Selective Photokilling of Human Pancreatic Cancer Cells Using Cetuximab-Targeted Mesoporous Silica Nanoparticles for Delivery of Zinc Phthalocyanine
Abstract
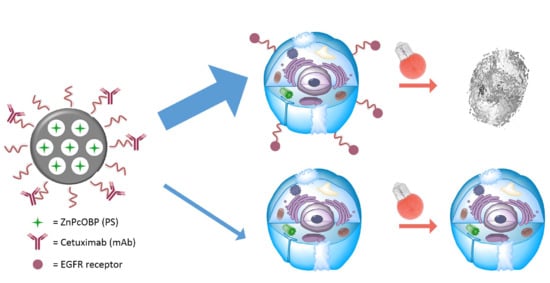
1. Introduction
2. Results
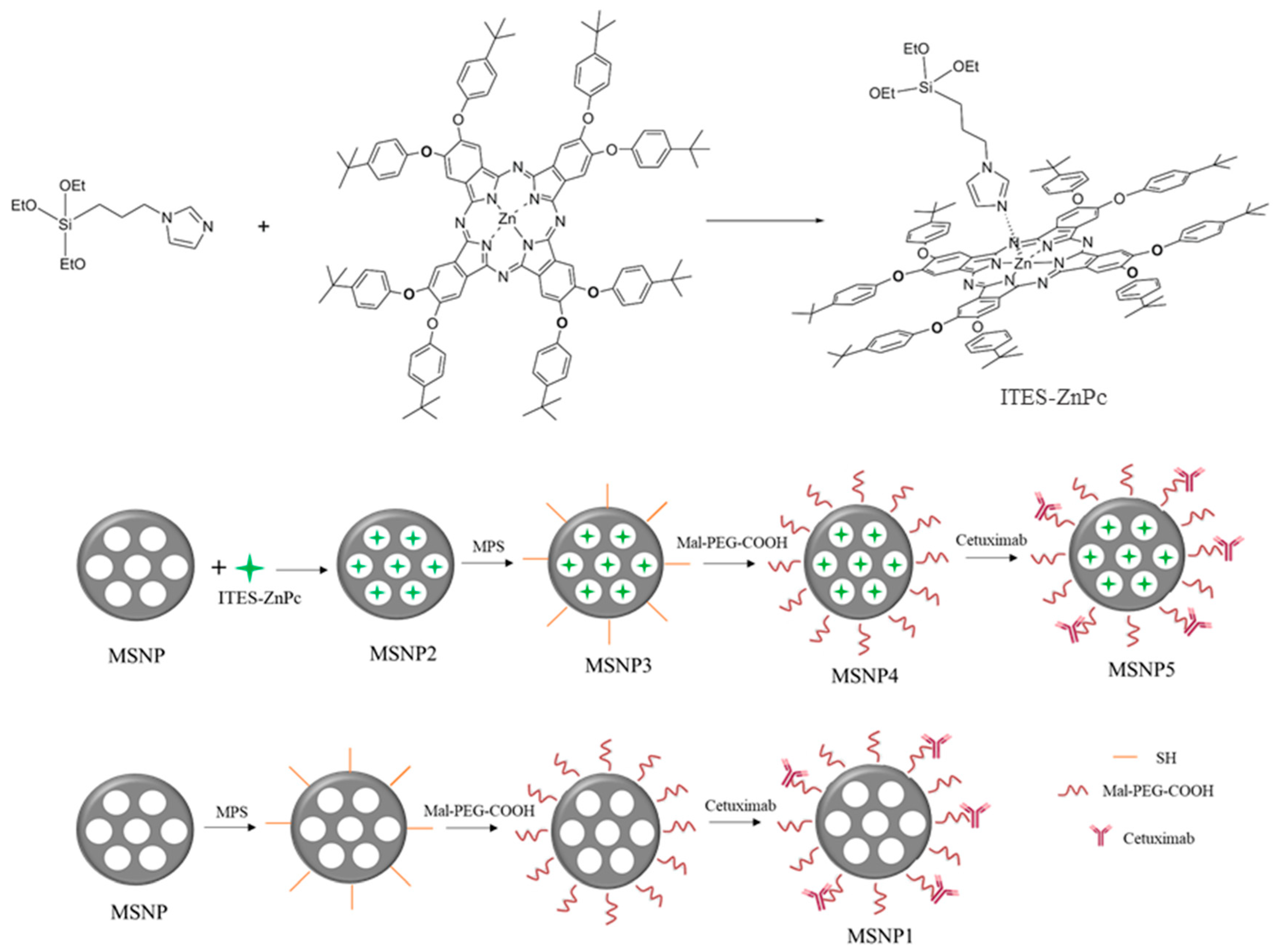
2.1. Synthesis of Zinc(II) 2,3,9,10,16,17,23,24-octa(tert-butylphenoxy))phthalocyaninato(2-)-N29,N30,N31,N32 (ZnPcOBP)
2.2. Preparation of Nanoparticles
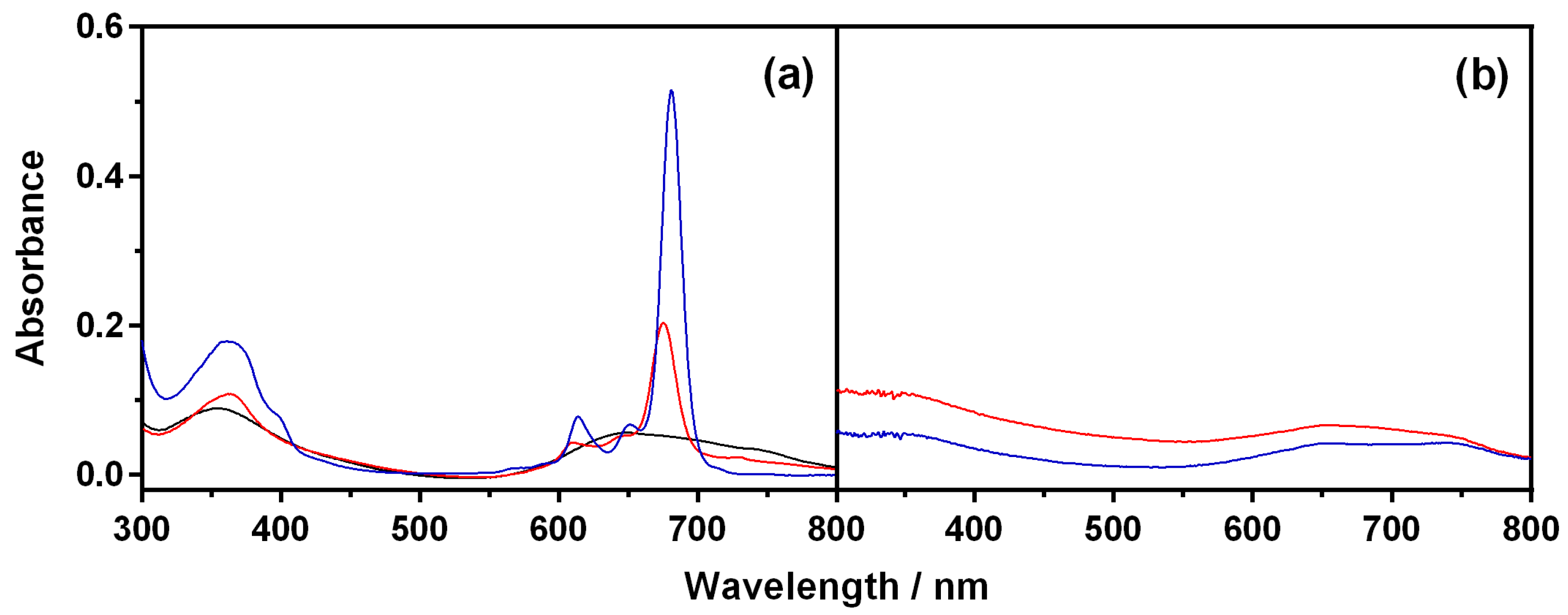
2.3. Characterization of Nanoparticles
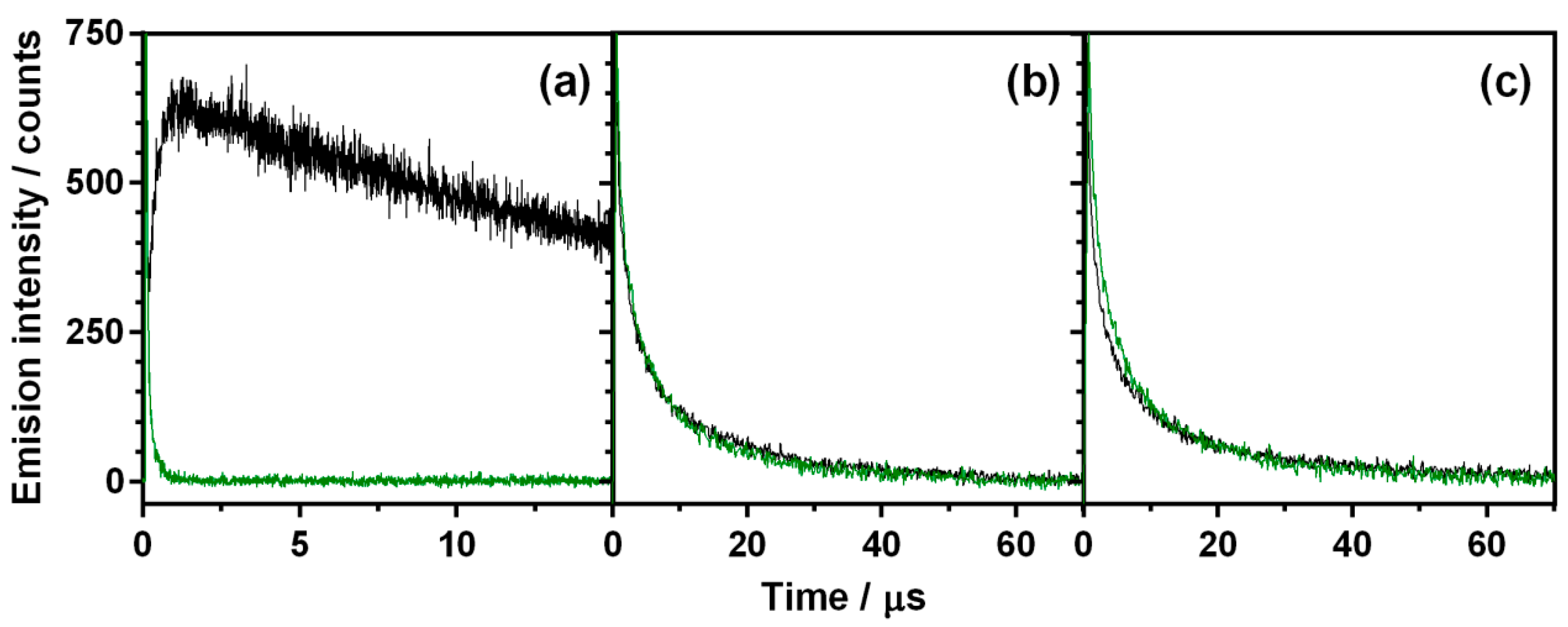
2.4. Singlet Oxygen Production
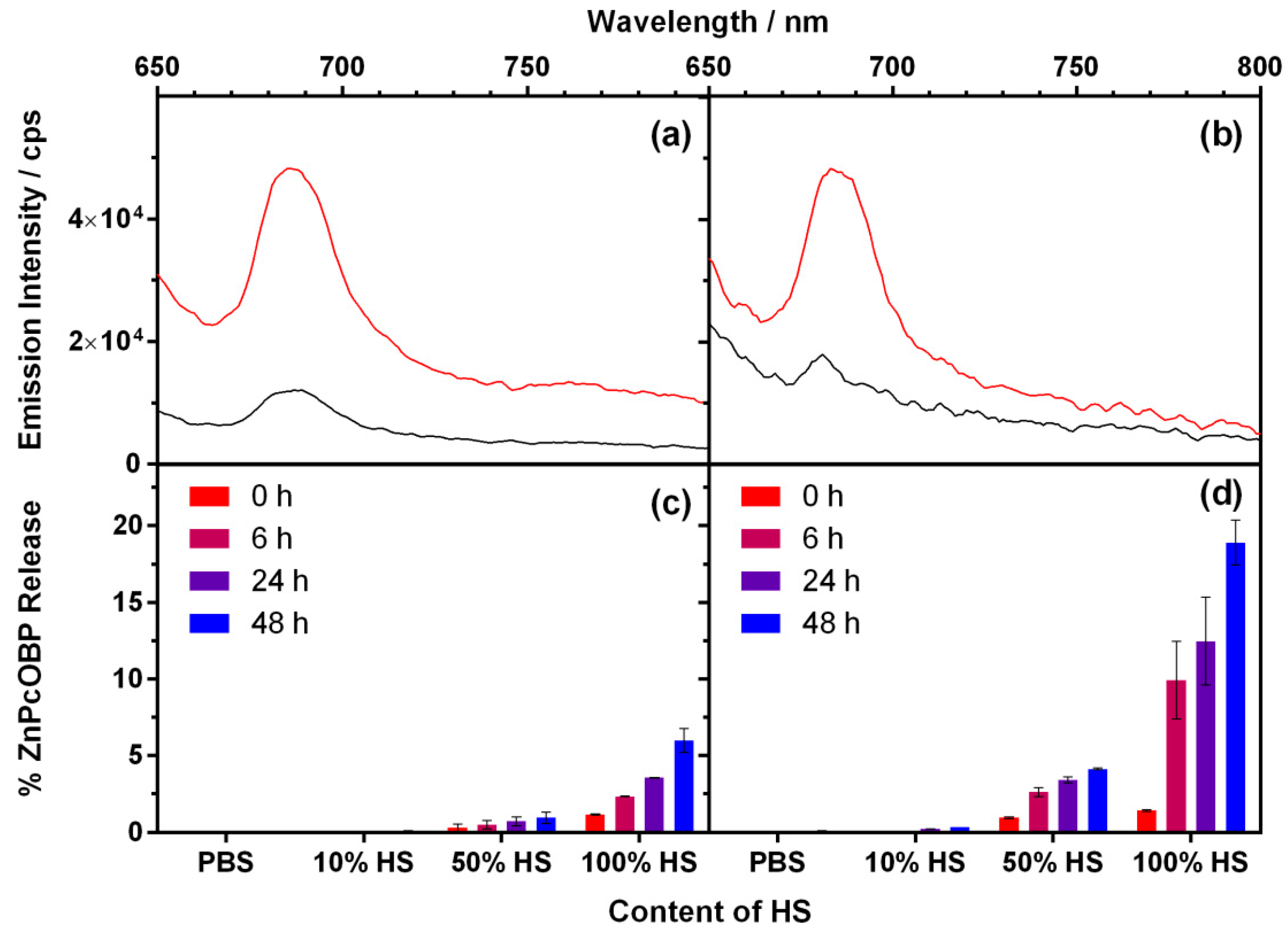
2.5. Release of ZnPcOBP from the Nanoparticles
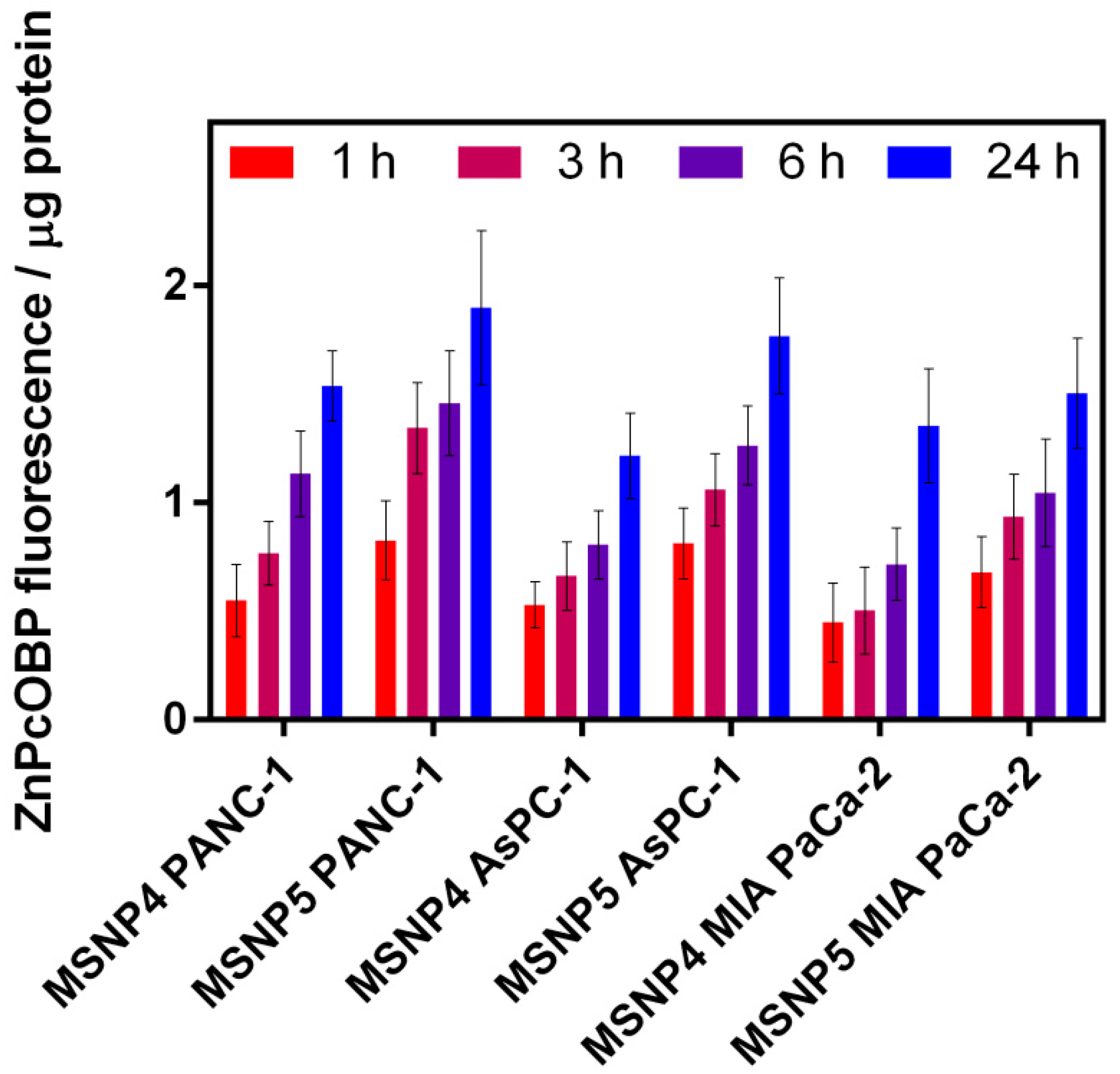
2.6. Cellular Uptake of MSNP4 and MSNP5
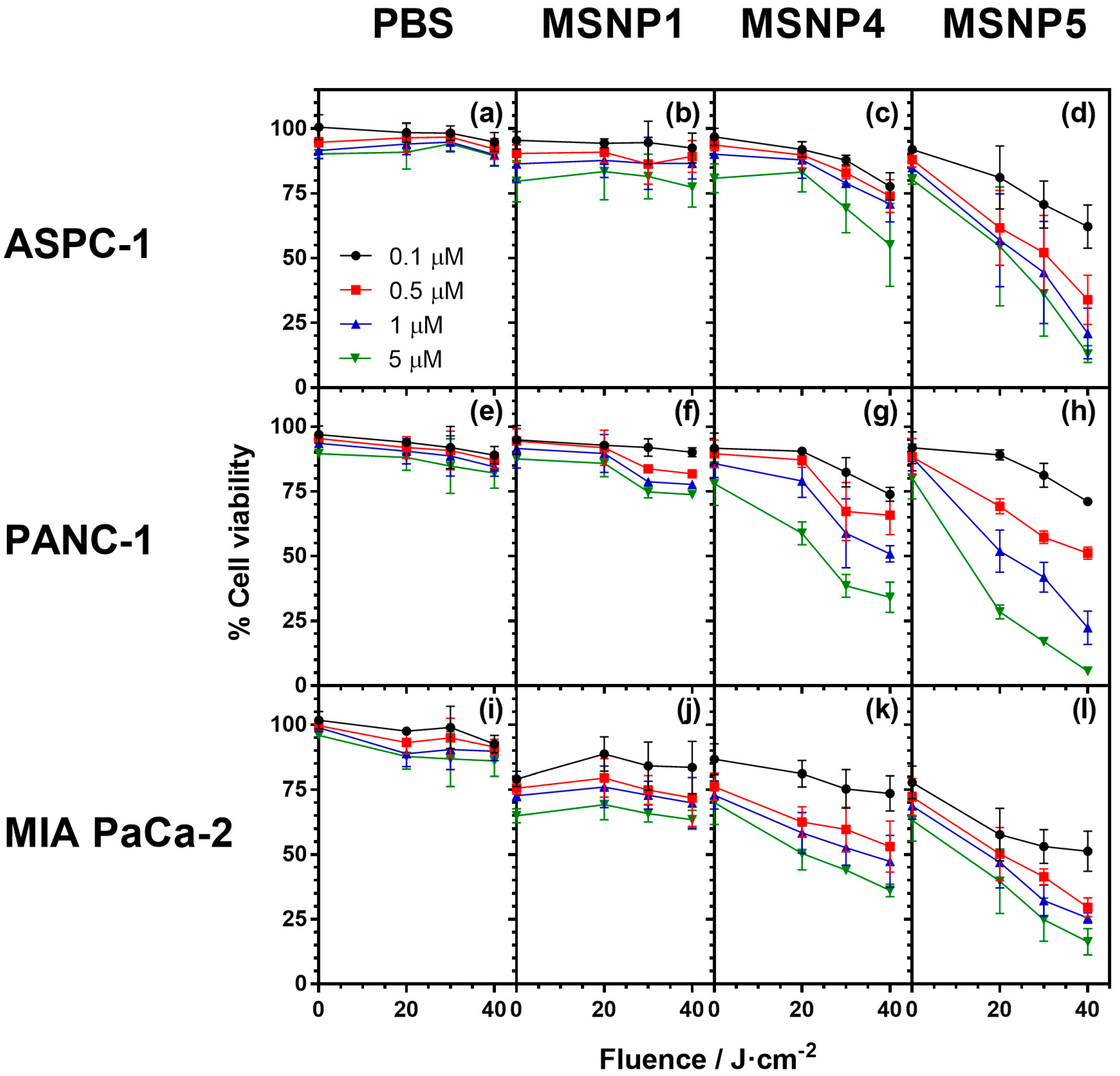
2.7. Cell Photocytotoxicity Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Derivatitzation of Mesoporous Silica Nanoparticles
4.2.1. Synthesis of Zinc (II) 2,3,9,10,16,17,23,24-octa(tert-butylphenoxy))phthalocyaninato (2-)-N29,N30,N31,N32 (ZnPcOBP)
4.2.2. Preparation of ITES-ZnPc Complex
4.2.3. Synthesis of Mesoporous Silica Nanoparticles and Encapsulation of ZnPcOBP (MSNP2)
4.2.4. Synthesis of MSNP3
4.2.5. Synthesis of MSNP4
4.2.6. Synthesis of MSNP5
4.2.7. Synthesis of MSNP1
4.2.8. Nanoparticle Characterization
4.3. Spectroscopic Techniques
4.4. Cellular Techniques
4.4.1. Cell Lines and Culture Conditions
4.4.2. Cellular uptake Assays
4.4.3. In Vitro Dark- and Phototoxicity Assays
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Borja-Cacho, D.; Jensen, E.H.; Saluja, A.K.; Buchsbaum, D.J.; Vickers, S.M. Molecular targeted therapies for pancreatic cancer. Am. J. Surg. 2008, 196, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, N.; Gnoni, A.; Brunetti, A.; Vincenti, L.; Santini, D.; Tonini, G.; Merchionne, F.; Maiello, E.; Lorusso, V.; Nardulli, P.; et al. Target therapies in pancreatic carcinoma. Curr. Med. Chem. 2014, 21, 948–965. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Da Silva, R.; Gravekamp, C.; Libutti, S.K.; Abraham, T.; Dadachova, E. Targeted radionuclide therapies for pancreatic cancer. Cancer Gene Ther. 2015, 22, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, S.; Rocha-Lima, C.M. Advanced or metastatic pancreatic cancer: Molecular targeted therapies. Mt. Sinai J. Med. J. Transl. Pers. Med. 2010, 77, 606–619. [Google Scholar] [CrossRef] [PubMed]
- P. Torres, M.; Chakraborty, S.; Souchek, J.; K. Batra, S. Mucin-based targeted pancreatic cancer therapy. Curr. Pharm. Des. 2012, 18, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Patterson, M.S. The physics, biophysics and technology of photodynamic therapy. Phys. Med. Biol. 2008, 53, R61–R109. [Google Scholar] [CrossRef] [PubMed]
- Nonell, S.; Flors, C. (Eds.) Singlet Oxygen: Applications in Biosciences and Nanosciences; The Royal Society of Chemistry: London, UK, 2016. [Google Scholar]
- Zorlu, Y.; Dumoulin, F.; Bouchu, D.; Ahsen, V.; Lafont, D. Monoglycoconjugated water-soluble phthalocyanines. Design and synthesis of potential selectively targeting PDT photosensitisers. Tetrahedron Lett. 2010, 51, 6615–6618. [Google Scholar] [CrossRef]
- Sekkat, N.; van den Bergh, H.; Nyokong, T.; Lange, N. Like a bolt from the blue: Phthalocyanines in biomedical optics. Molecules 2011, 17, 98–144. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Marrero, V.; Van de Winckel, E.; Anaya-Plaza, E.; Torres, T.; de la Escosura, A. Porphyrinoid biohybrid materials as an emerging toolbox for biomedical light management. Chem. Soc. Rev. 2018, 47, 7369–7400. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Kobayashi, N. Optically active porphyrin and phthalocyanine systems. Chem. Rev. 2016, 116, 6184–6261. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Plaza, E.; van de Winckel, E.; Mikkilä, J.; Malho, J.M.; Ikkala, O.; Gulías, O.; Bresolí-Obach, R.; Agut, M.; Nonell, S.; Torres, T.; et al. Photoantimicrobial biohybrids by supramolecular immobilization of cationic phthalocyanines onto cellulose nanocrystals. Chem. Eur. J. 2017, 23, 4320–4326. [Google Scholar] [CrossRef] [PubMed]
- Bechet, D.; Couleaud, P.; Frochot, C.; Viriot, M.L.; Guillemin, F.; Barberi-Heyob, M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008, 26, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Lin, W. Introduction: Nanoparticles in medicine. Chem. Rev. 2015, 115, 10407–10409. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.K.; Heo, J.; Shin, S.; Jeong, K.; Seo, Y.H.; Jang, W.D.; Park, C.R.; Park, S.Y.; Kim, S.; Kwon, I.C. Nanophotosensitizers toward advanced photodynamic therapy of Cancer. Cancer Lett. 2013, 334, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Zhou, H.Y. Molecularly targeted gemcitabine-loaded nanoparticulate system towards the treatment of EGFR overexpressing lung cancer. Biomed. Pharmacother. 2015, 70, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Bresolí-Obach, R.; Nos, J.; Mora, M.; Sagristà, M.L.; Ruiz-González, R.; Nonell, S. Anthracene-based fluorescent nanoprobes for singlet oxygen detection in biological media. Methods 2016, 109, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Couleaud, P.; Morosini, V.; Frochot, C.; Richeter, S.; Raehm, L.; Durand, J.O. Silica-based nanoparticles for photodynamic therapy applications. Nanoscale 2010, 2, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Monge-Fuentes, V.; Muehlmann, L.A.; de Azevedo, R.B. Perspectives on the application of nanotechnology in photodynamic therapy for the treatment of melanoma. Nano Rev. 2014, 5, 24381. [Google Scholar] [CrossRef] [PubMed]
- Planas, O.; Bresolí-Obach, R.; Nos, J.; Gallavardin, T.; Ruiz-González, R.; Agut, M.; Nonell, S. Synthesis, photophysical characterization, and photoinduced antibacterial activity of methylene blue-loaded amino- and mannose-targeted mesoporous silica nanoparticles. Molecules 2015, 20, 6284–6298. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.H.; Wu, S.H.; Yao, M.; Lu, C.W.; Lin, Y.S.; Hung, Y.; Mou, C.Y.; Chen, Y.C.; Huang, D.M. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials 2007, 28, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.Y. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, H.Y.; Yang, L.; Zhang, Z.; Ji, H. Cetuximab-modified mesoporous silica nano-medicine specifically targets EGFR-mutant lung cancer and overcomes drug resistance. Sci. Rep. 2016, 6, 25468. [Google Scholar] [CrossRef] [PubMed]
- Maree, S.E.; Nyokong, T. Syntheses and photochemical properties of octasubstituted phthalocyaninato zinc complexes. J. Porphyr. Phthalocyanines 2001, 5, 782–792. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, E.; Suardi-Murasecco, P.; Aminian-Saghafi, T.; Braun, A.M.; Hansen, H.J. 1H-Phenalen-1-one: Photophysical properties and singlet-oxygen production. Helv. Chim. Acta 1991, 74, 79–90. [Google Scholar] [CrossRef]
- Schmidt, R.; Tanielian, C.; Dunsbach, R.; Wolff, C. Phenalenone, a universal reference compound for the determination of quantum yields of singlet oxygen O2(1Δg) sensitization. J. Photochem. Photobiol. A Chem. 1994, 79, 11–17. [Google Scholar] [CrossRef]
- Martí, C.; Jürgens, O.; Cuenca, O.; Casals, M.; Nonell, S. Aromatic ketones as standards for singlet molecular oxygen photosensitization. Time-resolved photoacoustic and near-IR emission studies. J. Photochem. Photobiol. A Chem. 1996, 97, 11–18. [Google Scholar] [CrossRef]
- Li, M.Y.; Cline, C.S.; Koker, E.B.; Carmichael, H.H.; Chignell, C.F.; Bilski, P. Quenching of singlet molecular oxygen (1O2) by azide anion in solvent mixtures. Photochem. Photobiol. 2001, 74, 760–764. [Google Scholar] [CrossRef]
- Patra, C.R.; Bhattacharya, R.; Wang, E.; Katarya, A.; Lau, J.S.; Dutta, S.; Muders, M.; Wang, S.; Buhrow, S.A.; Safgren, S.L.; et al. Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent. Cancer Res. 2008, 68, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Ogunsipe, A.; Maree, D.; Nyokong, T. Solvent effects on the photochemical and fluorescence properties of zinc phthalocyanine derivatives. J. Mol. Struct. 2003, 650, 131–140. [Google Scholar] [CrossRef]
- Ince, M.; Gartmann, N.; Claessens, C.G.; Torres, T.; Bruhwiler, D. Synthesis of Subphthalocyanines as probes for the accessibility of silica nanochannels. Org. Lett. 2011, 13, 4918–4921. [Google Scholar] [CrossRef] [PubMed]
- Durgun, G.; Ocakoglu, K.; Ozcelik, S. Systematic tuning the hydrodynamic diameter of uniformed fluorescent silica nanoparticles. J. Phys. Chem. C 2011, 115, 16322–16332. [Google Scholar] [CrossRef]
- Chen, F.; Hong, H.; Zhang, Y.; Valdovinos, H.F.; Shi, S.; Kwon, G.S.; Theuer, C.P.; Barnhart, T.E.; Cai, W. In Vivo tumor targeting and image-guided drug delivery with antibody-conjugated, radiolabeled mesoporous silica nanoparticles. ACS Nano 2013, 7, 9027–9039. [Google Scholar] [CrossRef] [PubMed]
- Karra, N.; Nassar, T.; Ripin, A.N.; Schwob, O.; Borlak, J.; Benita, S. Antibody conjugated PLGA nanoparticles for targeted delivery of paclitaxel palmitate: Efficacy and biofate in a lung cancer mouse model. Small 2013, 9, 4221–4236. [Google Scholar] [CrossRef] [PubMed]
- Nonell, S.; Braslavsky, S.E. Time-resolved singlet oxygen detection. Methods Enzymol. 2000, 319, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Banzo, A.; Ragàs, X.; Kapusta, P.; Nonell, S. Time-resolved methods in biophysics. 7. Photon counting vs. analog time-resolved singlet oxygen phosphorescence detection. Photochem. Photobiol. Sci. 2008, 7, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- PierceTM BCA Protein Assay Kit. Available online: http://www.thermofisher.com/order/catalog/product/23225 (accessed on 24 August 2018).
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in cell biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| Title 1 | MSNP1 | MSNP3 | MSNP4 | MSNP5 |
|---|---|---|---|---|
| Size (nm) | 126 ± 8 | 262 ± 15 | 285 ± 27 | 303 ± 26 |
| Zeta Potential (mV) | −14 ± 4 | −27 ± 1 | −17 ± 5 | −9 ± 7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Er, Ö.; Colak, S.G.; Ocakoglu, K.; Ince, M.; Bresolí-Obach, R.; Mora, M.; Sagristá, M.L.; Yurt, F.; Nonell, S. Selective Photokilling of Human Pancreatic Cancer Cells Using Cetuximab-Targeted Mesoporous Silica Nanoparticles for Delivery of Zinc Phthalocyanine. Molecules 2018, 23, 2749. https://doi.org/10.3390/molecules23112749
Er Ö, Colak SG, Ocakoglu K, Ince M, Bresolí-Obach R, Mora M, Sagristá ML, Yurt F, Nonell S. Selective Photokilling of Human Pancreatic Cancer Cells Using Cetuximab-Targeted Mesoporous Silica Nanoparticles for Delivery of Zinc Phthalocyanine. Molecules. 2018; 23(11):2749. https://doi.org/10.3390/molecules23112749
Chicago/Turabian StyleEr, Özge, Suleyman Gokhan Colak, Kasim Ocakoglu, Mine Ince, Roger Bresolí-Obach, Margarita Mora, Maria Lluïsa Sagristá, Fatma Yurt, and Santi Nonell. 2018. "Selective Photokilling of Human Pancreatic Cancer Cells Using Cetuximab-Targeted Mesoporous Silica Nanoparticles for Delivery of Zinc Phthalocyanine" Molecules 23, no. 11: 2749. https://doi.org/10.3390/molecules23112749
APA StyleEr, Ö., Colak, S. G., Ocakoglu, K., Ince, M., Bresolí-Obach, R., Mora, M., Sagristá, M. L., Yurt, F., & Nonell, S. (2018). Selective Photokilling of Human Pancreatic Cancer Cells Using Cetuximab-Targeted Mesoporous Silica Nanoparticles for Delivery of Zinc Phthalocyanine. Molecules, 23(11), 2749. https://doi.org/10.3390/molecules23112749