Targeted Lignan Profiling and Anti-Inflammatory Properties of Schisandra rubriflora and Schisandra chinensis Extracts
Abstract
:1. Introduction
2. Results
2.1. Schisandra Rubriflora Lignan Profile
2.2. Schisandra Chinensis Lignan Profile
2.3. Anti-Inflammatory Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Plant Sample Extraction
4.3. UHPLC–MS/MS Lignan Targeted Profiling
4.4. Anti-Inflammatory Activity
4.4.1. Inhibitory Activity against 15-Lipooxygenase (15-LOX)
4.4.2. Inhibitory Activity against Cyclooxygenase-1 and Cyclooxygenase-2 (COX-1 and COX-2)
4.4.3. Inhibitory Activity against Phospholipases A2 (sPLA2)
4.4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Saunders, R.M.K. Monograph of Schisandra (Schisandraceae). In Systematic Botany Monographs; American Society of Plant Taxonomists: Laramie, WY, USA, 2000; Volume 58, pp. 1–146. ISBN 978-0912861586. [Google Scholar]
- Szopa, A.; Barnaś, M.; Ekiert, H. Phytochemical studies and biological activity of three Chinese Schisandra species (Schisandra sphenanthera, Schisandra henryi and Schisandra rubriflora): Current findings and future applications. Phytochem. Rev. 2018, 1–20. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines. Schisandrae chinensis fructus. In European Pharmacopoeia 9.0.; Council of Europe: Strasbourg Cedex, France, 2017. [Google Scholar]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Klimek, M.; Ekiert, H. Chinese magnolia vine (Schisandra chinensis)—therapeutic and cosmetic importance. Polish J. Cosmetol. 2016, 19, 274–284. [Google Scholar]
- European Directorate for the Quality of Medicines. Schisandrae chinensis fructus. In European Pharmacopoeia 6.0.; Council of Europe: Strasbourg Cedex, France, 2008. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Chemical Industry Press: Beijing, China, 2005. [Google Scholar]
- Committee of the Japanese Pharmacopoeia. Evaluation and Licensing Division Pharmaceuticals and Food Safety. In Japanese Pharmacopoeia; Bureau Ministry of Health, Labour and Welfare: Tokyo, Japan, 2006. [Google Scholar]
- Central Pharmaceutical Affairs Council of Korea. Korean Pharmacopoeia; Central Pharmaceutical Affairs Council of Korea: Seoul, Korea, 2002.
- Xu, L.; Grandi, N.; Del Vecchio, C.; Mandas, D.; Corona, A.; Piano, D.; Esposito, F.; Parolin, C.; Tramontano, E. From the traditional Chinese medicine plant Schisandra chinensis new scaffolds effective on HIV-1 reverse transcriptase resistant to non-nucleoside inhibitors. J. Microbiol. 2015, 53, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Upton, R.; Graff, A.; Jolliffe, G.; Länger, R.; Williamson, E. American Herbal Pharmacopoeia: Botanical Pharmacognosy—Microscopic Characterization of Botanical Medicines; CRC Press: Boca Raton, FL, USA, 2011; ISBN 1420073281. [Google Scholar]
- World Health Organization. Fructus Schisandrae. WHO Monographs on Selected Medicinal Plants; WHO: Geneva, Switzerland, 2007; Volume 3. [Google Scholar]
- Panossian, A.; Wikman, G. Pharmacology of Schisandra chinensis Bail.: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef]
- Hancke, J.L.; Burgos, R.A.; Ahumada, F. Schisandra chinensis (Turcz.) Baill. Fitoterapia 1999, 70, 451–471. [Google Scholar] [CrossRef]
- Li, G.; Zhao, J.; Tu, Y.; Yang, X.; Zhang, H.; Li, L. Chemical constituents of Schisandra rubriflora Rehd. et Wils. J. Integr. Plant Biol. 2005, 47, 362–367. [Google Scholar] [CrossRef]
- Mu, H.X.; Li, X.S.; Fan, P.; Yang, G.Y.; Pu, J.X.; Sun, H.D.; Hu, Q.F.; Xiao, W.L. Dibenzocyclooctadiene lignans from the fruits of Schisandra rubriflora and their anti-HIV-1 activities. J. Asian Nat. Prod. Res. 2011, 13, 393–399. [Google Scholar] [CrossRef]
- Xiao, W.L.; Wang, R.R.; Zhao, W.; Tian, R.R.; Shang, S.Z.; Yang, L.M.; Yang, J.H.; Pu, J.X.; Zheng, Y.T.; Sun, H.D. Anti-HIV-1 activity of lignans from the fruits of Schisandra rubriflora. Arch Pharm. Res. 2010, 33, 697–701. [Google Scholar] [CrossRef]
- Chen, M.; Kilgore, N.; Lee, K.H.; Chen, D.F. Rubrisandrins A and B, lignans and related anti-HIV compounds from Schisandra rubriflora. J. Nat. Prod. 2006, 69, 1697–1701. [Google Scholar] [CrossRef]
- Xiao, W.L.; Li, X.; Wang, R.R.; Yang, L.M.; Li, M.; Huang, S.X.; Pu, J.X.; Zheng, Y.T.; Li, R.T.; Sun, H.D. Triterpenoids from Schisandra rubriflora. J. Nat. Prod. 2007, 70, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, G.T. Anti-oxidant activity of dibenzocyclooctene lignans isolated from Schisandraceae. Planta Med. 1992, 58, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Opletal, L.; Sovová, H.; Bártlová, M. Dibenzo [a,c] cyclooctadiene lignans of the genus Schisandra: Importance, isolation and determination. J. Chromatogr. B 2004, 812, 357–371. [Google Scholar] [CrossRef]
- Chang, J.; Reiner, J.; Xie, J. Progress on the chemistry of dibenzocyclooctadiene lignans. Chem. Rev. 2005, 105, 4581–4609. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Han, N.; Yao, X.; Liu, Z.; Wang, Y.; Yang, J.; Yin, J. Structure-activity relationship study of dibenzocyclooctadiene lignans isolated from Schisandra chinensis on lipopolysaccharide-induced microglia activation. Planta Med. 2014, 80, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, X.; Xu, B.; Yang, P.; Liao, Z.; Morris-Natschke, S.L.; Lee, K.H.; Chen, D. Neglschisandrins E-F: Two new lignans and related cytotoxic lignans from Schisandra neglecta. Molecules 2013, 18, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Luo, Y.M.; Pu, J.X.; Li, X.N.; Lei, C.; Wang, R.R.; Zheng, Y.T.; Sun, H.D.; Li, R.T. Four new dibenzocyclooctadiene lignans from Schisandra rubriflora. Helv. Chim. Acta 2008, 91, 1053–1062. [Google Scholar] [CrossRef]
- Huyke, C.; Engel, K.; Simon-Haarhaus, B.; Quirin, K.-W.; Schempp, C. Composition and Biological Activity of Different Extracts from Schisandra sphenanthera and Schisandra chinensis. Planta Med. 2007, 73, 1116–1126. [Google Scholar] [CrossRef]
- Lim, H.; Son, K.H.; Bae, K.H.; Hung, T.M.; Kim, Y.S.; Kim, H.P. 5-Lipoxygenase-inhibitory constituents from Schisandra fructus and Magnolia flos. Phyther. Res. 2009, 23, 1489–1492. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Kim, Y.H.; Bae, D.S.; Um, B.H.; Pan, C.-H.; Kim, C.Y.; Lee, H.J.; Lee, J.K. Anti-Inflammatory Effects of Gomisin N, Gomisin J, and Schisandrin C Isolated from the Fruit of Schisandra chinensis. Biosci. Biotechnol. Biochem. 2010, 74, 285–291. [Google Scholar] [CrossRef]
- Ci, X.; Ren, R.; Xu, K.; Li, H.; Yu, Q.; Song, Y.; Wang, D.; Li, R.; Deng, X. Schisantherin A exhibits anti-inflammatory properties by down-regulating NF-κB and MAPK signaling pathways in lipopolysaccharide-treated RAW 264.7 cells. Inflammation 2010, 33, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Zhao, X.; Li, Z.; Wang, L.; Wang, Y. Study on main pharmacodynamic effects for Schisandra lignans based upon network pharmacology. Chin. J. Chin. Mater. Med. 2015, 40, 522–527. [Google Scholar]
- Guo, L.Y.; Hung, T.M.; Bae, K.H.; Shin, E.M.; Zhou, H.Y.; Hong, Y.N.; Kang, S.S.; Kim, H.P.; Kim, Y.S. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur. J. Pharmacol. 2008, 591, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.A. Lignans and neolignans. Nat. Prod. Rep. 1985, 193, 191–211. [Google Scholar] [CrossRef]
- Whiting, D.A. Lignans, neolignans, and related compounds. Nat. Prod. Rep. 1990, 7, 349–364. [Google Scholar] [CrossRef]
- Gottlieb, O.R. Chemosystematics of the Lauraceae. Phytochemistry 1972, 11, 1537–1570. [Google Scholar] [CrossRef]
- Li, L.; Ren, H.Y.; Yang, X.D.; Zhao, J.F.; Li, G.P.; Zhang, H. Bin Rubriflorin A and B, two novel partially saturated dibenzocyclooctene lignans from Schisandra rubriflora. Helv. Chim. Acta 2004, 87, 2943–2947. [Google Scholar] [CrossRef]
- Wales, M.E.; Madison, L.L.; Glaser, S.S.; Wild, J.R. Divergent allosteric patterns verify the regulatory paradigm for aspartate transcarbamylase. J. Mol. Biol. 1999, 294, 1387–1400. [Google Scholar] [CrossRef]
- Clematis–Źródło Dobrych Pnączy Spółka z o.o. spółka jawna. Available online: http://www.clematis.com.pl/pl/ (accessed on 3 October 2018).
Sample Availability: Samples of the compounds (all tested lignans) are available from the authors. |
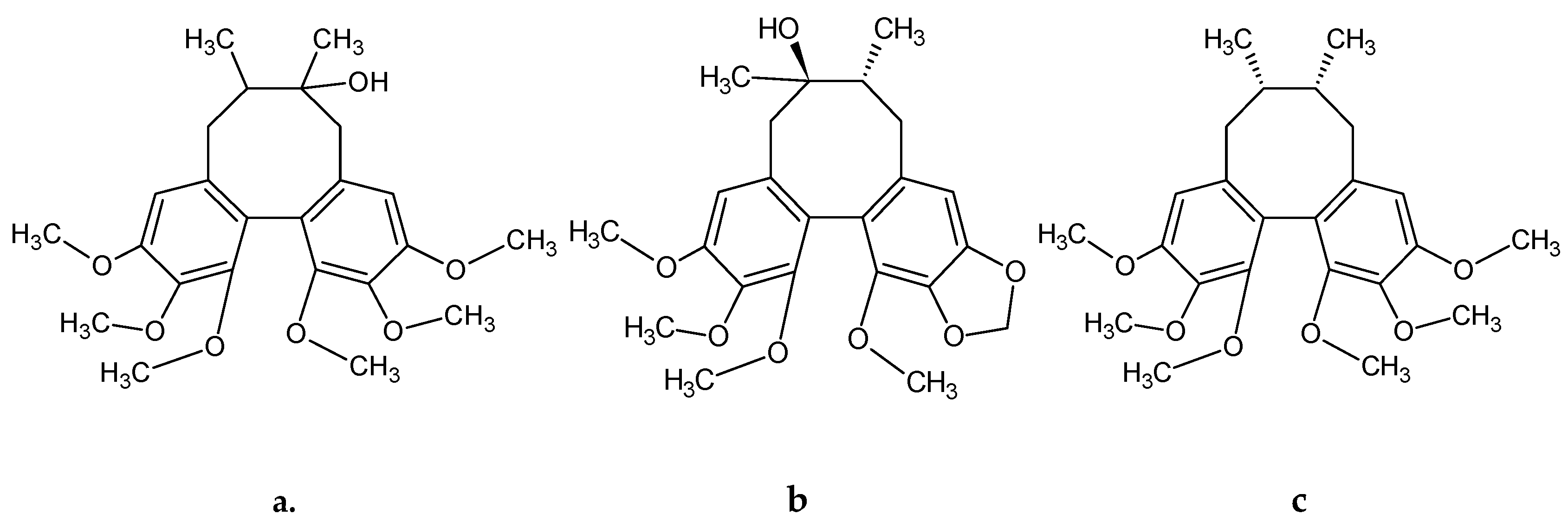
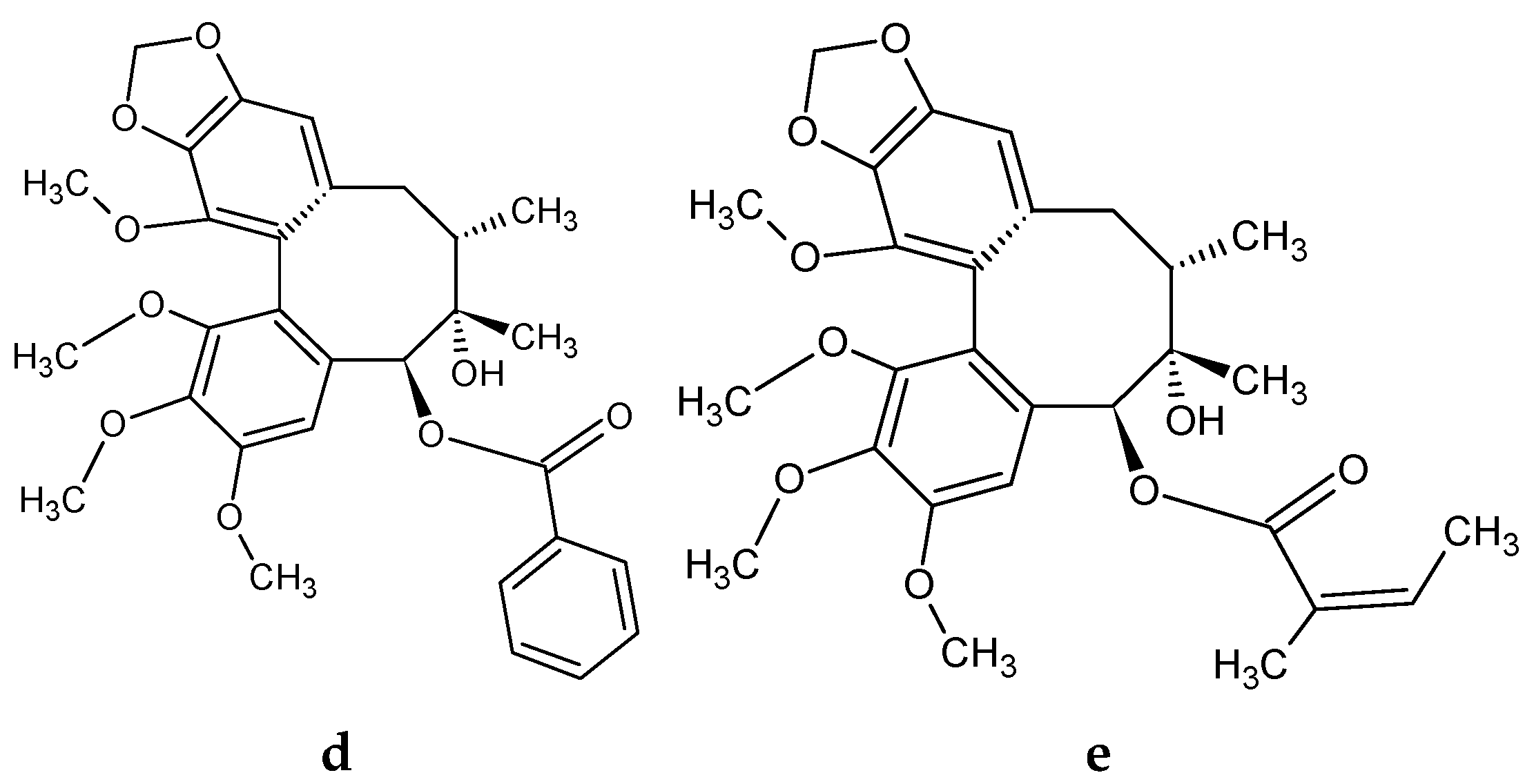
| Lignans | Fruits | Leaves | Shoots | ||
|---|---|---|---|---|---|
| F | M | F | M | ||
| Wulignan A1 | 19.39 ± 1.35 | 0.04 ± 0.001 | 0.04 ± 0.001 | 0.03 ± 0.002 | 0.07 ± 0.002 |
| Rubrisandrin A | 0.07 ± 0.001 | 0.06 ± 0.001 | 0.06 ± 0.001 | 0.06 ± 0.002 | 0.10 ± 0.001 |
| Rubriflorin A | traces | traces | traces | traces | traces |
| Schisandrin | 6.57 ± 0.35 | 8.15 ± 0.25 | 5.69 ± 0.13 | 4.01 ± 0.005 | 2.25 ± 0.01 |
| Gomisin D | 3.52 ± 0.16 | 16.45 ± 0.76 | 116.51 ± 24.28 | 8.25 ± 0.42 | 20.26 ± 0.44 |
| Gomisin J | 5.40 ± 0.27 | 0.97 ± 0.01 | 0.76 ± 0.03 | 0.46 ± 0.01 | 0.36 ± 0.004 |
| Gomisin A | 0.75 ± 0.01 | 6.40 ± 0.18 | 4.20 ± 0.07 | 2.99 ± 0.02 | 1.65 ± 0.01 |
| Gomisin G | 66.39 ± 12.37 | 11.13 ± 1.01 | 8.23 ± 0.12 | 5.25 ± 0.04 | 3.67 ± 0.02 |
| Licarin B | 1.98 ± 0.08 | 0.41 ± 0.06 | 0.24 ± 0.02 | 0.19 ± 0.04 | 0.12 ± 0.01 |
| Epigomisin O | 7.46 ± 0.32 | 10.62 ± 0.41 | 7.83 ± 0.12 | 4.91 ± 0.06 | 3.15 ± 0.01 |
| Gomisin O | 103.64 ± 26.25 | 22.90 ± 1.29 | 2.81 ± 0.01 | 12.07 ± 0.20 | 1.82 ± 0.01 |
| Mesodihydroguaiaretic acid | 1.03 ± 0.02 | 0.34 ± 0.01 | 0.32 ± 0.004 | 0.17 ± 0.001 | 0.16 ± 0.004 |
| Schisantherin A | 27.19 ± 3.00 | 226.80 ± 16.70 | 107.17 ± 1.66 | 84.35 ± 4.64 | 24.27 ± 1.51 |
| Schisantherin B | 118.07 ± 18.42 | 291.47 ± 51.98 | 104.28 ± 18.16 | 239.11 ± 38.00 | 169.04 ± 49.85 |
| Dehydroisoeugenol | 0.41 ± 0.004 | 0.73 ± 0.01 | 0.44 ± 0.01 | 0.33 ± 0.004 | 0.23 ± 0.01 |
| Schisanhenol | 268.02 ± 43.12 | 2.05 ± 0.06 | 2.73 ± 0.01 | 1.13 ± 0.003 | 2.53 ± 0.03 |
| Schisandrin A | 104.32 ± 10.11 | 0.22 ± 0.002 | 0.38 ± 0.004 | 0.12 ± 0.001 | 0.50 ± 0.002 |
| Fragransin A2 | 0.01 ± 0.002 | 0.01 ± 0.002 | Nd * | nd | 0.004 ± 0.002 |
| Pregomisin | 0.003 ± 0.001 | traces | traces | 0.01 ± 0.001 | traces |
| Gomisin N | 19.20 ± 0.66 | 1.58 ± 0.02 | 2.21 ± 0.03 | 0.81 ± 0.01 | 1.08 ± 0.01 |
| 6-O-Benzoylgomisin O | 35.28 ± 3.55 | 134.51 ± 5.91 | 564.62 ± 33.66 | 72.38 ± 4.77 | 52.18 ± 2.63 |
| Schisandrin C | 4.96 ± 0.12 | 0.44 ± 0.03 | 0.19 ± 0.02 | 0.28 ± 0.002 | 0.09 ± 0.01 |
| Angeloylgomisin H | 185.10 ± 27.55 | 100.83 ± 7.89 | 129.28 ± 13.66 | 105.80 ± 9.04 | 74.73 ± 1.54 |
| Angeloylgomisin O | 76.88 ± 4.55 | 17.21 ± 0.26 | 48.80 ± 3.66 | 17.25 ± 0.23 | 26.53 ± 0.46 |
| Total content | 1055.65 ± 152.26 | 853.33 ± 86.85 | 1106.80 ± 78.33 | 559.97 ± 57.50 | 384.80 ± 56.56 |
| Lignans | Fruits | Leaves | Shoots |
|---|---|---|---|
| Wulignan A1 | 0.15 ± 0.03 | 0.03 ± 0.001 | 0.04 ± 0.001 |
| Rubrisandrin A | 0.03 ± 0.002 | 0.04 ± 0.001 | 0.03 ± 0.001 |
| Rubriflorin A | 0.01 ± 0.001 | traces | 0.01 ± 0.001 |
| Schisandrin | 206.08 ± 22.32 | 32.51 ± 3.14 | 32.87 ± 4.14 |
| Gomisin D | 195.22 ± 15.63 | 9.62 ± 1.96 | 11.33 ± 1.12 |
| Gomisin J | 142.35 ± 19.12 | 18.06 ± 3.11 | 13.22 ± 0.54 |
| Gomisin A | 177.94 ± 20.14 | 73.82 ± 8.41 | 36.29 ± 2.41 |
| Gomisin G | 44.56 ± 5.44 | 12.18 ± 2.14 | 11.69 ± 1.44 |
| Licarin B | 0.37 ± 0.02 | 0.03 ± 0.001 | 0.03 ± 0.001 |
| Epigomisin O | 3.16 ± 0.09 | 1.01 ± 0.07 | 0.91 ± 0.80 |
| Gomisin O | 4.08 ± 1.21 | 5.35 ± 0.55 | 4.45 ± 0.12 |
| Mesodihydroguaiaretic acid | 0.46 ± 0.09 | 0.38 ± 0.06 | 0.42 ± 0.07 |
| Schisantherin A | 31.32 ± 3.25 | 3.86 ± 0.98 | 2.22 ± 0.14 |
| Schisantherin B | 185.82 ± 20.39 | 102.47 ± 4.87 | 35.27 ± 3.12 |
| Dehydroisoeugenol | 0.16 ± 0.05 | 0.36 ± 0.09 | 0.41 ± 0.04 |
| Schisanhenol | 9.60 ± 1.88 | 1.00 ± 0.07 | 0.91 ± 0.02 |
| Schisandrin A | 212.50 ± 18.45 | 17.74 ± 1.02 | 13.89 ± 1.21 |
| Fragransin A2 | nd | 0.02 ± 0.001 | 0.01 ± 0.001 |
| Pregomisin | traces | traces | traces |
| Gomisin N | 259.05 ± 30.88 | 55.06 ± 4.52 | 62.69 ± 4.98 |
| 6-O-Benzoylgomisin O | 33.64 ± 2.89 | 10.83 ± 2.01 | 7.48 ± 1.21 |
| Schisandrin C | 18.54 ± 2.15 | 37.20 ± 2.77 | 29.94 ± 4.23 |
| Angeloylgomisin H | 161.90 ± 15.65 | 47.34 ± 3.45 | 44.84 ± 2.27 |
| Angeloylgomisin O | 65.56 ± 5.99 | 4.67 ± 0.87 | 4.89 ± 0.84 |
| Total content | 1686.95 ± 185.67 | 433.59 ± 40.09 | 313.83 ± 28.70 |
| Plant Extracts | Concentration (μg/mL) | 15-LOX | COX-1 | COX-2 | sPLA2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Inh | SD | % Inh | SD | % Inh | SD | % Inh | SD | |||
| Schisandra rubriflora | Fruits | 175.0 | 1 | 0.1 | 32 | 3.5 | 52 | 5.7 | 62 | 2.5 |
| 17.5 | 22 | 1.5 | 71 | 7.8 | 48 | 5.2 | 25 | 1.0 | ||
| Leaves F | 175.0 | 38 | 2.7 | 96 | 10.6 | 90 | 9.9 | 64 | 2.6 | |
| 17.5 | 42 | 2.9 | 51 | 5.6 | 40 | 4.4 | 54 | 2.2 | ||
| Leaves M | 175.0 | 37 | 2.6 | 86 | 9.5 | 82 | 9.0 | 65 | 2.7 | |
| 17.5 | 38 | 2.7 | 58 | 6.4 | 49 | 5.4 | 55 | 2.3 | ||
| Schisandra chinensis | Fruits | 175.0 | no inhibition | 0.0 | 59 | 6.5 | 66 | 7.3 | 25 | 1.0 |
| 17.5 | 25 | 1.7 | 34 | 3.8 | 49 | 5.4 | 8 | 0.3 | ||
| Leaves | 175.0 | 31 | 2.2 | 69 | 7.6 | 77 | 8.4 | 49 | 2.0 | |
| 17.5 | 28 | 2.0 | 51 | 5.6 | 53 | 5.8 | 25 | 1.0 | ||
| Control inhibitor | NDGA | 30.2 (100 μM) | 23 | 2.0 | - | - | - | - | - | - |
| Ibuprofen | 2.1 (10 μM) | - | - | 23 | 2.5 | 21 | 2.0 | - | - | |
| Thioetheramide-PC | 73.6 (100 μM) | - | - | - | - | - | - | 91 | 4.0 | |
| Lignans | Concentration (μg/mL) | 15-LOX | COX-1 | COX-2 | sPLA2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Inh | SD | % Inh | SD | % Inh | SD | % Inh | SD | |||
| 6-O-Benzoylgomisin O | 1.75 | 18 | 1.2 | 35 | 4 | 47 | 5 | no inhibition | - | |
| 0.175 | 49 | 3.4 | 19 | 2 | 47 | 5 | no inhibition | - | ||
| Schisandrin | 1.75 | 31 | 2.0 | 62 | 5.6 | 54 | 4.9 | no inhibition | - | |
| 0.175 | 57 | 4.0 | 40 | 4.4 | 50 | 5.5 | no inhibition | - | ||
| Gomisin D | 1.75 | 31 | 1.8 | 42 | 4.2 | 62 | 6.4 | 3 | 0.1 | |
| 0.175 | 53 | 3.7 | 34 | 3.7 | 58 | 6.4 | 9 | 0.4 | ||
| Gomisin N | 1.75 | 16 | 1.1 | 42 | 4.3 | 58 | 7.0 | 6 | 0.3 | |
| 0.175 | 54 | 3.8 | 40 | 4.4 | 70 | 7.6 | no inhibition | - | ||
| Schisantherin A | 1.75 | 31 | 1.6 | 38 | 5.8 | 48 | 5.3 | 8 | 0.3 | |
| 0.175 | 55 | 3.8 | 74 | 8.1 | 31 | 3.4 | 1 | 0.0 | ||
| Average sample lignan composition (mix) | 1.75 | 20 | 1.4 | 61 | 6.7 | 43 | 4.7 | no inhibition | - | |
| 0.175 | 53 | 3.7 | 32 | 3.6 | 56 | 6.1 | 2 | 0.1 | ||
| Control inhibitor | NDGA | 30.2 (100 μM) | 23 | 2.0 | - | - | - | - | - | - |
| Ibuprofen | 2.1 (10 μM) | - | - | 23 | 2.5 | 21 | 2.0 | - | - | |
| Thioetheramide-PC | 73.6 (100μM) | - | - | - | - | - | - | 91 | 4.0 | |
| Lignans | Schisandra rubriflora | Schisandra chinensis | ||||||
|---|---|---|---|---|---|---|---|---|
| Fruits | Leaves | Shoots | Fruits | Leaves | Shoots | |||
| F | M | F | M | |||||
| Schisandrin | 6.57 | 8.15 | 5.69 | 4.01 | 2.25 | 206.08 | 32.51 | 32.87 |
| Gomisin D | 3.52 | 16.45 | 116.51 | 8.25 | 20.26 | 195.22 | 9.62 | 11.33 |
| Gomisin J | 5.40 | 0.97 | 0.76 | 0.46 | 0.36 | 142.35 | 18.06 | 13.22 |
| Gomisin A | 0.75 | 6.40 | 4.20 | 2.99 | 1.65 | 177.94 | 73.82 | 36.29 |
| Gomisin G | 66.39 | 11.13 | 8.23 | 5.25 | 3.67 | 44.56 | 12.18 | 11.69 |
| Schisantherin A | 27.19 | 226.80 | 107.17 | 84.35 | 24.27 | 31.32 | 3.86 | 2.22 |
| Schisantherin B | 118.07 | 291.47 | 104.28 | 239.11 | 169.04 | 185.82 | 102.47 | 35.27 |
| Schisanhenol | 268.02 | 2.05 | 2.73 | 1.13 | 2.53 | 9.60 | 1.00 | 0.91 |
| Schisandrin A | 104.32 | 0.22 | 0.38 | 0.12 | 0.50 | 212.50 | 17.74 | 13.89 |
| Gomisin N | 19.20 | 1.58 | 2.21 | 0.81 | 1.08 | 259.05 | 55.06 | 62.69 |
| 6-O-Benzoylgomisin O | 35.28 | 134.51 | 564.62 | 72.38 | 52.18 | 33.64 | 10.83 | 7.48 |
| Schisandrin C | 4.96 | 0.44 | 0.19 | 0.28 | 0.09 | 18.54 | 37.20 | 29.94 |
| Angeloylgomisin H | 185.10 | 100.83 | 129.28 | 105.80 | 74.73 | 161.90 | 47.34 | 44.84 |
| Total content | 1055.65 | 853.33 | 1106.80 | 559.97 | 384.80 | 1686.95 | 433.59 | 313.83 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szopa, A.; Dziurka, M.; Warzecha, A.; Kubica, P.; Klimek-Szczykutowicz, M.; Ekiert, H. Targeted Lignan Profiling and Anti-Inflammatory Properties of Schisandra rubriflora and Schisandra chinensis Extracts. Molecules 2018, 23, 3103. https://doi.org/10.3390/molecules23123103
Szopa A, Dziurka M, Warzecha A, Kubica P, Klimek-Szczykutowicz M, Ekiert H. Targeted Lignan Profiling and Anti-Inflammatory Properties of Schisandra rubriflora and Schisandra chinensis Extracts. Molecules. 2018; 23(12):3103. https://doi.org/10.3390/molecules23123103
Chicago/Turabian StyleSzopa, Agnieszka, Michał Dziurka, Angelika Warzecha, Paweł Kubica, Marta Klimek-Szczykutowicz, and Halina Ekiert. 2018. "Targeted Lignan Profiling and Anti-Inflammatory Properties of Schisandra rubriflora and Schisandra chinensis Extracts" Molecules 23, no. 12: 3103. https://doi.org/10.3390/molecules23123103
APA StyleSzopa, A., Dziurka, M., Warzecha, A., Kubica, P., Klimek-Szczykutowicz, M., & Ekiert, H. (2018). Targeted Lignan Profiling and Anti-Inflammatory Properties of Schisandra rubriflora and Schisandra chinensis Extracts. Molecules, 23(12), 3103. https://doi.org/10.3390/molecules23123103








