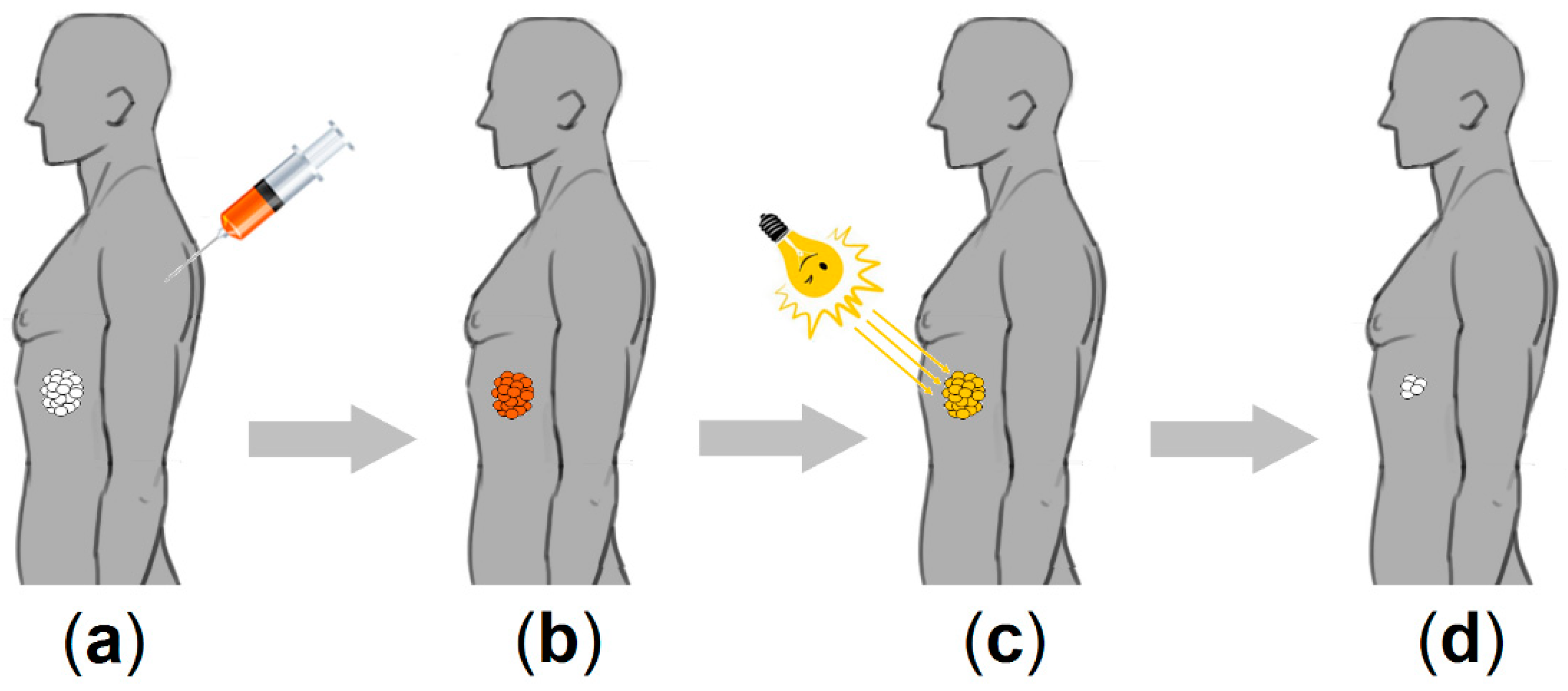
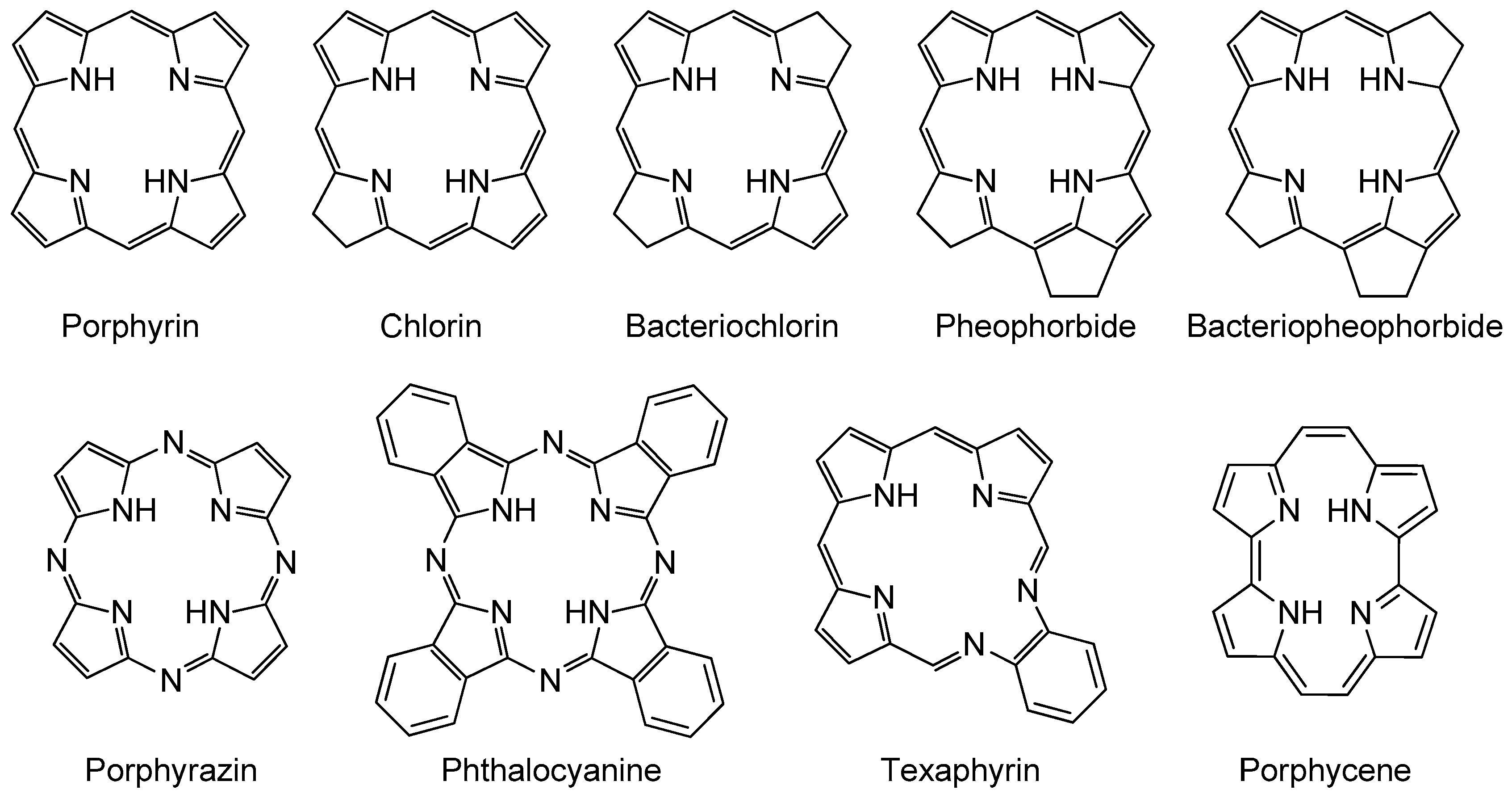
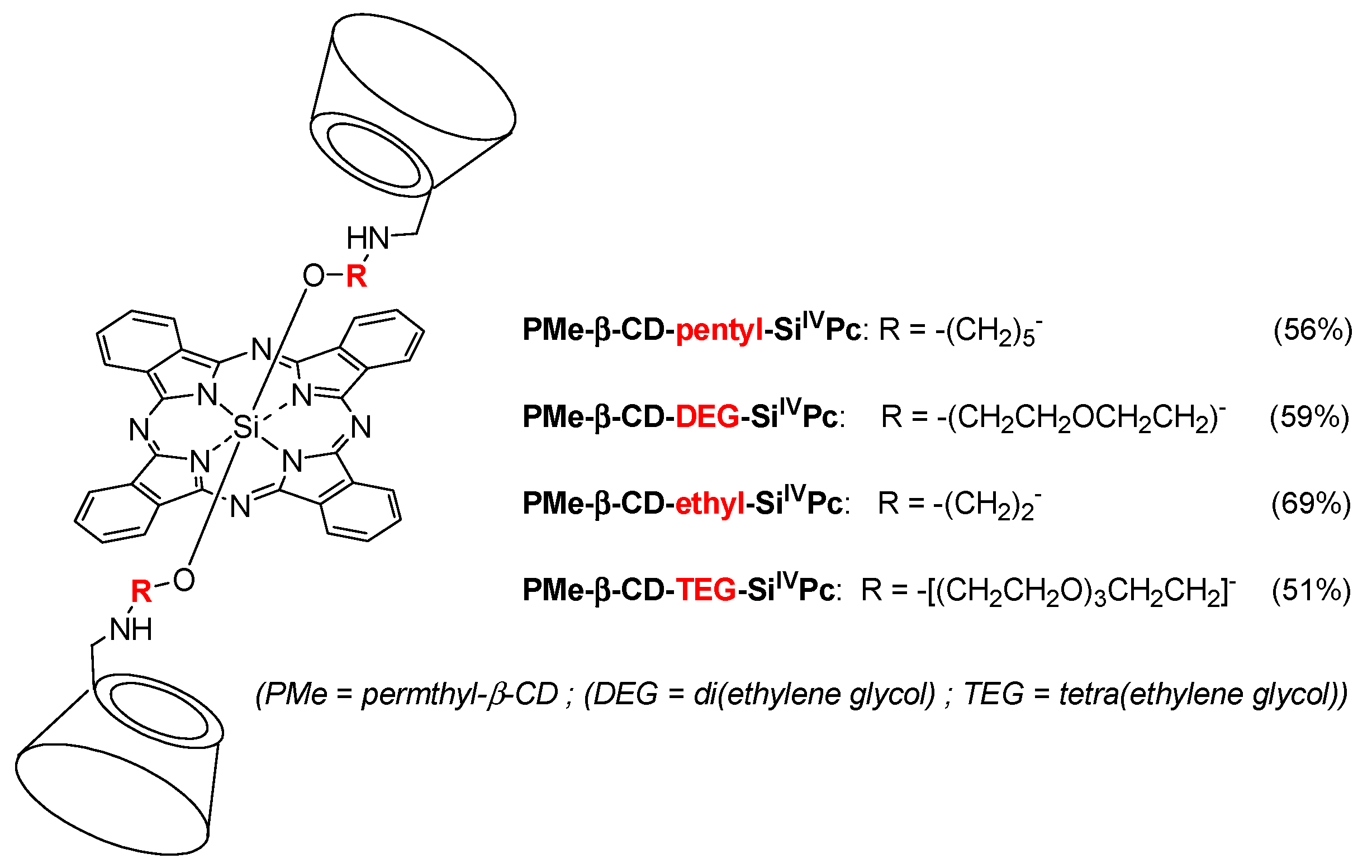2.2. Cyclodextrin–Photosensitizer Systems
CD–PS systems can be formed by three types of binding modes of PSs with CDs. This paragraph will focus on the CD–PS system formation by non-covalent binding (CD–PS inclusion complexes,
Section 2.2.1.), covalent binding (CD–PS conjugates,
Section 2.2.2.), and non-specific external binding (CD–PS nanoassemblies,
Section 2.2.3.)
2.2.1. Non-Covalent Cyclodextrin–Photosensitizer Inclusion Complexes
CDs are known as an attractive option for the PDT of cancers due to their ability to interact with a large variety of PSs, leading to the formation of non-covalent inclusion complexes. This part of the review focuses on the in vitro or/and in vitro biological effect of CD-PS inclusion complexes in anticancer PDT. However, with respect to articles involving the potential use of CD-PS inclusion complexes in anticancer PDT (without in vitro and/or in vivo biological studies), we can emphasize that several studies have estimated benefit of CD–PS inclusion complexes on the physicochemical properties of PSs. For porphyrinoid PSs, various studies showed that CD–PS inclusion complexes could (1) increase some of the photophysical properties of PSs (fluorescence intensity [
108,
109], tripling the lifetime in neutral aqueous solutions [
110],
1O
2 production [
111], and quantum yield [
112] in aqueous solutions), (2) reduce some drawbacks of PSs (such as poor water solubility [
113], self-aggregation [
111,
114], protonation of pyrrole nitrogens [
110], metalation [
110], thermal degradation [
115]), and (3) enhance drug delivery [
110,
115] and lipid membrane penetration [
116]. Similar results were also obtained for non-porphyrinoids PSs, i.e., the improvement of photophysical properties (fluorescence lifetime, emission, and quantum yield) [
117], drug delivery [
117,
118], and photostability [
119]. The studies that have presented an in vitro or/and in vivo biological anticancer PDT evaluation of CD–PS inclusion complexes are summarized below.
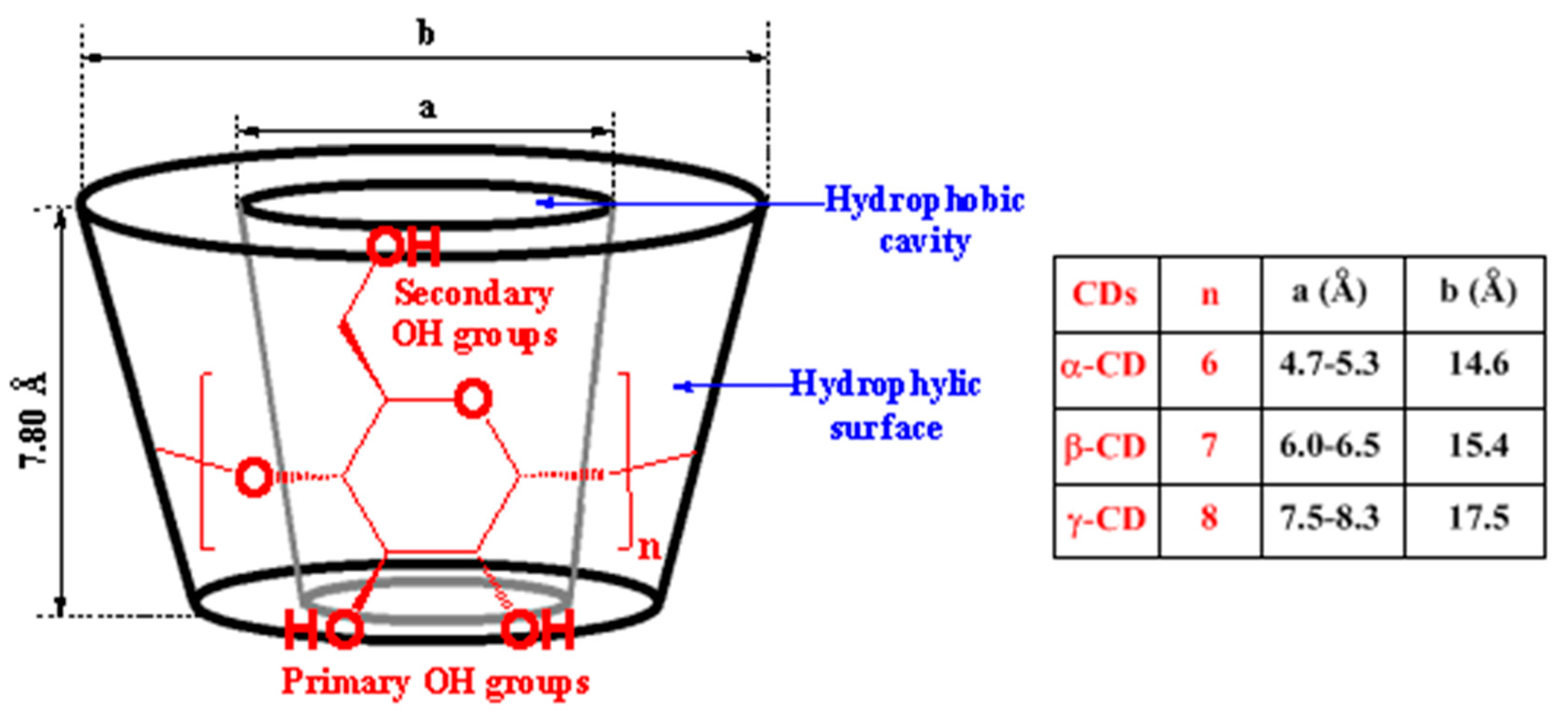
Generally, host–guest inclusion complexes between CDs and porphyrinoid PSs are formed by encapsulating the “aryl” substituent portion of the PS into the fairly large cavity of CD. Across all three natural types of CD, α-CD does not allow the formation of inclusion complexes due to its small cavity, while β-CD and γ-CD can generate inclusion complexes typically through the primary face of the β-CD cavity and the secondary face of the γ-CD (
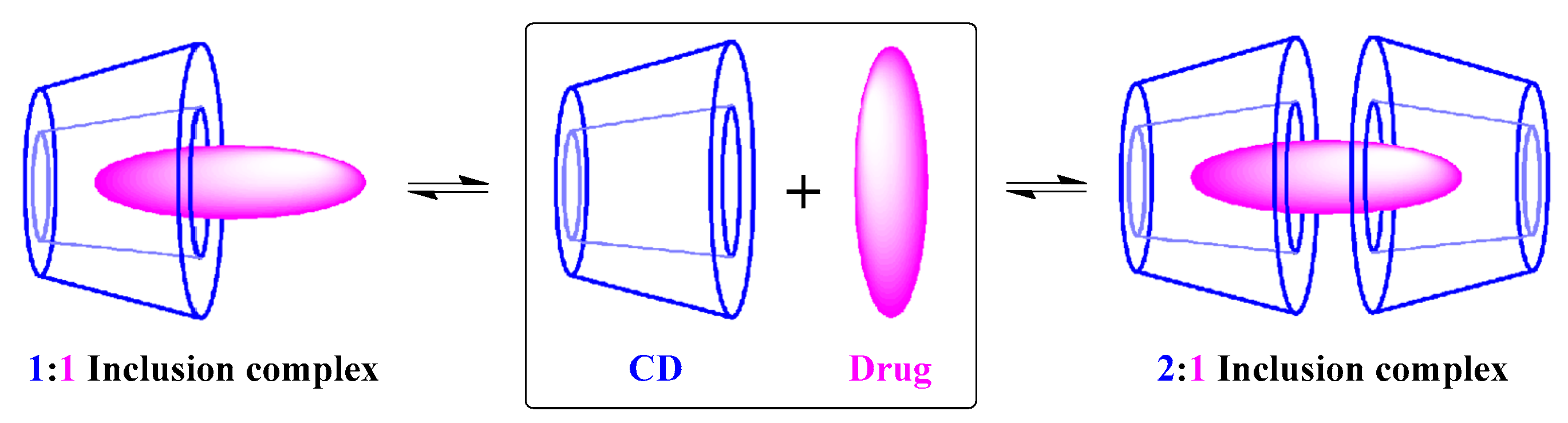
Figure 5). Different CD–PS inclusion complexes’ stoichiometry can be obtained, but 1:1 and 2:1 stoichiometries are the most common.
In 2003, Kolàrovà et al. [
120] studied the in vitro phototoxicity of two PSs models:
meso-tetrakis(4-sulphonatophenyl)porphyrin (TPPS
4) and its zinc metallocomplex (ZnTPPS
4) on G361 human melanoma cells in the presence and absence of 2-hydroxypropyl-β-cyclodextrin (HP-β-CD). Based on the biological studies performed on G361 human melanoma cells, the inclusion complexes of each PS with HP-β-CD showed a lower cytotoxicity and higher phototoxicity compared to the PSs alone. They already found that the most effective system was the HP-β-CD-ZnTPPS
4 inclusion complex, since the IC
50 value was 12.5 mg/mL at a light radiation dose of of 10 J/cm
2.
In 2005, the same team [
121] investigated the influence of the HP-β-CD-ZnTPPS
4 inclusion complex on the in vitro phototoxic properties of ZnTPPS
4 using the same G361 human melanoma cells. According to the cellular uptake studies, the prepared HP-β-CD-ZnTPPS
4 inclusion complex showed a good penetration through the cell membrane. Indeed, the inclusion complex accumulation was dependent of PS concentration and incubation time, and the best uptake was found at 3 µM of the inclusion complex after 48 h of incubation. The in vitro phototoxicity efficiency after 24 h of incubation of G361 cells with ZnTPPS
4 (10 µM) and HP-β-CD (1 mM) and under light irradiation (12.5 J/cm
2, 24 V/250 W) showed DNA breaks in G361 cells. The authors concluded that binding ZnTPPS
4 to HP-β-CD may improve its PDT efficiency against malign melanoma.
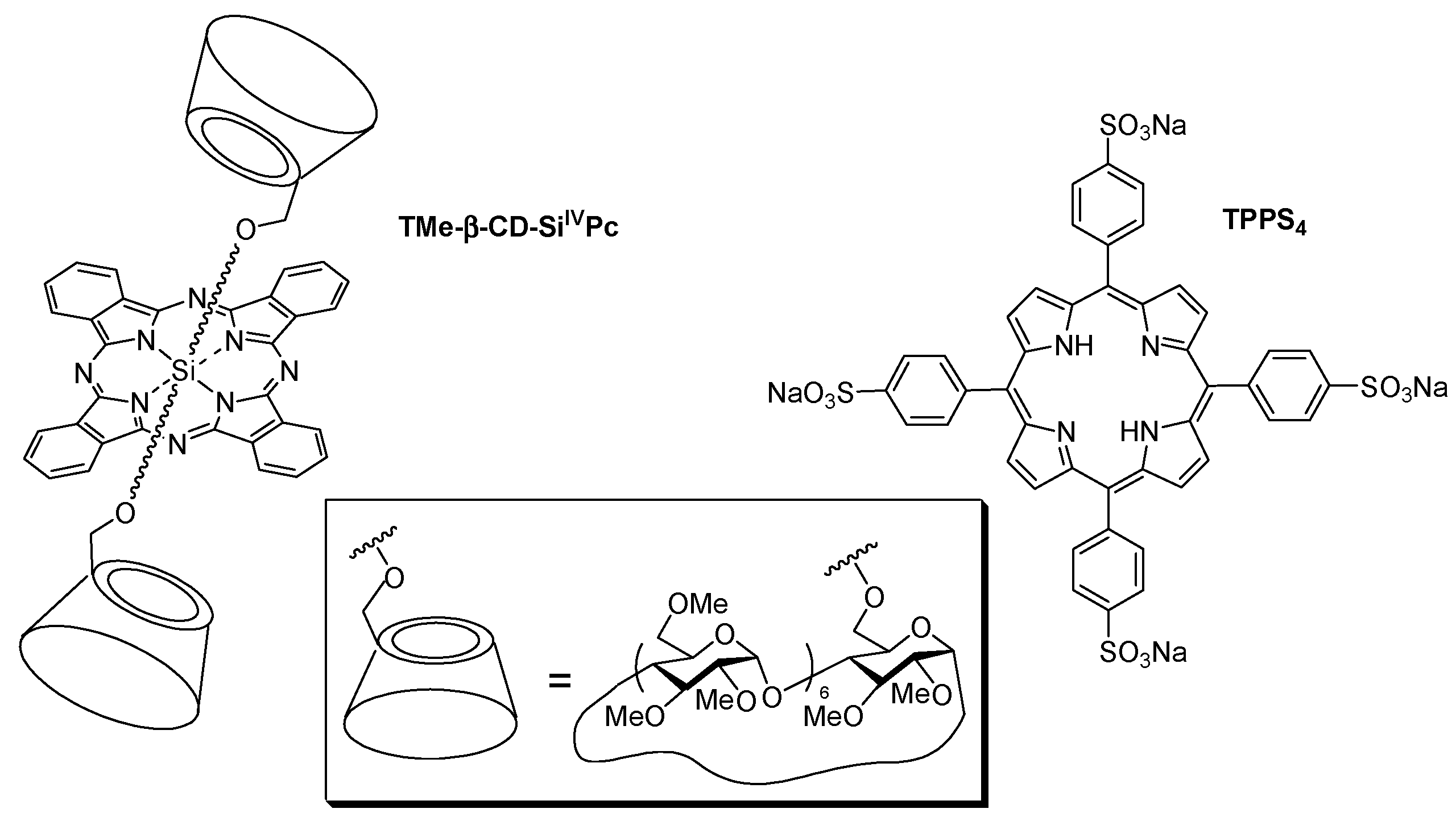
In 2007, Lo et al. [
122] studied the supramolecular hetero-arrays of tetrapyrrole derivatives held by host–guest interactions (1:1 stoichiometric ratio). The nanostructure contained heptakis(2,3,6-tri-
O-methyl)-β-cyclodextrin-conjugated silicon(IV) phthalocyanine (TMe-β-CD-Si
IVPc) complexed with the tetrasulfonated porphyrin (TPPS
4) (
Figure 6).
The authors studied briefly the in vitro PDT efficiency of TMe-β-CD-Si
IVPc and TPPS
4, and the 1:1 inclusion complex on HT29 human colon adenocarcinoma cells upon illumination with a red light (λ > 610 nm, total fluence = 48 J/cm
2;
Figure 7). They concluded that the porphyrin enhances the water solubility and facilitates the formulation of phthalocyanine through complex formation.
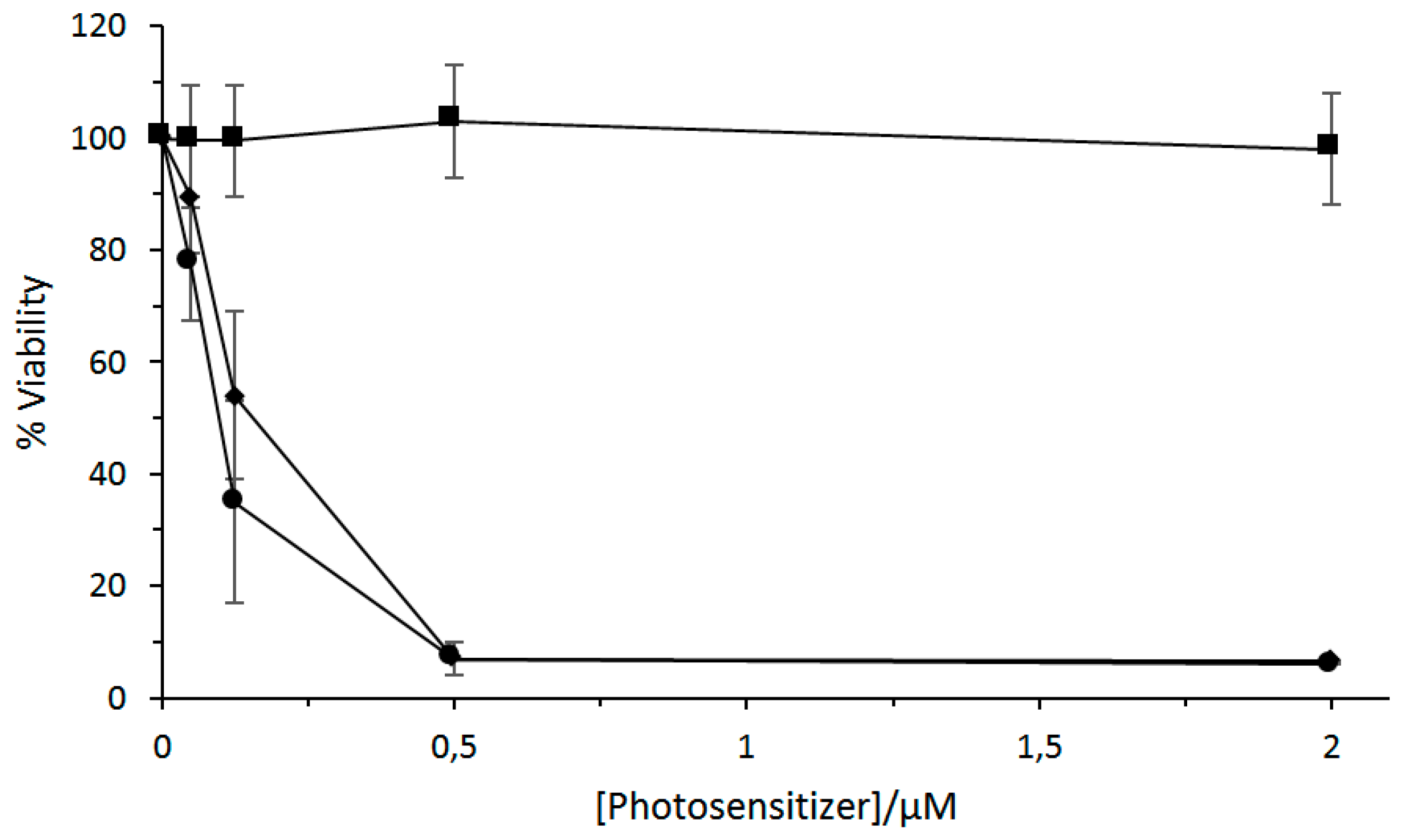
In 2011, Silva et al. [
123] studied a complex 2:1 of β-CD or HP-β-CD with a chloro-aluminum phthalocyanine (ClAlPc). The photophysical properties of the inclusion complexes in deuterated ethanol showed a fluorescence quantum yield of 0.38 and 0.09 for ClAlPc/β-CD and ClAlPc/HP-β-CD, respectively, and a
1O
2 quantum yield of 0.65 and 0.80 for ClAlPc/β-CD and ClAlPc/HP-β-CD, respectively; the authors concluded that the ClAlPc/Hp-β-CD inclusion complex should be the best candidate for PDT application. An in vitro study on J774 mouse macrophage tumor cells with a CIAlPc/HP-β-CD inclusion complex under various irradiation (70 mJ/cm
2, 140 mJ/cm
2, and 700 mJ/cm
2) indicated a decrease of the cell viability depending on the applied dose light (
Figure 8).
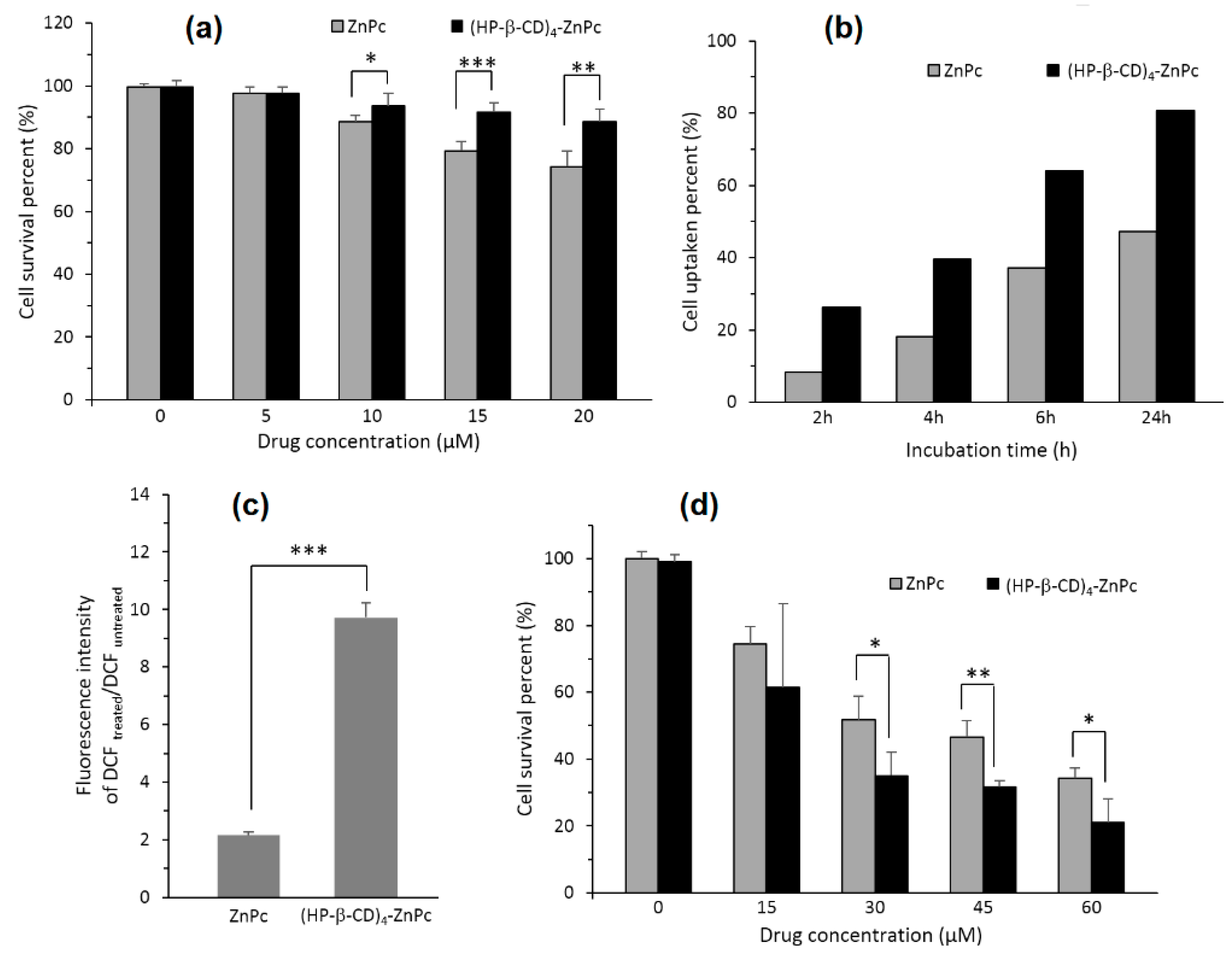
In 2014, Lu et al. [
124] prepared and studied a 4:1 inclusion complex of zinc phthalocyanine (ZnPc) with HP-β-CD ((HP-β-CD)
4-ZnPc) to improve the PDT efficiency of ZnPc by increasing the water solubility and decreasing the aggregation in the physiological environment of ZnPc. No obvious toxicity was observed on human cervical carcinoma (HeLa) cells at a high concentration of 80 µM. The inclusion complex exhibited superior
1O
2 production, intracellular ROS generation cellular uptake ability, and phototoxicity to cancer cells compared to free ZnPc. The first results are presented in
Figure 9.
Two years later, the same group [
125] studied the influence of the size of CD and synthesized 4:1 inclusion complexes: (α-CD)
4-ZnPc, (β-CD)
4-ZnPc, and (γ-CD)
4-ZnPc. Based on extracellular
1O
2 generation ability studies, (β-CD)
4-ZnPc appeared to be the best generator of
1O
2. The cellular uptake of the inclusion complexes was increased when compared with free ZnPc and particularly with (β-CD)
4-ZnPc. They also compared the PDT efficiency of these different compounds using Hela cells, and
Figure 10 shows the better in vitro PDT efficiency of the inclusion complexes compared to free ZnPc.
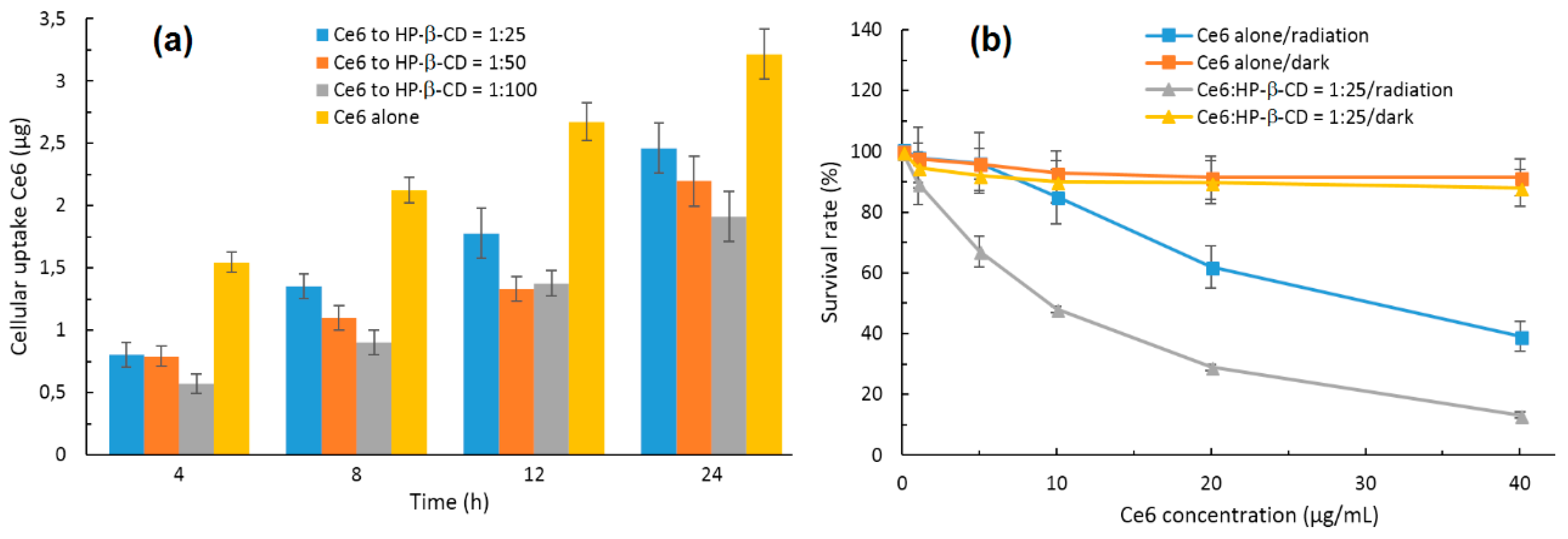
In 2015, Paul and his coworker [
126] described the incorporation of chlorin e6 (Ce6) into the HP-β-CD cavity, and a 1:1 stoichiometric ratio was found. The authors showed that the complexation of Ce6 with HP-β-CD enhanced the Ce6 solubility, decreased its aggregation in water, and enhanced its
1O
2 yield at the pH tumor environment compared to the free Ce6. However, the in vitro cellular uptake of the Ce6-HP-β-CD inclusion complex performed on human oral squamous carcinoma (OSC) indicated that Ce6-HP-β-CD with a higher HP-β-CD concentration decreased their cellular uptake due to the higher viscosity of the microenvironment in the presence of a significant amount of HP-β-CD. Finally, the in vitro phototoxicity studies of the Ce6-HP-β-CD inclusion complex (Ce6:HP-β-CD = 1:25, pH 6.2, 30 mW/cm
2 for 5 min) were performed on the OSC cells. The obtained results highlighted the important role of HP-β-CD in affecting the phototoxicity and PDT efficiency of Ce6 against tumors (
Figure 11). The authors concluded that CDs derivatives of larger size such HP-β-CD could be a successful formulation excipient to deliver monomeric Ce6 with improved PDT efficiency.
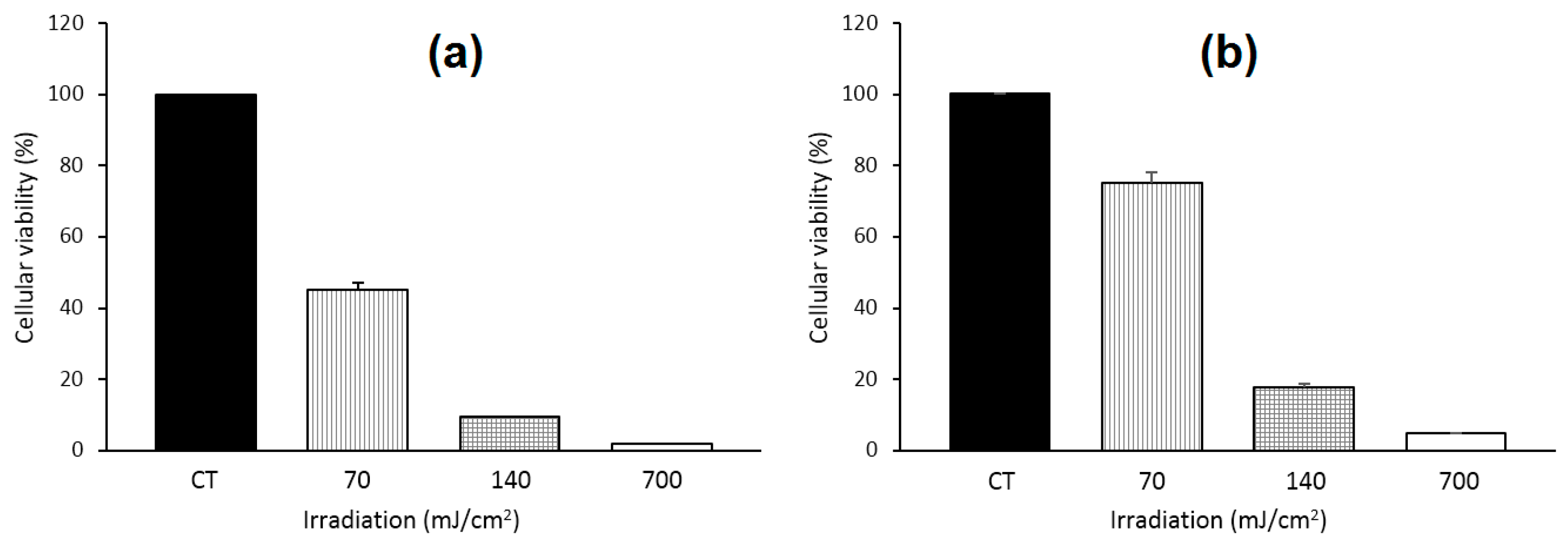
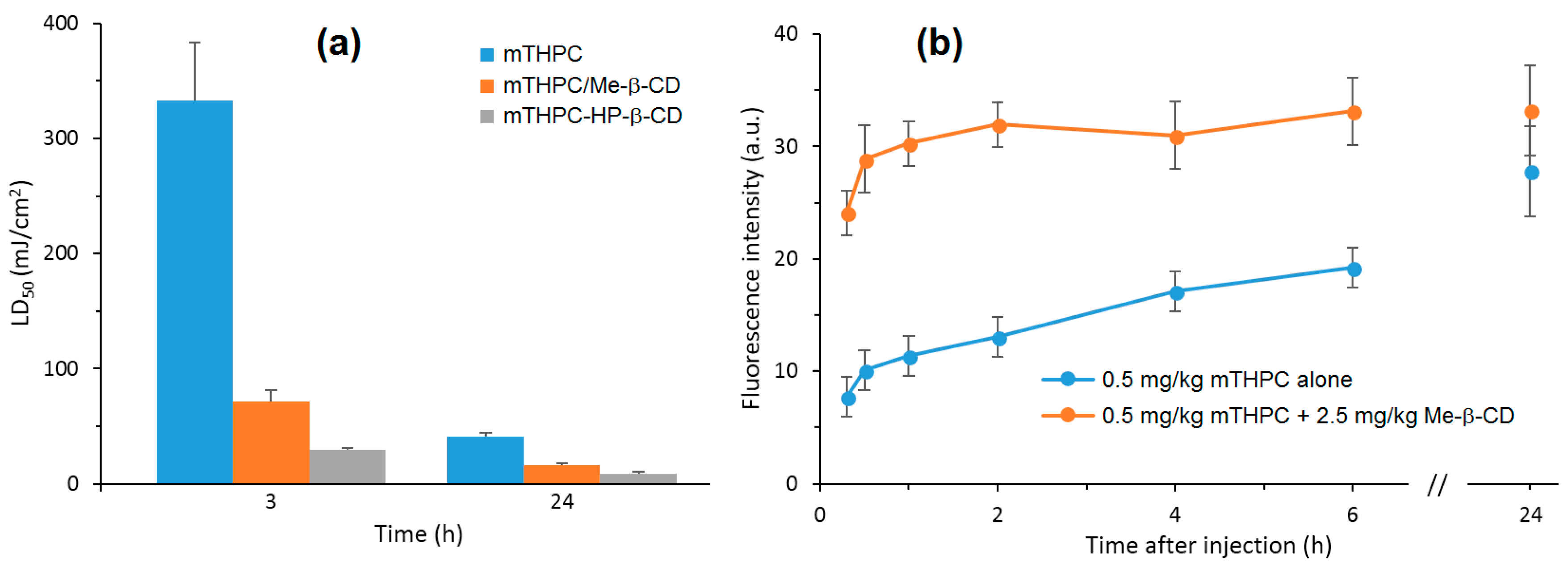
Recently, Yankovsky et al. [
127] studied the effect of two β-CDs derivatives, i.e., methyl-β-cyclodextrin (Me-β-CD) and HP-β-CD, at a wide range of concentrations on the in vitro and in vivo distribution of meta-tetra(hydroxyphenyl)chlorin (mTHPC). The authors found that the association of mTHPC with the β-CDs prevents its aggregation after introduction into blood, and enhanced its diffusion movement between biological structures. In addition, they demonstrated that the mTHPC distribution in blood serum and its accumulation in cellular culture medium were highly dependent on the β-CDs concentrations (maximal mTHPC accumulation at 10 µM of Me-β-CD and 200 µM of HP-β-CD) (
Figure 12a). Furthermore, photosensitization studies showed that the addition of β-CDs affect the intracellular distribution of mTHPC and enhance its photocytotoxicity effect toward HT29 human adenocarcinoma cultured cells inclusion complexes (Me-β-CD LD
50: 70 mJ/cm
2 and 14 mJ/cm
2 at 3 h and 24 h, and in the case of HP-β-CD 27 mJ/cm
2 and 8 mJ/cm
2) (
Figure 12a). Finally, the in vivo fluorescence kinetics studies and fluorescent imaging of the mTHPC distribution in different tissues (
Figure 12b) confirm that the use of β-CDs modifies the mTHPC distribution process in tumor-bearing animals with a decreased level of mTHPC in skin and muscles, and an increased mTHPC accumulation in tumor were observed.
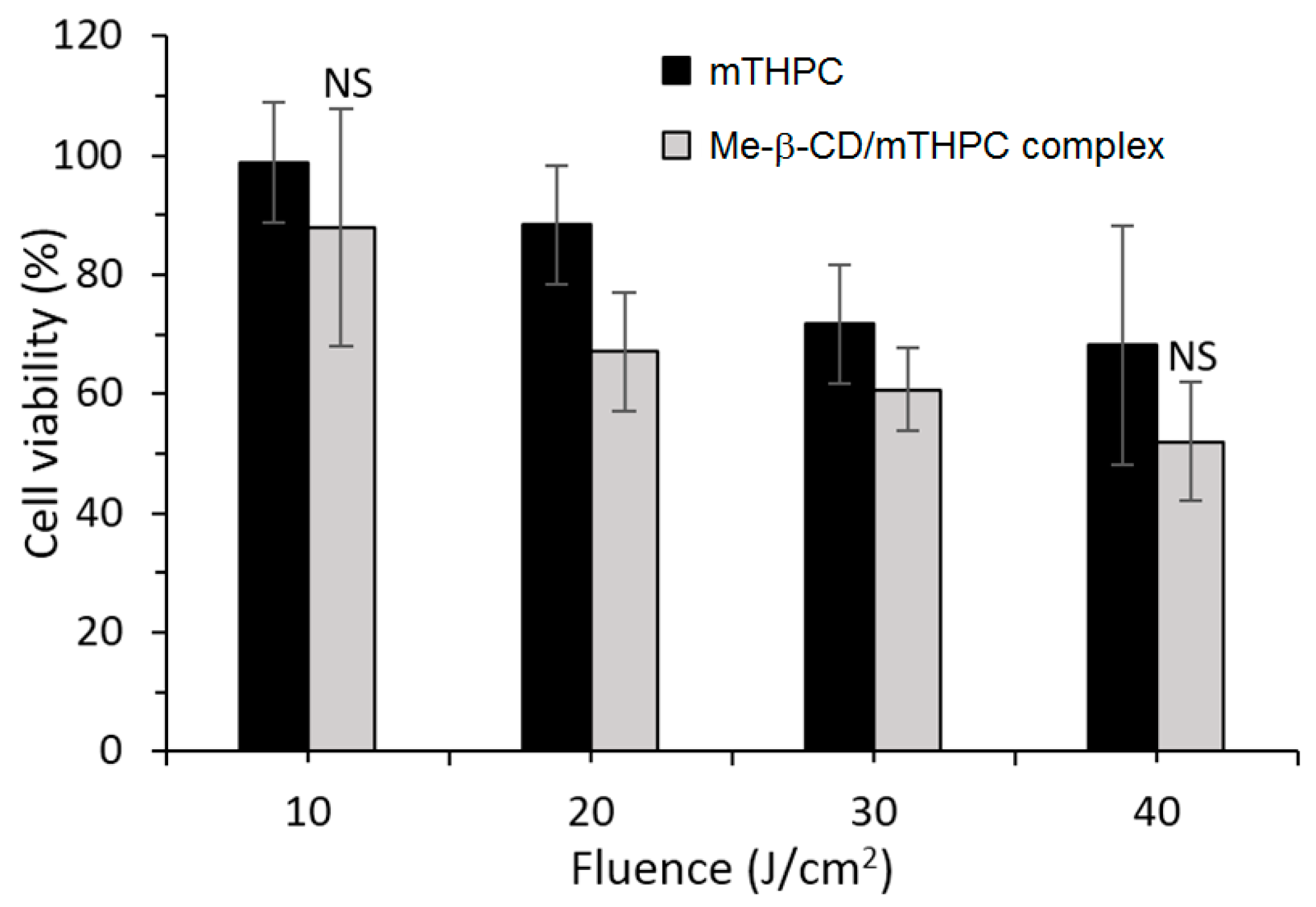
More recently, the same group published a work concerning the distribution and PDT efficiency of Me-β-CD or HP-β-CD encapsulating mTHPC on multicellular HT29 tumor spheroids [
128]. The 2:1 inclusion (Me-β-CD/mTHPC or HP-β-CD/mTHPC) induced showed two and three times higher mTHPC accumulation in spheroids than mTHPC alone. The authors highlighted the different distribution of the two inclusion complexes: whereas HP-β-CD/mTHPC accumulated at the spheroid periphery, Me-β-CD/mTHPC penetrated deeper, with a more homogeneous distribution into the spheroid. At a low light dose such as 20 J/cm
2, the Me-β-CD/mTHPC inclusion complex presented a phototoxicity that was 25% higher than mTHPC alone (
Figure 13).
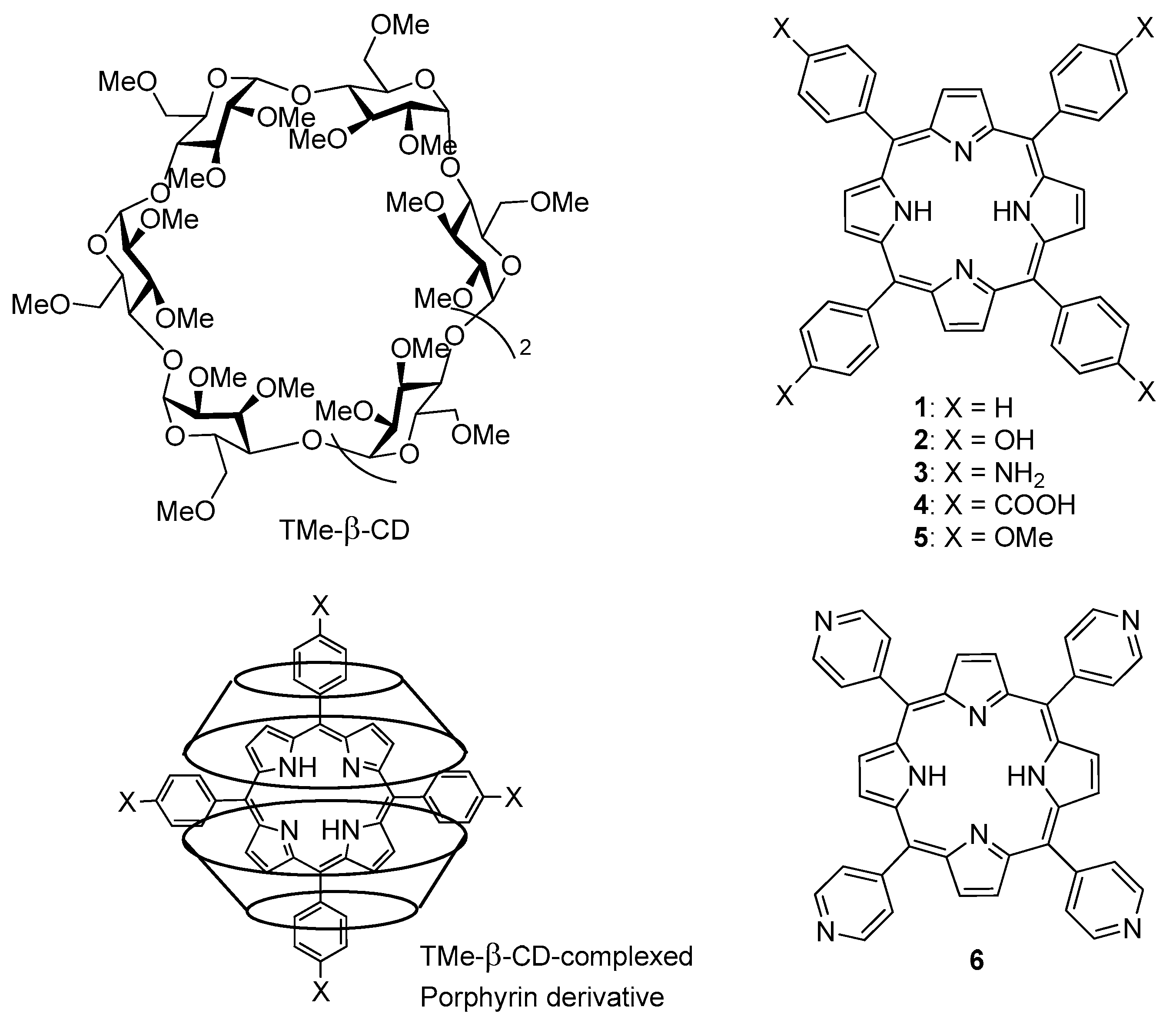
In 2017, Ikeda et al. [
129] described the PDT effect of 2:1 inclusion complexes
1–
6 of trimethyl-β-CD (TMe-β-CD) with different porphyrins, as described in
Figure 14.
For photostability reasons and 1O2 generation ability, only 2–4 were selected for in vitro PDT study. Inclusion complexes 1, 2, and 6 were unstable under light irradiation. Inclusion complexes 2–4 generated 1O2 in the order 3 > 2 ≈ 4. PDT experiments were done with the HeLa cell line after irradiation of 610–740 nm for 30 min under 9 mW/cm2 of light power. The order of PDT activity was 3 > 2 >> 4, suggesting that the PDT effect is more due to the higher intracellular uptake of inclusion complexes 2 and 3 (endocytosis) than 1O2 formation. The authors could check the formation of 1O2 in Hela cells by inclusion complexes 2 and 3, which presented 14 and 26 times higher PDT activity than Photofrin®.
Many of the approved PDT PSs for clinical use are porphyrinoid derivatives. However, their usefulness in PDT can sometimes be limited by various factors such as relatively poor water solubility and photostability. As a consequence, major efforts are made to develop new non-porphyrinoid PSs [
130].
In 2005, Bruzell et al. compared the PDT efficiency of different formulations of curcumin (curc) into DMSO, non-ionic micelles liposomes (LP), HP-β-CD, or alginate viscous solution [
131]. PDT efficiency was evaluated by two techniques: PI/Hoechst staining and fluorescence technique, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. By MTT assay, no effect of light could be observed after 350 nm to 550 nm of light irradiation (1.6 J/cm
2 and 3.6 J/cm
2 per well) of HP-β-CD/curcumin inclusion complex (5% HP-β-CD), whereas curcumin in HP-β-CD increased the apoptotic SM10-12 cells by 20-40-fold compared to non-irradiated cells or irradiated cells without curcumin (
Figure 15).
As a conclusion, except for viscous alginate solution, all of the formulations were suitable for using curcumin in damaging submandibular acinar cells. The phototoxic effect can be detected at low curcumin concentration (13.5 µM) with low light doses (1.6 J/cm
2 and 3.6 J/cm
2 per well) and a short incubation time (3 h). Unfortunately, the authors did not continue their study on anticancer PDT, and focused their efforts on antimicrobial PDT (see Valeron Bergh; Hjorth Tonnesen. [
113] and references cited therein.)
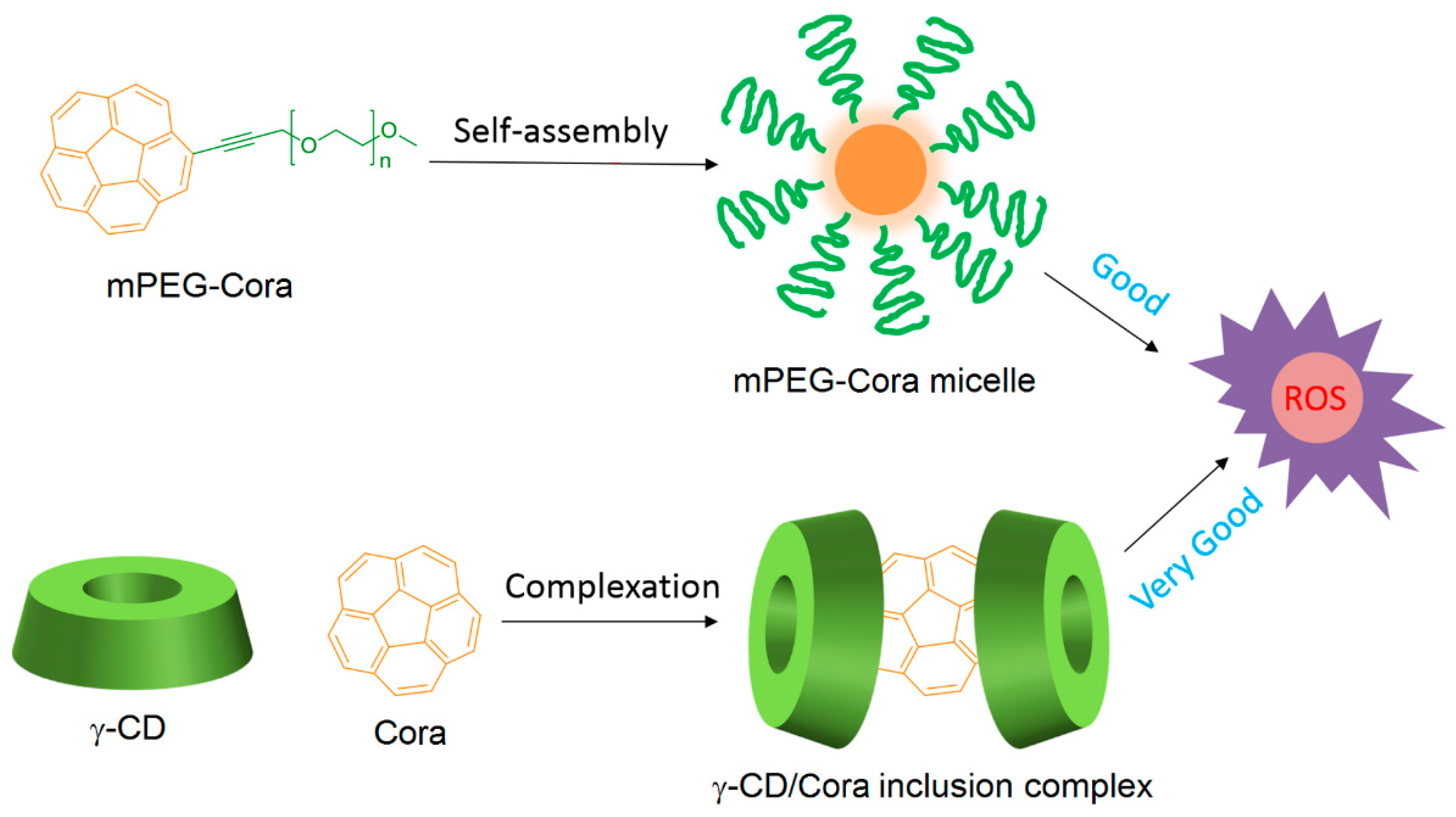
The non-porphyrinoid PS Corannulene (Cora) is known to produce ROS in a controlled manner, but the use of Cora is limited because of its low water solubility. Very recently, Zhang et al. [
132] designed two types of water-soluble Cora systems, i.e., methoxy poly(ethylene glycol)-corannulene (mPEG-Cora) micelle and a γ-CD/Cora inclusion complex (
Figure 16).
Subsequently, the vehicle effect on the ROS production by Cora contained in mPEG-Cora and γ-CD/Cora systems at the cellular level was studied using confocal laser scanning microscopy (365 nm, 95 mW/cm2, 20 min). It was found that both systems can produce ROS, but the γ-CD/Cora inclusion complex was the most effective system.
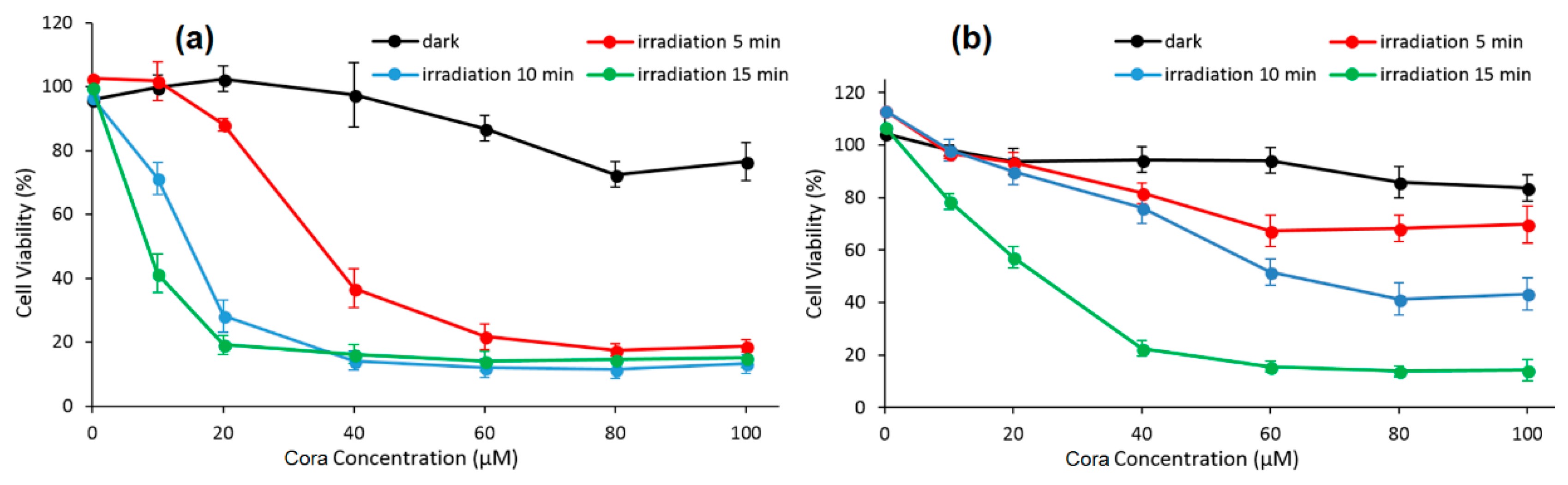
The PDT effect (type I, 365 nm, 95 mW/cm
2 for 5 min, 10 min, and 15 min) of each system on PC-3 cells were studied. At the same irradiation circumstance and Cora dose, the authors found that γ-CD/Cora inclusion complex could induce a higher extent of photocytotoxicity (e.g., for 15 min of irradiation, IC
50 of γ-CD/Cora = 9.2 ± 4.7 μM, IC
50 of m-PEG-Cora = 22.5 ± 2.6 μM), indicating more satisfactory therapeutic outcomes (
Figure 17).
Finally, the vehicles’ effect on the mitochondria targeting behavior of Cora was studied on PC-3 cells (365 nm, 95 mM/cm2, 20 min). It was found that the γ-CD/Cora complex has shown superior ability to deliver more Cora to mitochondria compared with the m-PEG-Cora micelle. The authors concluded that the CD complexation approach prevailed over the PEGylation method for PDT applications.
2.2.2. Cyclodextrin–Photosensitizer Conjugates
A second possible way to improve the photophysical properties of PSs for an anticancer PDT application would be to bind the CD and PS by a covalent link, i.e., the formation of a CD–PS conjugate. This binding mode has shown its potential validity for anticancer PDT application in various studies. This part of the review focuses on the in vitro or/and in vitro biological effect of CD–PS conjugates in anticancer PDT. However, with respect to articles involving the potential use of CD–PS conjugates in anticancer PDT (without in vitro and/or in vivo biological studies), we can emphasize the use of CD dimers as potential carriers with a
1O
2-responsive linker that would allow either the PS release [
133,
134,
135] or the PS concentration in the light beam and water solubility [
136]. In addition, the conjugation of β-CD with a PS via a non-cleavable ether bond showed an improvement of the water solubility and in vitro fluorescent intensity of PS [
137]. Other CD–PS conjugates with a dithienylethene linker have an enhanced water solubility and biocompatibility of PS, while also resulting in photo-controlled
1O
2 generation in aqueous solution [
138]. The studies that have presented an in vitro and/or in vivo biological anticancer PDT evaluation of CD–PS conjugates are summarized below.
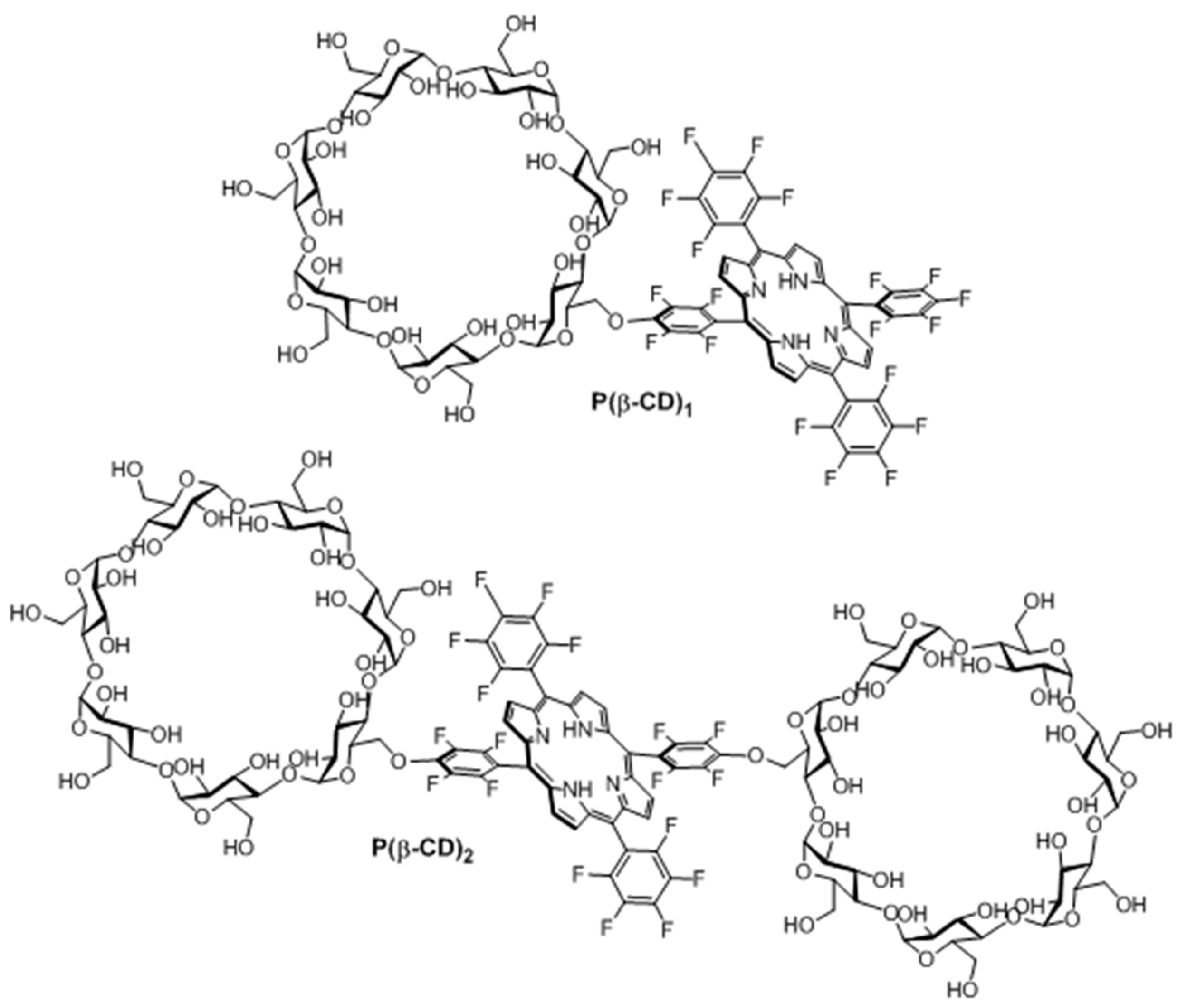
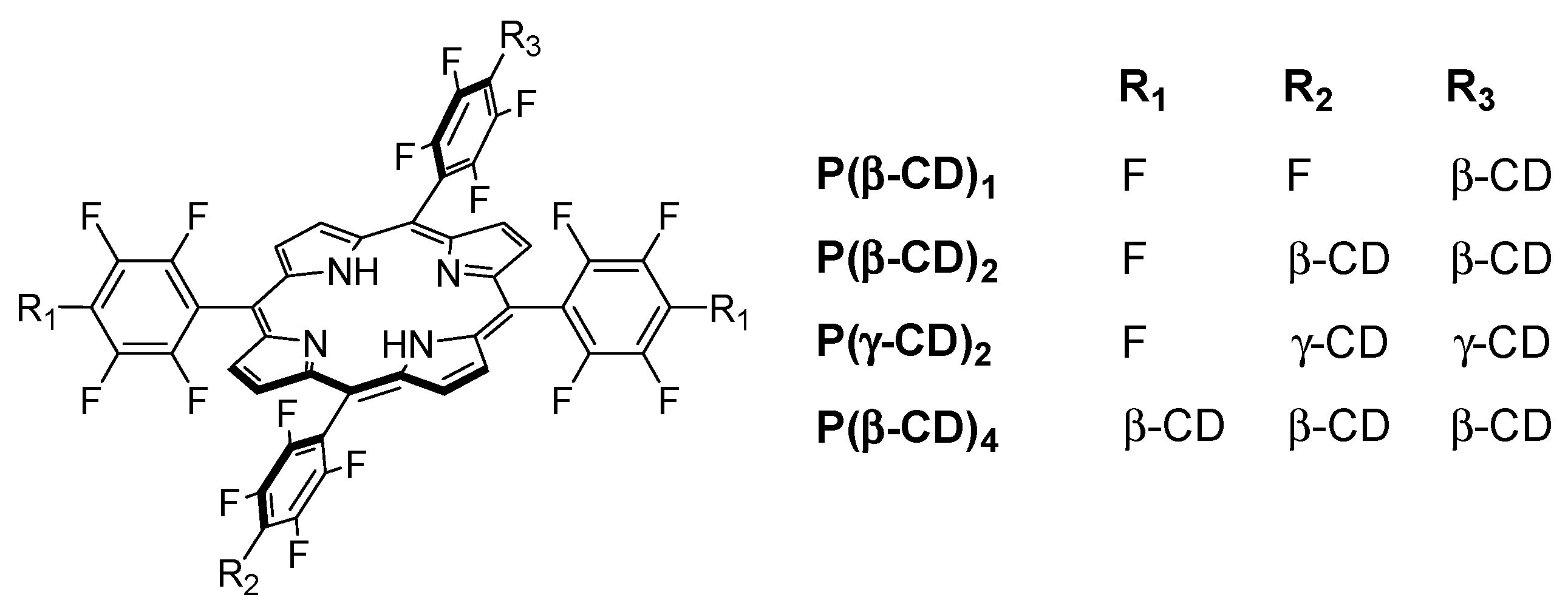
In 2006, Králová et al. [
139] synthesized two new perfluorinated porphyrin derivatives conjugated to one or two β-CD units (P(β-CD)
1 and P(β-CD)
2, as shown in
Figure 18).
With the aim of estimating the pharmacokinetic and photosensitizing properties of P(β-CD)1 and P(β-CD)2, in vitro studies were performed on different cell lines, i.e., human promyelotic leukemia (HL-60), mouse mammary carcinoma (4T1), mouse colon carcinoma (CT26.CL25), and human cervical carcinoma (HeLa). The phototoxicity of P(β-CD)1 and P(β-CD)2 at concentrations of 5 µM and 10 µM were investigated with HL-60 and 4T1 cells under irradiation at various doses of light (0 to 4.2 J/cm2). No dark cytotoxicity was detected; nevertheless, an increased cell death was seen in both cell lines when concentration and light dose increased. It was found that P(β-CD)2 was less efficient compared to P(β-CD)1. A similar effect was observed with the other cell lines (CT26.CL25 and HeLa), along with an increase in cell death over time. Furthermore, the accumulation in the tumor is higher and faster for P(β-CD)2.
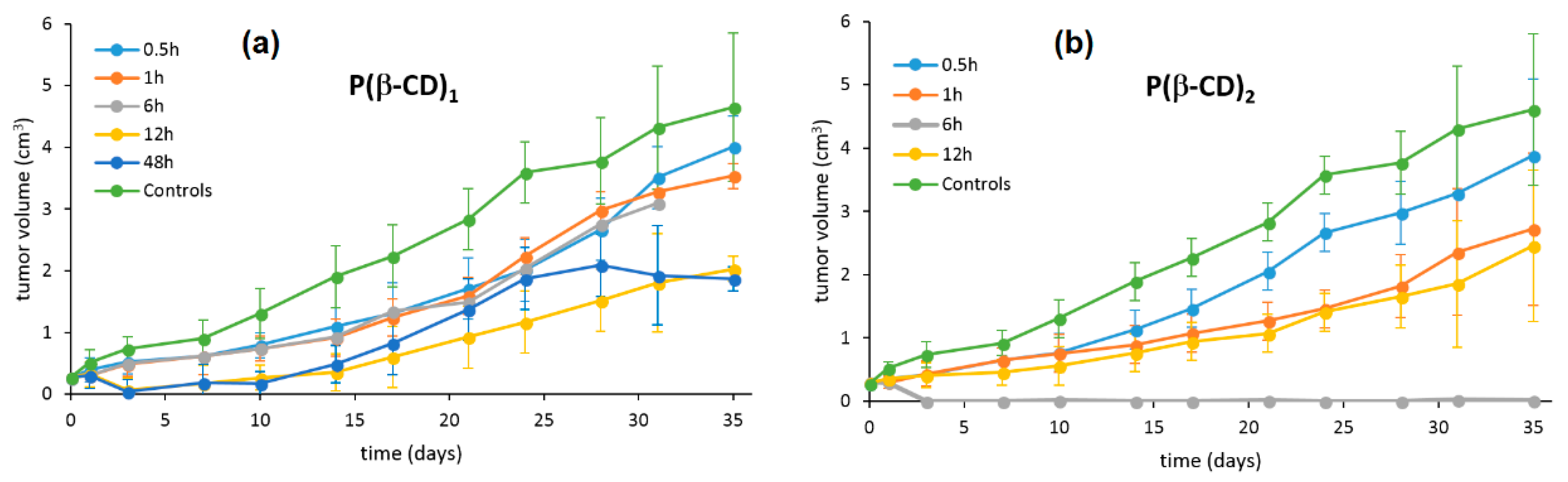
Finally, in vivo studies were realized using a 4T1 mouse-tumor model. P(β-CD)
1 and P(β-CD)
2 were injected into mice (5 mg/kg) and then exposed to light illumination (100 J/cm
2) at various times between 0.5 h and 35 h after injection. It was found that P(β-CD)
2 was the most efficient at totally inhibiting the tumor growth for a drug-light interval of 6 h (
Figure 19).
In 2010, the same team synthesized a new Lego-like system composed of perfluorinated porphyrin–CD conjugates (P(CD)
x) and various chemotherapy drugs [
140]. The perfluorinated porphyrins were first conjugated to CDs (β-CD or γ-CD) by covalent ether bond (
Figure 20) using the same strategy as described in their previous study [
139]. The chemotherapy drugs were then encapsulated into the cavity of CDs by non-covalent bond, i.e., inclusion complexes.
Due to the low affinity of P(β-CD)1 conjugate with the various chemotherapy drugs, the authors only investigated P(β-CD)2, P(γ-CD)2, and P(β-CD)4 conjugates for in vitro and in vivo studies using mouse mammary carcinoma (4T1) and human chronic myelogenous leukemia (K562) cell lines. The dark cytotoxicity and phototoxicity (λ = 500–520 nm, 4 J/cm2, 0.7 mW/cm2) of cells treated with chemotherapy drugs alone or their corresponding inclusion complexes with (P(CD)x conjugates were compared. They found that compared to the non-irradiated cells, the irradiated ones were much efficient in cancer cell killing. Furthermore, the authors observed a synergistic effect between PDT and a large fraction of chemotherapy drugs compared to chemotherapy or PDT alone.
Finally, in vivo studies were also done in a mouse cancer model. The P(β-CD)2, P(γ-CD)2, and P(β-CD)4 conjugates or their corresponding inclusion complexes with two chemotherapy drug models (doxorubicin and paclitaxel) were injected to the mouse followed by light irradiation (100 J/cm2, 200 mW/cm2). The highest decrease of tumor growth was observed for inclusion complexes of P(β-CD)2 and P(γ-CD)2 with chemotherapy drugs (paclitaxel and doxorubicin, respectively). These results are in agreement with those of in vitro studies indicating the usefulness of the synthesized conjugates for both targeted chemotherapy drug delivery and combined cancer therapy.
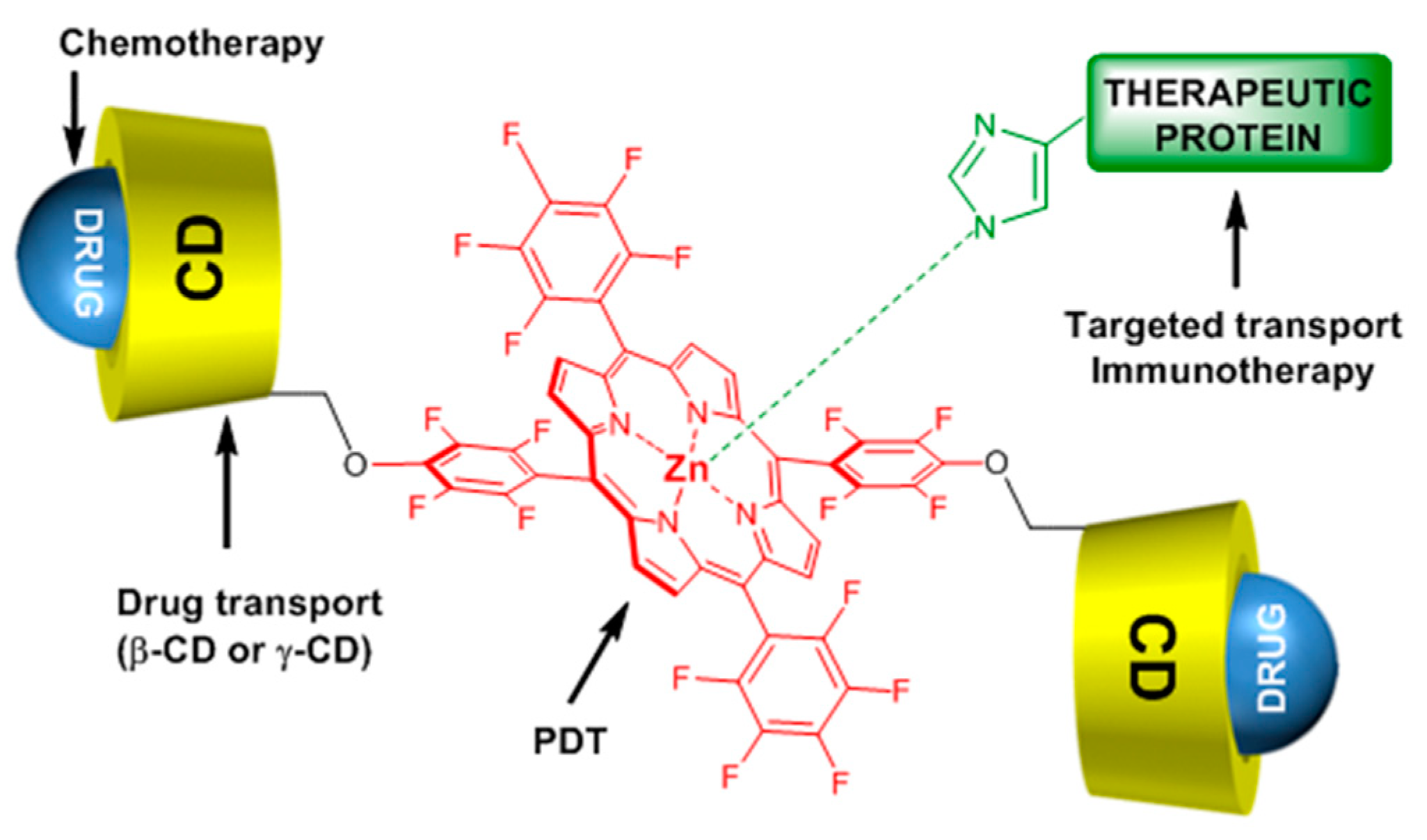
One year after and being encouraged by the results obtained in the last study [
140], the same team developed another “Lego”-like system based on a combination of therapeutic protein, metallo-cyclodextrin-porphyrin, and chemotherapy drugs in order to enhance the inhibition of tumor progress [
141]. The authors used the previous P(β-CD)
2 and P(γ-CD)
2 conjugates with the encapsulation of chemotherapy drugs (paclitaxel, doxorubicin), but this time by metalating the porphyrin core with Zn to coordinate therapeutic proteins (
Figure 21).
The authors prepared two types of Lego-like systems based on ZnP(β-CD)
2/paclitaxel/endoglobulin or ZnP(γ-CD)
2/doxorubicin/endoglobulin. The effect of combined therapy with both systems on the tumor volume of human amelanotic melanoma C32 in the in vivo nude mouse was studied. They found that compared to the ZnP(β-CD)
2/paclitaxel/endoglobulin system, the ZnP(γ-CD)
2/doxorubicin/endoglobulin system was most efficient in the case of PDT treatment alone (λ = 500–700 nm, 100 J/cm
2, 200 mW/cm
2) or combined therapy. Moreover, they found that by combining ZnP(β-CD)
2 or ZnP(γ-CD)
2 conjugates, the chemotherapy drug and endoglobulin enhanced the tumor destruction (
Figure 22).
In 2011, Ng et al. designed a new series of analogous complexes [
142,
143]. In the first study [
142], they studied the influence of the linker’s size (
Figure 23) on the photophysical properties and in vitro PDT activity.
All of the conjugates, except PMe-β-CD-ethyl-Si
IVPc due to a photoinduced electron transfer (PET) process, have the ability to greatly enhance the water solubility of the phtalocyanine core and reduce its self-aggregation in water. In vitro PDT activity using human colon adenocarcinoma (HT29) and human hepatocarcinoma (HepG2) cells was evaluated and summarized in
Table 4.
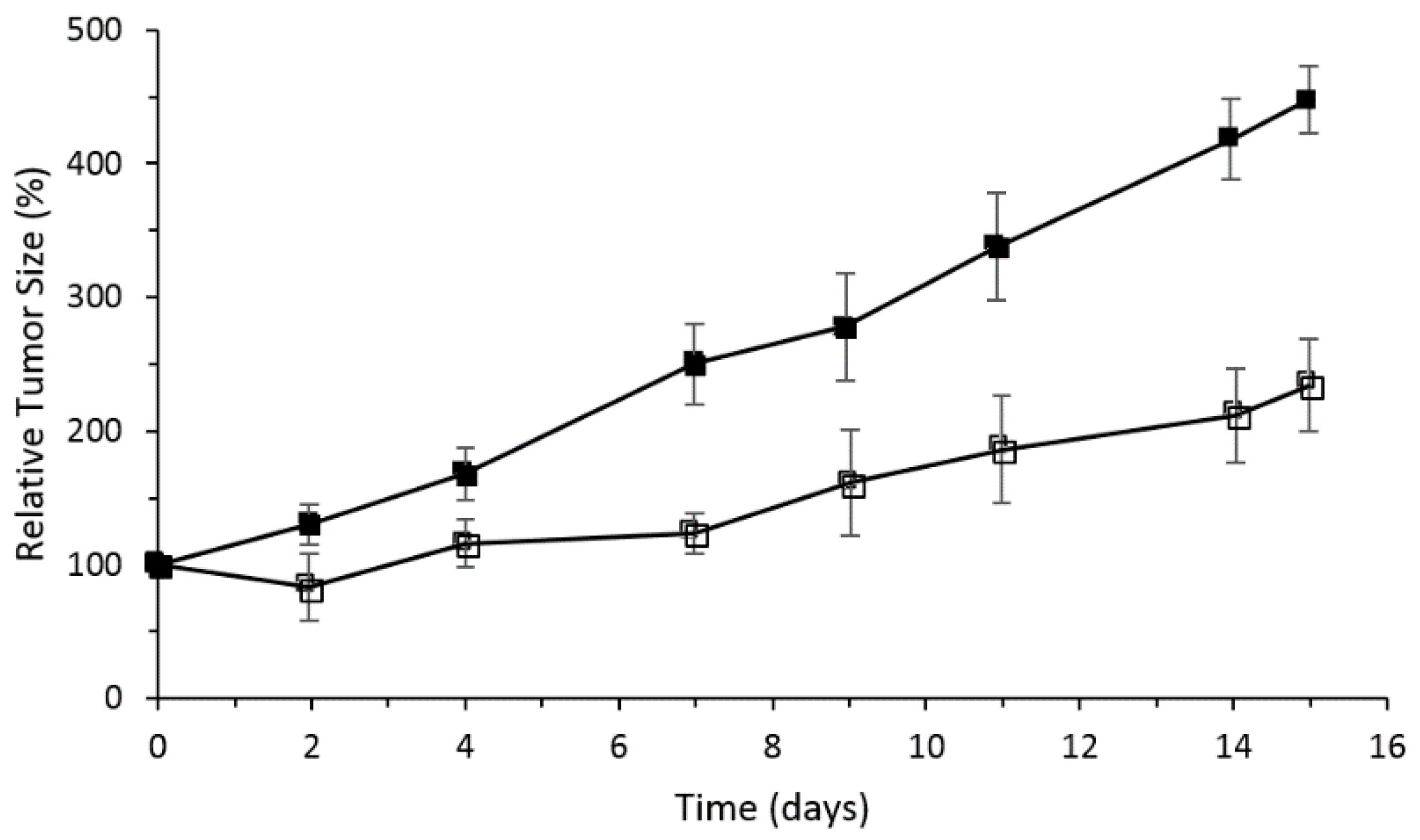
The PMe-β-CD-hexyl-Si
IVPc conjugate showed the best photocytotoxicity, which was explained by the difference in ROS production efficiency and cellular uptake by the lysosomes of the cells. It was shown by flow cytometry that cells in the early apoptotic state increase to 80% upon red light illumination. In vivo PDT activity was performed using nude mice with an HT29 tumor, PMe-β-CD-hexyl-Si
IVPc, and irradiation at 675 nm (30 J/cm
2).
Figure 24 shows the relative tumor size for 15 days, and clearly indicates that the PMe-β-CD-hexyl-Si
IVPc conjugate is a promising system for anticancer PDT.
In their second study [
143], the authors Lau; Lo; Fong; Ng reported the synthesis of unsymmetrical PMe-β-CD-Si
IVPc conjugates using Leng’s procedure [
122] (
Figure 25). The Q-band was sharp for PMe-β-CD-hexyl-Si
IVPc conjugates
1 and
4, and significantly broadened for PMe-β-CD-hexyl-Si
IVPc conjugates
2 and
3, suggesting that the sugar units are less effective at reducing the aggregation.
The in vitro studies were performed on HT29 and HepG2 cells with PMe-β-CD-Si
IVPc conjugates
1–
4. No dark cytotoxicity was observed for all of the compounds, but high cytotoxicity upon illumination (λ = 610 nm, 40 mW/cm
2, 48 J/cm
2) was highlighted. It was found also that the replacement of CD moieties in
1 by sugar or diamino groups enhance greatly the phototoxicity of the conjugates. The IC
50 value comparison of conjugates
1–
4 against HT29 and HepG2 cells are summarized in
Table 5.
The in vivo study was also performed by injection of PMe-β-CD-Si
IVPc conjugate
2 in the nude mice bearing (HT29) tumor using the same protocol conditions as described in Lau; Lo; Fong; Ng [
142]. Similarly, the same PDT effect was observed, indicating that the PMe-β-CD-hexyl-Si
IVPc conjugate
2 is a promising system for anticancer PDT.
In 2013, Aggelidou et al. [
144] described a new bimodal conjugate constituted of protoporphyrin IX (PpIX) covalently linked through amide bond to β-CD (PpIX+β-CD). Spectroscopy studies were used to confirm the formation of two conjugates (PpIX-β-CD “Major” and PpIX-2β-CD “Minor”). The photophysical studies of PpIX alone compared to conjugates revealed that the presence of β-CD in the conjugates enhanced the water solubility of PpIX. Furthermore, the authors found also that both conjugates have the ability to host an anticancer drug (
N-desmethyltamoxifen, NDMTAM.HCl) by its complexation in the empty cavity of β-CD, which shows that PpIX+βCD could efficaciously solubilize and transport NDMTAM.
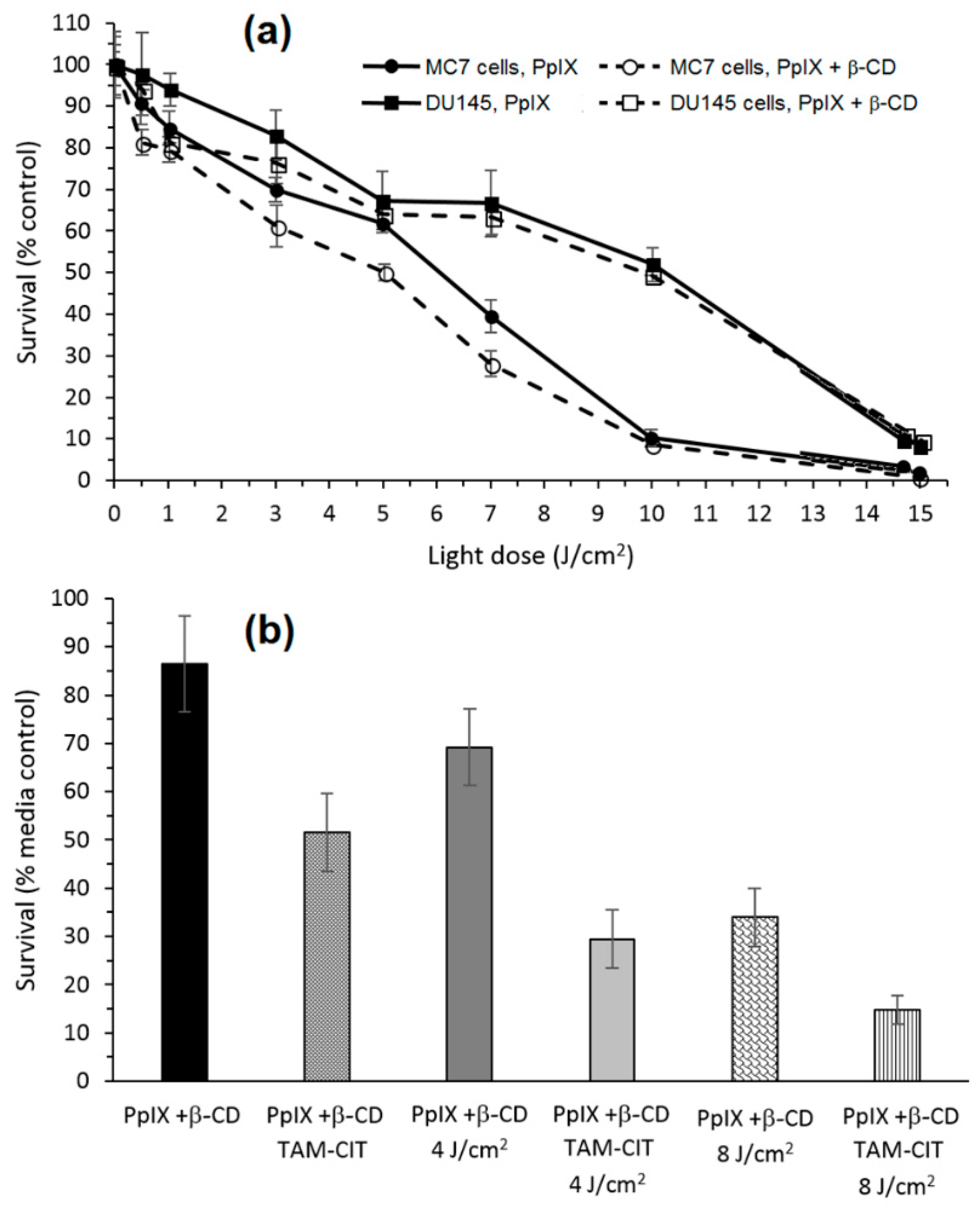
The in vitro phototoxic properties of PpIX+β-CD compared to PpIX alone were performed on human prostate carcinoma (DU145) and breast adenocarcinoma (MCF7) cell lines. PpIX+β-CD displayed less toxicity in the dark than PpIX (15% against 25%, respectively) in both cell lines, which could be explained by β-CD improving the solubility of PpIX, and thus reducing its ability to aggregate. The in vitro evaluation of the phototoxicity depending of the light dose (λ = 610 nm, 0 to 15 J/cm
2, 15 mW/cm
2) is given in
Figure 26a. There were no significant differences between PpIX and PpIX+β-CD, indicating that β-CD had no impact on the phototoxicity properties of PpIX.
Finally, the ability of PpIX+β-CD conjugates to host and transport a therapeutic molecule was investigated using NDMTAM. The PpIX+β-CD seemed to be a good drug carrier for the intracellular transport of this drug. Finally, the bimodal action of PpIX+β-CD complexed to tamoxifen citrate (TAM-CIT) was investigated using the MCF7 cell line (
Figure 26b). Under an irradiation of 4 J/cm
2, the PpIX+β-CD complexed to TAM-CIT had a toxicity of 70% against 30% for PpIX+β-CD alone, and under 8 J/cm
2, the toxicity was 85% against 67%. These observations highlighted that there was a synergistic effect of the toxicity of the chemotherapy drug and the phototoxicity of the PpIX+β-CD conjugates.
In the same year, Fraix et al. [
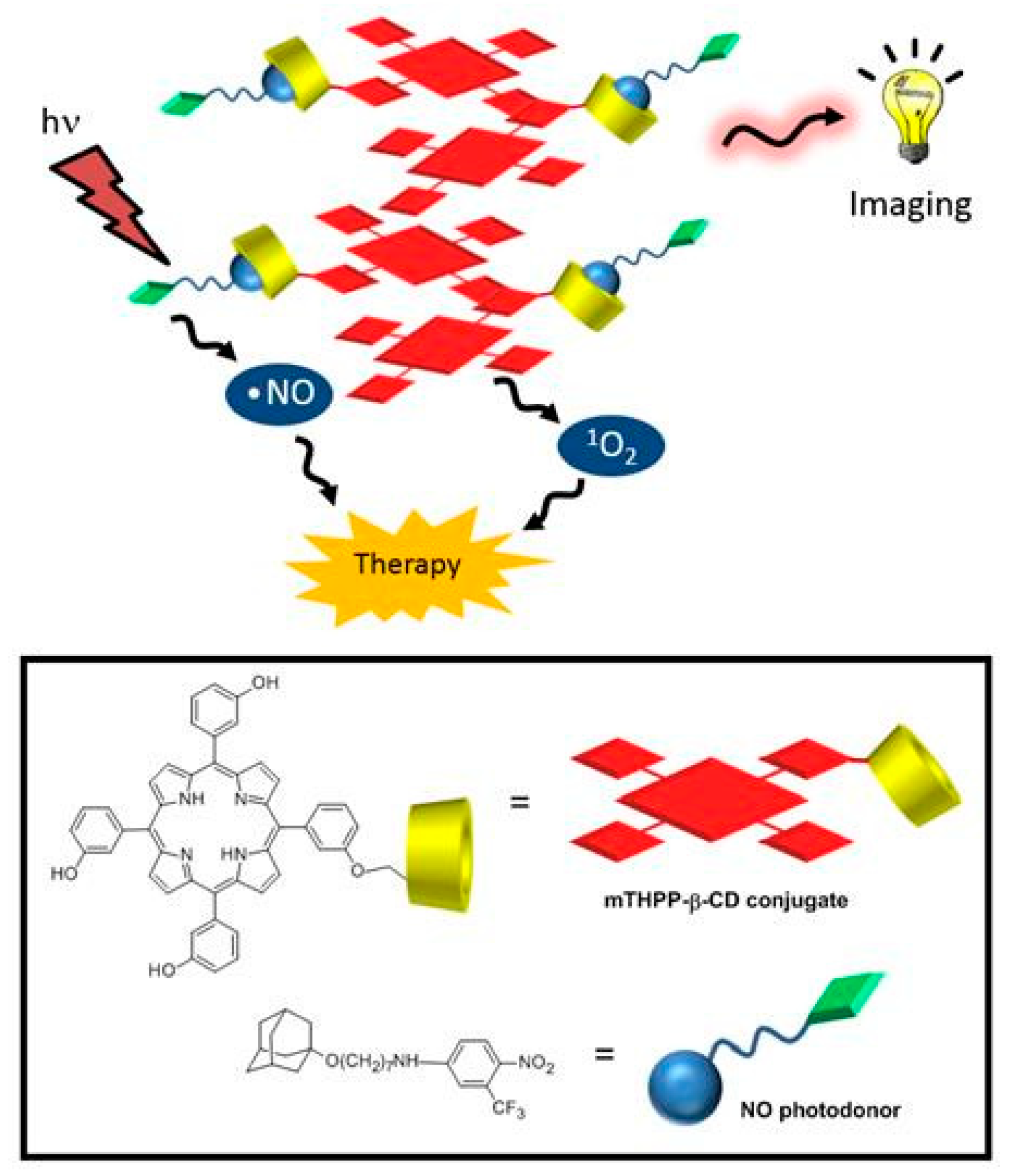
145] synthesized and investigated the properties of a new supramolecular assembly as a bimodal agent for PDT and imaging composed of
meta-(3-hydroxyphenyl)-porphyrin (mTHPP) conjugated to β-CD (mTHPP-β-CD conjugate) by an ether bond, and a nitric oxide photodonor (NO phodonor) tailored to fit the β-CD cavity (
Figure 27).
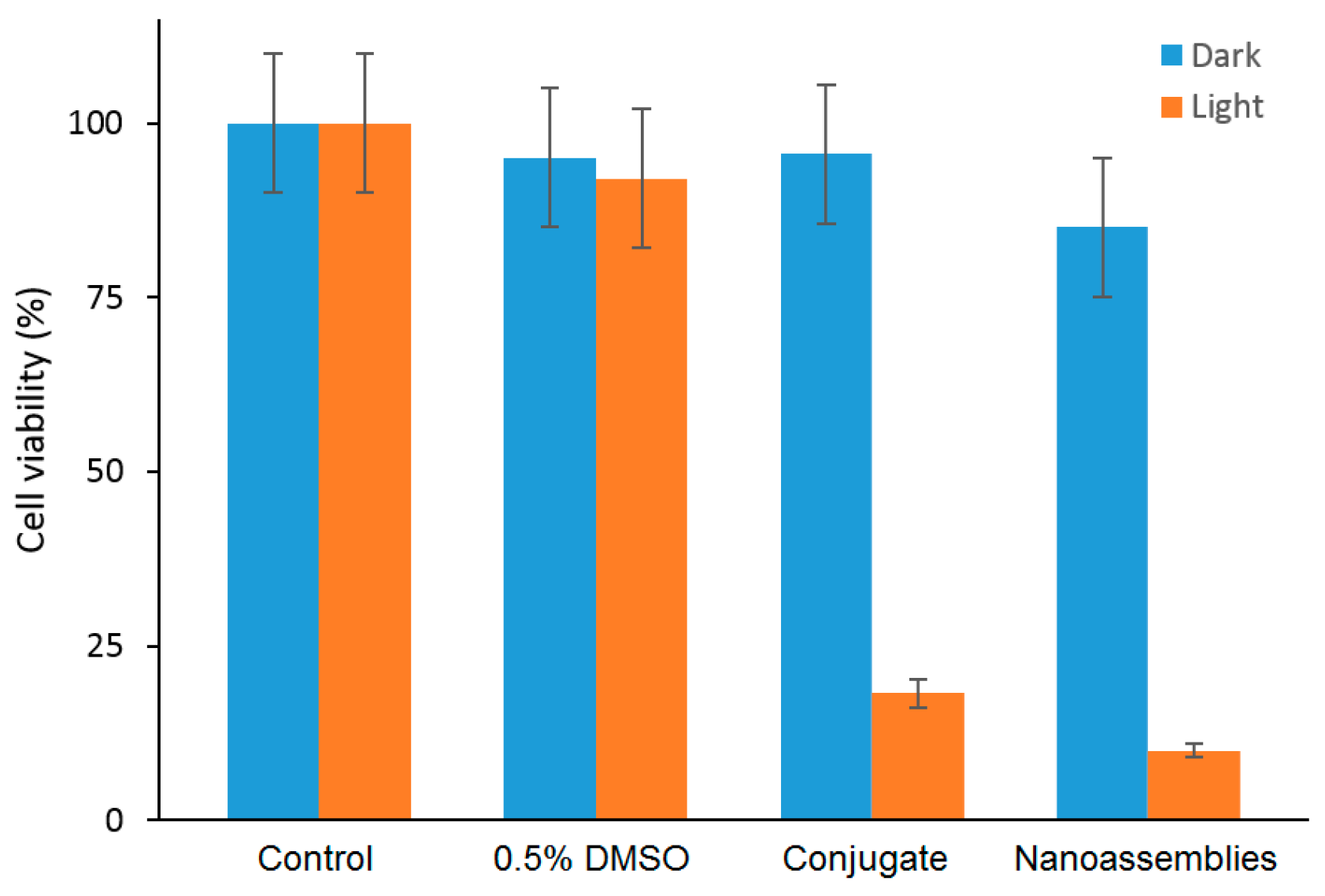
They found that compared to the free mTHPP, the mTHPP-β-CD conjugate has shown less aggregation in an aqueous environment, and formed small nanoassemblies with diameters of around 13 nm. Furthermore, the empty cavity of β-CD in the mTHPP-β-CD conjugate also had the ability to a host a nitroaniline derivative (NO photodonor) while maintaining the nanometer character of the aggregate and the fluorescence of the porphyrin core. In addition, bichromophoric nanoassemblies (β-CD-mTHPP/NO photodonor) were found to be able to generate both NO and 1O2 upon excitation with visible light (λexc > 400 nm).
By in vitro studies, the internalization in a human amelanotic melanoma cell line (A375) was evaluated for the conjugate and the bichromophoric nanaoassemblies at a concentration of 8 µM after 4 h of incubation, and the authors found a localization mainly in cytoplasm. The in vitro ability of the conjugate and the bichromophoric nanaoassemblies to induce A375 cell mortality upon light irradiation was compared. No dark toxicity was found, but a high phototoxicity was observed for both compounds, with a slight difference between them explained by the liberation of NO and
1O
2 for the nanoassemblies (
Figure 28).
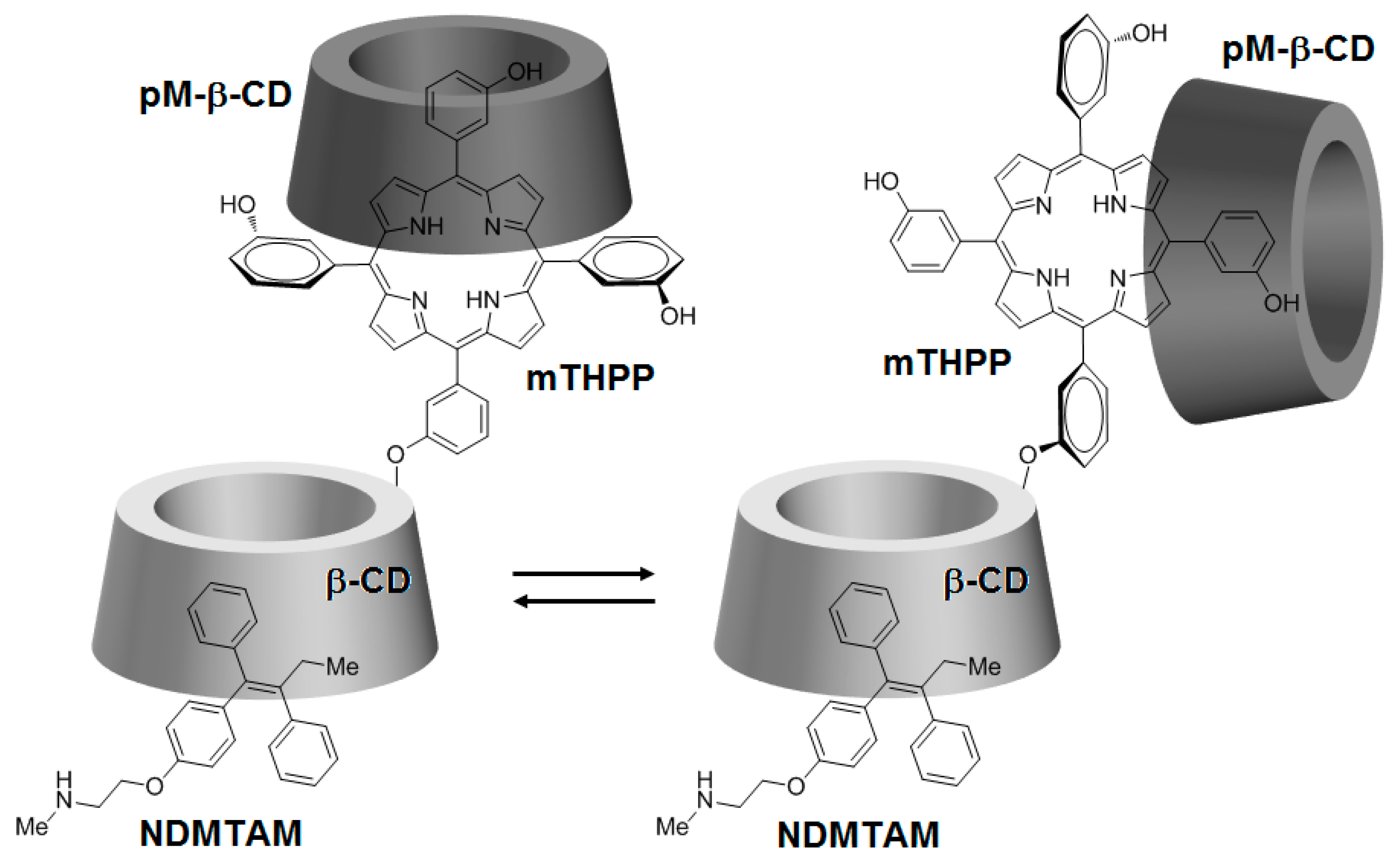
Being encouraged by the potential properties of the mTHPP-βCD conjugate, recently, the same team [
146] investigated the photochemical internalization (PCI) potential of the same conjugate with the NDMTAM drug as the guest molecule. In order to improve the water solubility of the mTHPP core in the conjugate, the authors encapsulated the mTHPP core in the empty cavity of heptakis(2,3,6-
O-methyl)-β-CD (pM-β-CD), leading to the formation of a new mTHPP-β-CD/pM-β-CD nanosystem. The obtained nanosystem has shown the ability to host the NDMTAM drug through either one of its unsubstituted phenyl groups (
Figure 29).
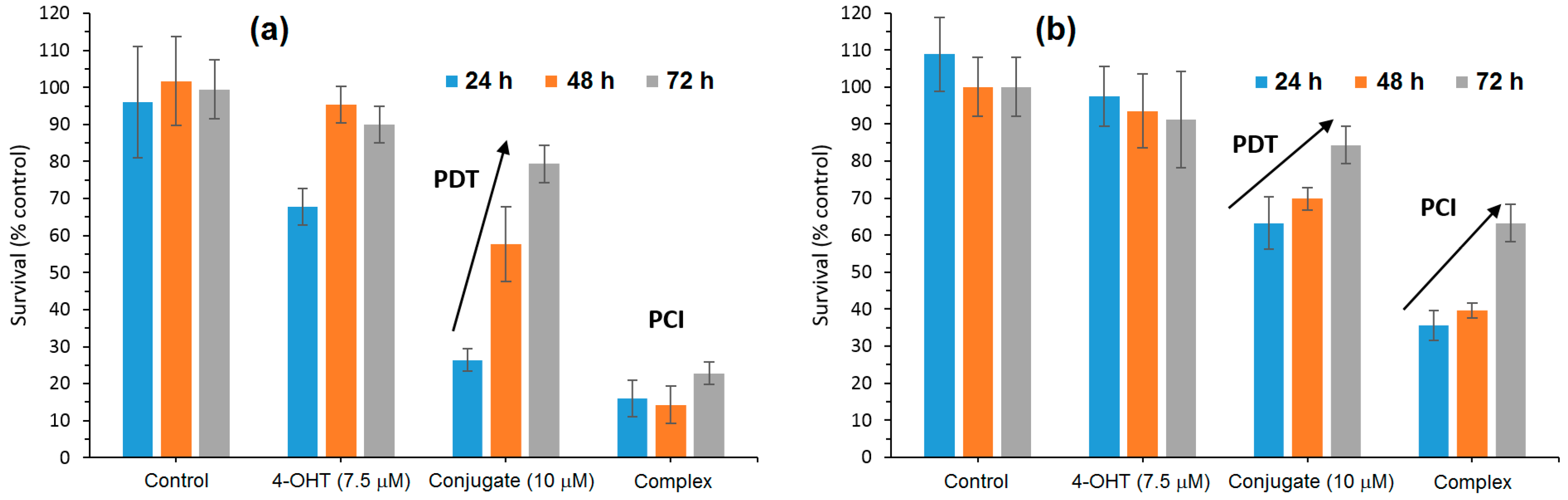
According to confocal microscopy studies, it was found that under irradiation, the porphyrin core of mTHPP-β-CD expedited endosomal membrane rupture and NDMTAM release into the cytosol. Furthermore, the authors also investigated the in vitro photocytotoxicity efficiency on breast human carcinoma (MDA-MB-231) and MCF7 cell lines of the mTHPP-β-CD conjugate, tamoxifen (4-OHT), and the resulting mTHPP-β-CD/4-OHT complex. Upon irradiation at LD
50 light doses, in the case of cells treated with the conjugate, phototoxicity around 70% was observed, which was annulled after 48 h and 72 h. Concerning cells irradiated after treatment with the complex, they showed a cell death of 80%, with no change even after 48 h and 72 h (
Figure 30).
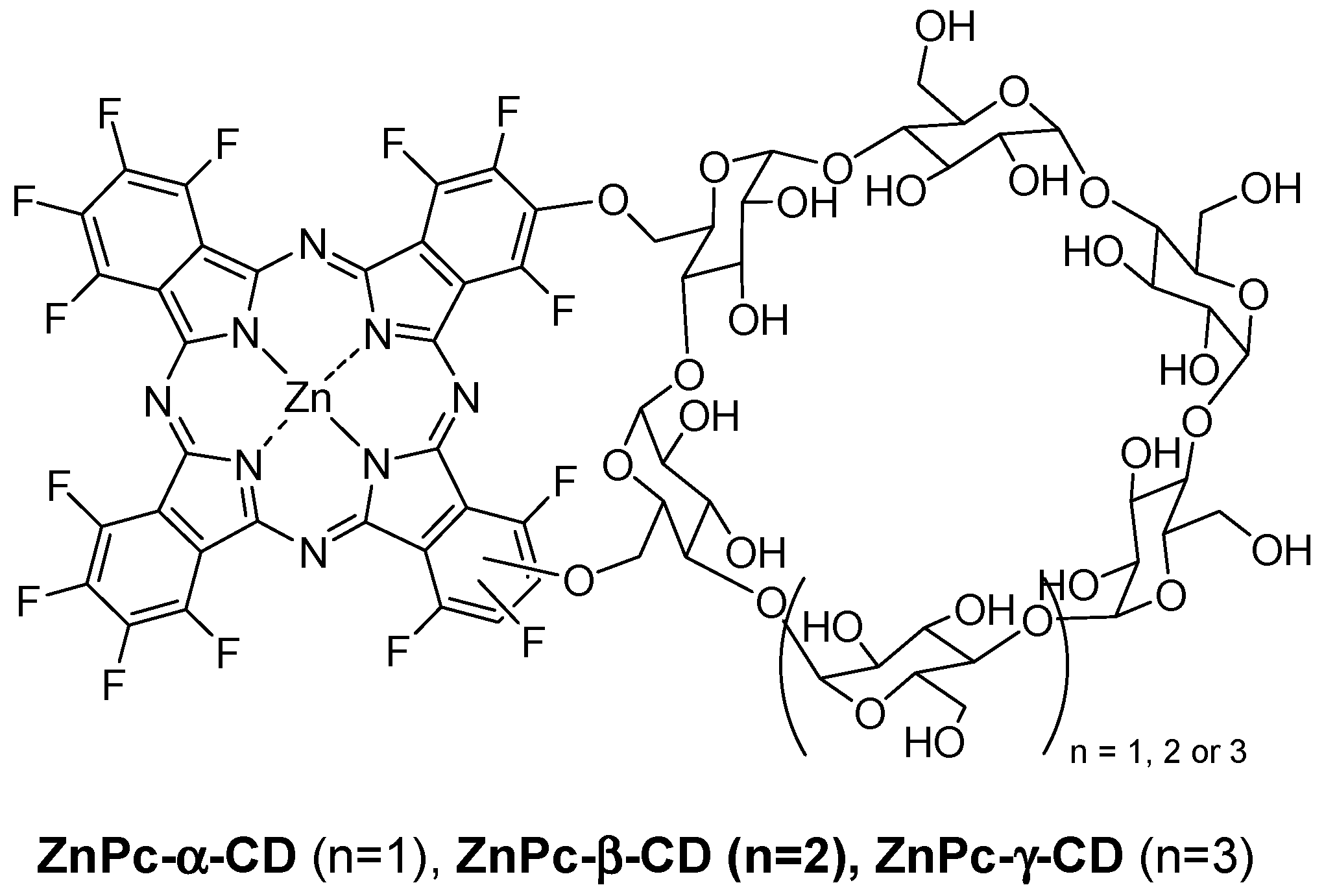
In 2014, Lourenço et al. described the synthesis and PDT properties of CD–PS conjugates (ZnPc-α-CD, ZnPc-β-CD, and ZnPc-γ-CD) constituted by a zinc perfluorinated phthalocyanine (ZnPc) covalently linked through ether bonds to various types of CDs (α-CD, β-CD, and γ-CD) (
Figure 31) [
147].
It was found that ZnPc-α-CD and ZnPc-γ-CD have a better solubility compared to ZnPc-β-CD due to the lesser solubility of the β-CD unity itself in water compared to α-CD and γ-CD. The ability of each ZnPc–CD conjugate to generate 1O2 was evaluated using 1,3-diphenylisobenzofuran as a probe. The authors found a similar effectiveness for ZnPc-α-CD and ZnPc-γ-CD conjugates compared to ZnPc alone, but ZnPc-β-CD seemed to be less effective, which was maybe due to the same reasons mentioned before.
The human cancer cell line derived from the transitional cell carcinoma of the bladder (UM-UC-3) was selected as a cancer cell model to study the in vitro photosensitizing efficiency. No dark toxicity was observed for the different conjugates at concentrations up to 10 µM and 4 h of incubation time. Under light irradiation (white or red-light source, 50 mW/cm2), after incubation with various concentrations of conjugates (0–1 µM), only ZnPc-α-CD and ZnPc-γ-CD conjugates have shown a phototoxic effect, and this effect was irradiation time and concentration-dependent. In addition, this effect was found to be more efficient under white-light irradiation compared to the red one. To better understand these results, the authors investigated the ROS production capacity of the conjugates under white or red-light irradiation using a 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) probe. They found that all of the conjugates produced ROS under white or red irradiation, and intracellular ROS production was higher in the case of ZnPc-α-CD and ZnPc-γ-CD conjugates. Indeed, they accumulated more in cells than ZnPc-β-CD, proving again their higher potential compared to ZnPc-β-CD for PDT.
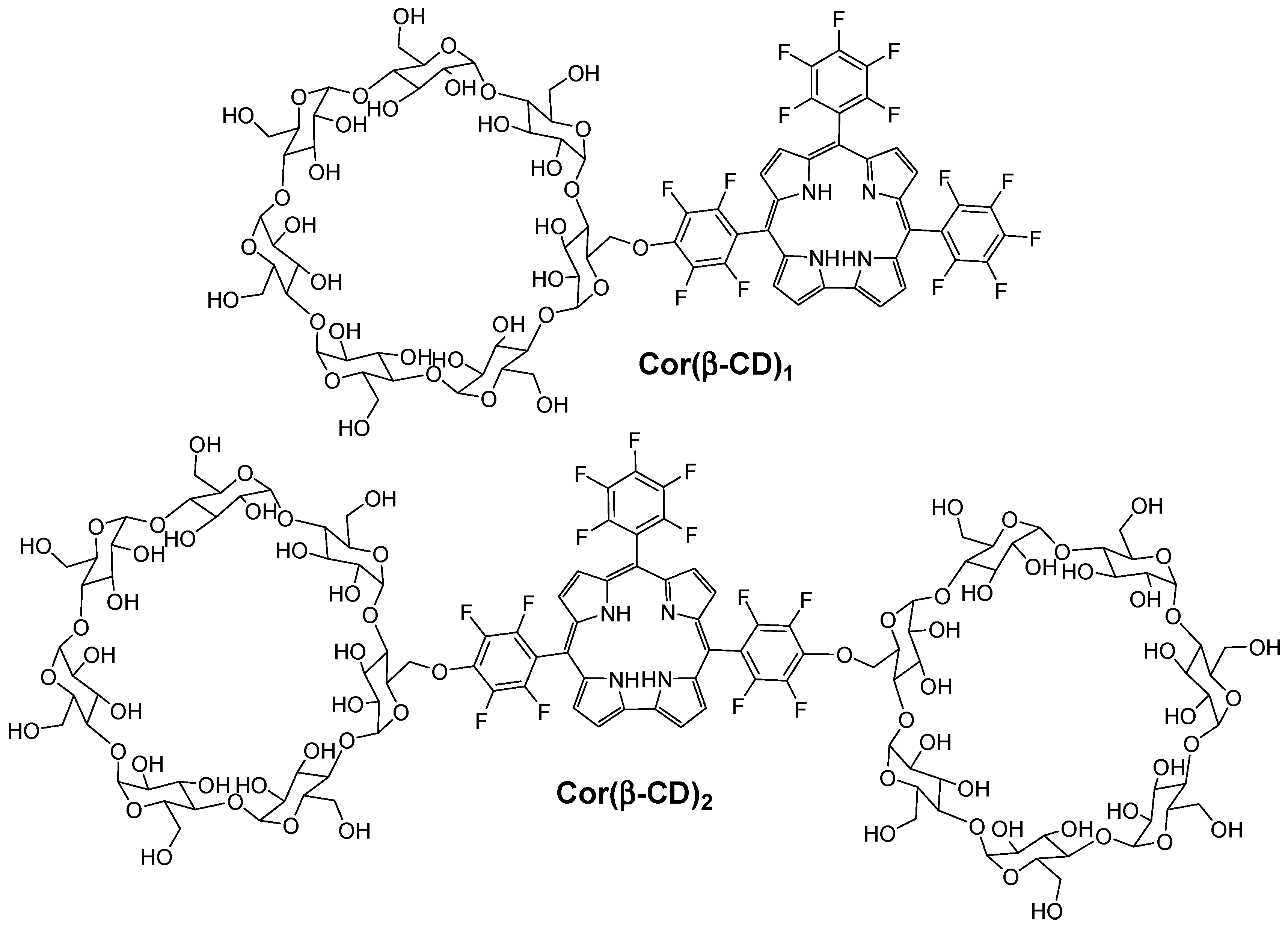
Recently, Barata et al. [
148] described and investigated the photosensitizing properties of corrole β-cyclodextrin conjugates (Cor(β-CD)
1 and Cor(β-CD)
2), which were composed of 5,10,15-tris(pentafluorophenyl)corrole (Cor) and one or two β-CD units (
Figure 32).
The authors found that the presence of β-CD units in both conjugates improved the hydrophicility of the corrole core. It was found also that all of the conjugates are highly photostable and able to produce 1O2.
In vitro studies were performed on human cervical cancer cell line (HeLa). The cytotoxicity of each compound (Cor(β-CD)
1, Cor(β-CD)
2, and Cor) was evaluated by using MTT assay at different concentrations (from 10
−7 M to 10
−4 M) in the dark. No cytotoxicity was found for both Cor and Cor(β-CD)
1 conjugate in contrast to the Cor(β-CD)
2 conjugate, which conduced to 14% of cytotoxicity in cells. Upon red-light irradiation (3 J/cm
2, 6 J/cm
2, 9 J/cm
2, and 12 J/cm
2, 5 mW/cm
2), all of the compounds showed concentration-dependent and light-dose dependent cell-destruction ability. Therefore, as shown in
Table 6, Cor seems to be the most effective compound to be used as a PS for PDT.
Due to the dark cytotoxicity of the Cor(β-CD)2 conjugate, the authors selected only Cor and Cor(β-CD)1 compounds for the next studies. According to the subcellular localization study, Cor was found to be mainly accumulated in lysosomes, whereas Cor(β-CD)1 conjugate accumulated in the Golgi apparatus. Finally, they investigated the PDT effect of Cor and Cor(β-CD)1 compounds on the cytoskeleton by combination of the injection of Cor or Cor(β-CD)1 compounds (10−5 M) upon red-light irradiation (12 J/cm2) on microtubules in HeLa cells. They observed changes on microtubules for both Cor and Cor(β-CD)1 compounds, but a higher PDT efficiency was observed in the case of Cor, which may be due to its localization in lysosomes.
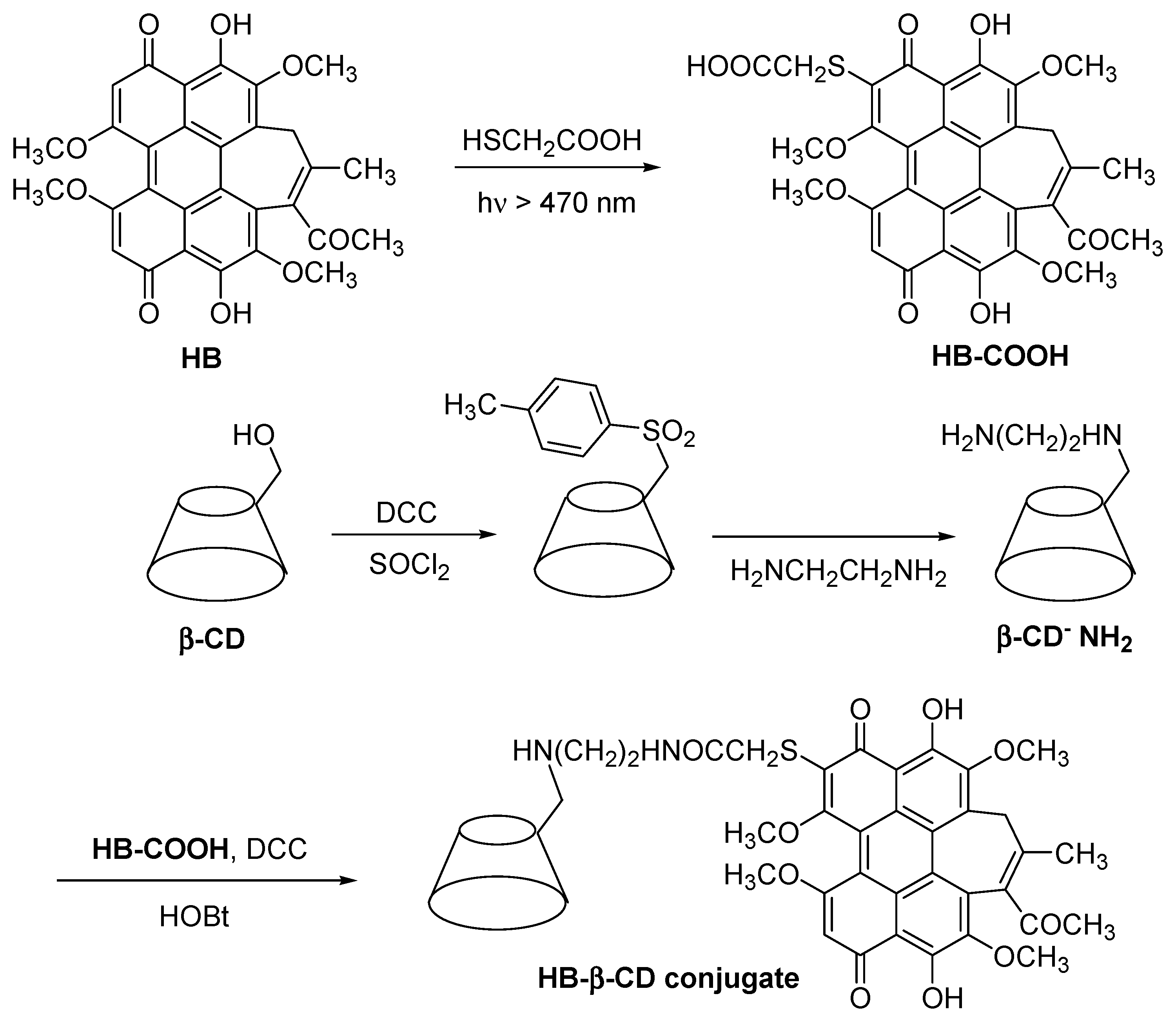
In 2002, Ou et al. [
149] synthesized a modified non-porphyrinoid PS (hypocrellin B, HB) conjugated to the β-CD (HB-β-CD conjugate) (
Figure 33), and studied its water solubility and PDT properties. They found that the HB-β-CD conjugate had a higher water solubility compared to free HB. They also proved that the HB-β-CD conjugate can produce different ROS such as O
2•−,
•OH, and
1O
2 species. Furthermore, the authors found that the β-CD unit present in the HB-β-CD conjugate reinforced their affinity for calf thymus DNA (CT DNA), leading to the stronger photodamage of CT DNA compared to free HB. The authors concluded that the introduction of β-CD units enhanced the water solubility and PDT properties of HB.
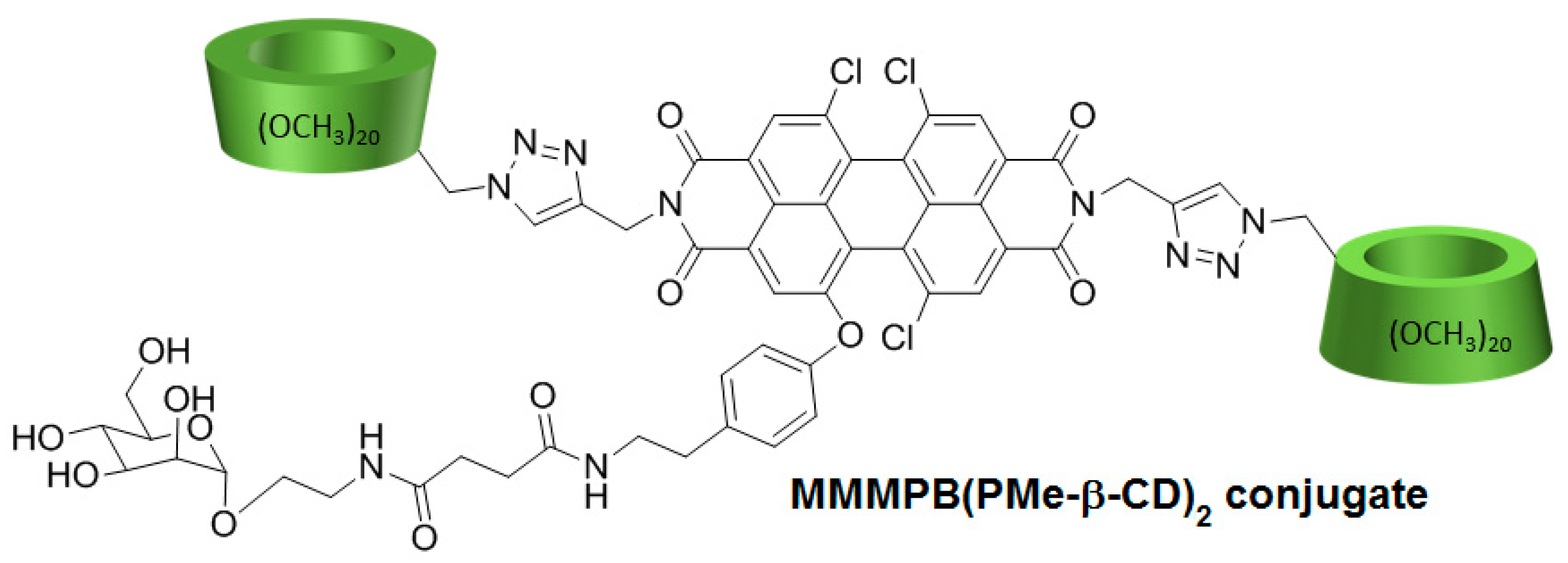
In 2017, Cao et al. [
150] developed a new PDT agent (MMMPB(PMe-β-CD)
2 conjugate) by conjugation of a non-porphyrinoid PS (a mono-mannose modified perylene bisimide, MMMPB) to two permethyl β-CDs (PMe-β-CDs) via click triazole links (
Figure 34), and evaluated its PDT properties.
In vitro studies were performed to investigate the PDT activity of the MMMPB(PMe-β-CD)2 conjugate using four cell lines (A549, Hela, MCF-7, and Hep G2) at various concentrations of MMMPB(PMe-β-CD)2 conjugate, TPPS4, and cisplatin as controls (0.2 µM, 1 µM, 5 µM, 25 µM, and 100 µM). The conjugate showed no dark toxicity, but was highly phototoxic under irradiation (20 mW/cm2) with better IC50 than controls. The ability to produce 1O2 was evaluated by using 1,3-diphenylisobenzofuran as a trap, and the production of 1O2 was highlighted. Fluorescence imaging was performed in MCF-7 cells by incubation with the conjugate (10.0 µM) to localize the distribution of the conjugate in cells, and it was found mainly in cytoplasm. They finally found that the cell death was induced with an apoptotic pathway.
2.2.3. Cyclodextrins-Photosensitizer Nanoassemblies
The last type of binding between CD and PS refers to CD–PS nanoassemblies. These nanoassemblies are supramolecular colloidal systems involving non-specific external links between the external sites of CDs and PSs. Chemical modifications of either narrow or wide edges of natural CDs by various substituents make them possible to produce ionic or nonionic CD derivatives that are capable of forming supramolecular buildings such as vesicles, micelles, or nanoparticles (NPs) that have the capacity to host PSs. With regard to the anticancer PDT application, it has been shown in vitro that this type of vector can improve PS administration and PDT efficacy [
151,
152]. However, to date, no in vivo PDT study has been devoted to PS–CD nanoassemblies.
Among the various types of CD derivatives, amphiphilic CDs are largely used to form supramolecular nanoassemblies, and have had many applications in the biomedical field [
153,
154,
155,
156,
157]. Researchers have developed the concept of amphiphilic CDs to adjust the hydrophobic/hydrophilic balance of their construction, leading to the formation of various types of supramolecular nanoassemblies (vesicles, micelles, NPs…) [
158,
159,
160,
161]. Amphiphilic CDs can be obtained by enzymatic pathways or by grafting various substituent groups via amino, amido, thio, ester, and ether bonds [
155].
All the studies described below are drawn from the work of Mazzaglia et al.
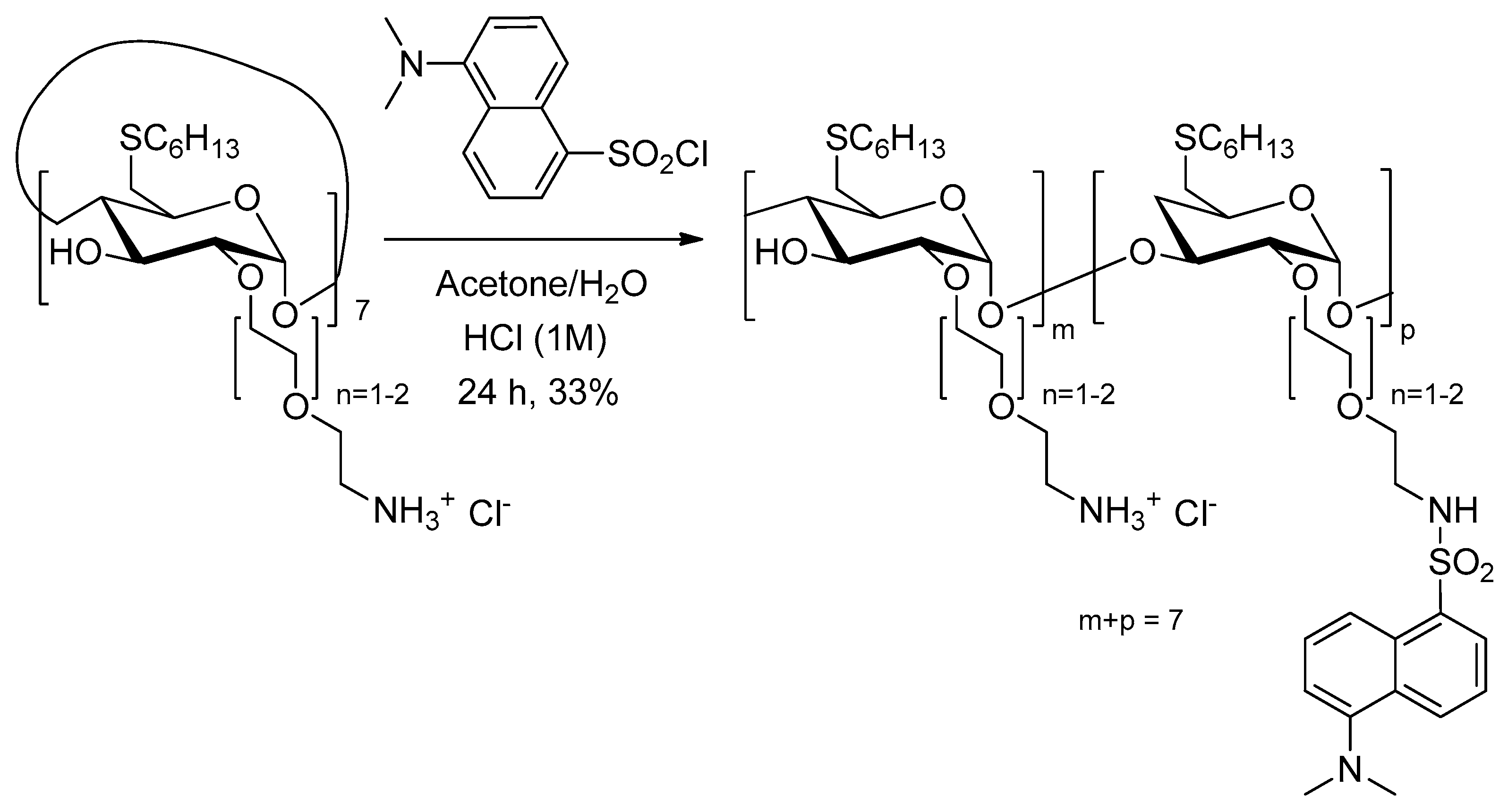
In two articles published in 2003 and 2005 [
162,
163], Mazzaglia et al. investigated the development of a new carrier–PS system TPPS
4/SC
6-β-CD-NH
2 composed of amphiphilic heptakis(2-
o-amino-
O-oligo(ethylene oxide)-6-hexylthio)-β-CD (SC
6-β-CD-NH
2,
Figure 35) self-assembled into vesicles and an encapsulated water-soluble porphyrin (TPPS
4). The authors showed that TPPS
4/SC
6-β-CD-NH
2 nanoaggregates produced
1O
2, but in lower amounts than free TPPS
4. However, it was found that SC
6-β-CD-NH
2 vesicles seemed to be an efficient carrier for intracellular PS delivery.
In 2006, Mazzaglia et al. [
164] investigated the PDT activity of TPPS
4/SC
6-β-CD-NH
2 nanoassemblies. It was found that they had a size ranging from 100 nm to 1000 nm, and that
1O
2 generation was highly dependent on the SC
6-β-CD-NH
2 concentration in nanoassemblies. Based on in vitro studies, the TPPS
4 internalization and PDT efficiency of nanoassemblies using HeLa cells were found to be highly dependent on the TPPS
4/SC
6-β-CD-NH
2 molar ratio. Molar ratio of 1:10 seemed to be the best ratio in terms of TPPS
4 internalization, which is the highest percentage of cells alive before irradiation and a considerably high percentage of cell death after irradiation (
Figure 36). The authors concluded that TPPS
4/SC
6-β-CD-NH
2 nanoassemblies can be used as potential candidates for PDT application.
In 2011, the same team [
165] used the above amphiphilic TPPS
4/SC
6-β-CD-NH
2 nanoassemblies covalently functionalized by the dansyl fluorophore. The resulting supramolecular system showed a good PS delivery while allowing a simultaneously detection of carrier and PS in tumor cells (
Figure 37).
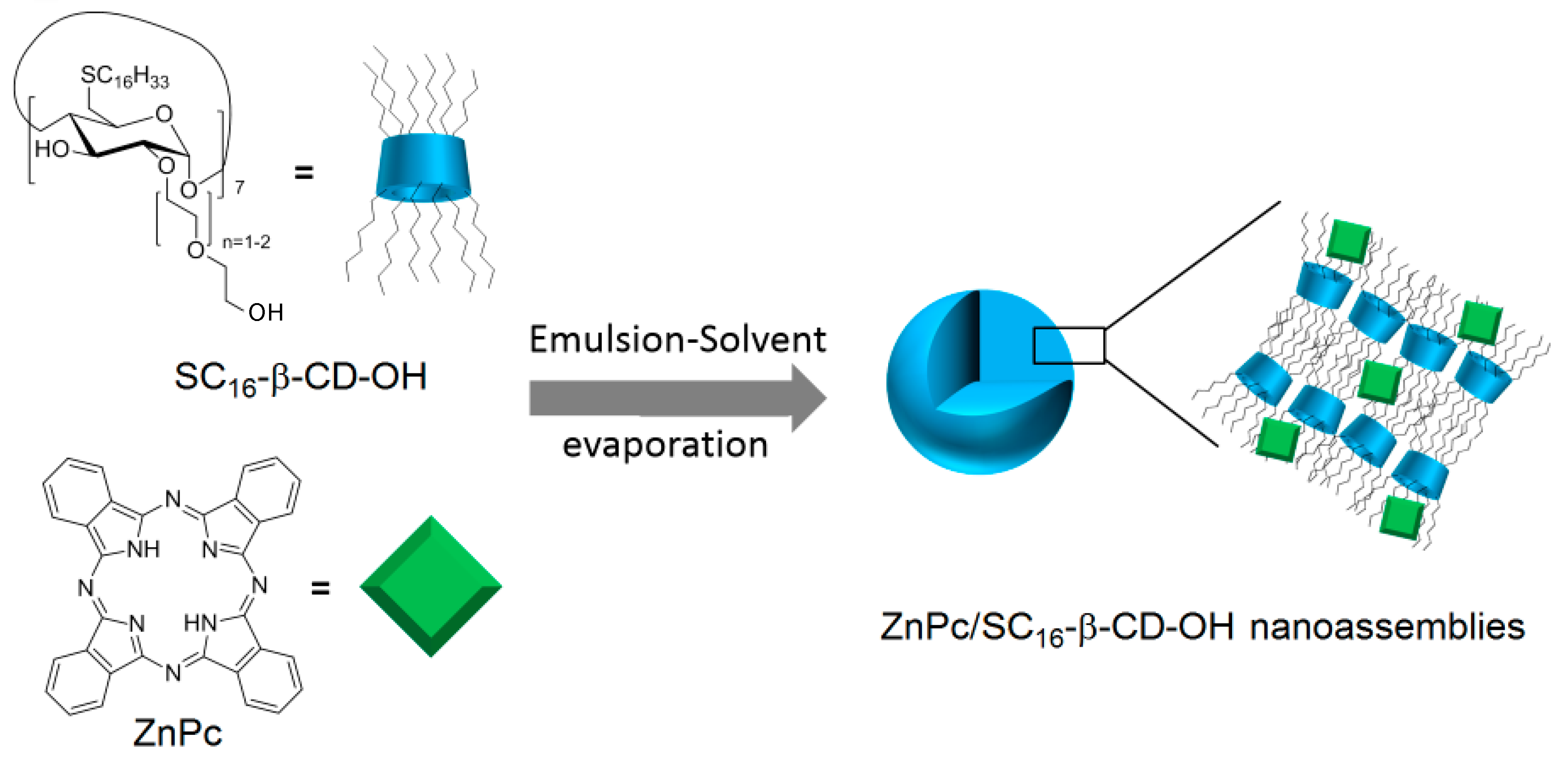
In 2014, Mazzaglia et al. [
166] synthesized the hydroxylated analog of SC
6-βCD-NH
2 (noted SC
16-βCD-OH), and developed a novel biodegradable phototherapeutic nanoassembly ZnPc/SC
16-β-CD-OH based on the self-assembly in aqueous media of heptakis(2-oligo(ethylene-oxide)-6-hexadecylthio-)-β-CD (SC
16-βCD-OH) and zinc-phthalocyanine (ZnPc) (
Figure 38). ZnPc/SC
16-β-CD-OH nanoassemblies have a hydrodynamic diameter of around 200 nm, and a shown ability to produce
1O
2.
The nanoassemblies’ formation was assessed by Dynamic Light Scattering (DLS), zeta potential,
1H NMR, TEM, and Scanning Near-Field Optical Luminescence (SNOL) spectroscopy. ZnPc/SC
16-β-CD-OH nanoassemblies have a hydrodynamic diameter of around 200 nm and a shown ability to produce
1O
2. Cellular uptake and cytotoxicity was investigated in HeLa cancer cells. The in vitro studies showed that ZnPc/SC
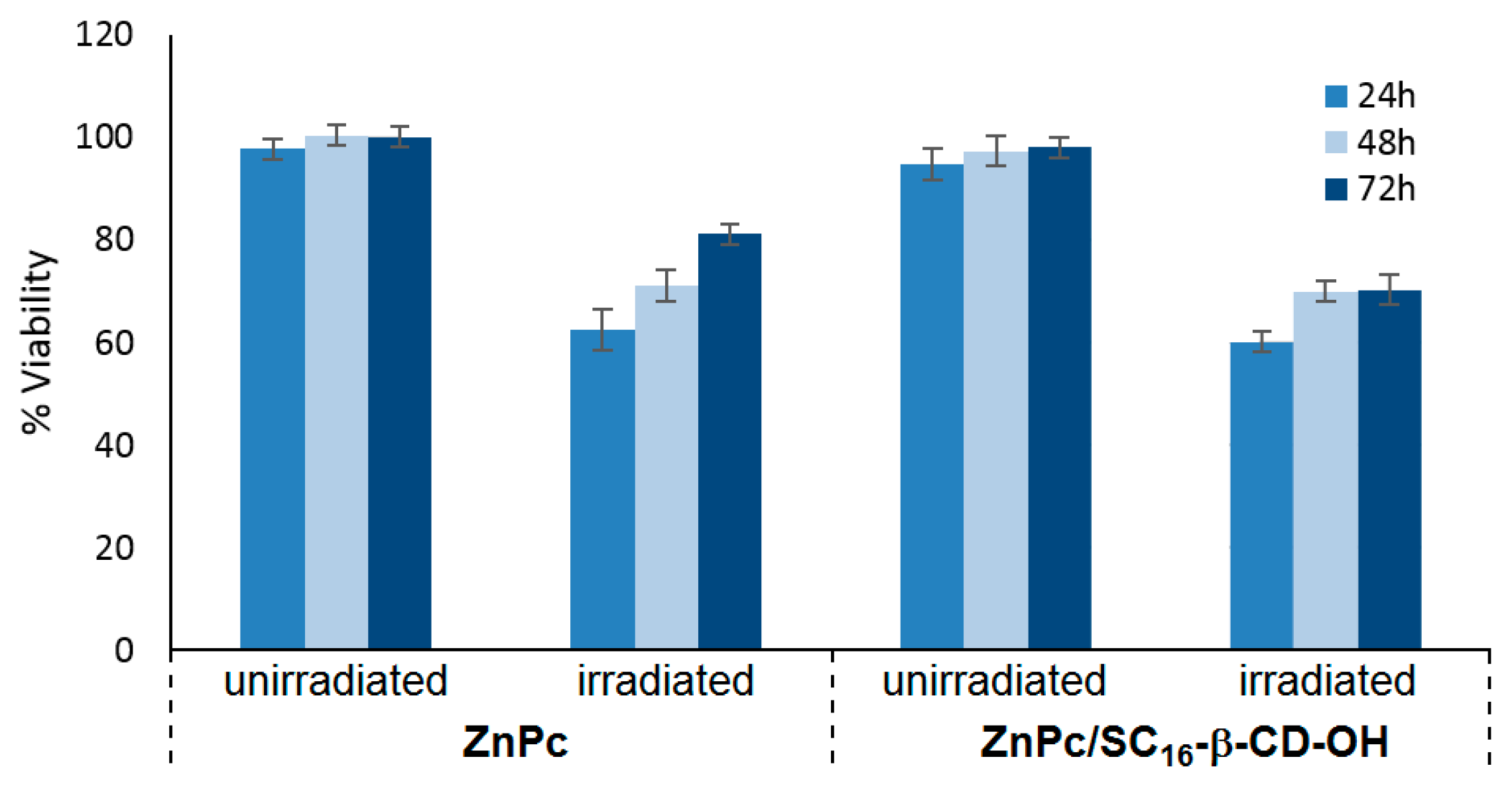
16-β-CD-OH can be internalized in HeLa cells at 37 °C, and their uptake was mediated by endocytosis, which is strongly temperature-dependent. Furthermore, the PDT efficiency of ZnPc/SC
16-β-CD-OH was performed using HeLa cells under irradiation (λ = 340 nm, 5 J/cm
2, 30 min), and ZnPc/SC
16-β-CD-OH showed a better phototoxic activity against HeLa cells than free ZnPc in DMSO (
Figure 39).
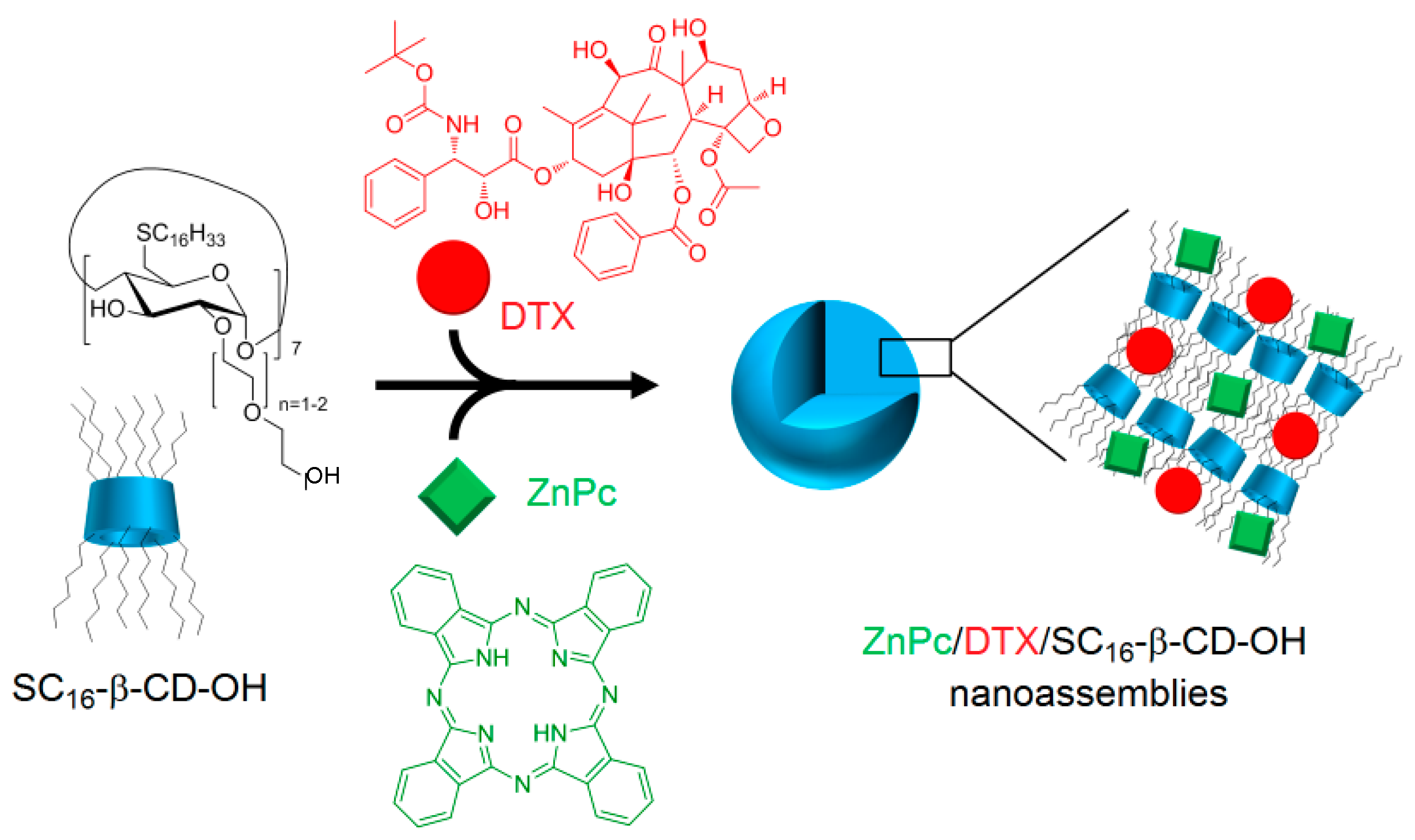
Encouraged by the promising results of their previous work, the authors investigated the possibility of using the same nanoasemblies for combined cancer therapies (PDT and chemotherapy) [
167]. The new nanoassemblies (hydrodynamic size of 200 nm) were formed as previously described [
166] in aqueous medium in the presence of docetaxel (DTX) as the chemotherapeutic drug (ZnPc/DTX/SC
16-β-CD-OH,
Figure 40).
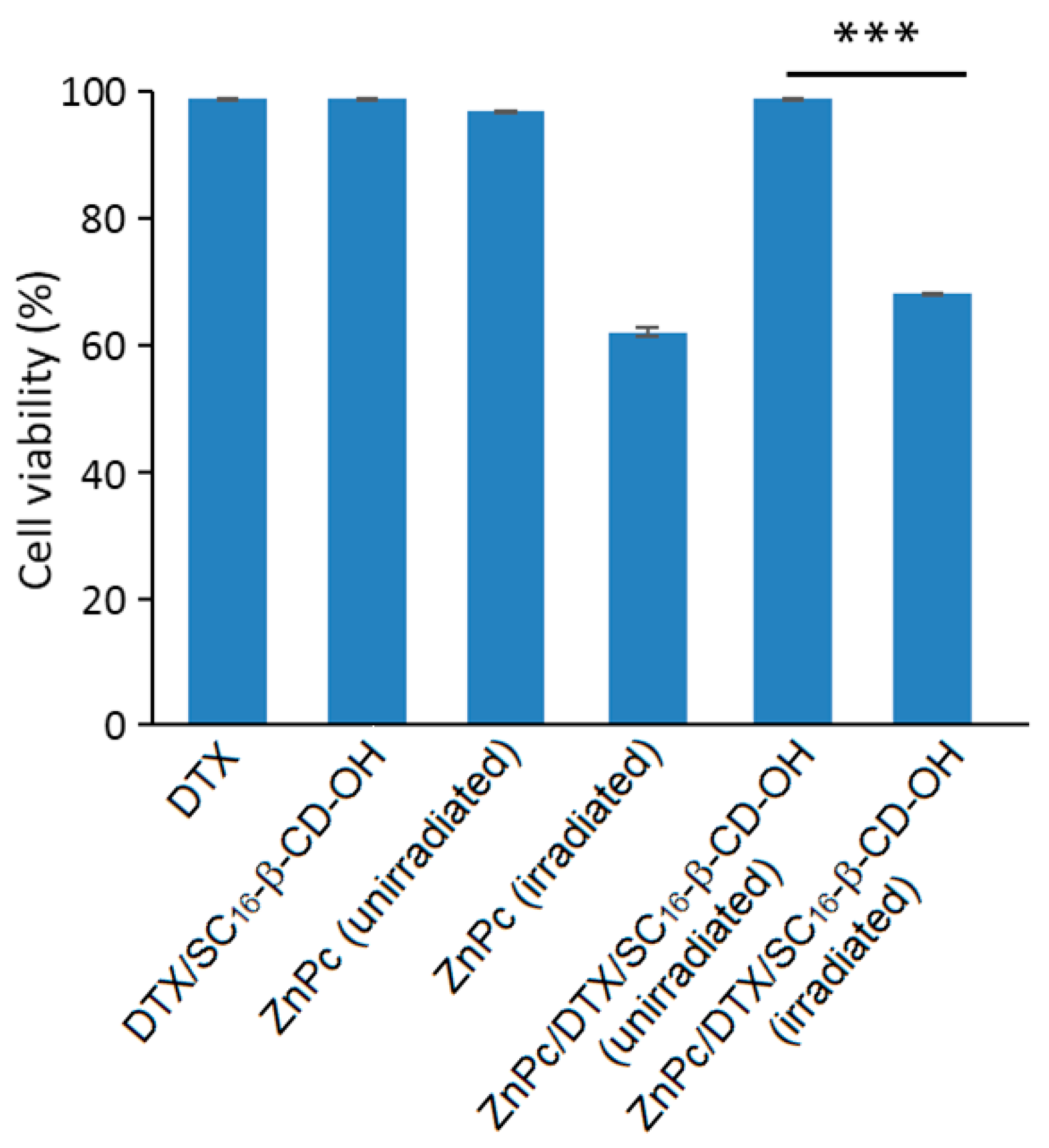
The new ZnPc/DTX/SC
16-β-CD-OH nanoassemblies were characterized as previously described [
166]. These NPs have a hydrodynamic diameter of around 200 nm, and no specific interaction with the SC
16-β-CD-OH cavity was shown. Finally, the PDT efficiency of ZnPc/DTX/SC
16-β-CD-OH NPs using HeLa cells was investigated and compared to free drugs. Based on in vitro studies using HeLa cells, no dark cytotoxicity was detected in all of the treated cells in the dark, but under irradiation (λ = 340 nm, 5 J/cm
2, 30 min), a similar phototoxic effect was observed for ZnPc/DTX/SC
16-β-CD-OH NPs, and exhibited a comparable phototoxic effect to that of irradiated free ZnPc in DMSO (
Figure 41).
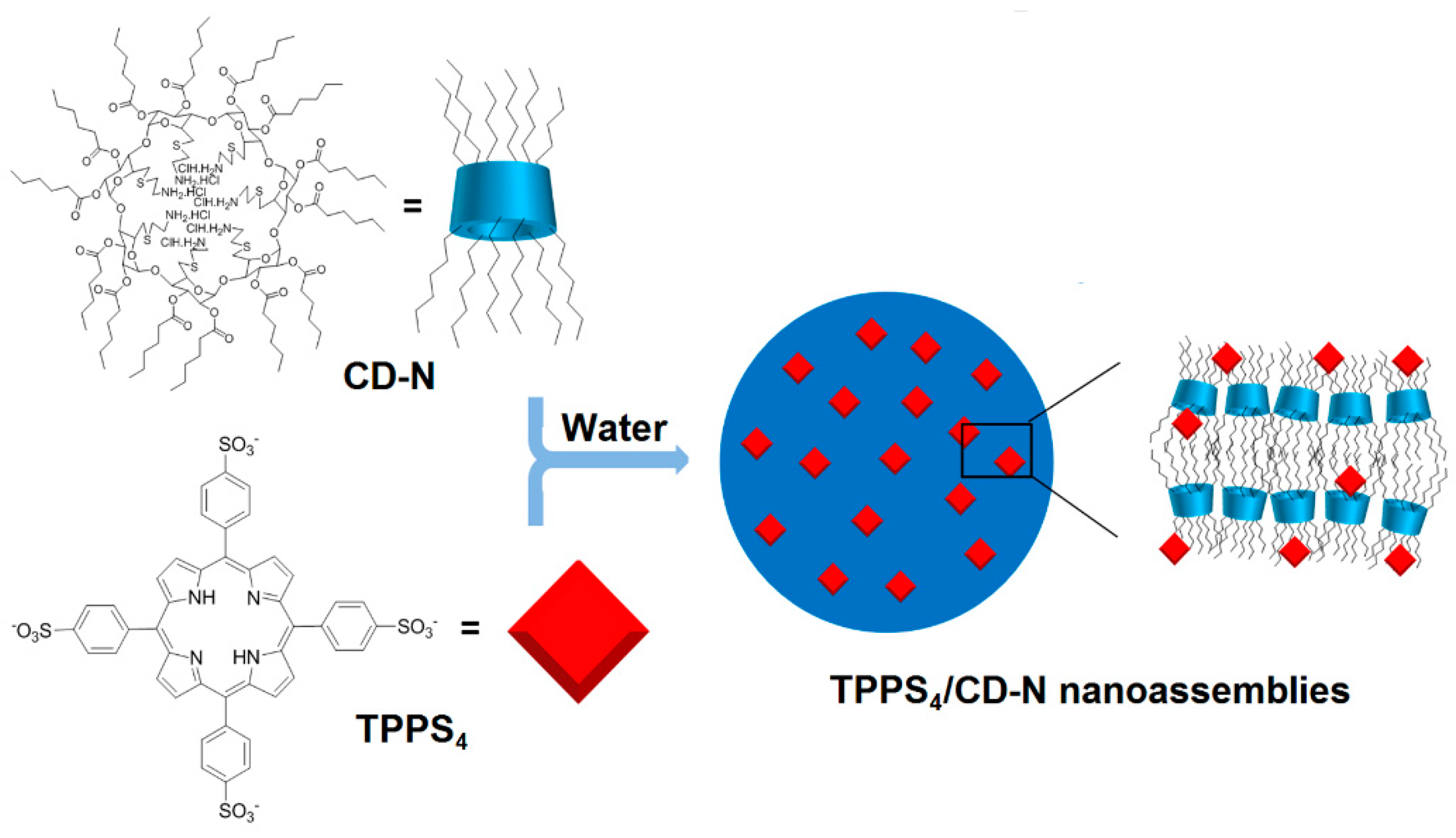
Finally, Mazzaglia et al. in 2017 [
168] described the elaboration of a novel nanophototherapeutic using the same emulsion–solvent evaporation procedure in a water environment as described in their previous studies [
162,
163,
165,
166,
167]. For this novel nanoassembly (hydrodynamic size of around 40 nm) and as reported in their previous work [
164], the authors used the same water-soluble anionic PS (TPPS
4), but this time with a new cationic amphiphilic CD, i.e., heptakis[6-(2-aminoethylthio)-6-deoxy-2,3-di-
O-hexanoyl] cyclomaltoheptaose (CD-N) (
Figure 42).
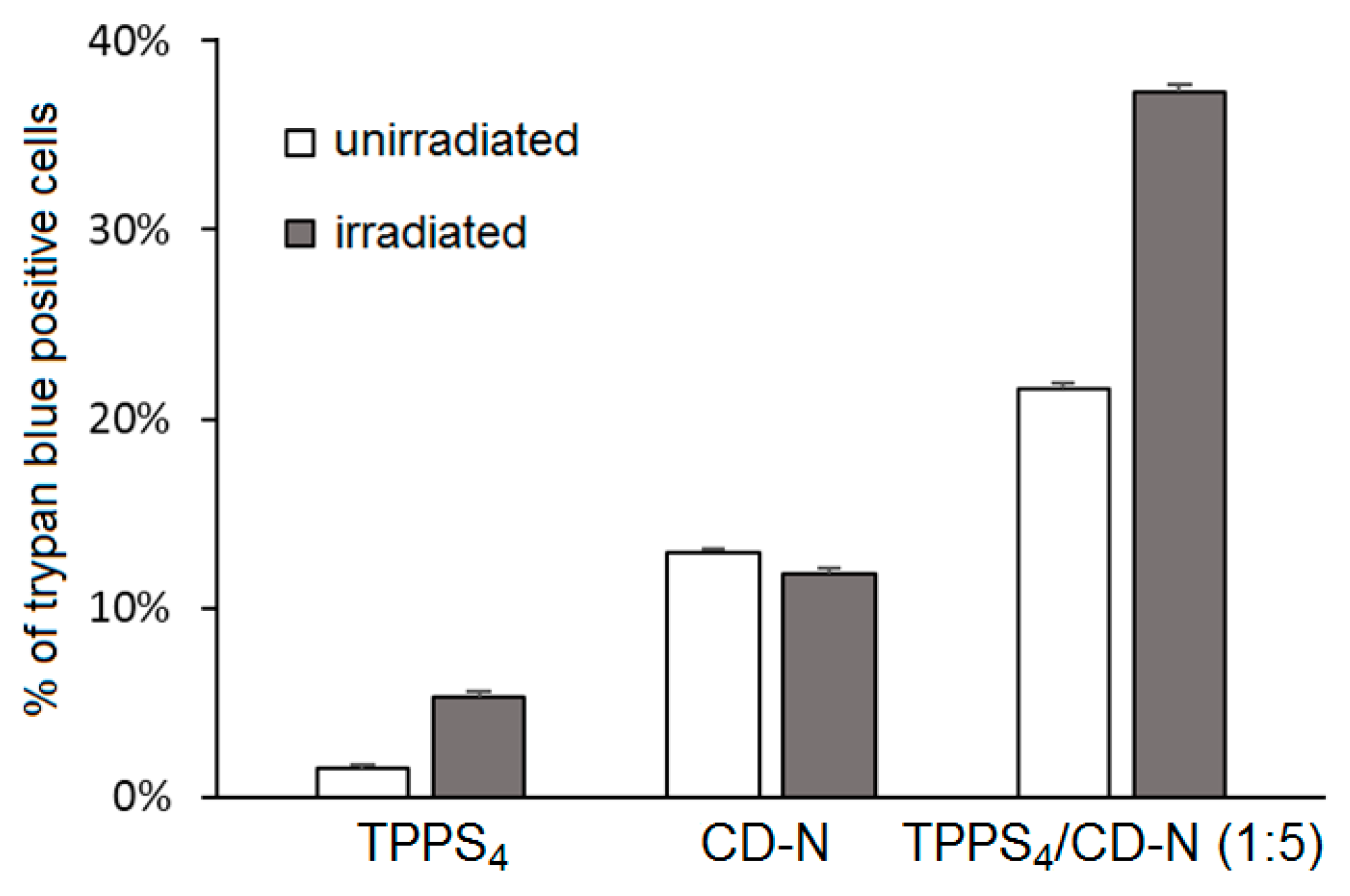
Cellular uptake was performed on HeLa cells treated with TPPS
4/CD-N nanoassemblies, and the authors showed that amphiphilic CD-N was able to promote the intracellular delivery of TPPS
4. The in vitro PDT efficiency of TPPS
4/CD-N nanoassemblies on the same cell lines model upon visible light irradiation (λ = 340 nm, 5 J/cm
2, 30 min) were also investigated. In the absence of light, higher dark cytotoxicity was observed in the case of cells treated with the TPPS
4/CD-N nanosystem compared to those treated with TPPS
4 alone, which may be due to a higher TPPS
4 uptake. Interestingly, the TPPS
4/CD-N nanosystem was found to be more efficient than free TPPS
4 to induce photodamage upon light irradiation (
Figure 43).
The development of supramolecular self-assemblies using amphiphilic CDs as drug delivery nanocarriers may suffer from the conjugate’s lack of stability [
169,
170,
171]. In fact, the chemical bonds that are often used to bind CDs to the drugs can be labile, and thus cause the premature loss of drugs [
156,
172]. To deal with this problem, Xiong et al. [
173] in 2017 used the high affinity of carboranes (CBs) for the β-CD and developed two stable amphiphilic supramolecular nanossemblies based on β-CDs and CBs, and loaded with PS (5-(4-hydroxy-phenyl)-10,15,20-triphenyl-porphyrin, TPP). Host–guest interactions between amphiphilic PEG-modified β-CD (PEG-β-CD) and octyl-carborane (C
8-CB) led to the formation of 1:1 and 2:1 inclusion complexes named PEG-β-CD/C
8-CB and 2PEG-β-CD/C
8-CB. These inclusion complexes self-assembled into spherical NPs and finally loaded with TPP to afford TPP@PEG-β-CD/C
8-CB and TPP@2PEG-β-CD/C
8-CB NPs with hydrodynamic sizes of 113 nm and 93 nm, respectively. The in vitro behavior of TPP@PEG-β-CD/C
8-CB NPs was investigated on human liver cancer cells (HepG2) using confocal microscopy and flow cytometry. The authors showed that NPs were mainly localized in the cytoplasm region, in close vicinity to the nuclei, and provided higher fluorescent signals compared to cells treated with free TPP or with the physical mixture of PEG-β-CD/C
8-CB and TPP. In vitro cell viability on normal human bronchial epithelial (BEAS-2B) cells, and HepG2 and HeLa cancer cells was investigated by MTT assay under irradiation (λ = 620 nm, 16 mW/cm
2). No dark cytotoxicity was observed, even at an NP concentration of 8 µg/mL. For TPP@PEG-β-CD/C
8-CB NPs, it was found that the viability of cancer cells (HepG2 and HeLa) after irradiation was 10 times lower compared to TPP alone or to the physical mixture of PEG-β-CD/C
8-CB and TPP. Finally, the stability in solution and the photostability of TPP@PEG-β-CD/C
8-CB NPs were evaluated, and the authors estimated that less than 5% of TPP leaked after five days in physiological solution (10% fetal bovine serum), and only a 5.2% absorbance decrease was observed after 90 min of irradiation.
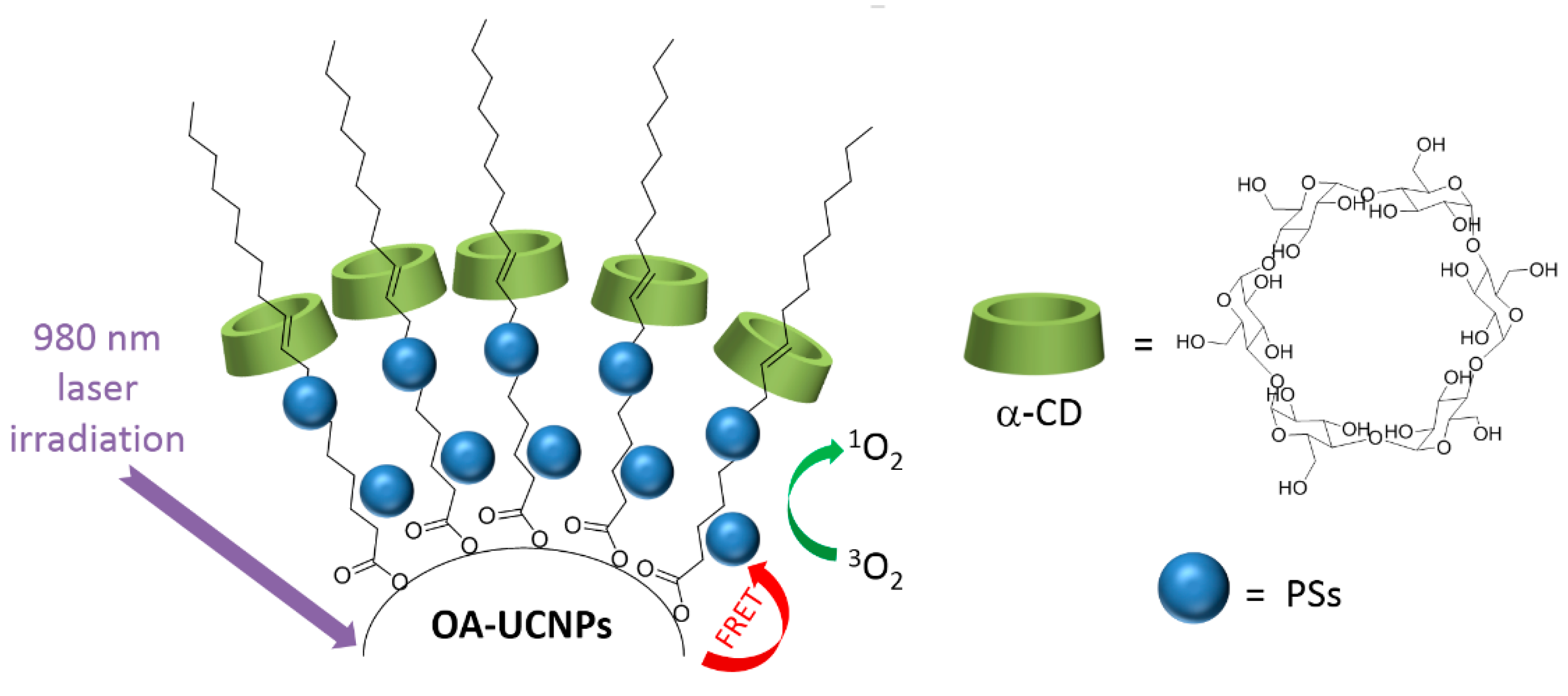
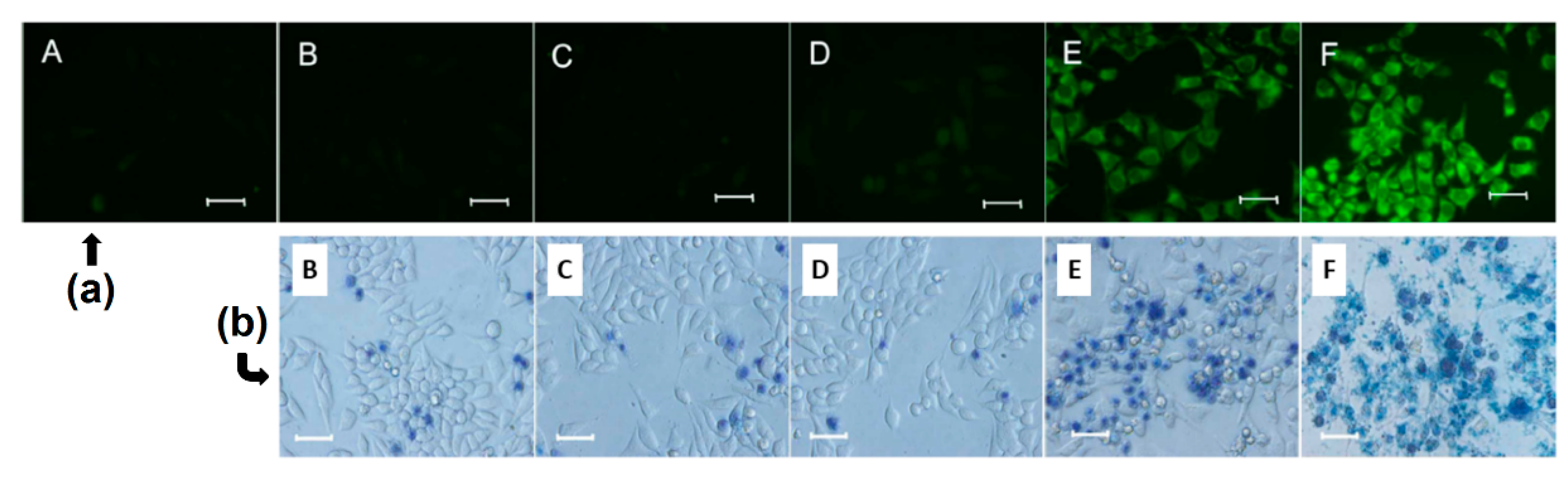
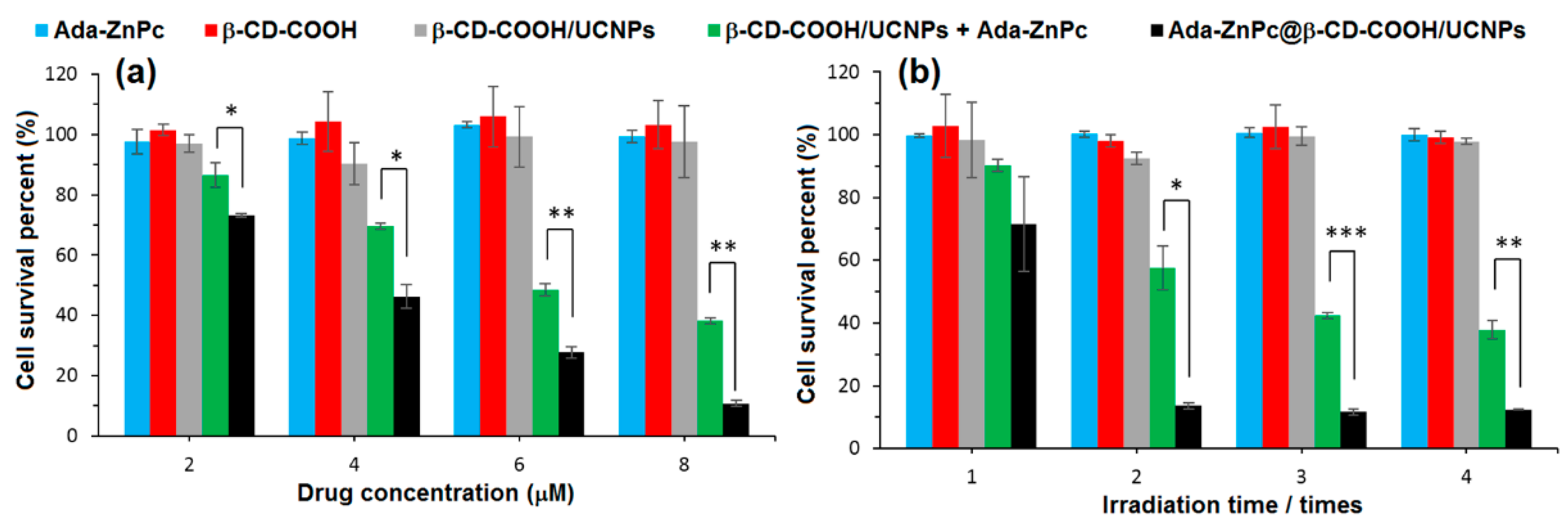
As complementary information and concerning the elaboration of the nanoassemblies of amphiphilic CDs and porphyrinoid PSs, we can mention also an article published in 2013 by Voskuhl et al. [
174]. The authors investigated the self-assembly of a supramolecular
1O
2 photosensitizing system based on host–guest interactions between an adamantane-functionalized ZnPc (Ada-ZnPc) and β-CD vesicles (β-CDVs). The resulted Ada-ZnPc/ β-CDV nanoassemblies allowed an increase of the
1O
2 photosensitizing ability of ZnPc, while preventing its self-aggregation. These results reflect the possible use of this supramolecular assembly as a biocompatible photoactive platform for the design of phototherapeutic agents. Concerning non-porphyrinoid PSs, only one article has been found in the literature. In 2016, Kauscher et al. [
175] described the immobilization of photoreactive squaraines (non-porphyrinoid PS) on a supramolecular amphiphilic CD nanoassemblies, and highlighted the
1O
2 production under irradiation and an improvement in the photochemical activity of squaraines. However, no in vitro biological study has yet been made of the impact of these nanoassemblies on the PDT efficiency.
Besides the supramolecular nanoassemblies based on amphiphilic CD, the researchers are studying new CD derivatives, and some works deal with the use of polymeric CDs for new stable and efficient buildings that can be used for PDT [
176].
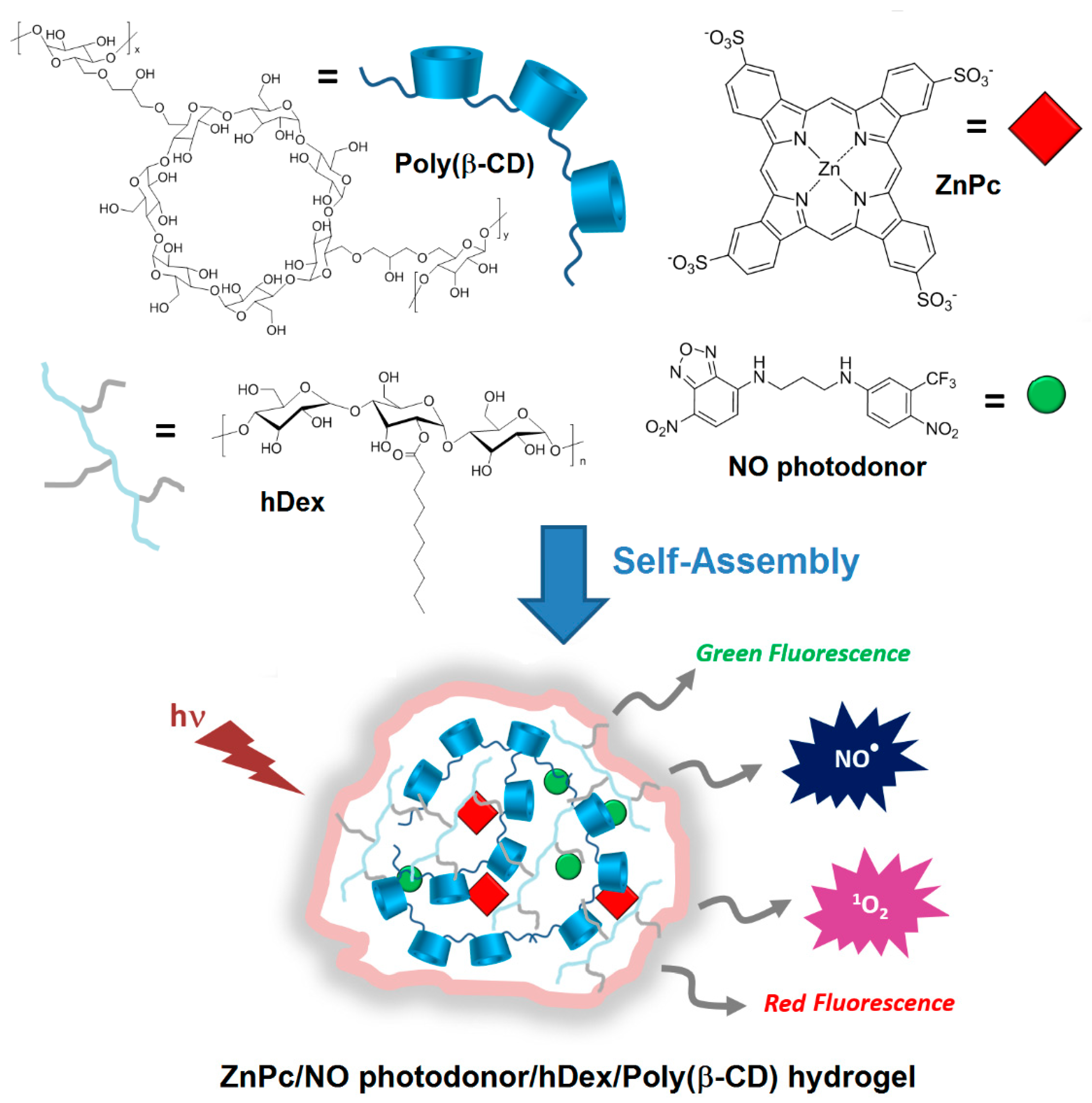
In 2014, Sortino et al. [
177] engineered supramolecular nanoassemblies that were composed of four different components, i.e., a poly(β-CD) polymer, a hydrophobically modified dextran (hDex), an anionic zinc phthalocyanine (ZnPc), and a NO photodonor. The assembly occurred spontaneously in aqueous medium forming a “Lock and Key” hydrogel, since the alkyl chains of hDex were included in the cavity of poly(β-CD) (
Figure 44). The authors described for the first time a hydrogel system that was suitable for (1) producing both red and green fluorescence signals, (2) photoreleasing simultaneously two cytotoxic species (
1O
2 and NO
•), and (3) inducing an amplified cancer cell death (
Figure 44).
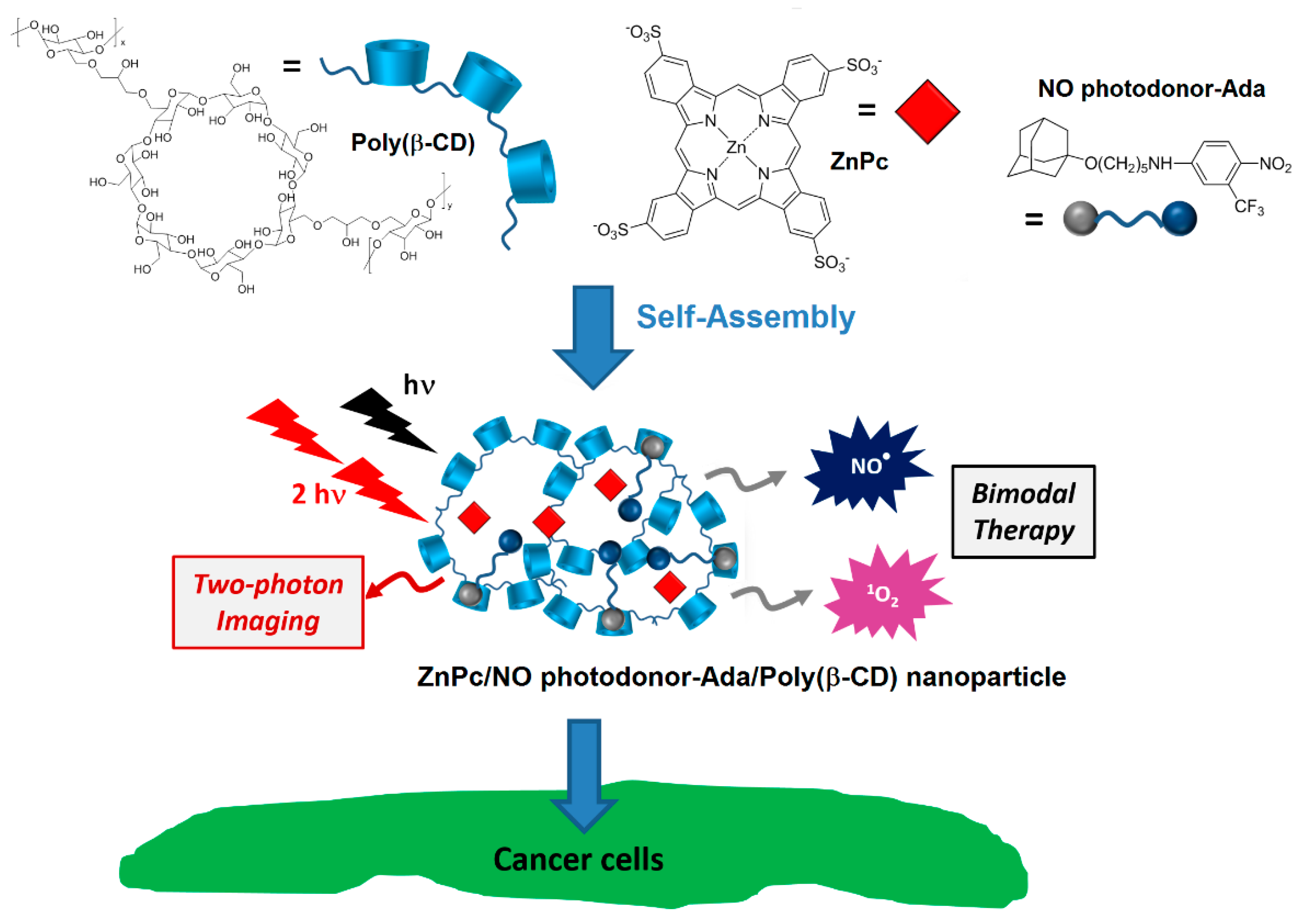
In that same year, Sortino et al. [
178] engineered supramolecular nanoassemblies composed of three different components, i.e., a poly(β-CD) polymer, an anionic zinc phthalocyanine (ZnPc), and an NO photodonor attached to an adamantane moiety (NO photodonor-Ada). The adamantane unit is known to form an inclusion complex with the cavity of β-CD. The self-assembly led to the formation of ZnPc/NO photodonor-Ada/Poly(β-CD) NPs with an average hydrodynamic diameter of 35 nm (
Figure 45).
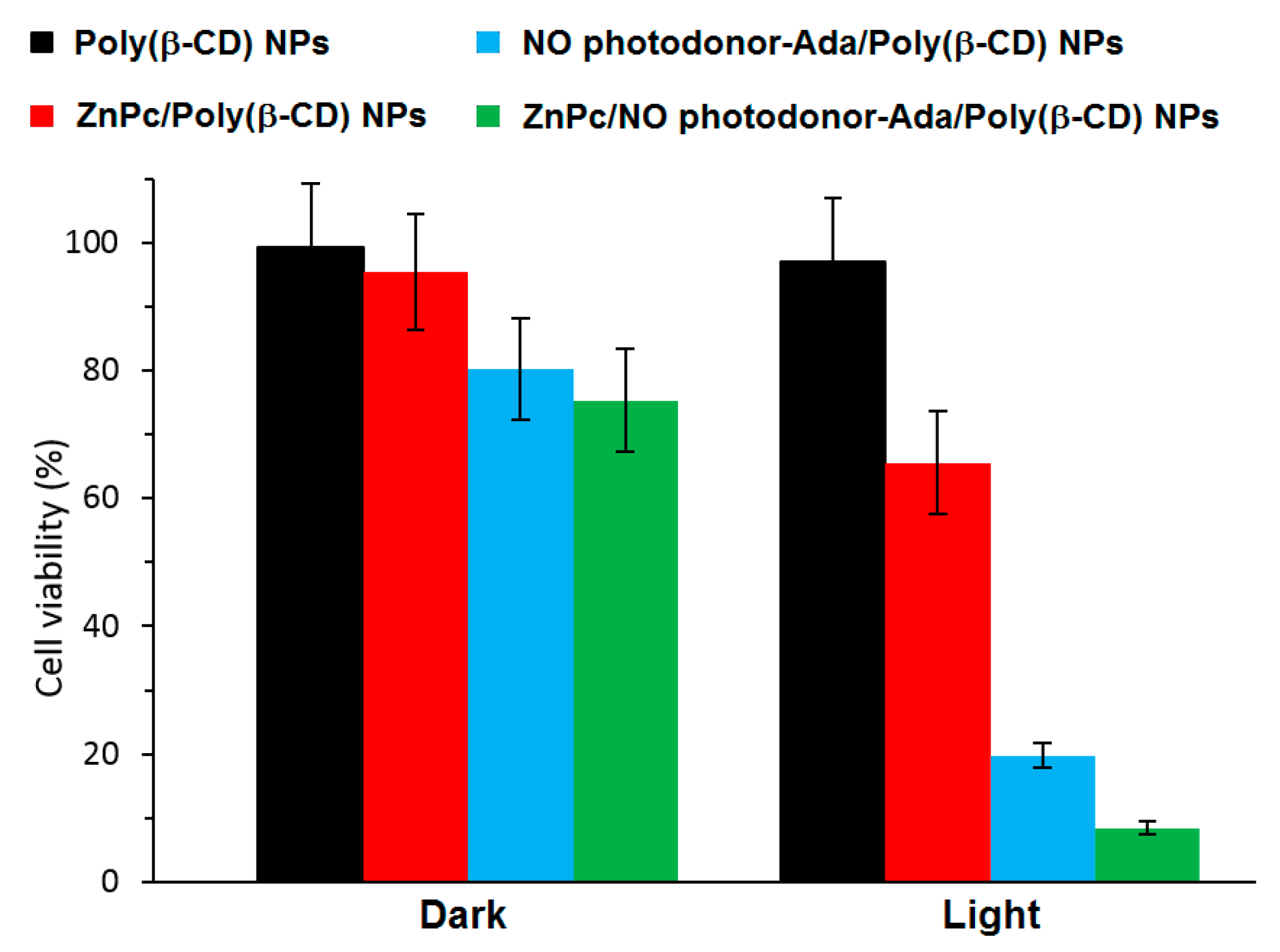
To validate the feasibility of ZnPc/NO photodonor-Ada/Poly(β-CD) NPs as bimodal phototherapeutic agents, an in vitro PDT study were performed using human squamous carcinoma (A431) cells (
Figure 46). The authors thereby showed that the irradiation of ZnPc/NO photodonor-Ada/Poly(β-CD) NPs with visible light (405-nm and 633-nm light to target the NO photodonor and ZnPc, respectively) triggered the simultaneous delivery of cytotoxic
1O
2 and NO
• species, resulting in an amplified cell photomortality due to the synergistic effect of both cytotoxic agents. It was shown also that ZnPc/NO photodonor-Ada/poly(β-CD) NPs could act as two photon emission (TPE) imaging agents.
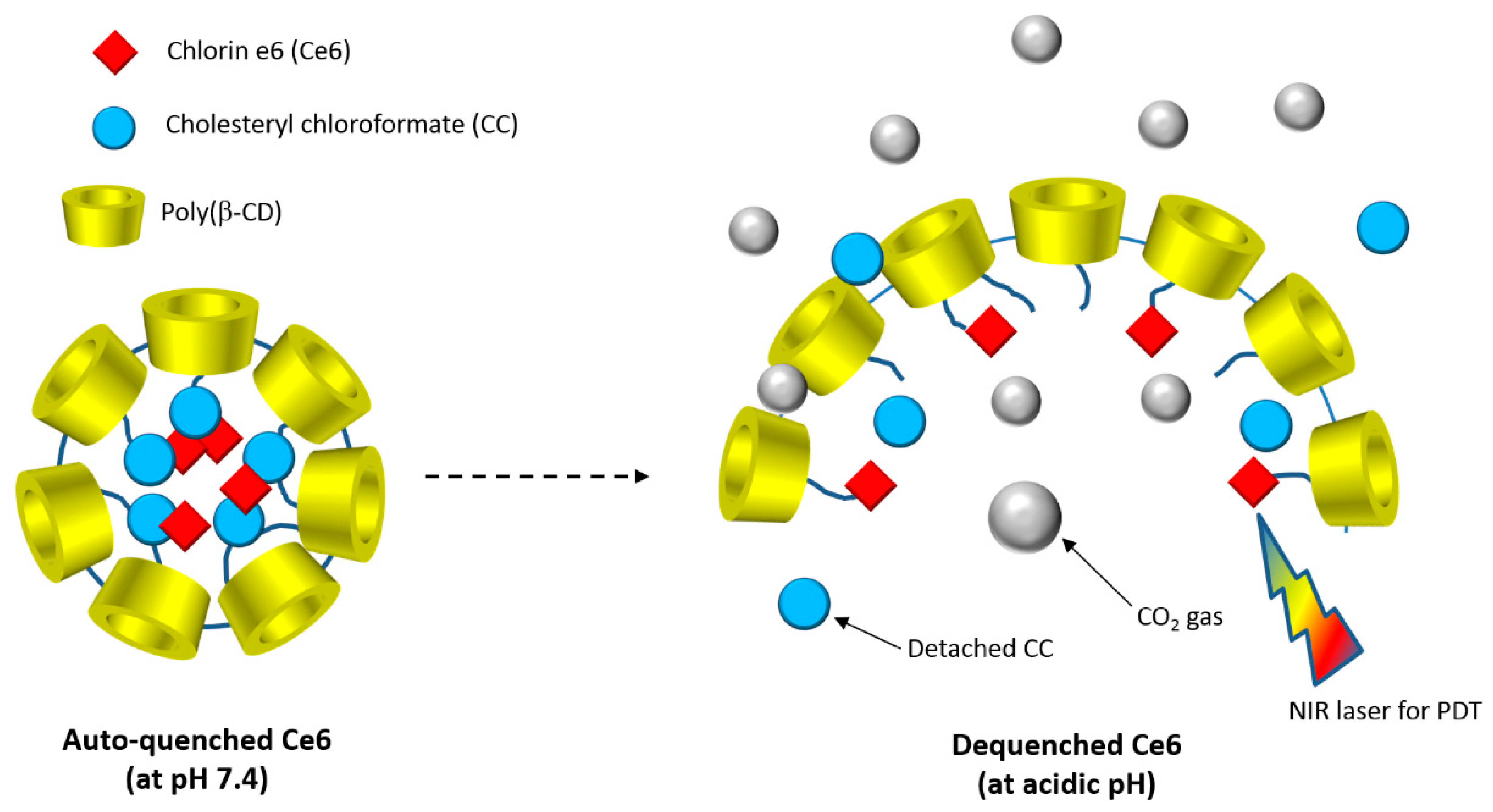
In 2015, Lee et al. [
179] developed a new biocompatible nanoassembly that was constituted of poly(β-CD) linked by carbonate bonds to cholesteryl chloroformate (CC) and chlorin e6 (Ce6). The self-assembly induced the formation of core-shell poly(β-CD)-
g-CC-
g-Ce6 NPs (average particle size of 61 nm) in which the hydrophobic CC and Ce6 core is enclosed with a layer of hydrophilic poly(β-CD) shell. The authors found that the poly(β-CD)-
g-CC-
g-Ce6 properties as drug carrier were highly pH-dependent. It was also found that at an acidic pH, poly(β-CD)-
g-CC-
g-Ce6 NPs disintegration happened due to the carbonate linkages cleavage producing CO
2 (
Figure 47).
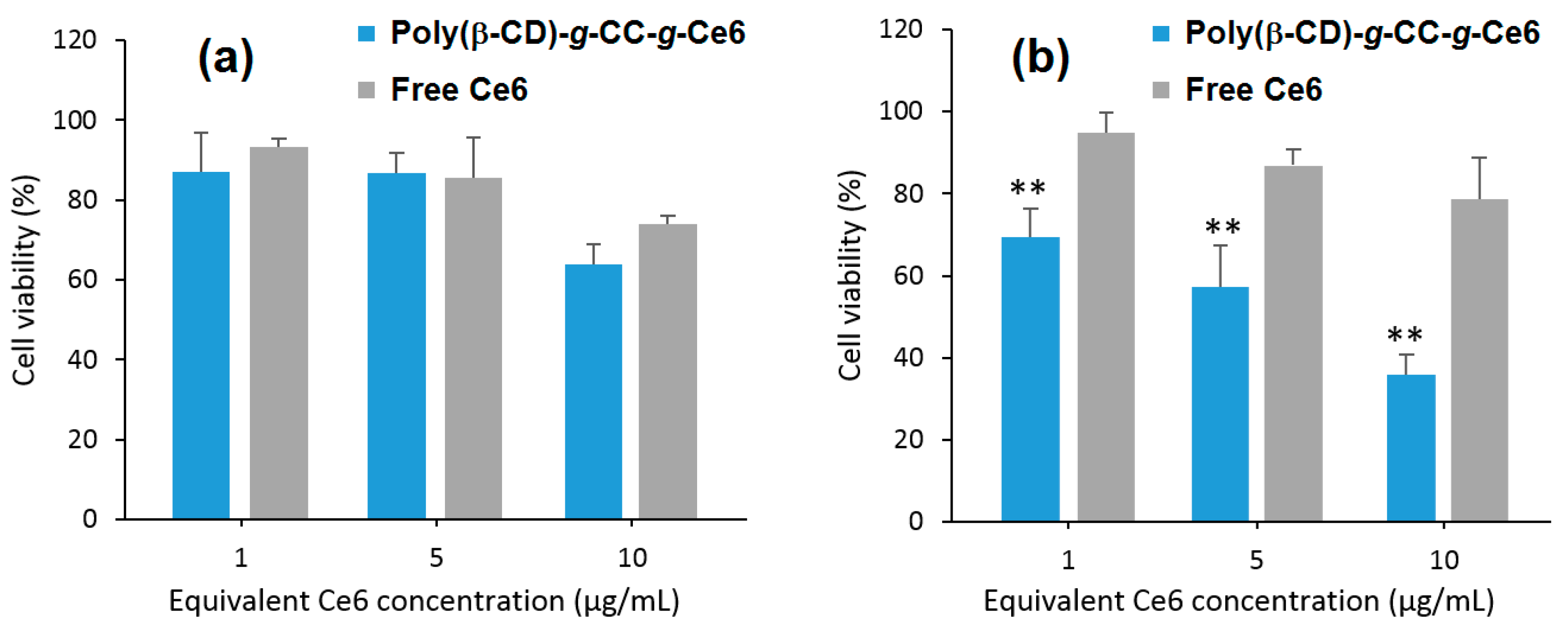
Furthermore, they found that upon irradiation (λ = 670 nm, 5.2 mW/cm
2 during 10 min), poly(β-CD)-
g-CC-
g-Ce6 NPs have the ability to produce more
1O
2 at acidic pH (tumor environment, pH 6.5) compared to the physiological pH (pH 7.4). The in vitro studies performed on the human nasopharyngeal epidermal carcinoma (KB) cell line showed that compared to Ce6 alone, a highest cellular uptake at acidic pH and mainly in nucleus for poly(β-CD)-
g-CC-
g-Ce6 NPs was observed. No dark cytotoxicity was detected for the poly(β-CD)-
g-CC-
g-Ce6 NPs. However, upon irradiation (λ = 670 nm, 5.2 mW/cm
2, during 10 min), poly(β-CD)-
g-CC-
g-Ce6 NPs exhibited stronger phototoxicity at pH 6.5 compared to that at pH 7.4 (
Figure 48). These results highlighted the potential usefulness of poly(β-CD)-
g-CC-
g-Ce6 NPs in anticancer PDT treatment.
As complementary information concerning the elaboration of nanoassemblies polymeric CDs and non-porphyrinoid PSs, only one article has been found in the literature. In 2014, Kirakci et al. [
180] described three supramolecular nanoassemblies composed by poly(β-CD) and octahedral molybdenum cluster complexes (non-porphyrinoid PSs). The resulting assemblies afforded hydrogel particles (hydrodynamic diameter from 160 nm to 240 nm) without adversely affecting the photophysical properties of the octahedral molybdenum cluster complexes (red luminescence and high quantum yield). The photophysical properties of these hydrogel particles were associated with the oxygen sensitivity of the luminescence, making them interesting for a usefulness as potential dual agents for PDT/boron neutron capture therapy.
Aside from the supramolecular CD–PS nanoassemblies based on PSs and chemically modified CDs, some teams wished to reverse this strategy by forming nanoassemblies with CDs and chemically modified PSs.
In 2015, Liu et al. [
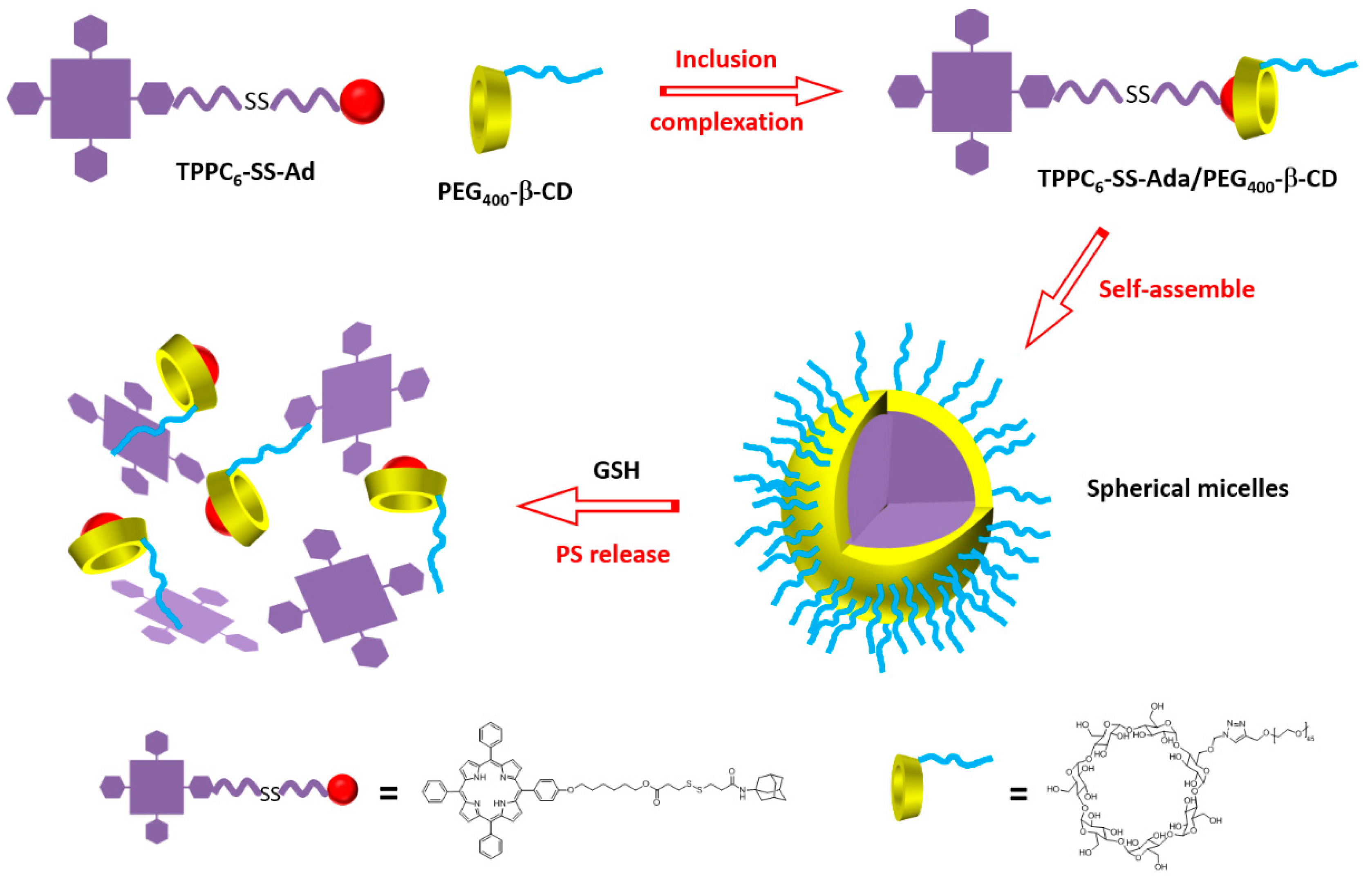
181] synthesized a supramolecular system using poly(ethylene glycol 400)-β-CD (PEG
400-β-CD) and a porphyrin derivative containing a disulfide bond (S-S) and an adamantane (Ada) group (TPPC
6-SS-Ada,
Figure 49).
The host–guest interactions between PEG
400-β-CD and TPPC
6-SS-Ada led to the formation of TPPC
6-SS-Ada/PEG
400-β-CD polypseudorotaxanes (PPRs) that were able to self-assemble into spherical micelles in aqueous solution (average particle size of around 72 nm,
Figure 50). The S–S linkage can be cleaved in reducing intracellular microenvironment. The high level of glutathione (GSH) in cytosol could allow the PS release.
For comparison, the authors also synthesized the TPPC6-Ada/PEG-β-CD analog without the disulfide bridge. The authors proved that the S–S bond could be cleaved upon the addition of GSH. Moreover, an in vitro study using MCF-7 cells showed that free porphyrin accumulated less than TPPC6-SS-Ada/PEG400-β-CD, which might be taken up through an endocytosis process. No dark toxicity could be observed for free porphyrin, TPPC6-SS-Ada/PEG-β-CD, and TPPC6-Ada/PEG400-β-CD micelles, even up to 100 mg/µL of porphyrin. The phototoxic effect upon irradiation with a visible light LED lamp (400 mW/cm2) for 20 min was the best for TPPC6-SS-Ada/PEG400-β-CD with an IC50 of 31 µg/mL. At 100 mg/µL of porphyrin, cell viability was 60% for TPPC6-Ada/PEG400-β-CD, and around 35% for TPPC6-SS-Ada/PEG400-β-CD. This better result is due to the release of the porphyrin upon cleavage of the S–S bond with GSH. The authors were the first to report the utilization of polypseudorotaxanes (PPRs) for PDT.
Based on the work above described about PPRs for PDT [
181], Tong et al. [
182] used a similar strategy in 2016 to develop GSH activatable PS-conjugated PPR nanocarriers (Ce6-SS-α-CD/PEG-
b-PMPC with an average size of 60 nm) for photodynamic theranostics. This time, the supramolecular system was based on host–guest interactions between PEG-
b-poly (2-methacryl-oyloxyethyl phosphorylcholine) (PEG-
b-PMPC) block copolymers and a chlorin e6 derivative containing a disulfide bond and α-CD (Ce6-SS-α-CD). The in vitro cellular redox activatable behavior of Ce6-SS-α-CD/PEG-
b-PMPC NPs was investigated on human oral epidermoid carcinoma (KB) cells using confocal microscopy and flow cytometry. The authors observed (1) a clear red fluorescence of Ce6 in KB cells without any treatment, indicating an efficient internalization of NPs, (2) the strongest intracellular Ce6 fluorescence in the presence of high GSH concentration, which became weakest in the presence of low GSH concentration. The in vitro study of KB cells under irradiation (λ = 660 nm, 50 mW/cm
2) showed a better phototoxic effect on cells treated with Ce6-SS-α-CD/PEG-
b-PMPC NPs compared to cells treated with free Ce6. Finally, in vivo fluorescence imaging-guided PDT of Ce6-SS-α-CD/PEG-
b-PMPC NPs in tumor-bearing mice (tumor size of 250 mm
3) highlighted an impressive PDT effect on the tumor size after 14 days of therapy. All of these results demonstrated the ability of Ce6-SS-α-CD/PEG-
b-PMPC NPs to exhibit redox activatable fluorescence signal and ROS generation for photodynamic theranostics.
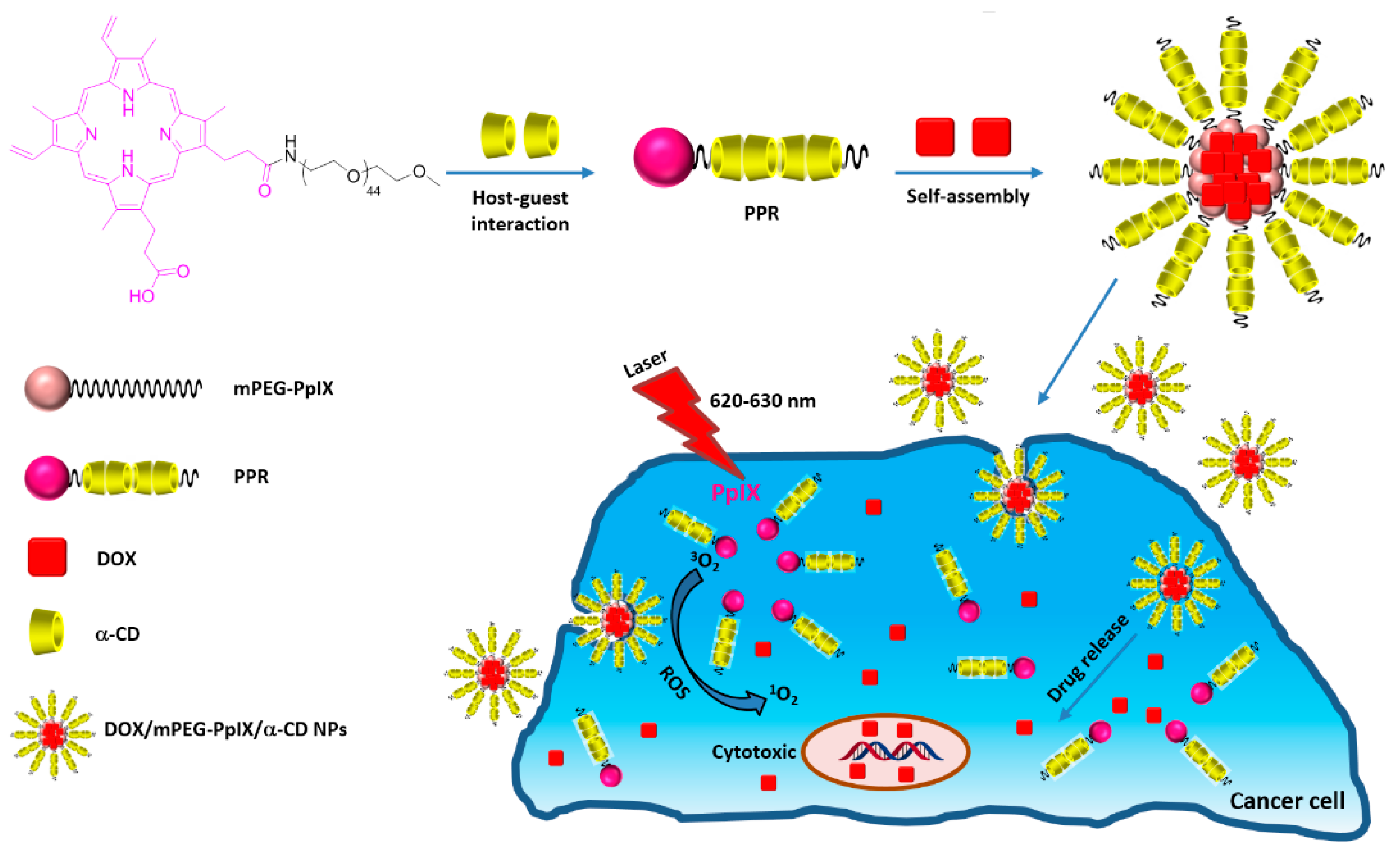
In 2017, Xu et al. [
183] developed polypseudorotaxane NPs (mPEG-PpIX/α-CD) based on self-assembly of mPEG-protoporphyrin IX (mPEG-PpIX) conjugate and α-CDs via host–guest interactions, followed by a chemotherapy drug (doxorubicin, DOX) encapsulation (DOX/mPEG-PpIX/α-CD NPs) with an average size of 89 nm, as shown in
Figure 51.
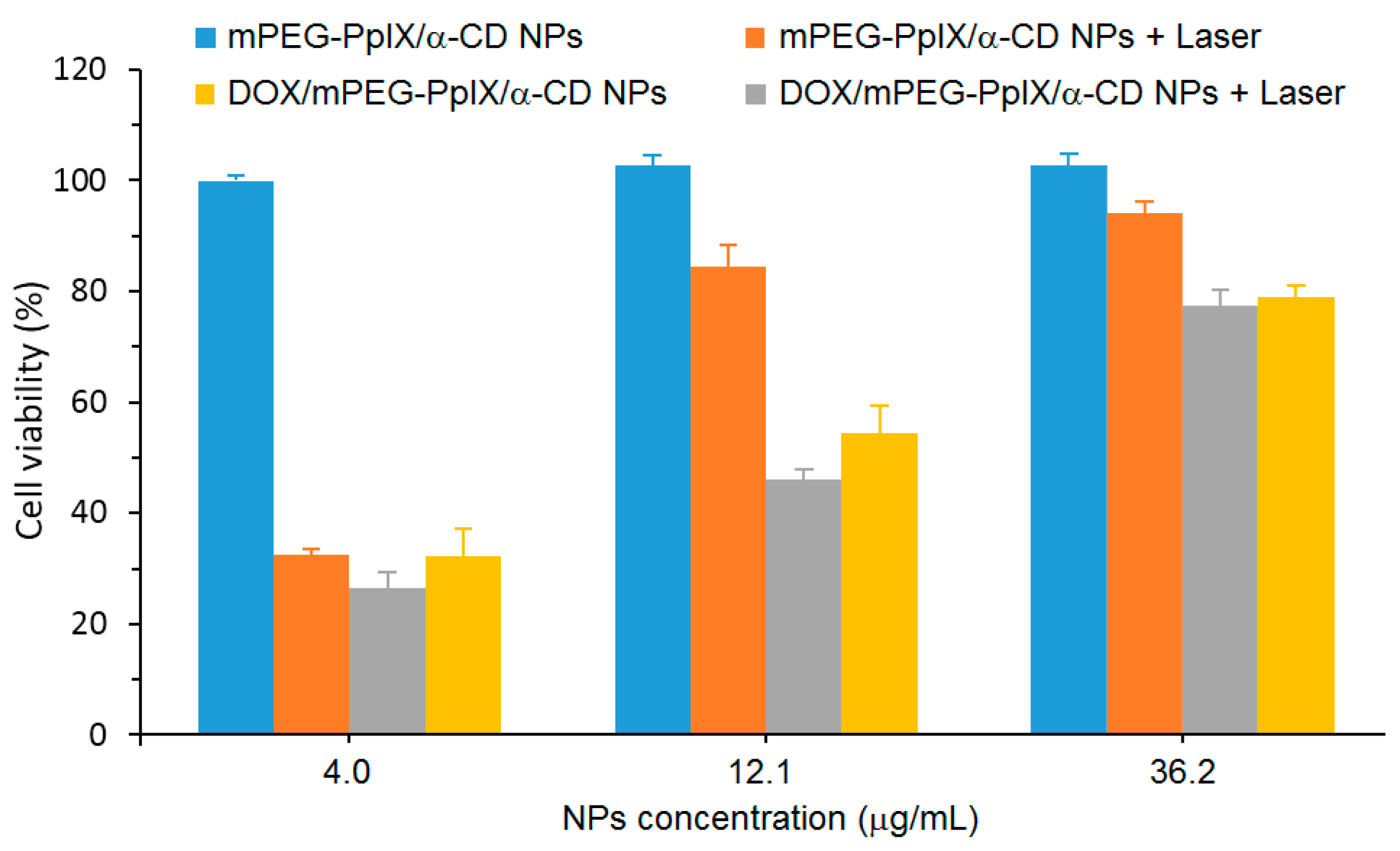
A cytotoxicity study of mPEG-PpIX/α-CD NPs using L929 fibroblasts cells was performed, and low dark cytotoxicity was found after the 48 h incubation of cells with mPEG-PpIX/α-CD NPs, even with a high concentration of NPs (80% cell viability at a NP concentration of 300 µg/mL). The in vitro PDT efficiency of mPEG-PpIX/α-CD and DOX/mPEG-PpIX/α-CD NPs was evaluated with different NP concentrations (4.0 µg/mL, 12.1 µg/mL, and 36.2 µg/mL) with diiode laser irradiation (λ = 620–630 nm) using HepG2 cells (
Figure 52). For mPEG-PpIX/α-CD NPs, no dark cytotoxicity was observed, whatever the concentration used. However, the cell viability upon irradiation was found to be NP concentration-dependent with a higher impact in the presence of DOX. These results revealed that DOX/mPEG-PpIX/α-CD NPs exhibited synergistic PDT/chemotherapy effects under laser irradiation.
As complementary information and concerning the elaboration of supramolecular nanoassemblies based on CDs and chemically modified PSs, we can also mention the work of Jin et al. [
184] in 2015. This work described the development of supramolecular hydrogels based on host–guest interactions between amphiphilic PS cored and α-CD leading to the formation of PPRs, which were subsequently self-assembled into hydrogels and loaded with DOX (chemotherapy drug). The resulted supramolecular DOX@PS/α-CD hydrogel exhibited efficient DOX release and
1O
2 generation upon light irradiation, which makes this promising for both cancer chemotherapy drug delivery systems and as a potential PDT agent.
Aside from the supramolecular CD–PS nanoassemblies based on PSs (or chemically modified PSs) and chemically modified CDs (or CDs), another strategy employed by Zhang et al. [
171] involved the use of platinium(IV) prodrug bridged β-CD dimer (Pt
IV(β-CD)
2) and 5,10,15,20-tetrakis(1-adamantyl-pyridinium-4-yl)porphyrin (TPyP-(Ada)
4). The authors investigated the formation of Pt
IV(β-CD)
2/TPyP-(Ada)
4 NPs (average size of around 100 nm) by host–guest interactions (stable 2:1 CD-PS inclusion complex) for chemo-photodynamic dual therapy against cisplatin-resistant cancer cells. The in vitro behavior of Pt
IV(β-CD)
2/TPyP-(Ada)
4 NPs was investigated on cisplatin-resistant human lung adenocarcinoma epithelial cells (A549R) using confocal microscopy and flow cytometry. Pt
IV(β-CD)
2/TPyP-(Ada)
4 NPs were internalized by A549R cells in the form of NPs, and a rapid spread of Pt
IV(β-CD)
2/TPyP-(Ada)
4 NPs happened in the whole cytoplasm under light irradiation. An in vitro cytotoxicity study on A549R cells in the dark or with light irradiation (λ = 430 nm, 10 mW/cm
2 for 2 min) showed that Pt
IV(β-CD)
2/TPyP-(Ada)
4 NPs were much more cytotoxic than cisplatin, even in the dark. The cytotoxicity of Pt
IV(β-CD)
2/TPyP-(Ada)
4 NPs was better under light irradiation, owing to the PDT effect (
Table 7). All of these results highlighted a further synergistic effect by the combination of PDT treatment.
2.4. Cyclodextrins with Fullerenes
Many researchers, in their quest for the development of a better suitable PS for anticancer PDT treatment, pursue their efforts and grapple with this challenge by trying to develop new original PSs. Among these, one can cite the use of fullerenes. Fullerenes are carbon molecules whose shape can be close to a sphere, an ellipsoid, a tube, or a ring. They are composed of hexagonal rings (such as graphite) but also pentagonal rings, which give the possibility of closed structures. Fullerenes are natural molecules (found in soot) that were identified in 1985 by Kroto et al. [
199] who received the Nobel Chemistry Prize in 1996 for this discovery. Fullerenes are extracted from the soot using a multistep procedure. After dissolution of the soot in appropriate organic solvents (solution containing up to 70% of C
60 ([60]Fullerene) and 15% of C
70 ([70]Fullerene), as well as other fullerenes), each fullerene fraction is separated using chromatography.
[60]Fullerene is the most stable form of fullerene whose composition includes 60 carbon atoms forming 20 hexagons and 12 pentagons, with a carbon atom at the top of each polygon and a bond at each side of the polygon. Each hexagon is adjacent to three hexagons and three pentagons, and each pentagon is surrounded by five hexagons (
Figure 61). [60]Fullerene has the same shape as a traditional football with sewn panels.
Over three decades have passed since the discovery of fullerenes, and the interest for medical applications generated by them over the years has increased considerably [
200,
201,
202,
203,
204]. Fullerenes combine many very interesting properties, making them good candidates for developing suitable PSs for PDT application [
205,
206,
207,
208]. These interesting properties include: (1) a higher photostability and a lower photobleaching compared to tetrapyrroles (porphyrinoid PSs) and synthetic dyes (non-porphyrinoid PSs); (2) both type I (free radicals) and type II (
1O
2) ROS generation compared to largely type II for tetrapyrroles; (3) an increase of both overall quantum yield and ROS production; (4) an extension of their absorption spectrum further into the red wavelengths; and (5) an ability to self-assemble into vesicles and functionalize with the aim of improving drug delivery. In spite of all these benefits, pristine [60]Fullerene is poorly soluble in water and biological media and self-assembles into nano-aggregates, limiting its photoactivity [
209]. Therefore, it appears obvious that the combination of fullerenes and CDs would be a reasonable alternative for developing ideal PSs for use in anticancer PDT treatment.
Between 2008–2017, Ikeda et al. focused on the application of cyclodextrin /fullerenes inclusion complexes in PDT [
210,
211,
212,
213,
214,
215,
216]. In 2008, Ikeda et al. synthesized lipid membrane-incorporated fullerenes (LMIC
x: x = 60 or 70) with high concentrations of fullerenes (C
x) through a simple and time-saving approach [
211]. The method was based on an exchange reaction where C
x was transferred from the C
x-γ-CD complex to the liposomes. The elaborated LMIC
60 and LMIC
70 possessed an average diameter of about 100 nm. The authors also proved the efficiency of their guest exchange method for the preparation of the lipid membrane-incorporated fullerenes (LMIC
x) in comparison to the conventional injection and premixing approaches [
212]. LMIC
60 and LMIC
70 prepared by the guest exchange approach displayed a higher PDT activity accompanied with a better stability and solubility as compared to those prepared by the premixing one (
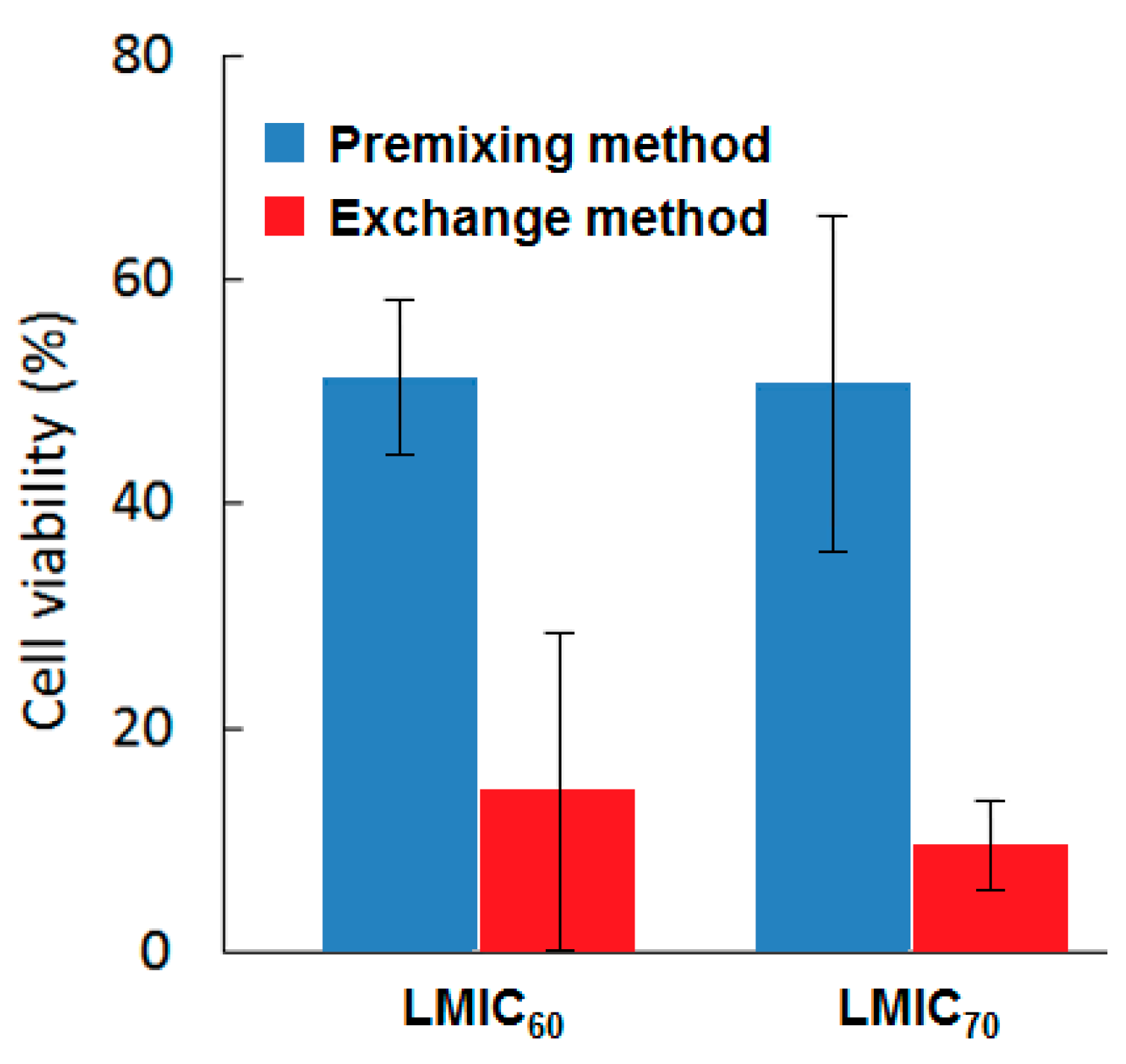
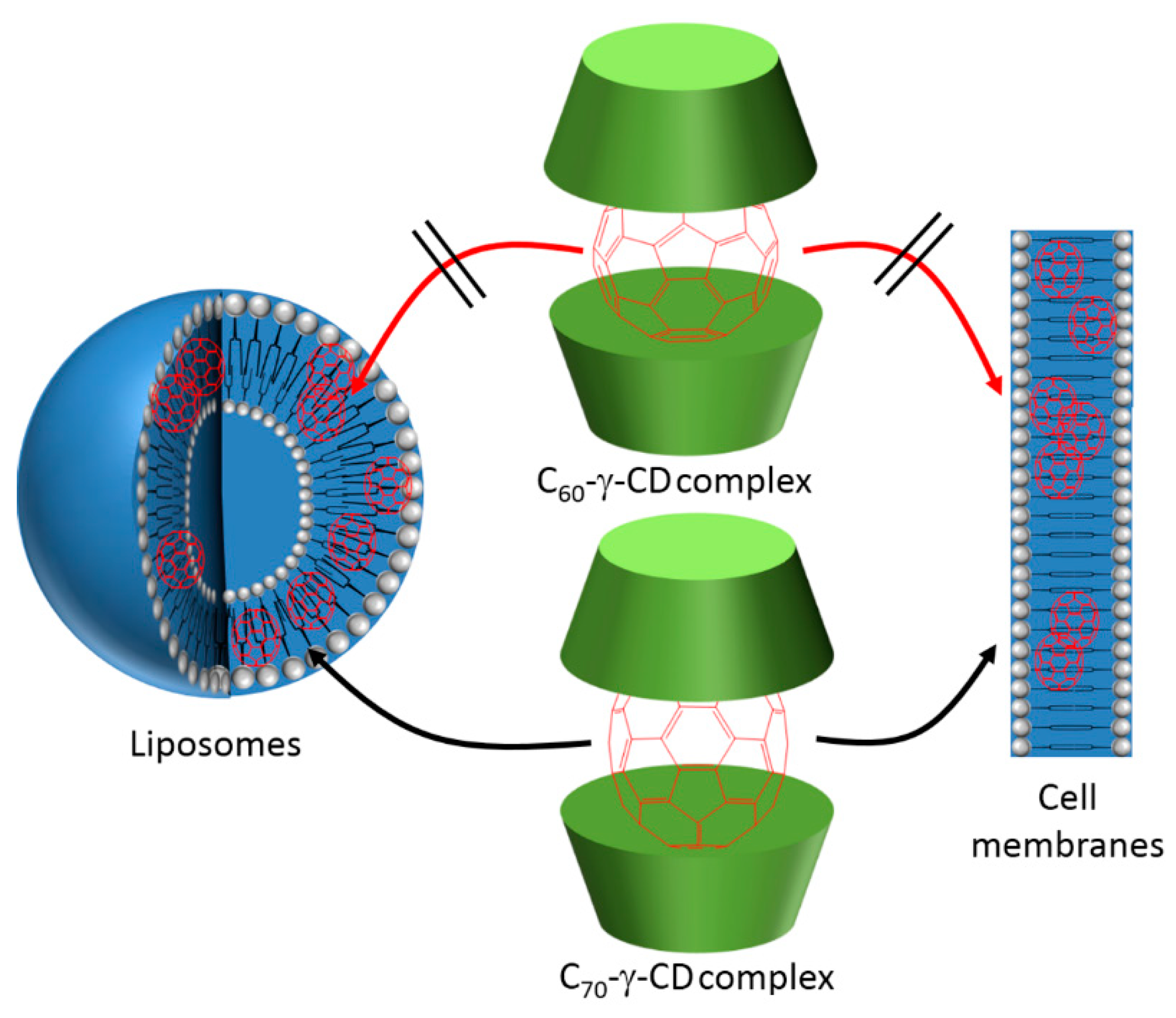
Figure 62). γ-CD were employed in this method to embrace the fullerene in the first step and facilitate the transfer of this fullerene from the cavity of the CD to the liposomes or lipid membrane, where only fullerene was the PS fulfilling the PDT effect.
To investigate the localization and the mode of internalization of the LMICx, only the liposomes were labeled by fluorochrome, and were incubated with HeLa cells. Fullerene was avoided, since it can quench fluorochrome. It appeared that only cationic liposomes were uptaken by the cells via endocytosis to be finally localized into the lysosomes. After 24 h of incubation with HeLa cells, both LMIC60 and LMIC70 revealed no dark toxicity. When excited at a wavelength between 350–500 nm, LMIC60 induced an 85% decrease of cell viability. However, when PDT was performed at a wavelength longer than 400 nm (400–740 nm), only LMIC70 was able to induce a significant decrease in the cell viability by 89% versus only 19% of reduction in the case of LMIC60. This difference in the performance was due to the greater production of 1O2 by LMIC60. Both LMIC60 and LMIC70 induced an early apoptotic cell death.
Ikeda et al. synthesized an inclusion complex that was constituted of fullerene C
x (x = 60 or 70) and γ-CD to implement the same concept but on the cellular membrane [
214] (
Figure 63). The intracellular uptake of both C
60 and C
70 from C
60-γ-CD and C
60-γ-CD inclusion complexes, respectively, was studied using human cervical HeLa cells ([C
60] = [C
70] = 20 µM). At 4 °C and 37 °C, C
60-γ-CD preserved its encapsulated structure, as no transfer of C
60 into the cells occurred. However, under the same conditions, C
70 from C
70-γ-CD were well incorporated into HeLa cells. Especially at 37 °C, 60% C
70-γ-CD was uptaken by the cells after only 5 min of incubation. The stability and solubility C
70-γ-CD was lower than that of the C
60-γ-CD complex. The incorporation mechanism was the direct exchange reaction from the C
70-γ-CD complex to the cell membrane. The fluorescence of rhodamine B 1,2-dihexadecanoyl-
sn-glycero-3-phosphoethanolamine (RhB-DHPE) that was used to stain the HeLa cells was only quenched in the presence of C
70-γ-CD. This result confirmed that C
70 and RhB-DHPE coexisted in the cellular membrane. Under visible light irradiation (λ = 400–700 nm, 54 mW/cm
2), the PDT efficiency against HeLa cells agreed with the results of the cellular uptake. In the dark, both complexes were not cytotoxic. Yet, as compared with the C
60-γ-CD complex and 5-Aminolevulinic acid (5-ALA) as a reference, only C
70-γ-CD triggered a drastic decrease of 75% of the cell viability, with only 1 µM of fullerene.
Encouraged and inspired by the good PDT activity obtained with LMIC
70 (λ > 400 nm) and C
70-γ-CD, the same team dedicated their efforts to improving this system for a higher stability and a more selective drug-delivery [
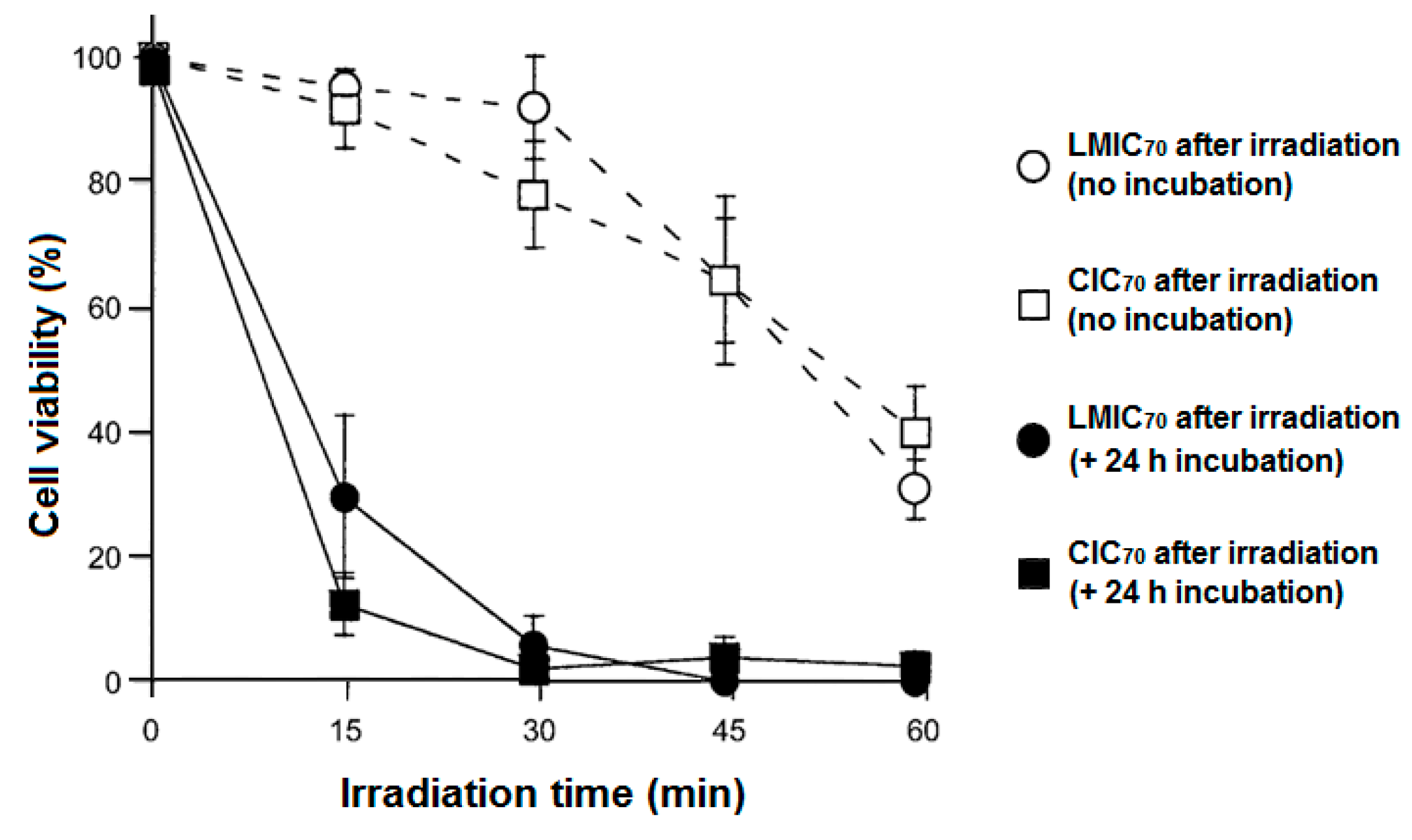
215]. In this study, their cerasome-incorporated C
70 (CIC
70) system was based on encapsulating the C
70 into a surface cross-linked liposomal bilayer covered with a polysiloxane surface (cerasome). This system was formed by the transfer of C
70 from the cavities of the γ-CD to occupy the vesicles of the cerasomes. The stability of the cerasomes was enhanced compared with the general liposomes by the deposition of silica on the surface of the cerasomes. C
70 acted as a fluorescence quencher in the presence of RhB, which confirmed the encapsulation of this fullerene in the lipid membrane of the cerasomes. A complete transfer of C
70 from the γ-CD cavities into the cerasomes was achieved at 25 °C after 1 min. The CIC
70 system possessed a diameter of about 170 nm, which was considered suitable for implementing an enhanced permeability and retention (EPR) effect. In addition, CIC
70 held high surface positive charges (+50.8 mV) that induced a good cellular uptake, yet was still slightly lower than that of LMIC
70 (+57.2 mV). The presence of C
70 improved the morphological stability of the cerasomes. For their application in PDT toward HeLa cells, CIC
70 appeared to be non-cytotoxic in darkness and induced a forceful PDT effect that was as good as that displayed by LMIC
70 under visible light irradiation (57 mW/cm
2, 400–740 nm) (
Figure 64).
On this basis, Ikeda et al. [
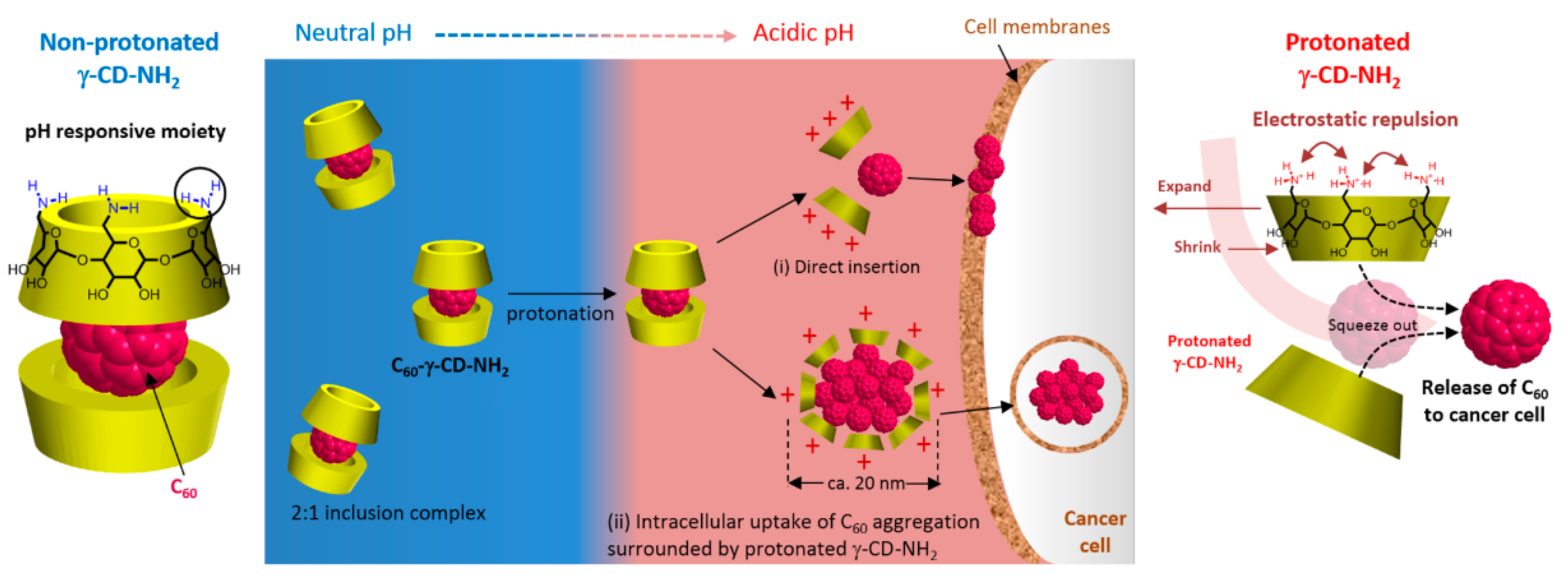
216] used a γ-CD derivative holding primary amine groups as pH responsive functions to carry their C
60 (C
60-γ-CD-NH
2). To validate the efficiency of the γ-CD-NH
2 carrier compared with γ-CD, the in vitro PDT activity of C
60-γ-CD-NH
2 and C
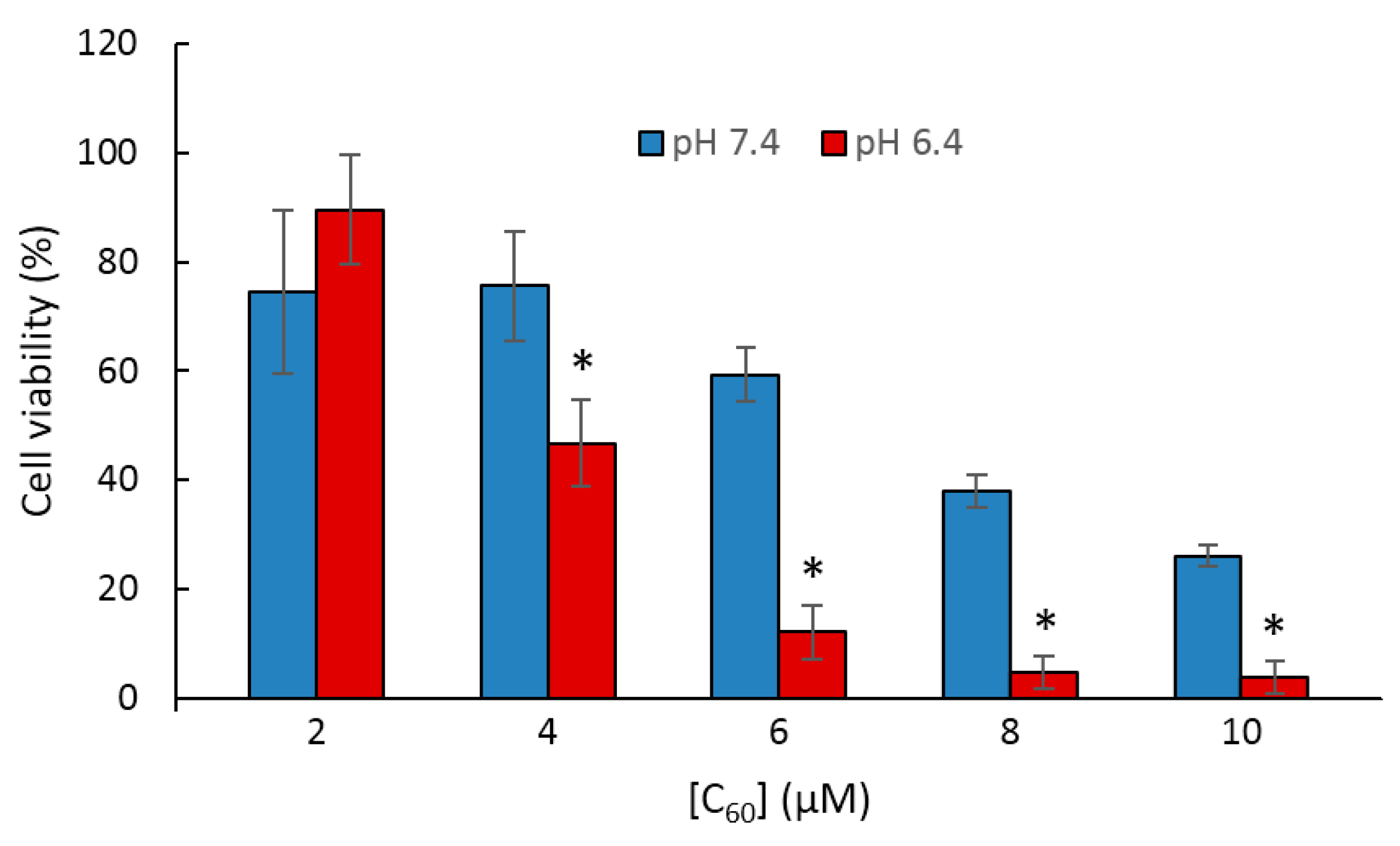
60-γ-CD toward HeLa cells under visible light illumination (400–500 nm) was assessed. The experiments were conducted at two pH values, 7.4 and 6.4, which correspond respectively to the extracellular pH of normal and neoplastic cells. Both C
60-γ-CD-NH
2 and C
60-γ-CD exhibited no dark toxicity, and negligible photoinduced toxicity was attained with C
60-γ-CD under both pH conditions. As for C
60-γ-CD-NH
2, it was photoactive in destroying HeLa cells at both pH values, with an enhanced activity witnessed at pH 6.4 (
Figure 65). Thus, it was revealed that as the pH started to become more acidic, the amine groups of the γ-CD-NH
2 became gradually protonated up to a critical pH, i.e., 6.7, where the C
60 was rapidly squeezed out of the γ-CD-NH
2 into the extracellular region. The released C
60 were rapidly uptaken by the cells through either endocytosis or direct insertion to the cellular membrane (
Figure 66). This γ-CD-NH
2 system represented a smart carrier of C
60 for a more efficient application in PDT.
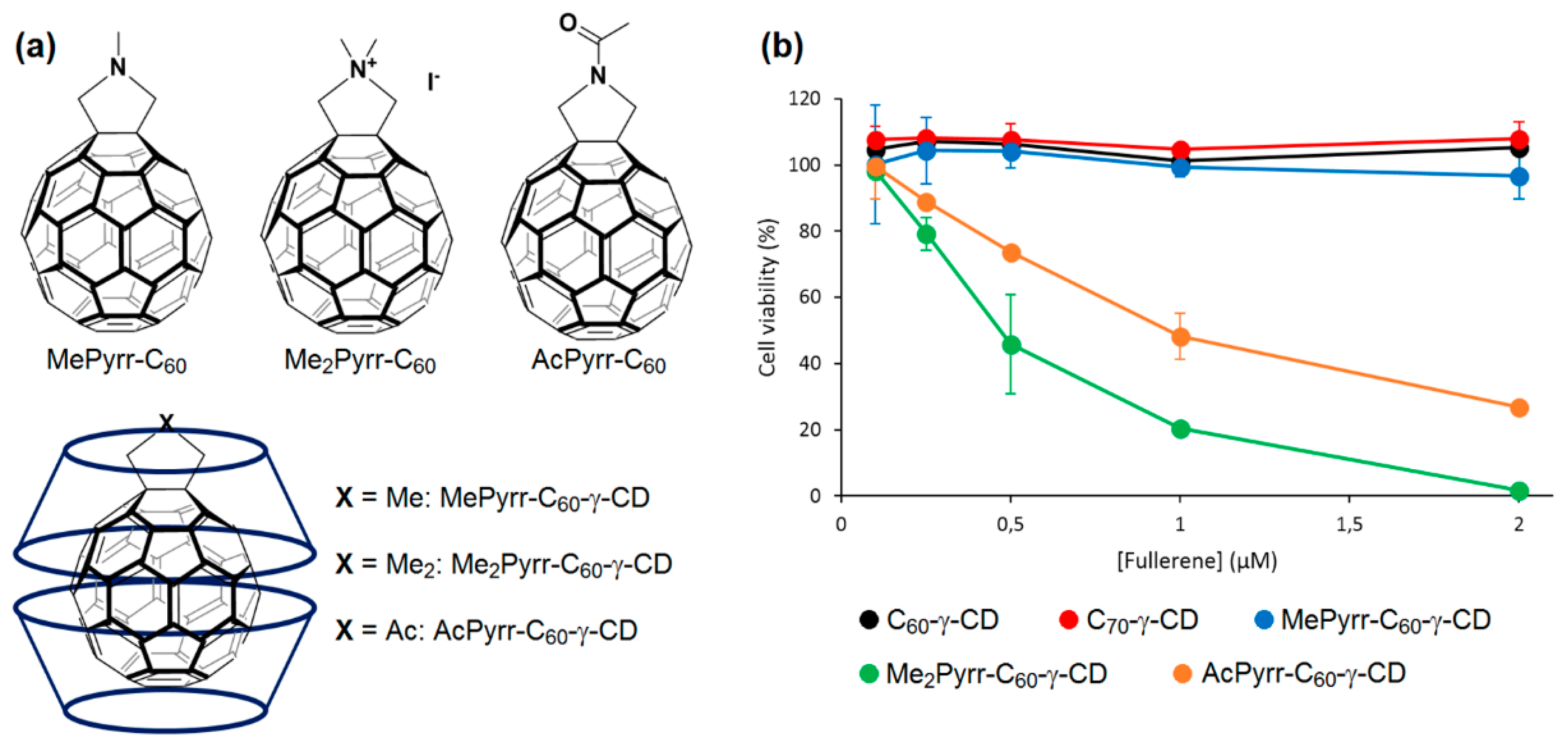
In 2013, Ikeda et al. addressed the problem of the stability and solubility of the C
x-γ-CD inclusion complexes that affected their performance in PDT [
213]. To form a stable inclusion complex, the authors used C
60 derivatives holding nitrogen atom-containing groups, namely
N-methylpyrrolidine (MePyrr, amino group),
N,
N-dimethylpyrrolidinium iodide (Me
2Pyrr, ammonium group), and
N-acetylpyrrolidine (AcPyrr, amide group) instead of C
60 (
Figure 67a). Compared with C
60-γ-CD, all of the γ-CD-complexed C
60 derivatives (MePyrr-C
60-γ-CD, Me
2Pyrr-C
60-γ-CD and AcPyrr-C
60-γ-CD) were found similarly water-soluble but more stable. In the absence of an irradiation source, the complexes were non-cytotoxic. The photoirradiation was performed at high wavelengths (610-720 nm). Under irradiation, Me
2Pyrr-C
60-γ-CD followed by AcPyrr-C
60-γ-CD complexes exhibited a superior PDT activity against HeLa cells as compared to Photofrin
®-γ-CD, C
60-γ-CD, C
70-γ-CD and MePyrr-γ-CD (
Figure 67b). This superior photoactivity, especially for Me
2Pyrr-C
60-γ-CD, was attributed to the increased cellular uptake caused by the cationic nature of the surface of this complex vs. the other two neutral complexes MePyrr-γ-CD and AcPyrr-C
60-γ-CD. In addition, higher levels of
1O
2 were elaborated by Me
2Pyrr-C
60-γ-CD. The suppressed PDT activity of MePyrr-γ-CD complex was imputed to the low
1O
2 production caused by the quenching effect of the triplet excited state of this complex by the lone pair of the electrons on the amino groups.
After they proved that using C
60 derivatives with the γ-CD improved the cellular uptake and therefore the PDT activity compared with C
60-γ-CD and C
60-γ-CD, Ikeda et al. used those γ-CD-complexed C
60 derivatives to prepare lipid membrane-incorporated C
60 derivatives by an exchange method [
210]. The C
60 derivatives were transferred from the two cavities of γ-CD into the lipid membranes of the liposomes. In a similar trend, Me
2Pyrr-C
60-γ-CD incorporated into the lipid membranes (Me
2Pyrr-LMIC
60) resulted in the higher PDT activity toward HeLa cells under excitation between 610–740 nm as compared to the other C
60 derivatives. The cationic nature of the incorporated Me
2Pyrr-C
60 did not induce any large effect on the surface potential of the Me
2Pyrr-LMIC
60 that might affect their internalization into the cells. Thus, the intercellular uptake of all of the tested liposomes was comparable. The source of this higher PDT activity of Me
2Pyrr-LMIC
60 was the greater ability to produce
1O
2, since even more
1O
2 was generated by Me
2Pyrr-LMIC
60 than that produced by the Me
2Pyrr-C
60-γ-CD inclusion complex.
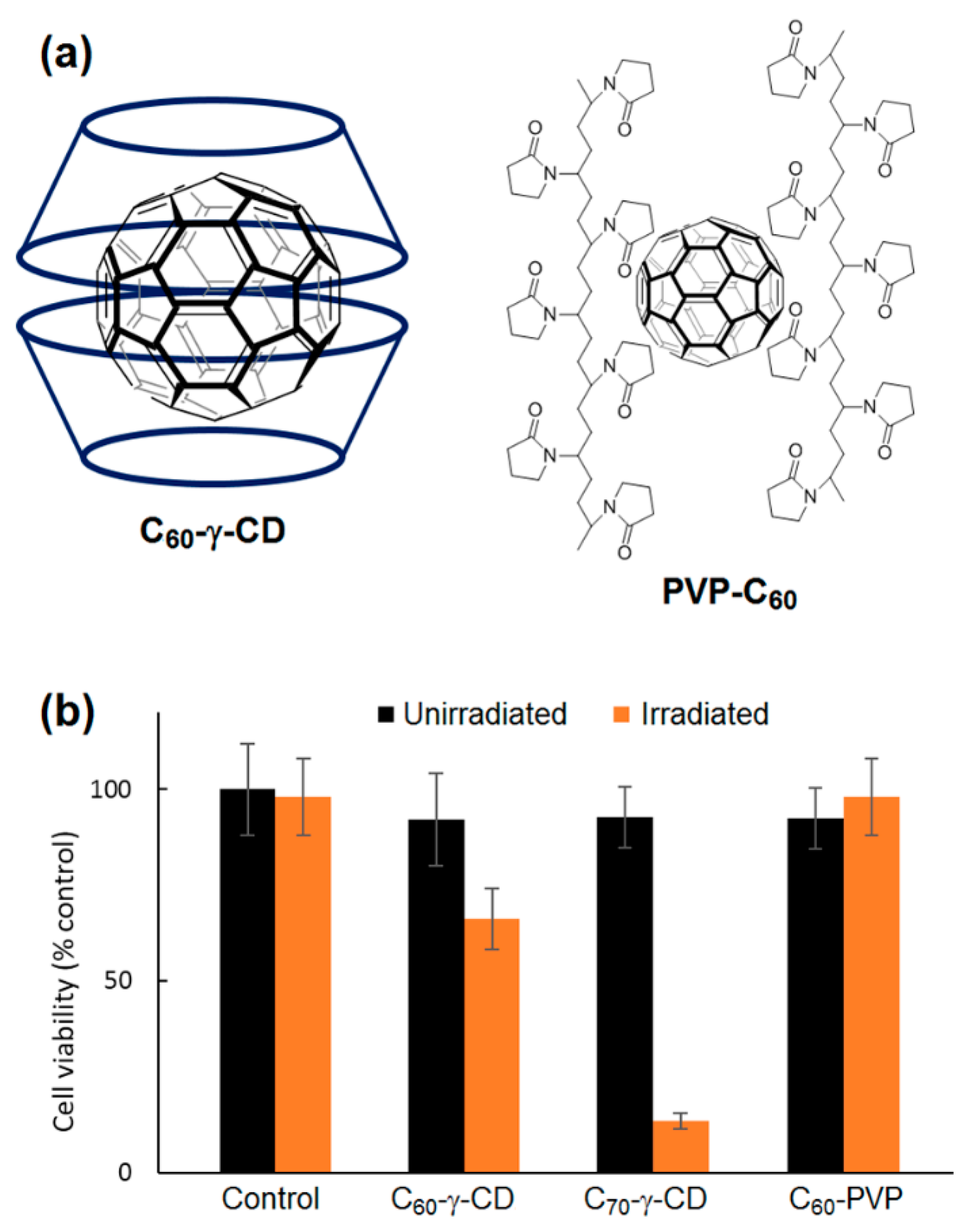
The good photophysical properties of fullerenes nominate them as potential PSs in PDT. However, their scarce solubility in aqueous media hinders their biological application. To overcome this issue, Iizumi et al. [
217] prepared water-soluble fullerene derivatives. The unmodified C
60 and C
70 were dispersed in water using γ-CD (C
60-γ-CD and C
70-γ-CD) or poly(vinylpyrrolidone) (C
60-PVP) (
Figure 68a). In C
60-γ-CD and C
70-γ-CD complexes, both fullerenes produced an appreciable amount of
1O
2 in water as compared to Rose Bengal (RB). Yet, the produced
1O
2 was less than that produced by fullerenes alone in organic solvents, most probably due to their inclusion into the cavities of γ-CD. This might have disrupted the efficient contact and accordingly the energy transfer between fullerenes and the molecular oxygen. Lower levels of
1O
2 were produced by C
60 in C
60-PVP. The assumption that C
60 tended to aggregate with the PVP dispersant while it formed a stable complex with γ-CD as monomers was possibly the reason behind the difference in
1O
2 generation. Dark toxicity and phototoxicity (λ = 633 nm, 3 mW/cm
2) were performed on the rat fibroblast cell line 5RP7 in the presence of C
60-γ-CD, C
70-γ-CD and C
60-PVP with fullerene concentration fixed at 10 µM. Slight dark toxicity was revealed in all cases. Nevertheless, and in coherence with the
1O
2 results, C
70-γ-CD exhibited a massive phototoxic impact leaving only 12% of the cells alive (
Figure 68b). Although C
60-γ-CD was proved analogous to C
70-γ-CD in terms of the
1O
2 generation, a much lower PDT effect was observed (66% of cell survival). This was most probably due the much lower extinction coefficient of C
60-γ-CD at 633 nm. Conversely, C
60-PVP mediated no cell death under irradiation. Thus, the phototoxicities of C
60 and C
70 were greatly influenced by their dispersed forms.
In 2011, Iohara et al. [
218] prepared C
60-HP-β-CD NPs by a co-grinding method. The authors compared the different properties of those NPs to C
60 dispersed in poly(vinylpyrrolidone) (PVP). It appeared that when HP-β-CD was used as a dispersant for C
60, a much lower mean particle diameter was attained (90 nm) than the one obtained in PVP (215 nm). This outcome agreed with the results obtained by Iizum et al. [
217].
1O
2 was already proved to be elaborated from C
60-HP-β-CD NPs through a type-II mechanism. Yet, in addition to
1O
2, the authors revealed the great aptitude of those NPs to generate multiple ROS, including HO
• and O
2•− under visible light irradiation through a type I reaction. The increase in the particle size due to the aggregation caused a restricted penetration of light that consequently led to a gradual loss of the capability of C
60-HP-β-CD NPs to produce ROS (
Figure 69). C
60-PVP and C
60 alone produced lower levels of HO
• and O
2•−, and barely generated
1O
2. In the dark, C
60, C
60-PVP, and C
60-HP-β-CD did not cause the death of HeLa cells, even at high concentrations. C
60 remained non-toxic, even under visible light irradiation. In contrary, C
60-PVP and C
60-HP-β-CD prompted an interesting dose-dependent phototoxic effect. Bringing up their high capacity to produce different ROS, it was not surprising that C
60-HP-β-CD NPs induced the most significant PDT effect among all. Those results assured the crucial role of HP-β-CD in elaborating such a stable and efficient system.
Many scientific efforts were devoted to render the fullerenes water-soluble for biological applications. Those attempts include the solubilization of those fullerenes by CDs through inclusion complexes, as previously presented by Ikeda et al. in their water-soluble host–guest fullerene–liposomes. On a similar track, Altaf et al. [
219] prepared their C
60-HSA NPs where the C
60 were transferred from the C
60/HP-β-CD NPs to the human serum albumin (HSA). The elaborated C
60-HSA NPs did not aggregate and exhibited a narrow size distribution and good dispersion stability. When linked to C
60, the fluorescence intensity of HSA decreased. C
60-HSA held a slightly improved high-affinity binding and a larger scavenging activity, and produced much more multiple ROS under visible light (
1O
2 and O
2•−) than HSA alone. HSA, C
60-HSA, and C
60-HP-β-CD did not exhibit dark cellular toxicity. However, the visible light-induced PDT effect of C
60-HSA NPs toward A549 cells was interesting, and analogous to that displayed by C
60-HP-β-CD NPs, and negligible in the case of HSA alone (
Figure 70). In conclusion, β-CD helped elaborate a potential drug delivery system for PDT.
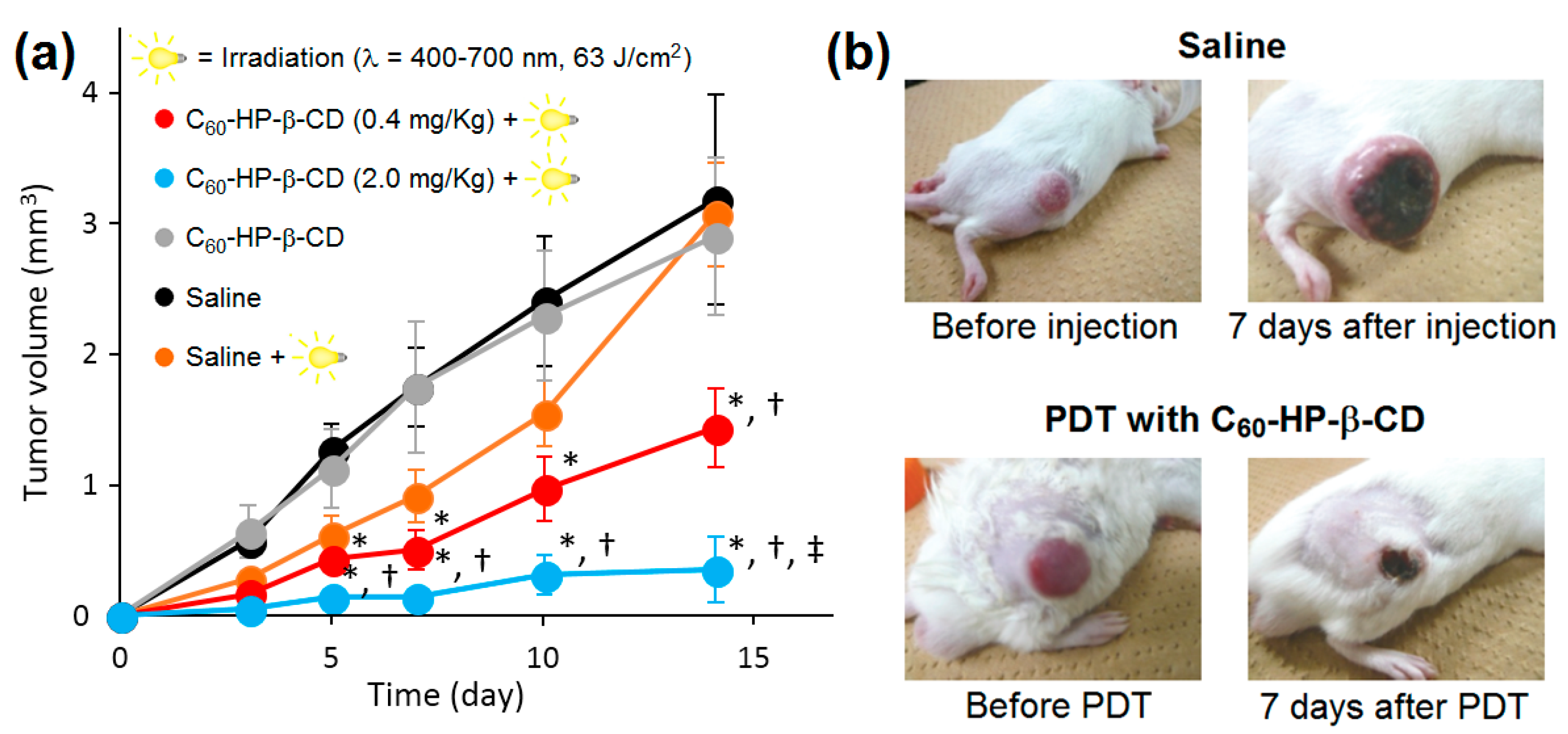
In vitro and in vivo experiments were also conducted on C
60/HP-β-CD NPs to assess their photosensitizing activity for PDT [
220]. In those NPs, C
60 partially occupied the hydrophobic cavity of CD. Under visible light irradiation, this system generated a high level of
1O
2 (φ
Δ = 0.96 in benzene) that even exceeded that produced by PpIX (φ
Δ = 0.56 in PBS). In addition, O
2•− was also produced by the C
60 component of the NPs. The generation of both types of ROS was dose-dependent, and it progressively and proportionally increased with the increase of the irradiation time and the energy of the light. In vitro, only when illuminated by visible light, C
60-HP-β-CD NPs caused remarkable photoinduced toxicity toward HeLa and A549 cells, with IC
50 values of 10 µM and 60 µM, respectively. C
60-HP-β-CD NPs acted better as PDT PS than 5-ALA, which was probably due to the greater ROS production. Moreover, the authors investigated the most appropriate parameters for an efficient in vivo PDT. In the presence of C
60-HP-β-CD NPs (2 mg/Kg of C
60), 12 times repeated visible light irradiation (λ = 400–700 nm, 350 mW/cm
2) for short period (15 s) with a total light dose of 63 J/cm
2 efficiently suppressed the growth of the sarcoma S-180 cells in ddY mice without causing any skin damage or major hyperthermia (
Figure 71).
Zhao et al. also addressed in their study the problem of the lack of solubility of fullerene in water [
221]. The authors prepared a supramolecular complex that was constituted of fullerene and γ-CD. This complex appeared to be water-soluble at room temperature, but tended to aggregate when heated at 85 °C (
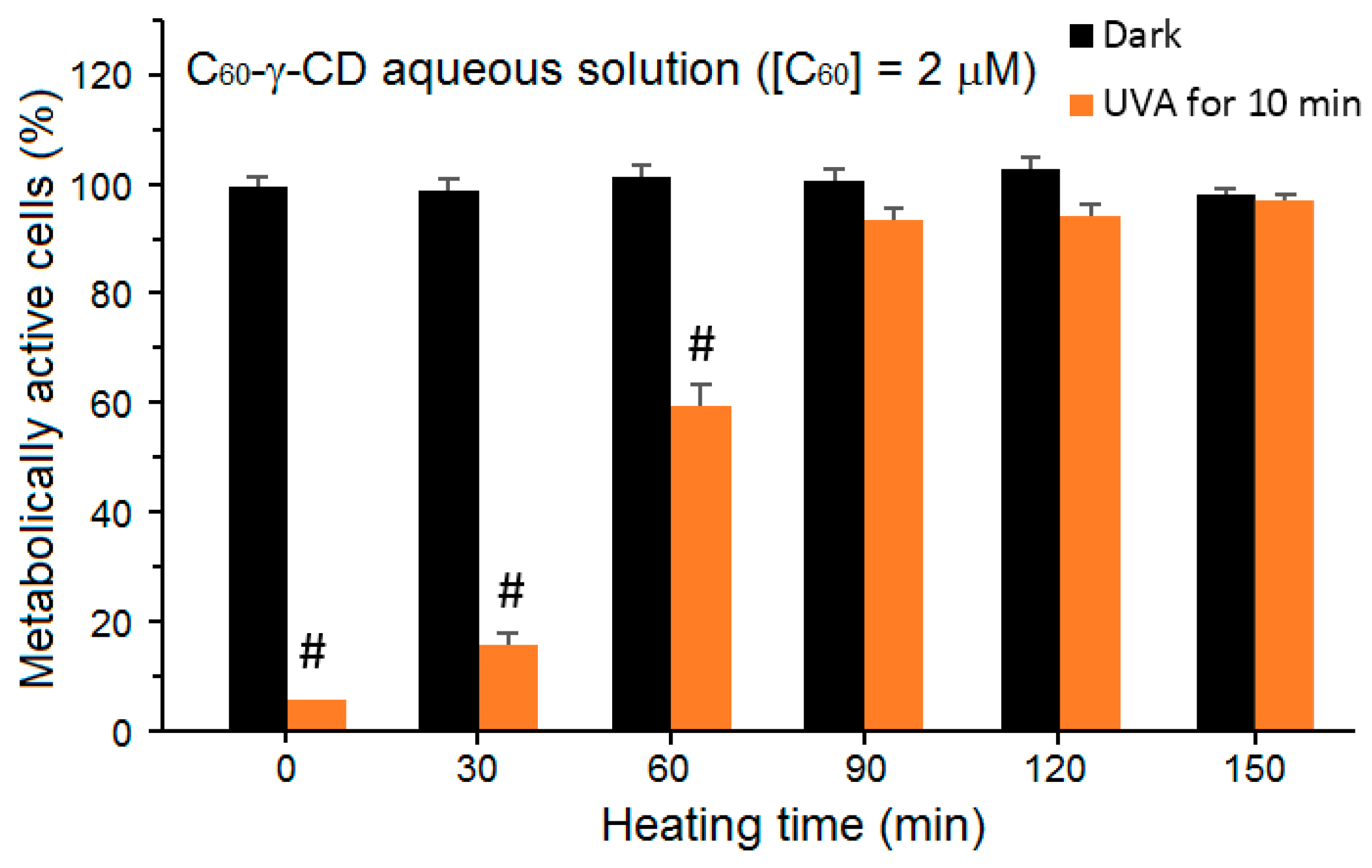
Figure 72). The aggregated complexes were much more incorporated into the lens epithelial cells HLE B-3. In darkness and under visible light illumination, the monomeric and aggregated C
60-γ-CD displayed no toxicity. Nevertheless, under UV-A irradiation, the viability of the HLE B-3 cells exposed to the monomeric C
60-γ-CD decreased sharply to less than 10% in a dose-dependent manner. As for the aggregated fullerenes, the PDT efficiency was gradually lost with the gradual growth of the aggregates, and finally vanished when the cells were treated with the largest aggregates attained after 150 min of heating at 85 °C. The production of
1O
2 followed the same trend, where the maximum amount was generated by C
60-γ-CD, and decreased proportionally with the increasing aggregate size (
Figure 73). After UVA irradiation, the
1O
2 was generated by C
60-γ-CD inside the cells, and it targeted the intracellular proteins in HLE B-3 cells. This outcome was confirmed by the presence of high amounts of protein peroxides in the cells, which led to their damage by the induced apoptosis.
In their approach, Wang et al. [
222] associated the fullerene C
60 to the outside of the β-CD molecule to yield a water-soluble exclusion complex rather than embedding it in the cavities. C
60 and β-CD were linked together via diaminotriethylene glycol as a hydrophilic spacer. Both the hydrophilic spacer and β-CD maintained an enhanced solubility of C
60 in water. The absorption profile and thus the basic structure of C
60 remained intact after being conjugated to β-CD. On the one hand, when this C
60-β-CD was introduced in different concentrations up to 200 µg/mL into the human neuroblastoma SH-SY5Y cells, a negligible dark toxicity was witnessed. On the other hand, under irradiation with visible light (λ ≥ 400 nm, 20 min), C
60-β-CD revealed a dose-dependent phototoxicity against the tumor cells (
Figure 74a). After investigating the in vitro cytotoxicity and phototoxicity of their C
60-β-CD, the authors aimed to assess the dispersion distribution of the C
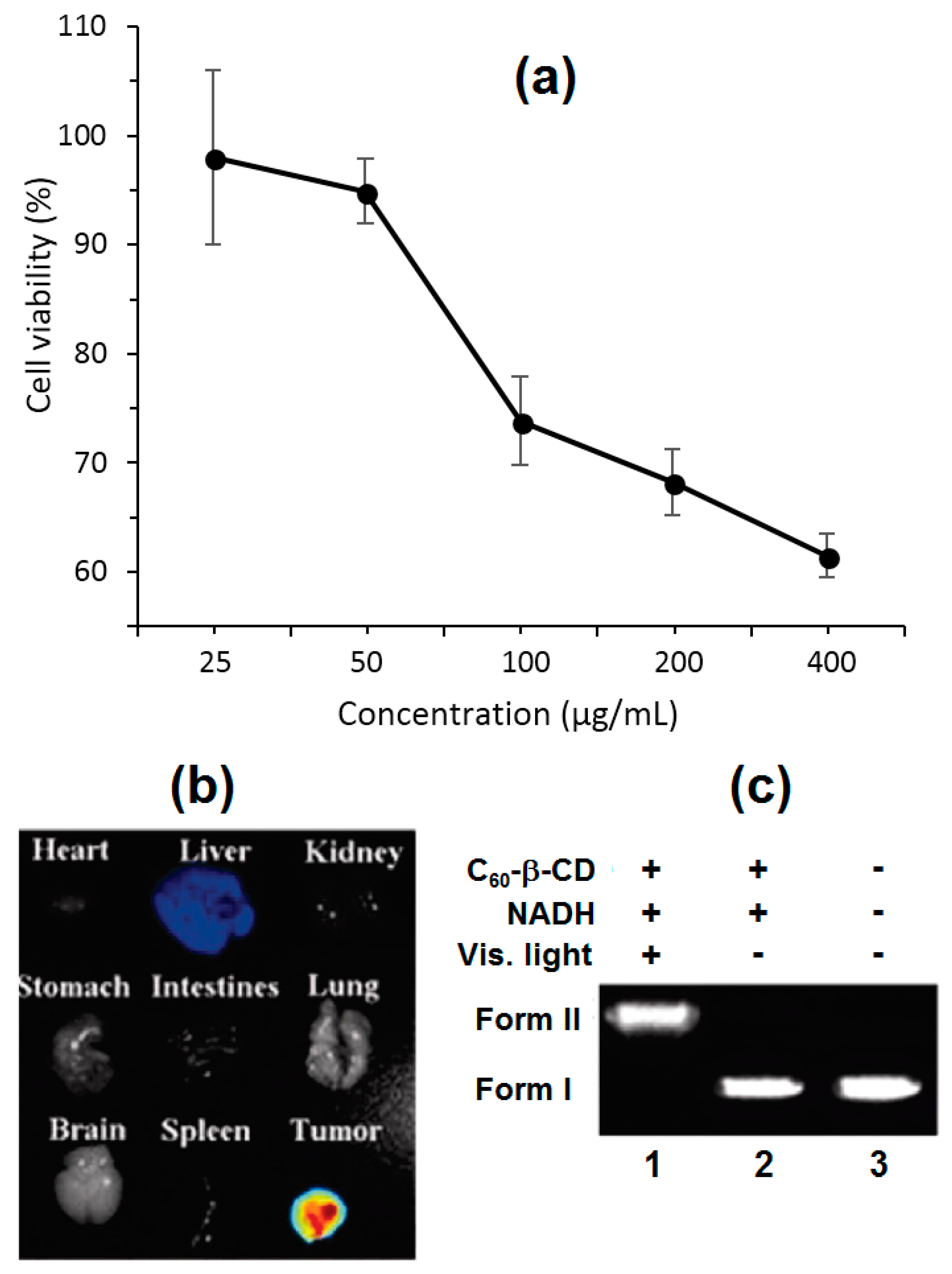
60-β-CD throughout the living body. For actions to serve this goal, they labeled C
60-β-CD with a NIR-dye, i.e., NIR-797, and injected it into hepatic H22 tumor-bearing mice. At 96 h post-injection, C
60-β-CD was massively localized in the tumor and scarcely in the liver (
Figure 74b). C
60-β-CD appeared to circulate throughout the blood with a half-life of about 4 h, leaving behind no acute or subacute toxicity in the different organs of the mice’s body. Only in the presence of visible light and nicotinamide adenine dinucleotide (NADH), which is a reducing agent that naturally assists in the photocleavage of DNA, was C
60-β-CD was active toward the cleavage of pBR322 plasmid DNA into form II (
Figure 74c). Both the ability of C
60-β-CD to cleave DNA and kill tumor cells by PDT were due to the action of ROS, precisely HO
• and O
2•−, which were significantly produced by the excited PS.
To overcome the water-solubility problem of fullerene C
60 and enhance its phototoxicity, Zhang et al. manipulated C
60 via the pre-used CD-functionalization tactic [
223]. However, instead of working with unmodified γ-CD, they used γ-CD polymer (γ-CD-P) to form their inclusion complex (C
60-γ-CD-P) (
Figure 75a). In this system, monomeric C
60 occupied the hydrophobic cavities of γ-CD-P. Similar to β-CD and γ-CD, γ-CD-P successfully rendered C
60 water-soluble, and the absorption spectrum of this inclusion complex displayed the characteristic peaks of C
60. However, those peaks were red-shifted as compared to the absorption peaks obtained from C
60-γ-CD-P. However, γ-CD-P appeared to be capable of forming inclusion complexes easier than γ-CD. In addition, C
60 in the C
60-γ-CD-P complex remained well-dispersed in water without forming aggregates, even at high concentrations of the complex due to the supramolecular interaction with γ-CD-P, which was an advantage over the other C
60 inclusion complexes with CD. The C
60-γ-CD-P complex efficiently produced
1O
2 only under UVA light, unlike C
60-β-CD, which can generate
1O
2 under both visible and UVA lights. Those complexes were studied in vitro (MTT assay) with mouse melanoma cell lines B16-F10. γ-CD-P increased the biocompatibility of C
60, as no cytotoxic was observed when the cells were exposed to different concentrations of C
60-γ-CD-P ([C
60] = 0.5–20 µM) after 48 h of incubation in the dark. Due to the non-aggregated nature of the C
60 enclosed into γ-CD-P and in agreement with the significant
1O
2 production, C
60-γ-CD-P exhibited a superior phototoxic effect than C
60 toward the tumor cells under UVA irradiation (
Figure 75b).