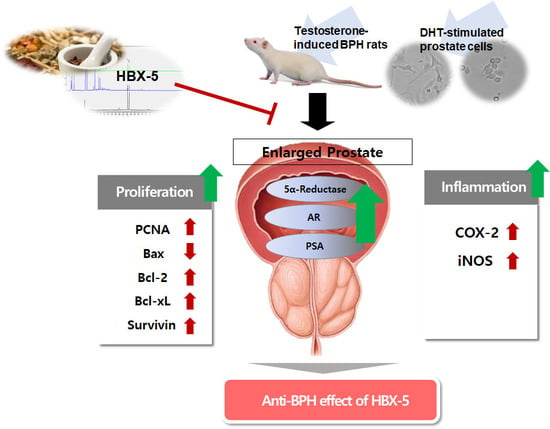
Anti-Proliferative Effects of HBX-5 on Progression of Benign Prostatic Hyperplasia
Abstract
1. Introduction
2. Results
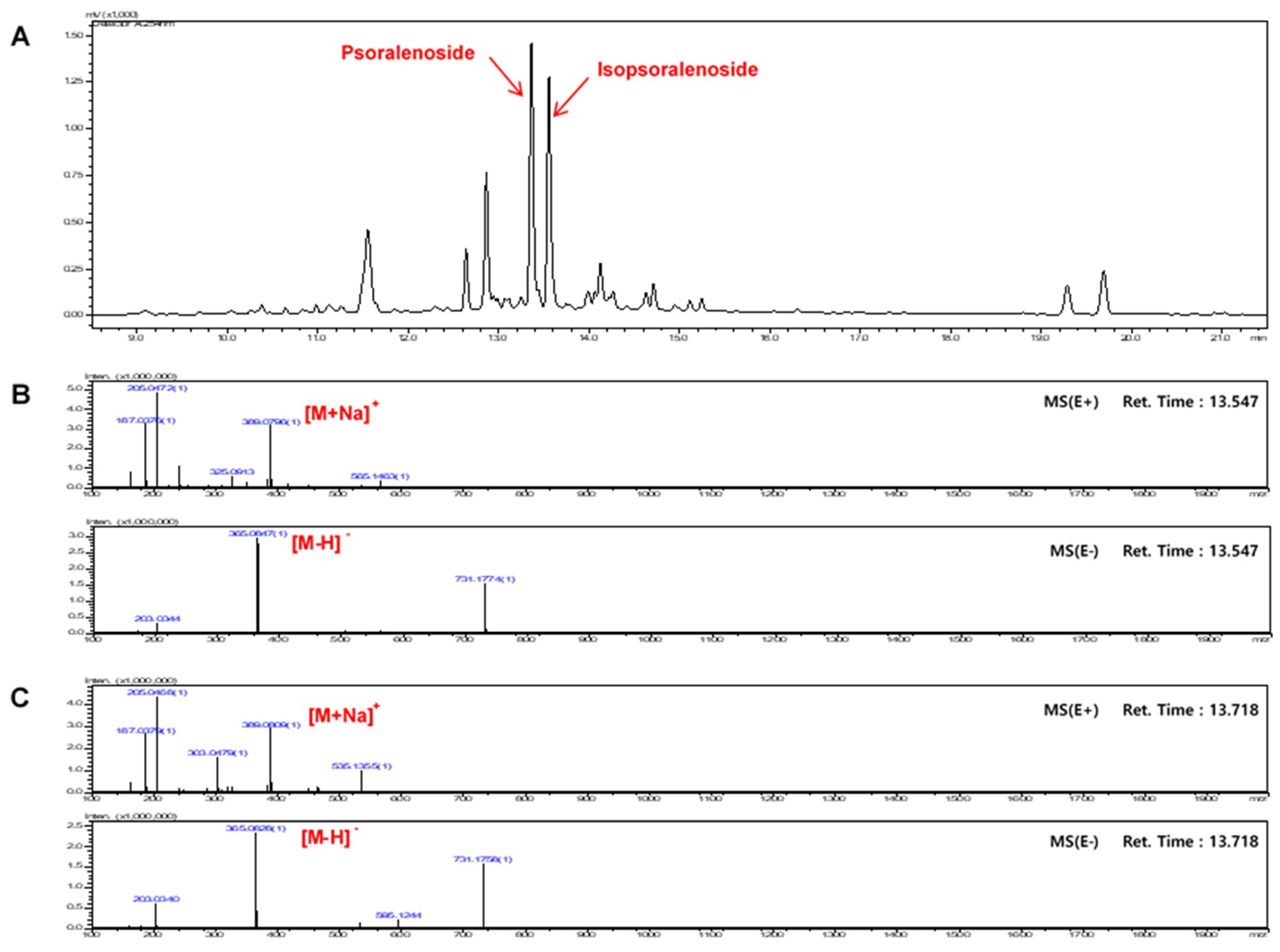
2.1. Optimization of Chromatographic Conditions
2.2. Calibration Curves, Limits of Detection, and Quantification
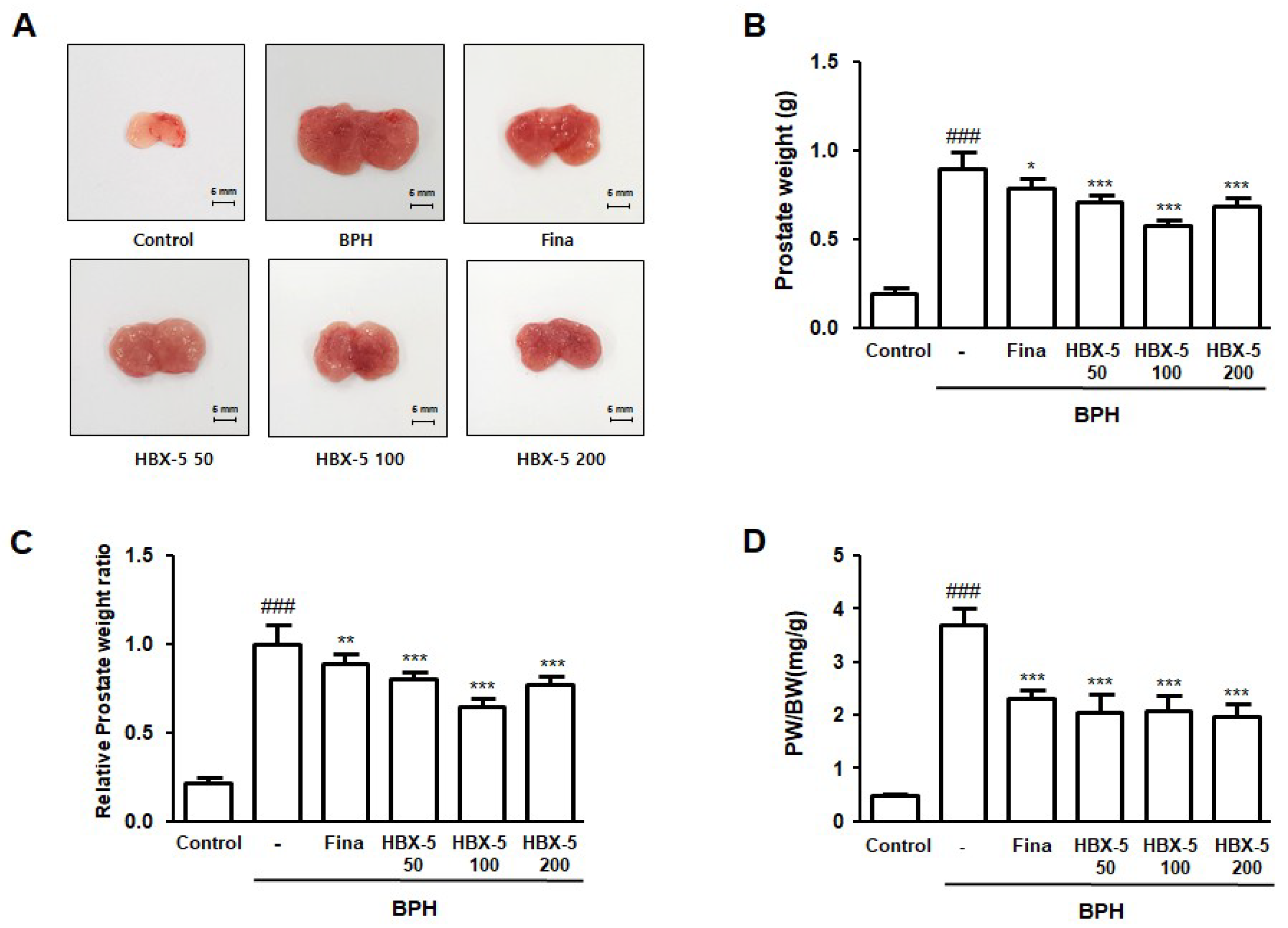
2.3. Effect of HBX-5 Administration on Prostate Weight in Rat with BPH Models
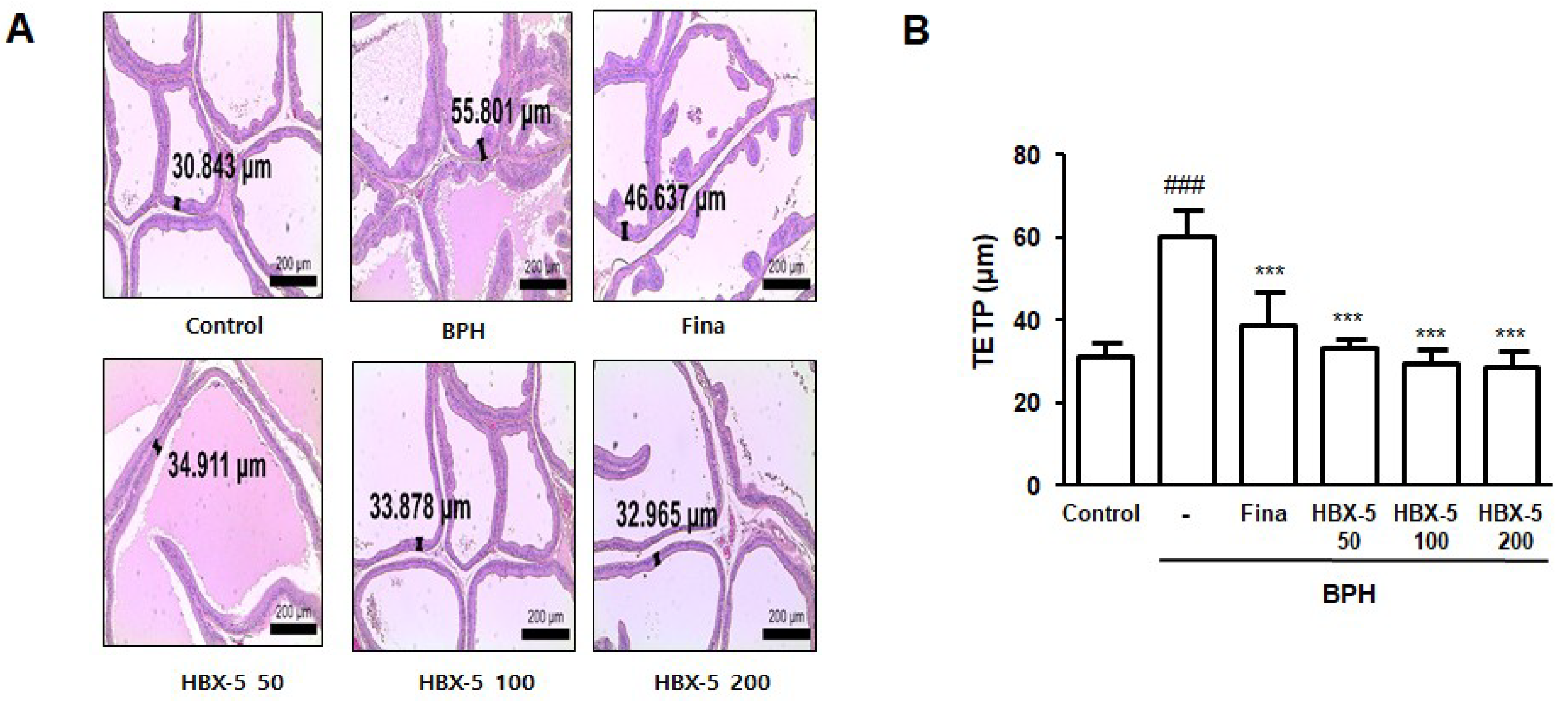
2.4. Effect of HBX-5 Administration on the Histological Alteration in Rats with BPH
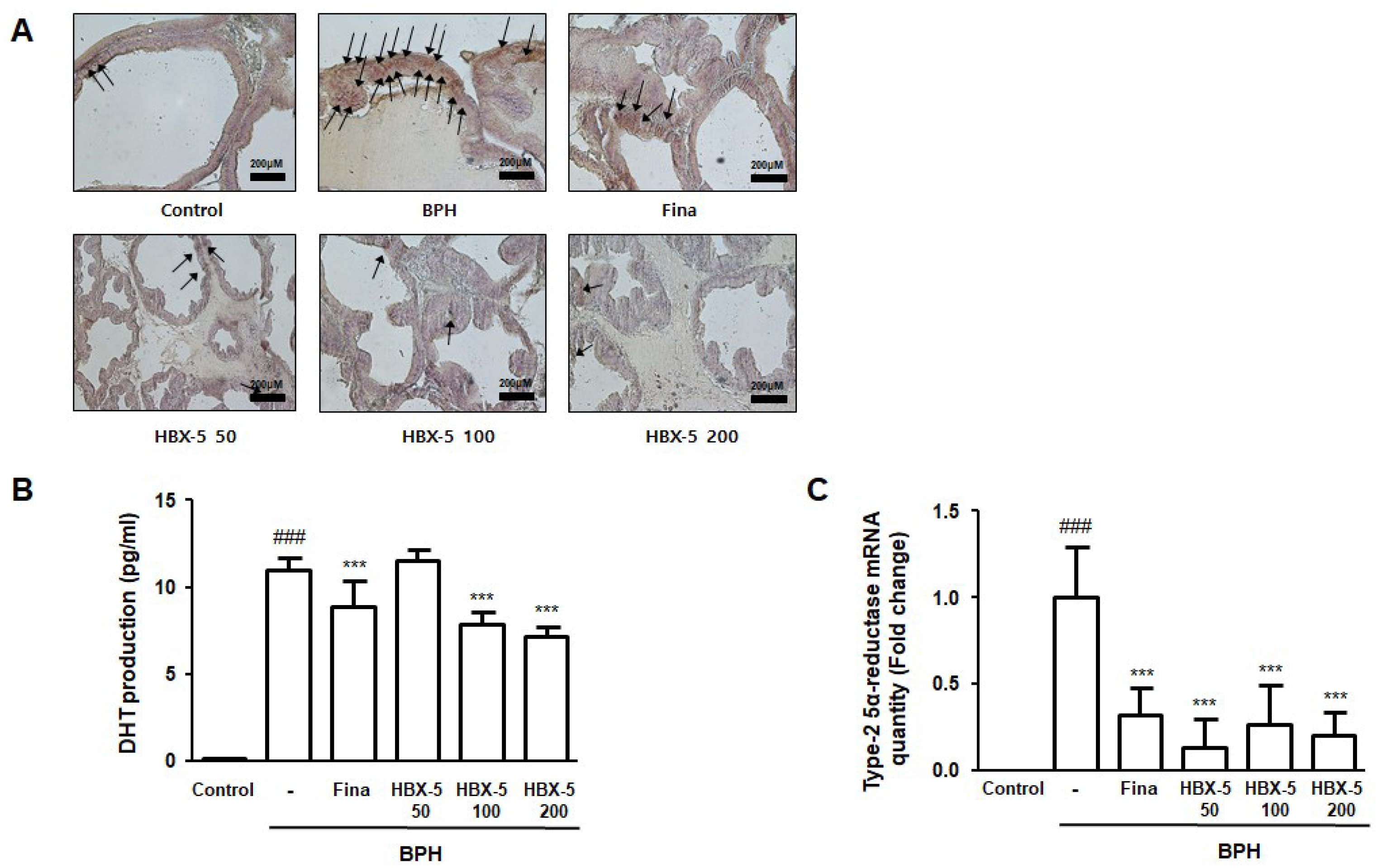
2.5. Effect of HBX-5 Administration on the Expression of AR, Type-2 5α-Reductase mRNA Expression, and Serum DHT Levels
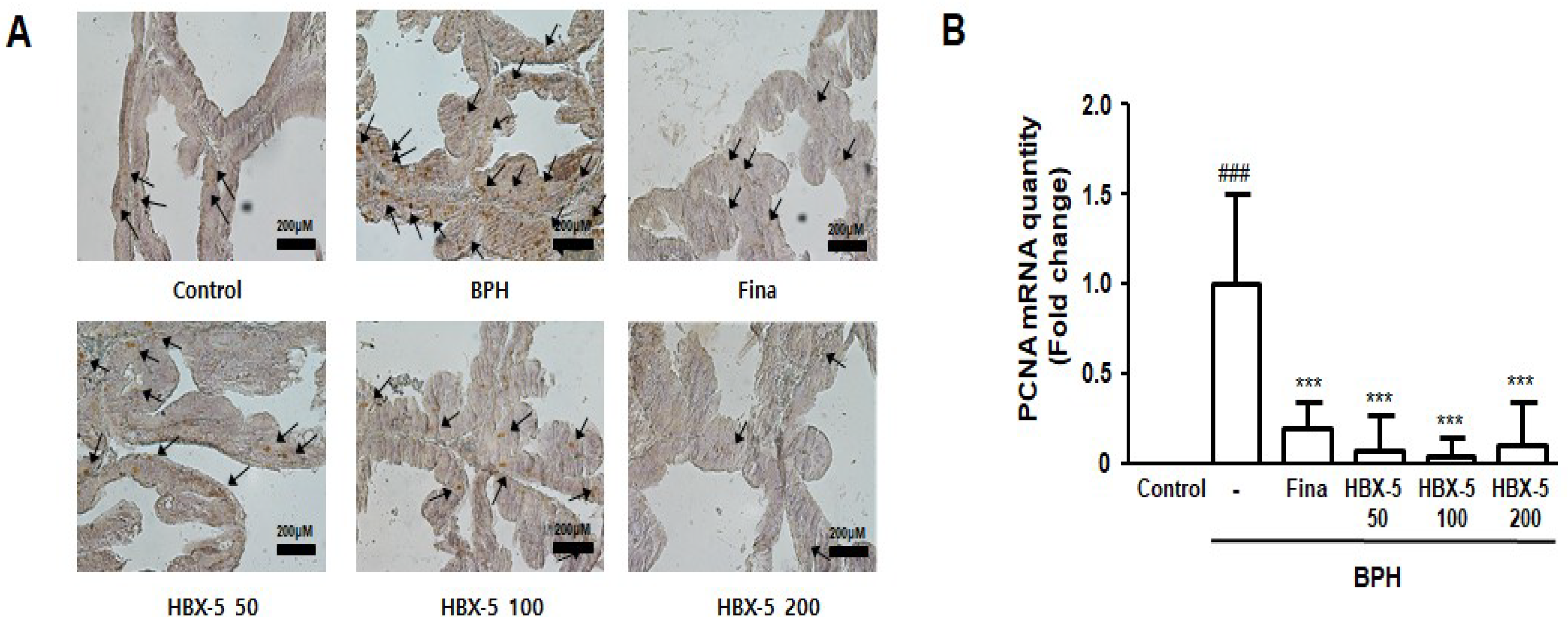
2.6. Effect of HBX-5 Administration on Prostatic Cell Proliferation
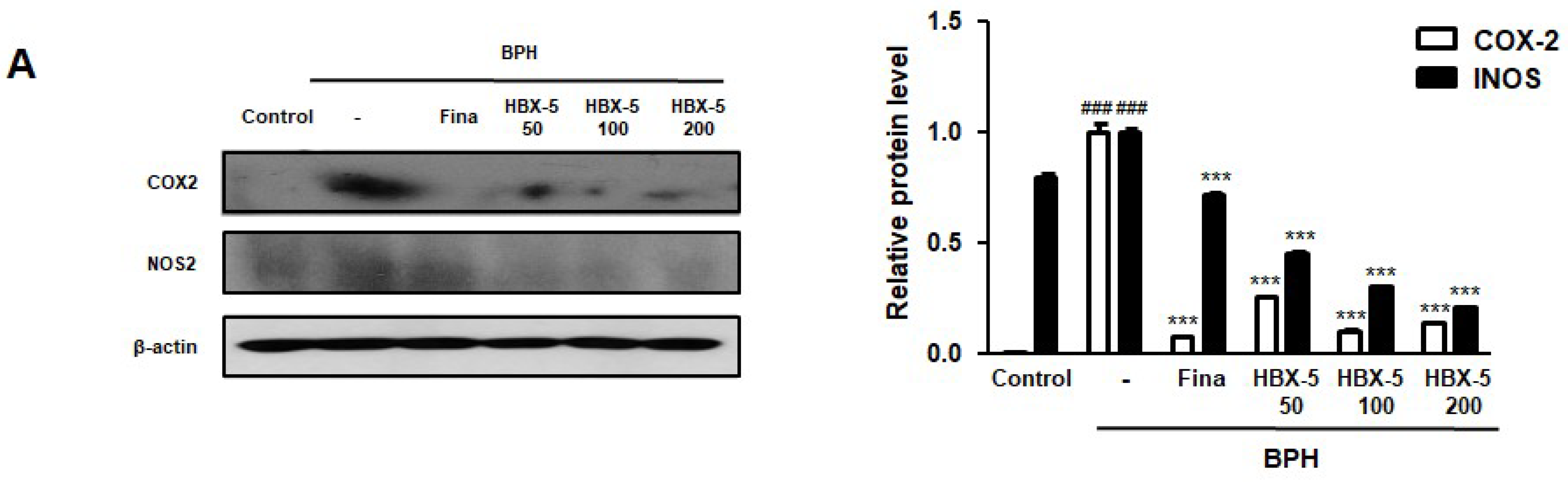
2.7. Effect of HBX-5 on the Expression of COX-2 and iNOS in the Prostatic Tissues of Rats with BPH Models
2.8. Effect of HBX-5 Administration on the Expression of Apoptosis-Related Proteins in the Prostatic Tissues of Rats with BPH Models
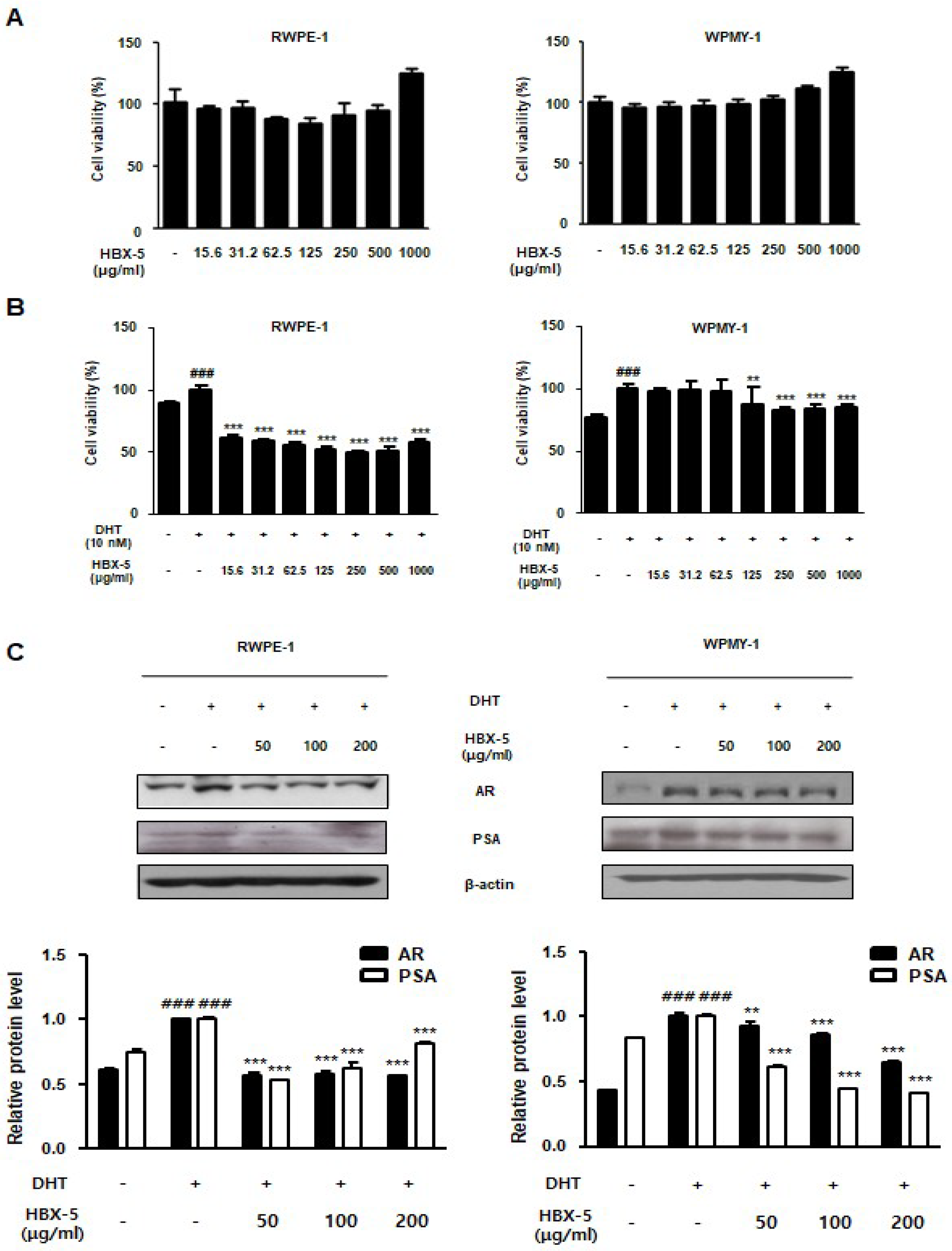
2.9. Effect of HBX-5 Administration on the BPH-Related Protein Expression in RWPE-1 and WPMY-1 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of HBX-5
4.3. Instruments
4.4. Sample Preparation for HPLC
4.5. Calibration Curves, Limits of Detection, and Quantification
4.6. UFLC-IT-TOF MS Analysis
4.7. Animals
4.8. Prostate Weight to Body Weight Ratio
4.9. Serum Concentrations of DHT Analysis
4.10. Histological Analysis
4.11. Quantitative Real-Time Polymerase Chain Reaction Analysis
4.12. Western Blot Analysis
4.13. Immunohistochemistry
4.14. Cell Culture and Sample Treatment
4.15. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roehrborn, C.G. Benign prostatic hyperplasia: An overview. Rev. Urol. 2005, 7, S3–S14. [Google Scholar] [PubMed]
- Briganti, A.; Capitanio, U.; Suardi, N.; Gallina, A.; Salonia, A.; Bianchi, M.; Tutolo, M.; Di Girolamo, V.; Guazzoni, G.; Rigatti, P.; et al. Benign prostatic hyperplasia and its aetiologies. Eur. Urol. Suppl. 2009, 8, 865–871. [Google Scholar] [CrossRef]
- Gandaglia, G.; Briganti, A.; Gontero, P.; Mondaini, N.; Novara, G.; Salonia, A.; Sciarra, A.; Montorsi, F. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (bph). BJU Int. 2013, 112, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Delongchamps, N.B.; de la Roza, G.; Chandan, V.; Jones, R.; Sunheimer, R.; Threatte, G.; Jumbelic, M.; Haas, G.P. Evaluation of prostatitis in autopsied prostates-is chronic inflammation more associated with benign prostatic hyperplasia or cancer? J. Urol. 2008, 179, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Zhang, Z.; Na, Y.; Guo, Y. Apoptosis profiles in benign prostatic hyperplasia: Close associations of cell kinetics with percent area density of histologic composition. Urology 2006, 68, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Lepor, H.; Kazzazi, A.; Djavan, B. Alpha-blockers for benign prostatic hyperplasia: The new era. Curr. Opin. Urol. 2012, 22, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lepor, H. Medical treatment of benign prostatic hyperplasia. Rev. Urol. 2011, 13, 20–33. [Google Scholar] [PubMed]
- Shi, H.; Zhou, C.; Li, Y.; Wang, G.; Sun, Y. Anti-inflammatory effect of aconitines. China J. Chin. Mater. Med. 1990, 15, 174–177, 192. [Google Scholar]
- Kang, D.G.; Choi, D.H.; Lee, J.K.; Lee, Y.J.; Moon, M.K.; Yang, S.N.; Kwon, T.O.; Kwon, J.W.; Kim, J.S.; Lee, H.S. Endothelial no/cgmp-dependent vascular relaxation of cornuside isolated from the fruit of cornus officinalis. Planta. Med. 2007, 73, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Chung, K.S.; An, H.J. Anti-proliferation effects of cistanches salsa on the progression of benign prostatic hyperplasia. Can. J. Physiol. Pharmacol. 2016, 94, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.M.; Chang, M.S.; Park, S.K. Effects of psoralea corylifolia on the camp-responsive element modulator (crem) expression and spermatogenesis in rats. J. Ethnopharmacol. 2008, 117, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ye, Q.; Tan, X.; Jiang, H.; Li, X.; Chen, K.; Kinghorn, A.D. Three new sesquiterpene glycosides from dendrobium nobile with immunomodulatory activity. J. Nat. Prod. 2001, 64, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.J.; Lu, R.K.; Yu, L.H. Effects of semen cuscutae, rhizoma curculiginis, radix morindae officinalis on human spermatozoan’s motility and membrane function in vitro. Chin. J. Integr. Tradit. West. Med. 1997, 17, 145–147. [Google Scholar]
- Yen, F.L.; Wu, T.H.; Lin, L.T.; Lin, C.C. Hepatoprotective and antioxidant effects of cuscuta chinensis against acetaminophen-induced hepatotoxicity in rats. J. Ethnopharmacol. 2007, 111, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.C.; Baquer, N.Z. Pharmacological effects of trigonella foenum-graecum l. In health and disease. Pharm. Biol. 2014, 52, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, Y.; Guo, S.; Gu, H.; Yuan, Q.; Xie, X. Testosterone-induced benign prostatic hyperplasia rat and dog as facile models to assess drugs targeting lower urinary tract symptoms. PLoS ONE 2018, 13, e0191469. [Google Scholar] [CrossRef] [PubMed]
- Mosli, H.H.; Esmat, A.; Atawia, R.T.; Shoieb, S.M.; Mosli, H.A.; Abdel-Naim, A.B. Metformin attenuates testosterone-induced prostatic hyperplasia in rats: A pharmacological perspective. Sci. Rep. 2015, 5, 15639. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Mizokami, A.; Lin, W.J.; Lai, K.P.; Chang, C. Androgen receptor roles in the development of benign prostate hyperplasia. Am. J. Pathol. 2013, 182, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Steers, W.D. 5α-reductase activity in the prostate. Urology 2001, 58, 17–24. [Google Scholar] [CrossRef]
- Kyprianou, N.; Tu, H.; Jacobs, S.C. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum. Pathol. 1996, 27, 668–675. [Google Scholar] [CrossRef]
- Wang, X.; Lin, W.J.; Izumi, K.; Jiang, Q.; Lai, K.P.; Xu, D.; Fang, L.Y.; Lu, T.; Li, L.; Xia, S.; et al. Increased infiltrated macrophages in benign prostatic hyperplasia (bph): Role of stromal androgen receptor in macrophage-induced prostate stromal cell proliferation. J. Biol. Chem. 2012, 287, 18376–18385. [Google Scholar] [CrossRef] [PubMed]
- Rick, F.G.; Schally, A.V.; Block, N.L.; Nadji, M.; Szepeshazi, K.; Zarandi, M.; Vidaurre, I.; Perez, R.; Halmos, G.; Szalontay, L. Antagonists of growth hormone-releasing hormone (ghrh) reduce prostate size in experimental benign prostatic hyperplasia. Proc. Natl. Acad. Sci. USA 2011, 108, 3755–3760. [Google Scholar] [CrossRef] [PubMed]
- Vela-Navarrete, R.; Escribano-Burgos, M.; Farre, A.L.; Garcia-Cardoso, J.; Manzarbeitia, F.; Carrasco, C. Serenoa repens treatment modifies bax/bcl-2 index expression and caspase-3 activity in prostatic tissue from patients with benign prostatic hyperplasia. J. Urol. 2005, 173, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Lilja, H. Biology of prostate-specific antigen. Urology 2003, 62, 27–33. [Google Scholar] [CrossRef]
- Siiteri, P.K.; Wilson, J.D. Dihydrotestosterone in prostatic hypertrophy. I. The formation and content of dihydrotestosterone in the hypertrophic prostate of man. J. Clin. Investig. 1970, 49, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Ishibe, T.; Hirayama, M.; Fukushige, M.; Takenaka, I.; Kazuta, M. Basic studies on the prostate of rat under various hormonal environment. Endocrinol. Jpn. 1965, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pandita, R.K.; Persson, K.; Hedlund, P.; Andersson, K.E. Testosterone-induced prostatic growth in the rat causes bladder overactivity unrelated to detrusor hypertrophy. Prostate 1998, 35, 102–108. [Google Scholar] [CrossRef]
- Lepor, H. Pathophysiology, epidemiology, and natural history of benign prostatic hyperplasia. Rev. Urol 2004, 6, S3–S10. [Google Scholar] [PubMed]
- Wu, X.; Gu, Y.; Li, L. The anti-hyperplasia, anti-oxidative and anti-inflammatory properties of qing ye dan and swertiamarin in testosterone-induced benign prostatic hyperplasia in rats. Toxicol. Lett. 2017, 265, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Uygur, M.C.; Gur, E.; Arik, A.I.; Altug, U.; Erol, D. Erectile dysfunction following treatments of benign prostatic hyperplasia: A prospective study. Andrologia 1998, 30, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Bauman, T.M.; Nicholson, T.M.; Abler, L.L.; Eliceiri, K.W.; Huang, W.; Vezina, C.M.; Ricke, W.A. Characterization of fibrillar collagens and extracellular matrix of glandular benign prostatic hyperplasia nodules. PLoS ONE 2014, 9, e109102. [Google Scholar] [CrossRef] [PubMed]
- Minutoli, L.; Rinaldi, M.; Marini, H.; Irrera, N.; Crea, G.; Lorenzini, C.; Puzzolo, D.; Valenti, A.; Pisani, A.; Adamo, E.B.; et al. Apoptotic pathways linked to endocrine system as potential therapeutic targets for benign prostatic hyperplasia. Int. J. Mol. Sci. 2016, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lim, H.J.; Kim, M.S.; Lee, M.S. Dietary supplements for benign prostatic hyperplasia: An overview of systematic reviews. Maturitas 2012, 73, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Braz. J. Med. Biol. Res. 2000, 33, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Fibbi, B.; Penna, G.; Morelli, A.; Adorini, L.; Maggi, M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int. J. Androl. 2010, 33, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Iacopino, F.; Angelucci, C.; Lama, G.; Zelano, G.; La Torre, G.; D’Addessi, A.; Giovannini, C.; Bertaccini, A.; Macaluso, M.P.; Martorana, G.; et al. Apoptosis-related gene expression in benign prostatic hyperplasia and prostate carcinoma. Anticancer Res. 2006, 26, 1849–1854. [Google Scholar] [PubMed]
- Tanguay, S.; Awde, M.; Brock, G.; Casey, R.; Kozak, J.; Lee, J.; Nickel, J.C.; Saad, F. Diagnosis and management of benign prostatic hyperplasia in primary care. Can. Urol. Assoc. J 2009, 3, S92–S100. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Yuan, Y.; Geng, H.; Xia, S.J. Influence of immune inflammation on androgen receptor expression in benign prostatic hyperplasia tissue. Asian. J. Androl. 2012, 14, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bergh, A.; Damber, J.E. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, bcl-2, and cell proliferation in the glandular epithelium. Prostate 2004, 61, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Novara, G.; Galfano, A.; Berto, R.B.; Ficarra, V.; Navarrete, R.V.; Artibani, W. Inflammation, apoptosis, and bph: What is the evidence? Eur. Urol. Suppl. 2006, 5, 401–409. [Google Scholar] [CrossRef]
- Banerjee, P.P.; Banerjee, S.; Brown, T.R. Bcl-2 protein expression correlates with cell survival and androgen independence in rat prostatic lobes. Endocrinology 2002, 143, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, P.J.; Dits, N.F.; Kokame, K.; Veldhoven, A.; van Weerden, W.M.; Bangma, C.H.; Trapman, J.; Jenster, G. Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res. 2006, 66, 5012–5020. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Peng, J.; He, H.; Wu, D.; Han, Z.; Bi, X.; Dai, Q. Ki-67 and pcna expression in prostate cancer and benign prostatic hyperplasia. Clin. Invest Med. 2008, 31, E8–E15. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
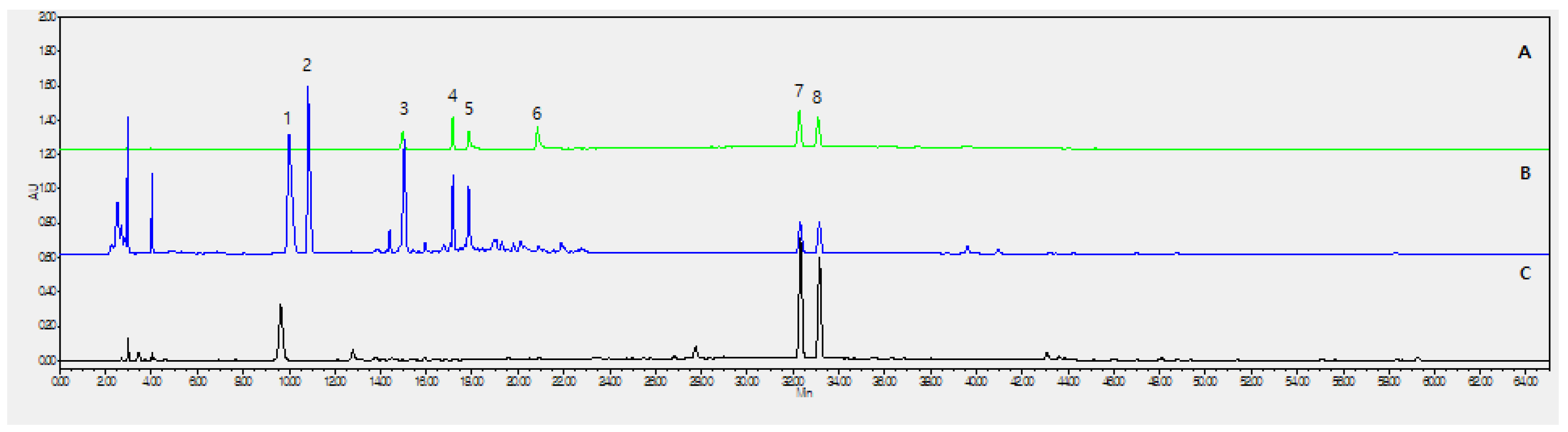

| No. | Analyte | Purity | Contents (mg/g) |
|---|---|---|---|
| (3) | Morroniside | 97.0% | 5.299 ± 0.530 |
| (4) | Loganin | 97.0% | 2.547 ± 0.245 |
| (5) | Echinacoside | 98.0% | 1.958 ± 0.168 |
| (6) | Acteoside | 99.0% | 0.554 ± 0.004 |
| (7) | Psoralen | 99.0% | 2.040 ± 0.011 |
| (8) | Isopsoralen (Angelicin) | 95.0% | 2.495 ± 0.025 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, B.-R.; Kim, H.-J.; Park, S.-K.; Kim, M.-S.; Lee, K.-H.; Yoon, I.-J.; An, H.-J. Anti-Proliferative Effects of HBX-5 on Progression of Benign Prostatic Hyperplasia. Molecules 2018, 23, 2638. https://doi.org/10.3390/molecules23102638
Jin B-R, Kim H-J, Park S-K, Kim M-S, Lee K-H, Yoon I-J, An H-J. Anti-Proliferative Effects of HBX-5 on Progression of Benign Prostatic Hyperplasia. Molecules. 2018; 23(10):2638. https://doi.org/10.3390/molecules23102638
Chicago/Turabian StyleJin, Bo-Ram, Hyo-Jung Kim, Sang-Kyun Park, Myoung-Seok Kim, Kwang-Ho Lee, Il-Joo Yoon, and Hyo-Jin An. 2018. "Anti-Proliferative Effects of HBX-5 on Progression of Benign Prostatic Hyperplasia" Molecules 23, no. 10: 2638. https://doi.org/10.3390/molecules23102638
APA StyleJin, B.-R., Kim, H.-J., Park, S.-K., Kim, M.-S., Lee, K.-H., Yoon, I.-J., & An, H.-J. (2018). Anti-Proliferative Effects of HBX-5 on Progression of Benign Prostatic Hyperplasia. Molecules, 23(10), 2638. https://doi.org/10.3390/molecules23102638