Time-Resolved Spectroscopic and Density Functional Theory Investigation of the Photogeneration of a Bifunctional Quinone Methide in Neutral and Basic Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
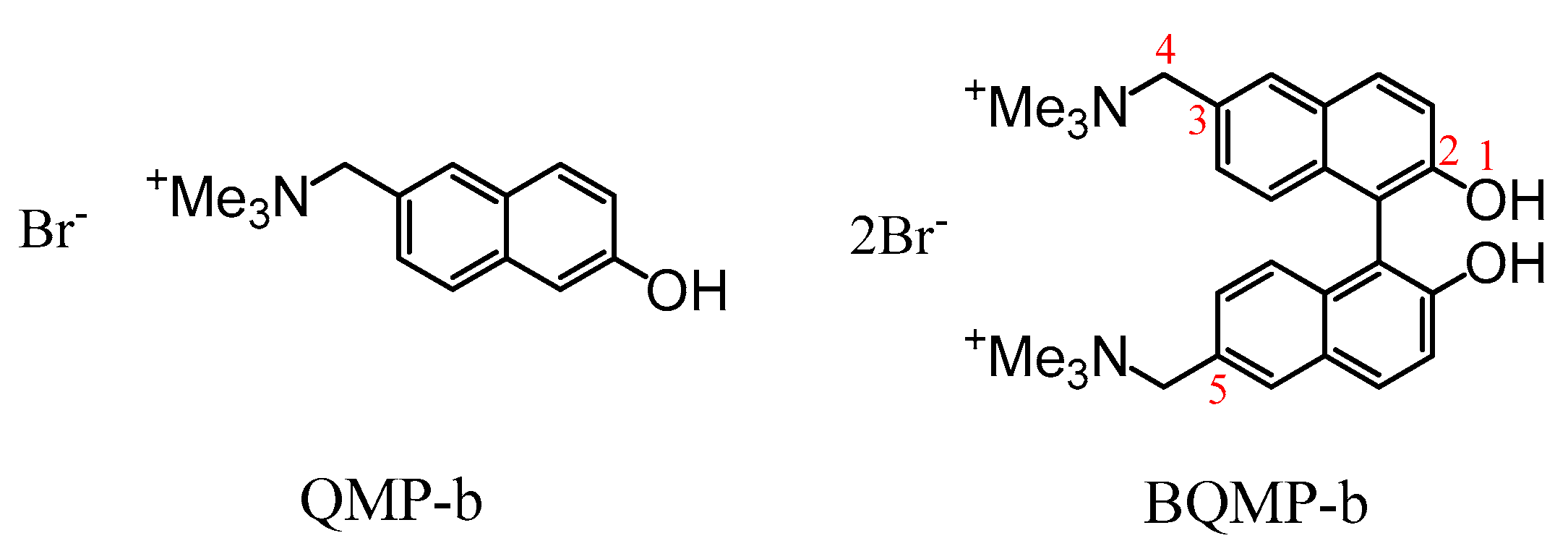
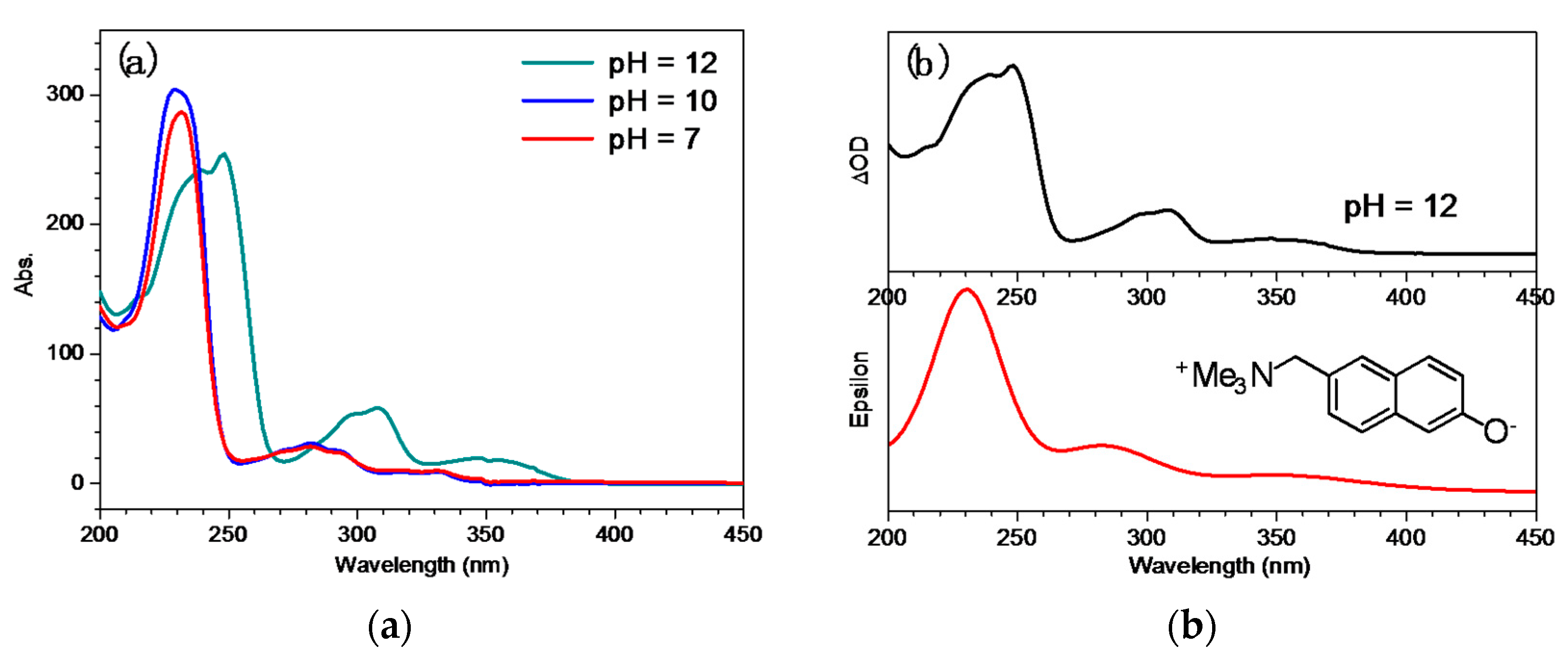
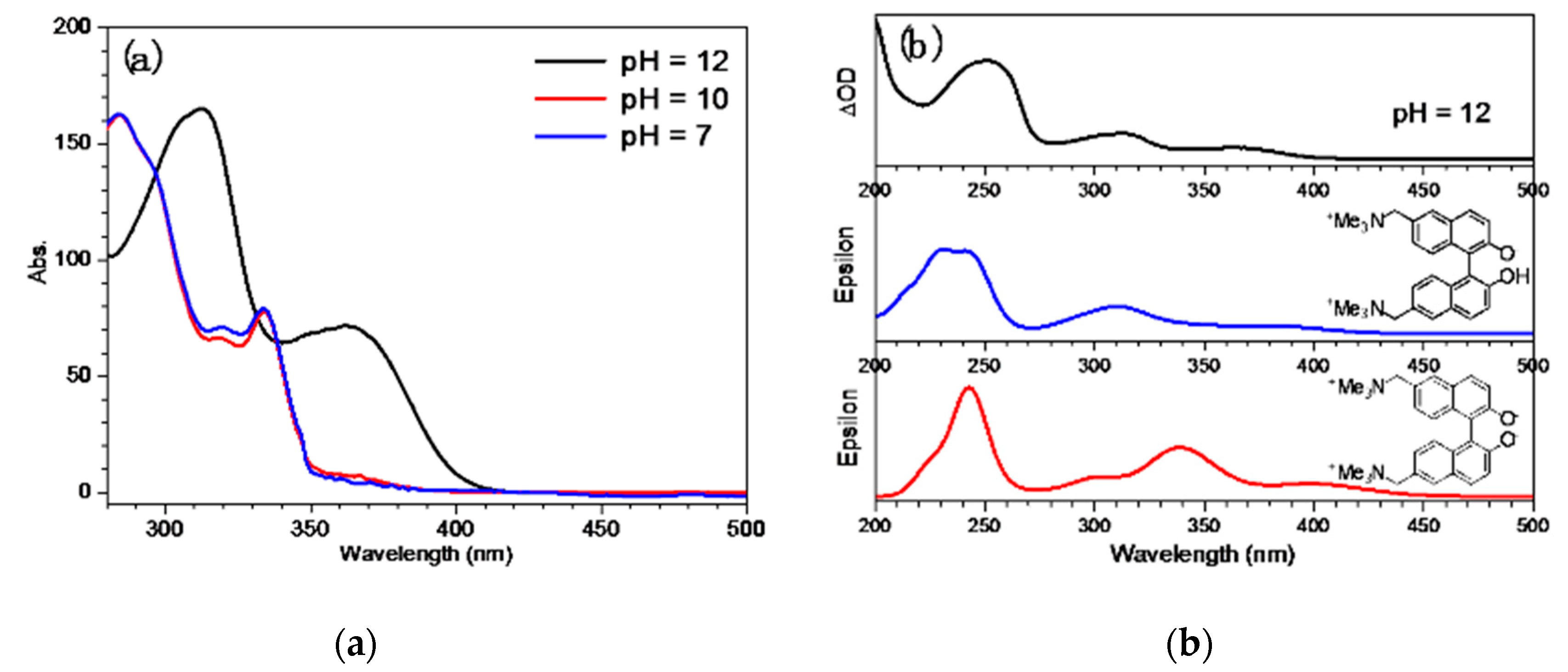
2.1. UV-Vis Spectra Study of QMP-b Containing One Naphthol Ring: The Ground State Form Present at pH = 7, 10, and 12 in Mixed Aqueous Solutions
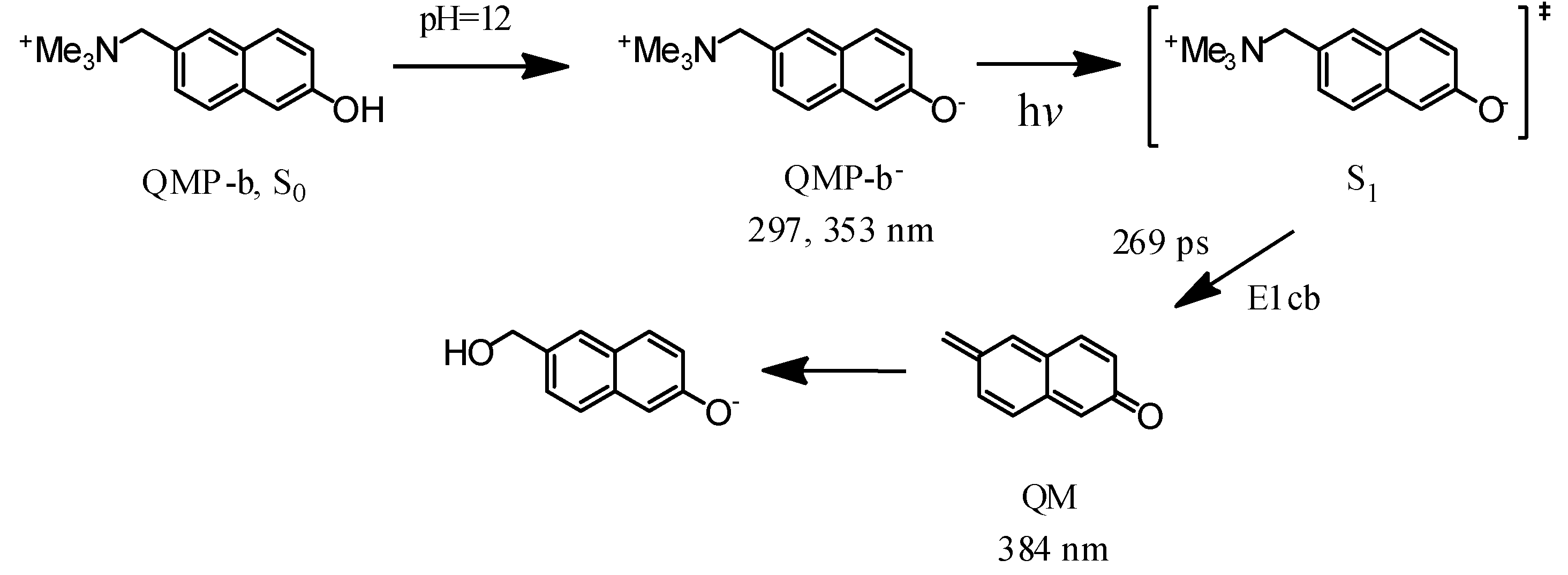
2.2. Fs-TA Study of QMP-b Containing One Naphthol Ring: Investigating the Early Time Processes after Photoexcitation in Varying pH Mixed Aqueous Solutions
2.3. UV-Vis Spectra Study of BQMP-b Containing Two Naphthol Rings: The Ground State Form Present at pH = 7, 10, and 12 in Mixed Aqueous Solutions
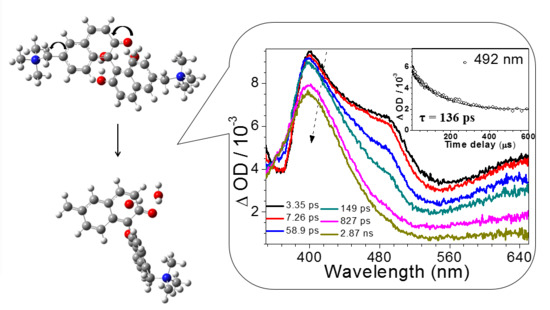
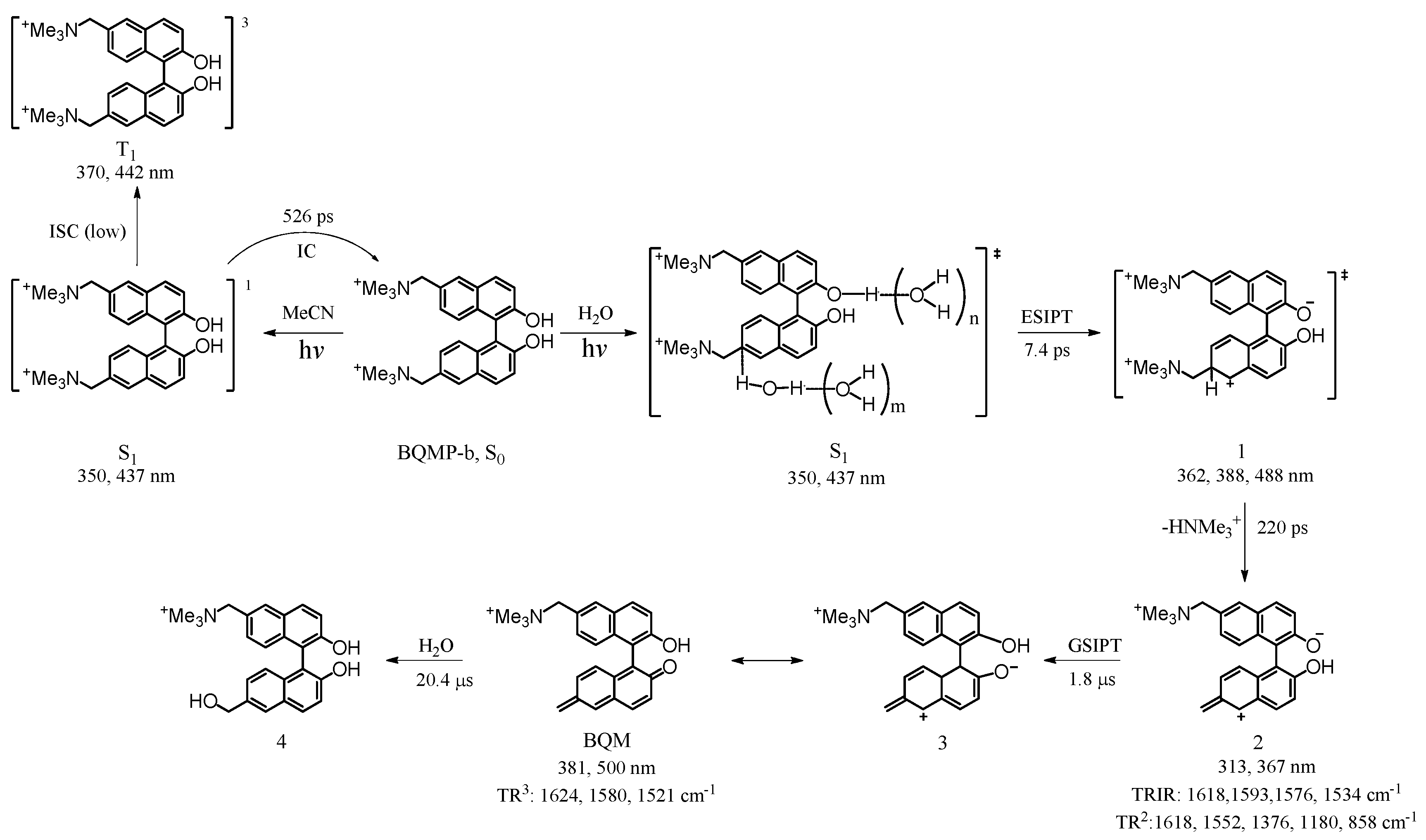
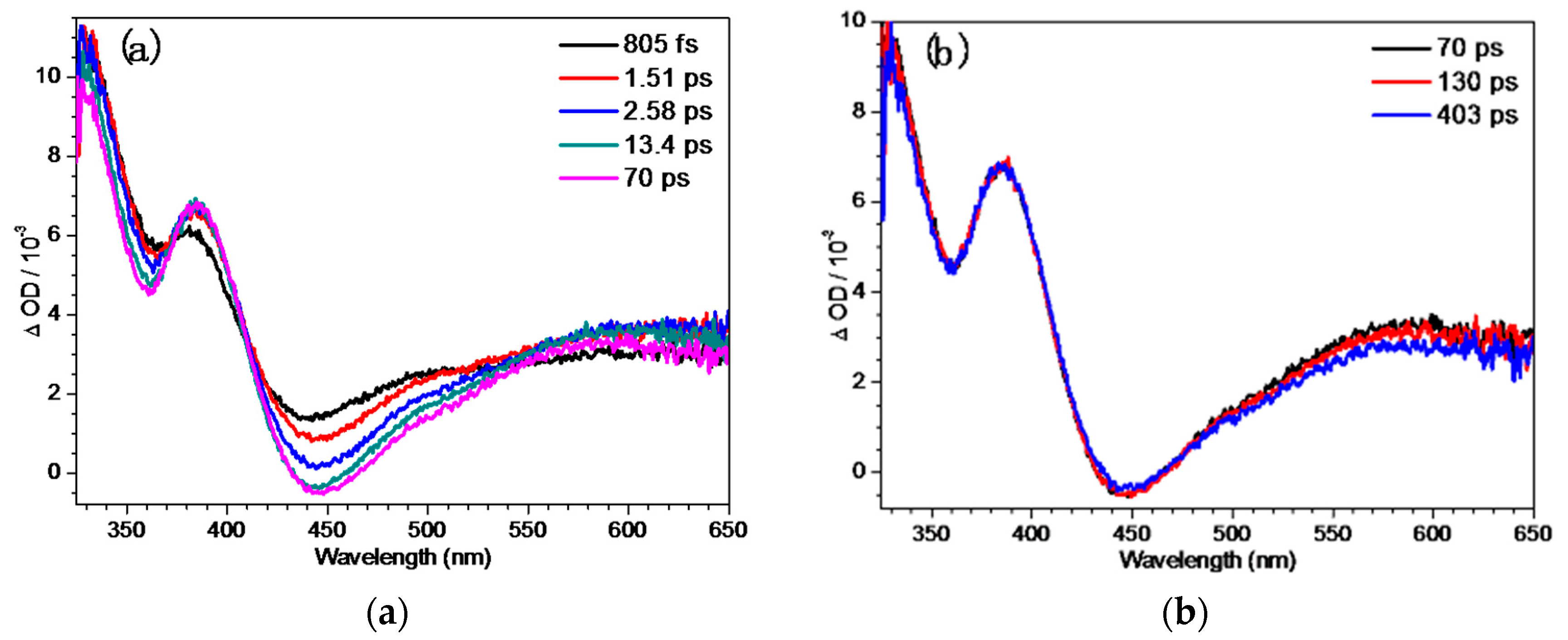
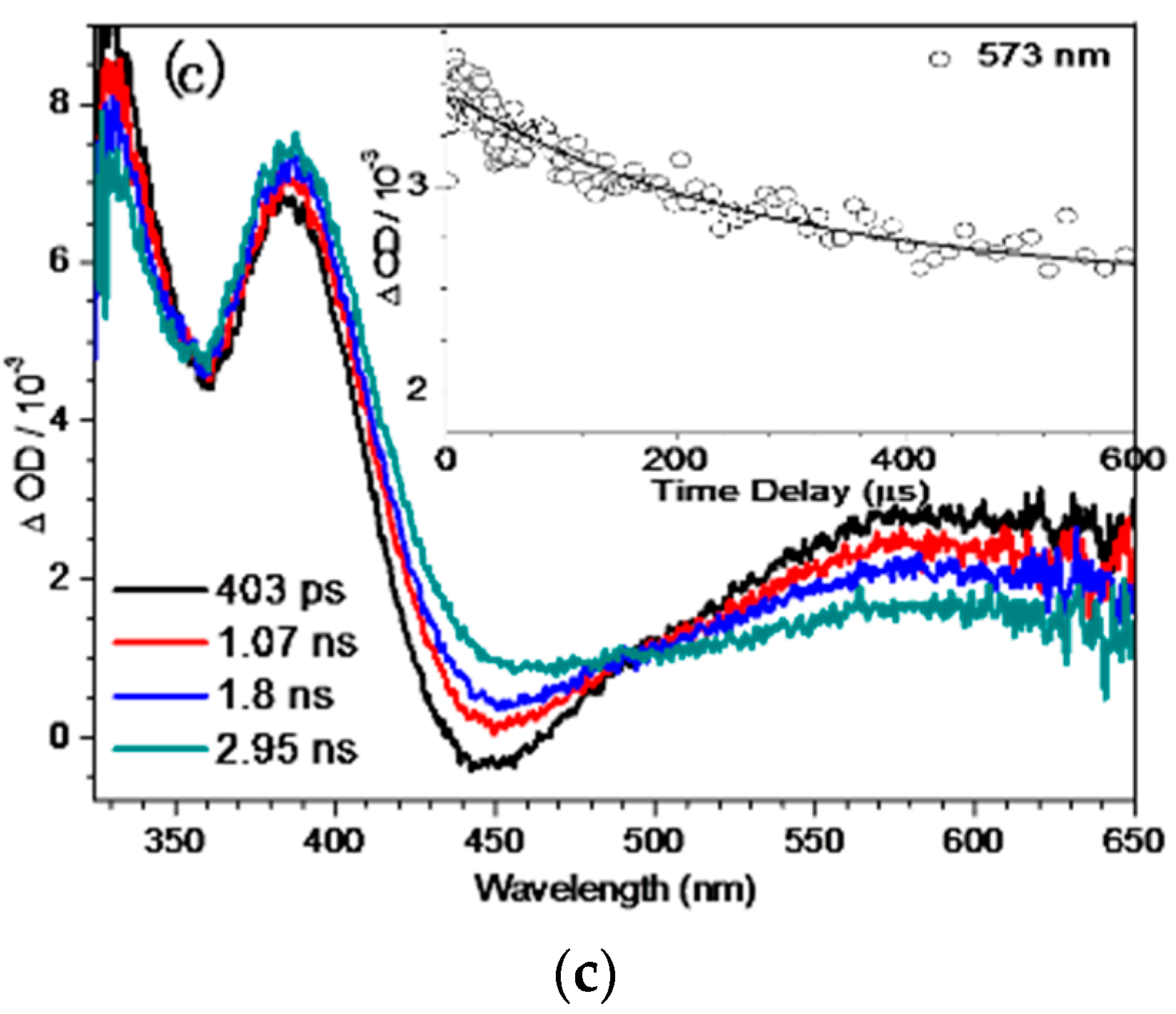
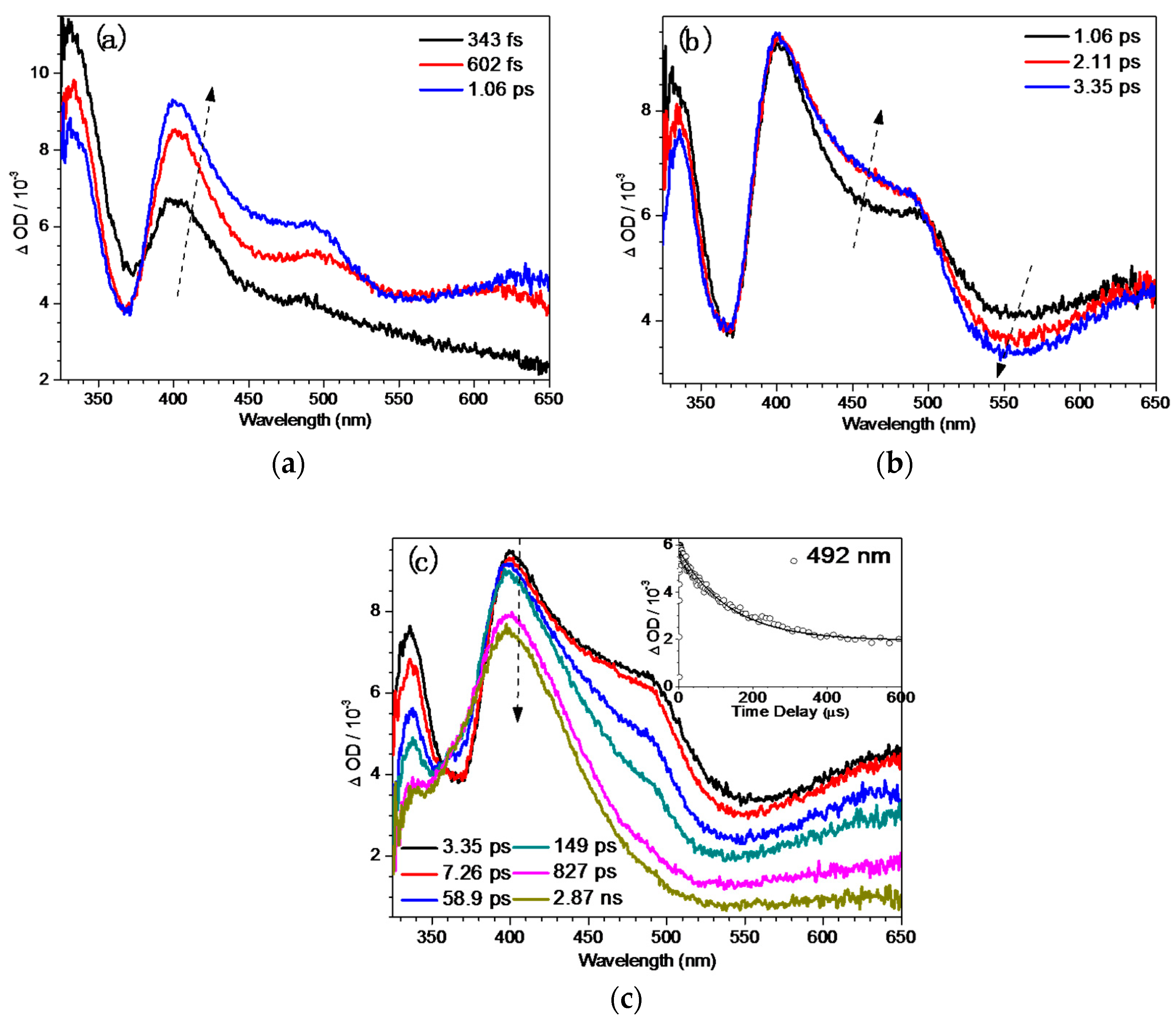
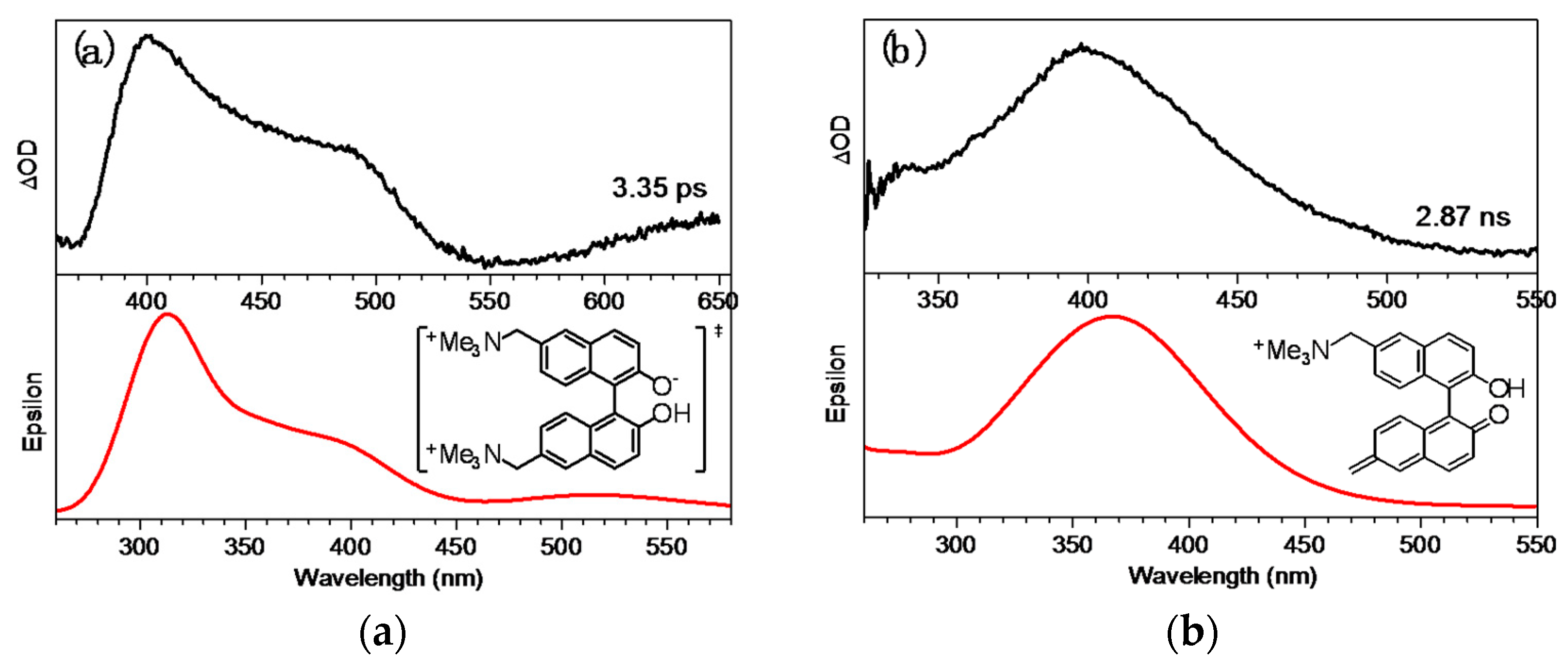
2.4. Fs-TA Study of BQMP-b Containing Two Naphthol Rings: Investigating the Early Time Processes after Photoexcitation in Varying pH Mixed Aqueous Solution
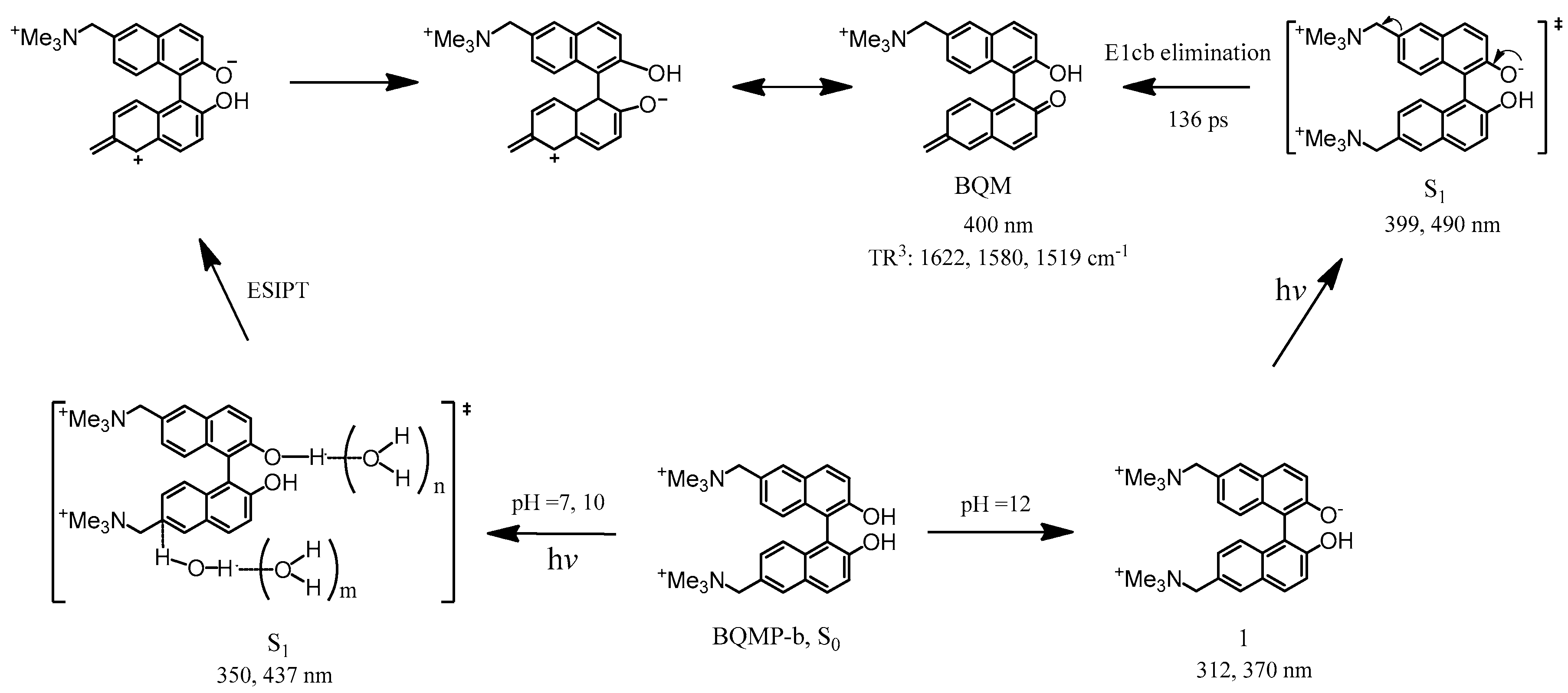
2.5. Ns-TR3 Study of BQMP-b Containing Two Naphthol Rings: Characterizing the Structure and Properties of Key Intermediates
3. Materials and Methods
3.1. Materials
3.2. Ns-TA and fs-TA Experiments
3.3. Ns-TR3 Experiments
3.4. DFT-Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angle, S.R.; Yang, W.J. Nucleophilic-addition of 2'-deoxynucleosides to the ortho-quinone methides 10-(acetyloxy) and 10-methoxy-3,4-dihydro-9(2h)-anthracenone. J. Org. Chem. 1992, 57, 1092–1097. [Google Scholar] [CrossRef]
- Egholm, M.; Koch, T.H. Coupling of the anthracycline antitumor drug menogaril to 2'-deoxyguanosine through reductive activation. J. Am. Chem. Soc. 1989, 111, 8291–8293. [Google Scholar] [CrossRef]
- Veldhuyzen, W.F.; Pande, P.; Rokita, S.E. A transient product of DNA alkylation can be stabilized by binding localization. J. Am. Chem. Soc. 2003, 125, 14005–14013. [Google Scholar] [CrossRef] [PubMed]
- Verga, D.; Nadai, M.; Doria, F.; Percivalle, C.; Di Antonio, M.; Palumbo, M.; Richter, S.N.; Freccero, M. Photogeneration and reactivity of naphthoquinone methides as purine selective DNA alkylating agents. J. Am. Chem. Soc. 2010, 132, 14625–14637. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Rokita, S.E. Reversible quinone methides alkylation of DNA. Chem. Res. Toxicol. 2010, 23, 270. [Google Scholar]
- Weinert, E.E.; Dondi, R.; Colloredo-Melz, S.; Frankenfield, K.N.; Mitchell, C.H.; Freccero, M.; Rokita, S.E. Substituents on quinone methides strongly modulate formation and stability of their nucleophilic adducts. J. Am. Chem. Soc. 2006, 128, 11940–11947. [Google Scholar] [CrossRef]
- Flegel, M.; Lukeman, M.; Huck, L.; Wan, P. Photoaddition of water and alcohols to the anthracene moiety of 9-(2'-hydroxyphenyl)anthracene via formal excited state intramolecular proton transfer. J. Am. Chem. Soc. 2004, 126, 7890–7897. [Google Scholar] [CrossRef]
- Chiang, Y.; Kresge, A.J.; Zhu, Y. Generation of o-quinone alpha-carbomethoxymethide by photolysis of methyl 2-hydroxyphenyldiazoacetate in aqueous solution. Phys. Chem. Chem. Phys. 2003, 5, 1039–1042. [Google Scholar] [CrossRef]
- Doria, F.; Richter, S.N.; Nadai, M.; Colloredo-Mels, S.; Mella, M.; Palumbo, M.; Freccero, M. BINOL-amino acid conjugates as triggerable carriers of DNA-targeted potent photocytotoxic agents. J. Med. Chem. 2007, 50, 6570–6579. [Google Scholar] [CrossRef]
- Huang, C.G.; Shukla, D.; Wan, P. Mechanism of photoisomerization of xanthene to 6h-dibenzo[B,D]pyran in aqueous-solution. J. Org. Chem. 1991, 56, 5437–5442. [Google Scholar] [CrossRef]
- Verga, D.; Richter, S.N.; Palumbo, M.; Gandolfi, R.; Freccero, M. Bipyridyl ligands as photoactivatable mono- and bis-alkylating agents capable of DNA cross-linking. Org. Biomol. Chem. 2007, 5, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Basaric, N.; Zabcic, I.; Mlinaric-Majerski, K.; Wan, P. Photochemical formation and chemistry of long-lived adamantylidene-quinone methides and 2-adamantyl cations. J. Org. Chem. 2010, 75, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Brousmiche, D.W.; Xu, M.S.; Lukeman, M.; Wan, P. Photohydration and photosolvolysis of biphenyl alkenes and alcohols via biphenyl quinone methide-type intermediates and diarylmethyl carbocations. J. Am. Chem. Soc. 2003, 125, 12961–12970. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Popik, V.V. Photochemical generation and the reactivity of o-naphthoquinone methides in aqueous solutions. J. Am. Chem. Soc. 2009, 131, 11892–11899. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.N.; Maggi, S.; Mels, S.C.; Palumbo, M.; Freccero, M. Binol quinone methides as bisalkylating and DNA cross-linking agents. J. Am. Chem. Soc. 2004, 126, 13973–13979. [Google Scholar] [CrossRef] [PubMed]
- Di Antonio, M.; Doria, F.; Richter, S.N.; Bertipaglia, C.; Mella, M.; Sissi, C.; Palumbo, M.; Freccero, M. Quinone methides tethered to naphthalene diimides as selective G-guadruplex alkylating agents. J. Am. Chem. Soc. 2009, 131, 13132–13141. [Google Scholar] [CrossRef] [PubMed]
- Du, L.L.; Zhang, X.T.; Xue, J.D.; Tang, W.J.; Li, M.D.; Lan, X.; Zhu, J.R.; Zhu, R.X.; Weng, Y.X.; Li, Y.L.; et al. Influence of water in the photogeneration and properties of a bifunctional quinone methide. J. Phys. Chem. B 2016, 120, 11132–11141. [Google Scholar] [CrossRef]
- Yan, Z.; Du, L.; Lan, X.; Zhang, X.; Phillips, D.L. Time-resolved spectroscopic and density functional theory investigation of the influence of the leaving group on the generation of a binol quinone methide. J. Mol. Struct. 2018, 1172, 102–107. [Google Scholar] [CrossRef]
- Isaks, M.; Yates, K.; Kalanderopoulos, P. Photohydration via intramolecular proton-transfer to carbon in electronically excited-states. J. Am. Chem. Soc. 1984, 106, 2728–2730. [Google Scholar] [CrossRef]
- Lukeman, M.; Veale, D.; Wan, P.; Munasinghe, V.R.N.; Corrie, J.E.T. Photogeneration of 1,5-naphthoquinone methides via excited-state (formal) intramolecular proton transfer (ESIPT) and photodehydration of 1-naphthol derivatives in aqueous solution. Can. J. Chem. 2004, 82, 240–253. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Beckstead, A.A.; Hu, Y.S.; Piao, X.J.; Bong, D.; Kohler, B. Excited-state dynamics of melamine and its lysine derivative investigated by femtosecond transient absorption spectroscopy. Molecules 2016, 21, 1645. [Google Scholar] [CrossRef] [PubMed]
- Percivalle, C.; La Rosa, A.; Verga, D.; Doria, F.; Mella, M.; Palumbo, M.; Di Antonio, M.; Freccero, M. Quinone methide generation via photoinduced electron transfer. J. Org. Chem. 2011, 76, 3096–3106. [Google Scholar] [CrossRef] [PubMed]
- Li, M.D.; Yan, Z.P.; Zhu, R.X.; Phillips, D.L.; Aparici-Espert, I.; Lhiaubet-Vallet, V.; Miranda, M.A. Enhanced drug photosafety by interchromophoric interaction owing to intramolecular charge separation. Chem. Eur. J. 2018, 24, 6654–6659. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.N.; Zhang, X.T.; Basaric, N.; Phillips, D.L. Direct observation of photoinduced ultrafast generation of singlet and triplet quinone methides in aqueous solutions and insight into the roles of acidic and basic sites in quinone methide formation. J. Am. Chem. Soc. 2017, 139, 18349–18357. [Google Scholar] [CrossRef] [PubMed]
- Alunni, S.; Tijskens, P. Study on the effect of the structure of the leaving group in the E1cb mechanism of base-promoted beta-elimination reactions from N-[2-(p-nitrophenyl)ethyl]alkylammonium ions. J. Org. Chem. 1995, 60, 8371–8374. [Google Scholar] [CrossRef]
- Thibblin, A. Leaving-group abilities in base-promoted elimination-reactions - discrimination between E1cb and E2 mechanisms. Chem. Scr. 1980, 15, 121–127. [Google Scholar]
- Cho, B.R.; Pyun, S.Y. Ketene-forming elimination reactions from aryl thienylacetates promoted by R2NH/R2NH2+ in 70 mol % MeCN(aq) effect of the beta-aryl group. J. Org. Chem. 2007, 72, 1098–1103. [Google Scholar] [CrossRef]
- Cheng, B.Y.; Wang, Y.N.; Li, T.R.; Lu, L.Q.; Xiao, W.J. Synthesis of polysubstituted pyrroles through a formal [4 + 1] cycloaddition/E1cb elimination/aromatization sequence of sulfur ylides and alpha,beta-unsaturated imines. J. Org. Chem. 2017, 82, 12134–12140. [Google Scholar] [CrossRef]
- Du, L.L.; Li, M.D.; Zhang, Y.F.; Xue, J.D.; Zhang, X.T.; Zhu, R.X.; Cheng, S.C.; Li, X.C.; Phillips, D.L. Photoconversion of beta-lapachone to alpha-lapachone via a protonation-assisted singlet excited state pathway in aqueous solution: A time-resolved spectroscopic study. J. Org. Chem. 2015, 80, 7340–7350. [Google Scholar] [CrossRef]
- Ma, J.N.; Zhang, X.T.; Basaric, N.; Wan, P.; Phillips, D.L. Observation of excited state proton transfer reactions in 2-phenylphenol and 2-phenyl-1-naphthol and formation of quinone methide species. Phys. Chem. Chem. Phys. 2015, 17, 9205–9211. [Google Scholar] [CrossRef]
- Chan, W.S.; Ma, C.S.; Kwok, W.M.; Zuo, P.; Phillips, D.L. Resonance raman spectroscopic and density functional theory study of benzoin diethyl phosphate. J. Phys. Chem. A 2004, 108, 4047–4058. [Google Scholar] [CrossRef]
- Du, L.L.; Zhu, R.X.; Xue, J.D.; Du, Y.; Phillips, D.L. Time-resolved spectroscopic and density functional theory investigation of the photochemistry of suprofen. J. Raman Spectrosc. 2015, 46, 117–125. [Google Scholar] [CrossRef]
- Santoro, F.; Jacquemin, D. Going beyond the vertical approximation with time-dependent density functional therory. Comput. Mol. Sci. 2016, 6, 460–486. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Du, L.; Lan, X.; Li, Y.; Wang, W.; Phillips, D.L. Time-Resolved Spectroscopic and Density Functional Theory Investigation of the Photogeneration of a Bifunctional Quinone Methide in Neutral and Basic Aqueous Solutions. Molecules 2018, 23, 3102. https://doi.org/10.3390/molecules23123102
Yan Z, Du L, Lan X, Li Y, Wang W, Phillips DL. Time-Resolved Spectroscopic and Density Functional Theory Investigation of the Photogeneration of a Bifunctional Quinone Methide in Neutral and Basic Aqueous Solutions. Molecules. 2018; 23(12):3102. https://doi.org/10.3390/molecules23123102
Chicago/Turabian StyleYan, Zhiping, Lili Du, Xin Lan, Yuanchun Li, Wenchao Wang, and David Lee Phillips. 2018. "Time-Resolved Spectroscopic and Density Functional Theory Investigation of the Photogeneration of a Bifunctional Quinone Methide in Neutral and Basic Aqueous Solutions" Molecules 23, no. 12: 3102. https://doi.org/10.3390/molecules23123102
APA StyleYan, Z., Du, L., Lan, X., Li, Y., Wang, W., & Phillips, D. L. (2018). Time-Resolved Spectroscopic and Density Functional Theory Investigation of the Photogeneration of a Bifunctional Quinone Methide in Neutral and Basic Aqueous Solutions. Molecules, 23(12), 3102. https://doi.org/10.3390/molecules23123102