Ethanolic Extract of Folium Sennae Mediates the Glucose Uptake of L6 Cells by GLUT4 and Ca2+
Abstract
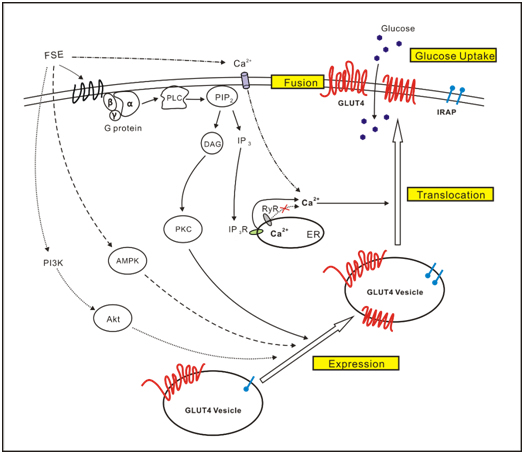
:1. Introduction
2. Results
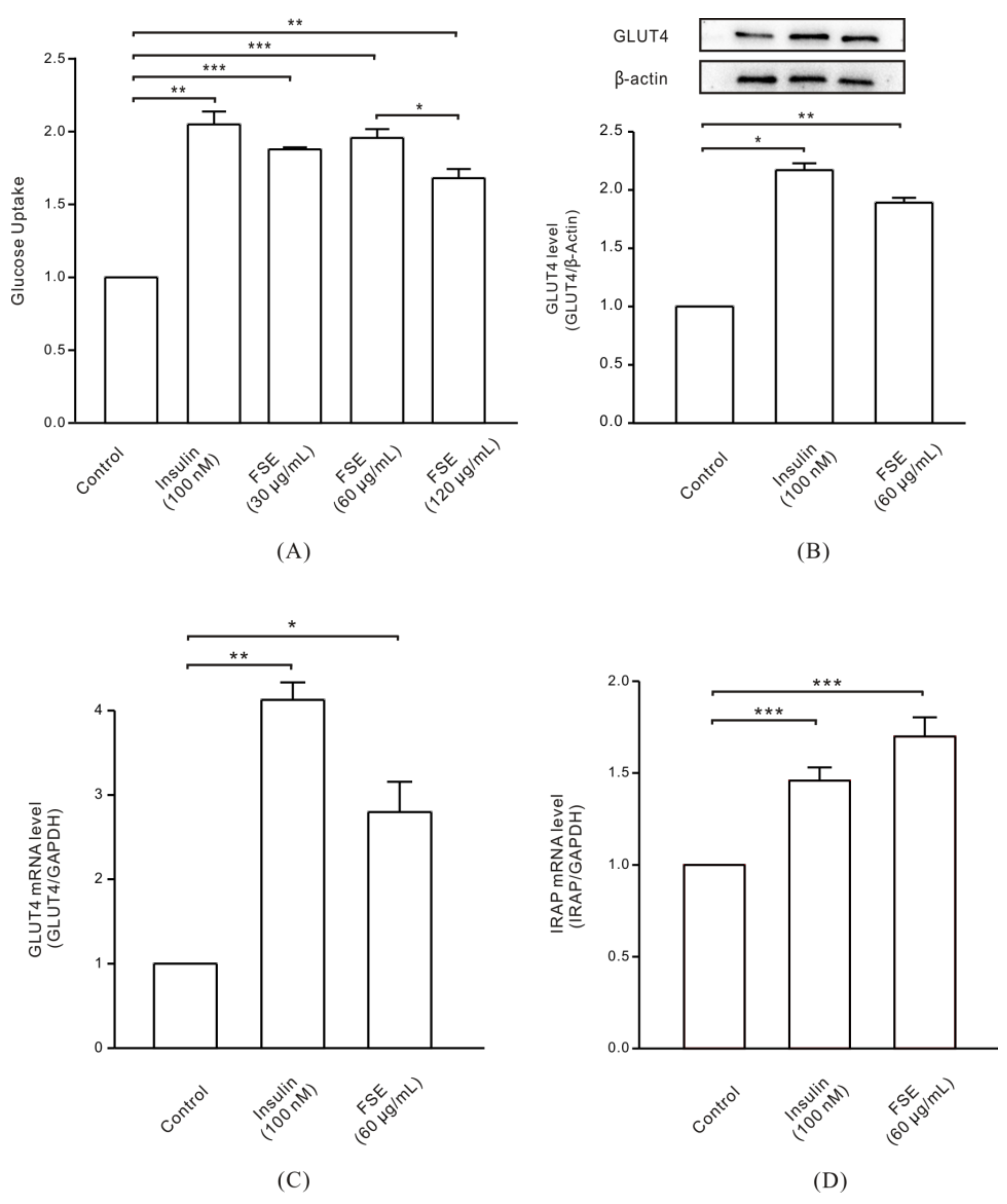
2.1. FSE Increases GLUT4 Expression Levels and Glucose Uptake in L6 Cells
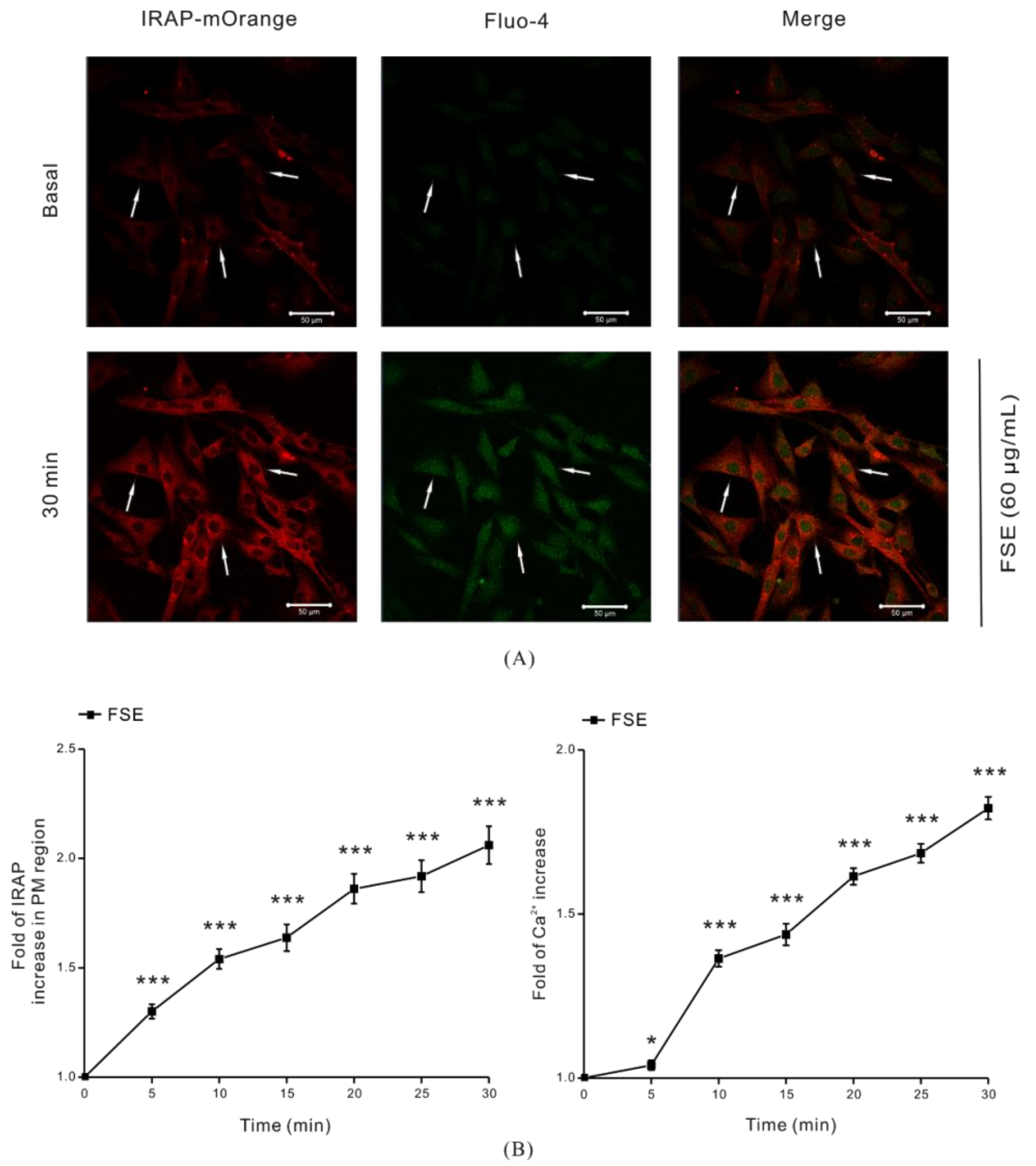
2.2. FSE Stimulates GLUT4 Translocation and Increases Intracellular Ca2+ Levels
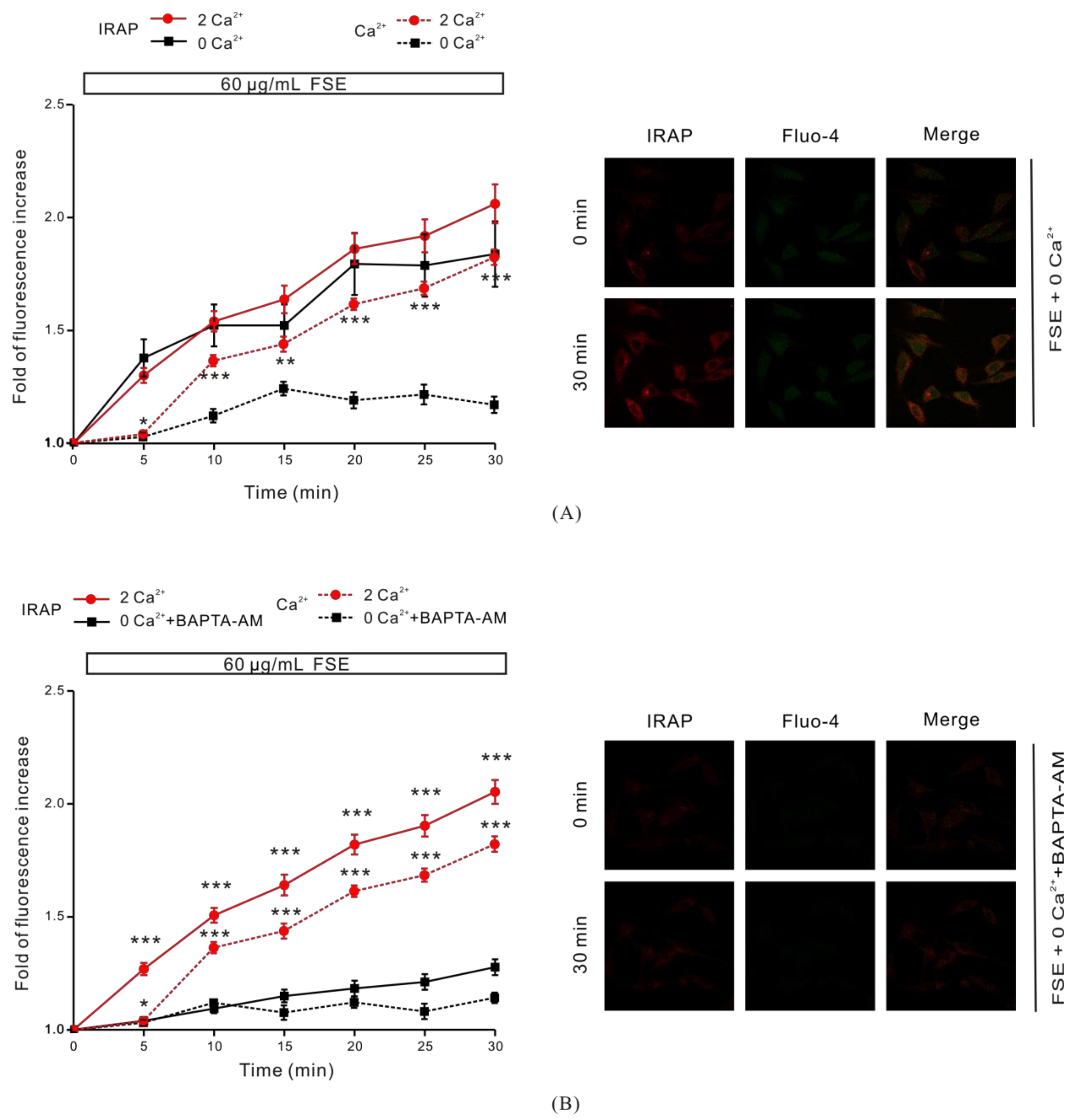
2.3. The Role of Cytosolic Ca2+ in FSE-Mediated GLUT4 Translocation
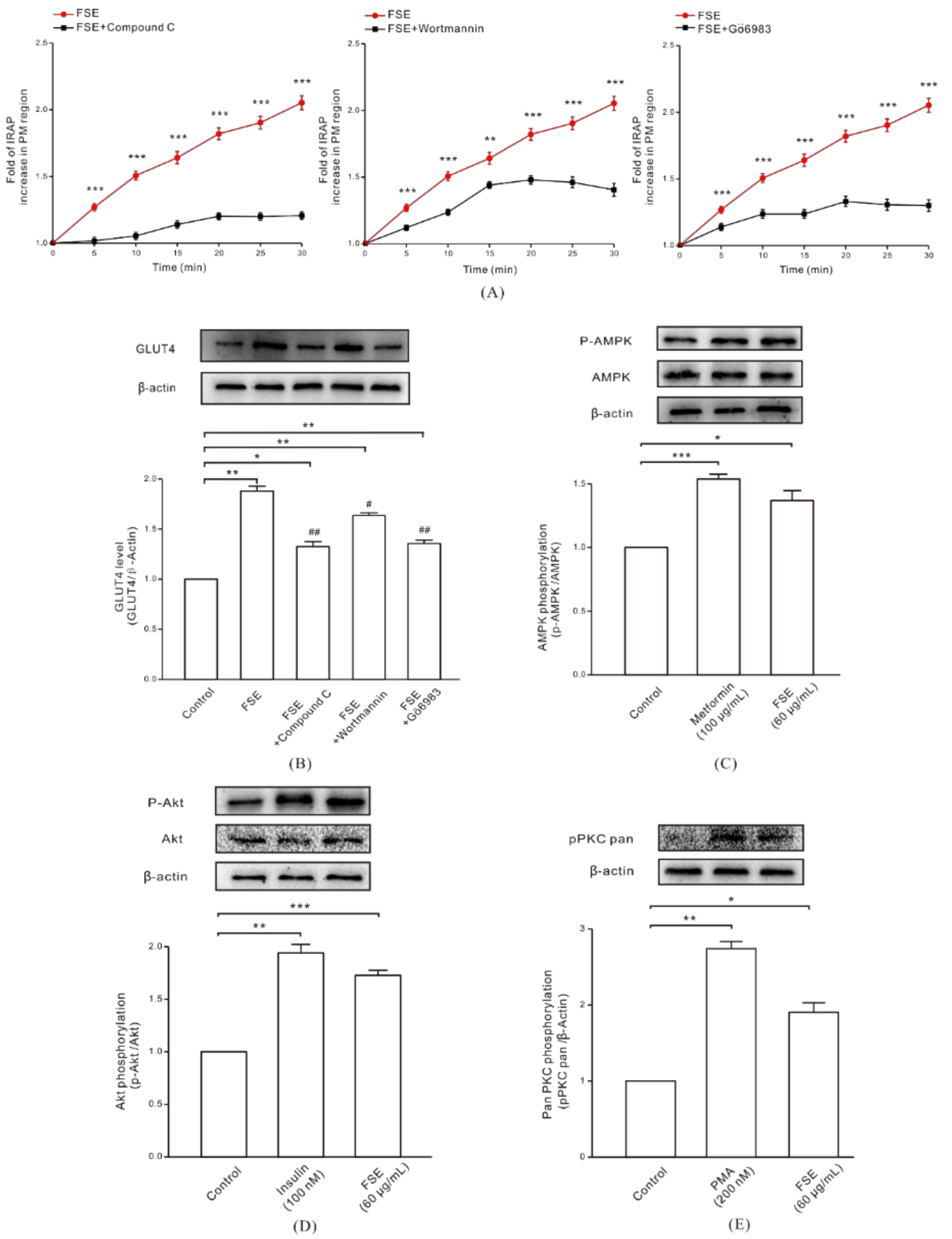
2.4. FSE Enhances GLUT4 Translocation and Expression through the AMPK, PI3K/Akt, and PKC Pathways
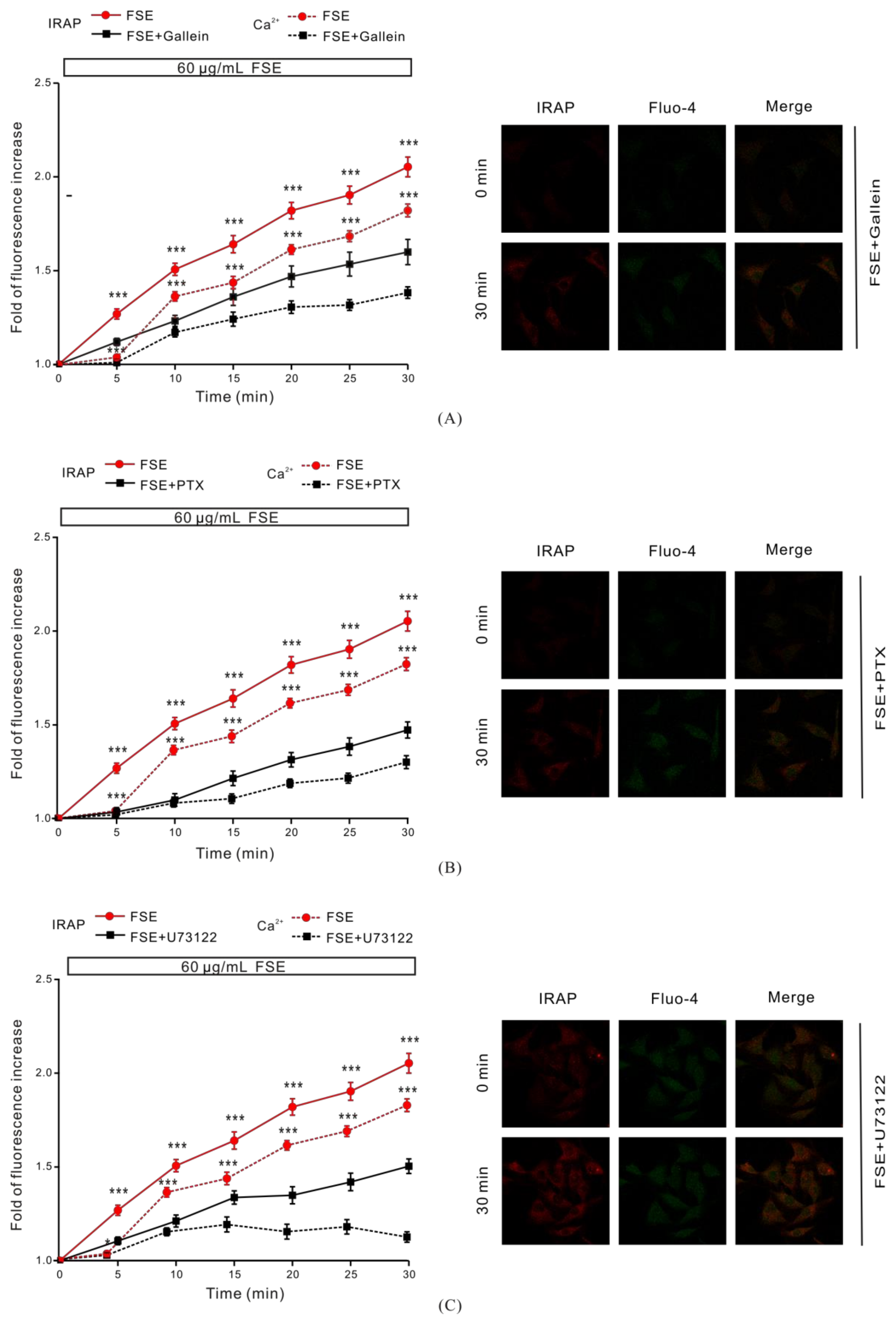
2.5. G Protein and PLC Regulate FSE-Mediated Intracellular Ca2+ Increases and GLUT4 Translocation
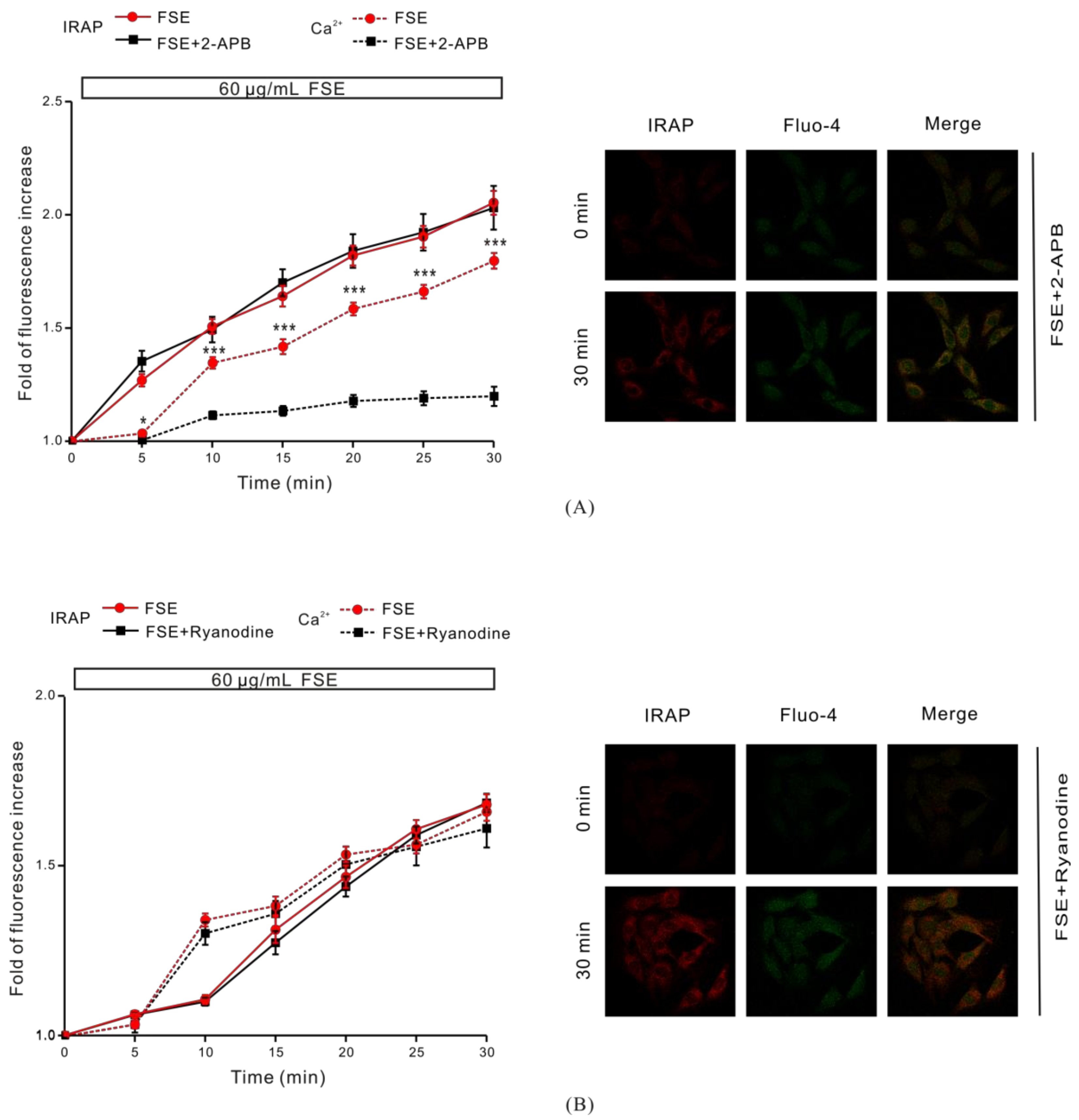
2.6. IP3R Is Involved in FSE-Triggered Intracellular Ca2+ Release
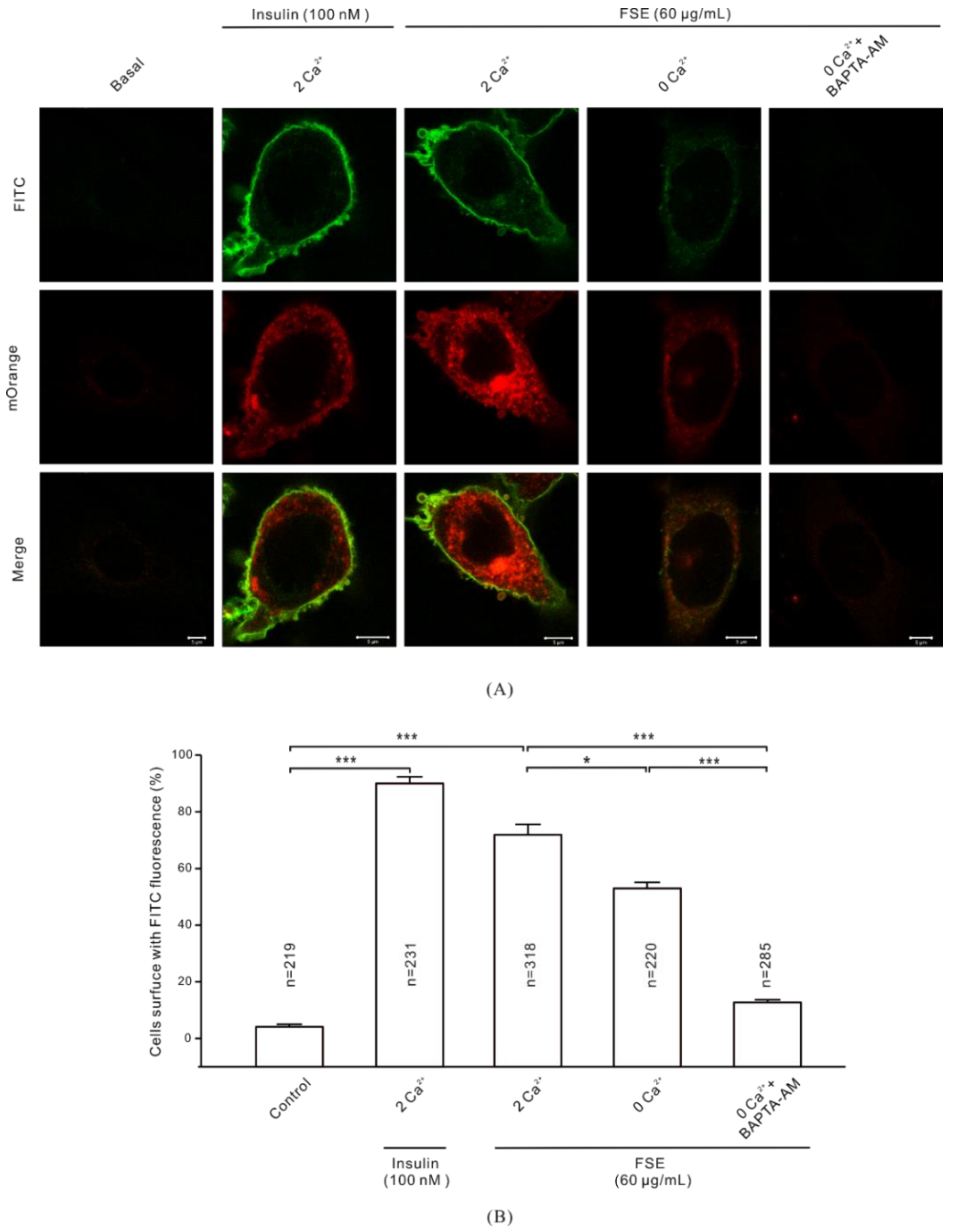
2.7. Ca2+ Is Required for GLUT4 Insertion into the PM
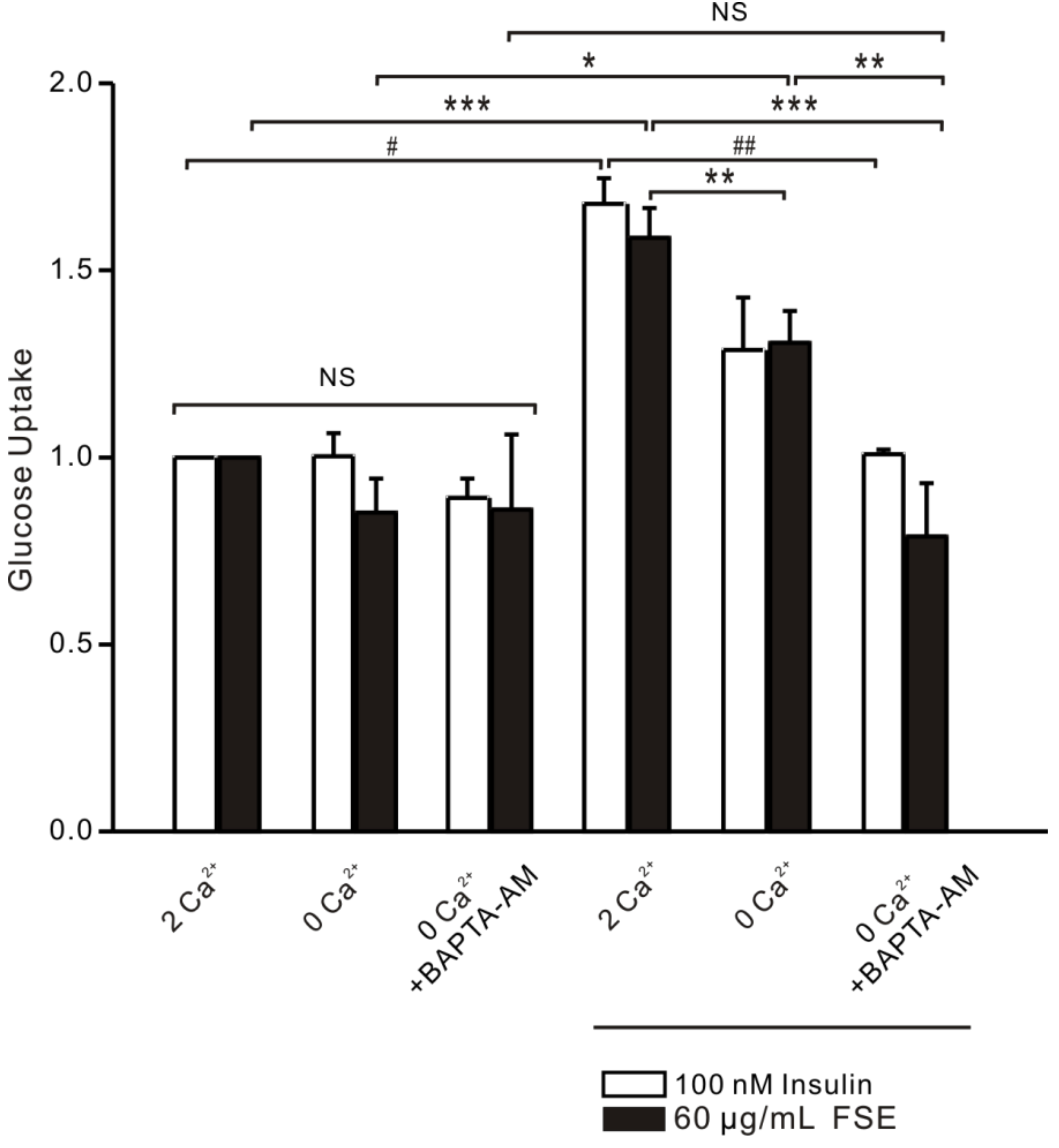
2.8. FSE-Induced Ca2+ Increases Improve Glucose Uptake in L6 Cells
3. Discussion
4. Materials and methods
4.1. Reagents and Solutions
4.2. Plant Collection and Preparation
4.3. L6 Cell Culture and Treatment
4.4. Amount of Glucose Uptake into Cells
4.5. Western Blotting Analyses
4.6. RT-PCR
4.7. Fluorescence Microscopy for Detection of IRAP Translocation and Ca2+
4.8. Immunofluorescence of GLUT4 Fusion with PM
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kang, O.H.; Shon, M.Y.; Kong, R.; Seo, Y.S.; Zhou, T.; Kim, D.Y.; Kim, Y.S.; Kwon, D.Y. Anti-diabetic effect of black ginseng extract by augmentation of AMPK protein activity and upregulation of GLUT2 and GLUT4 expression in db/db mice. BMC Complement. Altern. Med. 2017, 17, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhao, P.; Wan, D.; Zhou, Q.; Wang, C.; Shu, G.; Mei, Z.; Yang, X. Antidiabetic Effect of Methanolic Extract from Berberis julianae Schneid. via Activation of AMP-Activated Protein Kinase in Type 2 Diabetic Mice. Evid. Based Complement. Altern. Med. 2014, 2014, 106206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, X.; Liang, H.; Cai, H.; Hu, X.; Bian, Y.; Dong, L.; Ding, L.; Wang, L.; Yu, B.; et al. Semen Cassiae Extract Improves Glucose Metabolism by Promoting GlUT4 Translocation in the Skeletal Muscle of Diabetic Rats. Front. Pharmacol. 2018, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hao, J.; Tian, D.; Wen, Y.; Zhao, P.; Chen, H.; Lv, Y.; Yang, X. Antidiabetic Activity of a Flavonoid-Rich Extract From Sophora davidii (Franch.) Skeels in KK-Ay Mice via Activation of AMP-Activated Protein Kinase. Front. Pharmacol. 2018, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, Y.; Zhi, F.; Xing, Q.C.; Chen, Y.Z. The Effect of Sanggua Drink Extract on Insulin Resistance through the PI3K/AKT Signaling Pathway. Evid. Based Complement. Altern. Med. 2018, 2018, 9407945. [Google Scholar]
- Kamakura, R.; Son, M.J.; de Beer, D.; Joubert, E.; Miura, Y.; Yagasaki, K. Antidiabetic effect of green rooibos (Aspalathus linearis) extract in cultured cells and type 2 diabetic model KK-A(y) mice. Cytotechnology 2015, 67, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, K.J.; Koh, E.J.; Lee, B.Y. Gelidium elegans Extract Ameliorates Type 2 Diabetes via Regulation of MAPK and PI3K/Akt Signaling. Nutrients 2018, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Kadan, S.; Sasson, Y.; Saad, B.; Zaid, H. Gundelia tournefortii Antidiabetic Efficacy: Chemical Composition and GLUT4 Translocation. Evid. Based Complement. Altern. Med. 2018, 2018, 8294320. [Google Scholar] [CrossRef] [PubMed]
- Chanda, D.; Luiken, J.J.; Glatz, J.F. Signaling pathways involved in cardiac energy metabolism. FEBS Lett. 2016, 590, 2364–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaid, H.; Antonescu, C.N.; Randhawa, V.K.; Klip, A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem. J. 2008, 413, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Vlavcheski, F.; Baron, D.; Vlachogiannis, I.A.; MacPherson, R.E.K.; Tsiani, E. Carnosol Increases Skeletal Muscle Cell Glucose Uptake via AMPK-Dependent GLUT4 Glucose Transporter Translocation. Int. J. Mol. Sci. 2018, 19, 1321. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.C. Mechanisms of calcium-induced mitochondrial biogenesis and GLUT4 synthesis. Appl. Physiol. Nutr. Metab. 2007, 32, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, X.; Ishikura, S.; Zhang, D.; Gao, J.; Sun, Y.; Contreras-Ferrat, A.; Foley, K.P.; Lavandero, S.; Yao, Z.; et al. Ca2+ signals promote GLUT4 exocytosis and reduce its endocytosis in muscle cells. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E209–E224. [Google Scholar] [CrossRef] [PubMed]
- Lanner, J.T.; Katz, A.; Tavi, P.; Sandstrom, M.E.; Zhang, S.J.; Wretman, C.; James, S.; Fauconnier, J.; Lannergren, J.; Bruton, J.D.; et al. The role of Ca2+ influx for insulin-mediated glucose uptake in skeletal muscle. Diabetes 2006, 55, 2077–2083. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ferrat, A.E.; Toro, B.; Bravo, R.; Parra, V.; Vasquez, C.; Ibarra, C.; Mears, D.; Chiong, M.; Jaimovich, E.; Klip, A.; et al. An inositol 1,4,5-triphosphate (IP3)-IP3 receptor pathway is required for insulin-stimulated glucose transporter 4 translocation and glucose uptake in cardiomyocytes. Endocrinology 2010, 151, 4665–4677. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, N.; Tung, A.; Thong, F.; Klip, A. Muscle cell depolarization induces a gain in surface GLUT4 via reduced endocytosis independently of AMPK. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1276–E1286. [Google Scholar] [CrossRef] [PubMed]
- Waller, A.P.; Kalyanasundaram, A.; Hayes, S.; Periasamy, M.; Lacombe, V.A. Sarcoplasmic reticulum Ca2+ ATPase pump is a major regulator of glucose transport in the healthy and diabetic heart. Biochim. Biophys. Acta 2015, 1852, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ferrat, A.; Llanos, P.; Vasquez, C.; Espinosa, A.; Osorio-Fuentealba, C.; Arias-Calderon, M.; Lavandero, S.; Klip, A.; Hidalgo, C.; Jaimovich, E. Insulin elicits a ROS-activated and an IP(3)-dependent Ca(2)(+) release, which both impinge on GLUT4 translocation. J. Cell Sci. 2014, 127, 1911–1923. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Zhu, X.; Zhao, Y.; Zhang, D.; Pannu, A.; Chen, L.; Niu, W. PKC and Rab13 mediate Ca2+ signal-regulated GLUT4 traffic. Biochem. Biophys. Res. Commun. 2018, 495, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Loke, Y.K.; Furberg, C.D. Long-term risk of cardiovascular events with rosiglitazone: A meta-analysis. JAMA 2007, 298, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.P.; Duvuuri, V. Diabetes mellitus. Clin. Podiatr. Med. Surg. 2002, 19, 79–107. [Google Scholar] [CrossRef]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mangelinckx, S.; Adams, A.; Wang, Z.T.; Li, W.L.; De Kimpe, N. Natural flavonoids as potential herbal medication for the treatment of diabetes mellitus and its complications. Nat. Prod. Commun. 2015, 10, 187–200. [Google Scholar] [PubMed]
- Zaid, H.; Mahdi, A.A.; Tamrakar, A.K. Natural Active Ingredients for Diabetes and Metabolism Disorders Treatment. Evid. Based Complement. Altern. Med. 2016, 2016, 2965214. [Google Scholar] [CrossRef] [PubMed]
- Testa, R.; Bonfigli, A.R.; Genovese, S.; De Nigris, V.; Ceriello, A. The Possible Role of Flavonoids in the Prevention of Diabetic Complications. Nutrients 2016, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Roy, S.; Nandi, S.; Ukil, B.; Lyndem, L.M. Senna alexandrina Mill. induced ultrastructural changes on Hymenolepis diminuta. J. Parasit. Dis. 2017, 41, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Rama Reddy, N.R.; Mehta, R.H.; Soni, P.H.; Makasana, J.; Gajbhiye, N.A.; Ponnuchamy, M.; Kumar, J. Next Generation Sequencing and Transcriptome Analysis Predicts Biosynthetic Pathway of Sennosides from Senna (Cassia angustifolia Vahl.), a Non-Model Plant with Potent Laxative Properties. PLoS ONE 2015, 10, e0129422. [Google Scholar] [CrossRef] [PubMed]
- Muanda, F.N.; Bouayed, J.; Djilani, A.; Yao, C.; Soulimani, R.; Dicko, A. Chemical Composition and, Cellular Evaluation of the Antioxidant Activity of Desmodium adscendens Leaves. Evid. Based Complement. Altern. Med. 2011, 2011, 620862. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Ali, H.M. Bioactivities of Traditional Medicinal Plants in Alexandria. Evid. Based Complement. Altern. Med. 2018, 2018, 1463579. [Google Scholar] [CrossRef] [PubMed]
- Susunaga-Notario Adel, C.; Perez-Gutierrez, S.; Zavala-Sanchez, M.A.; Almanza-Perez, J.C.; Gutierrez-Carrillo, A.; Arrieta-Baez, D.; Lopez-Lopez, A.L.; Roman-Ramos, R.; Flores-Saenz, J.L.; Alarcon-Aguilar, F.J. Bioassay-guided chemical study of the anti-inflammatory effect of Senna villosa (Miller) H.S. Irwin & Barneby (Leguminosae) in TPA-induced ear edema. Molecules 2014, 19, 10261–10278. [Google Scholar] [PubMed]
- Pereira, R.M.; Ferreira-Silva, G.A.; Pivatto, M.; Santos Lde, A.; Bolzani Vda, S.; Chagas de Paula, D.A.; Oliveira, J.C.; Viegas Junior, C.; Ionta, M. Alkaloids derived from flowers of Senna spectabilis, (-)-cassine and (-)-spectaline, have antiproliferative activity on HepG2 cells for inducing cell cycle arrest in G1/S transition through ERK inactivation and downregulation of cyclin D1 expression. Toxicol. In Vitro 2016, 31, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Hishe, H.Z.; Ambech, T.A.; Hiben, M.G.; Fanta, B.S. Anti-nociceptive effect of methanol extract of leaves of Senna singueana in mice. J. Ethnopharmacol. 2018, 217, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Hiben, M.G.; Sibhat, G.G.; Fanta, B.S.; Gebrezgi, H.D.; Tesema, S.B. Evaluation of Senna singueana leaf extract as an alternative or adjuvant therapy for malaria. J. Tradit. Complement. Med. 2016, 6, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Thilagam, E.; Parimaladevi, B.; Kumarappan, C.; Mandal, S.C. Alpha-Glucosidase and alpha-amylase inhibitory activity of Senna surattensis. J. Acupunct. Meridian. Stud. 2013, 6, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ayinla, T.M.; Owoyele, V.B.; Yakubu, T.M. Effect of Ethanolic Leaf Extract of Senna Fistula on some Haematological Parameters, Lipid Profile and Oxidative Stress in Alloxan-induced Diabetic Rats. Niger. J. Physiol. Sci. 2015, 30, 87–93. [Google Scholar] [PubMed]
- Malematja, R.O.; Bagla, V.P. Potential Hypoglycaemic and Antiobesity Effects of Senna italica Leaf Acetone Extract. Evid. Based Complement. Altern. Med. 2018, 2018, 5101656. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.A.; Mateos, L.; Maioli, S.; Merino-Serrais, P. 27-Hydroxycholesterol impairs neuronal glucose uptake through an IRAP/GLUT4 system dysregulation. J. Exp. Med. 2017, 214, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, L.; Wang, D.; Zhang, X.; Li, J.; Ali, S.; Lu, J.; Zong, H.; Xu, X. Staurosporine as an agonist for induction of GLUT4 translocation, identified by a pH-sensitive fluorescent IRAP-mOrange2 probe. Biochem. Biophys. Res. Commun. 2016, 480, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Fan, J.; Bai, L.; Wang, Y.; Chen, Y.; Yang, L.; Chen, L.; Xu, T. Direct quantification of fusion rate reveals a distal role for AS160 in insulin-stimulated fusion of GLUT4 storage vesicles. J. Biol. Chem. 2008, 283, 8508–8516. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Chen, G.L.; Daskoulidou, N.; Xu, S.Z. The ryanodine receptor agonist 4-chloro-3-ethylphenol blocks ORAI store-operated channels. Br. J. Pharmacol. 2014, 171, 1250–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Rathore, S.S.; Davis, E.M.; Ouyang, Y.; Shen, J. Doc2b promotes GLUT4 exocytosis by activating the SNARE-mediated fusion reaction in a calcium- and membrane bending-dependent manner. Mol. Biol. Cell 2013, 24, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Gong, Z.; Mansuy-Aubert, V.; Zhou, Q.L.; Tatulian, S.A.; Sehrt, D.; Gnad, F.; Brill, L.M.; Motamedchaboki, K.; Chen, Y.; et al. C2 domain-containing phosphoprotein CDP138 regulates GLUT4 insertion into the plasma membrane. Cell Metab. 2011, 14, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Chen, Y. Antidiabetic Effects of a Chinese Herbal Medicinal Compound Sangguayin Preparation via PI3K/Akt Signaling Pathway in db/db Mice. Evid. Based Complement. Altern. Med. 2018, 2018, 2010423. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Chang, H. Wushenziye Formula Improves Skeletal Muscle Insulin Resistance in Type 2 Diabetes Mellitus via PTP1B-IRS1-Akt-GLUT4 Signaling Pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 4393529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, Y.; Liu, J.; Jin, M.; Qin, N.; Chen, Y.; Niu, W.; Duan, H. AMPK/AS160 mediates tiliroside derivatives-stimulated GLUT4 translocation in muscle cells. Drug Des. Dev. Ther. 2018, 12, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Beaton, N.; Rudigier, C.; Moest, H.; Muller, S.; Mrosek, N.; Roder, E.; Rudofsky, G.; Rulicke, T.; Ukropec, J.; Ukropcova, B.; et al. TUSC5 regulates insulin-mediated adipose tissue glucose uptake by modulation of GLUT4 recycling. Mol. Metab. 2015, 4, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Waller, A.P.; Burns, T.A.; Mudge, M.C.; Belknap, J.K.; Lacombe, V.A. Insulin resistance selectively alters cell-surface glucose transporters but not their total protein expression in equine skeletal muscle. J. Vet. Intern. Med. 2011, 25, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Werno, M.W.; Chamberlain, L.H. S-acylation of the Insulin-Responsive Aminopeptidase (IRAP): Quantitative analysis and Identification of Modified Cysteines. Sci. Rep. 2015, 5, 12413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigorash, B.B.; Suvorova, I.I.; Pospelov, V.A. AICAR-Dependent Activation of AMPK Kinase Is Not Accompanied by G1/S Block in Mouse Embryonic Stem Cells. Mol. Biol. (Mosk) 2018, 52, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Deng, Y.; Yue, Y.; Chen, W.; Zhang, Y.; Shi, G.; Wu, Z. Glutamine Enhances the Hypoglycemic Effect of Insulin in L6 Cells via Phosphatidylinositol-3-Kinase (PI3K)/Protein Kinase B (AKT)/Glucose Transporter 4 (GLUT4) Signaling Pathway. Med. Sci. Monit. 2018, 24, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, T. Dioleoylphosphoethanolamine Retains Cell Surface GLUT4 by Inhibiting PKCalpha-Driven Internalization. Cell. Physiol. Biochem. 2018, 46, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Litosch, I. Regulating G protein activity by lipase-independent functions of phospholipase C. Life Sci. 2015, 137, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, J.P.; Molero, J.C.; Clark, S.; Martin, S.; Meneilly, G.; James, D.E. The role of Ca2+ in insulin-stimulated glucose transport in 3T3-L1 cells. J. Biol. Chem. 2001, 276, 27816–27824. [Google Scholar] [CrossRef] [PubMed]
- Klip, A. The many ways to regulate glucose transporter 4. Appl. Physiol. Nutr. Metab. 2009, 34, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Kim, J.H.; Ghim, J.; Yoon, J.H.; Lee, A.; Kwon, Y.; Hyun, H.; Moon, H.Y.; Choi, H.S.; Berggren, P.O.; et al. Emodin regulates glucose utilization by activating AMP-activated protein kinase. J. Biol. Chem. 2013, 288, 5732–5742. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Bai, H.; Yin, S. Renoprotective effects of emodin against diabetic nephropathy in rat models are mediated via PI3K/Akt/GSK-3beta and Bax/caspase-3 signaling pathways. Exp. Ther. Med. 2017, 14, 5163–5169. [Google Scholar] [PubMed]
- Lee, M.S.; Sohn, C.B. Anti-diabetic properties of chrysophanol and its glucoside from rhubarb rhizome. Biol. Pharm. Bull. 2008, 31, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Muthusamy, V.S.; Sujatha, S.; Sangeetha, K.N.; Bharathi Raja, R.; Sudhagar, S.; Poornima Devi, N.; Lakshmi, B.S. Aloe emodin glycosides stimulates glucose transport and glycogen storage through PI3K dependent mechanism in L6 myotubes and inhibits adipocyte differentiation in 3T3L1 adipocytes. FEBS Lett. 2010, 584, 3170–3178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkhalidy, H.; Moore, W.; Zhang, Y.; McMillan, R. Small Molecule Kaempferol Promotes Insulin Sensitivity and Preserved Pancreatic beta-Cell Mass in Middle-Aged Obese Diabetic Mice. J. Diabetes Res. 2015, 2015, 532984. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, X.; Xiong, M.; Xu, X.; Zhen, L.; Chen, W.; Wang, Y.; Shen, J.; Zhao, P.; Liu, Q.H. Chloroquine Increases Glucose Uptake via Enhancing GLUT4 Translocation and Fusion with the Plasma Membrane in L6 Cells. Cell. Physiol. Biochem. 2016, 38, 2030–2040. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Furuhashi, K.; Son, M.J.; Toyozaki, M.; Yoshizawa, F.; Miura, Y.; Yagasaki, K. Antidiabetic effect of enterolactone in cultured muscle cells and in type 2 diabetic model db/db mice. Cytotechnology 2017, 69, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; He, J.; Yang, L.; Luo, Q.; Liu, Z. Histone Deacetylase-3 Modification of MicroRNA-31 Promotes Cell Proliferation and Aerobic Glycolysis in Breast Cancer and Is Predictive of Poor Prognosis. J. Breast Cancer 2018, 21, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Deng, S.; Han, Q.; Zhao, P.; Zhou, Q.; Zheng, S.; Ma, X.; Xu, C.; Yang, J.; Yang, X. Hypoglycemic Activity and the Potential Mechanism of the Flavonoid Rich Extract from Sophora tonkinensis Gagnep. in KK-Ay Mice. Front. Pharmacol. 2016, 7, 288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Khayat, Z.; Kishi, K.; Ebina, Y.; Klip, A. GLUT4 translocation by insulin in intact muscle cells: Detection by a fast and quantitative assay. FEBS Lett. 1998, 427, 193–197. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Ming, Q.; Qiu, J.; Tian, D.; Liu, J.; Shen, J.; Liu, Q.-H.; Yang, X. Ethanolic Extract of Folium Sennae Mediates the Glucose Uptake of L6 Cells by GLUT4 and Ca2+. Molecules 2018, 23, 2934. https://doi.org/10.3390/molecules23112934
Zhao P, Ming Q, Qiu J, Tian D, Liu J, Shen J, Liu Q-H, Yang X. Ethanolic Extract of Folium Sennae Mediates the Glucose Uptake of L6 Cells by GLUT4 and Ca2+. Molecules. 2018; 23(11):2934. https://doi.org/10.3390/molecules23112934
Chicago/Turabian StyleZhao, Ping, Qian Ming, Junying Qiu, Di Tian, Jia Liu, Jinhua Shen, Qing-Hua Liu, and Xinzhou Yang. 2018. "Ethanolic Extract of Folium Sennae Mediates the Glucose Uptake of L6 Cells by GLUT4 and Ca2+" Molecules 23, no. 11: 2934. https://doi.org/10.3390/molecules23112934
APA StyleZhao, P., Ming, Q., Qiu, J., Tian, D., Liu, J., Shen, J., Liu, Q.-H., & Yang, X. (2018). Ethanolic Extract of Folium Sennae Mediates the Glucose Uptake of L6 Cells by GLUT4 and Ca2+. Molecules, 23(11), 2934. https://doi.org/10.3390/molecules23112934