Synthesis of Novel FTY720 Analogs with Anticancer Activity through PP2A Activation
Abstract
1. Introduction
2. Results and Discussion
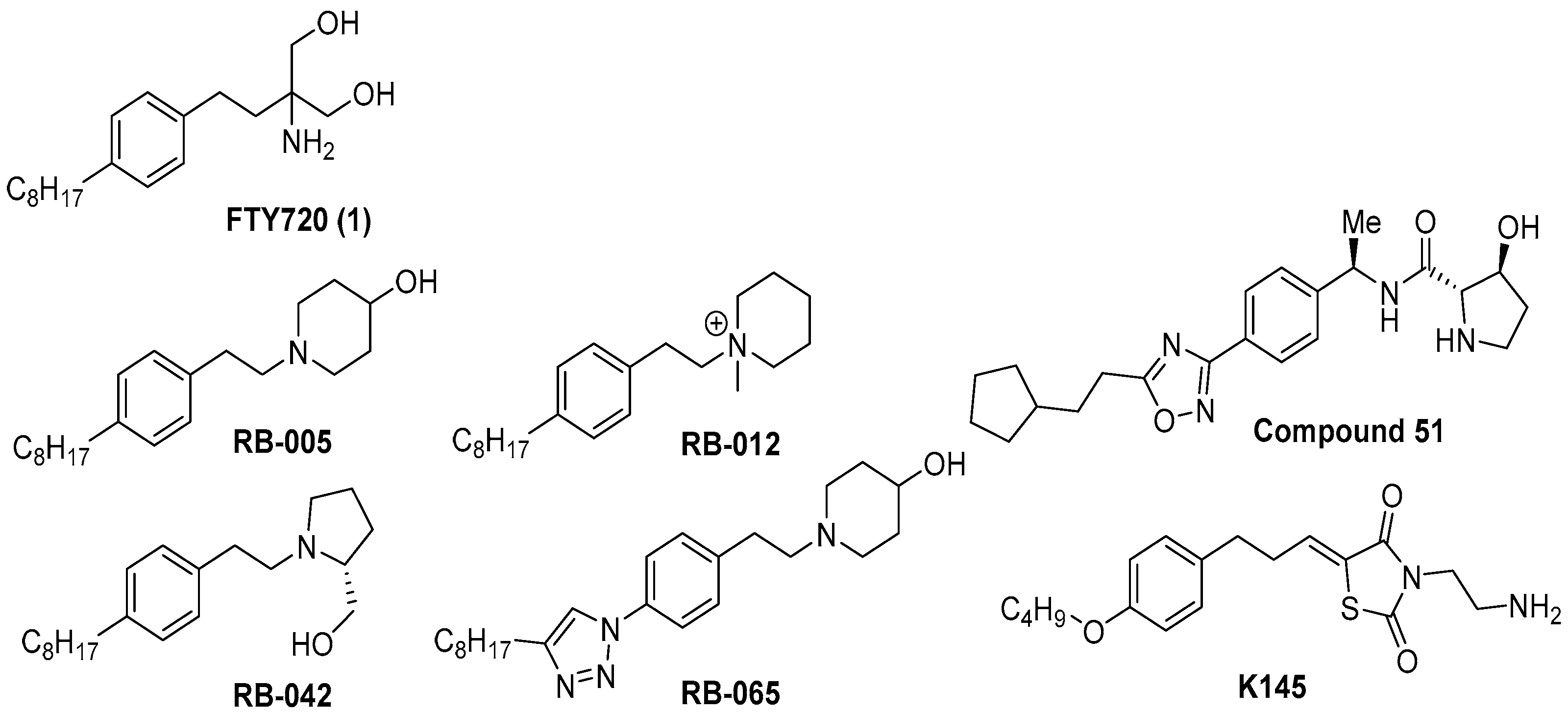
2.1. Chemical Synthesis
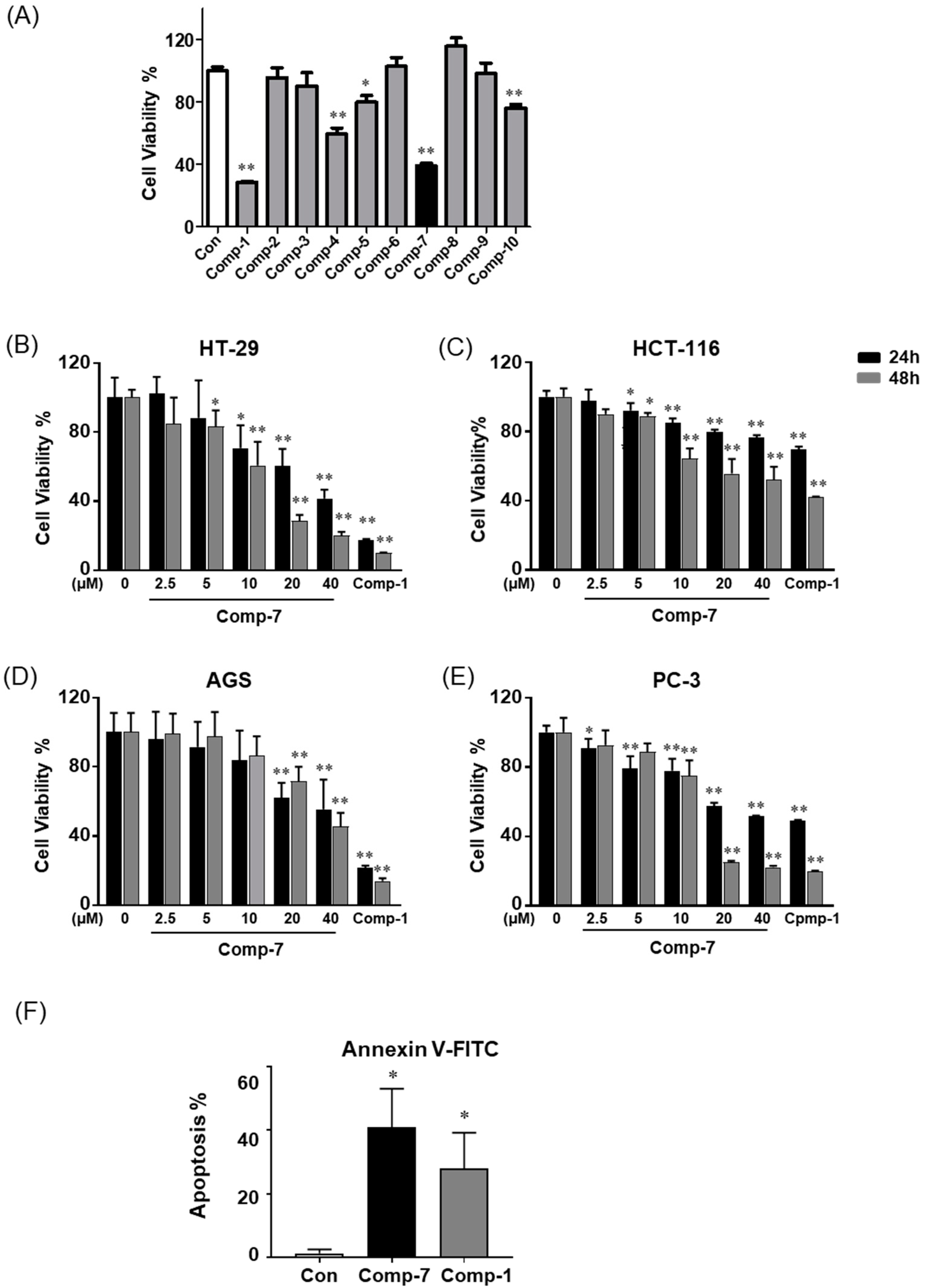
2.2. Compound 7 Inhibits the Growth of Human Cancer Cell Lines In Vitro
2.3. Effect of PF-543 and Compound 7 on SK1 Activity
2.4. Compoud 7 Effectively Activates PP2A and Decreases the Phosphorylation of ERK and AKT
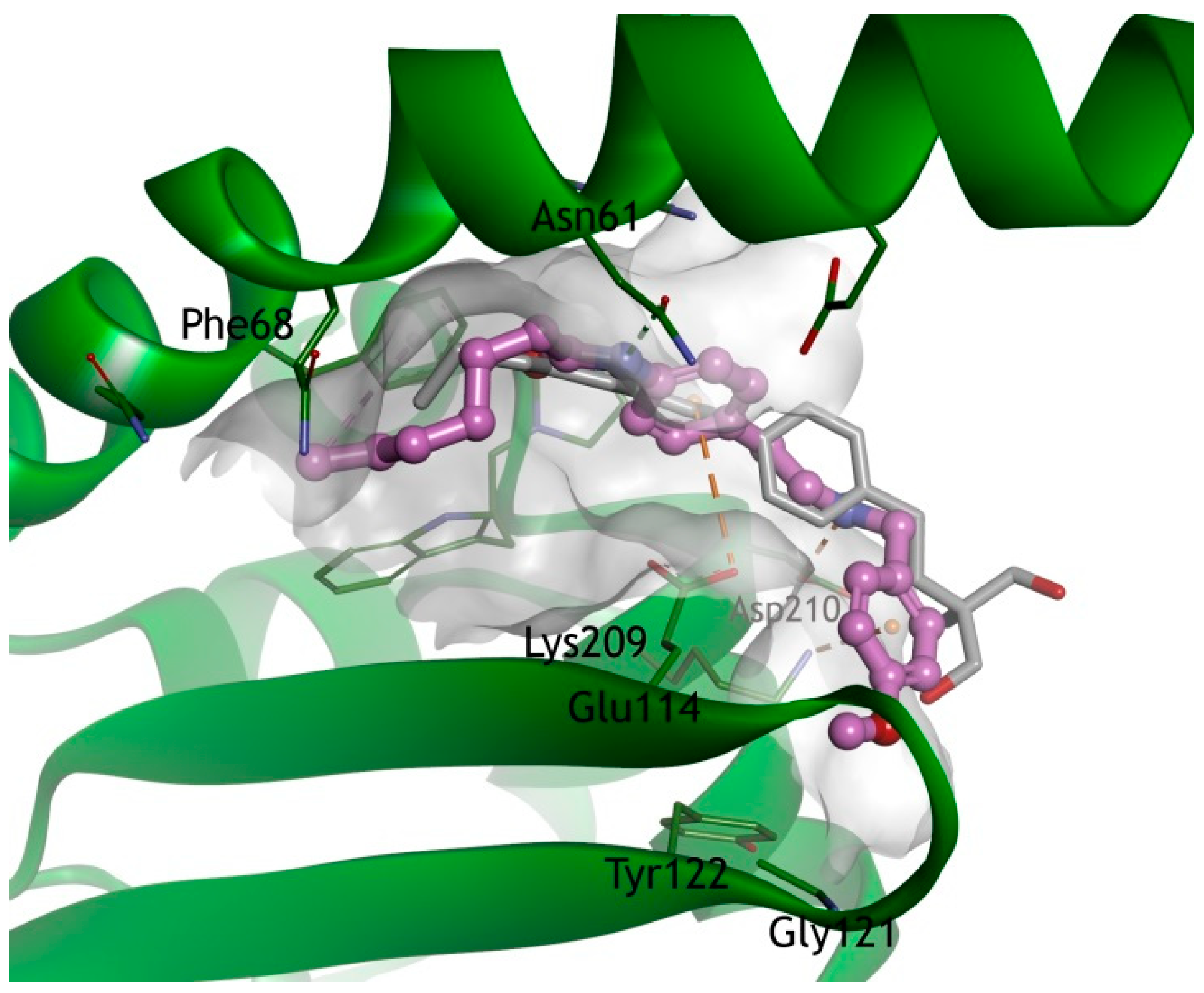
2.5. Molecular Modeling Studies of FTY720 and Compound 7 Against the Human I2PP2A/SET
3. Experimental Section
3.1. Synthesis in General
3.2. Synthesis
3.3. Chemicals, Reagents and Antibodies
3.4. Cancer Cell Line and Culture
3.5. MTT Cell Viability Assay
3.6. Annexin V Assay
3.7. PP2A Activity Assay
3.8. Immunoblotting
3.9. Molecular Modeling Studies
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pitson, S.M. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem. Sci. 2011, 36, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Goetzl, E.J.; Hla, T.; Igarashi, Y.; Lynch, K.R.; Moolenaar, W.; Pyne, S.; Tigyi, G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2002, 54, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Harikumar, K.B.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012, 22, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; McNaughton, M.; Boomkamp, S.; MacRitchie, N.; Evangelisti, C.; Martelli, A.M.; Jiang, H.R.; Ubhi, S.; Pyne, S. Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Adv. Biol. Regul. 2016, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Pitman, M.R.; Costabile, M.; Pitson, S.M. Recent advances in the development of sphingosine kinase inhibitors. Cell. Signal. 2016, 28, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.; Lima, S.; Maceyka, M.; Spiegel, S. Revisiting the sphingolipid rheostat: Evolving concepts in cancer therapy. Exp. Cell Res. 2015, 333, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Rossjohn, J.; Pellicci, D.G.; Patel, O.; Gapin, L.; Godfrey, D.I. Recognition of CD1d-restricted antigens by natural killer T cells. Nat. Rev. Immunol. 2012, 12, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Oaks, J.J.; Santhanam, R.; Walker, C.J.; Roof, S.; Harb, J.G.; Ferenchak, G.; Eisfeld, A.K.; Van Brocklyn, J.R.; Briesewitz, R.; Saddoughi, S.A.; et al. Antagonistic activities of the immunomodulator and PP2A-activating drug FTY720 (Fingolimod, Gilenya) in Jak2-driven hematologic malignancies. Blood 2013, 122, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.T.; Man, K.; Ho, J.W.; Sun, C.K.; Lee, T.K.; Zhao, Y.; Lo, C.M.; Poon, R.T.; Fan, S.T. Marked suppression of tumor growth by FTY720 in a rat liver tumor model: The significance of down-regulation of cell survival Akt pathway. Int. J. Oncol. 2007, 30, 375–380. [Google Scholar] [PubMed]
- Azuma, H.; Takahara, S.; Horie, S.; Muto, S.; Otsuki, Y.; Katsuoka, Y. Induction of apoptosis in human bladder cancer cells in vitro and in vivo caused by FTY720 treatment. J. Urol. 2003, 169, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.D.; Ji, X.J.; Cong, Z.X.; Zhu, J.H.; Zhou, Y. FTY720 for cancer therapy (Review). Oncol. Rep. 2013, 30, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, F.; Lim, K.G.; Loveridge, C.; Long, J.; Pitson, S.M.; Tigyi, G.; Bittman, R.; Pyne, S.; Pyne, N.J. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell. Signal. 2010, 22, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.G.; Tonelli, F.; Alossaimi, M.; Williamson, L.; Chan, E.; Gorshkova, I.; Berdyshev, E.; Bittman, R.; Pyne, N.J.; Pyne, S. The roles of sphingosine kinases 1 and 2 in regulating the Warburg effect in prostate cancer cells. Cell. Signal. 2013, 25, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Sun, C.; Valentine, W.J.; Shuyu, E.; Liu, J.; Tigyi, G.; Bittman, R. Chiral vinylphosphonate and phosphonate analogues of the immunosuppressive agent FTY720. J. Org. Chem. 2009, 74, 3192–3195. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.J.; MacRitchie, N.; Pyne, N.J.; Pyne, S.; Bittman, R. Synthesis of selective inhibitors of sphingosine kinase 1. Chem. Commun. 2013, 49, 2136–2138. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.J.; MacRitchie, N.; Anthony, N.G.; Mackay, S.P.; Pyne, S.; Pyne, N.J.; Bittman, R. Structure-activity relationships and molecular modeling of sphingosine kinase inhibitors. J. Med. Chem. 2013, 56, 9310–9327. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, J.M.; Coolen, C.; Goodwin, K.L.; Baek, D.J.; Bittman, R.; Samuel, M.S.; Pitson, S.M.; Lopez, A.F. Destabilisation of dimeric 14-3-3 proteins as a novel approach to anti-cancer therapeutics. Oncotarget 2015, 6, 14522–14536. [Google Scholar] [CrossRef] [PubMed]
- Bonandi, E.; Christodoulou, M.S.; Fumagalli, G.; Perdicchia, D.; Rastelli, G.; Passarella, D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Hirth, B.; Kane, J.L., Jr.; Liao, J.; Noson, K.D.; Yee, C.; Asmussen, G.; Fitzgerald, M.; Klaus, C.; Booker, M. Discovery of novel sphingosine kinase-1 inhibitors. Part 2. Bioorg. Med. Chem. Lett. 2010, 20, 4550–4554. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Guo, T.L.; Hait, N.C.; Allegood, J.; Parikh, H.I.; Xu, W.; Kellogg, G.E.; Grant, S.; Spiegel, S.; Zhang, S. Biological characterization of 3-(2-amino-ethyl)-5-[3-(4-butoxyl-phenyl)-propylidene]-thiazolidine-2,4-dione (K145) as a selective sphingosine kinase-2 inhibitor and anticancer agent. PLoS ONE 2013, 8, e56471. [Google Scholar] [CrossRef] [PubMed]
- Schnute, M.E.; McReynolds, M.D.; Kasten, T.; Yates, M.; Jerome, G.; Rains, J.W.; Hall, T.; Chrencik, J.; Kraus, M.; Cronin, C.N.; et al. Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. Biochem. J. 2012, 444, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Westermarck, J. Regulation of protein phosphatase 2A (PP2A) tumor suppressor function by PME-1. Biochem. Soc. Trans. 2016, 44, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, I.; Manso, R.; Rincón, R.; Caramés, C.; Senin, C.; Borrero, A.; Martínez-Useros, J.; Rodriguez, M.; Zazo, S.; Aguilera, O. PP2A inhibition is a common event in colorectal cancer and its restoration using FTY720 shows promising therapeutic potential. Mol. Cancer Ther. 2014, 13, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Muto, S.; Senda, M.; Akai, Y.; Sato, L.; Suzuki, T.; Nagai, R.; Senda, T.; Horikoshi, M. Relationship between the structure of SET/TAF-Ibeta/INHAT and its histone chaperone activity. Proc. Natl. Acad. Sci. USA 2007, 104, 4285–4290. [Google Scholar] [CrossRef] [PubMed]


| Compound | IC50 (µM) 24 h | |||
|---|---|---|---|---|
| HT-29 | HCT-116 | AGS | PC-3 | |
| Compound 7 | 10.39 | 7.335 | 11.04 | 7.456 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, J.; Ki, S.H.; Shin, S.M.; Kim, S.W.; Lee, J.-Y.; Jun, H.-S.; Lee, T.; Kim, S.; Baek, D.J.; Park, E.-Y. Synthesis of Novel FTY720 Analogs with Anticancer Activity through PP2A Activation. Molecules 2018, 23, 2750. https://doi.org/10.3390/molecules23112750
Shrestha J, Ki SH, Shin SM, Kim SW, Lee J-Y, Jun H-S, Lee T, Kim S, Baek DJ, Park E-Y. Synthesis of Novel FTY720 Analogs with Anticancer Activity through PP2A Activation. Molecules. 2018; 23(11):2750. https://doi.org/10.3390/molecules23112750
Chicago/Turabian StyleShrestha, Jitendra, Sung Hwan Ki, Sang Mi Shin, Seon Woong Kim, Joo-Youn Lee, Hee-Sook Jun, Taeho Lee, Sanghee Kim, Dong Jae Baek, and Eun-Young Park. 2018. "Synthesis of Novel FTY720 Analogs with Anticancer Activity through PP2A Activation" Molecules 23, no. 11: 2750. https://doi.org/10.3390/molecules23112750
APA StyleShrestha, J., Ki, S. H., Shin, S. M., Kim, S. W., Lee, J.-Y., Jun, H.-S., Lee, T., Kim, S., Baek, D. J., & Park, E.-Y. (2018). Synthesis of Novel FTY720 Analogs with Anticancer Activity through PP2A Activation. Molecules, 23(11), 2750. https://doi.org/10.3390/molecules23112750







