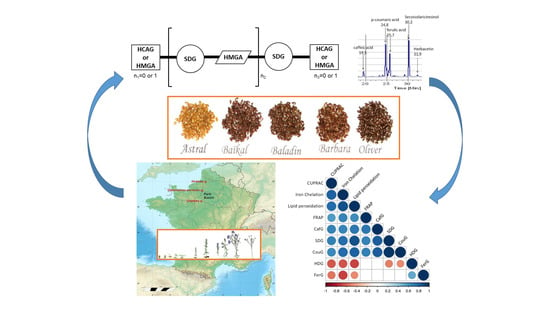
Insight into the Influence of Cultivar Type, Cultivation Year, and Site on the Lignans and Related Phenolic Profiles, and the Health-Promoting Antioxidant Potential of Flax (Linum usitatissimum L.) Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials and Cultivation
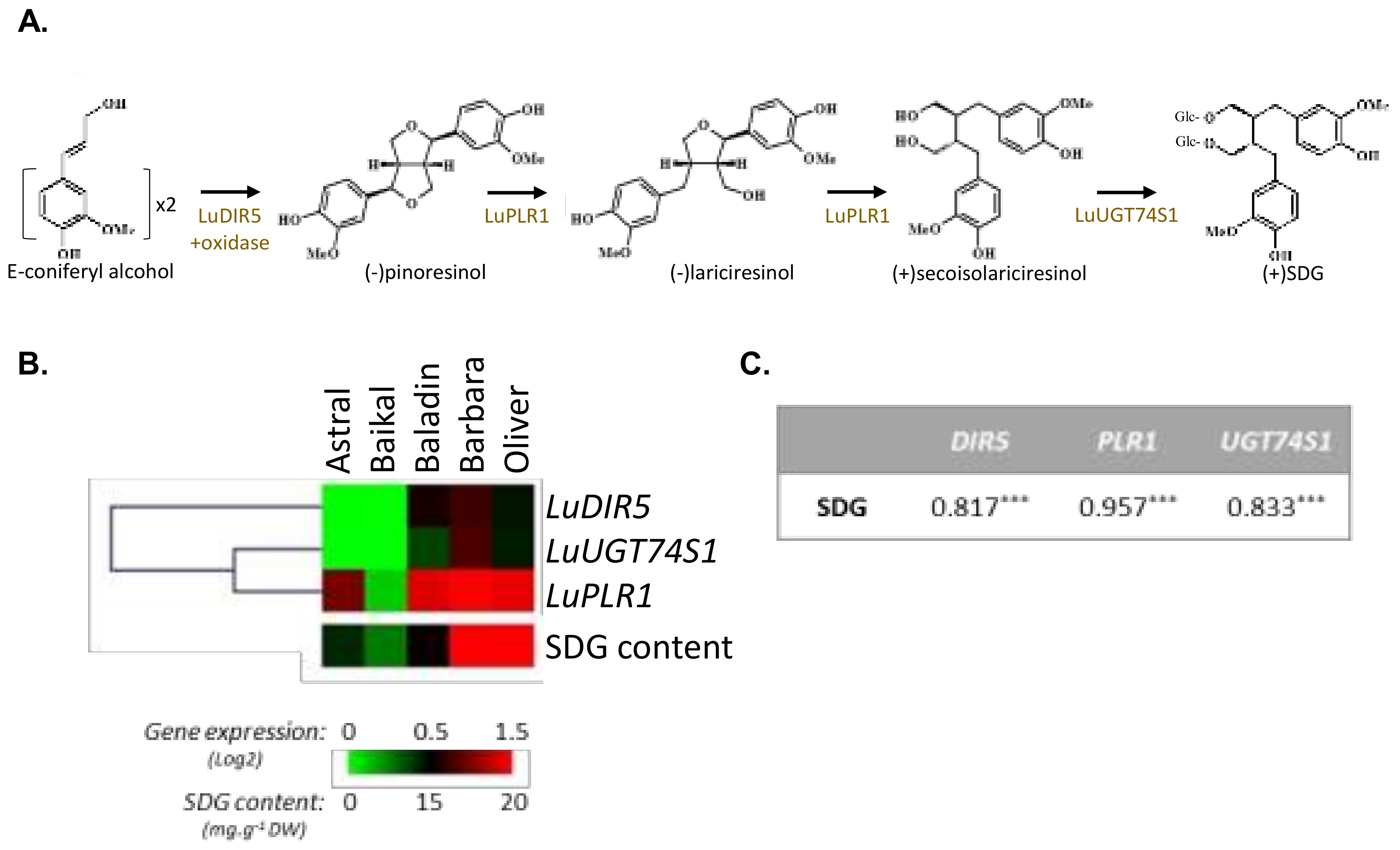
2.3. Gene Expression Analysis by RT-qPCR
2.4. Extraction, HPLC, and LC-ESI-MS Analysis
2.5. Determination of the Ferric-Reducing Antioxidant Power (FRAP)
2.6. Determination of Oxygen Radical Absorbance Capacity (ORAC)
2.7. Determination of the Iron-Chelating Capacity
2.8. Yeast Cells Cultivation and Treatments
2.9. Determination of Membrane Lipid Peroxidation Using Thiobarbituric Acid-Reactive Substances (TBARS) Assay
2.10. Statistical Treatment of Data
3. Results and Discussion
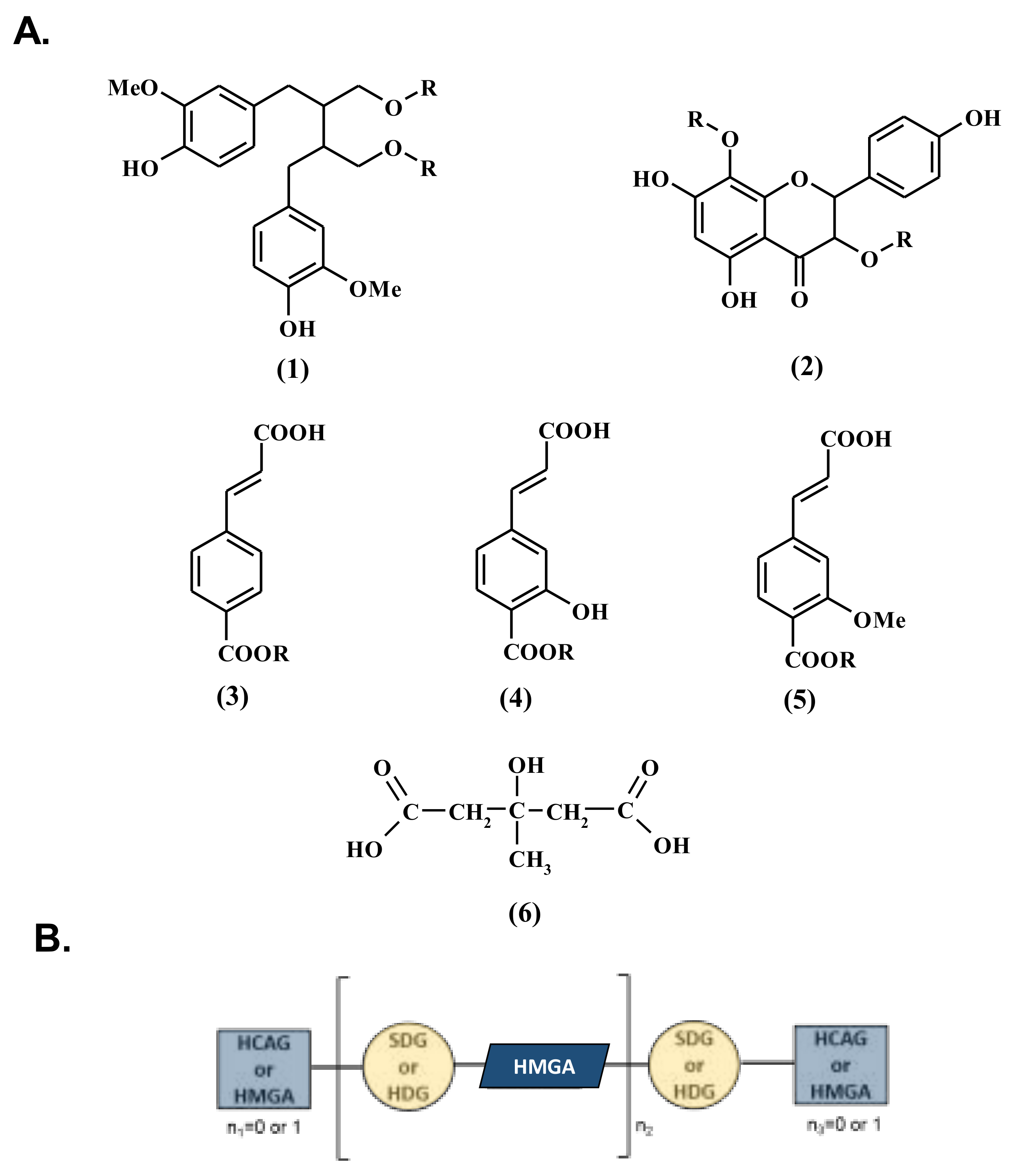
3.1. Influence of Genetic Variations on the Accumulation of the Main Constituents of the Lignan Macromolecule
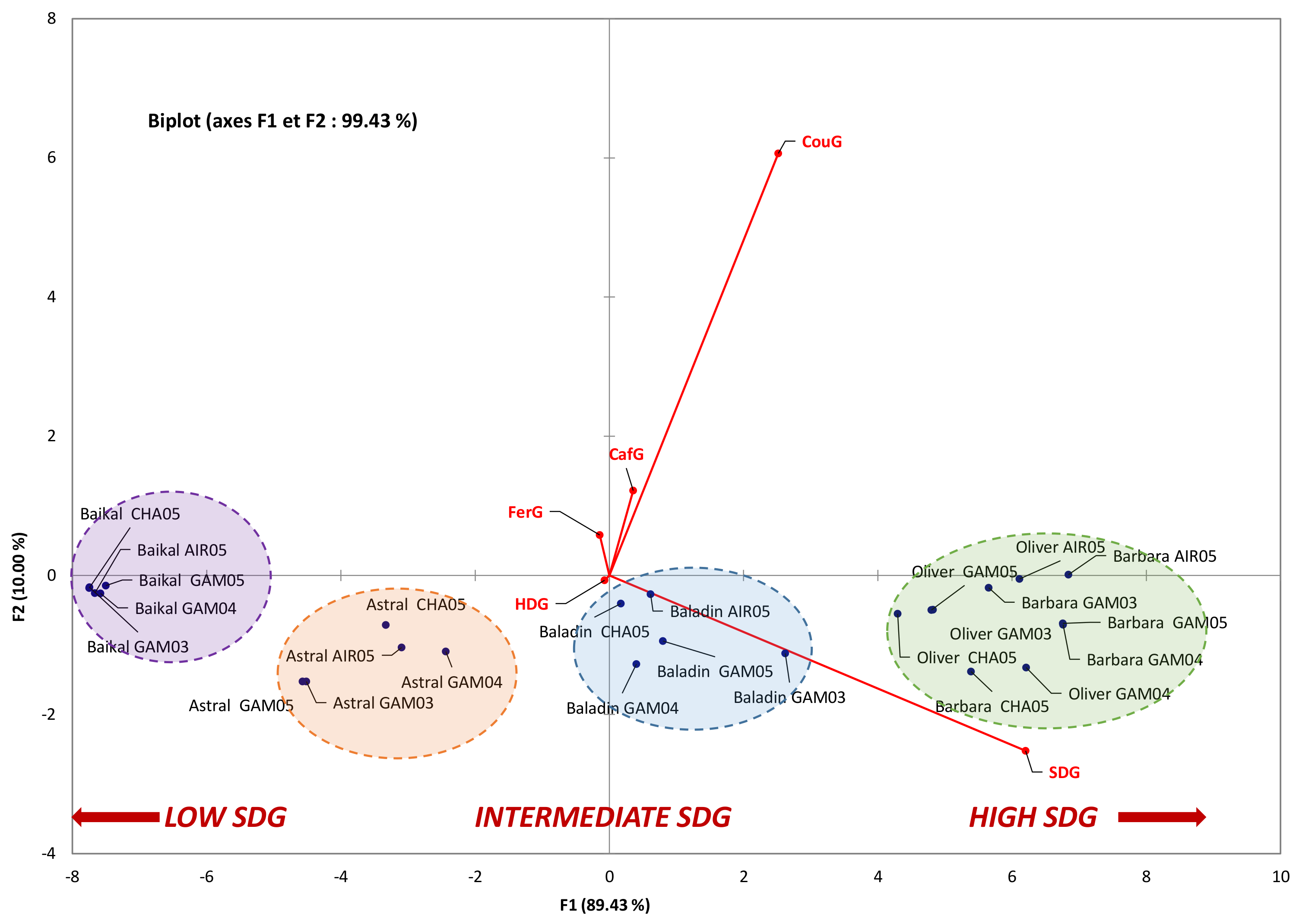
3.2. Influence of Geographic Parameters on the Accumulation of the Main Constituents of Lignan Macromolecule
3.3. Evaluation and Comparison of In Vitro and In Vivo Antioxidant Capacities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–919. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D. Flaxseed as a functional food source. J. Sci. Food Agric. 2001, 81, 889–894. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Peerlkamp, N.; Johnsson, P.; Andersson, R.; Andersson, R.E.; Lundgren, L.N.; Åman, P. An oligomer from flaxseed composed of secoisolariciresinoldiglucoside and 3-hydroxy-3-methyl glutaric acid residues. Phytochemistry 2001, 58, 587–590. [Google Scholar] [CrossRef]
- Struijs, K.; Vincken, J.-P.; Doeswijk, T.G.; Voragen, A.G.J.; Gruppen, H. The chain length of lignan macromolecule from flaxseed hulls is determined by the incorporation of coumaric acid glucosides and ferulic acid glucosides. Phytochemistry 2009, 70, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Westcott, N.; Muir, A. Flax seed lignan in disease prevention and health promotion. Phytochem. Rev. 2003, 2, 401–417. [Google Scholar] [CrossRef]
- McCann, M.J.; Gill, C.I.R.; McGlynn, H.; Rowland, I.R. Role of Mammalian Lignans in the Prevention and Treatment of Prostate Cancer Mark. Nutr. Cancer 2005, 52, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lainé, E.; Hano, C.; Lamblin, F.F. Phytoestrogens: Lignans; Knasmüller, S., DeMarini, D.M., Johnson, I., Gerhäuser, C., Eds.; WILEY-VCH: Weinheim, Germany, 2009; ISBN 9783527320585. [Google Scholar]
- Hano, C.; Renouard, S.; Molinié, R.; Corbin, C.; Barakzoy, E.; Doussot, J.; Lamblin, F.; Lainé, E. Flaxseed (Linum usitatissimum L.) extract as well as (+)-secoisolariciresinol diglucoside and its mammalian derivatives are potent inhibitors of α-amylase activity. Bioorg. Med. Chem. Lett. 2013, 23, 3007–3012. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K. Hydroxyl radical-scavenging property of secoisolariciresinol diglucoside (SDG) isolated from flax-seed. Mol. Cell. Biochem. 1997, 168, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Thompson, L.U. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol. Cell. Biochem. 1999, 202, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Hano, C.; Corbin, C.; Drouet, S.; Quéro, A.; Rombaut, N.; Savoire, R.; Molinié, R.; Thomasset, B.; Mesnard, F.; Lainé, E. The lignan (+)-secoisolariciresinol extracted from flax hulls is an effective protectant of linseed oil and its emulsion against oxidative damage. Eur. J. Lipid Sci. Technol. 2017, 119. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Mousavi, Y.; Clark, J.; Höckerstedt, K.; Hämäläinen, E.; Wähälä, K.; Mäkela, T.; Hase, T. Dietary phytoestrogen and cancer: In vitro and In vivo studies. J. Steroid Biochem. Mol. Biol. 1992, 41, 8012–8020. [Google Scholar] [CrossRef]
- Schoenrock, U.; Untiedt, S.; Kux, U.; Inoue, K. Application of Ferulic Acid Glucosides as Anti-irritants in Cosmetic and Topical Dermatological Preparations. German Patent AN 1997:708582, 1997. [Google Scholar]
- Kosinska, A.; Penkacik, K.; Wiczkowski, W.; Amarowicz, R. Presence of caffeic acid in flaxseed lignan macromolecule. Plant Foods Hum. Nutr. 2011, 66, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Beejmohun, V.; Grand, E.; Mesnard, F.; Fliniaux, M.A.; Kovensky, J. First synthesis of (1,2-13C2)-monolignol glucosides. Tetrahedron Lett. 2004, 45, 8745–8747. [Google Scholar] [CrossRef]
- Beejmohun, V.; Grand, E.; Lesur, D.; Mesnard, F.; Fliniaux, M.A.; Kovensky, J. Synthesis and purification of [1,2-13C2]coniferin. J. Label. Compd. Radiopharm. 2006, 49, 463–470. [Google Scholar] [CrossRef]
- Hano, C.; Martin, I.; Fliniaux, O.; Legrand, B.; Gutierrez, L.; Arroo, R.R.J.; Mesnard, F.; Lamblin, F.; Lainé, E. Pinoresinol-lariciresinol reductase gene expression and secoisolariciresinol diglucoside accumulation in developing flax (Linum usitatissimum) seeds. Planta 2006, 224, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Dalisay, D.S.; Kim, K.W.; Lee, C.; Yang, H.; Rübel, O.; Bowen, B.P.; Davin, L.B.; Lewis, N.G. Dirigent Protein-Mediated Lignan and Cyanogenic Glucoside Formation in Flax Seed: Integrated Omics and MALDI Mass Spectrometry Imaging. J. Nat. Prod. 2015, 78, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Huis, R.; Hawkins, S.; Neutelings, G. Selection of reference genes for quantitative gene expression normalization in flax (Linum usitatissimum L.). BMC Plant Biol. 2010, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Corbin, C.; Fidel, T.; Leclerc, E.A.; Barakzoy, E.; Sagot, N.; Falguiéres, A.; Renouard, S.; Blondeau, J.; Ferroud, C.; Doussot, J.; et al. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason. Sonochem. 2015, 26, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Mladěnka, P.; MacÁková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Bisquert, R.; Muñiz-Calvo, S.; Guillamón, J.M. Protective role of intracellular Melatonin against oxidative stress and UV radiation in Saccharomyces cerevisiae. Front. Microbiol. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hano, C.; Addi, M.; Fliniaux, O.; Bensaddek, L.; Duverger, E.; Mesnard, F.; Lamblin, F.; Lainé, E. Molecular characterization of cell death induced by a compatible interaction between Fusarium oxysporum f. sp. linii and flax (Linum usitatissimum) cells. Plant Physiol. Biochem. 2008, 46, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, A.; Fu, Y.B. Flax Genetic Diversity as the Raw Material for Future Success. In Proceedings of the International Conference on Flax and Other Bast Plants, Saskatoon, SK, Canada, 21–23 July 2008; pp. 270–279. [Google Scholar]
- Zimmermann, R.; Bauermann, U.; Morales, F. Effects of growing site and nitrogen fertilization on biomass production and lignan content of linseed (Linum usitatissimum L.). J. Sci. Food Agric. 2006, 86, 415–419. [Google Scholar] [CrossRef]
- Zimmermann, R.; Bauermann, U.; Spedding, C. Effects of nitrogen fertilisation and two growing sites on biomass production and lignan content of linseed (Linum usitatissimum L.): Second year. Acta Agron. Hungarica 2007, 55, 173–181. [Google Scholar] [CrossRef]
- Li, H.B.; Wong, C.C.; Cheng, K.W.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT-Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Struijs, K.; Vincken, J.P.; Verhoef, R.; van Oostveen-van Casteren, W.H.M.; Voragen, A.G.J.; Gruppen, H. The flavonoid herbacetin diglucoside as a constituent of the lignan macromolecule from flaxseed hulls. Phytochemistry 2007, 68, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Struijs, K.; Vincken, J.P.; Verhoef, R.; Voragen, A.G.J.; Gruppen, H. Hydroxycinnamic acids are ester-linked directly to glucosyl moieties within the lignan macromolecule from flaxseed hulls. Phytochemistry 2008, 69, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Westcott, N.D.; Muir, A.D. Variation in the concentration of the flax seed lignan concentration with variety, location and year. In Proceedings of the 56th Flax Institute of the United States Conference, Fargo, ND, USA, 20–22 March 1996; pp. 77–80. [Google Scholar]
- Johnsson, P.; Kamal-Eldin, A.; Lundgren, L.N.; Aman, P. HPLC method for analysis of secoisolariciresinol diglucoside in flaxseeds. J. Agric. Food Chem. 2000, 48, 5216–5219. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, C.; Kamal-Eldin, A.; Andersson, R.; Aman, P. High-performance liquid chromatographic analysis of secoisolariciresinol diglucoside and hydroxycinnamic acid glucosides in flaxseed by alkaline extraction. J. Chromatogr. A 2003, 1012, 151–159. [Google Scholar] [CrossRef]
- Ramsay, A.; Fliniaux, O.; Fang, J.; Molinie, R.; Roscher, A.; Grand, E.; Guillot, X.; Kovensky, J.; Fliniaux, M.A.; Schneider, B.; et al. Development of an NMR metabolomics-based tool for selection of flaxseed varieties. Metabolomics 2014, 10, 1258–1267. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Qiu, C.; Ye, Y.; Guo, X.; Chen, G.; Li, T.; Wang, Y.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles and health benefits in fiber and oil flaxseeds (Linum usitatissimum L.). Food Chem. 2017, 214, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Corbin, C.; Drouet, S.; Markulin, L.; Auguin, D.; Lainé, É.; Davin, L.B.; Cort, J.R.; Lewis, N.G.; Hano, C. A genome-wide analysis of the flax (Linum usitatissimum L.) dirigent protein family: From gene identification and evolution to differential regulation. Plant Mol. Biol. 2018, 97, 73–101. [Google Scholar] [CrossRef] [PubMed]
- von Heimendahl, C.B.I.; Schäfer, K.M.; Eklund, P.; Sjöholm, R.; Schmidt, T.J.; Fuss, E. Pinoresinol–lariciresinol reductases with different stereospecificity from Linum album and Linum usitatissimum. Phytochemistry 2005, 66, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Renouard, S.; Tribalatc, M.; Lamblin, F.; Mongelard, G.; Fliniaux, O.; Corbin, C.; Marosevic, D.; Pilard, S.; Demailly, H.; Gutierrez, L.; et al. RNAi-mediated pinoresinol lariciresinol reductase gene silencing in flax (Linum usitatissimum L.) seed coat: Consequences on lignans and neolignans accumulation. J. Plant Physiol. 2014, 171, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Ghose, K.; Selvaraj, K.; McCallum, J.; Kirby, C.W.; Sweeney-Nixon, M.; Cloutier, S.J.; Deyholos, M.; Datla, R.; Fofana, B. Identification and functional characterization of a flax UDP-glycosyltransferase glucosylating secoisolariciresinol (SECO) into secoisolariciresinol monoglucoside (SMG) and diglucoside (SDG). BMC Plant Biol. 2014, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Fofana, B.; Ghose, K.; McCallum, J.; You, F.M.; Cloutier, S. UGT74S1 is the key player in controlling secoisolariciresinol diglucoside (SDG) formation in flax. BMC Plant Biol. 2017, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.D.; Huang, K.; Wang, H.; Davin, L.B.; Lewis, N.G. Biosynthetic Pathway to the Cancer Chemopreventive Secoisolariciresinol Diglucoside-Hydroxymethyl Glutaryl Ester-Linked Lignan Oligomers in Flax (Linum usitatissimum) Seed. J. Nat. Prod. 2001, 2, 1388–1397. [Google Scholar] [CrossRef]
- Żuk, M.; Kulma, A.; Dymińska, L.; Szołtysek, K.; Prescha, A.; Hanuza, J.; Szopa, J. Flavonoid engineering of flax potentiate its biotechnological application. BMC Biotechnol. 2011, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Dave Oomah, B.; Mazza, G.; Kenaschuk, E.O. Flavonoid content of flaxseed. Influence of cultivar and environment. Euphytica 1996, 90, 163–167. [Google Scholar] [CrossRef]
- Saastamoinen, M.; Pihlava, J.M.; Eurola, M.; Klemola, A.; Jauhiainen, L.; Hietaniemi, V. Yield, SDG lignan, cadmium, lead, oil and protein contents of linseed (Linum usitatissimum L.) cultivated in trials and at different farm conditions in the south-western part of Finland. Agric. Food Sci. 2013, 22, 296–306. [Google Scholar] [CrossRef]
- Westcott, N.D.; Muir, A.D.; Lafond, G.; McAndrew, D.W.; May, W.; Irvine, B.; Grant, C.; Shirtliffe, S.; Bruulsema, T.W. Factors Affecting the Concentration of a Nutraceutical Lignan in Flaxseed. In Proceedings of the Symposium on Fertilizing Crops for Functional Food, Indianapolis, IN, USA, 11 November 2002; pp. 1–3. [Google Scholar]
- Podloucká, P.; Berka, K.; Fabre, G.; Paloncýová, M.; Duroux, J.L.; Otyepka, M.; Trouillas, P. Lipid bilayer membrane affinity rationalizes inhibition of lipid peroxidation by a natural lignan antioxidant. J. Phys. Chem. B 2013, 117, 5043–5049. [Google Scholar] [CrossRef] [PubMed]
- Donoso-Fierro, C.; Becerra, J.; Bustos-Concha, E.; Silva, M. Chelating and antioxidant activity of lignans from Chilean woods (Cupressaceae). Holzforschung 2009, 63, 559–563. [Google Scholar] [CrossRef]
- Fucassi, F.; Heikal, A.; Mikhalovska, L.I.; Standen, G.; Allan, I.U.; Mikhalovsky, S.V.; Cragg, P.J. Metal chelation by a plant lignan, secoisolariciresinol diglucoside. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 345–351. [Google Scholar] [CrossRef]
- Steels, E.L.; Learmonth, R.P.; Watson, K. Stress tolerance and membrane lipid unsaturation in Saccharomyces cerevisiae grown aerobically or anaerobically. Microbiology 1994, 140, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Wolak, N.; Kowalska, E.; Kozik, A.; Rapala-Kozik, M. Thiamine increases the resistance of baker’s yeast Saccharomyces cerevisiae against oxidative, osmotic and thermal stress, through mechanisms partly independent of thiamine diphosphate-bound enzymes. FEMS Yeast Res. 2014, 14, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
| Cultivar | Location_Year | SDG a | HDG a | FerG a | CouG a | CafG a |
|---|---|---|---|---|---|---|
| Astral | AIR_05 | 12.85 ± 0.14 | 0.98 ± 0.06 | 1.65 ± 0.06 | 5.88 ± 0.09 | 0.85 ± 0.04 |
| CHA_05 | 12.53 ± 0.11 | 1.05 ± 0.03 | 1.90 ± 0.08 | 6.05 ± 0.08 | 0.98 ± 0.06 | |
| GAM_03 | 11.73 ± 0.06 | 0.88 ± 0.04 | 1.58 ± 0.07 | 4.80 ± 0.08 | 1.23 ± 0.06 | |
| GAM_04 | 11.68 ± 0.09 | 1.10 ± 0.05 | 1.58 ± 0.04 | 4.78 ± 0.05 | 1.25 ± 0.05 | |
| GAM_05 | 13.48 ± 0.13 | 0.85 ± 0.01 | 1.57 ± 0.02 | 6.07 ± 0.22 | 0.87 ± 0.02 | |
| Barbara | AIR_05 | 21.68 ± 0.17 | 0.93 ± 0.06 | 1.95 ± 0.03 | 10.48 ± 0.12 | 1.83 ± 0.04 |
| CHA_05 | 20.88 ± 0.07 | 1.05 ± 0.03 | 2.18 ± 0.07 | 8.63 ± 0.65 | 1.63 ± 0.04 | |
| GAM_03 | 20.63 ± 0.10 | 0.95 ± 0.03 | 1.78 ± 0.03 | 9.95 ± 0.11 | 1.33 ± 0.06 | |
| GAM_04 | 21.85 ± 0.34 | 0.85 ± 0.03 | 1.78 ± 0.02 | 9.85 ± 0.26 | 1.53 ± 0.06 | |
| GAM_05 | 21.85 ± 0.81 | 0.88 ± 0.01 | 1.66 ± 0.01 | 9.84 ± 0.07 | 1.53 ± 0.01 | |
| Baladin | AIR_05 | 16.03 ± 0.11 | 1.15 ± 0.03 | 2.10 ± 0.05 | 7.88 ± 0.07 | 1.48 ± 0.07 |
| CHA_05 | 15.68 ± 0.19 | 1.18 ± 0.03 | 2.28 ± 0.07 | 7.58 ± 0.10 | 1.43 ± 0.09 | |
| GAM_03 | 18.20 ± 0.38 | 1.03 ± 0.03 | 2.03 ± 0.04 | 7.88 ± 0.12 | 1.33 ± 0.02 | |
| GAM_04 | 16.45 ± 0.13 | 1.08 ± 0.07 | 2.08 ± 0.02 | 7.33 ± 0.21 | 1.40 ± 0.08 | |
| GAM_05 | 16.20 ± 0.38 | 0.95 ± 0.01 | 1.92 ± 0.01 | 6.91 ± 0.04 | 1.19 ± 0.01 | |
| Baïkal | AIR_05 | 8.33 ± 0.13 | 1.03 ± 0.04 | 1.75 ± 0.07 | 4.85 ± 0.07 | 0.85 ± 0.01 |
| CHA_05 | 8.23 ± 0.07 | 1.18 ± 0.02 | 1.75 ± 0.05 | 4.85 ± 0.06 | 1.05 ± 0.03 | |
| GAM_03 | 8.23 ± 0.17 | 1.15 ± 0.05 | 1.88 ± 0.07 | 4.90 ± 0.07 | 0.80 ± 0.05 | |
| GAM_04 | 8.45 ± 0.10 | 1.05 ± 0.08 | 1.98 ± 0.03 | 4.98 ± 0.08 | 0.98 ± 0.04 | |
| GAM_05 | 8.40 ± 0.04 | 0.98 ± 0.01 | 1.79 ± 0.02 | 4.88 ± 0.05 | 0.83 ± 0.01 | |
| Oliver | AIR_05 | 21.00 ± 0.25 | 0.75 ± 0.03 | 1.55 ± 0.03 | 10.15 ± 0.09 | 1.90 ± 0.05 |
| CHA_05 | 19.50 ± 0.19 | 0.85 ± 0.03 | 1.33 ± 0.06 | 9.03 ± 0.26 | 1.75 ± 0.06 | |
| GAM_03 | 19.95 ± 0.11 | 0.98 ± 0.04 | 1.18 ± 0.06 | 9.38 ± 0.07 | 1.33 ± 0.06 | |
| GAM_04 | 19.93 ± 0.11 | 1.03 ± 0.07 | 1.03 ± 0.03 | 9.35 ± 0.09 | 1.45 ± 0.05 | |
| GAM_05 | 21.55 ± 0.37 | 0.83 ± 0.01 | 1.06 ± 0.01 | 9.12 ± 0.08 | 1.37 ± 0.01 | |
| F values | Cultivar (C) | 284.62 *** | 4.06 * | 19.18 *** | 95.33 *** | 14.76 *** |
| Location (L) | 0.02 | 1.21 | 1.04 | 0.13 | 0.61 | |
| Year (Y) | 0.03 | 2.09 | 0.68 | 0.02 | 0.48 | |
| C × L | 194.69 *** | 3.57 * | 21.90 *** | 75.96 *** | 11.94 *** | |
| C × Y | 198.99 *** | 4.58 * | 17.25 *** | 59.81 *** | 11.19 *** | |
| Y × L | 0.02 | 1.484 | 0.536 | 0.062 | 0.441 | |
| C × L × Y | 148.70 *** | 4.23 * | 16.18 *** | 51.26 *** | 9.62 *** |
| Variables | SDG | HDG | FerG | CouG | CafG | FRAP | ORAC | Iron Chelation | MDA inhibition |
|---|---|---|---|---|---|---|---|---|---|
| SDG | |||||||||
| HDG | −0.515 ** | ||||||||
| FerG | −0.231 ns | 0.510 ** | |||||||
| CouG | 0.966 *** | −0.476 * | −0.215 ns | ||||||
| CafG | 0.835 *** | −0.360 ns | −0.063 ns | 0.832 *** | |||||
| FRAP | 0.676 *** | −0.624 *** | −0.545 ** | 0.639 *** | 0.671 *** | ||||
| ORAC | 0.669 *** | −0.325 ns | −0.170 ns | 0.676 *** | 0.717 *** | 0.573 ** | |||
| Iron Chelation | 0.758 *** | −0.627 *** | −0.692 *** | 0.768 *** | 0.661 *** | 0.817 *** | 0.665 *** | ||
| MDA inhibition | 0.867 *** | −0.666 *** | −0.482 * | 0.806 *** | 0.721 *** | 0.774 *** | 0.617 *** | 0.875 *** |
| Cultivar | Location_Year | FRAP a | ORAC a | Iron Chelation b | MDA Inhibition c |
|---|---|---|---|---|---|
| Astral | AIR_05 | 252.55 ± 2.45 | 281.55 ± 11.16 | 9.57 ± 0.25 | 37.10 ± 0.28 |
| CHA_05 | 264.41 ± 10.28 | 317.87 ± 8.00 | 10.11 ± 0.41 | 36.95 ± 0.43 | |
| GAM_03 | 322.81 ± 1.41 | 263.13 ± 11.91 | 9.66 ± 0.12 | 38.47 ± 0.75 | |
| GAM_04 | 276.68 ± 4.33 | 312.34 ± 1.12 | 9.31 ± 0.53 | 37.71 ± 1.18 | |
| GAM_05 | 252.55 ± 5.23 | 263.92 ± 4.56 | 9.57 ± 0.22 | 38.17 ± 1.07 | |
| Barbara | AIR_05 | 332.55 ± 4.57 | 339.45 ± 1.95 | 11.97 ± 0.19 | 45.95 ± 2.26 |
| CHA_05 | 317.61 ± 9.33 | 341.29 ± 16.09 | 11.35 ± 0.72 | 44.58 ± 1.08 | |
| GAM_03 | 292.81 ± 5.84 | 329.45 ± 8.65 | 11.79 ± 0.06 | 43.05 ± 3.23 | |
| GAM_04 | 296.41 ± 8.20 | 336.55 ± 2.14 | 12.32 ± 0.53 | 46.41 ± 0.43 | |
| GAM_05 | 331.48 ± 5.23 | 309.45 ± 3.72 | 10.99 ± 0.28 | 45.65 ± 1.94 | |
| Baladin | AIR_05 | 217.35 ± 4.67 | 286.82 ± 2.79 | 8.69 ± 0.31 | 33.44 ± 2.16 |
| CHA_05 | 240.55 ± 6.31 | 334.18 ± 5.95 | 8.24 ± 0.44 | 33.28 ± 0.97 | |
| GAM_03 | 254.01 ± 2.30 | 307.61 ± 4.18 | 8.16 ± 0.43 | 32.06 ± 0.64 | |
| GAM_04 | 288.41 ± 11.27 | 321.82 ± 2.60 | 8.87 ± 0.06 | 28.85 ± 0.43 | |
| GAM_05 | 255.08 ± 14.24 | 281.03 ± 2.70 | 7.80 ± 0.59 | 34.50 ± 2.16 | |
| Baïkal | AIR_05 | 262.01 ± 3.21 | 278.66 ± 5.76 | 8.42 ± 0.37 | 25.34 ± 1.18 |
| CHA_05 | 274.95 ± 3.49 | 281.03 ± 2.79 | 8.16 ± 0.40 | 24.89 ± 1.19 | |
| GAM_03 | 227.61 ± 1.41 | 305.24 ± 2.32 | 7.18 ± 0.56 | 23.66 ± 1.62 | |
| GAM_04 | 243.75 ± 5.42 | 269.97 ± 4.09 | 7.62 ± 0.55 | 21.68 ± 3.13 | |
| GAM_05 | 243.78 ± 1.27 | 286.29 ± 2.51 | 8.07 ± 0.12 | 26.11 ± 0.86 | |
| Oliver | AIR_05 | 350.68 ± 0.81 | 368.66 ± 21.39 | 14.45 ± 0.25 | 47.02 ± 2.16 |
| CHA_05 | 306.28 ± 5.27 | 374.45 ± 6.42 | 14.54 ± 0.44 | 46.87 ± 1.19 | |
| GAM_03 | 302.41 ± 6.17 | 278.92 ± 5.39 | 13.74 ± 0.38 | 43.21 ± 0.75 | |
| GAM_04 | 355.75 ± 8.20 | 326.55 ± 2.70 | 14.72 ± 0.47 | 45.34 ± 2.37 | |
| GAM_05 | 343.08 ± 5.28 | 375.76 ± 7.91 | 14.10 ± 0.38 | 46.87 ± 1.40 | |
| F values | Genetic (C) | 11.91 *** | 5.37 *** | 188.00 *** | 161.33 *** |
| Location (L) | 0.02 | 1.05 | 0.04 | 0.03 | |
| Year (Y) | 0.06 | 0.15 | 0.04 | 0.08 | |
| C × L | 7.20 ** | 4.59 ** | 133.03 *** | 105.31 *** | |
| C × Y | 7.34 ** | 3.40 * | 133.16 *** | 130.60 *** | |
| Y × L | 0.03 | 0.64 | 0.04 | 0.06 | |
| C × L × Y | 4.91 * | 3.41 * | 114.80 *** | 109.27 *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garros, L.; Drouet, S.; Corbin, C.; Decourtil, C.; Fidel, T.; Lebas de Lacour, J.; Leclerc, E.A.; Renouard, S.; Tungmunnithum, D.; Doussot, J.; et al. Insight into the Influence of Cultivar Type, Cultivation Year, and Site on the Lignans and Related Phenolic Profiles, and the Health-Promoting Antioxidant Potential of Flax (Linum usitatissimum L.) Seeds. Molecules 2018, 23, 2636. https://doi.org/10.3390/molecules23102636
Garros L, Drouet S, Corbin C, Decourtil C, Fidel T, Lebas de Lacour J, Leclerc EA, Renouard S, Tungmunnithum D, Doussot J, et al. Insight into the Influence of Cultivar Type, Cultivation Year, and Site on the Lignans and Related Phenolic Profiles, and the Health-Promoting Antioxidant Potential of Flax (Linum usitatissimum L.) Seeds. Molecules. 2018; 23(10):2636. https://doi.org/10.3390/molecules23102636
Chicago/Turabian StyleGarros, Laurine, Samantha Drouet, Cyrielle Corbin, Cédric Decourtil, Thibaud Fidel, Julie Lebas de Lacour, Emilie A. Leclerc, Sullivan Renouard, Duangjai Tungmunnithum, Joël Doussot, and et al. 2018. "Insight into the Influence of Cultivar Type, Cultivation Year, and Site on the Lignans and Related Phenolic Profiles, and the Health-Promoting Antioxidant Potential of Flax (Linum usitatissimum L.) Seeds" Molecules 23, no. 10: 2636. https://doi.org/10.3390/molecules23102636
APA StyleGarros, L., Drouet, S., Corbin, C., Decourtil, C., Fidel, T., Lebas de Lacour, J., Leclerc, E. A., Renouard, S., Tungmunnithum, D., Doussot, J., Abassi, B. H., Maunit, B., Lainé, É., Fliniaux, O., Mesnard, F., & Hano, C. (2018). Insight into the Influence of Cultivar Type, Cultivation Year, and Site on the Lignans and Related Phenolic Profiles, and the Health-Promoting Antioxidant Potential of Flax (Linum usitatissimum L.) Seeds. Molecules, 23(10), 2636. https://doi.org/10.3390/molecules23102636