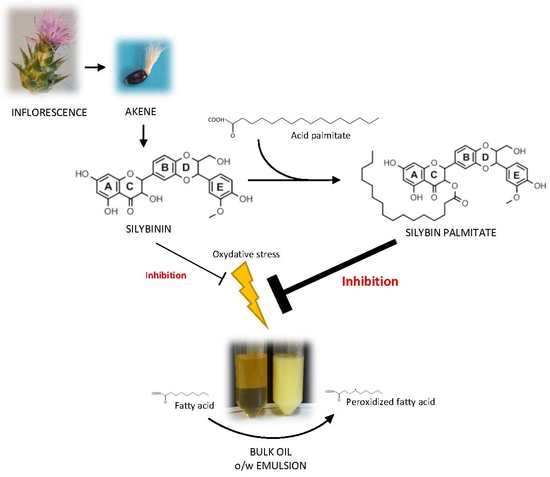
Selective Synthesis of 3-O-Palmitoyl-Silybin, a New-to-Nature Flavonolignan with Increased Protective Action against Oxidative Damages in Lipophilic Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis Protocol
2.2. Electrospray High Resolution Mass Spectrometry (ESI-HRMS) Study
2.3. NMR Analysis
2.4. Determination of Antioxidant Activity
2.5. Linseed Bulk Oil and Preparation of o/w Emulsion
2.6. Accelerated Degradation Protocol
2.7. Determination of Inhibition of Conjugated Diene Hydroperoxides (CD) Formation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Selective Synthesis of 3-O-palmitoyl-silybin
3.2. Structural Confirmation
3.3. Radical Scavenging and Anti-Lipoperoxidation Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Awasthi, S.K. Definition of standards of quality. In Book Prevention of Food Adulteration Act with Rules; Bhatnagar, J.P., Ed.; Ashoka Law House: New Delhi, India, 2000; pp. 699–707. [Google Scholar]
- Williams, G.M.; Iatropoulos, M.J.; Whysner, J. Safety Assessment of Butylated Hydroxyanisole and Butylated Hydroxytoluene as Antioxidant Food Additives. Food Chem. Toxicol. 1999, 37, 1027–1038. [Google Scholar] [CrossRef]
- Hano, C.; Corbin, C.; Drouet, S.; Quéro, A.; Rombaut, N.; Savoire, R.; Molinié, R.; Thomasset, B.; Mesnard, F.; Lainé, E. The lignan (+)-secoisolariciresinol extracted from flax hulls is an effective protectant of linseed oil and its emulsion against oxidative damage. Eur. J. Lipid Sci. Technol. 2017, 119. [Google Scholar] [CrossRef]
- Aditya, N.P.; Hamilton, I.E.; Norton, I.T. Amorphous nano-curcumin stabilized oil in water emulsion: Physico chemical characterization. Food Chem. 2017, 224, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed]
- Drouet, S.; Abbasi, B.H.; Falguières, A.; Ahmad, W.; Sumaira; Ferroud, C.; Doussot, J.; Vanier, J.R.; Lainé, E.; Hano, C. Single laboratory validation of a quantitative core shell-based LC separation for the evaluation of silymarin variability and associated antioxidant activity of pakistani ecotypes of milk thistle (Silybum marianum L.). Molecules 2018, 23, 904. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and chronic liver disease: A marriage of many years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, J.A.; Kim, M.; Kum, H.; Jung, E.; Park, D. Anti-Glycation activities of phenolic constituants from Silybum marianum (Milk Thistle) flower in vitro and on human explants. Molecules 2015, 20, 3549–3564. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rotter, S.; Ladurner, A.; Heiss, E.H.; Oberlies, N.H.; Dirsch, V.M.; Atanasov, A.G. Silymarin Constituents Enhance ABCA1 Expression in THP-1 Macrophages. Molecules 2016, 21, 55. [Google Scholar] [CrossRef] [PubMed]
- Anestopoulos, I.; Sfakianos, A.P.; Franco, R.; Chlichlia, K.; Panayiotidis, M.I.; Kroll, D.J.; Pappa, A. A novel role of Silibinin as a Putative Epigenetic Modulator in Human Prostate Carcinoma. Molecules 2017, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Szelenberger, R.; Saluk, J.; Nowak, P. Flavonolignans inhibit ADP induced blood platelets activation and aggregation in whole blood. Int. J. Biol. Macromol. 2017, 95, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Gažák, R.; Purchartová, K.; Marhol, P.; Živná, L.; Petr Sedmera; Valentová, K.; Kato, N.; Matsumura, H.; Kaihatsu, K.; Křena, V. Antioxidant and antiviral activities of silybin fatty acid conjugates. Eur. J. Med. Chem. 2010, 45, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Zarrelli, A.; Romanucci, V.; Della Greca, M.; De Napoli, L.; Previtera, L.; Di Fabio, G. New silybin scaffold for chemical diversification: Synthesis of novel 23-phosphodiester silybin conjugates. Synlett 2013, 24, 45–48. [Google Scholar] [CrossRef]
- Zarrelli, A.; Romanucci, V.; Tuccillo, C.; Federico, A.; Loguercio, C.; Gravante, R.; Di Fabio, G. New silibinin glyco-conjugates: Synthesis and evaluation of antioxidant properties. Bioorg. Med. Chem. Lett. 2014, 24, 5147–5149. [Google Scholar] [CrossRef] [PubMed]
- Quéro, A.; Molinié, R.; Mathiron, D.; Thiombiano, B.; Fontaine, J.X.; Brancourt, D.; Van Wuytswinkel, O.; Petit, E.; Demailly, H.; Mongelard, G.; et al. Metabolite profiling of developing Camelina sativa seeds. Metabolomics 2016, 12, 1–14. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Bhanger, M.I. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chem. 2007, 100, 246–254. [Google Scholar] [CrossRef]
- Gilles, V.; Vieira, M.A.; Lacerda, V., Jr.; Castro, E.V.R.; Santos, R.B.; Orestes, E.; Carneiro, J.W.M.; Greco, S.J. A New, Simple and Efficient Method of Steglich Esterification of Juglone with Long-Chain Fatty Acids: Synthesis of a New Class of Non-Polymeric Wax Deposition Inhibitors for Crude Oil. J. Braz. Chem. Soc. 2015, 26, 74–83. [Google Scholar] [CrossRef]
- Ma, Y.L.; Li, Q.M.; Van den Heuvel, H.; Claeys, M. Characterization of flavone and flavonol aglycones by collision-induced dissociation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 1997, 11, 1357–1364. [Google Scholar] [CrossRef]
- Ratz-Lyko, A.; Arct, J.; Pytkowska, K. Methods for evaluation of cosmetic antioxidant capacity. Skin Res. Technol. 2012, 18, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Meddeb, W.; Rezig, L.; Abderrabba, M.; Lizard, G.; Mejri, M. Tunisian milk thistle: An investigation of the chemical composition and the characterization of its cold-pressed seed oils. Int. J. Mol. Sci. 2017, 18, 2582. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.L.; Black, E.D.; Drolet, A.M. Use of polyamide oxidative fluorescence test on lipid emulsions: contrast in relative effectiveness of antioxidants in bulk versus dispersed systems. J. Agric. Food Chem. 1989, 37, 615–624. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.-W.; Kanner, J.; German, J.B. Interfacial Phenomena in the Evaluation of Antioxidants: Bulk Oils vs Emulsions. J. Agric. Food Chem. 1994, 42, 1054–1059. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Revisiting the Polar Paradox Theory: A Critical Overview. J. Agric. Food Chem. 2011, 59, 3499–3504. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The new compound 3-O-palmitoyl-silybin here described is available from the authors upon request. |
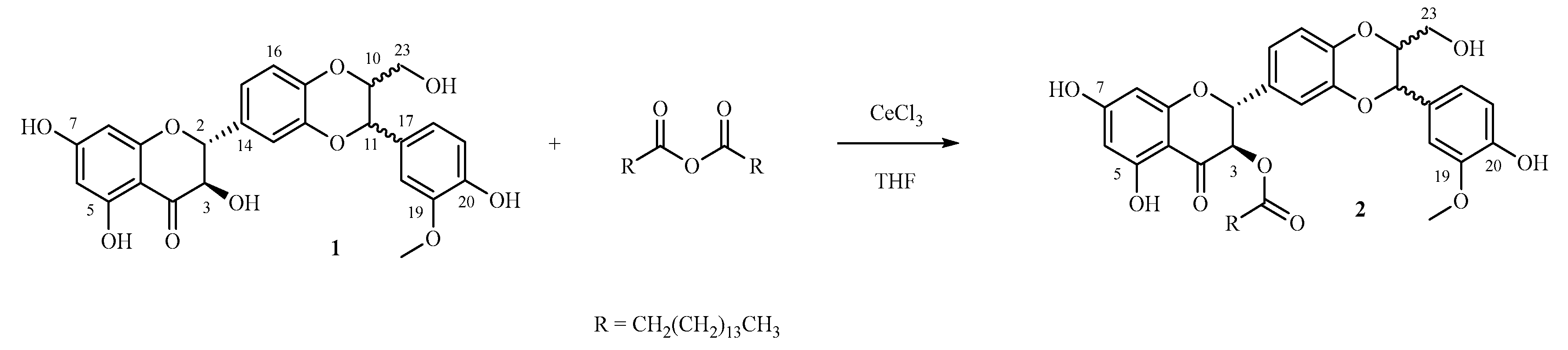

| NO | Proton | C |
|---|---|---|
| 2 | 5.50 d (11.8) | 79.8 |
| 3 | 5.95 d (11.8) | 71.6 |
| 4 | - | 191.4 |
| 4a | - | 101.1 |
| 5 | - | 163.2 |
| 6 | 5.95 d (2.1) | 96.4 |
| 7 | - | 167.4 |
| 8 | 5.92 d (2.1) | 95.4 |
| 8a | - | 162.5 |
| 10 | 4.15 ddd (7.5, 4.8, 2.7) | 78.2 |
| 4.12 ddd (7.5, 4.8, 2.7) | 78.1 | |
| 11 | 4.89 d (7.9) | 75.7 |
| 4.88 d (7.9) | 75.7 | |
| 12a | - | 144.1 |
| 13 | 7.12 dd (1.7, 2.2) | 116.6 |
| 14 | - | 128.5 |
| 15 | 7.01 dd (8.2, 2.2) | 121 |
| 7.00 dd (8.2, 2.2) | 121 | |
| 16 | 6.96 dd (8.2, 1.5) | 116.5 |
| 16a | - | 143.9 |
| 17 | - | 127.4 |
| 18 | 6.98 d (1.8) | 111.6 |
| 6.99 d (1.8) | 111.6 | |
| 19 | - | 147.7 |
| 20 | - | 150.9 |
| 21 | 6.80 d (8.2) | 115.2 |
| 6.79 d (8.2) | 115.2 | |
| 22 | 6.85 d (8.2, 1.8) | 120.3 |
| 6.83 d (8.2, 1.8) | 120.3 | |
| 23d | 3.54 nd | 60 |
| 23u | nd (water signal) | 60 |
| 19-Me | 3.78 s | 55.7 |
| 3.77 s | 55.7 | |
| 5-OH | 11.94 s | - |
| 7-OH | 11.03 br s | - |
| 20-OH | 9.15 s | - |
| 23-OH | 4.95 t (5.1) | - |
| CUPRAC * | FRAP * | |
|---|---|---|
| Silybin | 2.25 ± 0.09 a | 3.25 ± 0.76 c |
| Silybinyl palmitate | 3.50 ± 0.37 c | 1.86 ± 0.28 a,b |
| Ascorbic acid | 2.18 ± 0.19 b | 2.53 ± 0.18 b |
| Ascorbyl palmitate | 3.09 ± 0.09 b,c | 1.75 ± 0.18 a |
| BHA | 2.55 ± 0.14 a | 1.78 ± 0.32 a,b |
| Compound 1 | Linseed Bulk Oil 2 | o/w Emulsion 2 |
|---|---|---|
| Silybin | 56.83 ± 0.45 c | 51.90 ± 0.80 c |
| Silybinyl palmitate | 59.32 ± 0.42 d | 64.04 ± 0.31 d |
| Ascorbic acid | 32.51 ± 2.57 a | 26.73 ± 2.48 a |
| Ascorbyl palmitate | 41.67 ± 1.23 b | 53.11 ± 2.89 c |
| BHA | 29.06 ± 3.27 a | 43.34 ± 1.75 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drouet, S.; Doussot, J.; Garros, L.; Mathiron, D.; Bassard, S.; Favre-Réguillon, A.; Molinié, R.; Lainé, É.; Hano, C. Selective Synthesis of 3-O-Palmitoyl-Silybin, a New-to-Nature Flavonolignan with Increased Protective Action against Oxidative Damages in Lipophilic Media. Molecules 2018, 23, 2594. https://doi.org/10.3390/molecules23102594
Drouet S, Doussot J, Garros L, Mathiron D, Bassard S, Favre-Réguillon A, Molinié R, Lainé É, Hano C. Selective Synthesis of 3-O-Palmitoyl-Silybin, a New-to-Nature Flavonolignan with Increased Protective Action against Oxidative Damages in Lipophilic Media. Molecules. 2018; 23(10):2594. https://doi.org/10.3390/molecules23102594
Chicago/Turabian StyleDrouet, Samantha, Joël Doussot, Laurine Garros, David Mathiron, Solène Bassard, Alain Favre-Réguillon, Roland Molinié, Éric Lainé, and Christophe Hano. 2018. "Selective Synthesis of 3-O-Palmitoyl-Silybin, a New-to-Nature Flavonolignan with Increased Protective Action against Oxidative Damages in Lipophilic Media" Molecules 23, no. 10: 2594. https://doi.org/10.3390/molecules23102594
APA StyleDrouet, S., Doussot, J., Garros, L., Mathiron, D., Bassard, S., Favre-Réguillon, A., Molinié, R., Lainé, É., & Hano, C. (2018). Selective Synthesis of 3-O-Palmitoyl-Silybin, a New-to-Nature Flavonolignan with Increased Protective Action against Oxidative Damages in Lipophilic Media. Molecules, 23(10), 2594. https://doi.org/10.3390/molecules23102594