Evolution of Volatile Compounds and Spoilage Bacteria in Smoked Bacon during Refrigeration Using an E-Nose and GC-MS Combined with Partial Least Squares Regression
Abstract
:1. Introduction
2. Results and Discussion
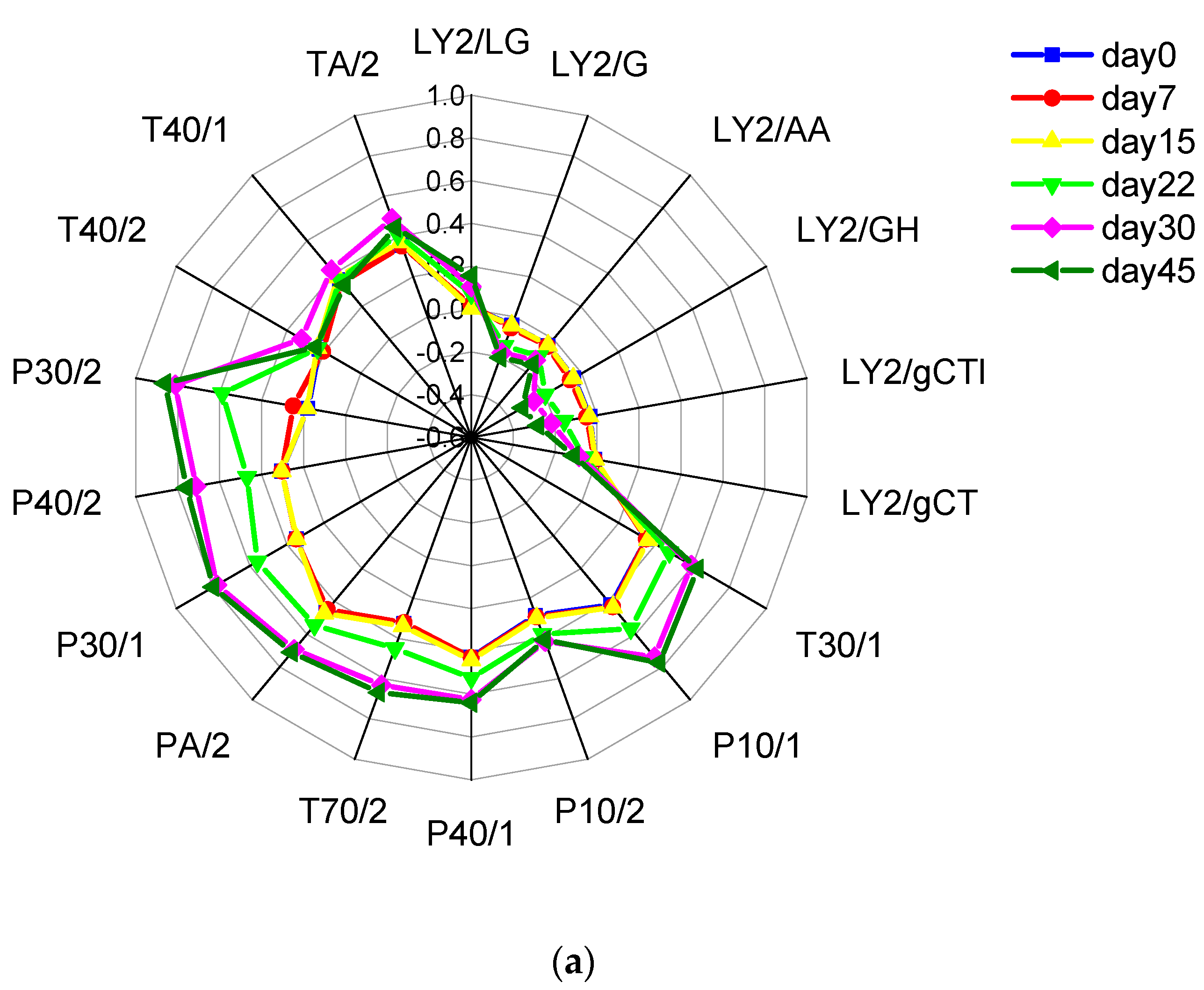
2.1. Results of the E-Nose Analysis
2.2. Microbiological Growth and Specific Spoilage Microorganisms
2.3. GC-MS Results for Bacon after Different Storage Times
2.4. Volatile Compound Evolution during Storage
2.5. Compounds Formed Related to Microbial Activity
2.6. BA Evolution during Storage
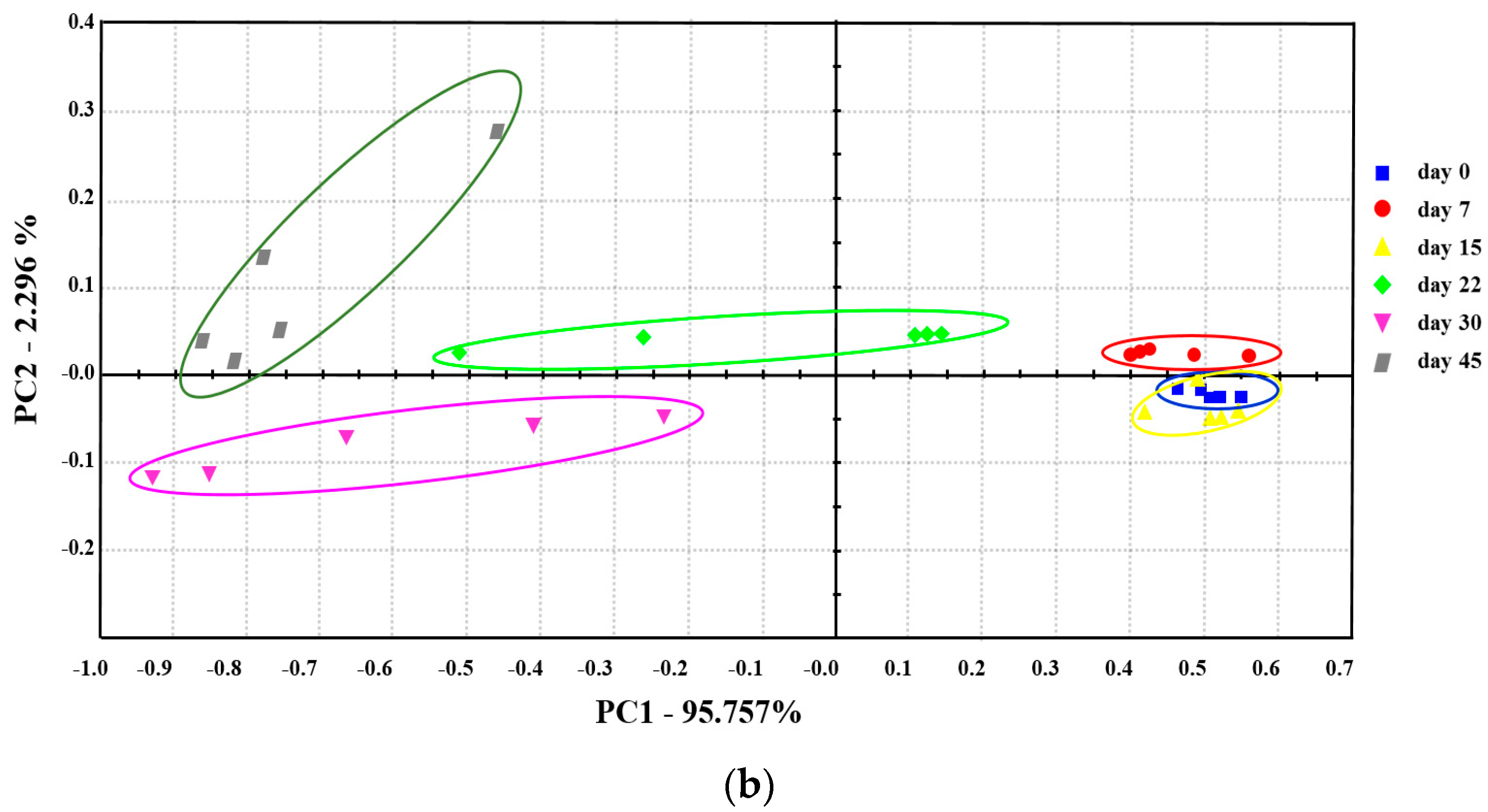
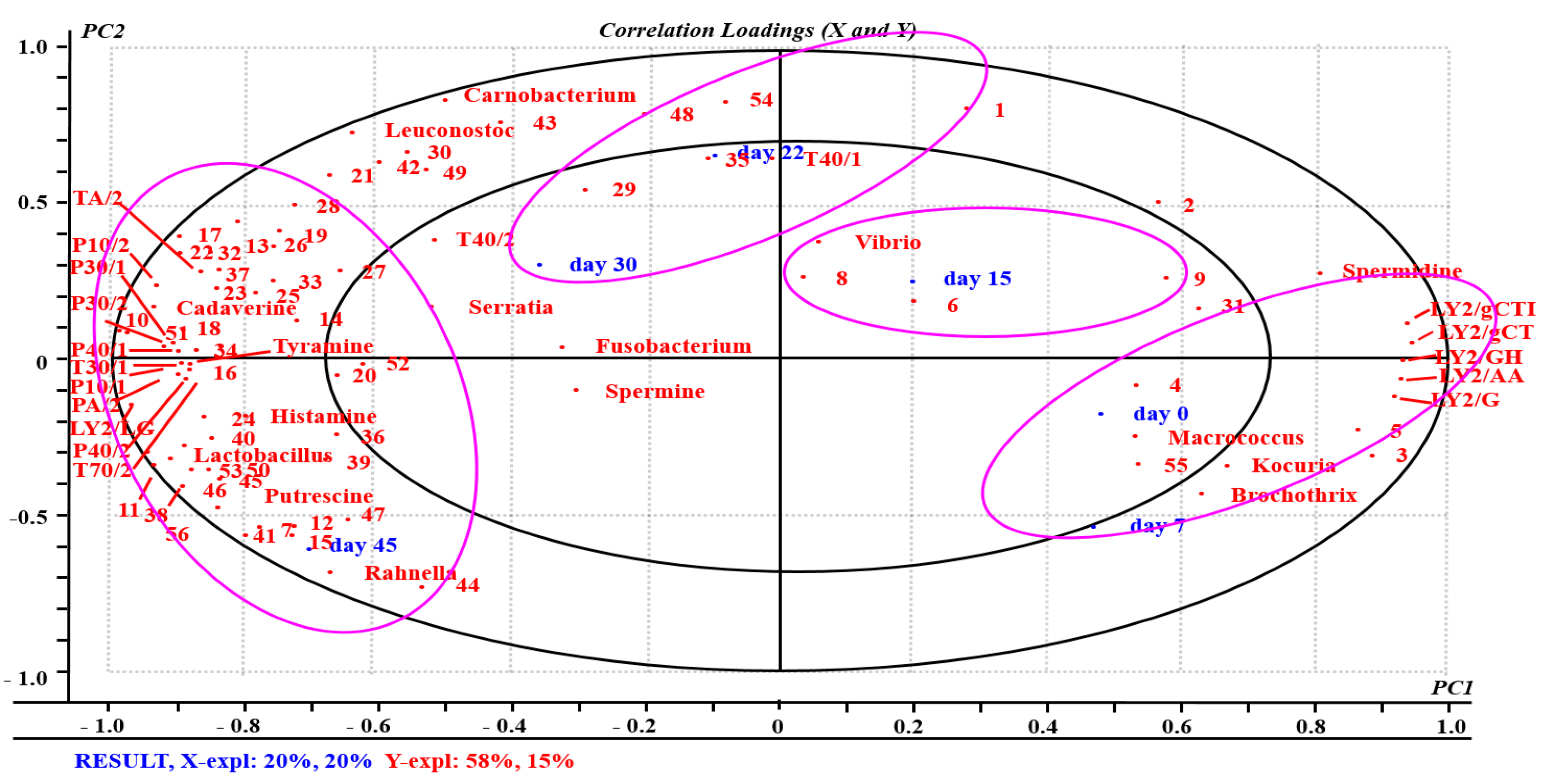
2.7. Relationships between Samples, VOCs, E-Nose Sensors, and Spoilage Bacteria
3. Materials and Methods
3.1. Sampling and Storage Conditions
3.2. Electronic Nose
3.3. Chemicals
3.4. SPME-GC-MS of Volatile Organic Compounds in Bacon
3.5. Calibration of Standard Curves
3.6. Biogenic Amine Determination
3.7. Microbiological Analyses
3.8. Quality Assurance/Quality Control (QA/QC)
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| VOCs | Volatile organic compounds |
| BAs | Biogenic amines |
| SPME | Solid-phase micro extraction |
| GC-MS | Gas chromatography-mass spectrometry |
| HPLC | High-performance liquid chromatography |
| SSOs | Specific spoilage microorganisms |
| e-nose | Electronic nose |
| PLSR | Partial least squares regression |
| LAB | Lactic acid bacteria |
| PCA | Principal component analysis |
| ANOVA | Analysis of variance |
| HTS | High-throughput sequencing |
| QA | Quality assurance |
| QC | Quality control |
| RSD | Relative standard deviation |
| LOD | Limits of detection |
| LOQ | Limits of quantification |
References
- Liu, X.; Huang, Z.; Jia, S.; Zhang, J.; Li, K.; Luo, Y. The roles of bacteria in the biochemical changes of chill-stored bighead carp (Aristichthys nobilis): Proteins degradation, biogenic amines accumulation, volatiles production, and nucleotides catabolism. Food Chem. 2018, 255, 174–181. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Montel, M.C.; Masson, F.; Talon, R. Bacterial role in flavour development. Meat Sci. 1998, 49, 111–123. [Google Scholar] [CrossRef]
- Huis in’t Veld, J.H. Microbial and biochemical spoilage of foods: An overview. Int. J. Food Microbiol. 1996, 33, 1–18. [Google Scholar] [CrossRef]
- Leroy, F.; Vasilopoulos, C.; Van Hemelryck, S.; Falony, G.; De Vuyst, L. Volatile analysis of spoiled, artisan-type, modified-atmosphere-packaged cooked ham stored under different temperatures. Food Microbiol. 2009, 26, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Samelis, J.; Kakouri, A.; Rementzis, J. Selective effect of the product type and the packaging conditions on the species of lactic acid bacteria dominating the spoilage microbial association of cooked meats at 4 degrees C. Food Microbiol. 2000, 17, 329–340. [Google Scholar] [CrossRef]
- Jørgensen, L.V.; Huss, H.H.; Dalgaard, P. Significance of volatile compounds produced by spoilage bacteria in vacuum-packed cold-smoked salmon (Salmo salar) analyzed by GC-MS and multivariate regression. J. Agric. Food Chem. 2001, 49, 2376–2381. [Google Scholar] [CrossRef]
- Carrapiso, A.I.; Jurado, Á.; Timón, M.L.; García, C. Odor-active compounds of iberian hams with different aroma characteristics. J. Agric. Food Chem. 2002, 50, 6453–6458. [Google Scholar] [CrossRef]
- Ercolini, D.; Ferrocino, I.; Nasi, A.; Ndagijimana, M.; Vernocchi, P.; La Storia, A.; Laghi, L.; Mauriello, G.; Guerzoni, M.E.; Villani, F. Monitoring of microbial metabolites and bacterial diversity in beef stored under different packaging conditions. Appl. Environ. Microbiol. 2011, 77, 7372–7381. [Google Scholar] [CrossRef]
- Gram, L.; Ravn, L.; Rasch, M.; Bruhn, J.B.; Christensen, A.B.; Givskov, M. Food spoilage—Interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002, 78, 79–97. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, Q.; Zhao, H.; Zhao, M.; Yang, B. Volatile compounds of cantonese sausage released at different stages of processing and storage. Food Chem. 2010, 121, 319–325. [Google Scholar] [CrossRef]
- Hernandezjover, T.; Izquierdopulido, M.; Veciananogues, M.T.; Marinefont, A.; Vidalcarou, M.C. Biogenic amine and polyamine contents in meat and meat products. J. Agric. Food Chem. 1997, 45, 2098–2102. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Spizzirri, U.G.; Bonesi, M.; Tundis, R.; Picci, N.; Restuccia, D. Influence of packaging conditions on biogenic amines and fatty acids evolution during 15months storage of a typical spreadable salami (‘nduja). Food Chem. 2016, 213, 115–122. [Google Scholar] [CrossRef] [PubMed]
- De Mey, E.; De Klerck, K.; De Maere, H.; Dewulf, L.; Derdelinckx, G.; Peeters, M.-C.; Fraeye, I.; Vander Heyden, Y.; Paelinck, H. The occurrence of n-nitrosamines, residual nitrite and biogenic amines in commercial dry fermented sausages and evaluation of their occasional relation. Meat Sci. 2014, 96, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Chenoll, E.; Macian, M.C.; Elizaquivel, P.; Aznar, R. Lactic acid bacteria associated with vacuum-packed cooked meat product spoilage: Population analysis by rDNA-based methods. J. Appl. Microbiol. 2007, 102, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Mataragas, M.; Skandamis, P.; Nychas, G.J.; Drosinos, E.H. Modeling and predicting spoilage of cooked, cured meat products by multivariate analysis. Meat Sci. 2007, 77, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.R.; Song, E.J.; Cho, Y.S.; Nam, Y.D.; Choi, Y.S.; Kim, D.O.; Seo, D.H.; Nam, T.G. Comparative evaluation of spoilage-related bacterial diversity and metabolite profiles in chilled beef stored under air and vacuum packaging. Food Microbiol. 2019, 77, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Buňková, L.; Buňka, F.; Klčovská, P.; Mrkvička, V.; Doležalová, M.; Kráčmar, S. Formation of biogenic amines by Gram-negative bacteria isolated from poultry skin. Food Chem. 2010, 121, 203–206. [Google Scholar] [CrossRef]
- Olafsdottir, G.; Jonsdottir, R.; Lauzon, H.L.; Luten, J.; Kristbergsson, K. Characterization of volatile compounds in chilled cod (Gadus morhua) fillets by gas chromatography and detection of quality indicators by an electronic nose. J. Agric. Food Chem. 2005, 53, 10140–10147. [Google Scholar] [CrossRef]
- Jaffrès, E.; Lalanne, V.; Macé, S.; Cornet, J.; Cardinal, M.; Sérot, T.; Dousset, X.; Joffraud, J.-J. Sensory characteristics of spoilage and volatile compounds associated with bacteria isolated from cooked and peeled tropical shrimps using SPME-GC-MS analysis. Int. J. Food Microbiol. 2011, 147, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Song, S.; Zhang, X.; Tan, C.; Karangwa, E. Effect of sterilization methods on ginger flavor beverage assessed by partial least squares regression of descriptive sensory analysis and gas chromatography–mass spectrometry. Eur. Food Res. Technol. 2013, 238, 247–257. [Google Scholar] [CrossRef]
- Blixt, Y.; Borch, E. Using an electronic nose for determining the spoilage of vacuum-packaged beef. Int. J. Food Microbiol. 1999, 46, 123–134. [Google Scholar] [CrossRef]
- Hansen, T.; Petersen, M.A.; Byrne, D.V. Sensory based quality control utilising an electronic nose and GC-MS analyses to predict end-product quality from raw materials. Meat Sci. 2005, 69, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Boothe, D.D.; Arnold, J.W. Electronic nose analysis of volatile compounds from poultry meat samples, fresh and after refrigerated storage. J. Sci. Food Agric. 2002, 82, 315–322. [Google Scholar] [CrossRef]
- Pennazza, G.; Fanali, C.; Santonico, M.; Dugo, L.; Cucchiarini, L.; Dachà, M.; D’Amico, A.; Costa, R.; Dugo, P.; Mondello, L. Electronic nose and GC–MS analysis of volatile compounds in tuber magnatum pico: Evaluation of different storage conditions. Food Chem. 2013, 136, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Cosio, M.S.; Ballabio, D.; Benedetti, S.; Gigliotti, C. Evaluation of different storage conditions of extra virgin olive oils with an innovative recognition tool built by means of electronic nose and electronic tongue. Food Chem. 2007, 101, 485–491. [Google Scholar] [CrossRef]
- Labreche, S.; Bazzo, S.; Cade, S.; Chanie, E. Shelf life determination by electronic nose: Application to milk. Sens. Actuators B Chem. 2005, 106, 199–206. [Google Scholar] [CrossRef]
- Siegmund, B.; Pfannhauser, W. Changes of the volatile fraction of cooked chicken meat during chill storing: Results obtained by the electronic nose in comparison to GC-MS and GC olfactometry. Z. Lebensm. Forsch. A 1999, 208, 336–341. [Google Scholar] [CrossRef]
- Arnold, J.W.; Senter, S.D. Use of digital aroma technology and SPME GC-MS to compare volatile compounds produced by bacteria isolated from processed poultry. J. Sci. Food Agric. 1998, 78, 343–348. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, X. A rapid and novel method for predicting nicotine alkaloids in tobacco through electronic nose and partial least-squares regression analysis. Anal. Methods 2016, 8, 1609–1617. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, M.; Niu, Y.; Chen, F.; Zhang, X.; Zhu, J.; Song, S.; Zhu, G. Contribution of chicken base addition to aroma characteristics of maillard reaction products based on gas chromatography-mass spectrometry, electronic nose, and statistical analysis. Food Sci. Biotechnol. 2015, 24, 411–419. [Google Scholar] [CrossRef]
- Soladoye, P.O.; Shand, P.J.; Aalhus, J.L.; Gariépy, C.; Juárez, M. Review: Pork belly quality, bacon properties and recent consumer trends. Can. J. Anim. Sci. 2015, 95, 325–340. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi-Varnamkhasti, M.; Mohtasebi, S.S.; Siadat, M.; Balasubramanian, S. Meat quality assessment by electronic nose (machine olfaction technology). Sensors 2009, 9, 6058–6083. [Google Scholar] [CrossRef] [PubMed]
- Peris, M.; Escuder-Gilabert, L. A 21st century technique for food control: Electronic noses. Anal. Chim. Acta 2009, 638, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Alpha MOS Electronic Noses. Available online: https://www.norlab.com/infocenter/207 (accessed on 9 September 2018).
- Li, X.; Li, C.; Ye, H.; Wang, Z.; Wu, X.; Han, Y.; Xu, B. Changes in the microbial communities in vacuum-packaged smoked bacon during storage. Food Microbiol. 2019, 77, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Hinrichsen, L.L.; Pedersen, S.B. Relationship among flavor, volatile compounds, chemical changes, and microflora in Italian-type dry-cured ham during processing. J. Agric. Food Chem. 1995, 43, 2932–2940. [Google Scholar] [CrossRef]
- Olivares, A.; Navarro, J.L.; Flores, M. Establishment of the contribution of volatile compounds to the aroma of fermented sausages at different stages of processing and storage. Food Chem. 2009, 115, 1464–1472. [Google Scholar] [CrossRef]
- Montanari, C.; Bargossi, E.; Gardini, A.; Lanciotti, R.; Magnani, R.; Gardini, F.; Tabanelli, G. Correlation between volatile profiles of italian fermented sausages and their size and starter culture. Food Chem. 2016, 192, 736–744. [Google Scholar] [CrossRef]
- Ansorena, D.; Gimeno, O.; Astiasaran, I.J. Analysis of volatile compounds by GC-MS of a dry fermented sausage: Chorizo de Pamplona. Food Res. Int. 2001, 34, 67–75. [Google Scholar] [CrossRef]
- Gamerschlag, R.; Möller, K.; Verbeeten, F. Determinants of voluntary CSR disclosure: Empirical evidence from Germany. Rev. Manag. Sci. 2010, 5, 233–262. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Rivas-Cañedo, A.; Juez-Ojeda, C.; Nuñez, M.; Fernández-García, E. Effects of high-pressure processing on the volatile compounds of sliced cooked pork shoulder during refrigerated storage. Food Chem. 2011, 124, 749–758. [Google Scholar] [CrossRef]
- Yu, A.-N.; Sun, B.-G.; Tian, D.-T.; Qu, W.-Y. Analysis of volatile compounds in traditional smoke-cured bacon (CSCB) with different fiber coatings using spme. Food Chem. 2008, 110, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Sabio, E.; Vidal-Aragón, M.; Bernalte, M.; Gata, J. Volatile compounds present in six types of dry-cured ham from south European countries. Food Chem. 1998, 61, 493–503. [Google Scholar] [CrossRef]
- Wang, S.; He, Y.; Wang, Y.; Tao, N.; Wu, X.; Wang, X.; Qiu, W.; Ma, M. Comparison of flavour qualities of three sourced eriocheir sinensis. Food Chem. 2016, 200, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Rubin, L.J.; D’Souza, L.A. Meat flavor volatiles: A review of the composition, techniques of analysis, and sensory evaluation. Crit. Rev. Food Sci. Nutr. 1986, 24, 141–243. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, J.A.; Hierro, E.M.; Bruna, J.M.; de la Hoz, L. Changes in the components of dry-fermented sausages during ripening. Crit. Rev. Food Sci. Nutr. 1999, 39, 329–367. [Google Scholar] [CrossRef] [PubMed]
- Kjällstrand, J.; Ramnäs, O.; Petersson, G. Methoxyphenols from burning of Scandinavian forest plant materials. Chemosphere 2000, 41, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Jerković, I.; Kovačević, D.; Šubarić, D.; Marijanović, Z.; Mastanjević, K.; Suman, K. Authentication study of volatile flavour compounds composition in Slavonian traditional dry fermented salami “kulen”. Food Chem. 2010, 119, 813–822. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, B.; Han, Q.; Xia, X.; Xu, L. The role of bacterial fermentation in lipolysis and lipid oxidation in Harbin dry sausages and its flavour development. LWT-Food Sci. Technol. 2017, 77, 389–396. [Google Scholar] [CrossRef]
- Toldra´, F. Proteolysis and lipolysis in flavour development of dry-cured meat products. Meat Sci. 1998, 49, 101–110. [Google Scholar] [CrossRef]
- Zotta, T.; Ricciardi, A.; Ianniello, R.G.; Storti, L.V.; Glibota, N.A.; Parente, E. Aerobic and respirative growth of heterofermentative lactic acid bacteria: A screening study. Food Microbiol. 2018, 76, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J. Observations on the succession dynamics of lactic acid bacteria populations in chill-stored vacuum-packaged beef. Int. J. Food Microbiol. 2004, 90, 273–282. [Google Scholar] [CrossRef]
- Martín, A.; Benito, M.J.; Aranda, E.; Ruiz-Moyano, S.; Córdoba, J.J.; Córdoba, M.G. Characterization by volatile compounds of microbial deep spoilage in Iberian dry-cured ham. J. Food Sci. 2010, 75, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Joffraud, J.J.; Leroi, F.; Roy, C.; Berdagué, J.L. Characterization of volatile compounds produced by bacteria isolated from cold-smoked salmon flora. Inter. J. Food Microbiol. 2001, 66, 175–184. [Google Scholar] [CrossRef]
- Cui, S.; Wang, J.; Yang, L.; Wu, J.; Wang, X. Qualitative and quantitative analysis on aroma characteristics of ginseng at different ages using E-nose and GC-MS combined with chemometrics. J. Pharm. Biomed. Anal. 2015, 102, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Cmg, S.; Mba, G. Bioactive amines in chicken breast and thigh after slaughter and during storage at 4 ± 1 °C and in chicken-based meat products. Food Chem. 2002, 78, 241–248. [Google Scholar]
- Kalač, P. Biologically active polyamines in beef, pork and meat products: A review. Meat Sci. 2006, 73, 1–11. [Google Scholar] [CrossRef]
- Dainty, R.H.; Edwards, R.A.; Hibbard, C.M.; Ramantanis, S.V. Bacterial sources of putrescine and cadaverine in chill stored vacuum-packaged beef. J. Appl. Bacteriol. 2010, 61, 117–123. [Google Scholar] [CrossRef]
- Lonvaud-Funel, A. Biogenic amines in wines: Role of lactic acid bacteria. FEMS Microbiol. Lett. 2001, 199, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Bover-Cid, S.; Izquierdo-Pulido, M.; Carmen Vidal-Carou, M. Changes in biogenic amine and polyamine contents in slightly fermented sausages manufactured with and without sugar. Meat Sci. 2001, 57, 215–221. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, X.; Xiao, Z.; Song, S.; Eric, K.; Jia, C.; Yu, H.; Zhu, J. Characterization of odor-active compounds of various cherry wines by gas chromatography–mass spectrometry, gas chromatography–olfactometry and their correlation with sensory attributes. J. Chromatogr. B 2011, 879, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, X.; Hayat, K.; Huang, M.; Liu, P.; Karangwa, E.; Gu, F.; Jia, C.; Xia, S.; Xiao, Z.; Niu, Y. Contribution of beef base to aroma characteristics of beeflike process flavour assessed by descriptive sensory analysis and gas chromatography olfactometry and partial least squares regression. J. Chromatogr. Coruña 2010, 1217, 7788–7799. [Google Scholar] [CrossRef]
- Tian, H.; Zhan, P.; Li, W.; Zhang, X.; He, X.; Ma, Y.; Guo, Z.; Zhang, D. Contribution to the aroma characteristics of mutton process flavor from oxidized suet evaluated by descriptive sensory analysis, gas chromatography, and electronic nose through partial least squares regression. Eur. J. Lipid Sci. Technol. 2014, 116, 1522–1533. [Google Scholar] [CrossRef]
- Dainty, R.H.; Mackey, B.M. The relationship between the phenotypic properties of bacteria from chill-stored meat and spoilage processes. J. Appl. Bacteriol. 1992, 73, 103–114. [Google Scholar] [CrossRef]
- Ercolini, D.; Russo, F.; Torrieri, E.; Masi, P.; Villani, F. Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl. Environ. Microbiol. 2006, 72, 4663–4671. [Google Scholar] [CrossRef]
- Tsigarida, E.; Nychas, G.-J.E. Ecophysiological attributes of a lactobacillus sp. and a Pseudomonas sp. on sterile beef fillets in relation to storage temperature and film permeability. J. Appl. Microbiol. 2001, 90, 696–705. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Paramithiotis, S.; Kagkli, D.M.; Nychas, G.-J.E. Lactic acid bacteria population dynamics during minced beef storage under aerobic or modified atmosphere packaging conditions. Food Microbiol. 2010, 27, 1028–1034. [Google Scholar] [CrossRef] [Green Version]
- Alpha MOS. STREE Electrochemical Sensor Technology. Available online: http://www.alphamos-cn.com/ (accessed on 9 September 2018).
- Ngapo, T.M.; Vachon, L. Biogenic amine concentrations and evolution in “chilled” Canadian pork for the Japanese market. Food Chem. 2017, 233, 500–506. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Shu, C.; Chen, H.; Xiao, Z. Comparison of aroma-active compounds and sensory characteristics of durian (Durio zibethinusl.) wines using strains ofsaccharomyces cerevisiaewith odor activity values and partial least-squares regression. J. Agric. Food Chem. 2015, 63, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Mah, J.-H.; Hwang, H.-J. Biogenic amine formation and bacterial contribution in fish, squid and shellfish. Food Chem. 2009, 116, 87–95. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Spoilage Bacteria | Storage Time/Days | Pearson’s Correlation Coefficients | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 15 | Day 22 | Day 30 | Day 45 | r | p | |
| Leuconostoc | 0.91 ± 0.44 c | 1.74 ± 0.44 c | 26.72 ± 15.09 b | 53.73 ± 18.97 a | 40.26 ± 21.17 a,b | 27.49 ± 12.85 b | 0.616 | 0.193 |
| Lactobacillus | 2.62 ± 1.16 c | 3.76 ± 1.85 c | 6.25 ± 3.76 c | 9.45 ± 8.48 b,c | 18.70 ± 6.90 b | 41.94 ± 16.14 a | 0.934 ** | 0.006 |
| Vibrio | 5.91 ± 4.80 b | 5.42 ± 2.51 b | 30.69 ± 30.27 a | 10.96 ± 16.22 a,b | 8.08 ± 14.84 a,b | 8.44 ± 17.32 a,b | −0.022 | 0.968 |
| Brochothrix | 2.43 ± 0.73 a,b | 12.76 ± 20.75 a | 1.48 ± 0.48 a,b | 2.41 ± 2.77 a,b | 0.17 ± 0.07 b | 0.33 ± 0.21 b | −0.527 | 0.282 |
| Serratia | 0.34 ± 0.33 | 0.47 ± 0.34 | 0.20 ± 0.07 | 0.80 ± 1.19 | 6.28 ± 12.89 | 1.57 ± 2.83 | 0.48 | 0.335 |
| Kocuria | 13.19 ± 6.90 a | 4.48 ± 2.07 b | 1.56 ± 1.15 b | 0.33 ± 0.22 b | 0.38 ± 0.14 b | 0.36 ± 0.33 b | −0.76 | 0.079 |
| Macrococcus | 8.58 ± 9.59 a | 0.97 ± 0.29 b | 0.27 ± 0.25 b | 0.01 ± 0.00 b | 0.15 ± 0.15 b | 0.01 ± 0.00 b | −0.657 | 0.156 |
| Carnobacterium | 1.00 ± 0.37 c | 1.51 ± 0.47 c | 9.66 ± 6.62 a,b | 12.94 ± 8.14 a | 12.15 ± 6.38 a,b | 5.52 ± 1.29 b,c | 0.48 | 0.335 |
| Rahnella | 0.12 ± 0.07 | 0.48 ± 0.55 | 0.05 ± 0.03 | 0.13 ± 0.13 | 0.27 ± 0.07 | 2.78 ± 5.73 | 0.745 | 0.089 |
| Fusobacterium | 1.02 ± 1.18 | 0.89 ± 1.45 | 0.16 ± 0.20 | 0.09 ± 0.14 | 3.58 ± 0.36 | 0.91 ± 1.79 | 0.261 | 0.618 |
| Others | 63.88 ± 2.93 a | 67.51 ± 19.52 a | 22.98 ± 14.39 b | 9.15 ± 3.49 b | 9.97 ± 2.61 b | 10.65 ± 1.82 b | −0.826 * | 0.043 |
| No | Compound | Standard Curve | r2 | Validation Range (μg kg−1) |
|---|---|---|---|---|
| 1 | 3-Methylbutanal | y = 1.11x + 0.035 | 0.987 | 0.1–5 |
| 2 | Hexanal | y = 1.84x − 0.019 | 0.991 | 10–100 |
| 3 | Furfural | y = 0.94x + 0.083 | 0.986 | 10–100 |
| 4 | Heptanal | y = 1.1x + 0.004 | 0.992 | 1–50 |
| 5 | 5-Methyl-2-furancarboxaldehyde | y = 0.88x + 0.031 | 0.982 | 1–50 |
| 6 | Octanal | y = 0.73x − 0.041 | 0.984 | 1–100 |
| 7 | Phenylacetaldehyde | y = 1.13x + 0.051 | 0.992 | 1–50 |
| 8 | Nonanal | y = 2.12x + 0.006 | 0.979 | 20–500 |
| 9 | Decanal | y = 0.95x − 0.081 | 0.981 | 1–50 |
| 10 | Ethanol | y = 1.21x + 0.065 | 0.986 | 50–1000 |
| 11 | 1-Propanol | y = 0.95x − 0.027 | 0.988 | 50–1000 |
| 12 | 2-Butanol | y = 1.17x + 0.094 | 0.987 | 20–500 |
| 13 | 3-Methyl-1-Butanol | y = 1.18x + 0.028 | 0.992 | 1–10 |
| 14 | Propylene glycol | y = 1.24x + 0.007 | 0.987 | 1–50 |
| 15 | 1-Pentanol | y = 0.81x + 0.057 | 0.981 | 1–50 |
| 16 | 2-Furanmethanol | y = 1.15x − 0.025 | 0.979 | 20–500 |
| 17 | 1-Hexanol | y = 2.28x + 0.068 | 0.973 | 1–50 |
| 18 | Phenol | y = 1.51x + 0.029 | 0.986 | 10–100 |
| 19 | 2-Methylphenol | y = 0.86x + 0.021 | 0.992 | 10–100 |
| 20 | 4-Methylphenol (p-cresol) | y = 0.98x + 0.009 | 0.978 | 10–100 |
| 21 | 3-Methylphenol | y = 0.88x + 0.059 | 0.979 | 1–50 |
| 22 | 2-Methoxyphenol | y = 1.17x − 0.061 | 0.984 | 20–500 |
| 23 | 2,6-Dimethylphenol | y = 1.17x - 0.068 | 0.991 | 1–10 |
| 24 | 2-Methoxy-3-methylphenol | y = 0.88x + 0.069 | 0.992 | 1–10 |
| 25 | Creosol | y = 0.84x + 0.029 | 0.994 | 20–500 |
| 26 | 4-Ethyl-2-methoxyphenol | y = 0.97x − 0.019 | 0.984 | 10–100 |
| 27 | 2-Methoxy-4-vinylphenol | y = 2.13x − 0.029 | 0.977 | 1–10 |
| 28 | 2,6-Dimethoxyphenol | y = 1.19x − 0.012 | 0.975 | 10–100 |
| 29 | Eugenol | y = 1.16x + 0.068 | 0.987 | 1–10 |
| 30 | trans-Isoeugenol | y = 0.96x + 0.043 | 0.984 | 1–10 |
| 31 | 1-Hydroxy-2-propanone | y = 1.15x + 0.042 | 0.986 | 1–50 |
| 32 | 2-Cyclopentenone | y = 0.93x − 0.051 | 0.989 | 1–10 |
| 33 | 2-Methyl-2-cyclopentenone | y = 0.82x − 0.067 | 0.978 | 10–100 |
| 34 | 3-Methyl-2-cyclopentenone | y = 1.19x + 0.018 | 0.983 | 10–100 |
| 35 | 3,4-Dimethyl-2-cyclopentenone | y = 0.99x + 0.085 | 0.984 | 0.1–5 |
| 36 | 2-Hydroxy-3-methyl-2-cyclopentenone | y = 0.96x − 0.027 | 0.991 | 10–100 |
| 37 | 2,3-Dimethyl-2-cyclopentenone | y = 0.85x + 0.047 | 0.978 | 10–100 |
| 38 | 3-Ethyl-2-cyclopentenone | y =1.17x + 0.018 | 0.979 | 1–50 |
| 39 | 3-Ethyl-2-hydroxy-2-cyclopentenone | y = 1.26x − 0.069 | 0.992 | 1–50 |
| 40 | 2,3,4-Trimethylpentane | y = 1.14x + 0.068 | 0.986 | 1–50 |
| 41 | 2,3,3-Trimethylpentane | y = 0.79x − 0.028 | 0.985 | 1–50 |
| 42 | Octane | y = 0.86x + 0.021 | 0.985 | 1–50 |
| 43 | Decane | y = 0.88x + 0.068 | 0.977 | 1–50 |
| 44 | Cyclooctane | y = 1.18x − 0.012 | 0.978 | 1–50 |
| 45 | 2,2,8-Trimethyldecane | y = 0.096x + 0.035 | 0.986 | 1–50 |
| 46 | 3-Methyl-3-heptene | y = 0.99x − 0.039 | 0.982 | 1–10 |
| 47 | (Z)-2-Octene | y = 0.86x + 0.017 | 0.981 | 1–10 |
| 48 | Styrene | y = 0.94x − 0.008 | 0.994 | 1-10 |
| 49 | α-Pinene | y = 1.27x + 0.058 | 0.989 | 0.1–5 |
| 50 | D-Limonene | y = 1.17.x − 0.059 | 0.995 | 1–10 |
| 51 | Acetic acid | y = 0.86x + 0.029 | 0.981 | 20–500 |
| 52 | Butanoic acid | y = 0.84x − 0.046 | 0.993 | 1–50 |
| 53 | Propanoic acid | y = 0.97x + 0.027 | 0.976 | 0.1–5 |
| 54 | Benzoic acid | y = 0.86x + 0.038 | 0.977 | 1–50 |
| 55 | 2-Furylmethylketone | y = 0.98x − 0.017 | 0.988 | 1–10 |
| 56 | Ethyl hexanoate | y = 1.18.x + 0.051 | 0.992 | 1–10 |
| Code A | Compound | RI B (Calculated) | Identification C | Storage Time/Days | Pearson’s Correlation Coefficients | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 15 | Day 22 | Day 30 | Day 45 | r | p | ||||
| Aldehydes | |||||||||||
| 1 | 3-Methylbutanal | 703 | MS, RI, Sta | 1.89 ± 0.35 a,D | - | 1.58 ± 0.55 b | 1.82 ± 0.35 a,b | 1.42 ± 0.35 c | - | −0.373 | 0.467 |
| 2 | Hexanal | 803 | MS, RI, Sta | 97.61 ± 10.77 a | 38.98±4.89 c | 103.2 ± 6.02 a | 70.91 ± 7.86 b | 47.01 ± 10.08 c | 24.38 ± 3.19 d | −0.644 | 0.167 |
| 3 | Furfural | 839 | MS, RI, Sta | 84.36 ± 7.13 a | 72.74 ± 4.03 b | 72.36 ± 8.49 b | 13.25 ± 2.05 c | - | - | −0.900 ** | 0.007 |
| 4 | Heptanal | 904 | MS, RI, Sta | 33.7 ± 5.60 a | 0.71 ± 0.13 c | 8.22 ± 1.52 b | - | 3.4 ± 0.86 c | - | −0.639 | 0.172 |
| 5 | 5-Methyl-2-Furan-carboxaldehyde | 969 | MS, RI, Sta | 14.72 ± 2.26 b | 15.62 ± 1.54 a,b | 17.45 ± 0.90 a | 2.9 ± 0.35 c | - | - | −0.844 * | 0.035 |
| 6 | Octanal | 1006 | MS, RI, Sta | 88.70 ± 7.82 a | 24.89 ± 3.10 e | 79.15 ± 6.67 b | 53.53 ± 6.11 c | 40.92 ± 3.37 d | 53.8 ± 6.94 c | −0.299 | 0.566 |
| 7 | Phenyl-acetaldehyde | 1052 | MS, RI, Sta | 1.49 ± 0.21 b | - | - | 2.88 ± 0.60 b | 1.87 ± 0.56 b | 36.98 ± 6.27 a | 0.779 | 0.068 |
| 8 | Nonanal | 1107 | MS, RI, Sta | 234.35 ± 28.98 b | 59.21 ± 7.11 e | 384.74 ± 27.84 a | 161.42 ± 20.22 d | 151.19 ± 16.09 d | 189.73 ± 19.80 c | −0.069 | 0.897 |
| 9 | Decanal | 1209 | MS, RI, Sta | 13.72 ± 2.67 b | 3.94 ± 0.38 c | 17.65 ± 1.56 a | 2.54 ± 0.33 c | 2.08 ± 0.38 c | - | −0.65 | 0.162 |
| 570.54 ± 41.07 b | 216.09 ± 6.69 d | 684.35 ± 40.11 a | 309.25 ± 29.37 c | 247.87 ± 19.55 | 304.89 ± 23.41 c | −0.421 | 0.406 | ||||
| Alcohols | |||||||||||
| 10 | Ethanol | <700 | MS, RI, Sta | 5.07 ± 0.85 d | 24.61 ± 2.72 d | 123.68 ± 12.65 c | 265.77 ± 39.00 b | 414.96 ± 52.38 a | 525.24 ± 78.14 a | 0.982 ** | 0 |
| 11 | 1-Propanol | <700 | MS, RI, Sta | - | - | - | 75.73 ± 10.402 c | 160.68 ± 20.27 b | 381.37 ± 48.94 a | 0.930 ** | 0.007 |
| 12 | 2-Butanol | <700 | MS, RI, Sta | - | - | 16.83 ± 2.93 b | 13.54 ± 3.66 b | - | 212.07 ± 32.92 a | 0.77 | 0.073 |
| 13 | 3-Methyl-1-butanol | 735 | MS, RI, Sta | - | - | 6.06 ± 0.86 b,c | 5.07 ± 1.16 c | 10.44 ± 1.24 a | 7.56 ± 1.69 b | 0.819 * | 0.046 |
| 14 | Propylene Glycol | 753 | MS, Sta | 5.47 ± 0.42 d | 1.94 ± 0.26 e | 12.66 ± 2.85 b | 11.51 ± 2.14 b,c | 6.81 ± 1.02 c,d | 17.32 ± 3.25 a | 0.74 | 0.093 |
| 15 | 1-Pentanol | 767 | MS, RI, Sta | 0.59 ± 0.15 b | - | - | 2.58 ± 0.13 b | - | 37.43 ± 3.14 a | 0.764 | 0.077 |
| 16 | 2-Furanmethanol | 861 | MS, RI, Sta | 27.39 ± 5.05 c | 28.39 ± 2.66 c | 63.5 ± 3.70 b | 66.29 ± 11.99 b | 66.83 ± 9.28 b | 113.76 ± 13.50 a | 0.982 ** | 0.003 |
| 17 | 1-Hexanol | 871 | MS, RI, Sta | 1.37 ± 0.18 c | 1.14 ± 0.12 c | 15.41 ± 1.29 b | 38.12 ± 2.62 a | 37.23 ± 7.35 a | 41.24 ± 6.73 a | 0.905 ** | 0.013 |
| 39.89 ± 4.73 d | 56.08 ± 1.62 d | 238.15 ± 16.41 d | 478.61 ± 41.06 c | 696.95 ± 61.40 b | 1335.99 ± 106.72 a | 0.976 ** | 0.001 | ||||
| Phenols | |||||||||||
| 18 | Phenol | 990 | MS, RI, Sta | 10.33 ± 2.98 c | 12.4 ± 2.01 c | 18.62 ± 2.97 c | 28.51 ± 4.10 b | 49.11 ± 9.57 a | 52.62 ± 6.54 a | 0.956 ** | 0.003 |
| 19 | 2-Methylphenol | 1064 | MS, RI, Sta | 16.41 ± 2.04 b | 19.1 ± 1.48 b | 37.76 ± 4.63 a | 41.73 ± 6.39 a | 33.58 ± 5.52 a | 41.74 ± 8.47 a | 0.796 | 0.058 |
| 20 | 4-Methylphenol (p-cresol) | 1086 | MS, RI, Sta | 41.52 ± 4.48 c | 34.74 ± 3.78 c | 70.97 ± 7.45. a,b | 45.12 ± 5.20 c | 54.16 ± 12.28 b | 76.45 ± 7.96 a | 0.706 | 0.117 |
| 21 | 3-Methylphenol | 1088 | MS, RI, Sta | 2.11 ± 0.37 d | - | 12.1 ± 1.42 c | 45.13±4.26 a | 22.99 ± 3.49 b | 26.09 ± 4.39 b | 0.644 | 0.168 |
| 22 | 2-Methoxyphenol | 1092 | MS, RI, Sta | 134.97 ± 16.79 d | 118.61 ± 11.19 d | 280.97 ± 26.87 c | 293.44 ± 21.90 b,c | 331.51 ± 44.66 a,b | 372.9 ± 47.34 a | 0.919 ** | 0.01 |
| 23 | 2,6-Dimethyl-phenol | 1114 | MS, RI, Sta | - | - | - | - | - | 7.23 ± 0.83 | 0.758 | 0.081 |
| 24 | 2-Methoxy-3-methylphenol | 1191 | MS, Sta | 1.16 ± 0.25 d | 1.34 ± 0.16 d | 4.98 ± 0.75 b | 3.49 ± 0.32 b,c | 3.1 ± 0.83 c | 8.19 ± 0.99 a | 0.850 * | 0.032 |
| 25 | Creosol | 1194 | MS, RI, Sta | 49.2 ± 6.94 d | 34.45 ± 3.68 d | 95.11 ± 18.12 b,c | 108.1 ± 9.56 b | 69.6 ± 9.31 c | 123.38 ± 18.34 a | 0.768 | 0.074 |
| 26 | 4-Ethyl-2-methoxyphenol | 1282 | MS, RI, Sta | 18.29 ± 2.57 d | 13.72 ± 1.98 d | 35.96 ± 3.68 b,c | 41.85 ± 6.86 a,b | 29.86 ± 3.16 c | 42.23 ± 9.39 a | 0.759 | 0.08 |
| 27 | 2-Methoxy-4-vinylphenol | 1319 | MS, RI, Sta | 5.16 ± 1.09 c | 2.17 ± 0.44 d | 7.92 ± 1.24 a | 8.03 ± 1.51 a,b | 5.47 ± 0.77 b,c | 8.66 ± 1.46 a | 0.613 | 0.196 |
| 28 | 2,6-Dimethoxy-phenol | 1356 | MS, Sta | 26.45 ± 2.63 b | 16.67 ± 1.81 b | 52.52 ± 8.82 a | 54.18 ± 8.64 a | 42.58 ± 6.82 a | 53.58 ± 9.49 a | 0.699 | 0.122 |
| 29 | Eugenol | 1362 | MS, RI, Sta | 1.25 ± 0.15 d | 0.29 ± 0.06 d | 2.55 ± 0.30 c | 12.66 ± 1.53 a | - | 4.4 ± 0.63 b | 0.251 | 0.631 |
| 30 | trans-Isoeugenol | 1458 | MS, RI, Sta | 1.27 ± 0.15 c | - | 3.48 ± 0.73 a | 3.05 ± 0.47 a,b | 2.98 ± 0.89 a,b | 2.51 ± 0.37 b | 0.539 | 0.27 |
| 308.12 ± 20.85 c | 253.48 ± 8.10 c | 622.94 ± 37.94 b | 685.31 ± 22.89 b | 644.94 ± 79.80 b | 819.97 ± 87.33 a | 0.894 * | 0.016 | ||||
| Ketones | |||||||||||
| 31 | 1-Hydroxy-2-propanone | 669 | MS, RI, Sta | 17.23 ± 2.23 b | 12.6 ± 2.64 b,c | 41.36 ± 6.70 a | 9.21 ± 0.94 c | - | - | −0.548 | 0.26 |
| 32 | 2-Cyclopentenone | 839 | MS, RI, Sta | - | - | - | 8.46 ± 1.41 | 7.9 ± 0.96 | 8.55 ± 1.84 | 0.847 * | 0.033 |
| 33 | 2-Methyl-2-cyclo-pentenone | 910 | MS, RI, Sta | 17.3 ± 2.41 c | 15.59 ± 1.62 c | 42.62 ± 6.15 b | 43.09 ± 6.86 b | 38.02 ± 4.25 b | 58.92 ± 5.99 a | 0.895 * | 0.016 |
| 34 | 3-Methyl-2-cyclo-pentenone | 971 | MS, RI, Sta | 19.03 ± 2.31 c | 20.6 ± 2.30 b,c | 34.75 ± 4.76 a | 25.02 ± 4.11 b | 36.99 ± 8.94 a | 40.73 ± 3.54 a | 0.867 * | 0.025 |
| 35 | 3,4-Dimethyl-2-cyclopentenone | 1027 | MS, Sta | - | - | - | 3.47 ± 0.75 | - | - | 0.065 | 0.902 |
| 36 | 2-Hydroxy-3-methyl-2-cyclo-pentenone | 1034 | MS, RI, Sta | 15.23 ± 2.22 c | 3.66 ± 0.51 d | 40.12 ± 5.12 b | - | 36.85 ± 9.58 b | 61.47 ± 7.86 a | 0.708 | 0.115 |
| 37 | 2,3-Dimethyl-2-cyclopentenone | 1042 | MS, RI, Sta | 17.43 ± 1.69 c | 16.12 ± 1.65 c | 40.2 ± 6.53 b | 43.6 ± 7.56 b | 33.79 ± 4.66 b | 52.96 ± 6.07 a | 0.855 * | 0.03 |
| 38 | 3-Ethyl-2-cyclopentenone | 1080 | MS, Sta | 3 ± 0.32 c | 3.43 ± 0.30 c | 6.53 ± 1.47 b,c | 6.12 ± 0.98 b,c | 7.52 ± 1.32 b | 17.91 ± 2.63 a | 0.916 * | 0.01 |
| 39 | 3-Ethyl-2-hydroxy-2-cyclo-pentenone | 1100 | MS, RI, Sta | 12.25 ± 1.59 c | 8.03 ± 2.62 d | 17.45 ± 2.04 b | 11.3 ± 1.64 c | 12.49 ± 1.83 c | 26.83 ± 4.06 a | 0.732 | 0.098 |
| 101.47 ± 3.70 e | 80.01 ± 7.17 f | 223.03 ± 22.43 b | 146.8 ± 11.78 d | 173.56 ± 25.92 c | 267.38 ± 15.01 a | 0.807 | 0.052 | ||||
| Alkanes | |||||||||||
| 40 | 2,3,4-Trimethyl-Pentane | 752 | MS, Sta | - | - | 3.11 ± 0.62 c | 6.81 ± 1.77 b | 1.42 ± 0.30 c | 15.85 ± 2.77 a | 0.833 * | 0.04 |
| 41 | 2,3,3-Trimethyl-pentane | 759 | MS, Sta | - | - | - | - | 3.37 ± 0.69 | 33.35 ± 4.28 | 0.801 | 0.055 |
| 42 | Octane | 801 | MS, RI, Sta | 2.54 ± 0.33 c | - | 17.39 ± 2.94 b | 37.18 ± 5.44 a | 16.16 ± 2.54 b | 20.49 ± 3.47 b | 0.572 | 0.236 |
| 43 | Decane | 1000 | MS, RI, Sta | 19.72 ± 1.99 c | 3.48 ± 0.48 d | 13.4 ± 2.24 c | 72.29 ± 7.58 a | 48.09 ± 7.18 b | 22.49 ± 3.45 c | 0.343 | 0.506 |
| 44 | Cyclooctane | 1075 | MS, Sta | 1.24 ± 0.23 c | 3.12 ± 0.41 b | 4.41 ± 1.46 b | - | - | 15.02 ± 1.82 a | 0.631 | 0.179 |
| 23.5 ± 1.94 e | 6.6 ± 0.74 f | 38.31 ± 1.32 d | 116.28 ± 11.83 a | 69.03 ± 6.12 c | 107.2 ± 5.38 b | 0.701 | 0.12 | ||||
| Terpene compounds | |||||||||||
| 45 | 2,2,8-Trimethyl-Decane | 792 | MS, Sta | - | - | 5.04 ± 1.23 b | 0.99 ± 0.53 c | 4.28 ± 0.49 b | 18.03 ± 3.03 a | 0.850 * | 0.032 |
| 46 | 3-Methyl-3-heptene | 798 | MS, Sta | - | - | - | - | 3.62 ± 0.75 | 8.13 ± 1.17 | 0.855 * | 0.019 |
| 47 | (Z)-2-Octene | 807 | MS, RI, Sta | - | - | - | - | - | 5.15 ± 0.79 | 0.758 | 0.081 |
| 48 | Styrene | 898 | MS, RI, Sta | - | - | - | 10.44 ± 1.65 | 3.57 ± 0.34 | - | 0.172 | 0.745 |
| 49 | α-Pinene | 940 | MS, RI, Sta | - | - | 0.91 ± 0.23 b | 0.92 ± 0.14 b | 1.95 ± 0.38 a | 0.55 ± 0.12 c | 0.523 | 0.287 |
| 50 | D-Limonene | 1036 | MS, RI, Sta | - | 0.88 ± 0.17 c | - | - | 5.74 ± 0.83 b | 8 ± 1.42 a | 0.867 * | 0.025 |
| - | 0.88 ± 0.17 e | 5.95 ± 1.09 d | 15.82 ± 2.09 c | 19.16 ± 0.60 b | 39.87 ± 3.97 a | 0.975 ** | 0.001 | ||||
| Organic acids | |||||||||||
| 51 | Acetic acid | <700 | MS, RI, Sta | 24.78 ± 3.14 e | 10.72 ± 2.71 f | 44.88 ± 6.66 d | 64.73 ± 9.02 c | 100.71 ± 11.31 b | 126.69 ± 16.48 a | 0.964 ** | 0.002 |
| 52 | Butanoic acid | 890 | MS, RI, Sta | 9.89 ± 0.85 c | 2.62 ± 0.50 d | 9.36 ± 1.87 c | 3.68 ± 0.92 d | 22.57 ± 2.97 a | 16.22 ± 1.10 b | 0.6 | 0.208 |
| 53 | Propanoic acid | 985 | MS, RI, Sta | - | - | - | - | 0.64 ± 0.17 | 1.10 ± 0.19 | 0.890 * | 0.017 |
| 54 | Benzoic acid | 1549 | MS, RI, Sta | 12.26 ± 2.56 b,c | 1.79 ± 0.20 e | 15.22 ± 3.47 a.b | 16.71 ± 4.48 a | 9.73 ± 1.98 c,d | 8.26 ± 1.74 d | 0.008 | 0.989 |
| 46.93 ± 4.70 f | 19.39 ± 2.87 e | 69.46 ± 7.13 d | 85.12 ± 7.60 c | 133.65 ± 13.43 b | 152.27 ± 14.41 a | 0.935 ** | 0.006 | ||||
| Others | |||||||||||
| 55 | 2-Furylmethyl-ketone | 914 | MS, RI, Sta | - | 11.26 ± 1.21 | 9.11 ± 1.15 | - | - | - | −0.437 | 0.386 |
| 56 | Ethyl hexanoate | 998 | MS, RI, Sta | - | - | - | 2.97 ± 0.90 c | 5.05 ± 1.09 b | 14.28 ± 1.67 a | 0.920 ** | 0.009 |
| - | 11.26 ± 1.21 b | 9.11 ± 1.15 c | 2.97 ± 0.90 e | 5.05 ± 1.09 d | 14.28 ± 1.67 a | 0.523 | 0.287 | ||||
| 1090.45 ± 47.93 d | 643.79 ± 13.25 e | 1891.29 ± 127.58b c | 1840.16 ± 51.26 c | 1990.21 ± 207.91 b | 3041.85 ± 140.65 a | 0.918 ** | 0.01 | ||||
| Biogenic Amines | Storage Time/Days | Pearson’s Correlation Coefficients | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 15 | Day 22 | Day 30 | Day 45 | r | p | |
| Tryptamine | - | - | - | - | - | - | - | - |
| Phenylethylamine | - | - | - | - | - | - | - | - |
| Putrescine | 2.81 ± 0.57 c,d | 2.39 ± 0.36 d | 3.83 ± 0.58 b,c | 2.81 ± 0.70 c,d | 4.14 ± 0.47 b | 11.69 ± 1.86 a | 0.827 * | 0.042 |
| Cadaverine | 5.75 ± 0.53 c | 4.23 ± 0.46 d | 5.34 ± 0.60 c | 7.50 ± 0.93 b | 8.76 ± 1.11 a | 9.50 ± 0.94 a | 0.892 * | 0.017 |
| Histamine | 1.38 ± 0.31 c | 1.12 ± 0.15 c | 1.89 ± 0.27 b | 1.16 ± 0.17 c | 2.17 ± 0.29 a,b | 2.38 ± 0.43 a | 0.768 | 0.075 |
| Tyramine | 4.02 ± 0.79 c | 4.51 ± 0.93 c | 4.21 ± 0.53 c | 9.94 ± 1.15 b | 14.06 ± 1.93 a | 15.94 ± 2.48 a | 0.940 * | 0.005 |
| Spermidine | 2.22 ± 0.45 a,b | 2.45 ± 0.42 a | 2.20 ± 0.32 a,b | 2.22 ± 0.30 a,b | 2.29 ± 0.31 a,b | 1.85 ± 0.15 b | −0.716 | 0.110 |
| Spermine | 6.26 ± 0.98 | 7.40 ± 0.77 | 6.23 ± 0.58 | 7.02 ± 1.05 | 7.16 ± 1.12 | 6.93 ± 0.69 | 0.336 | 0.515 |
| Total | 22.44 ± 1.22 d | 22.10 ± 3.90 d | 23.70 ± 1.47 d | 30.65 ± 2.05 c | 38.58 ± 3.93 b | 48.29 ± 3.83 a | 0.963 ** | 0.002 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhu, J.; Li, C.; Ye, H.; Wang, Z.; Wu, X.; Xu, B. Evolution of Volatile Compounds and Spoilage Bacteria in Smoked Bacon during Refrigeration Using an E-Nose and GC-MS Combined with Partial Least Squares Regression. Molecules 2018, 23, 3286. https://doi.org/10.3390/molecules23123286
Li X, Zhu J, Li C, Ye H, Wang Z, Wu X, Xu B. Evolution of Volatile Compounds and Spoilage Bacteria in Smoked Bacon during Refrigeration Using an E-Nose and GC-MS Combined with Partial Least Squares Regression. Molecules. 2018; 23(12):3286. https://doi.org/10.3390/molecules23123286
Chicago/Turabian StyleLi, Xinfu, Jiancai Zhu, Cong Li, Hua Ye, Zhouping Wang, Xiang Wu, and Baocai Xu. 2018. "Evolution of Volatile Compounds and Spoilage Bacteria in Smoked Bacon during Refrigeration Using an E-Nose and GC-MS Combined with Partial Least Squares Regression" Molecules 23, no. 12: 3286. https://doi.org/10.3390/molecules23123286






