Pilloin, A Flavonoid Isolated from Aquilaria sinensis, Exhibits Anti-Inflammatory Activity In Vitro and In Vivo
Abstract
1. Introduction
2. Results
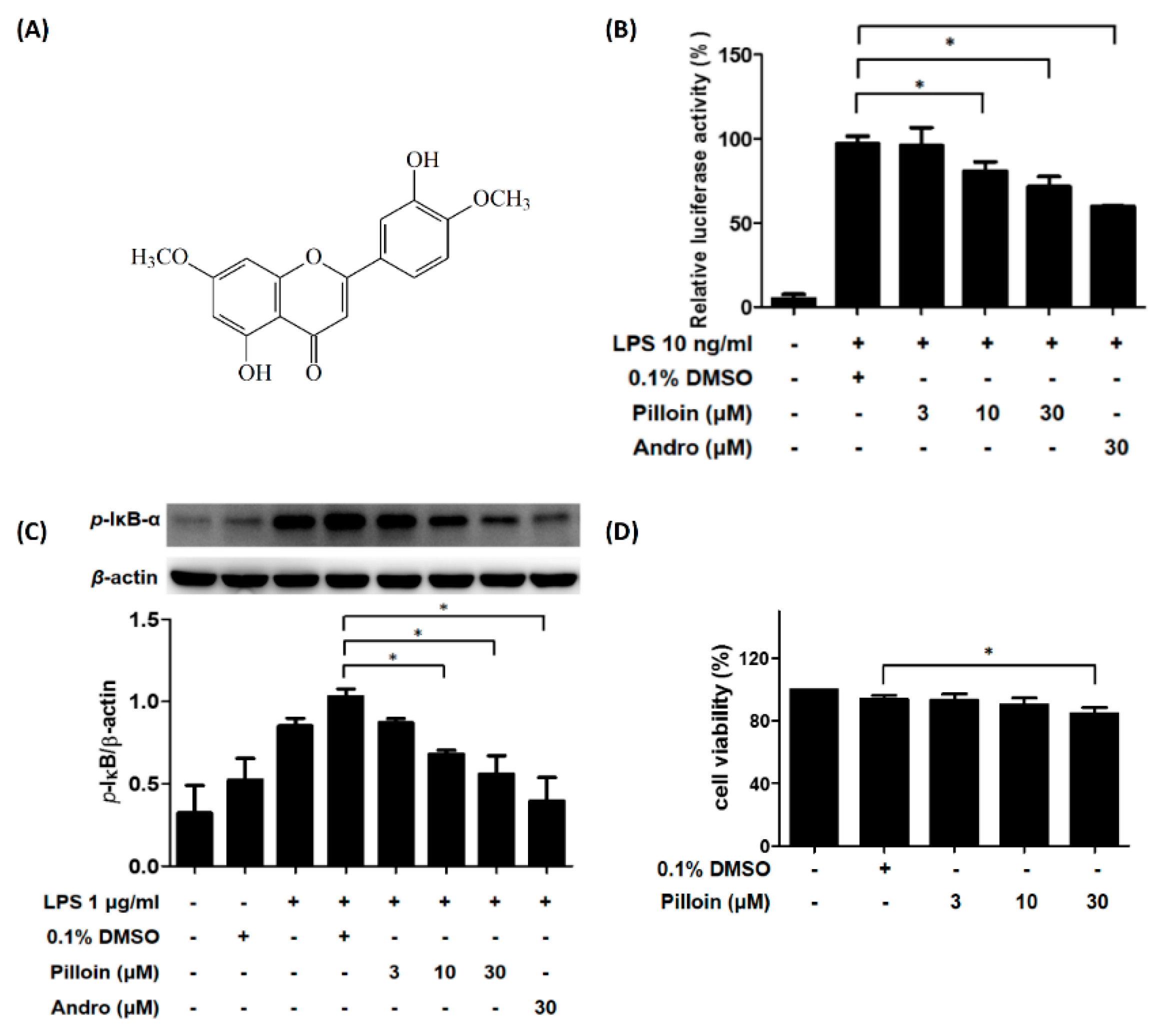
2.1. Isolation and Identification of Pilloin from A. sinensis
2.2. Pilloin Suppresses NF-κB Activity in LPS-Induced RAW 264.7 Macrophages
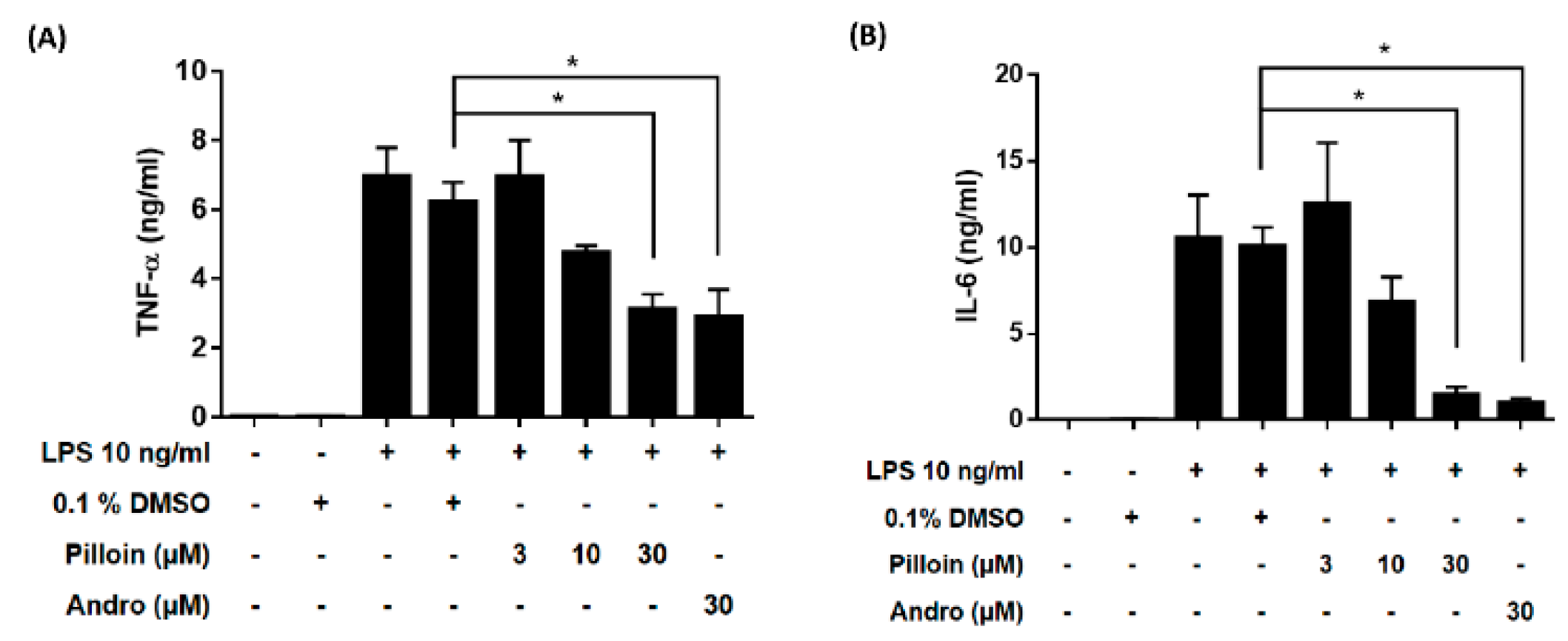
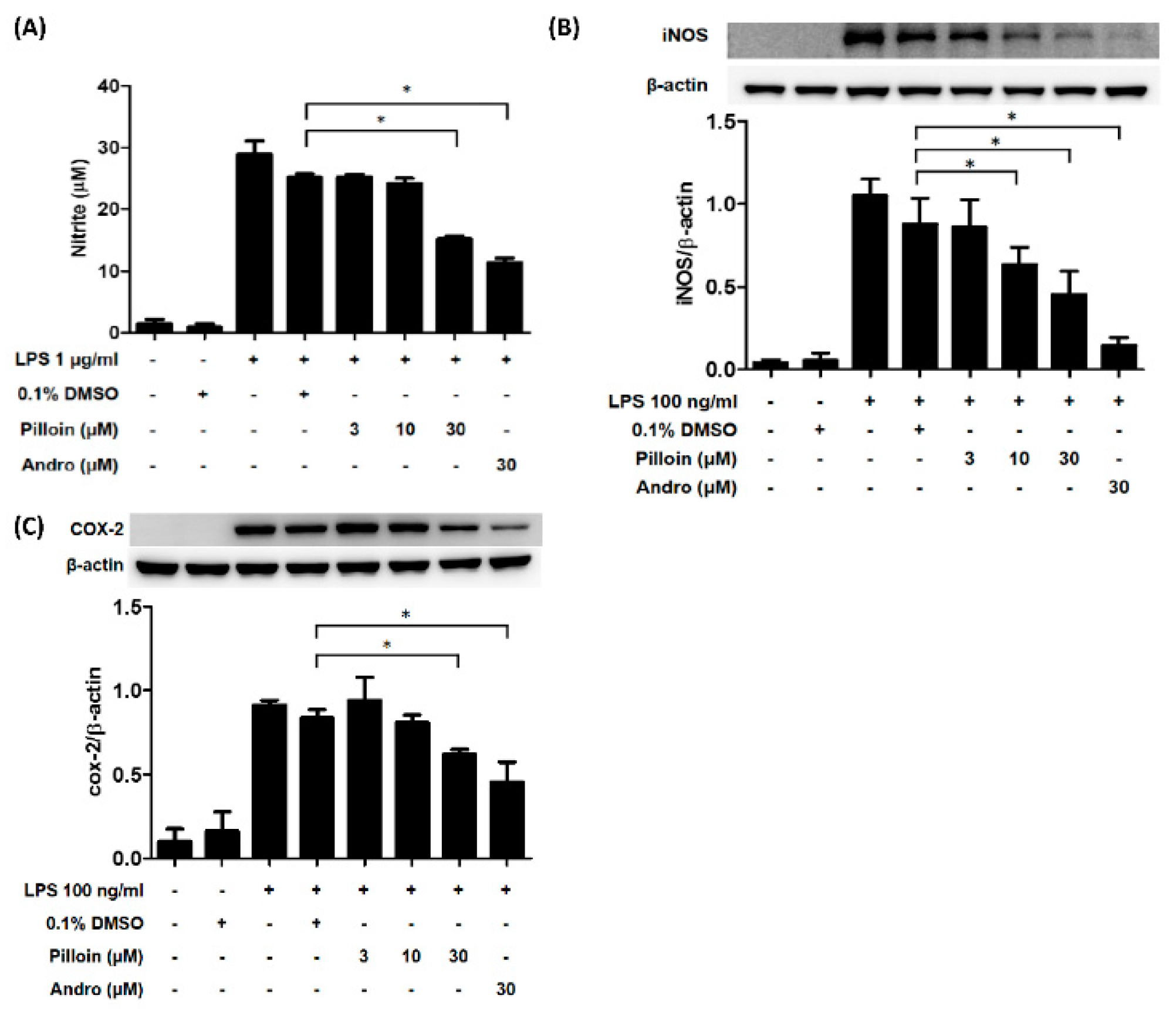
2.3. Pilloin Suppresses the Production of Pro-Inflammatory Molecules Induced by LPS
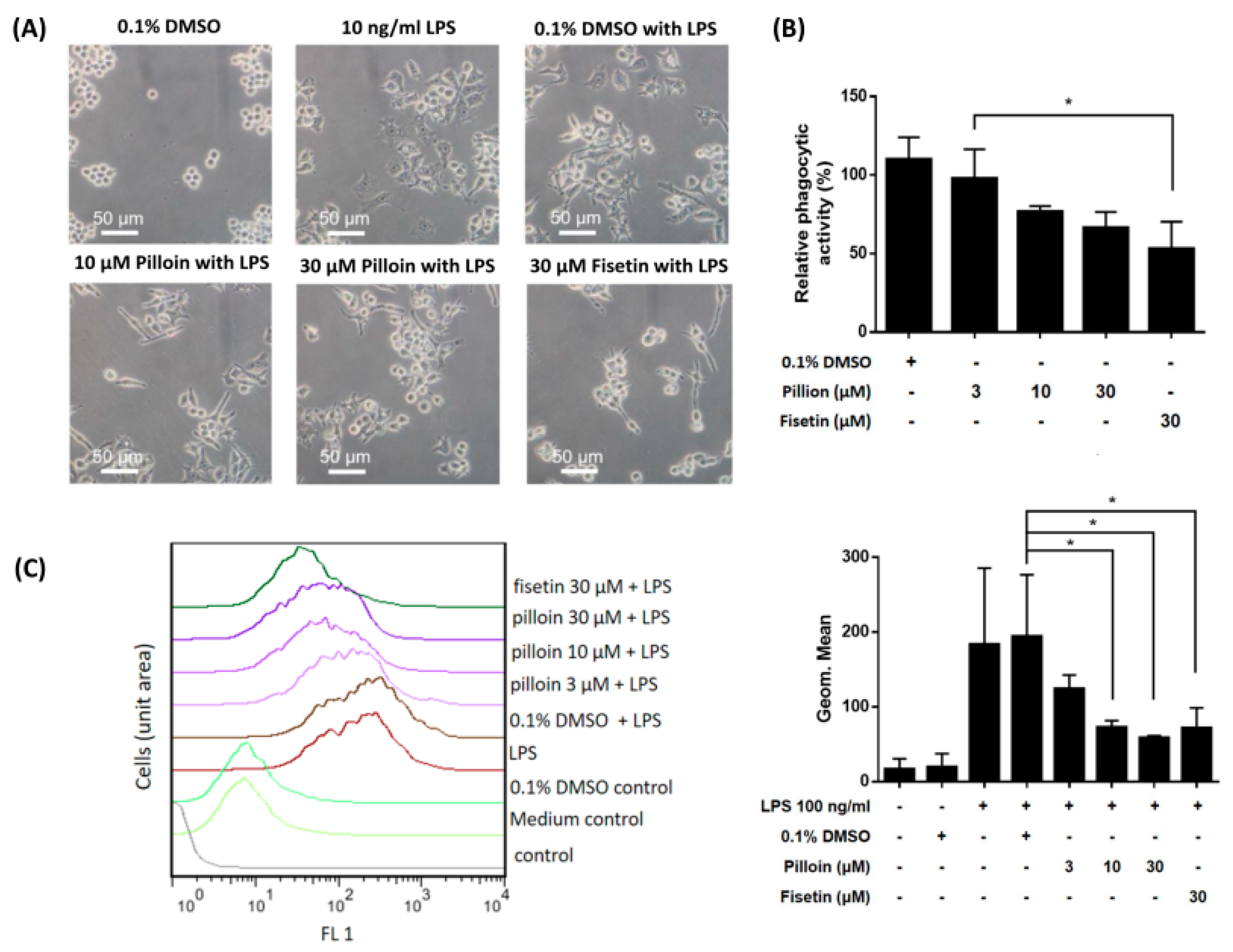
2.4. Pilloin Suppresses LPS-Induced Morphological Alterations, Phagocytic Activity and ROS Elevation in RAW 264.7 Macrophages
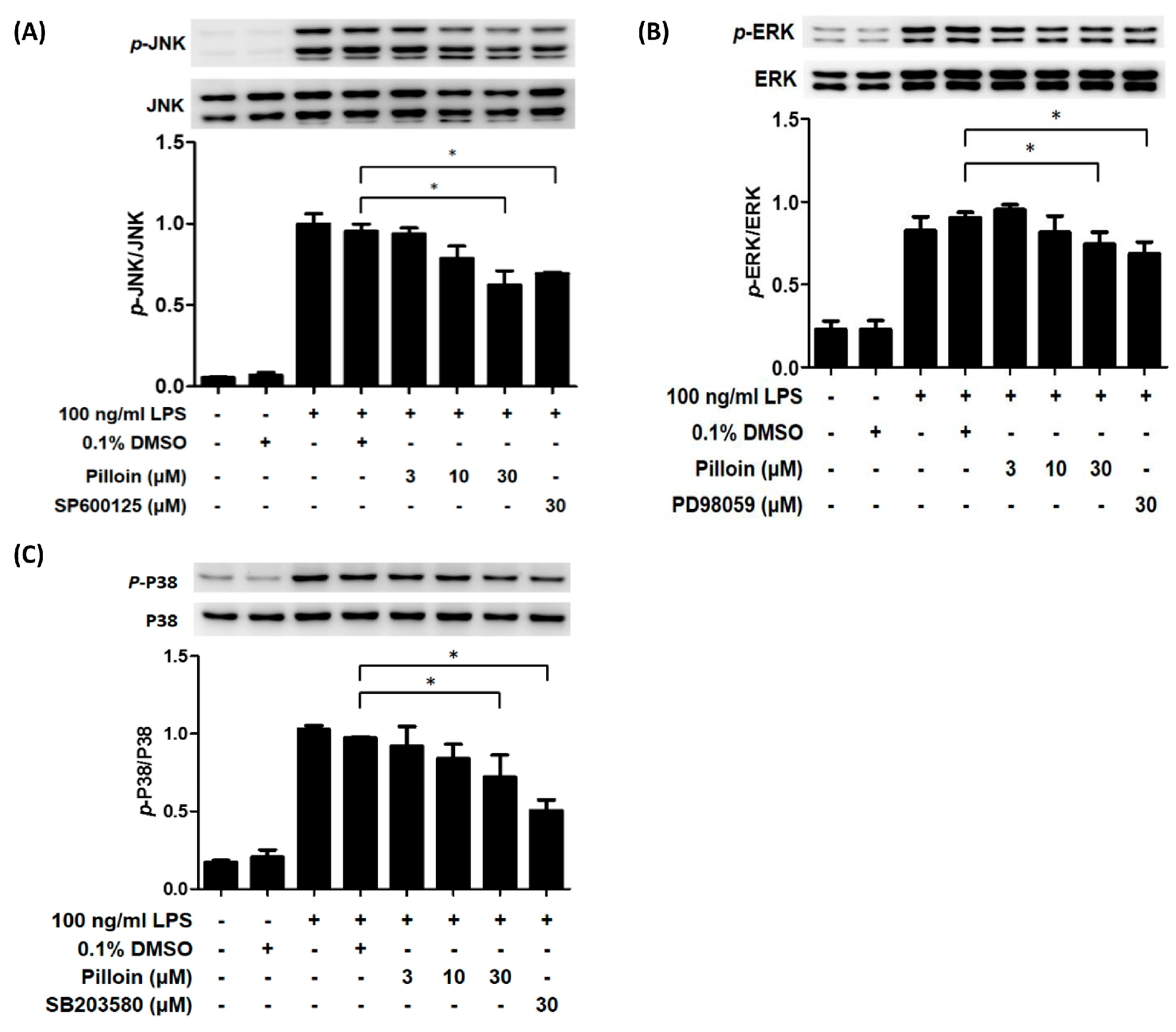
2.5. Pilloin Inhibits Mitogen-Activated Protein Kinase (MAPK)-Mediated Signalling Pathways
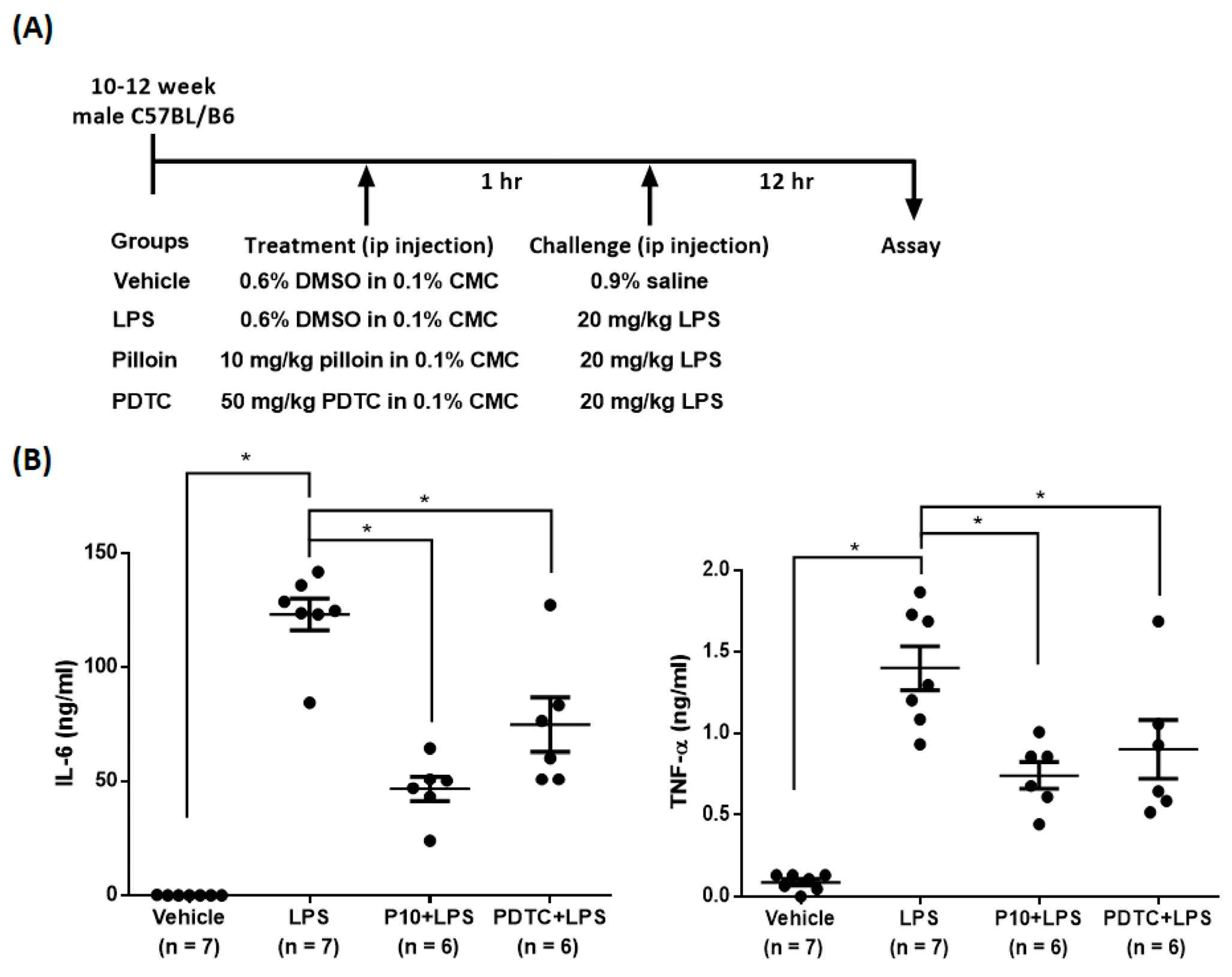
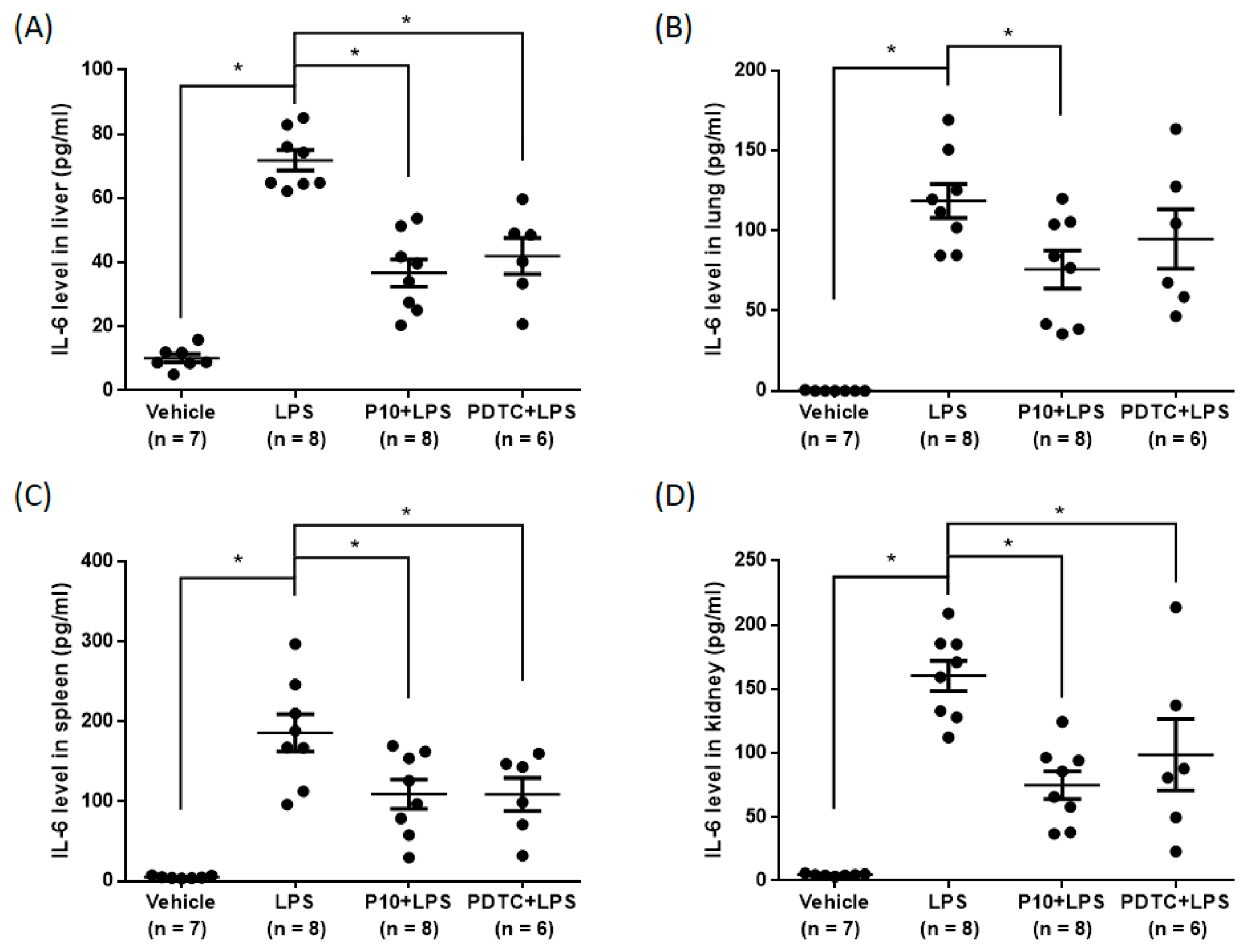
2.6. Pilloin Attenuates LPS-Induced Cytokine Expression In Vivo
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. General Experimental Instruments
4.3. Extraction and Isolation of Pilloin from A. sinensis
4.4. Physical and Spectroscopic Data of Pilloin
4.5. Cell Culture
4.6. Luciferase Reporter Assay
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Determination of NO Production
4.9. MTT Assay
4.10. Western Blot
4.11. ROS Detection
4.12. Phagocytosis Assay
4.13. LPS-Induced Inflammatory Animal Model
4.14. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-Q.; Dai, Y.; Yang, Y.; Huang, C.; Meng, X.-M.; Wu, B.-M.; Li, J. Emerging role of microRNAs in regulating macrophage activation and polarization in immune response and inflammation. Immunology 2016, 148, 237–248. [Google Scholar] [PubMed]
- Ye, H.; Wang, Y.; Jenson, A.B.; Yan, J. Identification of inflammatory factor TNF-α inhibitor from medicinal herbs. Exp. Mol. Pathol. 2016, 100, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, Q.; Yang, T.; Ding, W.; Zhao, Y. Cellular metabolism and macrophage functional polarization. Int. Rev. Immunol. 2015, 34, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 2012, 28, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.P.; Warren, H.S. Strategies to improve drug development for sepsis. Nat. Rev. Drug Discov. 2014, 13, 741–758. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- Schulert, G.S.; Grom, A.A. Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu. Rev. Med. 2015, 66, 145–159. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Caamaño, J.; Hunter, C.A. NF-κB family of transcription factors: Central regulators of innate and adaptive immune functions. Clin. Microbiol. Rev. 2002, 15, 414–429. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Lin, C.-H.; Hung, S.-K.; Chou, J.-H.; Chi, C.-W.; Fu, S.-L. Fisetin inhibits lipopolysaccharide-induced macrophage activation and dendritic cell maturation. J. Agric. Food Chem. 2010, 58, 10831–18039. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Hung, S.K.; Fu, S.L. Immunosuppressive effects of fisetin in ovalbumin-induced asthma through inhibition of NF-κB activity. J. Agric. Food Chem. 2011, 59, 10496–10504. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.F.; Ye, B.Q.; Li, Y.D.; Wang, J.G.; He, X.J.; Lin, X.; Yao, X.; Ma, D.; Slungaard, A.; Hebbel, R.P.; et al. Andrographolide attenuates inflammation by inhibition of NF-κB activation through covalent modification of reduced cysteine 62 of p50. J. Immunol. 2004, 173, 4207–4217. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Chang, H.H.; Chung, Y.H.; Lee, T.Y. Andrographolide acts as an anti-inflammatory agent in LPS-stimulated RAW264.7 macrophages by inhibiting STAT3-mediated suppression of the NF-κB pathway. J. Ethnopharmacol. 2011, 135, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Hoensch, H.P.; Oertel, R. The value of flavonoids for the human nutrition: Short review and perspectives. Clin. Nutri. Exp. 2015, 3, 8–14. [Google Scholar] [CrossRef]
- Cheng, J.T.; Wu, X.D.; Li, Y.; Han, Y.Q.; Dong, L.B.; Zhao, Q.S. Two new tirucallane triterpenoids from the leaves of Aquilaria sinensis. Arch. Pharm. Res. 2013, 36, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, S.A.; Roussrs, V.; Wiemer, D.F. New prenylated phenolics from Piper auruum. Phytochemistry 1987, 26, 2367–2370. [Google Scholar] [CrossRef]
- Shan, J.; Wang, X.; Ma, Y.; Yang, R.; Li, X.; Jin, Y. Flavonoids from leaves of Murraya panaculata L. (I.). Zhongguo Yaoxue Zazhi 2010, 45, 1910–1912. [Google Scholar]
- Liang, S.; Tian, J.-M.; Feng, Y.; Liu, X.-H.; Xiong, Z.; Zhang, W.-D. Flavonoids from Daphne aurantiaca and their inhibitory activities against Nitric Oxide production. Chem. Pharm. Bull. 2011, 59, 653–656. [Google Scholar] [CrossRef]
- Herz, W.; Sosa, V.E. Sesquiterpene lactones and other constituents of Arnica acaulis. Phytochemistry 1988, 27, 155–159. [Google Scholar] [CrossRef]
- Nunez-Alarcon, J. Pilloin, a new flavone from Ovidia Pillo-Pillo. J. Org. Chem. 1971, 36, 3829–3838. [Google Scholar] [CrossRef]
- Fu, S.L.; Hsu, Y.H.; Lee, P.Y.; Hou, W.C.; Hung, L.C.; Lin, C.H.; Chen, C.M.; Huang, Y.J. Dioscorin isolated from Dioscorea alata activates TLR4-signaling pathways and induces cytokine expression in macrophages. Biochem. Biophys. Res. Commun. 2006, 339, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Linehan, E.; Dombrowski, Y.; Snoddy, R.; Fallon, P.G.; Kissenpfennig, A.; Fitzgerald, C.D. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell 2014, 13, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.C.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; Mcguire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013, 27, 669–684. [Google Scholar] [PubMed]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Ando, S.; Toguchida, I.; Yoshikawa, M. Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg. Med. Chem. 2003, 11, 1995–2000. [Google Scholar] [CrossRef]
- Grael, C.F.F.; Kanashiro, A.; Kabeya, L.M.; Jordão, C.O.; Takeara, R.; Gobbo-Neto, L.; Polizello, A.C.M.; Lucisano-Valim, Y.M.; Lopes, N.P.L.; Lopes, J.L.C. In vitro study of antioxidant and scavenger properties of phenolic cpmpounds from Lychnophora species. Quim Nova 2010, 33, 867–870. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Matsuda, N.; Hattori, Y. Systemic inflammatory response syndrome (SIRS): Molecular pathophysiology and gene therapy. J. Pharmacol. Sci. 2006, 101, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Adcock, I.M. NF-κB: A pivotal role in asthma and a new target for therapy. Trends Pharmacol. Sci. 1997, 18, 46–50. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Ahmed, K.M.; Dong, S.; Fan, M.; Li, J.J. Nuclear factor-kappaB p65 inhibits mitogen-activated protein kinase signaling pathway in radioresistant breast cancer cells. Mol. Cancer Res. 2006, 4, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Y.H.; Lin, W.W. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology 1999, 97, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Boomer, J.S.; Green, J.M.; Hotchkiss, R.S. The changing immune system in sepsis: Is individualized immuno-modulatory therapy the answer? Virulence 2014, 5, 45–56. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Toledo-Arruda, A.C.; Mernak, M.; Barrosa, K.H.; Martins, M.A.; Tibério, I.F.L.C.; Prado, C.M. Structure-activity association of flavonoids in lung diseases. Molecules 2014, 19, 3570–3595. [Google Scholar] [CrossRef]
- Walle, T. Methylation of dietary flavones increases their metabolic stability and chemopreventive effects. Int. J. Mol. Sci. 2009, 10, 5002–5019. [Google Scholar] [CrossRef]
- Michelis, F.; Tiligada, E.; Skaltsa, H.; Lazari, D.; Skaltsounis, A.-L.; Delitheos, A. Effects of the flavonoid pilloin Isolated from Marrubium cylleneum on mitogen-induced lymphocyte transformation. Pharm. Biol. 2002, 40, 245–248. [Google Scholar] [CrossRef]
- Wang, S.L.; Hwang, T.L.; Chung, M.I.; Sung, P.J.; Shu, C.W.; Cheng, M.J.; Chen, J.J. New Flavones, a 2-(2-Phenylethyl)-4H-chromen-4-one derivative, and anti-inflammatory constituents from the stem barks of Aquilaria sinensis. Molecules 2015, 20, 20912–20925. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of Nitric Oxide production in biological systems by using Griess reaction assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
- Liu, S.H.; Lin, C.H.; Liang, F.P.; Chen, P.F.; Kuo, C.D.; Alam, M.M.; Mait, B.; Hung, S.K.; Chi, C.W.; Sun, C.M.; et al. Andrographolide downregulates the v-Src and Bcr-Abl oncoproteins and induces Hsp90 cleavage in the ROS-dependent suppression of cancer malignancy. Biochem. Pharmacol. 2014, 87, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.P. Animal models of sepsis. Virulence 2014, 5, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.M.; Shin, D.M.; Lee, H.M.; Kim, J.J.; Kim, S.W.; Jin, H.S.; Yang, C.S.; Park, K.A.; Chanda, D.; Kim, D.K.; et al. The orphan nuclear receptor SHP acts as a negative regulator in inflammatory signaling triggered by Toll-like receptors. Nat. Immunol. 2011, 12, 742–752. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-C.; Wang, S.-L.; Wu, M.-Y.; Liao, C.-H.; Lin, C.-H.; Chen, J.-J.; Fu, S.-L. Pilloin, A Flavonoid Isolated from Aquilaria sinensis, Exhibits Anti-Inflammatory Activity In Vitro and In Vivo. Molecules 2018, 23, 3177. https://doi.org/10.3390/molecules23123177
Tsai Y-C, Wang S-L, Wu M-Y, Liao C-H, Lin C-H, Chen J-J, Fu S-L. Pilloin, A Flavonoid Isolated from Aquilaria sinensis, Exhibits Anti-Inflammatory Activity In Vitro and In Vivo. Molecules. 2018; 23(12):3177. https://doi.org/10.3390/molecules23123177
Chicago/Turabian StyleTsai, Yun-Chen, Sin-Ling Wang, Mei-Yao Wu, Chia-Huei Liao, Chao-Hsiung Lin, Jih-Jung Chen, and Shu-Ling Fu. 2018. "Pilloin, A Flavonoid Isolated from Aquilaria sinensis, Exhibits Anti-Inflammatory Activity In Vitro and In Vivo" Molecules 23, no. 12: 3177. https://doi.org/10.3390/molecules23123177
APA StyleTsai, Y.-C., Wang, S.-L., Wu, M.-Y., Liao, C.-H., Lin, C.-H., Chen, J.-J., & Fu, S.-L. (2018). Pilloin, A Flavonoid Isolated from Aquilaria sinensis, Exhibits Anti-Inflammatory Activity In Vitro and In Vivo. Molecules, 23(12), 3177. https://doi.org/10.3390/molecules23123177






