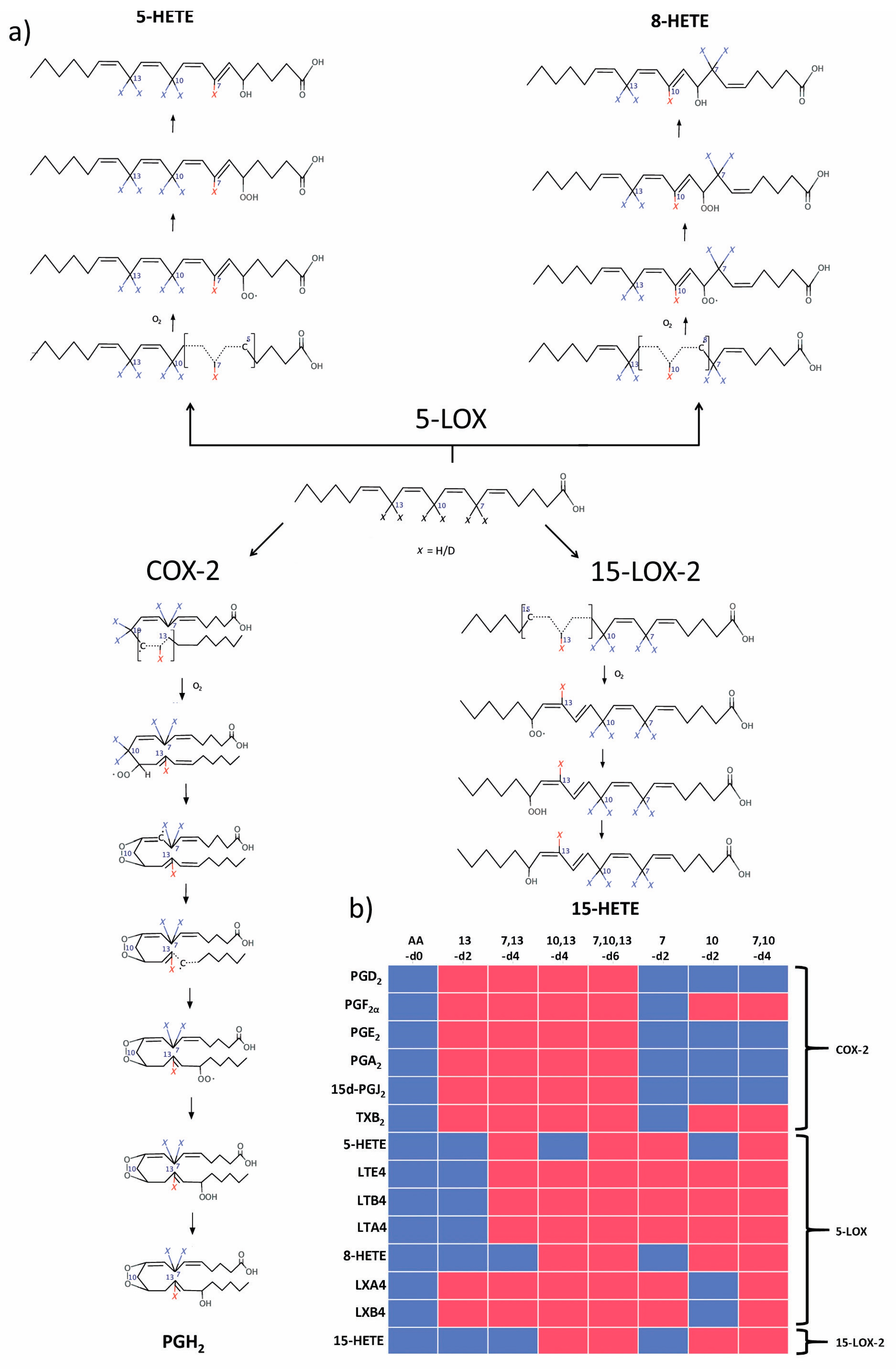
2.3. 5-LOX Kinetic Parameters for AA Isotopologues, Estimated by 5-HETE and 8-HETE Products
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Arachidonic Acid Library
4.3. COX-2 Kinetic Assays
4.4. 5-LOX Kinetic Assays
4.5. 15-LOX-2 Kinetic Assays
Author Contributions
Funding
Conflicts of Interest
References
- Shchepinov, M.S. Reactive oxygen species, isotope effect, essential nutrients, and enhanced longevity. Rejuv. Res. 2007, 10, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Berbée, J.F.P.; Mol, I.M.; Milne, G.L.; Pollock, E.; Hoeke, G.; Lütjohann, D.; Monaco, C.; Rensen, P.C.N.; van der Ploeg, L.H.T.; Shchepinov, M.S. Deuterium-reinforced polyunsaturated fatty acids protect against atherosclerosis by lowering lipid peroxidation and hypercholesterolemia. Atherosclerosis 2017, 264, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, A.Y.; Tsui, H.S.; Milne, G.L.; Shmanai, V.V.; Bekish, A.V.; Fomich, M.A.; Pham, M.N.; Nong, Y.; Murphy, A.N.; Clarke, C.F.; et al. Isotope-reinforced polyunsaturated fatty acids protect mitochondria from oxidative stress. Free Radic. Biol. Med. 2015, 82, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.; Lamberson, C.R.; Xu, L.; To, R.; Tsui, H.S.; Shmanai, V.V.; Bekish, A.V.; Awad, A.M.; Marbois, B.N.; Cantor, C.R.; et al. Small amounts of isotope-reinforced polyunsaturated fatty acids suppress lipid autoxidation. Free Radic. Biol. Med. 2012, 53, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Breyer, R.M.; Bagdassarian, C.K.; Myers, S.A.; Breyer, M.D. Prostanoid receptors: Subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 661–690. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, D.V.; Grabeklis, S.; Goriainov, S.V.; Chistyakov, V.V.; Sergeeva, M.G.; Reiser, G. Astrocytes synthesize primary and cyclopentenone prostaglandins that are negative regulators of their proliferation. Biochem. Biophys. Res. Commun. 2018, 500, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.S.; Rokach, J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2015, 1851, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Folco, G.; Murphy, R.C. Transcellular biosynthesis of eicosanoids. Pharmacol. Rep. 2010, 62, 503–510. [Google Scholar] [CrossRef]
- Patrignani, P.; Patrono, C. Cyclooxygenase inhibitors: From pharmacology to clinical read-outs. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2015, 1851, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Steinhilber, D.; Hofmann, B. Recent Advances in the Search for Novel 5-Lipoxygenase Inhibitors. Basic Clin. Pharmacol. Toxicol. 2014, 114, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of inflammation: What controls its onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Navratil, A.R.; Shchepinov, M.S.; Dennis, E.A. Lipidomics Reveals Dramatic Physiological Kinetic Isotope Effects during the Enzymatic Oxygenation of Polyunsaturated Fatty Acids Ex Vivo. J. Am. Chem. Soc. 2018, 140, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; Garavito, R.M.; DeWitt, D.L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 1996, 271, 33157–33160. [Google Scholar] [CrossRef]
- Moreno, J.J. New aspects of the role of hydroxyeicosatetraenoic acids in cell growth and cancer development. Biochem. Pharmacol. 2009, 77, 1–10. [Google Scholar] [CrossRef]
- Brash, A.R.; Boeglin, W.E.; Chang, M.S. Discovery of a second 15S-lipoxygenase in humans. Proc. Natl. Acad. Sci. 1997, 94, 6148–6152. [Google Scholar] [CrossRef]
- Rådmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2015, 1851, 331–339. [Google Scholar] [CrossRef]
- Shimizu, T.; Rådmark, O.; Samuelsson, B. Enzyme with dual lipoxygenase activities catalyzes leukotriene A4 synthesis from arachidonic acid. Proc. Natl. Acad. Sci. USA 1984, 81, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Kumar, R.B.; Balagunaseelan, N.; Hamberg, M.; Jegerschöld, C.; Rådmark, O.; Haeggström, J.Z.; Rinaldo-Matthis, A. Kinetic investigation of human 5-lipoxygenase with arachidonic acid. Bioorganic Med. Chem. Lett. 2016, 26, 3547–3551. [Google Scholar] [CrossRef]
- Gilroy, D.; De Maeyer, R. New insights into the resolution of inflammation. Semin. Immunol. 2015, 27, 161–168. [Google Scholar] [CrossRef]
- Wu, G.; Lü, J.M.; Van Der Donk, W.A.; Kulmacz, R.J.; Tsai, A.L. Cyclooxygenase reaction mechanism of prostaglandin H synthase from deuterium kinetic isotope effects. J. Inorg. Biochem. 2011, 105, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.-L.; Kulmacz, R.J. Prostaglandin H synthase: Resolved and unresolved mechanistic issues. Arch. Biochem. Biophys. 2010, 493, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, M.; Strokin, M.; Reiser, G. Regulation of intracellular calcium levels by polyunsaturated fatty acids, arachidonic acid and docosahexaenoic acid, in astrocytes: Possible involvement of phospholipase A2. Reprod. Nutr. Dev. 2005, 45, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Suardíaz, R.; Jambrina, P.G.; Masgrau, L.; González-Lafont, À.; Rosta, E.; Lluch, J.M. Understanding the Mechanism of the Hydrogen Abstraction from Arachidonic Acid Catalyzed by the Human Enzyme 15-Lipoxygenase-2. A Quantum Mechanics/Molecular Mechanics Free Energy Simulation. J. Chem. Theory Comput. 2016, 12, 2079–2090. [Google Scholar] [CrossRef]
- Wecksler, A.T.; Kenyon, V.; Garcia, N.K.; Deschamps, J.D.; Van Der Donk, W.A.; Holman, T.R. Kinetic and structural investigations of the allosteric site in human epithelial 15-lipoxygenase-2. Biochemistry 2009, 48, 8721–8730. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Banthiya, S.; Van Leyen, K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2015, 1851, 308–330. [Google Scholar] [CrossRef]
- Sun, L.; Xu, Y.-W.; Han, J.; Liang, H.; Wang, N.; Cheng, Y. 12/15-Lipoxygenase metabolites of arachidonic acid activate PPARγ: A possible neuroprotective effect in ischemic brain. J. Lipid Res. 2015, 56, 502–514. [Google Scholar] [CrossRef]
- Shah, R.; Shchepinov, M.S.; Pratt, D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci. 2018, 4, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Zesiewicz, T.; Heerinckx, F.; De Jager, R.; Omidvar, O.; Kilpatrick, M.; Shaw, J.; Shchepinov, M.S. Randomized, clinical trial of RT001: Early signals of efficacy in Friedreich’s ataxia. Mov. Disord. 2018, 33, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; McGinley, C.M.; Van Der Donk, W.A. Synthesis of Site-Specifically Labeled Arachidonic Acids as Mechanistic Probes for Prostaglandin, H. Synthase. Org. Lett. 2004, 6, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Fomich, M.A.; Bekish, A.V.; Vidovic, D.; Lamberson, C.R.; Lysenko, I.L.; Lawrence, P.; Brenna, J.T.; Sharko, O.L.; Shmanai, V.V.; Shchepinov, M.S. Full Library of (Bis–allyl)-deuterated Arachidonic Acids: Synthesis and Analytical Verification. ChemistrySelect 2016, 1, 4758–4764. [Google Scholar] [CrossRef]
- Kulmacz, R.J.; Lands, W.E.M. Requirements for hydroperoxide by the cyclooxygenase and peroxidase activities of prostaglandin H. synthase. Prostaglandins 1983, 25, 531–540. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

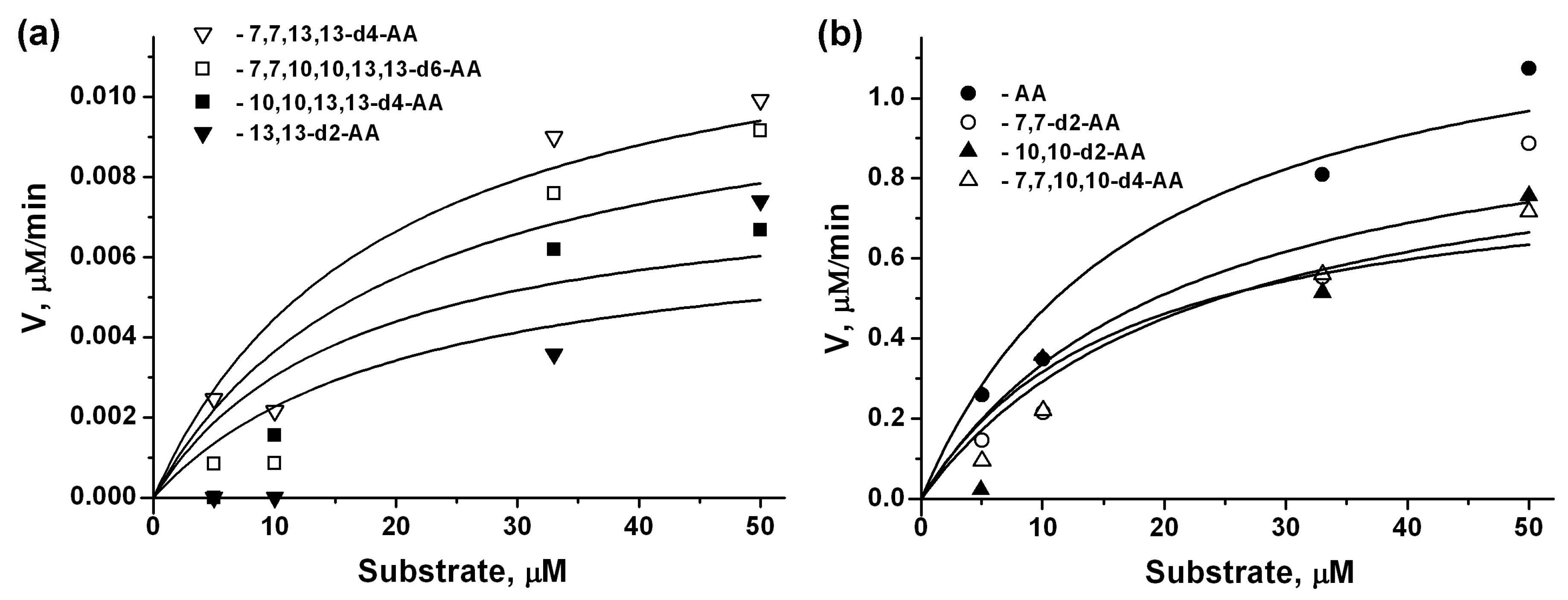
| COX-2 * | 15-LOX-2 ** | |||||
|---|---|---|---|---|---|---|
| AA Isotopologues | Vmax (µM/min) | KM (µM) | Isotope Effect Calculated from Hkcat/Dkcat | Vmax (µM/min) | KM (µM) | Isotope Effect Calculated from Hkcat/Dkcat |
| AA | 11.07 (1.1) *** | 10.8 (4.1) | 1.316 (0.099) | 18.0 (3.6) | ||
| 7,7-d2-AA | 9.11 (0.7) | 15.4 (3.2) | 1 | 1.056 (0.088) | 21.4 (4.3) | 1 |
| 10,10-d2-AA | 7.53 (0.41) | 12.7 (2.8) | 1 | 0.973 (0.077) | 23.2 (4.6) | 1 |
| 13,13-d2-AA | 0.44 (0.21) | 10.7 (3.5) | 25 | 0.007 (0.001) | 20.9 (4.2) | 188 |
| 7,7,10,10-d4-AA | 8.61 (0.82) | 15.8 (2.9) | 1 | 0.844 (0.063) | 16.6 (3.3) | 2 |
| 7,7,13,13-d4-AA | 0.58 (0.25) | 12.2 (3.1) | 19 | 0.013 (0.001) | 19.1 (3.8) | 101 |
| 10,10,13,13-d4-AA | 0.83 (0.22) | 17.6 (3.7) | 13 | 0.008 (0.001) | 16.4 (3.3) | 165 |
| 7,7,10,10,13,13-d6-AA | 1.07 (0.31) | 13.8 (3.6) | 10 | 0.011 (0.001) | 20.1 (4.0) | 120 |
| 5-HETE | 8-HETE | Product Ratio | |||||
|---|---|---|---|---|---|---|---|
| Vmax (µM/min) | KM (µM) | Isotope Effect Calculated from Hkcat/Dkcat. | Vmax (µM/min) | KM (µM) | Isotope Effect Calculated from Hkcat/Dkcat. | (5-HETE/8-HETE),% | |
| AA | 1.151 (0.21) | 17.3 (3.5) | - | 0.025 (0.008) | 22.9 (4.6) | - | 98/2 |
| 7,7-d2-AA | 0.020 (0.01) | 22.4 (4.5) | 58 | 0.023 (0.007) | 21.7 (4.3) | 1 | 51/49 |
| 10,10-d2-AA | 0.933 (0.15) | 15.9 (3.2) | 1 | n/a | n/a | - | 100/0 |
| 13,13-d2-AA | 1.154 (0.12) | 19.8 (4.0) | 1 | 0.043 (0.004) | 25.2 (5.0) | 0.5 | 98/2 |
| 7,7,10,10-d4-AA | 0.037 (0.015) | 16.6 (3.3) | 31 | n/a | n/a | - | 100/0 |
| 7,7,13,13-d4-AA | 0.093 (0.015) | 25.6 (5.1) | 12 | 0.054 (0.015) | 20.4 (4.1) | 0.5 | 60/40 |
| 10,10,13,13-d4-AA | 0.665 (0.09) | 21.7 (4.3) | 2 | n/a | n/a | - | 100/0 |
| 7,7,10,10,13,13-d6-AA | 0.040 (0.02) | 23.5 (4.7) | 29 | n/a | n/a | - | 100/0 |
| Analyte | AA | 7,7-AA-d2 | 10,10-AA-d2 | 13,13-AA-d2 | 7,7,10,10-AA-d4 | 7,7,13,13-AA-d4 | 10,10,13,13-AA-d4 | 7,7,10,10,13,13-AA-d6 |
|---|---|---|---|---|---|---|---|---|
| 5-HETE | 319-115 | 320-115 | 321-115 | 321-115 | 322-115 | 322-115 | 322-115 | 324-115 |
| 8-HETE | 319-155 | 321-157 | 320-155 | 321-155 | 323-157 | 323-157 | 323-157 | 324-157 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chistyakov, D.V.; Filimonov, I.S.; Azbukina, N.V.; Goriainov, S.V.; Chistyakov, V.V.; Fomich, M.A.; Bekish, A.V.; Shmanai, V.V.; Sergeeva, M.G.; Shchepinov, M.S. Deuterated Arachidonic Acids Library for Regulation of Inflammation and Controlled Synthesis of Eicosanoids: An In Vitro Study. Molecules 2018, 23, 3331. https://doi.org/10.3390/molecules23123331
Chistyakov DV, Filimonov IS, Azbukina NV, Goriainov SV, Chistyakov VV, Fomich MA, Bekish AV, Shmanai VV, Sergeeva MG, Shchepinov MS. Deuterated Arachidonic Acids Library for Regulation of Inflammation and Controlled Synthesis of Eicosanoids: An In Vitro Study. Molecules. 2018; 23(12):3331. https://doi.org/10.3390/molecules23123331
Chicago/Turabian StyleChistyakov, Dmitry V., Ivan S. Filimonov, Nadezhda V. Azbukina, Sergei V. Goriainov, Viktor V. Chistyakov, Maksim A. Fomich, Andrei V. Bekish, Vadim V. Shmanai, Marina G. Sergeeva, and Mikhail S. Shchepinov. 2018. "Deuterated Arachidonic Acids Library for Regulation of Inflammation and Controlled Synthesis of Eicosanoids: An In Vitro Study" Molecules 23, no. 12: 3331. https://doi.org/10.3390/molecules23123331
APA StyleChistyakov, D. V., Filimonov, I. S., Azbukina, N. V., Goriainov, S. V., Chistyakov, V. V., Fomich, M. A., Bekish, A. V., Shmanai, V. V., Sergeeva, M. G., & Shchepinov, M. S. (2018). Deuterated Arachidonic Acids Library for Regulation of Inflammation and Controlled Synthesis of Eicosanoids: An In Vitro Study. Molecules, 23(12), 3331. https://doi.org/10.3390/molecules23123331





