Melamine Sponge Functionalized with Urea-Formaldehyde Co-Oligomers as a Sorbent for the Solid-Phase Extraction of Hydrophobic Analytes
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis Optimization
2.1.1. Effect of Formaldehyde-to-Urea Ratio
2.1.2. Effect of Concentrations of Urea and Formaldehyde
2.1.3. Number of Synthesized Sponge Cubes per Batch
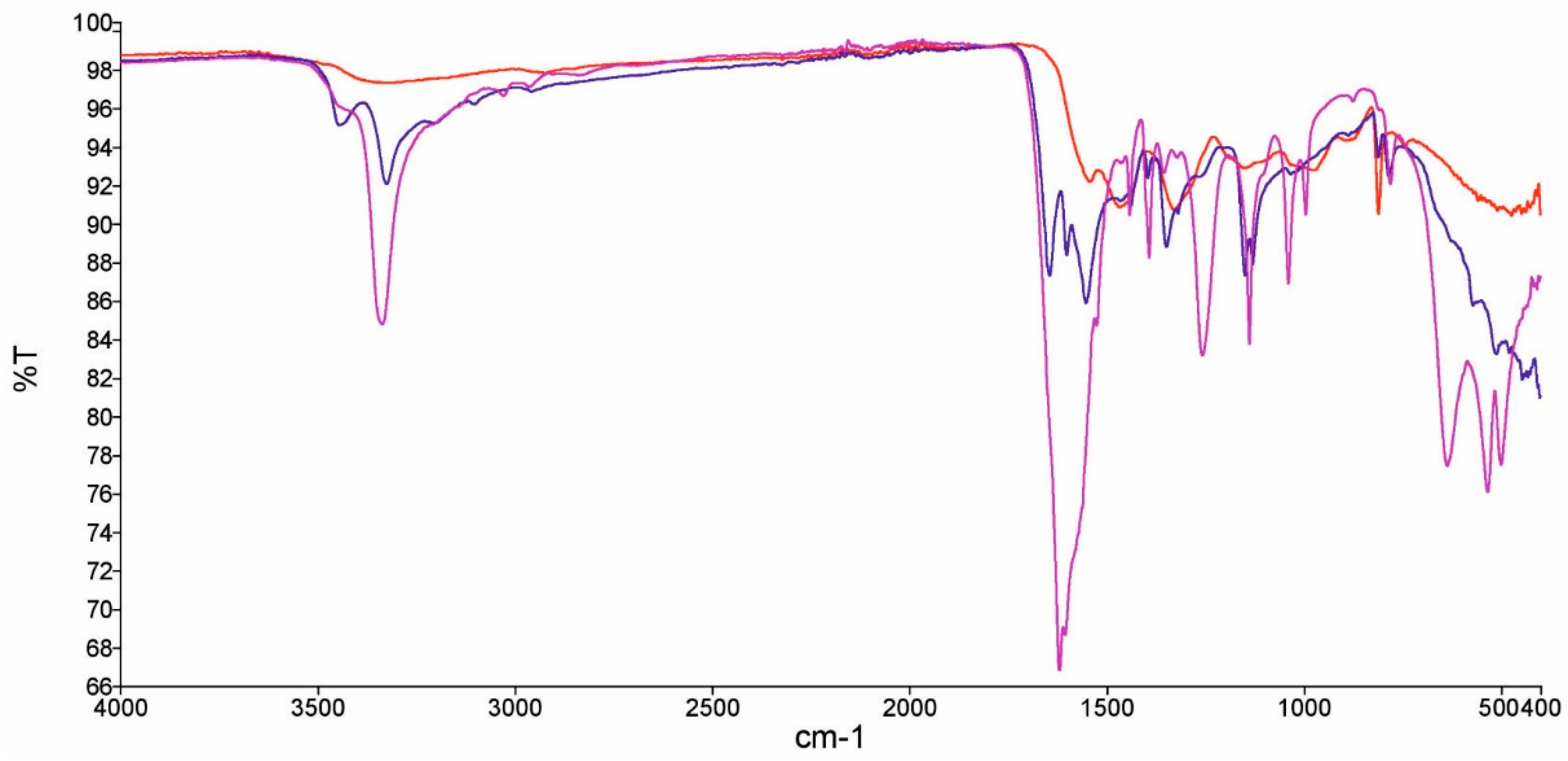
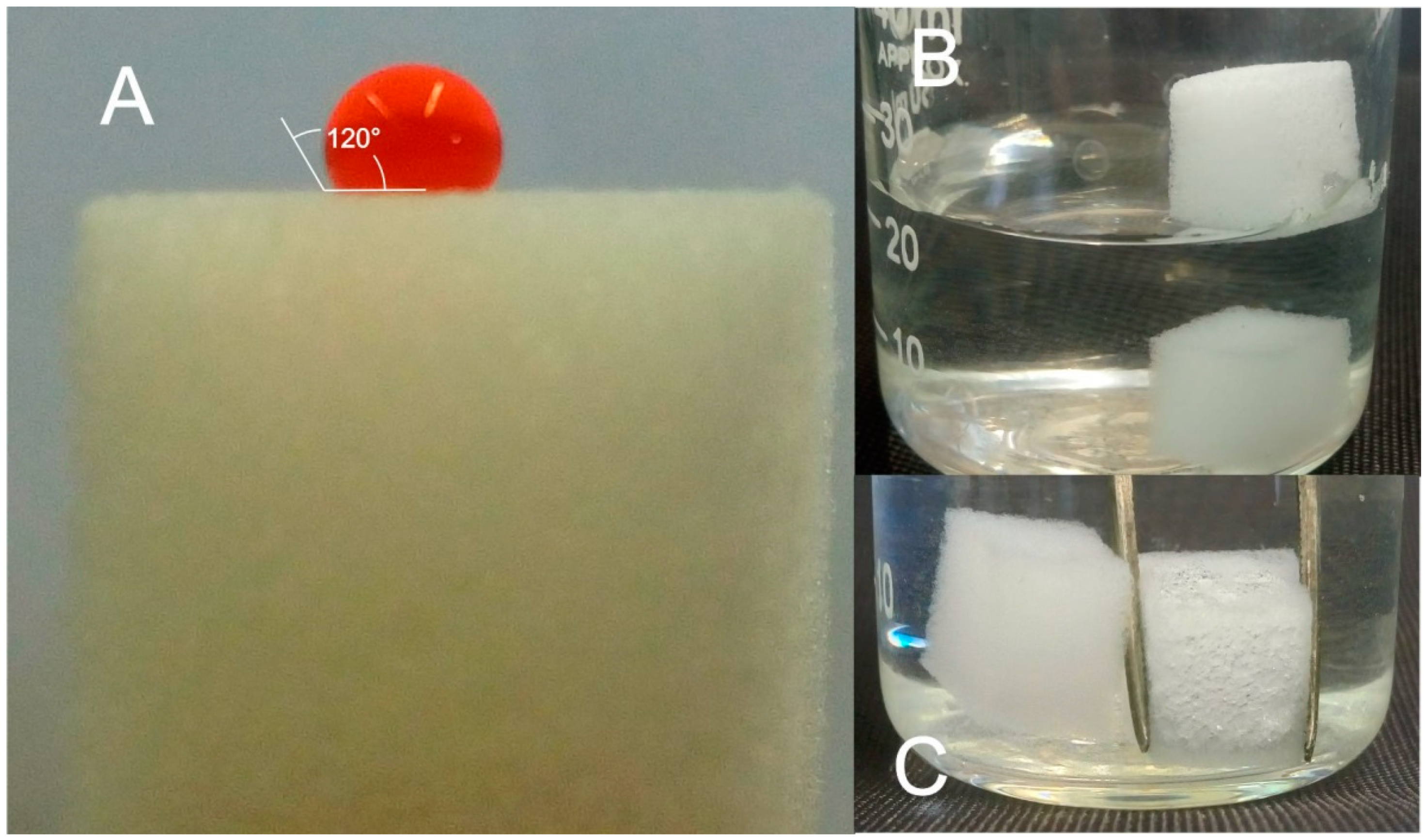
2.2. Characterization of MUF Sponges
2.3. Optimization of the Proposed Procedure
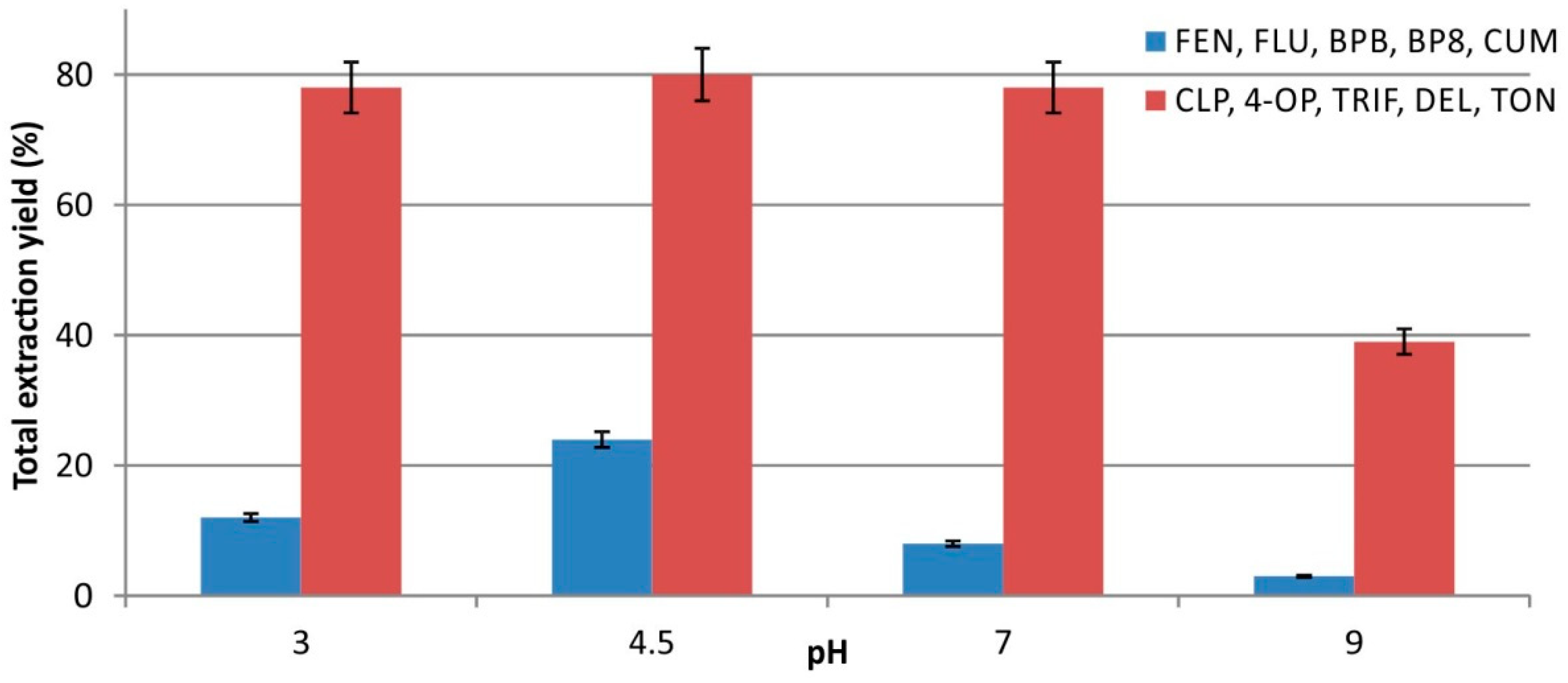
2.3.1. Effect of Sample pH and Ionic Strength on Extraction Yield
2.3.2. Effect of Adsorbent Quantity and Sample Volume
2.3.3. Elution Conditions
2.4. Method Validation
2.5. Analysis of Real Samples
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Synthesis of Urea-Formaldehyde Sponges
3.3. Apparatus
3.4. SPE Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chatzimitakos, T.; Stalikas, C. Carbon-Based Nanomaterials Functionalized with Ionic Liquids for Microextraction in Sample Preparation. Separations 2017, 4, 14. [Google Scholar] [CrossRef]
- Buszewski, B.; Szultka, M. Past, Present, and Future of Solid Phase Extraction: A Review. Crit. Rev. Anal. Chem. 2012, 42, 198–213. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review (Part I). Trends Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Modern trends in solid phase extraction: New sorbent media. Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Pham, V.H.; Dickerson, J.H. Superhydrophobic Silanized Melamine Sponges as High Efficiency Oil Absorbent Materials. ACS Appl. Mater. Interfaces 2014, 6, 14181–14188. [Google Scholar] [CrossRef] [PubMed]
- Reyssat, M.; Richard, D.; Clanet, C.; Quéré, D. Dynamical superhydrophobicity. Faraday Discuss. 2010, 146, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Vinhal, J.O.; Lima, C.F.; Cassella, R.J. Polyurethane foam loaded with sodium dodecylsulfate for the extraction of ‘quat’ pesticides from aqueous medium: Optimization of loading conditions. Ecotoxicol. Environ. Saf. 2016, 131, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Moawed, E.A.; Abulkibash, A.B.; El-Shahat, M.F. Synthesis of tannic acid azo polyurethane sorbent and its application for extraction and determination of atrazine and prometryn pesticides in foods and water samples. Environ. Nanotechnol. Monit. Manag. 2015, 3, 61–66. [Google Scholar] [CrossRef]
- Moawed, E.A.; Radwan, A.M. Application of acid modified polyurethane foam surface for detection and removing of organochlorine pesticides from wastewater. J. Chromatogr. B 2017, 1044–1045, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Deng, Y.; Tong, Z.; Wang, C. Multifunctional foams derived from poly(melamine formaldehyde) as recyclable oil absorbents. J. Mater. Chem. A 2014, 2, 9994–9999. [Google Scholar] [CrossRef]
- Hou, K.; Jin, Y.; Chen, J.; Wen, X.; Xu, S.; Cheng, J.; Pi, P. Fabrication of superhydrophobic melamine sponges by thiol-ene click chemistry for oil removal. Mater. Lett. 2017, 202, 99–102. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, G.; Ouyang, Y.; Liang, Y.; Deng, Y.; Wang, C. Simple fabrication of multi-functional melamine sponges. Mater. Lett. 2017, 190, 119–122. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.; Zhang, X.; Lei, Z.; Chen, C.; Deng, Y.; Wang, C. Polyethylenimine and dithiocarbamate decorated melamine sponges for fast copper(II) ions removal from aqueous solution. Appl. Surf. Sci. 2018, 445, 471–477. [Google Scholar] [CrossRef]
- Deng, C.-H.; Gong, J.-L.; Zhang, P.; Zeng, G.-M.; Song, B.; Liu, H.-Y. Preparation of melamine sponge decorated with silver nanoparticles-modified graphene for water disinfection. J. Colloid Interface Sci. 2017, 488, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Chen, G.; Zeng, G.; Chen, A.; He, K.; Huang, Z.; Hu, L.; Shi, J.; Li, H.; Yuan, L.; et al. Superhydrophobic kaolinite modified graphene oxide-melamine sponge with excellent properties for oil-water separation. Appl. Clay Sci. 2018, 163, 63–71. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, Q.; Wang, X.; Xie, H.; Chen, Y. Recycle and reusable melamine sponge coated by graphene for highly efficient oil-absorption. Colloids Surf. A 2016, 488, 93–99. [Google Scholar] [CrossRef]
- Chen, J.; You, H.; Xu, L.; Li, T.; Jiang, X.; Li, C.M. Facile synthesis of a two-tier hierarchical structured superhydrophobic-superoleophilic melamine sponge for rapid and efficient oil/water separation. J. Colloid Interface Sci. 2017, 506, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Chatzimitakos, T.; Samanidou, V.; Stalikas, C.D. Graphene-functionalized melamine sponges for microextraction of sulfonamides from food and environmental samples. J. Chromatogr. A 2017, 1522, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chatzimitakos, T.G.; Stalikas, C.D. Melamine sponge decorated with copper sheets as a material with outstanding properties for microextraction of sulfonamides prior to their determination by high-performance liquid chromatography. J. Chromatogr. A 2018, 1554, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Philbrook, A.; Blake, C.J.; Dunlop, N.; Easton, C.J.; Keniry, M.A.; Simpson, J.S. Demonstration of co-polymerization in melamine–urea–formaldehyde reactions using 15N NMR correlation spectroscopy. Polymer 2005, 46, 2153–2156. [Google Scholar] [CrossRef]
- Cao, J.; Yan, H.; Shen, S.; Bai, L.; Liu, H.; Qiao, F. Hydrophilic molecularly imprinted melamine-urea-formaldehyde monolithic resin prepared in water for selective recognition of plant growth regulators. Anal. Chim. Acta 2016, 943, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Dunky, M. Urea–formaldehyde (UF) adhesive resins for wood. Int. J. Adhes. Adhes. 1998, 18, 95–107. [Google Scholar] [CrossRef]
- Li, T.; Cao, M.; Liang, J.; Xie, X.; Du, G. New Mechanism Proposed for the Base-Catalyzed Urea–Formaldehyde Condensation Reactions: A Theoretical Study. Polymers 2017, 9, 203. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Pierson, S.A.; Anderson, J.L.; Stalikas, C.D. Enhanced magnetic ionic liquid-based dispersive liquid-liquid microextraction of triazines and sulfonamides through a one-pot, pH-modulated approach. J. Chromatogr. A 2018, 1571, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wells, M. Handling large volume samples: Applications of SPE to environmental matrices. In Solid-Phase Extraction: Principles, Techniques and Applications; CRC Press: Boca Raton, FL, USA, 2000; pp. 97–119. ISBN 9780824700218. [Google Scholar]
- Chatzimitakos, T.; Binellas, C.; Maidatsi, K.; Stalikas, C. Magnetic ionic liquid in stirring-assisted drop-breakup microextraction: Proof-of-concept extraction of phenolic endocrine disrupters and acidic pharmaceuticals. Anal. Chim. Acta 2016, 910, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Lempart, A.M.; Kudlek, E.A.; Lempart, M.; Dudziak, M. The Presence of Compounds from the Personal Care Products Group in Swimming Pool Water. J. Ecol. Eng. 2018, 19, 29–37. [Google Scholar] [CrossRef]
- González-Mariño, I.; Quintana, J.B.; Rodríguez, I.; Cela, R. Simultaneous determination of parabens, triclosan and triclocarban in water by liquid chromatography/electrospray ionisation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, V.; Carlucci, M.; Ettorre, V.; Cotellese, R.; Palumbo, P.; Fontana, A.; Siani, G.; Carlucci, G. Dispersive magnetic solid phase extraction exploiting magnetic graphene nanocomposite coupled with UHPLC-PDA for simultaneous determination of NSAIDs in human plasma and urine. J. Pharm. Biomed. Anal. 2018, 161, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Benijts, T.; Dams, R.; Lambert, W.; De Leenheer, A. Countering matrix effects in environmental liquid chromatography–electrospray ionization tandem mass spectrometry water analysis for endocrine disrupting chemicals. J. Chromatogr. A 2004, 1029, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Xue, N.; Li, F.; Hou, H.; Li, B. Occurrence of endocrine-disrupting pesticide residues in wetland sediments from Beijing, China. Environ. Toxicol. Chem. 2009, 27, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors.. |
| Compound | Category | Structure | Log P | pKa | Quantification Wavelength | Retention Time (min) |
|---|---|---|---|---|---|---|
| Fenbufen (FEN) | Non-steroidal anti-inflammatory drugs | 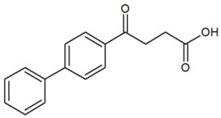 | 3.13 | 4.22 | 285 | 7.2 |
| Flurbiprofen (FLU) | 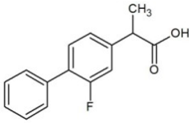 | 4.11 | 4.42 | 254 | 17.2 | |
| Benzophenone-8 (BP8) | Benzophenones | 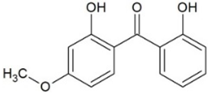 | 3.93 | 7.11 | 285 | 12.0 |
| Butylparaben (BPB) | Parabens | 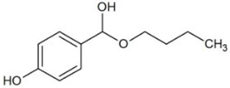 | 3.46 | 8.47 | 254 | 9.8 |
| Cumylphenol (CUM) | Phenols | 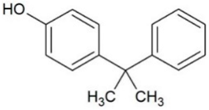 | 4.17 | 10.0 | 275 | 15.3 |
| 4-octylphenol (4-OP) | 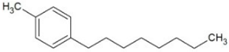 | 5.66 | 10.15 | 275 | 24.8 | |
| Chlorpyrifos (CLP) | Pesticides | 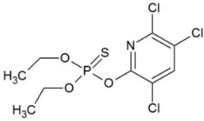 | 4.77 | - | 285 | 18.4 |
| Trifluralin (TRIF) | 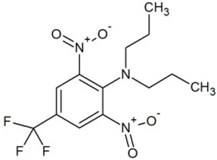 | 5.41 | - | 285 | 20.6 | |
| Deltamethrin (DEL) | 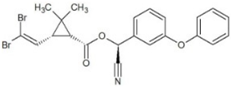 | 6.20 | 10.62 | 275 | 31.1 | |
| Tonalide (TON) | Musks |  | 5.70 | - | 254 | 27.2 |
| Compound | Linear Equation | Coefficient of Determination (R2) | LOD (μg L−1) | EF | E% | RSD (%) | Relative Recoveries (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Within-Day (n = 5) | Between-Day (n = 3 × 5) | 3 × LOQ | 10 × LOQ | ||||||
| Fenbufen | y = 31,481x + 1117 | 0.9998 | 0.02 | 22 | 96 | 7.0 | 7.4 | 94 | 96 |
| Butylparaben | y = 30,462x + 642 | 0.9993 | 0.01 | 23 | 96 | 6.7 | 7.4 | 95 | 98 |
| Benzophenone-8 | y = 30,121x + 832 | 0.9992 | 0.02 | 27 | 96 | 7.1 | 7.8 | 96 | 99 |
| Cumylphenol | y = 30,432x + 667 | 0.9991 | 0.02 | 23 | 96 | 6.4 | 7.1 | 95 | 98 |
| Flurbiprofen | y = 29,010x + 1844 | 0.9995 | 0.02 | 25 | 96 | 6.7 | 8.4 | 93 | 97 |
| Chlorpyrifos | y = 7498x + 1091 | 0.9993 | 0.09 | 24 | 96 | 5.6 | 6.1 | 94 | 96 |
| Trifluralin | y = 30,967x + 967 | 0.9996 | 0.02 | 25 | 96 | 6.9 | 8.0 | 97 | 99 |
| 4-octylphenol | y = 30,367x + 784 | 0.9997 | 0.02 | 24 | 96 | 6.4 | 7.3 | 98 | 100 |
| Tonalide | y = 29,633x + 518 | 0.9998 | 0.01 | 23 | 96 | 7.3 | 8.2 | 92 | 98 |
| Deltamethrin | y = 2321x + 418 | 0.9994 | 0.33 | 30 | 97 | 5.7 | 7.1 | 96 | 98 |
| Analytes | Matrix | Method | Material | Sample Volume (mL) | Elution Volume (mL) | Equipment | RR (%) | RSD (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| BP8 | Swimming pool water | SPE | C18 | 100 | 3 | GC-MS | - | <3% (CV) | [27] |
| BPB | River water | SPE | Oasis HLB | 500 | 4 | HPLC-MS/MS | 92.9 | 15.5 | [28] |
| FEN and FLU | Plasma and urine | MSPE | G/Fe3O4 | 5 | 0.5 | HPLC-PDA | 97.5–102 | 2–4.2 | [29] |
| CUM and 4-OP | Water | SPE | Oasis HLB | 500 | 6 | HPLC-MS/MS | - | - | [30] |
| CLP, DEL and TRIF | Wetland sediments | SPE | Oasis HLB | 500 | 5 | GC-ECD | 69–101 | <15 | [31] |
| FEN, BPB, BP8, CUM, FLU, CLP, TRIF, 4-OP, TON and DEL | Lake water | SPE | MUF | 25 | 5 | HPLC-PDA | 92–100 | 5.6–8.4 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Valverde, M.T.; Chatzimitakos, T.; Lucena, R.; Cárdenas, S.; Stalikas, C.D. Melamine Sponge Functionalized with Urea-Formaldehyde Co-Oligomers as a Sorbent for the Solid-Phase Extraction of Hydrophobic Analytes. Molecules 2018, 23, 2595. https://doi.org/10.3390/molecules23102595
García-Valverde MT, Chatzimitakos T, Lucena R, Cárdenas S, Stalikas CD. Melamine Sponge Functionalized with Urea-Formaldehyde Co-Oligomers as a Sorbent for the Solid-Phase Extraction of Hydrophobic Analytes. Molecules. 2018; 23(10):2595. https://doi.org/10.3390/molecules23102595
Chicago/Turabian StyleGarcía-Valverde, María Teresa, Theodoros Chatzimitakos, Rafael Lucena, Soledad Cárdenas, and Constantine D. Stalikas. 2018. "Melamine Sponge Functionalized with Urea-Formaldehyde Co-Oligomers as a Sorbent for the Solid-Phase Extraction of Hydrophobic Analytes" Molecules 23, no. 10: 2595. https://doi.org/10.3390/molecules23102595
APA StyleGarcía-Valverde, M. T., Chatzimitakos, T., Lucena, R., Cárdenas, S., & Stalikas, C. D. (2018). Melamine Sponge Functionalized with Urea-Formaldehyde Co-Oligomers as a Sorbent for the Solid-Phase Extraction of Hydrophobic Analytes. Molecules, 23(10), 2595. https://doi.org/10.3390/molecules23102595








