Phytochemical and Analytical Characterization of Novel Sulfated Coumarins in the Marine Green Macroalga Dasycladus vermicularis (Scopoli) Krasser
Abstract
:1. Introduction
2. Results
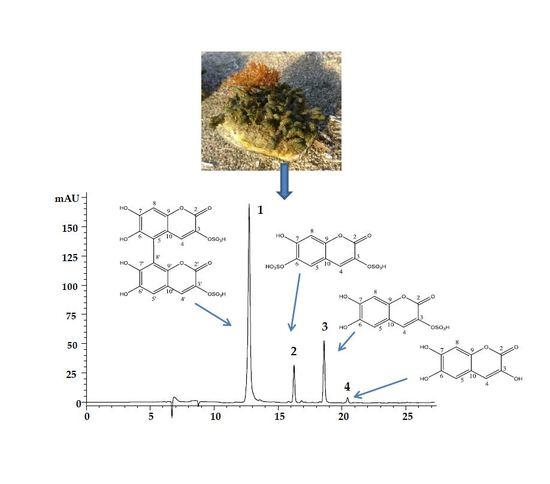
2.1. Isolation and Identification of the Coumarins
2.2. HPLC-Method Development
2.3. Method Validation
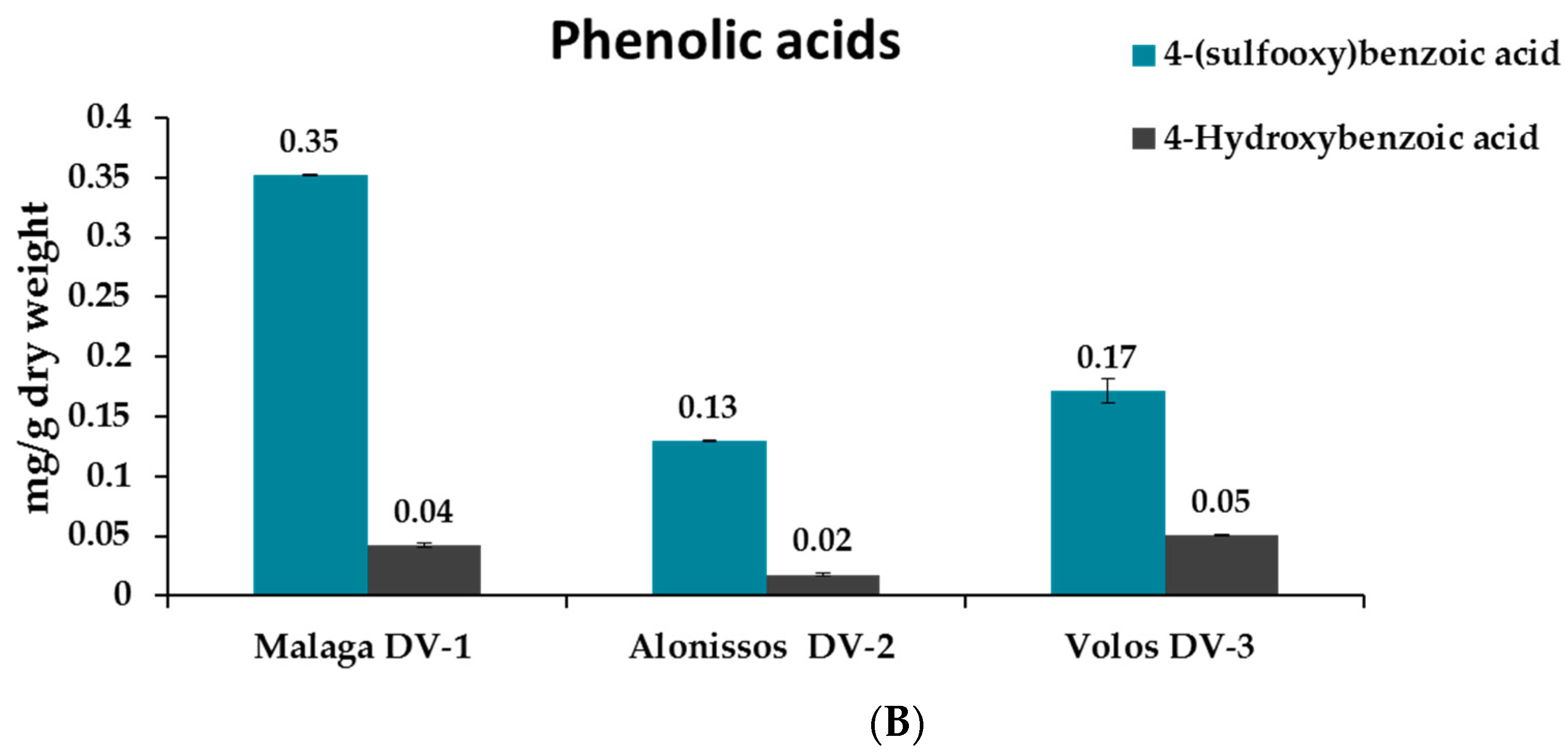
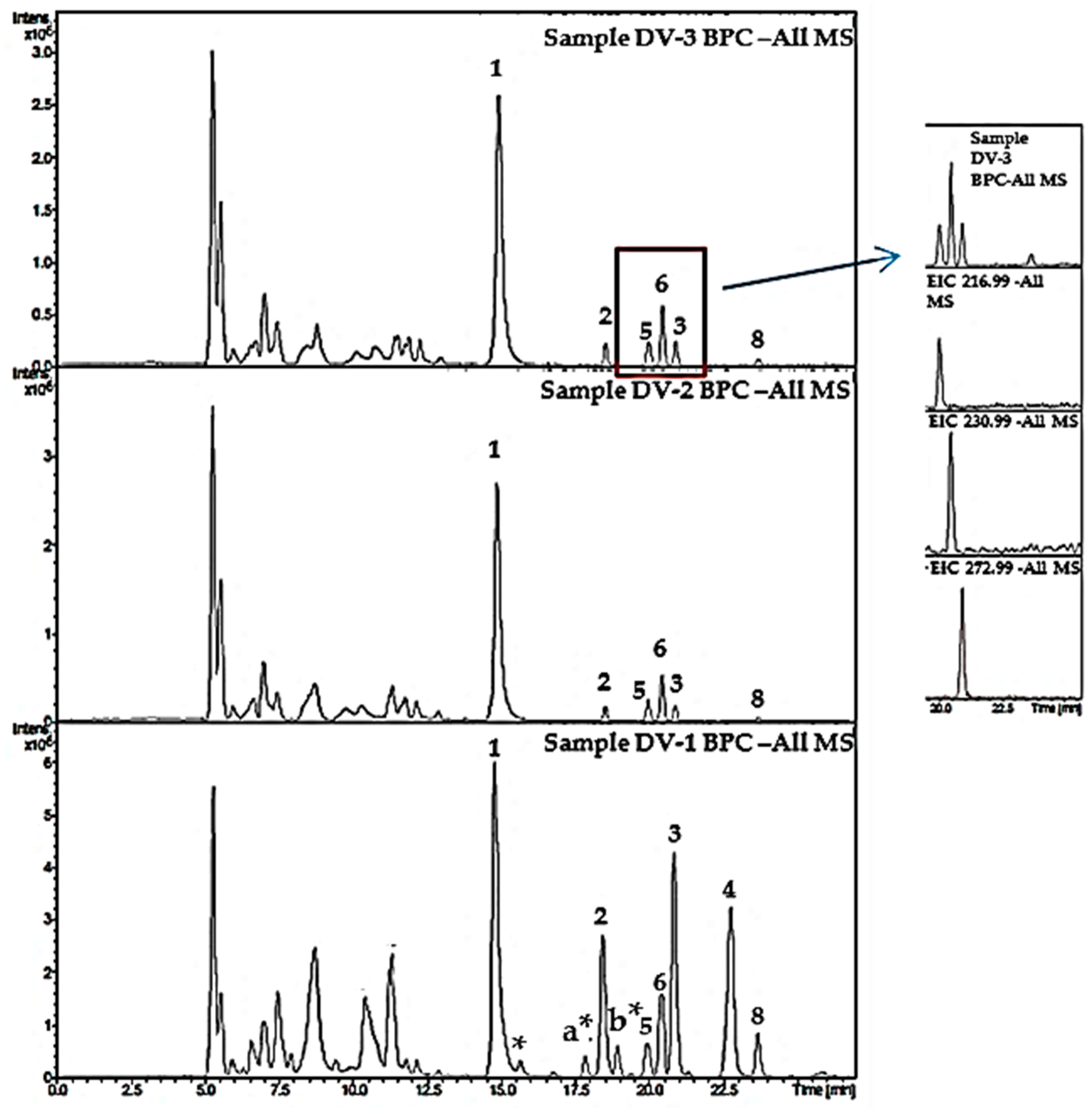
2.4. Analysis of Samples
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Algal Material
4.3. Instrumentation
4.4. Isolation and Structural Analysis of Coumarins
4.5. Sample Preparation
4.6. Analytical Conditions
4.7. Synthesis of 4-(sulfooxy)benzoic Acid and Synthesis of 4-(sulfooxy)phenylacetic Acid
4.8. Synthesis of 3,6,7-trihydroxycoumarin
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guiry, M.D.; Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Galway, Ireland; Available online: http://www.algaebase.org (accessed on 18 October 2018).
- Carrillo, J.M.A. Algunas observaciones sobre la distribucion vertical de las algas en la Isla del Hierro (canarias). Vieraea. Fol. Sci. Biol. Canar. 1980, 10, 3–16. [Google Scholar]
- Menzel, D.; Kazlauskas, R.; Reichelt, J. Coumarins in the Siphonalean Green Algal Family Dasycladaceae Kutzing (Chlorophyceae). Bot. Mar. 1983, 26, 23–29. [Google Scholar] [CrossRef]
- Gomez, I.; Pérez-Rodríguez, E.; Viñegla, B.; Figueroa, F.L.; Karsten, U. Effects of solar radiation on photosynthesis, UV-absorbing compounds and enzyme activities of the green alga Dasycladus vermicularis from southern Spain. J. Photochem. Photobiol. B 1998, 47, 46–57. [Google Scholar] [CrossRef]
- Perez-Rodriguez, E.; Aguilera, J.; Gomez, I.; Figueroa, F.L. Excretion of coumarins by the Mediterranean green alga Dasycladus vermicularis in response to environmental stress. Mar. Biol. 2001, 139, 633–639. [Google Scholar] [CrossRef]
- Perez-Rodriguez, E.; Aguilera, J.; Figueroa, F.L. Tissular localization of coumarins in the green alga Dasycladus vermicularis (Scopoli) Krasser: A photoprotective role? J. Exp. Bot. 2003, 54, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Coneva, V.; Chitwood, D.H. Plant architecture without multicellularity: Quandaries over patterning and the soma-germline divide in siphonous algae. Front. Plant. Sci. 2015, 6, 287. [Google Scholar] [CrossRef] [PubMed]
- Welling, M.; Pohnert, G.; Küppert, F.C.; Ross, C. Rapid Biopolymerisation During Wound Plug Formation in Green Algae. J. Adhes. 2009, 85, 825–838. [Google Scholar] [CrossRef]
- Welling, M.; Ross, C.; Pohnert, G.A. Desulfatation-Oxidation Cascade Activates Coumarin-Based Cross-Linkers in the Wound Reaction of the Giant Unicellular Alga Dasycladus vermicularis. Angew. Chem.-Int. E 2011, 50, 7691–7694. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.; Vreeland, V.; Waite, J.H.; Jacobs, R.S. Rapid assembly of a wound plug: Stage one of a two-stage wound repair mechanism in the giant unicellular chlorophyte Dasycladus vermicularis (Chlorophyceae). J. Phycol. 2005, 41, 46–54. [Google Scholar] [CrossRef]
- Sever, M.J.; Weisser, J.T.; Monahan, J.; Srinivasan, S.; Wilker, J.J. Metal-mediated cross-linking in the generation of a marine-mussel adhesive. Angew. Chem.-Int. E. 2004, 43, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Thoms, C.; Schupp, P.J. Activated chemical defense in marine sponges—A case study on Aplysinella rhax. J. Chem. Ecol. 2008, 34, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Kurth, C.; Cavas, L.; Pohnert, G. Sulfation mediates activity of zosteric acid against biofilm formation. Biofouling 2015, 31, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Kurth, C.; Welling, M.; Pohnert, G. Sulfated phenolic acids from Dasycladales siphonous green algae. Phytochemtry 2015, 117, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Haensel, R.; Sticher, O. Pharmakognosie Phytopharmazie, 9th ed.; Springer Medizin Verlag GmbH: Heidelberg, Germany, 2010; pp. 419–420. [Google Scholar]
- Bailly, F.; Maurin, C.; Teissier, E.; Vezin, H.; Cotelle, P. Antioxidant properties of 3-hydroxycoumarin derivatives. Bioorg. Med. Chem. 2004, 12, 5611–5618. [Google Scholar] [CrossRef] [PubMed]
- ICH Harmonization for Better Health. Available online: http://www.ich.org/products/guidelines.html (accessed on 6 August 2018).
- Zhu, J.J.; Jiang, J.G. Pharmacological and Nutritional Effects of Natural Coumarins and Their Structure-Activity Relationships. Mol. Nutr. Food Res. 2018, 62, 1701073. [Google Scholar] [CrossRef] [PubMed]
- Kayser, O.; Kolodziej, H. Antibacterial Activity of Simple Coumarins: Structural Requirements for Biological Activity. Naturforschung C 1999, 54, 169. [Google Scholar] [CrossRef]
- Aihara, K.; Higuchi, T.; Hirobe, M. 3-Hydroxycoumarins: First direct preparation from coumarins using a Cu2(+)-ascorbic acid-O2 system, and their potent bioactivities. Biochem. Biophys. Res. Commun. 1990, 168, 169–175. [Google Scholar] [CrossRef]
- Verespy, S.; Metha, A.Y.; Afosah, D.; Al-Horani, R.A.; Desai, U.R. Allosteric partial Inhibition of Monomeric Proteases. Sulfated Coumarins Induce Regulation, not just Inhibition of Thrombin. Sci. Rep. 2016, 6, 24043. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, X.; Wang, J.; Zhang, Z.; Gao, B.; Shi, S.; Wang, X.; Li, J.; Tu, P. Simultaneous characterization of fifty coumarins from the roots of Angelica dahurica by off-line two-dimensional high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Phytochem. Anal. 2014, 25, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, F.; Ai, Y.; Ma, W.; Bian, Q.; Lee, D.; Dai, R. Simultaneous determination of seven coumarins by UPLC-MS/MS: Application to a comparative pharmacokinetic study in normal and arthritic rats after administration of Huo Luo Xiao Ling Dan or single herb. J. Chromatogr. B 2015, 991, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Li, G.J.; Wu, H.J.; Wang, Y.; Hung, W.L.; Rouseff, R.L. Determination of citrus juice coumarins, furanocoumarins and methoxylated flavones using solid phase extraction and HPLC with photodiode array and fluorescence detection. Food Chem. 2019, 15, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Schoenwaelder, M.E.A. The Biology of Phenolic Containing Vesicles. Algae 2008, 23, 163–175. [Google Scholar] [CrossRef]
- Cotelle, P.; Vezin, H. EPR of free radicals formed from 3-hydroxyesculetin and related derivatives. Res. Chem. Intermed. 2003, 29, 365–377. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds/fractions are available from the authors upon request. |



| Position | Dasycladin A (1) in D2O | HMBC C | Dasycladin B (2) in D2O | HMBC C | ||
|---|---|---|---|---|---|---|
| δH | δC, Type | δH | δC, Type | |||
| 2 | 163.3, C | 161.0, C | ||||
| 3 | 136.1, C | 134.1, C | ||||
| 4 | 7.40 (s) | 134.4, CH | 2, 3, 5, 9 | 7.94 (s) | 133.3, CH | 2, 3, 5, 9 |
| 5 | 118.3, C | 7.64 (s) | 122.4, CH | 4, 6, 7, 9 | ||
| 6 | 144.1, C | 137.6, C | ||||
| 7 | 152.7, C | 153.0, C | ||||
| 8 | 7.10 (s) | 106.8, CH | 6, 7, 9, 10 | 7.05 (s) | 105.1, CH | 6, 7, 9, 10 |
| 9 | 150.2, C | 151.1, C | ||||
| 10 | 113.5, C | 112.1, C | ||||
| 2′ | 163.0, C | |||||
| 3′ | 135.8, C | |||||
| 4′ | 7.96 (s) | 135.8, CH | 2′, 3′, 5′, 9′ | |||
| 5′ | 7.28 (s) | 115.8, CH | 4′, 6′, 7′, 9′ | |||
| 6′ | 145.7, C | |||||
| 7′ | 151.5, C | |||||
| 8′ | 111.2, C | |||||
| 9′ | 147.7, C | |||||
| 10′ | 113.9, C | |||||
| Parameter | 1 (Dasycladin A) | 4 (thyc) | 5 (SBA) | 6 (SPA) | 7 (4-OH-PAA) | 8 (4-OH-BA) |
|---|---|---|---|---|---|---|
| Regr. Equation | Y = 22.953x − 7.904 | Y = 67.354x − 296.52 | Y = 19.684x + 22.158 | Y = 0.652x − 1.475 | Y = 97.057x + 14.771 | Y = 2.110x + 2.757 |
| σ rel of the slope | 0.09 | 0.683 | 0.116 | 0.175 | 0.049 | 0.339 |
| R | 0.9999 | 0.9986 | 0.9999 | 1.000 | 1.000 | 1.000 |
| Range (μg/mL) | 440–0.859 | 445–6.953 | 629–1.229 | 1154–18.031 | 124.75–0.975 | 483–3.770 |
| LOD 1 | 0.192 | 0.589 | 0.039 | 1.939 | 0.014 | 1.045 |
| LOQ 2 | 0.581 | 1.784 | 0.117 | 5.876 | 0.044 | 3.168 |
| Substance | Accuracy 1 | Precision 2 | |||||
|---|---|---|---|---|---|---|---|
| High Spike | Medium Spike | Low Spike | Day 1 | Day 2 | Day 3 | Intra-Day | |
| 1 | 99.49 | 95.63 | 101.89 | 7.49 | 1.57 | 1.38 | 1.86 |
| 2 | - | - | - | 2.22 | 4.43 | 6.65 | 3.71 |
| 3 | - | - | - | 6.39 | 4.37 | 2.81 | 5.99 |
| 4 | 97.49 | 97.87 | 91.34 | - | - | - | - |
| 5 | 102.62 | 99.58 | 99.40 | - | - | - | - |
| 6 | 98.70 | 103.23 | 100.72 | - | - | - | - |
| 7 | 97.45 | 104.06 | 103.77 | - | - | - | - |
| 8 | 102.10 | 99.50 | 98.85 | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartmann, A.; Ganzera, M.; Karsten, U.; Skhirtladze, A.; Stuppner, H. Phytochemical and Analytical Characterization of Novel Sulfated Coumarins in the Marine Green Macroalga Dasycladus vermicularis (Scopoli) Krasser. Molecules 2018, 23, 2735. https://doi.org/10.3390/molecules23112735
Hartmann A, Ganzera M, Karsten U, Skhirtladze A, Stuppner H. Phytochemical and Analytical Characterization of Novel Sulfated Coumarins in the Marine Green Macroalga Dasycladus vermicularis (Scopoli) Krasser. Molecules. 2018; 23(11):2735. https://doi.org/10.3390/molecules23112735
Chicago/Turabian StyleHartmann, Anja, Markus Ganzera, Ulf Karsten, Alexsander Skhirtladze, and Hermann Stuppner. 2018. "Phytochemical and Analytical Characterization of Novel Sulfated Coumarins in the Marine Green Macroalga Dasycladus vermicularis (Scopoli) Krasser" Molecules 23, no. 11: 2735. https://doi.org/10.3390/molecules23112735
APA StyleHartmann, A., Ganzera, M., Karsten, U., Skhirtladze, A., & Stuppner, H. (2018). Phytochemical and Analytical Characterization of Novel Sulfated Coumarins in the Marine Green Macroalga Dasycladus vermicularis (Scopoli) Krasser. Molecules, 23(11), 2735. https://doi.org/10.3390/molecules23112735