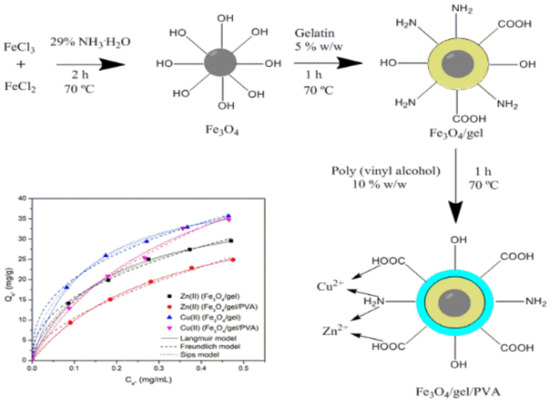
Adsorption of Cu(II) and Zn(II) Ions from Aqueous Solution by Gel/PVA-Modified Super-Paramagnetic Iron Oxide Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
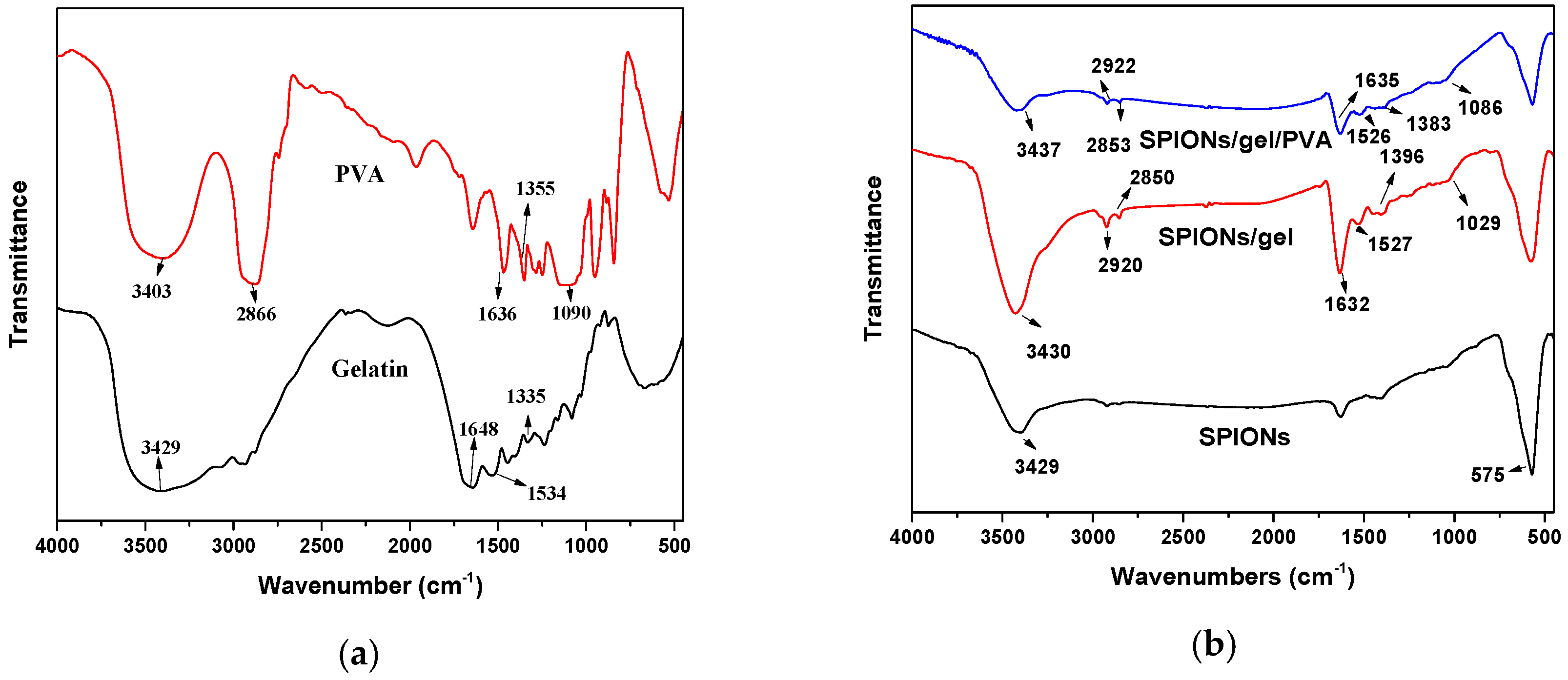
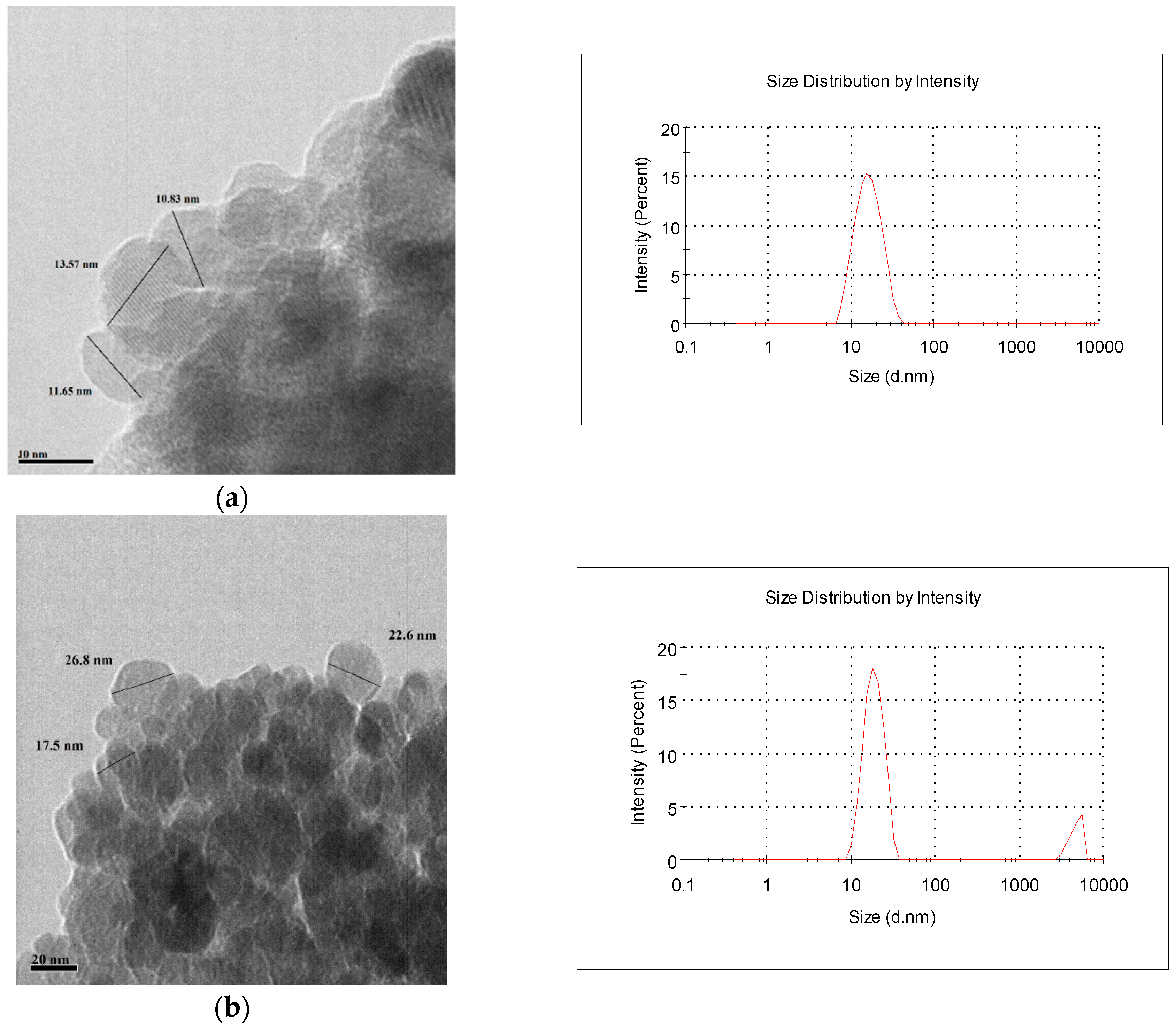
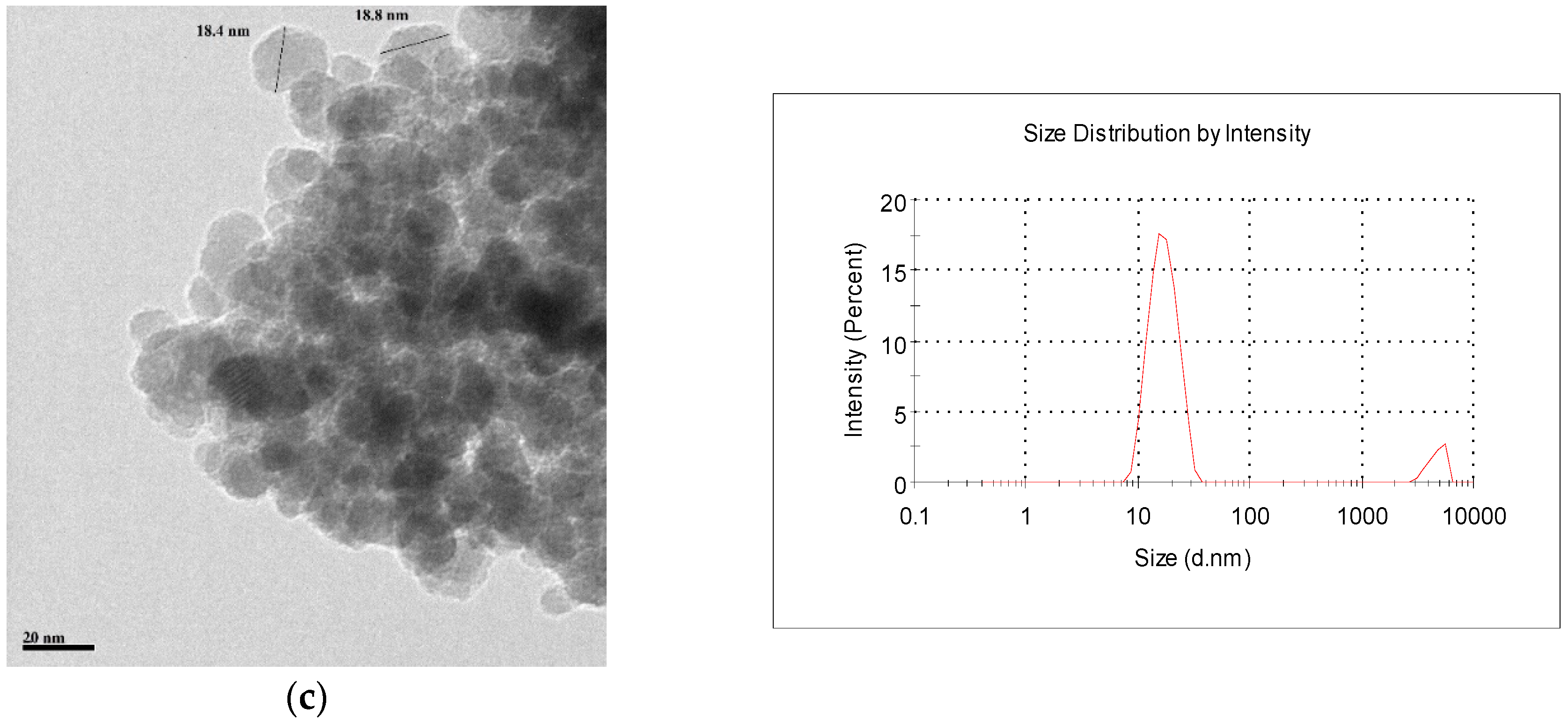
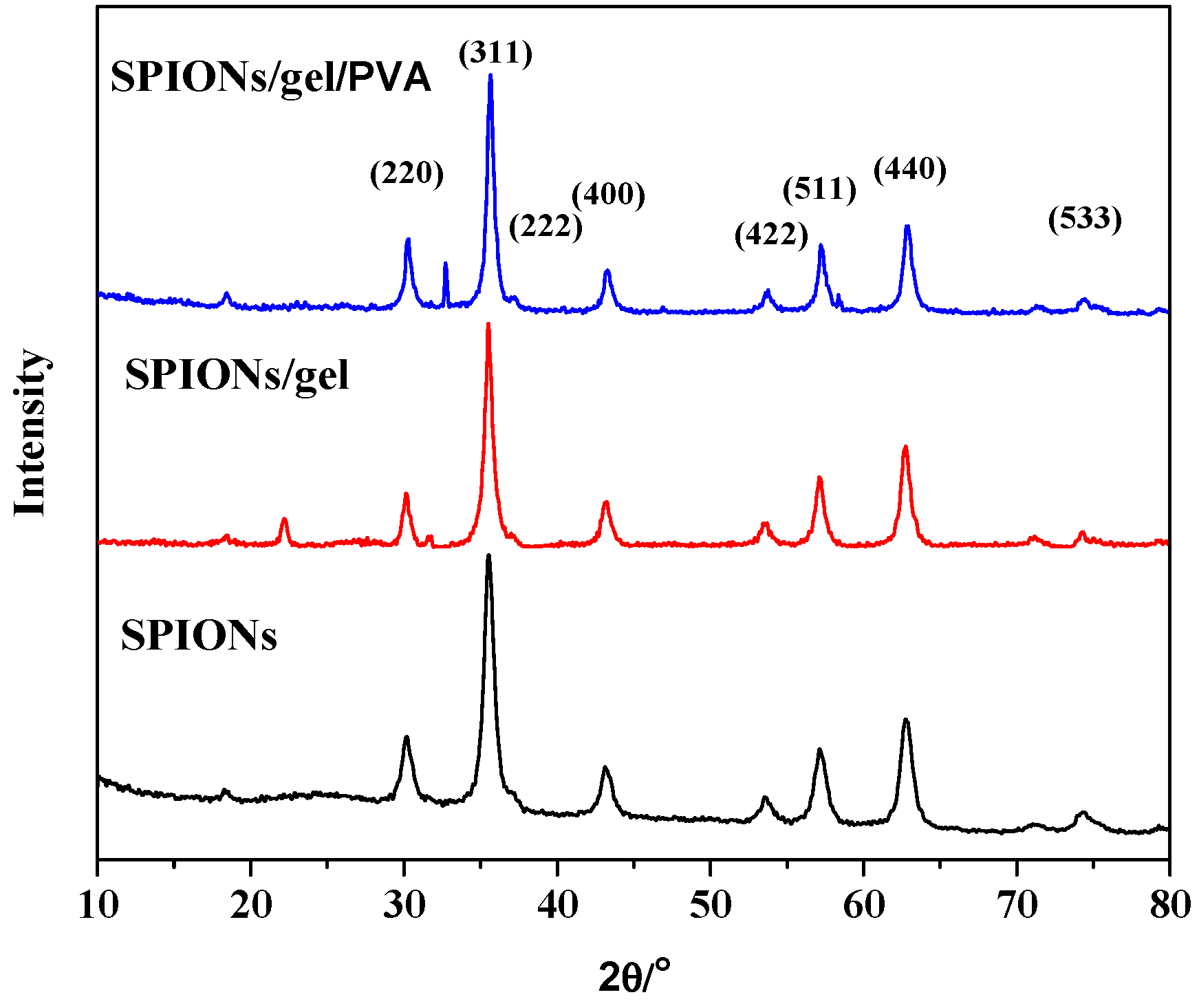
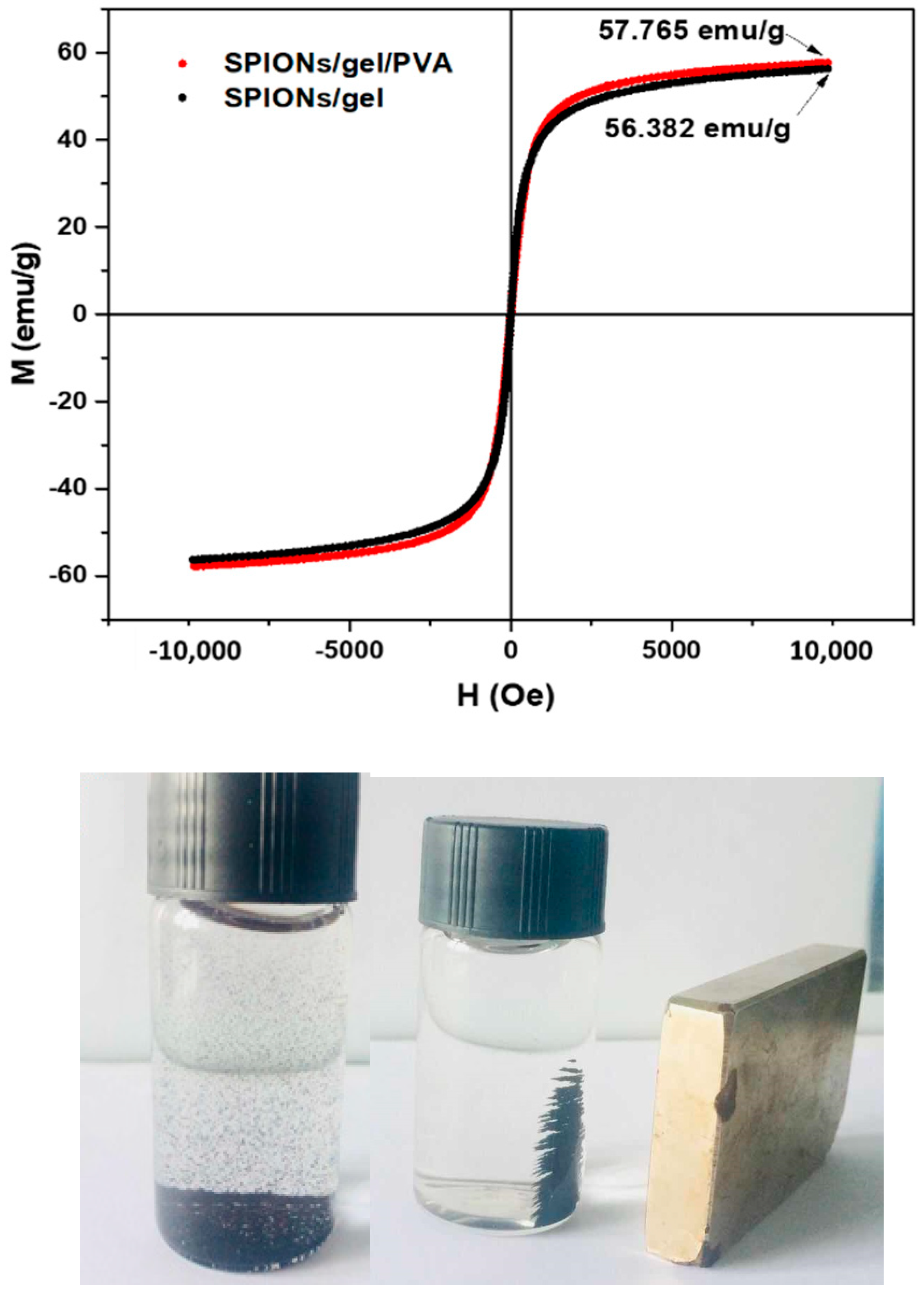
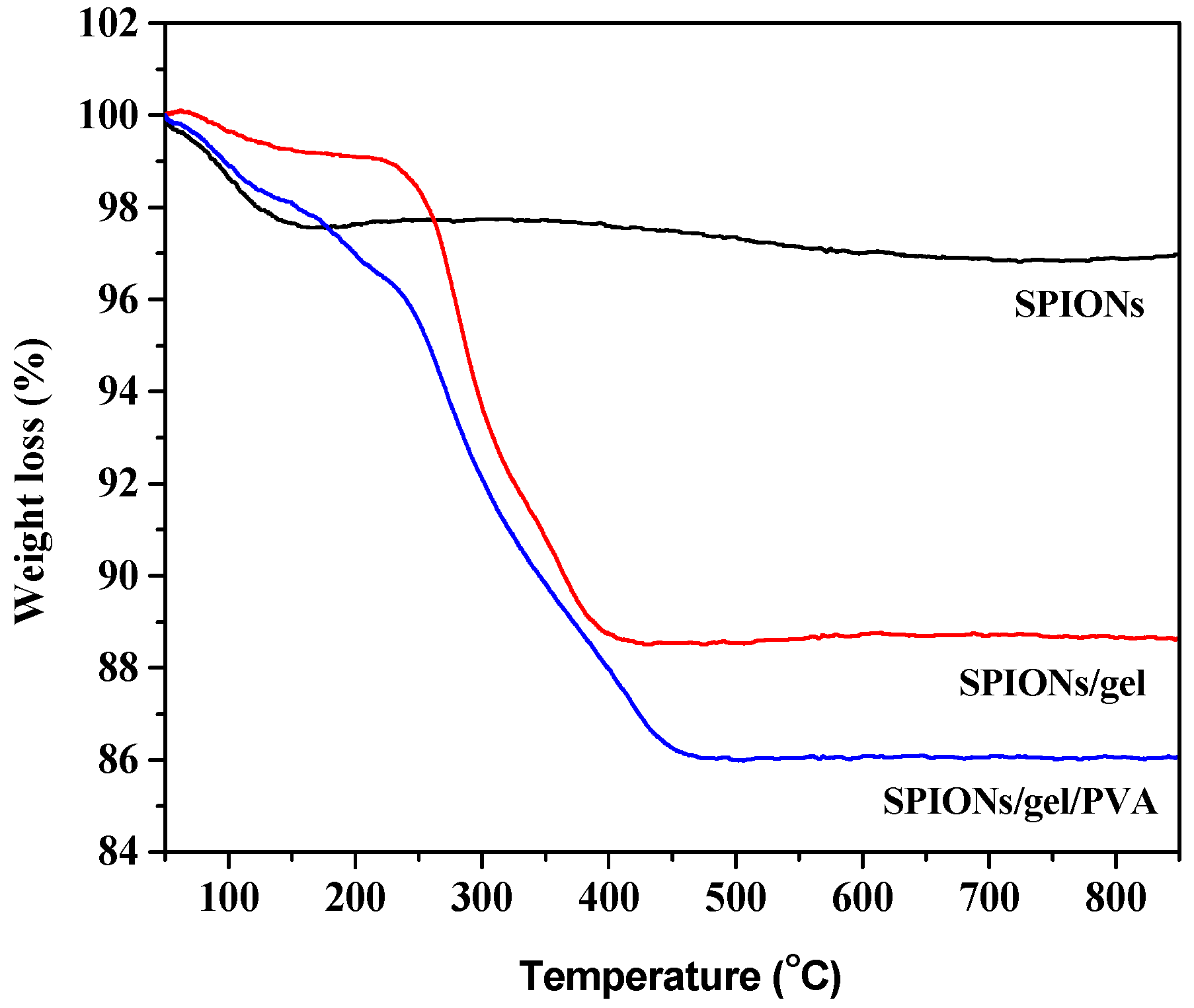
2.1. Characterization of the Adsorbents
2.2. Adsorption
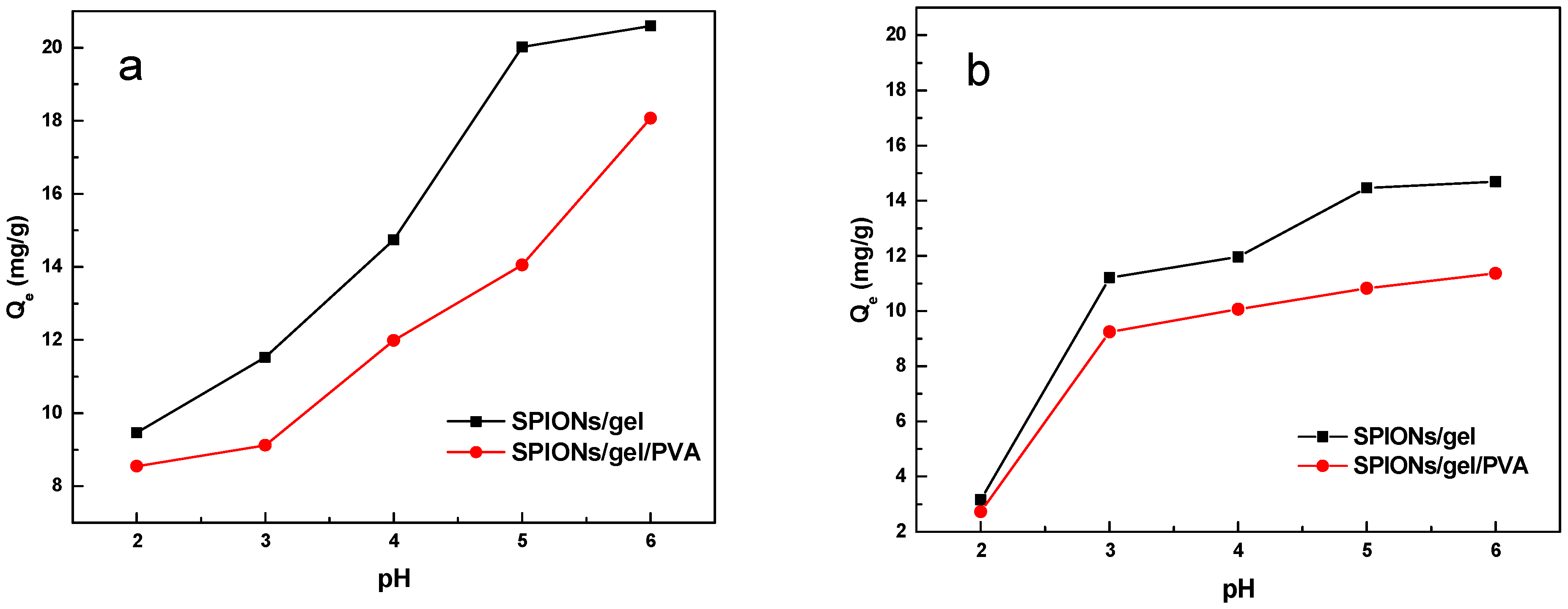
2.2.1. Effect of pH
2.2.2. Adsorption Kinetics
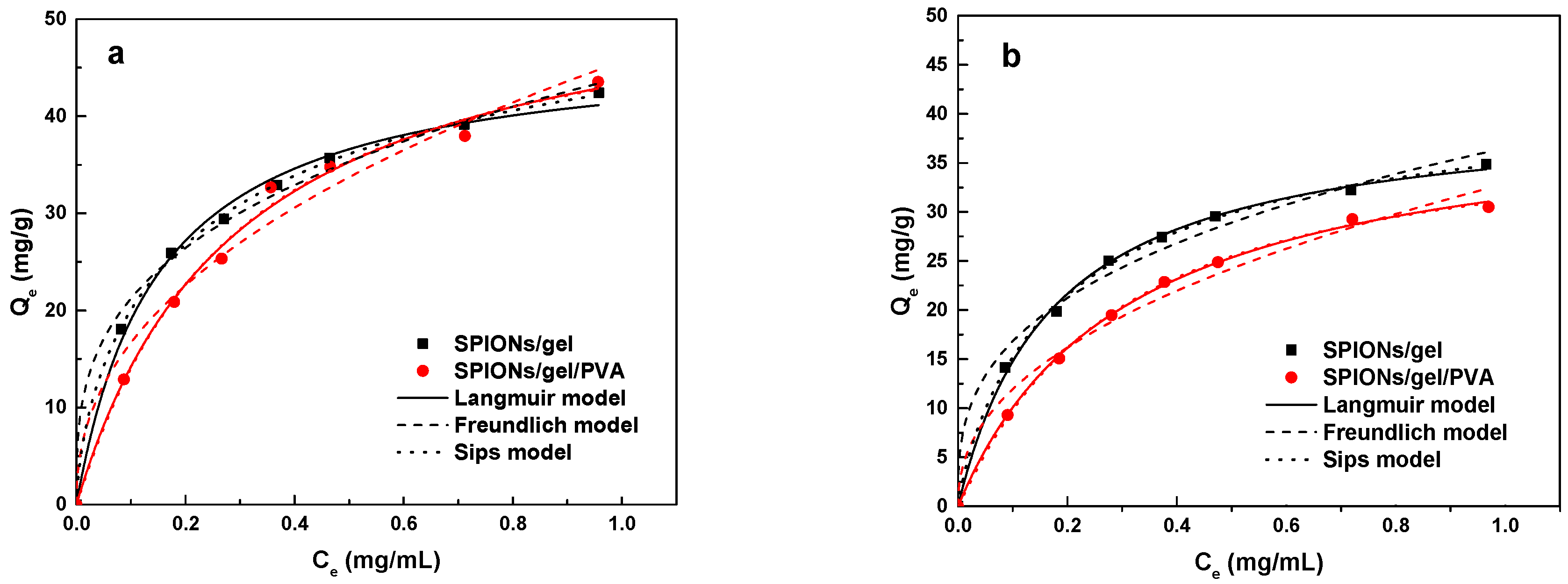
2.2.3. Adsorption Isotherms
2.2.4. Regeneration
3. Experimental
3.1. Materials
3.2. Synthesis of SPIONs/Gel/PVA
3.3. Characterization of Adsorbent
3.4. Adsorption
3.4.1. Effect of pH
3.4.2. Adsorption Kinetics
3.4.3. Adsorption Isotherms
3.4.4. Regeneration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lingamdinne, L.P.; Chang, Y.Y.; Yang, J.K.; Singh, J.; Choi, E.H.; Shiratani, M.; Koduru, J.R.; Attri, P. Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem. Eng. J. 2017, 307, 74–84. [Google Scholar] [CrossRef]
- Ramavandi, B.; Asgari, G. Comparative study of sun-dried and oven-dried Malva sylvestris biomass for high-rate Cu(II) removal from wastewater. Process. Saf. Environ. Prot. 2018, 116, 61–73. [Google Scholar] [CrossRef]
- Turan, N.G.; Elevli, S.; Mesci, B. Adsorption of copper and zinc ions on illite: Determination of the optimal conditions by the statistical design of experiments. Appl. Clay Sci. 2011, 52, 392–399. [Google Scholar] [CrossRef]
- Keochaiyom, B.; Wan, J.; Zeng, G.; Huang, D.; Xue, W.; Hu, L.; Huang, C.; Zhang, C.; Cheng, M. Synthesis and application of magnetic chlorapatite nanoparticles for zinc (II), cadmium (II) and lead (II) removal from water solutions. J. Colloid Interface Sci. 2017, 505, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. Waste biomass adsorbents for copper removal from industrial wastewater—A review. J. Hazard. Mater 2013, 263, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Hui, B.; Zhang, Y.; Ye, L. Structure of PVA/gelatin hydrogel beads and adsorption mechanism for advanced Pb(II) removal. J. Ind. Eng. Chem. 2015, 21, 868–876. [Google Scholar] [CrossRef]
- Lv, L.; Chen, N.; Feng, C.; Gao, Y.; Li, M. Xanthate-modified magnetic chitosan/poly (vinyl alcohol) adsorbent: Preparation, characterization, and performance of Pb(II) removal from aqueous solution. J. Taiwan Inst. Chem. Eng. 2017, 78, 485–492. [Google Scholar] [CrossRef]
- Gaffer, A.; Al Kahlawy, A.A.; Aman, D. Magnetic zeolite-natural polymer composite for adsorption of chromium (VI). Egypt. J. Pet. 2017, 26, 995–999. [Google Scholar] [CrossRef]
- Zhu, F.; Li, L.; Xing, J. Selective adsorption behavior of Cd(II) ion imprinted polymers synthesized by microwave-assisted inverse emulsion polymerization: Adsorption performance and mechanism. J. Hazard. Mater. 2017, 321, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Row, K.H. Magnetic Solid-phase Extraction with Fe3O4/Molecularly Imprinted Polymers Modified by Deep Eutectic Solvents and Ionic Liquids for the Rapid Purification of Alkaloid Isomers (Theobromine and Theophylline) from Green Tea. Molecules 2017, 22, 1061. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Y.; Fu, Y.Q.; Jiang, R.; Yao, J.; Xiao, L.; Zeng, G.M. Novel magnetic chitosan/poly(vinyl alcohol) hydrogel beads: Preparation, characterization and application for adsorption of dye from aqueous solution. Bioresour. Technol. 2012, 105, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Xu, X.; Liu, X.; Wang, Y.; Hayat, T.; Alsaedi, A.; Meng, Y.; Li, J. Highly enhanced adsorption performance of U(VI) by non-thermal plasma modified magnetic Fe3O4 nanoparticles. J. Colloid Interface Sci. 2018, 513, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Liu, Y.G.; Liu, S.B.; Shu, D.; Zeng, G.M.; Hu, X.J.; Tan, X.F.; Jiang, L.H.; Yan, Z.L.; Cai, X.X. Cu(II)-influenced adsorption of ciprofloxacin from aqueous solutions by magnetic graphene oxide/nitrilotriacetic acid nanocomposite: Competition and enhancement mechanisms. Chem. Eng. J. 2017, 319, 219–228. [Google Scholar] [CrossRef]
- Tang, P.; Shen, J.; Hu, Z.; Bai, G.; Wang, M.; Peng, B.; Shen, R.; Linghu, W. High-efficient scavenging of U(VI) by magnetic Fe3O4@gelatin composite. J. Mol. Liq. 2016, 221, 497–506. [Google Scholar] [CrossRef]
- Saarai, A.; Kasparkova, V.; Sedlacek, T.; Saha, P. On the development and characterisation of crosslinked sodium alginate/gelatine hydrogels. J. Mech. Behav. Biomed. Mater. 2013, 18, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Rajzer, I.; Menaszek, E.; Kwiatkowski, R.; Planell, J.A.; Castano, O. Electrospun gelatin/poly(epsilon-caprolactone) fibrous scaffold modified with calcium phosphate for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Rajan, M.; Raj, V.; Al-Arfaj, A.A.; Murugan, A.M. Hyaluronidase enzyme core-5-fluorouracil-loaded chitosan-PEG-gelatin polymer nanocomposites as targeted and controlled drug delivery vehicles. Int. J. Pharm. 2013, 453, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Erden, P.E.; Kaçar, C.; Öztürk, F.; Kılıç, E. Amperometric uric acid biosensor based on poly(vinylferrocene)-gelatin-carboxylated multiwalled carbon nanotube modified glassy carbon electrode. Talanta 2015, 134, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Hayeeye, F.; Sattar, M.; Chinpa, W.; Sirichote, O. Kinetics and thermodynamics of Rhodamine B adsorption by gelatin/activated carbon composite beads. Colloids Surf. A 2017, 513, 259–266. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Ma, X.; Zhang, J.; Yao, J. Molecular interactions in gelatin/chitosan composite films. Food Chem. 2017, 235, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Y.G.; Liu, S.B.; Liu, H.Y.; Zeng, G.M.; Tan, X.F.; Yang, C.P.; Ding, Y.; Yan, Z.L.; Cai, X.X. Sorption performance and mechanisms of arsenic(V) removal by magnetic gelatin-modified biochar. Chem. Eng. J. 2017, 314, 223–231. [Google Scholar] [CrossRef]
- Wong, E.T.; Chan, K.H.; Idris, A. Kinetic and equilibrium investigation of Cu(II) removal by Co(II)-doped iron oxide nanoparticle-immobilized in PVA–alginate recyclable adsorbent under dark and photo condition. Chem. Eng. J. 2015, 268, 311–324. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.; Wang, J. Removal of Co2+ from radioactive wastewater by polyvinyl alcohol (PVA)/chitosan magnetic composite. Prog. Nucl. Energy 2014, 71, 172–178. [Google Scholar] [CrossRef]
- Nithya, K.; Sathish, A.; Kumar, P.S.; Ramachandran, T. Fast kinetics and high adsorption capacity of green extract capped superparamagnetic iron oxide nanoparticles for the adsorption of Ni(II) ions. J. Ind. Eng. Chem. 2018, 59, 230–241. [Google Scholar] [CrossRef]
- Yang, L.; Tian, J.; Meng, J.; Zhao, R.; Ma, J.; Jin, T. Modification and Characterization of Fe3O4 Nanoparticles for Use in Adsorption of Alkaloids. Molecules 2018, 23, 562. [Google Scholar] [CrossRef] [PubMed]
- Dil, N.N.; Sadeghi, M. Free radical synthesis of nanosilver/gelatin-poly (acrylic acid) nanocomposite hydrogels employed for antibacterial activity and removal of Cu(II) metal ions. J. Hazard. Mater. 2018, 351, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.K.; Park, H.K.; Nam, C.W.; Kim, S.D.; Kim, J. Facile synthesis and characterization of γ-AlOOH/PVA composite granules for Cr(VI) adsorption. J. Ind. Eng. Chem. 2018, 60, 485–492. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, L.; Zhou, W.; Lu, R.; Gao, H.; Zhang, S. Superior adsorption capacity of Fe3O4@nSiO2@mSiO2 core-shell microspheres for removal of congo red from aqueous solution. J. Mol. Liq. 2016, 219, 88–94. [Google Scholar] [CrossRef]
- Yan, E.; Cao, M.; Jiang, J.; Gao, J.; Jiang, C.; Ba, X.; Yang, X.; Zhang, D. A novel adsorbent based on magnetic Fe3O4 contained polyvinyl alcohol/chitosan composite nanofibers for chromium (VI) removal. Solid State Sci. 2017, 72, 94–102. [Google Scholar] [CrossRef]
- Fakhre, N.A.; Ibrahim, B.M. The use of new chemically modified cellulose for heavy metal ion adsorption. J. Hazard. Mater. 2018, 343, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Wen, J.; Hu, Y.; Tan, X. Adsorption of Cu2+ and Cd2+ from aqueous solution by novel electrospun poly(vinyl alcohol)/graphene oxide nanofibers. RSC Adv. 2016, 6, 79641–79650. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Karim, K.J.A.; Ibrahim, W.A.W.; Jume, B.H. Equilibrium, kinetic and mechanism studies of Cu(II) and Cd(II) ions adsorption by modified chitosan bead. Int. J. Biol. Macromol. 2018, 116, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Ojemaye, M.O.; Okoh, O.O.; Okoh, A.I. Adsorption of Cu2+ from aqueous solution by a novel material; azomethine functionalized magnetic nanoparticles. Sep. Purif. Technol. 2017, 183, 204–215. [Google Scholar] [CrossRef]
- Mokadem, Z.; Mekki, S.; Saïdi-Besbes, S.; Agusti, G.; Elaissari, A.; Derdour, A. Triazole containing magnetic core-silica shell nanoparticles for Pb2+, Cu2+ and Zn2+ removal. Arab. J. Chem. 2017, 10, 1039–1051. [Google Scholar] [CrossRef]
- Ghasemi, N.; Ghasemi, M.; Moazeni, S.; Ghasemi, P.; Alharbi, N.S.; Gupta, V.K.; Agarwal, S.; Burakova, I.V.; Tkachew, A.G. Zn(II) removal by amino-functionalized magnetic nanoparticles: Kinetics, isotherm, and thermodynamic aspects of adsorption. J. Ind. Eng. Chem. 2018, 62, 302–310. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Ma, P.; Bai, H.; Dong, W.; Chen, M. Preparation of polyvinyl alcohol/chitosan hydrogel compounded with graphene oxide to enhance the adsorption properties for Cu(II) in aqueous solution. J. Polym. Res. 2015, 22, 150. [Google Scholar] [CrossRef]
- Wang, X.; Huang, K.; Chen, Y.; Liu, J.; Chen, S.; Cao, J.; Mei, S.; Zhou, Y.; Jing, T. Preparation of dumbbell manganese dioxide/gelatin composites and their application in the removal of lead and cadmium ions. J. Hazard. Mater. 2018, 350, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhu, L.; Wang, J.; Yue, T.; Li, R.; Li, Z. Adsorption of Cd(II) and Pb(II) by in situ oxidized Fe3O4 membrane grafted on 316 L porous stainless steel filter tube and its potential application for drinking water treatment. J. Environ. Manag. 2017, 196, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.S.; Carvalho, J.O.; Bezerra, R.D.S.; Silva, M.S.; Ferreira, F.J.L.; Osajima, J.A.; Filho, E.C.S. Potential of Cellulose Functionalized with Carboxylic Acid as Biosorbent for the Removal of Cationic Dyes in Aqueous Solution. Molecules 2018, 23, 743. [Google Scholar] [CrossRef] [PubMed]
- Mirzabe, G.H.; Keshtkar, A.R. Application of response surface methodology for thorium adsorption on PVA/Fe3O4/SiO2/APTES nanohybrid adsorbent. J. Ind. Eng. Chem. 2015, 26, 277–285. [Google Scholar] [CrossRef]
- Hui, C.; Guo, X.; Sun, P.; Khan, R.A.; Zhang, Q.; Liang, Y.; Zhao, Y.H. Removal of nitrite from aqueous solution by Bacillus amyloliquefaciens biofilm adsorption. Bioresour. Technol. 2018, 248, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Lee, D.S.; Lee, M.W.; Woo, S.H. Enhanced adsorption of congo red from aqueous solutions by chitosan hydrogel beads impregnated with cetyl trimethyl ammonium bromide. Bioresour. Technol. 2009, 100, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, J.; Wang, J. Competitive adsorption of Pb(II), Cu(II) and Zn(II) onto xanthate-modified magnetic chitosan. J. Hazard. Mater. 2012, 221, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Nieboer, E.; Richardson, D.H.S. The replacement of the bobdescript term heavy metals by a biologocally and chemically significant classification of metal ions. Environ. Pollut. 1980, 1, 3–26. [Google Scholar] [CrossRef]
- Wen, T.; Wang, J.; Li, X.; Huang, S.; Chen, Z.; Wang, S.; Hayat, T.; Alsaedi, A.; Wang, X. Production of a generic magnetic Fe3O4 nanoparticles decorated tea waste composites for highly efficient sorption of Cu(II) and Zn(II). JECE 2017, 5, 3656–3666. [Google Scholar] [CrossRef]
- Mirzaeinejad, M.; Mansoori, Y.; Amiri, M. Amino functionalized ATRP-prepared polyacrylamide-g-magnetite nanoparticles for the effective removal of Cu(II) ions: Kinetics investigations. Mater. Chem. Phys. 2018, 205, 195–205. [Google Scholar] [CrossRef]
- Xie, Y.; Yuan, X.; Wu, Z.; Zeng, G.; Jiang, L.; Peng, X.; Li, H. Adsorption behavior and mechanism of Mg/Fe layered double hydroxide with Fe3O4-carbon spheres on the removal of Pb(II) and Cu(II). J. Colloid Interface Sci. 2018, 536, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Li, L.Y.; Hsieh, C.T.; Juang, R.S. Co-precipitation of magnetic Fe3O nanoparticles onto carbon nanotubes for removal of copper ions from aqueous solution. J. Taiwan Inst. Chem. Eng. 2018, 82, 56–63. [Google Scholar] [CrossRef]
- Wondracek, M.H.P.; Jorgetto, A.O.; Silva, A.C.P.; Ivassechen, J.D.R.; Schneider, J.F.; Saeki, M.J.; Pedrosa, V.A.; Yoshito, W.K.; Colauto, F.; Ortiz, W.A.; et al. Synthesis of mesoporous silica-coated magnetic nanoparticles modified with 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole and its application as Cu(II) adsorbent from aqueous samples. Appl. Surf. Sci. 2016, 367, 533–541. [Google Scholar] [CrossRef]
- Vismara, E.; Bongio, C.; Coletti, A.; Edelman, R.; Serafini, A.; Mauri, M.; Simonutti, R.; Bertini, S.; Urso, E.; Assaraf, Y.G.; et al. Albumin and Hyaluronic Acid-Coated Superparamagnetic Iron Oxide Nanoparticles Loaded with Paclitaxel for Biomedical Applications. Molecules 2017, 22, 1030. [Google Scholar] [CrossRef] [PubMed]
- Habila, M.A.; Alothman, Z.A.; El-Toni, A.M.; Labis, J.P.; Khan, A.; Al-Matghany, A.; Elafifi, H.E. One-Step Carbon Coating and Polyacrylamide Functionalization of Fe3O4 Nanoparticles for Enhancing Magnetic Adsorptive-Remediation of Heavy Metals. Molecules 2017, 22, 2074. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, J.; Wen, Y.; Li, H.; Ma, J.; Fu, D. Fast and effective removal of cadmium ion from water using chitosan encapsulated magnetic Fe3O4 nanoparticles. Desalin. Water Treat. 2015, 57, 8540–8548. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds SPIONs/gel and SPIONs/gel/PVA are available from the authors. |


| Metal | Adsorbents | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| Qt (mg/g) | K1 (1/min) | R2 | Qt (mg/g) | K2 (g/(mg·min)) | R2 | ||
| Cu(II) | SPIONs/gel | 18.834 | 0.055 | 0.992 | 20.779 | 0.004 | 0.991 |
| SPIONs/gel/PVA | 13.886 | 0.043 | 0.992 | 15.55 | 0.003 | 0.994 | |
| Zn(II) | SPIONs/gel | 14.109 | 0.137 | 0.986 | 14.888 | 0.019 | 0.998 |
| SPIONs/gel/PVA | 11.101 | 0.059 | 0.983 | 12.146 | 0.008 | 0.986 | |
| Isotherm | Parameters | Cu(II) | Zn(II) | ||
|---|---|---|---|---|---|
| SPIONs/Gel | SPIONs/Gel/PVA | SPIONs/Gel | SPIONs/Gel/PVA | ||
| Langmuir model | Qmax (mg/g) | 47.594 | 56.051 | 40.559 | 40.865 |
| KL (mL/mg) | 6.66 | 3.397 | 5.727 | 3.267 | |
| R2 | 0.995 | 0.993 | 0.997 | 0.999 | |
| Freundlich model | KF ((mg/g)(mL/mg)1/n) | 43.991 | 45.658 | 36.515 | 32.866 |
| 1/n | 0.3164 | 0.4373 | 0.3367 | 0.4399 | |
| R2 | 0.993 | 0.976 | 0.986 | 0.979 | |
| Sips model | Qmax (mg/g) | 60.422 | 54.823 | 43.974 | 38.976 |
| KS (mL/mg)1/n | 2.382 | 3.713 | 3.854 | 3.961 | |
| 1/n | 0.6816 | 1.0319 | 0.8632 | 1.071 | |
| R2 | 0.999 | 0.991 | 0.998 | 0.999 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolgormaa, A.; Lv, C.-j.; Li, Y.; Yang, J.; Yang, J.-x.; Chen, P.; Wang, H.-p.; Huang, J. Adsorption of Cu(II) and Zn(II) Ions from Aqueous Solution by Gel/PVA-Modified Super-Paramagnetic Iron Oxide Nanoparticles. Molecules 2018, 23, 2982. https://doi.org/10.3390/molecules23112982
Dolgormaa A, Lv C-j, Li Y, Yang J, Yang J-x, Chen P, Wang H-p, Huang J. Adsorption of Cu(II) and Zn(II) Ions from Aqueous Solution by Gel/PVA-Modified Super-Paramagnetic Iron Oxide Nanoparticles. Molecules. 2018; 23(11):2982. https://doi.org/10.3390/molecules23112982
Chicago/Turabian StyleDolgormaa, Anudari, Chang-jiang Lv, Yin Li, Jian Yang, Jun-xing Yang, Peng Chen, Hong-peng Wang, and Jun Huang. 2018. "Adsorption of Cu(II) and Zn(II) Ions from Aqueous Solution by Gel/PVA-Modified Super-Paramagnetic Iron Oxide Nanoparticles" Molecules 23, no. 11: 2982. https://doi.org/10.3390/molecules23112982
APA StyleDolgormaa, A., Lv, C.-j., Li, Y., Yang, J., Yang, J.-x., Chen, P., Wang, H.-p., & Huang, J. (2018). Adsorption of Cu(II) and Zn(II) Ions from Aqueous Solution by Gel/PVA-Modified Super-Paramagnetic Iron Oxide Nanoparticles. Molecules, 23(11), 2982. https://doi.org/10.3390/molecules23112982