Abstract
Nucleoside antibiotics are uridine-derived natural products that inhibit the bacterial membrane protein MraY. MraY is a key enzyme in the membrane-associated intracellular stages of peptidoglycan biosynthesis and therefore considered to be a promising, yet unexploited target for novel antibacterial agents. Muraymycins are one subclass of such naturally occurring MraY inhibitors. As part of structure-activity relationship (SAR) studies on muraymycins and their analogues, we now report on novel derivatives with different attachment of one characteristic structural motif, i.e., the aminoribose moiety normally linked to the muraymycin glycyluridine core unit. Based on considerations derived from an X-ray co-crystal structure, we designed and synthesised muraymycin analogues having the aminoribose attached (via a linker) to either the glycyluridine amino group or to the uracil nucleobase. Reference compounds bearing the non-aminoribosylated linker units were also prepared. It was found that the novel aminoribosylated analogues were inactive as MraY inhibitors in vitro, but that the glycyluridine-modified reference compound retained most of the inhibitory potency relative to the unmodified parent muraymycin analogue. These results point to 6′-N-alkylated muraymycin analogues as a potential novel variation of the muraymycin scaffold for future SAR optimisation.
1. Introduction
Bacterial infections with pathogens resistant against established antimicrobial drugs are an emerging concern in current and future healthcare [1,2]. In order to address this issue, new antibacterial drug candidates are urgently needed. These should ideally display new or previously unexploited modes of action to avoid cross resistance with existing drugs. One way to reach this goal might be the identification of new targets within bacterial pathways which are blocked by existing antibiotics [3]. Inhibition of the formation of the bacterial cell wall, i.e., of peptidoglycan biosynthesis, is a highly attractive mode of action for antimicrobial drugs as there is no human counterpart to this bacterial process [3,4]. Therefore, inhibitors of peptidoglycan biosynthesis can be anticipated to be highly selective with very limited toxicity to human host cells. Within peptidoglycan biosynthesis, the bacterial membrane enzyme MraY (translocase I) represents a hitherto unexploited target [5,6]. MraY mediates the first membrane-associated step of the intracellular section of peptidoglycan formation. Thus, it catalyses the reaction of uridine diphosphate N-acetylmuramic acid (UDP-MurNAc) pentapeptide (‘Park’s nucleotide’) 1 with the isoprenoid membrane anchor undecaprenyl phosphate 2 to give membrane-bound lipid I 3 (Scheme 1) [7,8,9,10,11,12].
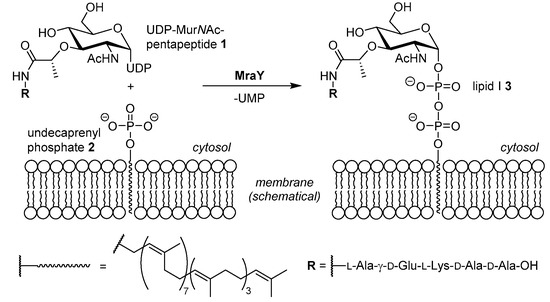
Scheme 1.
The reaction mediated by the bacterial membrane enzyme MraY (translocase I). UDP = uridine diphosphate, UMP = uridine monophosphate. The exact composition of the pentapeptide unit (residue R) can vary among different bacteria [12].
A large family of antibacterially active natural products is known to inhibit MraY. These microbial secondary metabolites have in common that they are uridine-derived nucleoside analogues, and hence, they are often referred to as ‘nucleoside antibiotics’. These nucleoside antibiotics are divided into a range of structurally different subclasses, e.g., muraymycins, caprazamycins, liposidomycins, mureidomycins, pacidamycins, capuramycins and tunicamycins [13,14,15,16]. We have set up a long-term research program on muraymycins and their synthetic analogues in order to develop them into potential antibacterial drug candidates. Muraymycins were originally reported as a collection of 19 structurally related secondary metabolites from Streptomyces [17]. Just recently, some previously unknown members of the muraymycin subclass were isolated from the producing strains [18]. The muraymycin scaffold consists of a (5′S,6′S)-glycyluridine (GlyU) unit (i.e., a so-called ‘high-carbon’ nucleoside) and a urea peptide moiety, both connected by a short alkyl linker. The urea peptide contains the non-proteinogenic amino acid epicapreomycidine as a cyclic arginine derivative (Figure 1). Most of the naturally occurring muraymycins are 5′-O-aminoribosylated in the GlyU core unit (structure Z in Figure 1).
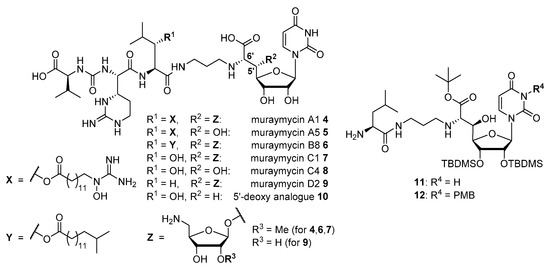
Figure 1.
Selected naturally occurring muraymycins 4–9 [17,18], the previously reported synthetic 5′-deoxy analogue 10 [19] and other previously reported muraymycin analogues 11 and 12 [20].
Muraymycins have been formally divided into four subclasses based on the exact structure of the central l-leucine unit: subclasses A-C contain a (3S)-3-hydroxyl-l-leucine that is derivatised with fatty acyl moieties in the A- and B-series. In the A-series, one can find ω-substituted fatty acyl structures with a terminal (N-hydroxyl-)guanidine group (e.g., muraymycins A1 4 and A5 5, Figure 1). In contrast, the B-series contains branched alkyl chains in this position (e.g., muraymycin B8 6). The C-series (e.g., muraymycin C1 7 and C4 8) is not O-acylated at the 3-hydroxyl-l-leucine, and the D-series (e.g., muraymycin D2 9) contains proteinogenic non-hydroxylated l-leucine at this position.
Methodology for the total synthesis of muraymycins has been developed [15], even though synthetic access to the 5′-O-aminoribosylated ‘high-carbon’ GlyU core structure as well as the epicapreomycidine and the (3S)-3-hydroxyl-l-leucine units is challenging. Relevant contributions to this field have been reported by Ichikawa and Matsuda [21,22], Kurosu [23,24], and our group [25,26,27,28]. Structure-activity relationship (SAR) studies on muraymycin analogues have already been performed and also included structurally simplified derivatives [15,19,20,29,30,31,32,33,34]. Some of these findings are subsequently highlighted. Our group has described 5′-defunctionalised (‘5′-deoxy’) analogues of the uridine-derived GlyU core unit, i.e., derivatives not only lacking the aminoribosyl moiety (Z in Figure 1), but any substituent in the 5′-position [19,34,35,36]. For instance, in case of the 5′-deoxy analogue 10 of muraymycin C4 8 (Figure 1), it was shown that the structurally simplified muraymycin congener 10 still was a rather potent inhibitor of MraY in vitro (IC50 = 95 ± 19 nM for 10) [19,33]. Shortly after the isolation of the naturally occurring muraymycins, Yamashita et al. reported the synthesis and biological activity of truncated 5′-epi-muraymycin analogues 11 and 12 (Figure 1) [20]. These compounds were found to inhibit the growth of some Gram-positive pathogens in cellulo and MraY in vitro (using a coupled MraY-MurG assay). This was remarkable as 11 and 12 still contained synthetic protecting groups, i.e., para-methoxybenzyl (PMB) at the uracil-N-3 (only for 12), tert-butyldimethylsilyl (TBDMS) at 2′-O and 3′-O, and a tert-butyl ester moiety. Deprotection of the compounds led to a loss of bioactivity. Recently, we have re-investigated some naturally occurring muraymycins for their activities as MraY inhibitors [33]. We demostrated that the absence of the fatty acid moiety (such as in 7–9) leads to a loss of antimicrobial activity in cellulo (most likely due to impaired cellular uptake of the non-lipophilised muraymycins), but retention of MraY inhibition in vitro. The 5′-O-aminoribosylated muraymycin natural products were found to be extremely potent MraY inhibitors, with IC50 values in the pM range [33].
MraY is an integral membrane protein, which makes its overexpression as well as structural biology studies challenging. Nevertheless, methodology for the overexpression and isolation of MraY has been described [37,38,39]. For in vitro assays of MraY activity and inhibition, an efficient fluorescence-based method using a dansylated derivative of Park’s nucleotide 1 is established [33,34,40,41,42,43]. Based on an in silico topology model, it had been predicted that MraY would be composed of ten transmembrane helices and five cytosolic loops [44]. This proposed architecture was experimentally confirmed when Lee reported the first X-ray crystal structure of MraY from the extremophile Aquifex aeolicus [45]. Following this structure of ligand-free MraY, the same group accomplished the first X-ray crystal structure of MraY (again from Aquifex aeolicus) in complex with an inhibitor, i.e., with muraymycin D2 9 (PDB 5CKR, 2.95 Å resolution) [46,47]. A comparison of this co-crystal structure with the structure of the aforementioned ligand-free apo enzyme [45] revealed a major conformational change upon inhibitor binding, i.e., a significant conformational plasticity of the enzyme. Another co-crystal structure of MraY from C. bolteae in complex with the nucleoside antibiotic tunicamycin has also been reported [48].
The conformational plasticity of MraY (vide supra) and the complex structures of nucleoside antibiotics make computer-aided design for the development of new nucleosidic MraY inhibitors very challenging. We have managed to obtain proposed binding modes for several naturally occurring muraymycins by in silico modelling, based on the co-crystal structure of MraY with muraymycin D2 9 [33]. However, it is precluded to apply such a procedure for synthetic muraymycin analogues with more pronounced structural differences to natural product 9.
With respect to the complex and synthetically challenging structures of naturally occurring muraymycins, one general long-term goal of our research is to identify bioactive, structurally simplified muraymycin analogues with improved chemical tractability. In this context, we would like to utilise the insights provided by the co-crystal structure of MraY with muraymycin D2 9. As in silico modelling on this system is associated with major hurdles (vide supra), we aim to derive information from the co-crystal structure that inspires the design of novel muraymycin analogues. Subsequent synthesis and biological testing can then probe the initial proposal. In this work, we report on one example of such an approach, i.e., the design, synthesis and biological evaluation of aminoribosylated, but structurally simplified muraymycin analogues.
2. Results
2.1. Design of Target Structures
For the design of the target structures of this study, we have inspected the co-crystal structure of MraY from Aquifex aeolicus in complex with the inhibitor muraymycin D2 9 [46,47] more closely. This particularly concerned the interaction of the 5′-O-aminoribosylated GlyU core unit of 9 with MraY. The complex reveals a fairly specific set of interactions for the aminoribose moiety, including a network of hydrogen bonds and an electrostatic interaction of the ribose-5″-amino group with Asp-193 (Figure 2a). This finding is in line with the significant contribution of the aminoribose unit to MraY inhibition: while 5′-O-aminoribosylated naturally occurring muraymycins were pM inhibitors of MraY in vitro, inhibitory potency dropped to the nM range for 5′-defunctionalised synthetic analogue 10 (vide supra) [33]. Another very specific set of interactions can be found for the uracil nucleobase, which is accommodated in a pocket with hydrogen bonding (with Asn-255, Asp-196, Lys-70) and π-stacking (with Phe-262).
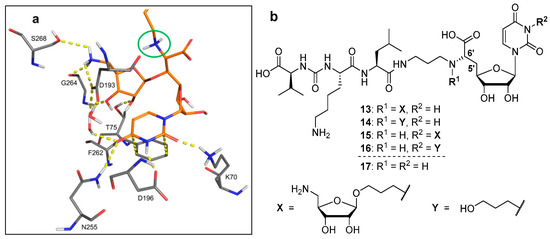
Figure 2.
(a) Insights into the X-ray co-crystal structure of MraY from Aquifex aeolicus in complex with muraymycin D2 9 (PDB 5CKR) [46,47]: protein-inhibitor interactions at the nucleoside binding site and its close proximity (hydrocarbon scaffold of inhibitor 9 in orange, green circle highlights 6′-N). (b) Target structures 13–16 of this study with previously reported muraymycin analogue 17 [34].
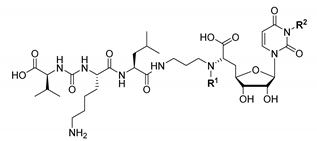
In this work, our principle approach was to attempt a different linkage of the aminoribosyl unit to the muraymycin scaffold, i.e., a connection not via the 5′-hydroxyl group. Synthetic access to the 5′-aminoribosylated GlyU core unit is not trivial and involves multi-step routes [22,24,25,49,50,51,52]. Ideally, the novel aminoribosylated analogues would combine an improved chemical tractability with strong MraY inhibitory potencies due to interactions of the aminoribose motif with the protein. These considerations have led to the design of target structures 13–16 (Figure 2b), which were derived from the previously reported simplified muraymycin analogue 17 [34]. In 17, the epicapreomycidine unit is replaced with proteinogenic l-lysine, the central l-leucine is not β-hydroxylated (as in muraymycin D2 9), and the 5′-defunctionalised version of the GlyU core is employed (as in analogue 10, vide supra). This design had given a moderate, but notable inhibitory activity of 17 towards MraY from S. aureus (IC50 = 2.5 ± 0.6 μM) [34]. In this proof-of-principle study, we aimed to investigate if chemically tractable muraymycin analogue 17 can be decorated with the aminoribose unit, connected to the scaffold via a linker.
A connection site at the muraymycin scaffold in fair proximity to the native 5′-position was preferable. Therefore, we decided to link the aminoribose moiety to the 6′-amino group of the 5′-deoxy-GlyU core. The X-ray co-crystal structure revealed the protonated 6′-amino group of the inhibitor to be oriented in a way that the two hydrogen atoms point towards the α-face of the β-configured aminoribose unit (Figure 2a). It was estimated that a short alkyl linker might be sufficient for bridging the distance to the β-face of the aminoribose moiety. Hence, the connection of the aminoribose to the 6′-amino group was attempted with a three-carbon linker, thus leading to the first 6′-substituted target structure 13 (Figure 2b). As a reference compound, we planned to also synthesise 14, which contains the 6′-linker unit (terminated with a polar hydroxyl group), but not the aminoribose motif. In search for an alternative attachment site at the muraymycin scaffold, we then considered the uracil nucleobase. The highly specific uracil binding pocket of MraY (vide supra) seems to preclude functionalisation of the uracil base. On the other hand, uracil-N-3-p-methoxybenzylated analogue 12 (see Figure 1) had been reported to be an MraY-inhibiting antibiotic (vide supra) [20]. We speculated that this might be a consequence of a slightly different binding mode of N-3-substituted muraymycin derivatives to MraY, which would explain the apparent contradiction to the insights from the X-ray co-crystal structure. In order to probe this hypothesis, we have therefore designed uracil-N-3-substituted target structure 15 (Figure 2b). Again, the three-carbon linker was envisioned to potentially bridge the distance to the aminoribose binding pocket. As for the first target structure, a non-aminoribosylated reference compound 16 with the attached linker motif was also planned to be synthesised and evaluated.
2.2. Synthesis of Aminoribosylated Muraymycin Analogues and Reference Compounds
For the synthesis of aminoribosylated target compounds 13 and 15, suitably protected aminoribose-containing building blocks were required. Starting from d-ribose 18, we therefore prepared anomerically pure 2,3-pentylidene-protected 5-azido-5-deoxy-β-ribosyl fluoride 19 according to established procedures (Scheme 2) [49,50,51,52]. With 19 as a glycosyl donor and homoallylic alcohol as acceptor, riboside 20 was obtained in 65% yield. The pronounced β-selectivity resulted from steric shielding of the α-face [49,50]. A sequence of Staudinger reduction of the azide and Boc protection then afforded butenyl riboside 21 in 98% yield.
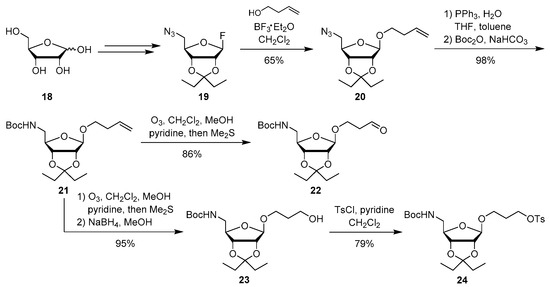
Scheme 2.
Synthesis of aminoribosyl-linker building blocks 22 and 24.
Ozonolysis of the double bond in 21 furnished aldehyde 22 (86% yield, Scheme 2) as a reagent for the introduction of the aminoribosyl-linker unit by reductive amination. For an alternative attachment of the aminoribosyl-linker moiety by nucleophilic substitution, butenyl riboside 21 was converted in a different manner. It also underwent ozonolysis, but the resultant aldehyde was not purified and directly reduced to alcohol 23 in 95% yield. Tosylation of the hydroxyl group then gave 24 (79% yield) as a reactive derivative for nucleophilic substitions.
For the synthesis of target compound 13, we started the construction of the muraymycin scaffold from the diastereomerically pure, protected (6′S)-5′-deoxy nucleosyl amino acid 25, which had been prepared from uridine according to previously reported protocols [35,36]. A reductive amination reaction of aldehyde 22 with uridine derivative 25 gave the N-alkylated product 26 in 76% yield (Scheme 3). Another reductive amination of 26 with l-leucine-derived aldehyde 27 (which had been prepared as described before [20]) then furnished tertiary amine 28 in 70% yield. Remarkably, the order of the two reductive aminations (first with 22, then with 27) was crucial. Attempts to reverse the order, i.e., to perform the reductive amination with aldehyde 27 first, resulted in a failure of the second reductive amination reaction. We speculate that this might be owed to increased steric hindrance of the 6′-amino group in the latter case.
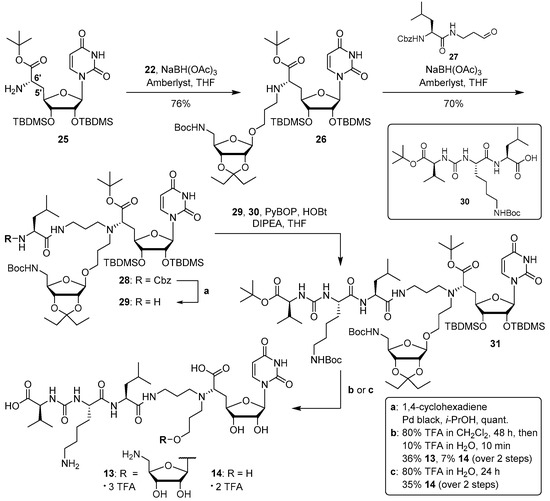
Scheme 3.
Synthesis of target structures 13 and 14.
The synthesis of target structure 13 was completed in the following manner. Hydrogenolysis of the Cbz protecting group in 28 gave primary amine 29 in quantitative yield (Scheme 3). Coupling of 29 with the urea dipeptide building block 30 (which had been synthesised as reported before [34]) was accomplished with (benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate (PyBOP) and 1-hydroxybenzotriazole (HOBt) to afford the protected full-length muraymycin analogue 31. This compound was directly subjected to global acidic deprotection. Surprisingly, usual standard conditions for this transformation (80% trifluoroacetic acid (TFA) in water [19,22,31,34,36]) did not only lead to the desired removal of all protecting groups, but also to the hydrolysis of the glycosidic bond at the aminoribose moiety. We assume that the reduced steric hindrance at the anomeric center of the aminoribosyl unit, relative to 5′-O-aminoribosylated muraymycins, might account for this unexpected hurdle. In order to synthesise 13, we carefully optimised this acidic deprotection step, including various concentrations of TFA and different solvents. Best results were achieved using 80% TFA in dichloromethane for 48 h, followed by 10% TFA in water for just 10 min. This protocol gave, after purification by semipreparative HPLC, 13 (as tris-TFA salt) in 36% yield and reference compound 14 (as bis-TFA salt) in 7% yield, respectively, each over two steps from 29. A more efficient way to obtain non-aminoribosylated reference 14 was the treatment of 31 with 80% aqueous TFA, which afforded 14 in 35% yield over two steps from 29 (Scheme 3).
For the synthesis of target compound 15, we employed the previously reported protected 5′-deoxy muraymycin building block 32 [36] as starting material. Selective alkylation of 32 with tosylate 24 at the uracil-N-3-position was achieved in 70% yield (product 33, Scheme 4). The position of the newly introduced aminoribosyl-linker unit was unambigously proven by 2D NMR spectroscopy. The pronounced regioselectivity of this reaction (uracil-N-3 vs. 6′-amino group) was probably owed to the significant steric shielding of the electronically more nucleophilic 6′-amino functionality.
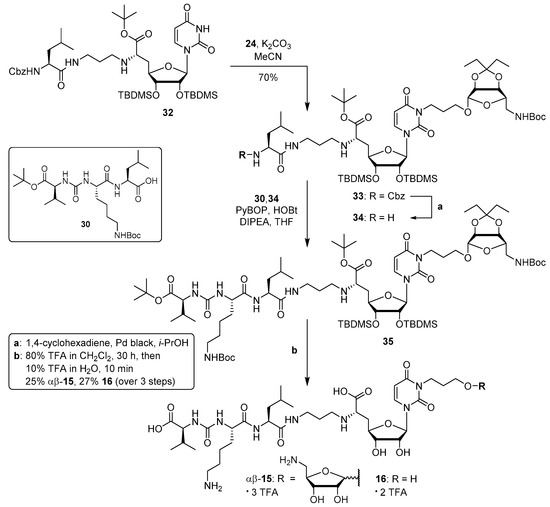
Scheme 4.
Synthesis of target structures 15 and 16.
Hydrogenolysis of the Cbz protecting group in 33 then gave primary amine 34, which was used in the next step in crude form. Coupling of 34 with the urea dipeptide building block 30 [34] was achieved with PyBOP and HOBt to afford the protected full-length muraymycin analogue 35. This compound was directly subjected to global acidic deprotection. Again, a range of conditions was studied in order to control the potential hydrolytic cleavage of the aminoribosyl unit. Best results were obtained using 80% TFA in dichloromethane for 30 h, followed by 10% TFA in water for just 10 min. This procedure gave, after purification by semipreparative HPLC, 15 (as tris-TFA salt) in 25% yield and reference compound 16 (as bis-TFA salt) in 27% yield, respectively, each over three steps from 33. Remarkably, the acidic deprotection also led to epimerisation at the anomeric center of the aminoribosyl moiety, thus furnishing an inseparable 1:1 mixture of the α- and the β-anomer. Hence, this compound is from now on referred to as αβ-15 (Scheme 4). At this stage, it was decided not to attempt a stereocontrolled synthesis of 15, but to evaluate the properties of αβ-15 as an MraY inhibitor first.
2.3. Biological Evaluation
All target structures 13–16 were tested in vitro as potential inhibitors of the bacterial target enzyme MraY. For this, we employed the fluorescence-based assay for MraY activity (vide supra), which utilises a dansylated derivative of Park’s nucleotide 1 and MraY from S. aureus (recombinantly overexpressed in E. coli) [33,34,40,41,42,43]. None of the tested muraymycin analogues showed interfering autofluorescence at the used excitation wavelength (λex = 355 nm). The obtained inhibitory activities (IC50 values) are listed in Table 1. Muraymycin analogues 13–16 were also investigated for their antibacterial activities in cellulo against E. coli, but turned out not to be active (MIC > 50 μg/mL).

Table 1.
In vitro inhibitory activities of muraymycin analogues 13–16 against MraY from S. aureus.
3. Discussion
We have accomplished the synthesis of the four envisioned target muraymycin analogues 13–16 in an efficient manner, even though 15 was obtained as an inseparable 1:1 mixture of anomers (αβ-15). Strategies for the regioselective introduction of an aminoribosyl-linker substituent at either the 6′-amino group or the uracil-N-3 were developed. One unexpected finding was the hydrolytic lability of the aminoribose glycosidic bond under acidic deprotection conditions which had been successful for 5′-O-aminoribosylated muraymycins. This probably correlated with a reduced steric hindrance at the aminoribosyl-linker motif. However, this hurdle could be utilised for an efficient synthesis of the two non-aminoribosylated analogues 14 and 16. Both en route to 13 and 15, acidic deprotection conditions could be adjusted in a way that enabled the isolation of the aminoribosylated target structures as well as 14 and 16, thus avoiding separate synthetic routes towards these two reference compounds. In the synthesis of αβ-15, the higher amount of deribosylated product 16 formed in the acidic deprotection step and the epimerisation at the anomeric center indicated that the aminoribosyl unit in 15 was probably even less sterically shielded than the corresponding structural motif in 6′-substituted congener 13.
The in vitro evaluation of 13–16 as MraY inhibitors revealed that only reference compound 14 showed notable inhibitory potency in the relevant concentration range (<100 μM). With an IC50 value of 17 ± 9 μM, it was just slightly less active than the previously reported muraymycin analogue 17 (IC50 = 2.5 ± 0.6 μM) [34]. The non-activity of 6′-substituted aminoribosylated analogue 13 indicated that the design concept inspired by the X-ray co-crystal structure, i.e., a bridging of the aminoribose unit to the 6′-position, did not work out. However, the activity of 14 demonstrated that a substitution of the 6′-position towards a tertiary amine does not necessarily lead to a major loss of activity. It is possible that the selected three-carbon linker is not ideal to place the 6′-connected aminoribose unit in the corresponding binding pocket of MraY. It has to be emphasised that a more exact in silico modelling of an approriate linker architecture is hampered by the pronounced conformational plasticity of MraY and the size and conformational flexibility of muraymycin-derived inhibitors. This leaves the identification of suitable linkers to experimental approaches such as the one reported in this work.
The inactivity of both 15 and 16 confirmed what had been derived from the X-ray co-crystal structure, i.e., that a substitution at the uracil-N-3 is precluded by the highly specific interaction of this motif with MraY. While this effect might be assigned to steric bulk of the substituent in the case of 15, the non-activity of 16 (bearing a much smaller N-3 substituent) suggests that any derivatisation of the uracil-N-3 might lead to a loss of inhibitory activity. This is in contrast to the reported bioactivity of muraymycin analogue 12 (see Figure 1) [20] though. It cannot be ruled out that 12 might bind to MraY in a different binding mode, but this remains to be studied in future work. However, it was our goal to probe the concept of uracil-N-3 derivatisation, and this aim was successfully accomplished as the reported results generally discourage uracil-N-3 substitution.
As target structures 13, 15 and 16 did not show notable potency as MraY inhibitors, it was not surprising that they were also antibacterially inactive in cellulo against E. coli. In the case of MraY-inhibiting analogue 14, the missing antibacterial activity requires further discussion. We had found out in our previous work that there might be a ‘threshold’ of inhibitory potency towards MraY for observing antibacterial activity in cellulo [34]. For instance, analogue 10 (as a nM MraY inhibitor) shows some rather moderate activity against the growth of E. coli [19], while 17 (as a low-μM MraY inhibitor) was inactive against the same strains [34]. From these observations, we had concluded that a low-nM (or stronger) inhibitory potency against MraY seems to be a prerequisite for reasonable antibacterial activity [34]. As target compound 14 was a slightly weaker MraY inhibitor than 17, it is not surprising that it was devoid of antibacterial activity. It needs to be taken into account though that the polar muraymycin scaffold impairs cellular uptake, thus making the penetration of the bacterial membrane a bottleneck for antibacterial activity. This hurdle might be overcome, for instance, by lipophilisation of the polar muraymycin structure. However, it should be pointed out that the primary goal of this study was to further elucidate MraY inhibition and not to obtain novel analogues with strong antibacterial potencies.
4. Materials and Methods
4.1. Synthesis of Aminoribosylated Muraymycin Analogues and Reference Compounds
General Methods: All chemicals were purchased from standard suppliers. Reactions involving oxygen and/or moisture sensitive reagents were carried out under an atmosphere of argon using anhydrous solvents. Anhydrous solvents were obtained in the following manner. CH2Cl2, MeCN and THF were purchased in HPLC quality, dried with a solvent purification system (MBRAUN MB SPS 800) and stored over activated molecular sieves (3 Å (CH2Cl2, MeCN) or 4 Å (THF)). Pyridine was dried over CaH2, distilled and stored over activated molecular sieves (4 Å). MeOH was degassed, dried and stored over activated molecular sieves (3 Å). i-PrOH was dried over calcium sulfate hemihydrate and stored over activated molecular sieves (3 Å). All other solvents were of technical quality and distilled prior to use, and deionised water was used throughout. Column chromatography was carried out on silica gel 60 (0.040–0.063 mm, 230–400 mesh ASTM, VWR, Darmstadt, Germany) under flash conditions except where indicated. TLC was performed on aluminium plates precoated with silica gel 60 F254 (VWR, Darmstadt, Germany). Visualisation of the spots was carried out using UV light (254 nm) and/or staining under heating (H2SO4 staining solution: 4 g vanillin, 25 mL conc. H2SO4, 80 mL AcOH, and 680 mL MeOH; KMnO4 staining solution: 1 g KMnO4, 6 g K2CO3, and 1.5 mL 1.25 M NaOH solution, all dissolved in 100 mL H2O; ninhydrin staining solution: 0.3 g ninhydrin, 3 mL AcOH, and 100 mL 1-butanol). Analytical HPLC was performed on a Thermo Scientific system (ThermoFisher Scientific, Dreieich, Germany) equipped with an MWD detector (250.0), an ESI mass spectrometer and an EC 125/3 column (5 × 100 mm) containing reversed phase silica gel NUCLEODURTM 100–5 C18ec (5 μm, Macherey-Nagel, Düren, Germany). Method 1: eluent A water (+0.1% TFA), eluent B MeCN (+0.1% TFA); 0–19 min gradient of B (5–100%), 19–22 min 100% B, 22–23 min gradient of B (100–5%), 23–25 min 5% B; flow 0.8 mL/min. Semipreparative HPLC was performed on an Agilent Technologies 1200 Series system (Agilent Technologies, Waldbronn, Germany) equipped with an MWD detector (254.16/280.16) and a LiChroCartTM column (10 × 250 mm) containing reversed phase silica gel PurospherTM RP18e (5 μm, VWR). Method 1: eluent A water (+0.1% TFA), eluent B MeCN (+0.1% TFA); 0–36 min gradient of B (5–100%), 36–44 min 100% B, 44–44.1 min gradient of B (100–1%); flow 3 mL/min. Method 2: eluent A water (+0.1% TFA), eluent B MeCN (+0.1% TFA); 0–36 min gradient of B (5–60%), 36–40 min gradient of B (60–100%), 40–44 min 100% B, 44–44.1 min gradient of B (100–1%); flow 3 mL/min. Method 3: eluent A water (+0.1% TFA), eluent B MeCN (+0.1% TFA); 0–36 min gradient of B (5–70%), 36–40 min gradient of B (70–100%), 40–44 min 100% B, 44–44.1 min gradient of B (100–1%); flow 3 mL/min. 500 MHz-1H, 126 MHz-13C, as well as 282 MHz-19F NMR spectra were recorded on Bruker AVANCE-500 and AVANCE-300 spectrometers (Bruker, Bremen, Germany). All 13C and 19F NMR spectra are 1H-decoupled. All spectra were recorded at room temperature except where indicated otherwise and were referenced internally to solvent reference frequencies. Chemical shifts (δ) are quoted in ppm and coupling constants (J) are reported in Hz. Assignment of signals was carried out using H,H-COSY, HSQC, and HMBC spectra obtained on the spectrometers mentioned above. The numbering of atoms of muraymycin target structures is depicted in the Supplementary Materials (Figure S1). Mass spectra of small molecules were measured on a Finnigan LCQ ion-trap mass spectrometer or on a Bruker microTOF spectrometer (Bruker, Bremen, Germany). High resolution spectra were measured on a Bruker 7 Tesla Fourier transform ion cyclotron resonance (FTICR) mass spectrometer (Bruker, Bremen, Germany). Infrared spectroscopy (IR) was performed on a Bruker Vertex 70 spectrometer equipped with an integrated ATR unit (PlatinumATRTM, Bruker). Wavenumbers (ν) are quoted in cm−1. UV spectroscopy was carried out on an Cary 100 spectrophotometer (Agilent Technologies, Waldbronn, Germany). Wavelengths of maximum absorption (λmax) are reported in nm. Optical rotations were recorded on a Krüss Optronic polarimeter with a Na source using a 5 cm cell (Krüss Optronic, Hamburg, Germany). Melting points (m.p.) were measured on a Büchi instrument (Büchi, Essen, Germany) and are not corrected.
6′-N-Substituted aminoribosylated muraymycin analogue (13): To a solution of protected urea dipeptide 30 (6.1 mg, 14 μmol) in THF (2.5 mL), HOBt (1.9 mg, 14 μmol), PyBOP (7.1 mg, 14 μmol) and DIPEA (4.7 μL, 27 μmol) were added and the mixture was stirred at rt for 30 min. At 0 °C, a solution of protected 6′-N-substituted aminoribosylated truncated muraymycin analogue 29 (15.2 mg, 13.7 μmol) in THF (2 mL) was added dropwise, and the reaction mixture was stirred at 0 °C for 1 h and at rt for 3 h. The solvent was evaporated under reduced pressure, and the resultant crude product was purified by column chromatography (97:3, CH2Cl2-MeOH) to give 31 as a colourless solid (20.4 mg) that was directly used in the subsequent deprotection step. A solution of the protected 6′-N-substituted aminoribosylated muraymycin analogue 31 (16.0 mg) in TFA (80% in CH2Cl2, 5.0 mL) was stirred at rt for 48 h. The mixture was then diluted with CH2Cl2 (20 mL) and the solvent was evaporated under reduced pressure. The resultant residue was dissolved in TFA (10% in water, 5.0 mL), the mixture was stirred at rt for 10 min and then diluted with water (20 mL). The solvent was evaporated under reduced pressure, and the resultant crude product was purified by semipreparative HPLC (method 1) to give 13 (tris-TFA salt) as a colourless solid (5.0 mg, 36% over 2 steps from 29) and 14 (bis-TFA salt) as a colourless solid (0.75 mg, 7% over 2 steps from 29). 13: 1H NMR (500 MHz, D2O): δ [ppm] = 0.90 (d, J = 6.1 Hz, 3 H, 5‴-Ha), 0.95 (d, J = 5.2 Hz, 3H, 5‴-Hb), 0.96 (d, J = 6.2 Hz, 3H, 4v-Ha), 1.00 (d, J = 6.8 Hz, 3H, 4v-Hb), 1.41–1.54 (m, 2H, 4iv-H), 1.56–1.77 (m, 6H, 3‴-H, 4‴-H, 3iv-Ha, 5iv-H), 1.70–1.86 (m, 1H, 3iv-Hb), 1.95–2.06 (m, 3H, 2″-H, 2vi-Ha), 2.06–2.15 (m, 1H, 2vi-Hb), 2.16–2.25 (m, 2H, 5′-Ha, 3v-H), 2.43–2.49 (m, 1H, 5′-Hb), 3.03 (dd, J = 7.6, 7.6 Hz, 2H, 6iv-H), 3.09 (dd, J = 11.5, 11.1 Hz, 1H, 5vii-Ha), 3.16–3.27 (m, 2H, 1″-Ha, 3″-Ha), 3.27–3.34 (m, 2H, 1″-Hb, 1vi-Ha), 3.34–3.40 (m, 2H, 3″-Hb, 1vi-Hb), 3.42 (dd, J = 13.3, 2.4 Hz, 1H, 5vii-Hb), 3.61–3.67 (m, 1H, 3vi-Ha), 3.88 (ddd, J = 6.9, 4.3, 3.9 Hz, 1H, 3vi-Hb), 4.07 (dd, J = 9.6, 3.1 Hz, 1H, 6′-H), 4.10–4.19 (m, 5H, 3′-H, 2iv-H, 2v-H, 2vii-H, 4vii-H), 4.22 (dd, J = 6.3, 4.8 Hz, 1H, 3vii-H), 4.26 (dd, J = 7.7, 6.6 Hz, 1H, 4′-H), 4.30 (dd, J = 10.0, 4.7 Hz, 1H, 2‴-H), 4.55 (dd, J = 4.9, 4.5 Hz, 1H, 2′-H), 5.03 (s, 1H, 1vii-H), 5.76 (d, J = 4.5 Hz, 1H, 1′-H), 5.92 (d, J = 8.1 Hz, 1H, 5-H), 7.69 (d, J = 8.1 Hz, 1H, 6-H). 13C NMR (126 MHz, D2O): δ [ppm] = 16.96 (Ca-4v), 18.49 (Cb-4v), 20.58 (Ca-5‴), 21.95 (C-4iv), 22.12 (Cb-5‴), 24.13 (C-2″), 24.19 (C-2vi), 24.40 (C-4‴), 26.25 (C-5iv), 29.46 (C-5′), 29.92 (C-3v), 30.68 (C-3iv), 36.14 (C-3″), 39.21 (C-6iv), 39.52 (C-3‴), 43.11 (C-5vii), 49.65 (C-1″), 50.64 (C-1vi), 52.47 (C-2‴), 54.24 (C-2iv), 58.89 (C-2v), 63.30 (C-6′), 65.62 (C-3vi), 72.36 (C-3vii), 72.42 (C-2′), 73.08 (C-3′), 74.05 (C-2vii), 78.26 (C-4vii), 80.52 (C-4′), 92.19 (C-1′), 102.29 (C-5), 107.52 (C-1vii), 116.33 (q, 1JCF = 291.5 Hz, F3CCOO), 143.23 (C-6), 151.28 (C-2), 159.51 (NC(=O)N), 162.97 (q, 2JCF = 35.5 Hz, F3CCOO), 166.09 (C-4), 171.57 (C-7′), 174.76 (C-1‴), 175.45 (C-1iv), 176.71 (C-1v). 19F NMR (282 MHz, D2O): δ [ppm] = −75.57 (s, CF3). HRMS (ESI+): calcd.: 466.7504 [M+2H]2+, found: 466.7504. IR (ATR): ν = 3066, 2962, 1668, 1556, 1464, 1199, 1131, 1030, 835, 799, 721, 551. UV (MeCN): λmax = 260. optical rotation: [α]D20 = −11.3 (c = 1.3, H2O). m.p. = 145 °C. Analytical HPLC: tR = 6.7 min (method 1). Semipreparative HPLC: tR = 12.3 min (method 1). 14: see below for analytical data.
6′-N-Substituted non–aminoribosylated muraymycin analogue (14): Protected 6′-N-substituted aminoribosylated muraymycin analogue 31 was prepared as described above. A solution of 31 (21.2 mg) in TFA (80% in water, 6.0 mL) was stirred at rt for 24 h. The mixture was then diluted with water (20 mL) and the solvent was evaporated under reduced pressure. The resultant crude product was purified by semipreparative HPLC (method 2) to give 14 (tris-TFA salt) as a colourless solid (5.1 mg, 35% over 2 steps from 29). 1H NMR (500 MHz, D2O): δ [ppm] = 0.80 (d, J = 6.1 Hz, 3H, 5‴-Ha), 0.85 (d, J = 4.9 Hz, 3H, 5‴-Hb), 0.86 (d, J = 6.1 Hz, 3H, 4v-Ha), 0.90 (d, J = 6.7 Hz, 3H, 4v-Hb), 1.31–1.43 (m, 2H, 4iv-H), 1.46–1.67 (m, 6H, 3‴-H, 4‴-H, 3iv-Ha, 5iv-H), 1.69–1.76 (m, 1H, 3iv-Hb), 1.84–1.95 (m, 4H, 2″-H, 2vi-H), 2.10 (dqq, J = 6.7, 6.6, 6.1 Hz, 1H, 3v-H), 2.14 (ddd, J = 14.0, 10.7, 3.0 Hz, 1H, 5′-Ha), 2.37–2.42 (m, 1H, 5′-Hb), 3.03 (dd, J = 7.5, 7.5 Hz, 2H, 6iv-H), 3.07–3.19 (m, 2H, 1″-Ha, 3″-Ha), 3.19–3.27 (m, 3H, 1″-Hb, 3″-Hb, 1vi-Ha), 3.29–3.35 (m, 1H, 1vi-Hb), 3.60–3.68 (m, 2H, 3vi-H), 4.00–4.10 (m, 4H, 3′-H, 6′-H, 2iv-H, 2v-H), 4.15–4.22 (m, 1H, 4′-H, 2‴-H), 4.44 (dd, J = 5.1, 4.4 Hz, 1H, 2′-H), 5.67 (d, J = 4.4 Hz, 1H, 1′-H), 5.82 (d, J = 8.1 Hz, 1H, 5-H), 7.59 (d, J = 8.1 Hz, 1H, 6-H). 13C NMR (126 MHz, D2O): δ [ppm] = 16.94 (Ca-4v), 18.47 (Cb-4v), 20.61 (Ca-5‴), 22.01 (C-4iv), 22.09 (Cb-5‴), 24.22 (C-2″), 24.38 (C-4‴), 26.21 (C-2vi), 26.24 (C-5iv), 29.34 (C-5′), 29.92 (C-3v), 30.74 (C-3iv), 36.11 (C-3″), 39.21 (C-6iv), 39.54 (C-3‴), 49.87 (C-1″), 50.97 (C-1vi), 52.50 (C-2‴), 54.08 (C-2iv), 58.84 (C-2v), 59.13 (C-3vi), 62.70 (C-6′), 72.47 (C-2′), 73.02 (C-3′), 80.50 (C-4′), 92.01 (C-1′), 102.29 (C-5), 116.33 (q, 1JCF = 291.5 Hz, F3CCOO), 143.11 (C-6), 151.30 (C-2), 159.48 (NC(=O)N), 162.97 (q, 2JCF = 35.4 Hz, F3CCOO), 166.10 (C-4), 171.35 (C-7′), 174.73 (C-1‴), 175.35 (C-1iv), 176.70 (C-1v). 19F NMR (282 MHz, D2O): δ [ppm] = −75.59 (s, CF3). HRMS (ESI+): calcd.: 401.2213 [M+2H]2+, found: 401.2220. IR (ATR): ν = 2962, 1670, 1556, 1464, 1389, 1262, 1199, 1133, 1065, 834, 800, 768. UV (MeCN): λmax = 260. optical rotation: [α]D20 = +6.7 (c = 0.8, H2O). m.p. = 150 °C. Analytical HPLC: tR = 6.9 min (method 1). Semipreparative HPLC: tR = 16.5 min (method 2).
Uracil-N-3-substituted aminoribosylated muraymycin analogue (αβ-15) and uracil-N-3-substituted non-aminoribosylated muraymycin analogue (16): To a solution of the protected uracil-N-3-substituted aminoribosylated truncated N-Cbz-muraymycin analogue 33 (27.4 mg, 22.0 μmol) in i-PrOH (3 mL), 1,4-cyclohexadiene (21.0 μL, 226 μmol) and Pd black (10.0 mg, 94.0 μmol) were added and the mixture was stirred at rt for 1 h. It was then filtered through a syringe filter, and the syringe filter was washed with i-PrOH (4 × 5 mL). The solvent of the combined filtrates was evaporated under reduced pressure to give 34 as a colourless wax (24.0 mg) that was directly used in the subsequent peptide coupling step. To a solution of protected urea dipeptide 30 (6.3 mg, 14 μmol) in THF (2.5 mL), HOBt (2.0 mg, 14 μmol), PyBOP (7.4 mg, 14 μmol) and DIPEA (4.8 μL, 28 μmol) were added and the mixture was stirred at rt for 30 min. At 0 °C, a solution of protected uracil-N-3–substituted aminoribosylated truncated muraymycin analogue 34 (16.0 mg) in THF (2 mL) was added dropwise, and the reaction mixture was stirred at 0 °C for 1 h and at rt for 3 h. The solvent was evaporated under reduced pressure, and the resultant crude product was purified by column chromatography (97:3, CH2Cl2-MeOH) to give 35 as a colourless solid (18.4 mg) that was directly used in the subsequent deprotection step. A solution of the protected uracil-N-3–substituted aminoribosylated muraymycin analogue 35 (20.0 mg) in TFA (80% in CH2Cl2, 6.0 mL) was stirred at rt for 30 h. The mixture was then diluted with CH2Cl2 (20 mL) and the solvent was evaporated under reduced pressure. The resultant residue was dissolved in TFA (10% in water, 5.0 mL), the mixture was stirred at rt for 10 min and then diluted with water (20 mL). The solvent was evaporated under reduced pressure, and the resultant crude product was purified by semipreparative HPLC (method 3) to give αβ-15 (tris-TFA salt) as a colourless solid (5.1 mg, 1:1 mixture of α,β-anomers, 25% over 3 steps from 33) and 16 (tris-TFA salt) as a colourless solid (4.5 mg, 27% over 3 steps from 33). αβ-15: 1H NMR (500 MHz, D2O): δ [ppm] = 0.86 (d, J = 6.0 Hz, 3H, 5‴-Ha), 0.86 (d, J = 6.0 Hz, 3H, 5‴-Ha), 0.92 (d, J = 5.9 Hz, 3H, 5‴-Hb), 0.92 (d, J = 5.9 Hz, 3H, 5‴-Hb), 0.93 (2 d, J = 6.9 Hz, 3H, 4v-Ha), 0.93 (2 d, J = 6.9 Hz, 3H, 4v-Ha), 0.96 (d, J = 6.8 Hz, 3H, 4v-Hb), 0.96 (d, J = 6.8 Hz, 3H, 4v-Hb), 1.35–1.51 (m, 2 × 2 H, 2 × 4iv-H), 1.52–1.74 (m, 2 × 6 H, 2 × 3‴-H, 2 × 4‴-H, 2 × 3iv-Ha, 2 × 5iv-H), 1.74–1.84 (m, 2 × 1 H, 2 × 3iv-Hb), 1.86–1.98 (m, 2 × 4 H, 2 × 2″-H, 2 × 2vi-H), 2.16 (dqq, J = 6.9, 6.8, 5.4 Hz, 1H, 3v-H), 2.16 (dqq, J = 6.9, 6.8, 5.4 Hz, 1H, 3v-H), 2.32 (ddd, J = 15.0, 11.3, 5.7 Hz, 1H, 5′-Ha), 2.32 (ddd, J = 15.0, 11.3, 5.7 Hz, 1H, 5′-Ha), 2.45 (ddd, J = 15.0, 6.4, 2.5 Hz, 1H, 5′-Hb), 2.45 (ddd, J = 15.0, 6.4, 2.5 Hz, 1H, 5′-Hb), 2.99 (dd, J = 7.6, 7.6 Hz, 2H, 6iv-H), 2.99 (dd, J = 7.6, 7.6 Hz, 2H, 6iv-H), 3.04–3.13 (m, 2 × 3H, 2 × 1″-Hb, 2 × 5vii-Ha), 3.21–3.28 (m, 2 × 1H, 2 × 3″-Ha), 3.29–3.42 (m, 2 × 2H, 2 × 3″-Hb, 2 × 5vii-Hb), 3.56 (dt, J = 10.2, 6.0, 1H, β-3vi-Ha), 3.63 (dt, J = 10.4, 6.2 Hz, 1H, α-3vi-Ha), 3.79–3.87 (m, 2 × 1H, 2 × 3vi-Hb), 3.94 (dd, J = 11.3, 6.4 Hz, 1H, 6′-H), 3.94 (dd, J = 11.3, 6.4 Hz, 1H, 6′-H), 3.97–4.04 (m, 2 × 3H, 2 × 1vi-H, α-3vii-H, β-2vii-H), 4.04–4.09 (m, 2 × 2H, 2 × 3′-H, 2 × 2v-H), 4.09–4.20 (m, 1 × 3H, 1 × 4H, 2 × 4′-H, 2 × 2iv-H, α-2vii-H, β-3vii-H, β-4vii-H), 4.20–4.28 (m, 1 × 1H, 1 × 2H, 2 × 2‴-H, α-4vii-H), 4.34–4.39 (m, 2 × 1H, 2 × 2′-H), 4.98 (s, 1H, β-1vii-H), 5.15 (d, J = 4.3 Hz, 1H, α-1vii-H), 5.79 (d, J = 3.5 Hz, 1H, 1′-H), 5.81 (d, J = 3.5 Hz, 1H, 1′-H), 5.94 (d, J = 8.1 Hz, 1H, 5-H), 5.94 (d, J = 8.1 Hz, 1H, 5-H), 7.64 (d, J = 8.1 Hz, 1H, 6-H), 7.64 (d, J = 8.1 Hz, 1H, 6-H). 13C NMR (126 MHz, D2O): δ [ppm] = 16.91 (2 × Ca-4v), 18.43 (2 × Cb-4v), 20.58 (2 × Ca-5‴), 21.98 (2 × C-4iv), 22.04 (2 × Cb-5‴), 24.32 (2 × C-4‴), 25.56 (2 × C-2″), 26.20 (2 × C-5iv), 26.65, 26.89 (α-C-2vi, β-C-2vi), 29.87 (2 × C-3v), 30.70 (2 × C-3iv), 32.67 (2 × C-5′), 35.93 (2 × C-3″), 38.96, 39.04 (α-C-1vi, β-C-1vi), 39.16 (2 × C-6iv), 39.42 (2 × C-3‴), 41.37, 43.11 (α-C-5vii, β-C-5vii), 44.24 (2 × C-1″), 52.51 (2 × C-2‴), 54.02 (2 × C-2iv), 58.82 (2 × C-2v), 59.32 (2 × C-6′), 66.53 (β-C-3vi), 66.67 (α-C-3vi), 70.54 (β-C-3vii), 70.69 (α-C-3vii), 72.52 (α-C-2vii) 72.88, 72.92 (2 × C-2′, 2 × C-3′), 74.07 (β-C-2vii), 78.07 (β-C-4vii), 78.79 (α-C-4vii), 79.29, 79,35 (α-C-4′, β-C-4′), 91.79, 92.16 (α-C-1′, β-C-1′), 101.64, 101.68 (α-C-5, β-C-5), 102.31 (α-C-1vii), 107.51 (β-C-1vii), 116.29 (q, 1JCF = 291.5 Hz, F3CCOO), 116.29 (q, 1JCF = 291.5 Hz, F3CCOO), 140.20, 140.31 (α-C-6, β-C-6), 151.52, 151.57 (α-C-2, β-C-2), 159.46 (2 × NC(=O)N), 162.96 (q, 2JCF = 35.5 Hz, F3CCOO), 162.96 (q, 2JCF = 35.5 Hz, F3CCOO), 165.09, 165.15 (α-C-4, β-C-4), 171.80, 171.85 (α-C-7′, β-C-7′), 174.85 (2 × C-1‴), 175.40 (2 × C-1iv), 176.69 (2 × C-1v). 19F NMR (282 MHz, D2O): δ [ppm] = −75.57 (s, CF3), –75.57 (s, CF3). HRMS (ESI+): calcd.: 466.7504 [M+2H]2+, found: 466.7507. IR (ATR): ν = 3293, 3082, 2961, 1649, 1553, 1462, 1182, 1129, 835, 799, 721. UV (MeCN): λmax = 262. optical rotation: [α]D20 = +29.3 (c = 0.7, H2O). m.p. = 156 °C. Analytical HPLC: tR = 6.5 min (method 1). Semipreparative HPLC: tR = 14.4 min (method 3). 16: 1H NMR (500 MHz, D2O): δ [ppm] = 0.90 (d, J = 5.6 Hz, 3H, 5‴-Ha), 0.94–0.97 (m, 6H, 5‴-Hb, 4v-Ha), 0.99 (d, J = 6.5 Hz, 3H, 4v-Hb), 1.41–1.53 (m, 2H, 4iv-H), 1.55–1.76 (m, 6H, 3‴-H, 4‴-H, 3iv-Ha, 5iv-H), 1.78–1.85 (m, 1H, 3iv-Hb), 1.88 (tt, J = 7.1, 6.4 Hz, 2H, 2vi-H), 1.94 (ddt, J = 7.0, 6.9, 6.6 Hz, 2H, 2″-H), 2.19 (dqq, J = 6.7, 6.5, 5.9 Hz, 1H, 3v-H), 2.31 (ddd, J = 15.1, 8.9, 6.3 Hz, 1H, 5′-Ha), 2.47 (ddd, J = 15.1, 5.8, 1.8 Hz, 1H, 5′-Hb), 3.03 (dd, J = 7.5, 7.5 Hz, 2H, 6iv-H), 3.07–3.15 (m, 2H, 1″-H), 3.28 (ddd, J = 13.3, 6.9, 6.4 Hz, 1H, 3″-Ha), 3.34 (ddd, J = 13.3, 7.0, 6.4 Hz, 1H, 3″-Hb), 3.67 (t, J = 6.4 Hz, 2H, 3vi-H), 3.90 (dd, J = 6.3, 5.8 Hz, 1H, 6′-H), 4.01 (t, J = 7.1 Hz, 2H, 1vi-H), 4.08–4.13 (m, 2H, 3′-H, 2v-H), 4.15 (dd, J = 7.6, 6.0 Hz, 1H, 2iv-H), 4.19 (ddd, J = 8.9, 7.2, 1.8 Hz, 1H, 4′-H), 4.28 (dd, J = 9.3, 4.7 Hz, 1H, 2‴-H), 4.43 (dd, J = 3.7, 2.7 Hz, 1H, 2′-H), 5.81 (d, J = 2.7 Hz, 1H, 1′-H), 5.97 (d, J = 8.1 Hz, 1H, 5-H), 7.67 (d, J = 8.1 Hz, 1H, 6-H). 13C NMR (126 MHz, D2O): δ [ppm] = 16.94 (Ca-4v), 18.48 (Cb-4v), 20.64 (Ca-5‴), 22.00 (C-4iv), 22.07 (Cb-5‴), 24.36 (C-4‴), 25.62 (C-2″), 26.23 (C-5iv), 29.34 (C-2vi), 29.94 (C-3v), 30.76 (C-3iv), 32.87 (C-5′), 35.93 (C-3″), 38.67 (C-1vi), 39.20 (C-6iv), 39.50 (C-3‴), 44.26 (C-1″), 52.54 (C-2‴), 54.00 (C-2iv), 58.91 (C-2v), 59.32 (C-3vi), 59.93 (C-6′), 72.81 (C-2′), 72.90 (C-3′), 79.80 (C-4′), 92.49 (C-1′), 101.66 (C-5), 116.33 (q, 1JCF = 291.9 Hz, F3CCOO), 140.55 (C-6), 151.55 (C-2), 159.46 (NC(=O)N), 162.99 (q, 2JCF = 35.5 Hz, F3CCOO), 165.19 (C-4), 172.13 (C-7′), 174.85 (C-1‴), 175.35 (C-1iv), 176.79 (C-1v). 19F NMR (282 MHz, D2O): δ [ppm] = −75.59 (s, CF3). HRMS (ESI+): calcd.: 401.2213 [M+2H]2+, found: 401.2217. IR (ATR): ν = 3306, 2961, 1645, 1554, 1462, 1199, 1181, 1130, 1084, 836, 800, 721. UV (MeCN): λmax = 262. optical rotation: [α]D20 = +27.0 (c = 0.6, H2O). m.p. = 185 °C. Analytical HPLC: tR = 6.8 min (method 1). Semipreparative HPLC: tR = 16.7 min (method 3).
Protected but-3-enyl β-d-5-azido-5-deoxyriboside (20): To a solution of protected β-d-ribosyl fluoride 19 (50.0 mg, 20.0 μmol) [49,50,51,52] in CH2Cl2 (2 mL) at 0 °C, molecular sieves (4 Å) and a solution of homoallylic alcohol (18.0 mg, 31.0 μmol) in CH2Cl2 (0.5 mL) were added and the mixture was stirred at 0 °C for 1 h. Boron trifluoride etherate (38 μL, 30 mmol, 0.2 M in CH2Cl2) was added and the mixture was stirred at 0 °C for further 2 h. It was then quenched with sat. NaHCO3 solution, warmed to rt, and filtered. The residue was washed with CH2Cl2 (3 × 30 mL). The combined organics were washed with brine (100 mL) and dried over Na2SO4. The solvent was evaporated under reduced pressure, and the resultant crude product was purified by column chromatography (9:1, iso-hexane–EtOAc) to give 20 as a colourless oil (40.0 mg, 65%). 1H NMR (500 MHz, C6D6): δ [ppm] = 0.80 (t, J = 7.5 Hz, 3H, 1′-H), 0.99 (t, J = 7.5 Hz, 3H, 5′-H), 1.42 (q, J = 7.5 Hz, 2H, 2′-H), 1.70 (q, J = 7.5 Hz, 2H, 4′-H), 2.06–2.17 (m, 2H, 2″-H), 2.70 (dd, J = 12.5, 6.4 Hz, 1H, 5-Ha), 3.02 (dd, J = 12.5, 8.1 Hz, 1H, 5-Hb), 3.12 (dt, J = 9.5, 6.6 Hz, 1H, 1″-Ha), 3.63 (dt, J = 9.5, 6.7 Hz, 1H, 1″-Hb), 4.17 (dd, J = 6.0, 0.7 Hz, 1H, 3-H), 4.29 (ddd, J = 8.1, 6.4, 0.7 Hz, 1H, 4-H), 4.44 (d, J = 6.0 Hz, 1H, 2-H), 4.98–5.04 (m, 2H, 4″-H), 5.08 (s, 1H, 1-H), 5.68 (ddt, J = 17.1, 10.3, 6.8 Hz, 1H, 3″-H). 13C NMR (126 MHz, C6D6): δ [ppm] = 7.47 (C-5′), 8.29 (C-1′), 29.13 (C-4′), 29.41 (C-2′), 34.03 (C-2″), 53.57 (C-5), 67.03 (C-1″), 82.52 (C-3), 85.62 (C-2), 85.74 (C-4), 109.00 (C-1), 116.43 (C-4″), 116.63 (C-3′), 135.06 (C-3″). HRMS (ESI+): calcd.: 320.1581 [M+Na]+, found: 320.1525. IR (ATR): ν = 2974, 2939, 2882, 2100, 1464, 1360, 1273, 926, 847. optical rotation: [α]D20 = −47.5 (c = 1.7, CHCl3). TLC: Rf = 0.49 (9:1, iso-hexane–Et2O).
Protected but-3-enyl β-d-5-amino-5-deoxyriboside (21): To a solution of protected but-3-enyl β-d-5-azido-5-deoxyriboside 20 (123 mg, 0.414 mmol) in THF/toluene (1:1, 6 mL), PPh3 (326 mg, 1.24 mmol) and water (373 μL, 20.7 mmol) were added and the mixture was stirred at 50 °C for 13 h. After cooling to rt, di–tert–butyldicarbonate (181 mg, 0.827 mmol) and NaHCO3 (70.0 mg, 0.830 mmol) were added and the mixture was stirred at rt for 1.5 h. It was then diluted with EtOAc (150 mL), washed with water (150 mL) and brine (150 mL) and dried over Na2SO4. The solvent was evaporated under reduced pressure, and the resultant crude product was purified by column chromatography (9:1, iso-hexane–EtOAc) to give 21 as a colourless oil (150 mg, 98%). 1H NMR (500 MHz, C6D6): δ [ppm] = 0.78 (t, J = 7.5 Hz, 3H, 1′-H), 0.99 (t, J = 7.4 Hz, 3H, 5′-H), 1.41 (q, J = 7.5 Hz, 2H, 2′-H), 1.43 (s, 9H, OC(CH3)3), 1.69 (q, J = 7.4 Hz, 2H, 4′-H), 2.08–2.20 (m, 2H, 2″-H), 3.11 (dt, J = 9.4, 6.5 Hz, 1H, 1″-Ha), 3.20–3.30 (m, 2H, 5-H), 3.53 (dt, J = 9.4, 6.7 Hz, 1H, 1″-Hb), 4.31 (dd, J = 6.0, 6.0 Hz, 1H, 4-H), 4.46 (d, J = 5.9 Hz, 1H, 3-H), 4.53 (d, J = 5.9 Hz, 1H, 2-H), 4.96 (s, 1H, NH), 4.98–5.05 (m, 2H, 4″-H), 5.08 (s, 1H, 1-H), 5.70 (ddt, J = 17.1, 10.3, 6.8 Hz, 1H, 3″-H). 13C NMR (126 MHz, C6D6): δ [ppm] = 7.75 (C-5′), 8.60 (C-1′), 28.49 (OC(CH3)3), 29.38 (C-4′), 29.62 (C-2′), 34.30 (C-2″), 44.18 (C-5), 67.43 (C-1″), 78.88 (OC(CH3)3), 82.79 (C-3), 86.45 (C-2), 86.76 (C-4), 109.32 (C-1), 116.58 (C-3′), 116.79 (C-4″), 135.06 (C-3″), 156.01 (Boc-C=O). HRMS (ESI+): calcd.: 394.2200 [M+Na]+, found: 394.2213. IR (ATR): ν = 2974, 1716, 1699, 1514, 1365, 1248, 1167, 924, 872. optical rotation: [α]D20 = −30.5 (c = 1.9, CHCl3). TLC: Rf = 0.15 (9:1, iso-hexane–Et2O).
Protected 3-oxopropyl β-d-5-amino-5-deoxyriboside (22): Ozone was bubbled through a solution of protected but-3-enyl β-d-5-amino-5-deoxyriboside 21 (106 mg, 0.269 mmol) in CH2Cl2 (3 mL), MeOH (26 mL) and pyridine (87 μL, 1.1 mmol) at −78 °C for 15 min. Nitrogen was then bubble through the solution at −78 °C for 45 min, and dimethyl sulfide (198 μL, 2.69 mmol) was added. The reaction mixture was stirred overnight and slowly warmed to rt during this period. The solvent was evaporated under reduced pressure, and the resultant crude product was purified by column chromatography (7:3, iso-hexane–EtOAc) to give 22 as a colourless oil (87.0 mg, 86%). 1H NMR (500 MHz, C6D6, 70 °C): δ [ppm] = 0.79 (t, J = 7.4 Hz, 3H, 1′-H), 0.96 (t, J = 7.4 Hz, 3H, 5′-H), 1.40–1.48 (m, 2H, 2′-H), 1.43 (s, 9H, OC(CH3)3), 1.68 (q, J = 7.4 Hz, 2H, 4′-H), 2.01–2.13 (m, 2H, 2″-H), 3.12–3.19 (m, 2H, 5-H), 3.23 (ddd, J = 10.1, 6.6, 5.3 Hz, 1H, 1″-Ha), 3.68 (ddd, J = 10.1, 6.8, 5.4 Hz 1H, 1″-Hb), 4.29 (dd, J = 6.4, 6.3 Hz, 1H, 4-H), 4.42 (d, J = 6.1 Hz, 1H, 3-H), 4.45 (d, J = 6.1 Hz, 1H, 2-H), 4.84 (s, 1H, NH), 4.99 (s, 1H, 1-H), 9.33 (dd, J = 1.6, 1.5 Hz, 1H, 3″-H). 13C NMR (126 MHz, C6D6, 70 °C): δ [ppm] = 7.19 (C-5′), 7.89 (C-1′), 28.06 (OC(CH3)3), 29.21 (C-4′), 29.33 (C-2′), 43.20 (C-2″), 44.04 (C-5), 61.30 (C-1″), 78.66 (OC(CH3)3), 82.47 (C-3), 86.01 (C-2), 86.45 (C-4), 109.15 (C-1), 116.42 (C-3′), 155.61 (Boc-C=O), 198.24 (C-3″). HRMS (ESI+): calcd.: 396.1993 [M+Na]+, found: 396.2005. IR (ATR): ν = 1713, 1695, 1518, 1365, 1257, 1167, 1011, 924, 791. optical rotation: [α]D20 = −17.6 (c = 1.7, CHCl3). TLC: Rf = 0.15 (7:3, iso-hexane–EtOAc).
Protected 3-hydroxypropyl β-d-5-amino-5-deoxyriboside (23): Ozone was bubbled through a solution of protected but-3-enyl β-d-5-amino-5-deoxyriboside 21 (70.0 mg, 19.0 μmol) in CH2Cl2 (3 mL), MeOH (26 mL) and pyridine (61 μL, 0.75 mmol) at −78 °C for 30 min. Dimethyl sulfide (138 μL, 1.88 mmol) was then added. The reaction mixture was stirred overnight and slowly warmed to rt during this period. The solvent was evaporated under reduced pressure, and the resultant crude aldehyde 22 was dissolved in MeOH (10 mL). At 0 °C, sodium borohydride (34.0 mg, 1.90 mmol) was added and the mixture was stirred at rt for 30 min. After addition of sat. NH4Cl solution, the aqueous layer was extracted with EtOAc (3 × 30 mL). The combined organics were washed with water (2 × 20 mL) and dried over Na2SO4. The solvent was evaporated under reduced pressure, and the resultant crude product was purified by column chromatography (65:35, iso-hexane-EtOAc) to give 23 as a colourless oil (66.0 mg, 95%). 1H NMR (500 MHz, C6D6): δ [ppm] = 0.80 (t, J = 7.5 Hz, 3H, 1′-H), 0.99 (t, J = 7.5 Hz, 3H, 5′-H), 1.38–1.47 (m, 2H, 2′-H), 1.43 (s, 9H, OC(CH3)3), 1.51–1.59 (m, 2H, 2″-H), 1.69 (q, J = 7.4 Hz, 2H, 4′-H), 3.14 (ddd, J = 9.6, 5.5, 5.5 Hz, 1H, 1″-Ha), 3.19–3.26 (m, 2H, 5-H), 3.44–3.51 (m, 1H, 3″-Ha), 3.51–3.58 (m, 1H, 3″-Hb), 3.72–3.79 (m, 1H, 1″-Hb), 4.32 (dd, J = 6.6, 5.9 Hz, 1H, 4-H), 4.41 (d, J = 6.0 Hz, 1H, 3-H), 4.50 (d, J = 6.0 Hz, 1H, 2-H), 5.05 (s, 1H, NH), 5.10 (s, 1H, 1-H). 13C NMR (126 MHz, C6D6): δ [ppm] = 7.76 (C-5′), 8.60 (C-1′), 28.48 (OC(CH3)3), 29.39 (C-4′), 29.63 (C-2′), 32.60 (C-2″), 44.42 (C-5), 59.43 (C-3″), 64.87 (C-1″), 79.09 (OC(CH3)3), 82.76 (C-3), 86.42 (C-2), 86.65 (C-4), 109.38 (C-1), 116.64 (C-3′), 156.23 (Boc-C=O). HRMS (ESI+): calcd.: 398.2149 [M+Na]+, found: 398.2130. IR (ATR): ν = 2936, 1689, 1365, 1250, 1167, 1088, 1012, 972, 924. optical rotation: [α]D20 = −30.2 (c = 1.7, CHCl3). TLC: Rf = 0.12 (7:3, iso-hexane–EtOAc).
Protected 3-(tosyloxy)propyl β-d-5-amino-5-deoxyriboside (24): To a solution of protected 3-hydroxypropyl β-d-5-amino-5-deoxyriboside 23 (77.0 mg, 0.205 mmol) in CH2Cl2 (5 mL) at 0 °C, pyridine (38.0 μL, 0.472 mmol) and tosyl chloride (50.8 mg, 0.267 mmol) were added. The mixture was slowly warmed to rt and then stirred at rt for 3 d. Water (20 mL) was added, and the aqueous layer was extracted with CH2Cl2 (2 × 30 mL). The combined organics were washed with brine (30 mL) and dried over Na2SO4. The solvent was evaporated under reduced pressure, and the resultant crude product was purified by column chromatography (4:1, iso-hexane–EtOAc) to give 24 as a colourless oil (86.0 mg, 79%). 1H NMR (500 MHz, C6D6): δ [ppm] = 0.78 (t, J = 7.5 Hz, 3H, 1′-H), 0.97 (t, J = 7.5 Hz, 3H, 5′-H), 1.41 (q, J = 7.5 Hz, 2H, 2′-H), 1.43 (s, 9H, OC(CH3)3), 1.44–1.52 (m, 2H, 2″-H), 1.67 (q, J = 7.5 Hz, 2H, 4′-H), 1.88 (s, 3H, aryl–CH3), 2.92 (ddd, J = 9.8, 6.3, 6.3 Hz, 1H, 1″-Ha), 3.02–3.10 (m, 1H, 3″-Ha), 3.12–3.20 (m, 1H, 3″-Hb), 3.37 (ddd, J = 9.8, 5.7, 5.7 Hz, 1H, 1″-Hb), 3.90–3.99 (m, 2H, 5-H), 4.22 (dd, J = 6.3, 6.3 Hz, 1H, 4-H), 4.33 (d, J = 6.0 Hz, 1H, 3-H), 4.38 (d, J = 6.0 Hz, 1H, 2-H), 4.67 (dd, J = 5.4, 5.4 Hz, 1H, NH), 4.85 (s, 1H, 1-H), 6.77 (d, J = 8.2 Hz, 2H, 3‴-H, 5‴-H), 7.77 (d, J = 8.2 Hz, 2H, 2‴-H, 6‴-H). 13C NMR (126 MHz, C6D6): δ [ppm] = 7.74 (C-5′), 8.60 (C-1′), 21.18 (aryl–CH3), 28.46 (OC(CH3)3), 29.26 (C-2″), 29.38 (C-4′), 29.65 (C-2′), 44.14 (C-5), 63.25 (C-3″), 67.13 (C-1″), 79.04 (OC(CH3)3), 82.70 (C-3), 86.20 (C-2), 86.75 (C-4), 109.05 (C-1), 116.61 (C-3′), 128.20 (C-2‴, C-6‴), 129.87 (C-3‴, C-5‴), 134.49 (C-4‴), 144.24 (C-1‴), 155.94 (NC(=O)O). HRMS (ESI+): calcd.: 552.2238 [M+Na]+, found: 552.2237. IR (ATR): ν = 1713, 1364, 1174, 1095, 924, 837, 814, 752, 663. UV (MeCN): λmax = 225, 262, 273. optical rotation: [α]D20 = −22.8 (c = 2.0, CHCl3). TLC: Rf = 0.18 (4:1, iso-hexane–EtOAc).
Protected 6′-N-substituted aminoribosylated (6′S)-nucleosyl amino acid (26): To a solution of the protected (6′S)-nucleosyl amino acid 25 (83.0 mg, 0.142 mmol) [35,36] in THF (6 mL) over molecular sieves (4 Å), aldehyde 22 (63.0 mg, 0.169 mmol) was added and the mixture was stirred at rt for 24 h. Amberlyst™ 15 (6.6 mg, 31 μmol) and sodium triacetoxyborohydride (60.0 mg, 0.284 mmol) were added, and the mixture was stirred at rt for 22 h. It was then filtered, the filtrate was diluted with EtOAc (100 mL) and washed with sat. Na2CO3 solution (100 mL). The aqueous layer was extracted with EtOAc (3 × 50 mL), and the combined organics were dried over Na2SO4. The solvent was evaporated under reduced pressure, and the resultant crude product was purified by column chromatography (97.5:2.5, CH2Cl2-MeOH) to give 26 as a colourless solid (101 mg, 76%). 1H NMR (500 MHz, CD3OD): δ [ppm] = 0.09 (s, 3H, SiCH3), 0.10 (s, 3H, SiCH3), 0.12 (s, 3H, SiCH3), 0.13 (s, 3H, SiCH3), 0.87 (t, J = 7.5 Hz, 3H, 1iv-H), 0.89 (t, J = 7.5 Hz, 3H, 5iv-H), 0.91 (s, 9H, SiC(CH3)3), 0.93 (s, 9H, SiC(CH3)3), 1.44 (s, 9H, NC(=O)OC(CH3)3), 1.49 (s, 9H, C(=O)OC(CH3)3), 1.58 (q, J = 7.5 Hz, 2H, 2iv-H), 1.67 (q, J = 7.5 Hz, 2H, 4iv-H), 1.70–1.82 (m, 2H, 2″-H), 1.86–1.94 (m, 1H, 5′-Ha), 2.00–2.08 (m, 1H, 5′-Hb), 2.58–2.65 (m, 1H, 1″-Ha), 2.65–2.72 (m, 1H, 1″-Hb), 3.17 (d, J = 7.2 Hz, 2H, 5‴-H), 3.34 (dd, J = 9.2, 4.7 Hz, 1H, 6′-H), 3.41–3.47 (m, 1H, 3″-Ha), 3.74–3.80 (m, 1H, 3″-Hb), 3.91 (dd, J = 4.6, 4.4 Hz, 1H, 3′-H), 4.04–4.10 (m, 1H, 4′-H), 4.16 (dd, J = 7.2, 7.2 Hz, 1H, 4‴-H), 4.33 (dd, J = 4.4, 4.1 Hz, 1H, 2′-H), 4.60 (d, J = 6.0 Hz, 1H, 3‴-H), 4.67 (d, J = 6.0 Hz, 1H, 2‴-H), 5.01 (s, 1H, 1‴-H), 5.75 (d, J = 8.1 Hz, 1H, 5-H), 5.78 (d, J = 4.1 Hz, 1H, 1′-H), 7.65 (d, J = 8.1 Hz, 1H, 6-H). 13C NMR (126 MHz, CD3OD): δ [ppm] = −4.40 (SiCH3), –4.39 (SiCH3), –4.39 (SiCH3), −3.98 (SiCH3), 7.75 (C-5iv), 8.72 (C-1iv), 18.88 (SiC(CH3)3), 18.94 (SiC(CH3)3, 26.40 (SiC(CH3)3), 26.47 (SiC(CH3)3), 28.47 (NC(=O)OC(CH3)3), 28.79 (C(=O)OC(CH3)3), 29.92 (C-4iv), 30.31 (C-2iv), 30.54 (C-2″), 38.00 (C-5′), 44.63 (C-5‴), 46.10 (C-1″), 60.70 (C-6′), 67.13 (C-3″), 75.92 (C-2′), 76.60 (C-3′), 80.28 (NC(=O)OC(CH3)3), 82.57 (C(=O)OC(CH3)3), 82.92 (C-4′), 83.83 (C-3‴), 87.09 (C-2‴), 87.20 (C-4‴), 91.87 (C-1′), 103.04 (C-5), 110.00 (C-1‴), 117.68 (C-3iv), 142.68 (C-6), 152.15 (C-2), 158.31 (Boc-C=O), 166.01 (C-4), 174.84 (C-7′). HRMS (ESI+): calcd.: 943.5490 [M+H]+, found: 943.5486. IR (ATR): ν = 2931, 2858, 1692, 1561, 1366, 1251, 1157, 1090, 836, 776. UV (MeCN): λmax = 261. optical rotation: [α]D20 = −15.0 (c = 1.0, CHCl3). m.p. = 62 °C. TLC: Rf = 0.30 (94:6, CH2Cl2-MeOH).
Protected 6′-N-substituted aminoribosylated truncated N-Cbz-muraymycin analogue (28): To a solution of the protected 6′-N-substituted aminoribosylated (6′S)–nucleosyl amino acid 26 (88.0 mg, 93.3 μmol) in THF (6 mL) over molecular sieves (4 Å), aldehyde 27 (33.0 mg, 103 μmol) [20] was added and the mixture was stirred at rt for 39 h. Amberlyst™ 15 (4.3 mg, 21 μmol) and sodium triacetoxyborohydride (39.3 mg, 187 μmol) were added, and the mixture was stirred at rt for 15 h. More aldehyde 27 (33.0 mg, 103 μmol) and, after 4 h, more sodium triacetoxyborohydride (39.3 mg, 187 μmol) was added. The mixture was stirred at rt for 18 h, then more sodium triacetoxyborohydride (39.3 mg, 187 μmol) was added and stirring at rt was continued for 2 h. The mixture was then filtered, the filtrate was diluted with EtOAc (100 mL) and washed with sat. Na2CO3 solution (70 mL). The aqueous layer was extracted with EtOAc (3 × 50 mL), and the combined organics were dried over Na2SO4. The solvent was evaporated under reduced pressure, and the resultant crude product was purified by column chromatography (1. 98.2:1.8→98:2, CH2Cl2-MeOH, 2. 98:2, CH2Cl2-MeOH) to give 28 as a colourless solid (81.6 mg, 70%). 1H NMR (500 MHz, CD3OD): δ [ppm] = 0.06 (s, 3H, SiCH3), 0.10 (s, 3H, SiCH3), 0.13 (s, 3H, SiCH3), 0.15 (s, 3H, SiCH3), 0.86 (t, J = 7.5 Hz, 3H, 1vii-H), 0.88–0.93 (m, 3H, 5vii-H), 0.90 (s, 9H, SiC(CH3)3), 0.94 (s, 9H, SiC(CH3)3), 0.92–0.97 (m, 6H, 5‴-H), 1.44 (s, 9H, NC(=O)OC(CH3)3), 1.48 (s, 9H, C(=O)OC(CH3)3), 1.51–1.57 (m, 2H, 3‴-H), 1.57 (q, J = 7.5 Hz, 2H, 2vii-H), 1.67 (q, J = 7.5 Hz, 2H, 4vii-H), 1.62–1.75 (m, 5H, 2″-H, 4‴-H, 2v-H), 1.77–1.85 (m, 1H, 5′-Ha), 2.17–2.27 (m, 1H, 5′-Hb), 2.52–2.62 (m, 2H, 1″-Ha, 1v-Ha), 2.64–2.67 (m, 2H, 1″-Hb, 1v-Hb), 3.13–3.19 (m, 2H, 5vi-H), 3.22 (dd, J = 6.7, 6.7 Hz, 2H, 3″-H), 3.40–3.47 (m, 1H, 3v-Ha), 3.50 (dd, J = 10.6, 3.5 Hz, 1H, 6′-H), 3.66–3.73 (m, 1H, 3v-Hb), 3.94 (dd, J = 4.5, 4.1 Hz, 1H, 3′-H), 3.96–4.02 (m, 1H, 4′-H), 4.12 (dd, J = 8.9, 7.7 Hz, 1H, 2‴-H), 4.15 (dd, J = 7.3, 6.8 Hz, 1H, 4vi-H), 4.40 (dd, J = 4.8, 4.5 Hz, 1H, 2′-H), 4.59 (d, J = 6.0 Hz, 1H, 3vi-H), 4.67 (d, J = 6.0 Hz, 1H, 2vi-H), 5.01 (s, 1H, 1vi-H), 5.07 (d, J = 12.5 Hz, 1H, 1iv-Ha), 5.11 (d, J = 12.5 Hz, 1H, 1iv-Hb), 5.76 (d, J = 8.1 Hz, 1H, 5-H), 5.78 (d, J = 4.8 Hz, 1H, 1′-H), 7.24–7.38 (m, 5H, 3iv-H, 4iv-H, 5iv-H, 6iv-H, 7iv-H,), 7.61 (d, J = 8.1 Hz, 1H, 6-H). 13C NMR (126 MHz, CD3OD): δ [ppm] = −4.43 (SiCH3), –4.31 (SiCH3), –4.23 (SiCH3), −3.98 (SiCH3), 7.80 (C-5vii), 8.74 (C-1vii), 18.86 (SiC(CH3)3), 19.00 (SiC(CH3)3), 22.06 (Ca-5‴), 23.48 (Cb-5‴), 25.95 (C-4‴), 26.39 (SiC(CH3)3), 26.49 (SiC(CH3)3), 28.68 (C(=O)OC(CH3)3), 28.81 (NC(=O)OC(CH3)3), 29.26 (C-2″), 29.93 (C-4vii), 30.13 (C-2v), 30.33 (C-2vii), 35.38 (C-5′), 38.59 (C-3″), 42.36 (C-3‴), 44.67 (C-5vi), 49.28 (C-1v), 49.97 (C-1″), 55.12 (C-2‴), 61.91 (C-6′), 66.82 (C-3v), 67.73 (C-1iv), 75.63 (C-2′), 76.96 (C-3′), 80.27 (NC(=O)OC(CH3)3), 82.60 (C(=O)OC(CH3)3), 83.35 (C-4′), 83.88 (C-2vi), 87.03 (C-3vi), 87.10 (C-4vi), 91.76 (C-1′), 103.23 (C-5), 110.00 (C-1vi), 117.67 (C-3vii), 128.88, 129.04, 129.49 (C-3iv, C-4iv, C-5iv, C-6iv C-7iv), 138.18 (C-2iv), 142.84 (C-6), 152.15 (C-2), 158.28, 158.34 (Cbz–C=O, Boc-C=O), 165.94 (C-4), 172.89, 175.19 (C-7′, C-1‴). HRMS (ESI+): calcd.: 1247.7277 [M+H]+, found: 1247.7252. IR (ATR): ν = 2929, 2857, 1692, 1366, 1258, 1153, 1087, 1043, 836, 777. UV (MeCN): λmax = 260. optical rotation: [α]D20 = −5.0 (c = 1.0, CHCl3). m.p. = 75 °C. TLC: Rf = 0.26 (96:4, CH2Cl2-MeOH).
Protected 6′-N-substituted aminoribosylated truncated muraymycin analogue (29): To a solution of the protected 6′-N-substituted aminoribosylated truncated N-Cbz–muraymycin analogue 28 (28.2 mg, 22.6 μmol) in i-PrOH (3 mL), 1,4–cyclohexadiene (21.0 μL, 226 μmol) and Pd black (10.0 mg, 94.0 μmol) were added and the mixture was stirred at rt for 1.5 h. It was then filtered through a syringe filter, and the syringe filter was washed with i-PrOH (4 × 5 mL). The solvent of the combined filtrates was evaporated under reduced pressure to give 29 as a colourless wax (25.2 mg, quant.). 1H NMR (500 MHz, CD3OD): δ [ppm] = 0.07 (s, 3 H, SiCH3), 0.10 (s, 3H, SiCH3), 0.13 (s, 3H, SiCH3), 0.15 (s, 3H, SiCH3), 0.87 (t, J = 7.5 Hz, 3H, 1vi-H), 0.89 (t, J = 7.5 Hz, 3H, 5vi-H), 0.90 (s, 9H, SiC(CH3)3), 0.95 (s, 9H, SiC(CH3)3), 0.95–1.02 (m, 6H, 5‴-H), 1.45 (s, 9H, NC(=O)OC(CH3)3), 1.49 (s, 9H, C(=O)OC(CH3)3), 1.58 (q, J = 7.5 Hz, 2H, 2vi-H), 1.67 (q, J = 7.5 Hz, 2H, 4vi-H), 1.63–1.77 (m, 7H, 2″-H, 3‴-H, 4‴-H, 2iv-H), 1.77–1.85 (m, 1H, 5′-Ha), 2.17–2.26 (m, 1H, 5′-Hb), 2.56–2.65 (m, 2H, 1″-Ha, 1iv-Ha), 2.66–2.78 (m, 2H, 1″-Hb, 1iv-Hb), 3.13–3.26 (m, 3H, 3″-Ha, 5v-H), 3.33–3.41 (m, 1H, 3″-Hb), 3.42–3.49 (m, 1H, 3iv-Ha), 3.51 (dd, J = 10.5, 3.5 Hz, 1H, 6′-H), 3.68–3.75 (m, 1H, 3iv-Hb), 3.76–3.81 (m, 1H, 2‴-H), 3.95 (dd, J = 4.4, 4.1 Hz, 1H, 3′-H), 3.96–4.01 (m, 1H, 4′-H), 4.13–4.19 (m, 1H, 4v-H), 4.43 (dd, J = 4.8, 4.4 Hz, 1H, 2′-H), 4.58–4.62 (m, 1H, 3v-H), 4.68 (d, J = 6.0 Hz, 1H, 2v-H), 4.99–5.02 (m, 1H, 1v-H), 5.76 (d, J = 4.8 Hz, 1H, 1′-H), 5.77 (d, J = 8.0 Hz, 1H, 5-H), 7.60 (d, J = 8.0 Hz, 1H, 6-H). 13C NMR (126 MHz, CD3OD): δ [ppm] = −4.43 (SiCH3), –4.34 (SiCH3), –4.27 (SiCH3), –3.98 (SiCH3), 7.79 (C-5vi), 8.73 (C-1vi), 18.86 (SiC(CH3)3), 19.00 (SiC(CH3)3), 22.29 (Ca-5‴), 23.08 (Cb-5‴), 25.56 (C-4‴), 26.38 (SiC(CH3)3), 26.48 (SiC(CH3)3), 28.67 (C(=O)OC(CH3)3), 28.81 (NC(=O)OC(CH3)3), 29.42 (C-2″), 29.93 (C-4vi), 30.06 (C-2iv), 30.32 (C-2vi), 35.10 (C-5′), 39.07 (C-3″), 41.99 (C-3‴), 44.63 (C-5v), 50.05 (C-1iv), 50.27 (C-1″), 53.25 (C-2‴), 62.04 (C-6′), 66.83 (C-3iv), 75.61 (C-2′), 76.99 (C-3′), 80.30 (NC(=O)OC(CH3)3), 82.69 (C(=O)OC(CH3)3), 83.29 (C-4′), 83.89 (C-2v), 87.04 (C-3v), 87.12 (C-4v), 92.13 (C-1′), 103.15 (C-5), 109.91 (C-1v), 117.71 (C-3vi), 142.95 (C-6), 152.09 (C-2), 158.32 (Boc-C=O), 166.04 (C-4), 170.68, 172.93 (C-7′, C-1‴). HRMS (ESI+): calcd.: 1113.6897 [M+H]+, found: 1113.6909. IR (ATR): ν = 2961, 2929, 1695, 1366, 1258, 1149, 1085, 1014, 867, 797. UV (MeCN): λmax = 260. optical rotation: [α]D20 = −53.8 (c = 1.3, CHCl3). TLC: Rf = 0.10 (95:5, CH2Cl2-MeOH).
Protected uracil-N-3-substituted aminoribosylated truncated N-Cbz-muraymycin analogue (33): To a solution of protected truncated muraymycin analogue 32 (81.0 mg, 91.0 μmol) [36] in MeCN (10 mL), tosylate 24 (48.2 mg, 91.0 μmol) and K2CO3 (13.8 mg, 100 μmol) were added. Over a period of 8 h, the mixture was stirred and slowly heated to reflux, and then it was stirred under reflux for 16 h. After cooling to rt, it was diluted with EtOAc (150 mL) and washed with water (100 mL). The organic layer was dried over Na2SO4, and the solvent was evaporated under reduced pressure. The resultant crude product was purified by column chromatography (98:2, CH2Cl2-MeOH) to give 33 as a colourless solid (79.1 mg, 70%). 1H NMR (500 MHz, CD3OD): δ [ppm] = 0.11 (s, 3H, SiCH3), 0.11 (s, 3H, SiCH3), 0.11 (s, 3H, SiCH3), 0.13 (s, 3H, SiCH3), 0.87–0.92 (m, 3H, 5vii-H), 0.88 (t, J = 7.5 Hz, 3H, 1vii-H), 0.89–0.96 (m, 6H, 5‴-H), 0.91 (s, 9H, SiC(CH3)3), 0.93 (s, 9H, SiC(CH3)3), 1.44 (s, 9H, NC(=O)OC(CH3)3), 1.49 (s, 9H, C(=O)OC(CH3)3), 1.51–1.56 (m, 2H, 3‴-H), 1.58 (q, J = 7.5 Hz, 2H, 2vii-H), 1.64–1.72 (m, 3H, 2″-H, 4‴-H), 1.67 (q, J = 7.5 Hz, 2H, 4vii-H), 1.83–1.90 (m, 2H, 2v-H), 1.88–1.96 (m, 1H, 5′-Ha), 2.02–2.10 (m, 1H, 5′-Hb), 2.52–2.59 (m, 1H, 1″-Ha), 2.60–2.68 (m, 1H, 1″-Hb), 3.13–3.18 (m, 2H, 5vi-H), 3.21–3.29 (m, 2H, 3″-H), 3.25 (dd, J = 9.7, 4.3 Hz, 1H, 6′-H), 3.39–3.46 (m, 1H, 3v-Ha), 3.72–3.78 (m, 1H, 3v-Hb), 3.85 (dd, J = 5.3, 4.3 Hz, 1H, 3′-H), 4.00 (dd, J = 6.9, 6.9 Hz, 2H, 1v-H) 4.07–4.13 (m, 2H, 4′-H, 2‴-H), 4.16 (dd, J = 7.1, 7.1 Hz, 1H, 4vi-H), 4.31 (dd, J = 4.3, 3.6 Hz, 1H, 2′-H), 4.55 (d, J = 6.0 Hz, 1H, 3vi-H), 4.66 (d, J = 6.0 Hz, 1H, 2vi-H), 5.00 (s, 1H, 1vi-H), 5.05–5.12 (m, 2H, 1iv-H), 5.80 (d, J = 3.6 Hz, 1H, 1′-H), 5.84 (d, J = 8.0 Hz, 1H, 5-H), 7.25–7.38 (m, 5H, C-3iv, C-4iv, C-5iv, C-6iv, C-7iv), 7.64 (d, J = 8.1 Hz, 1H, 6-H). 13C NMR (126 MHz, CD3OD): δ [ppm] = −4.47 (SiCH3), –4.41 (SiCH3), –4.17 (SiCH3), –3.93 (SiCH3), 7.77 (C-5vii), 8.81 (C-1vii), 18.91 (SiC(CH3)3), 18.93 (SiC(CH3)3), 22.01 (Ca-5‴), 23.44 (Cb-5‴), 25.94 (C-4‴), 26.47 (SiC(CH3)3), 26.48 (SiC(CH3)3), 28.47 (C(=O)OC(CH3)3), 28.67 (C-2v), 28.78 (NC(=O)OC(CH3)3), 29.91 (C-4vii), 30.35 (C-2vii), 30.73 (C-2″), 37.95 (C-5′), 38.20 (C-3″), 40.04 (C-1v), 42.28 (C-3‴), 44.62 (C-5vi), 46.09 (C-1″), 55.23 (C-2‴), 60.75 (C-6′), 67.11 (C-3v), 67.72 (C-1iv), 75.21 (C-2′), 76.52 (C-3′), 80.27 (NC(=O)OC(CH3)3), 82.24 (C-4′), 83.03 (C(=O)OC(CH3)3), 83.84 (C-2vi), 87.05 (C-3vi), 87.15 (C-4vi), 92.75 (C-1′), 102.41 (C-5), 110.14 (C-1vi), 117.64 (C-3vii), 128.88, 129.04, 129.48 (C-3iv, C-4iv, C-5iv, C-6iv, C-7iv), 138.18 (C-2iv), 140.52 (C-6), 152.33 (C-2), 158.32, 158.35 (Boc-C=O, Cbz–C=O), 164.83 (C-4), 174.56, 175.49 (C-7′, C-1‴). HRMS (ESI+): calcd.: 1247.7277 [M+H]+, found: 1247.7292. IR (ATR): ν = 2959, 2930, 2857, 1708, 1663, 1258, 1156, 1091, 802, 778. UV (MeCN): λmax = 262. optical rotation: [α]D20 = −4.5 (c = 1.1, CHCl3). m.p. = 66 °C. TLC: Rf = 0.13 (96:4, CH2Cl2-MeOH).
4.2. Overexpression of MraY from S. aureus
The overexpression of MraY was performed as described before [33,34,43].
4.3. Fluorescence-Based MraY Assay
The assay was performed as described before [33,34,43].
5. Conclusions
In summary, we have successfully synthesised chemically tractable aminoribosylated muraymycin analogues 13 and 15 alongside the non-aminoribosylated reference compounds 14 and 16. The design of the aminoribosylated target structures, particularly of 13, has been inspired by an available X-ray co-crystal structure of the bacterial target protein MraY in complex with the naturally occurring inhibitor muraymycin D2 9. Constrasting the expectations, analogue 13 (with attachment of the aminoribosyl-linker unit to the 6′-amino group) was not active as an MraY inhibitor, but 6′-substituted non-aminoribosylated reference compound 14 was. The finding that uracil-N-3-derivatised analogues 15 and 16 were inactive as MraY inhibitors was in good agreement with predictions derived from the aforementioned co-crystal structure, but somewhat in contrast to the reported bioactivity of previously described muraymycin analogue 12. The reason for this will be investigated in the context of future work, when we will synthesise and study muraymycin analogues 11 and 12 again to elucidate their interaction with MraY.
Taken together, the results for 13 and 14 suggest that the concept to derivatise the 6′-amino group of the muraymycin scaffold should be explored further in future work. In particular, it needs to be investigated if the introduction of more bulky substituents (such as an aminoribose unit) at this position is generally precluded, or if an approriate choice of linker length and architecture might furnish bioactive analogues. This will lead to valuable further SAR insights for muraymycins and will support the development of optimised analogues of this promising class of antibacterials. Work along this line is on the way in our laboratories.
Supplementary Materials
The following are available online at http://www.mdpi.com/1420-3049/23/12/3085/s1, Figure S1, copies of NMR spectra.
Author Contributions
Conceptualization, C.D.; Formal analysis, D.W., S.K. and C.D.; Funding acquisition, C.D.; Investigation, D.W. and S.K.; Project administration, C.D.; Supervision, C.D.; Writing–original draft, D.W. and C.D.; Writing, review & editing, D.W., S.K. and C.D.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, grant DU 1095/5-1) and the Konrad-Adenauer-Stiftung (doctoral fellowship to D.W.). The APC was funded by the Deutsche Forschungsgemeinschaft (DFG, grant DU 1095/5-1).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taubes, G. The bacteria fight back. Science 2008, 321, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Shlaes, D. Fix the antibiotics pipeline. Nature 2011, 472, 32. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C. Where will new antibiotics come from? Nat. Rev. Microbiol. 2003, 1, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.B.; Gomez-Castellanos, J.R.; Henry, L.; Ducho, C.; McDonough, M.A.; Schofield, C.J. The enzymes of β-lactam biosynthesis. Nat. Prod. Rep. 2013, 30, 21–107. [Google Scholar] [CrossRef] [PubMed]
- Dini, C. MraY Inhibitors as Novel Antibacterial Agents. Curr. Top. Med. Chem. 2005, 5, 1221–1236. [Google Scholar] [CrossRef] [PubMed]
- Bugg, T.D.H.; Lloyd, A.J.; Roper, D.I. Phospho-MurNAc-pentapeptide translocase (MraY) as a target for antibacterial agents and antibacterial proteins. Infect. Disord. Drug Targets 2006, 6, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Struve, W.G.; Neuhaus, F.C. Evidence for an initial acceptor of UDP-NAc-muramyl-pentapeptide in the synthesis of bacterial mucopeptide. Biochem. Biophys. Res. Commun. 1965, 18, 6–12. [Google Scholar] [CrossRef]
- Anderson, J.S.; Matsuhashi, M.; Haskin, M.A.; Strominger, J.L. Lipid-phosphoacetylmuramyl-pentapeptide and lipid-phosphodisaccharide-pentapeptide: Presumed membrane transport intermediates in cell wall synthesis. Proc. Natl. Acad. Sci. USA 1965, 53, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Heydanek, M.G., Jr.; Struve, W.G.; Neuhaus, F.C. Initial state in peptidoglycan synthesis. III. Kinetics and uncoupling of phospho-N-acetylmuramyl-pentapeptide translocase (uridine 5′-phosphate). Biochemistry 1969, 8, 1214–1221. [Google Scholar] [CrossRef]
- Ikeda, M.; Wachi, M.; Jung, H.K.; Ishino, F.; Matsuhashi, M. The Escherichia coli mraY gene encoding UDP-N-acetylmuramoyl-pentapeptide: Undecaprenyl-phosphate phospho-N-acetylmuramoyl-pentapeptide transferase. J. Bacteriol. 1991, 173, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Boyle, D.S.; Donachie, W.D. MraY is an essential gene for cell growth in Escherichia coli. J. Bacteriol. 1998, 180, 6429–6432. [Google Scholar] [PubMed]
- Vollmer, W.; Blanot, D.; Pedro, M.A.D. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.; Goss, R.J.M.; Kimura, K.; Bugg, T.D.H. Antimicrobial nucleoside antibiotics targeting cell wall assembly: Recent advances in structure–function studies and nucleoside biosynthesis. Nat. Prod. Rep. 2010, 27, 279–304. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Yamaguchi, M.; Matsuda, A. Antibacterial nucleoside natural products inhibiting phospho-MurNAc-pentapeptide translocase; chemistry and structure-activity relationship. Curr. Med. Chem. 2015, 22, 3951–3979. [Google Scholar] [CrossRef] [PubMed]
- Wiegmann, D.; Koppermann, S.; Wirth, M.; Niro, G.; Leyerer, K.; Ducho, C. Muraymycin nucleoside-peptide antibiotics: Uridine-derived natural products as lead structures for the development of novel antibacterial agents. Beilstein J. Org. Chem. 2016, 12, 769–795. [Google Scholar] [CrossRef] [PubMed]
- Bugg, T.D.H.; Rodolis, M.T.; Mihalyi, A.; Jamshidi, S. Inhibition of phospho-MurNAc-pentapeptide translocase (MraY) by nucleoside natural product antibiotics, bacteriophage ϕX174 lysis protein E, and cationic antibacterial peptides. Bioorg. Med. Chem. 2016, 24, 6340–6347. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.A.; Barbieri, L.R.; Carter, G.T.; Lenoy, E.; Lotvin, J.; Petersen, P.J.; Siegel, M.M.; Singh, G.; Williamson, R.T. Structures of the muraymycins, novel peptidoglycan biosynthesis inhibitors. J. Am. Chem. Soc. 2002, 124, 10260–10261. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wang, X.; Koppermann, S.; Thorson, J.S.; Ducho, C.; Van Lanen, S.G. Antibacterial muraymycins from mutant strains of Streptomyces sp. NRRL 30471. J. Nat. Prod. 2018, 81, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Spork, A.P.; Büschleb, M.; Ries, O.; Wiegmann, D.; Boettcher, S.; Mihalyi, A.; Bugg, T.D.H.; Ducho, C. Lead structures for new antibacterials: stereocontrolled synthesis of a bioactive muraymycin analogue. Chem. Eur. J. 2014, 20, 15292–15297. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Norton, E.; Petersen, P.J.; Rasmussen, B.A.; Singh, G.; Yang, Y.; Mansour, T.S.; Ho, D.M. Muraymycins, novel peptidoglycan biosynthesis inhibitors: Synthesis and SAR of their analogues. Bioorg. Med. Chem. Lett. 2003, 13, 3345–3350. [Google Scholar] [CrossRef]
- Tanino, T.; Hirano, S.; Ichikawa, S.; Matsuda, A. Synthetic study of muraymycins using Ugi-four component reaction. Nucleic Acids Symp. Ser. 2008, 52, 557–558. [Google Scholar] [CrossRef] [PubMed]
- Tanino, T.; Ichikawa, S.; Shiro, M.; Matsuda, A. Total synthesis of (−)-muraymycin D2 and its epimer. J. Org. Chem. 2010, 75, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Aleiwi, B.A.; Schneider, C.M.; Kurosu, M. Synthesis of ureido-muraymycidine derivatives for structure activity relationship studies of muraymycins. J. Org. Chem. 2012, 77, 3859–3867. [Google Scholar] [CrossRef] [PubMed]
- Mitachi, K.; Aleiwi, B.A.; Schneider, C.M.; Siricilla, S.; Kurosu, M. Stereocontrolled total synthesis of muraymycin D1 having a dual mode of action against Mycobacterium tuberculosis. J. Am. Chem. Soc. 2016, 138, 12975–12980. [Google Scholar] [CrossRef] [PubMed]
- Spork, A.P.; Koppermann, S.; Dittrich, B.; Herbst-Irmer, R.; Ducho, C. Efficient synthesis of the core structure of muraymycin and caprazamycin nucleoside antibiotics based on a stereochemically revised sulfur ylide reaction. Tetrahedron: Asymmetry 2010, 21, 763–766. [Google Scholar] [CrossRef]
- Ries, O.; Ochmann, A.; Ducho, C. Synthesis of N-alkyl-N-hydroxyguanidines: A comparative study using different protecting group strategies. Synthesis 2011, 2357–2368. [Google Scholar]
- Büschleb, M.; Granitzka, M.; Stalke, D.; Ducho, C. A biomimetic domino reaction for the concise synthesis of capreomycidine and epicapreomycidine. Amino Acids 2012, 43, 2313–2328. [Google Scholar] [CrossRef] [PubMed]
- Ries, O.; Büschleb, M.; Granitzka, M.; Stalke, D.; Ducho, C. Amino acid motifs in natural products: Synthesis of O-acylated derivatives of (2S,3S)-3-hydroxyleucine. Beilstein J. Org. Chem. 2014, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-I.; Li, Z.; Francisco, G.D.; McDonald, L.A.; Davis, R.A.; Singh, G.; Yang, Y.; Mansour, T.S. Muraymycins, novel peptidoglycan biosynthesis inhibitors: Semisynthesis and SAR of their derivatives. Bioorg. Med. Chem. Lett. 2002, 12, 2341–2344. [Google Scholar] [CrossRef]
- Tanino, T.; Ichikawa, S.; Al-Dabbagh, B.; Bouhss, A.; Oyama, H.; Matsuda, A. Synthesis and biological evaluation of muraymycin analogues active against anti-drug-resistant bacteria. ACS Med. Chem. Lett. 2010, 1, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Tanino, T.; Al-Dabbagh, B.; Mengin-Lecreulx, D.; Bouhss, A.; Oyama, H.; Ichikawa, S.; Matsuda, A. Mechanistic analysis of muraymycin analogues: A guide to the design of MraY inhibitors. J. Med. Chem. 2011, 54, 8421–8439. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, Y.; Tanino, T.; Sekiguchi, M.; Yonezawa, S.; Sakagami, M.; Takahashi, F.; Togame, H.; Tanaka, Y.; Takemoto, H.; Ichikawa, S.; et al. Expansion of antibacterial spectrum of muraymycins toward Pseudomonas aeruginosa. ACS Med. Chem. Lett. 2014, 5, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Koppermann, S.; Cui, Z.; Fischer, P.D.; Wang, X.; Ludwig, J.; Thorson, J.S.; Van Lanen, S.G.; Ducho, C. Insights into the target interaction of naturally occurring muraymycin nucleoside antibiotics. ChemMedChem 2018, 13, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Spork, A.P.; Koppermann, S.; Schier (née Wohnig), S.; Linder, R.; Ducho, C. Analogues of muraymycin nucleoside antibiotics with epimeric uridine-derived core structures. Molecules 2018, 23, 2868. [Google Scholar] [CrossRef] [PubMed]
- Spork, A.P.; Ducho, C. Novel 5′-deoxy nucleosyl amino acid scaffolds for the synthesis of muraymycin analogues. Org. Biomol. Chem. 2010, 8, 2323–2326. [Google Scholar] [CrossRef] [PubMed]
- Spork, A.P.; Wiegmann, D.; Granitzka, M.; Stalke, D.; Ducho, C. Stereoselective synthesis of uridine-derived nucleosyl amino acids. J. Org. Chem. 2011, 76, 10083–10098. [Google Scholar] [CrossRef] [PubMed]
- Bouhss, A.; Crouvoisier, M.; Blanot, D.; Mengin-Lecreulx, D. Purification and characterization of the bacterial MraY translocase catalyzing the first membrane step of peptidoglycan biosynthesis. J. Biol. Chem. 2004, 279, 29974–29980. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Münch, D.; Schneider, T.; Sahl, H.-G.; Bouhss, A.; Ghoshdastider, U.; Wang, J.; Dötsch, V.; Wang, X.; Bernhard, F. Preparative scale cell-free production and quality optimization of MraY homologues in different expression modes. J. Biol. Chem. 2011, 286, 38844–38853. [Google Scholar] [CrossRef] [PubMed]
- Henrich, E.; Ma, Y.; Engels, I.; Münch, D.; Otten, C.; Schneider, T.; Henrichfreise, B.; Sahl, H.-G.; Dötsch, V.; Bernhard, F. Lipid Requirements for the enzymatic activity of MraY translocases and in vitro reconstitution of the lipid II synthesis pathway. J. Biol. Chem. 2016, 291, 2535–2546. [Google Scholar] [CrossRef] [PubMed]
- Brandish, P.E.; Burnham, M.K.; Lonsdale, J.T.; Southgate, R.; Inukai, M.; Bugg, T.D.H. Slow binding inhibition of phospho-N-acetylmuramyl-pentapeptide-translocase (Escherichia coli) by mureidomycin A. J. Biol. Chem. 1996, 271, 7609–7614. [Google Scholar] [CrossRef] [PubMed]
- Brandish, P.E.; Kimura, K.-I.; Inukai, M.; Southgate, R.; Lonsdale, J.T.; Bugg, T.D.H. Modes of action of tunicamycin, liposidomycin B, and mureidomycin A: Inhibition of phospho-N-acetylmuramyl-pentapeptide translocase from Escherichia coli. Antimicrob. Agents Chemother. 1996, 40, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Stachyra, T.; Dini, C.; Ferrari, P.; Bouhss, A.; van Heijenoort, J.; Mengin-Lecreulx, D.; Blanot, D.; Biton, J.; Le Beller, D. Fluorescence detection-based functional assay for high-throughput screening for MraY. Antimicrob. Agents Chemother. 2004, 48, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Wohnig, S.; Spork, A.P.; Koppermann, S.; Mieskes, G.; Gisch, N.; Jahn, R.; Ducho, C. Total synthesis of dansylated Park’s nucleotide for high-throughput MraY assays. Chem. Eur. J. 2016, 22, 17813–17819. [Google Scholar] [CrossRef] [PubMed]
- Bouhss, A.; Mengin-Lecreulx, D.; Le Beller, D.; Van Heijenoort, J. Topological analysis of the MraY protein catalysing the first membrane step of peptidoglycan synthesis. Mol. Microbiol. 1999, 34, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.C.; Zhao, J.; Gillespie, R.A.; Kwon, D.-Y.; Guan, Z.; Hong, J.; Zhou, P.; Lee, S.-Y. Crystal structure of MraY, an essential membrane enzyme for bacterial cell wall synthesis. Science 2013, 341, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.C.; Mashalidis, E.H.; Tanino, T.; Kim, M.; Matsuda, A.; Hong, J.; Ichikawa, S.; Lee, S.-Y. Structural insights into inhibition of lipid I production in bacterial cell wall synthesis. Nature 2016, 533, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Koppermann, S.; Ducho, C. Natural Products at Work: Structural Insights into Inhibition of the Bacterial Membrane Protein MraY. Angew. Chem. Int. Ed. 2016, 55, 11722–11724. [Google Scholar] [CrossRef] [PubMed]
- Hakulinen, J.K.; Hering, J.; Brändén, G.; Chen, H.; Snijder, A.; Ek, M.; Johansson, P. MraY–antibiotic complex reveals details of tunicamycin mode of action. Nat. Chem. Biol. 2017, 13, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Ichikawa, S.; Matsuda, A. Total Synthesis of Caprazol, a Core Structure of the Caprazamycin Antituberculosis Antibiotics. Angew. Chem. Int. Ed. 2005, 44, 1854–1856. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Ichikawa, S.; Matsuda, A. Development of a highly β-selective ribosylation reaction without using neighboring group participation: total synthesis of (+)-caprazol, a core structure of caprazamycins. J. Org. Chem. 2007, 72, 9936–9946. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Wang, L.; Abe, H.; Ravi, G.; Masuda, T.; Watanabe, T.; Shibasaki, M. Catalytic asymmetric total synthesis of (+)-caprazol. Org. Lett. 2014, 16, 3364–3367. [Google Scholar] [CrossRef] [PubMed]
- Wiegmann, D.; Spork, A.P.; Niro, G.; Ducho, C. Ribosylation of an acid-labile glycosyl acceptor as a potential key step for the synthesis of nucleoside antibiotics. Synlett 2018, 29, 440–446. [Google Scholar] [CrossRef]
Sample Availability: Samples of the target compounds are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).