A Comprehensive Study on the Biological Activity of Elderberry Extract and Cyanidin 3-O-Glucoside and Their Interactions with Membranes and Human Serum Albumin
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Content by HPLC/LC-MS Method
2.2. Packing Order and Fluidity of Membrane
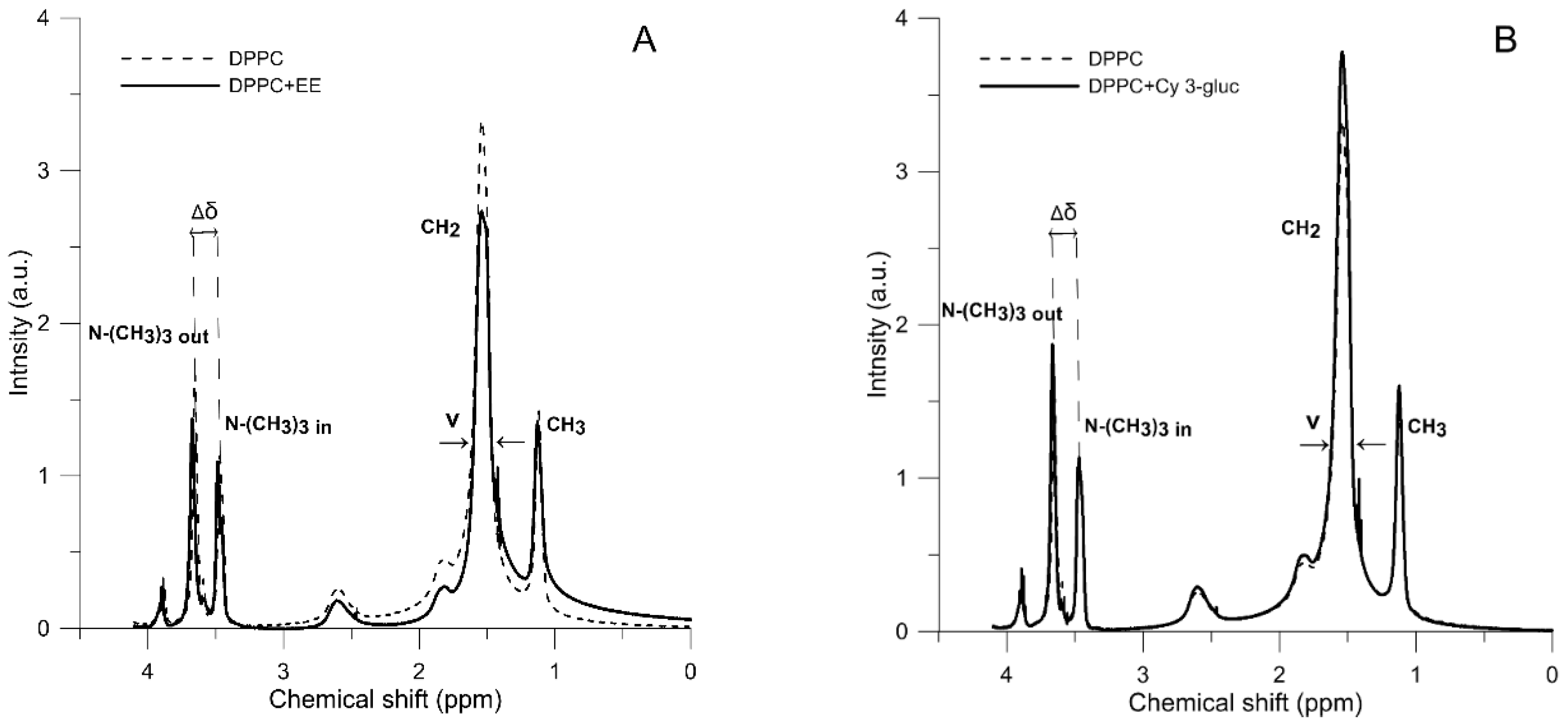
2.3. 1H-NMR Measurements
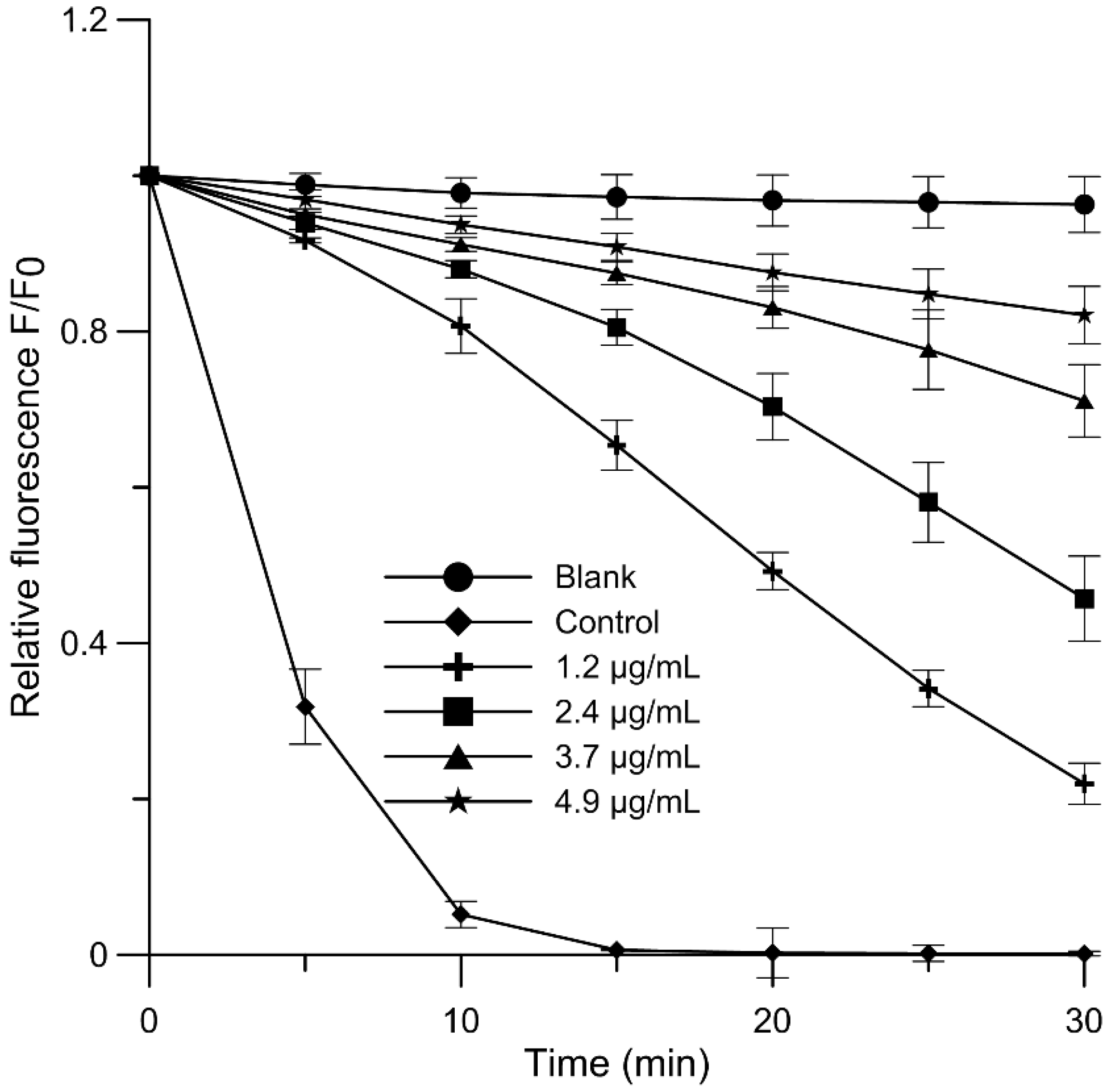
2.4. Antioxidant Activity and Free-Radical Scavenging Activity
2.5. Cyclooxygenase Activity
2.6. Microcapsulation of Elderberry
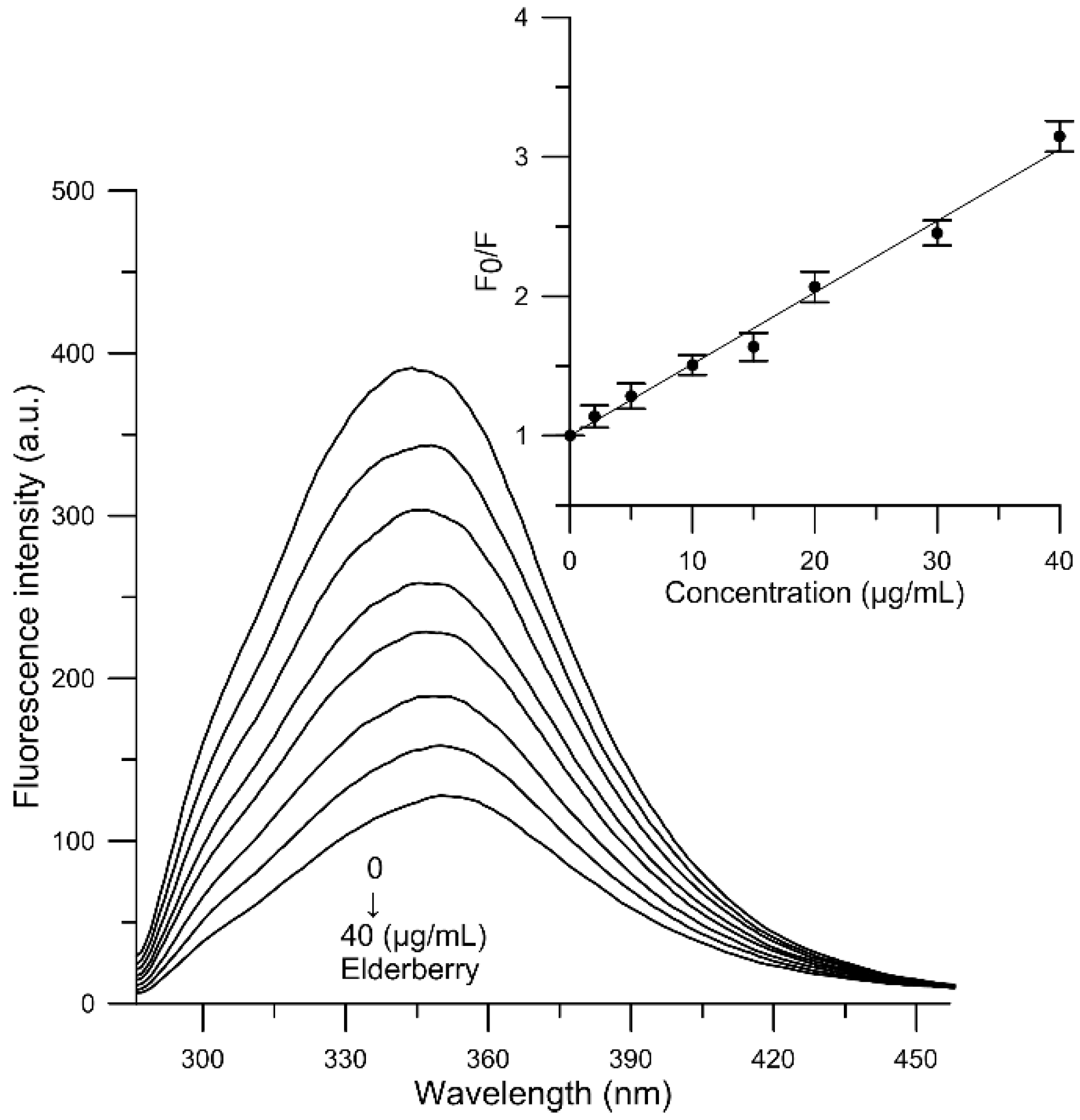
2.7. Fluorescence Quenching of Human Serum Albumin
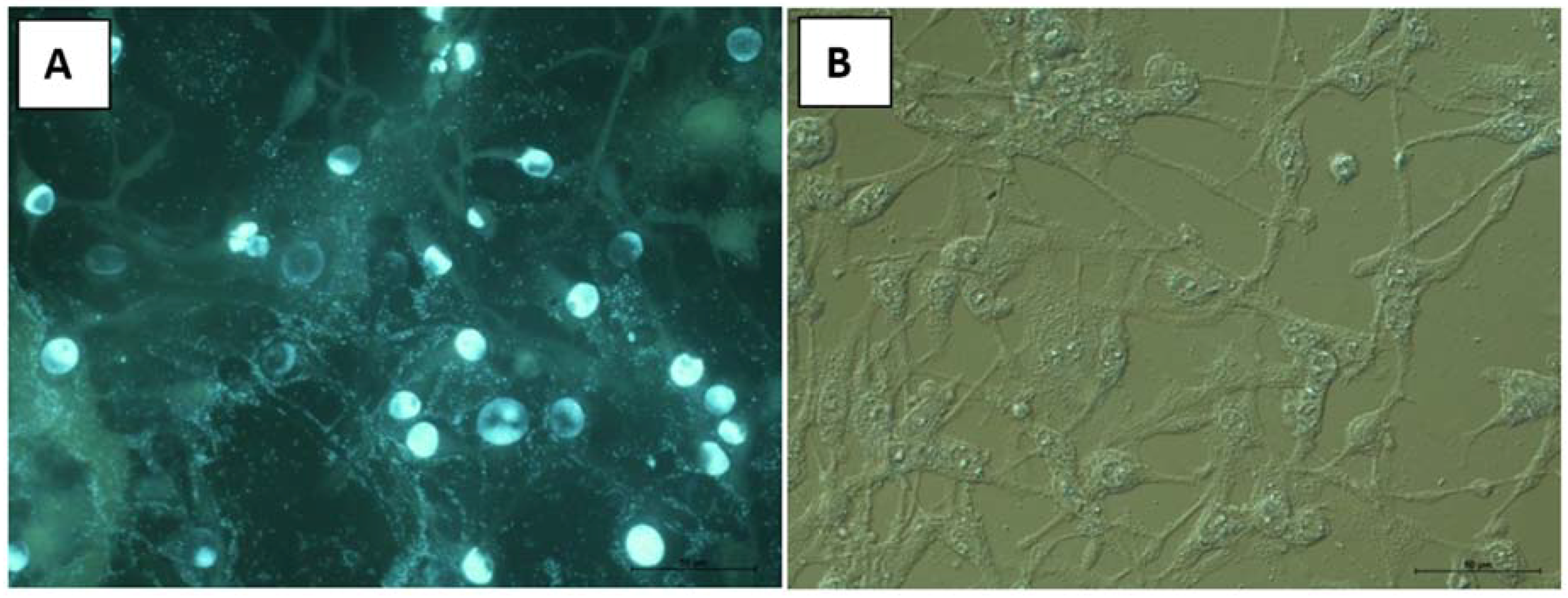
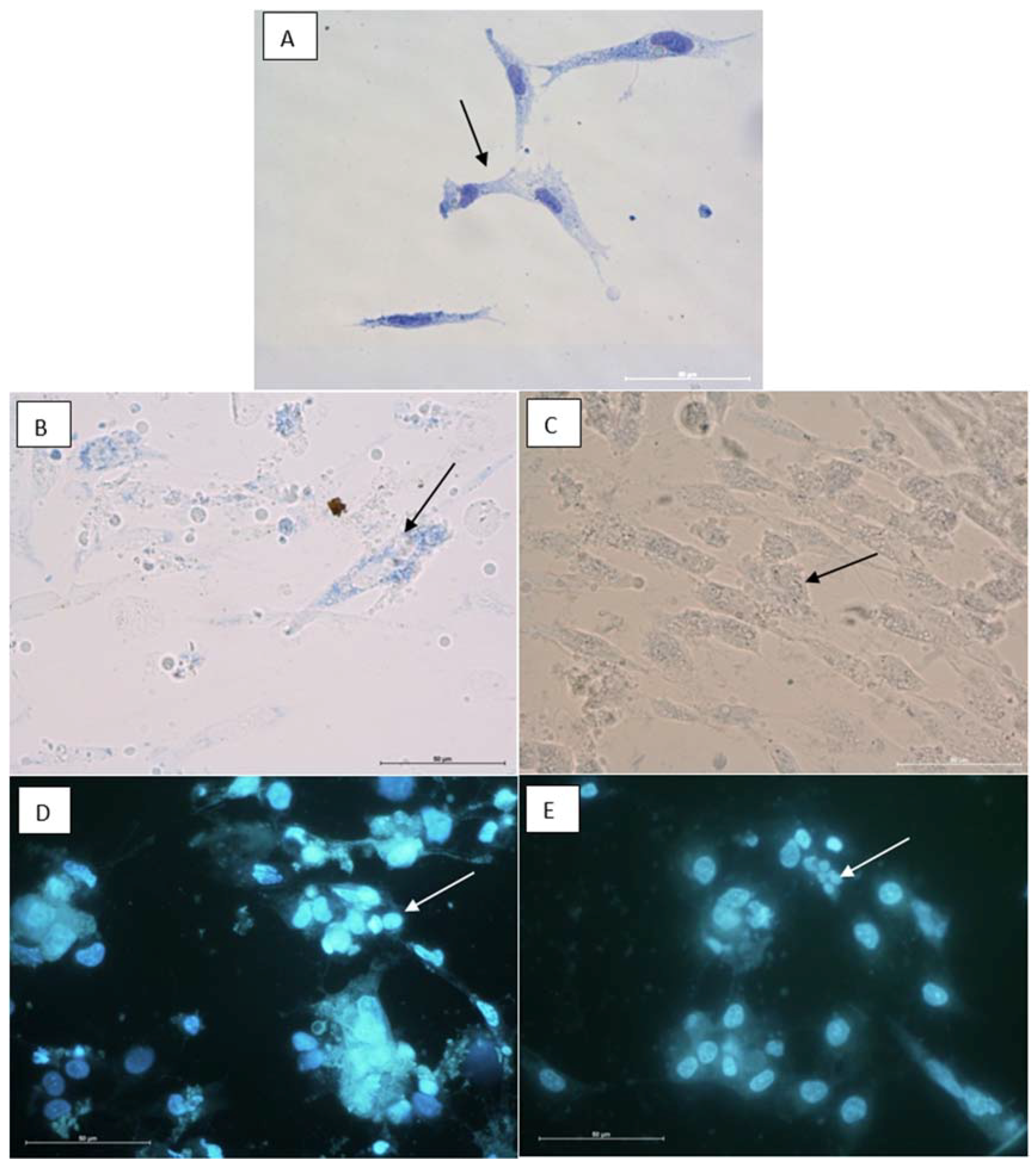
2.8. Cytotoxic Properties on BHK-21 and CEF Cell Lines
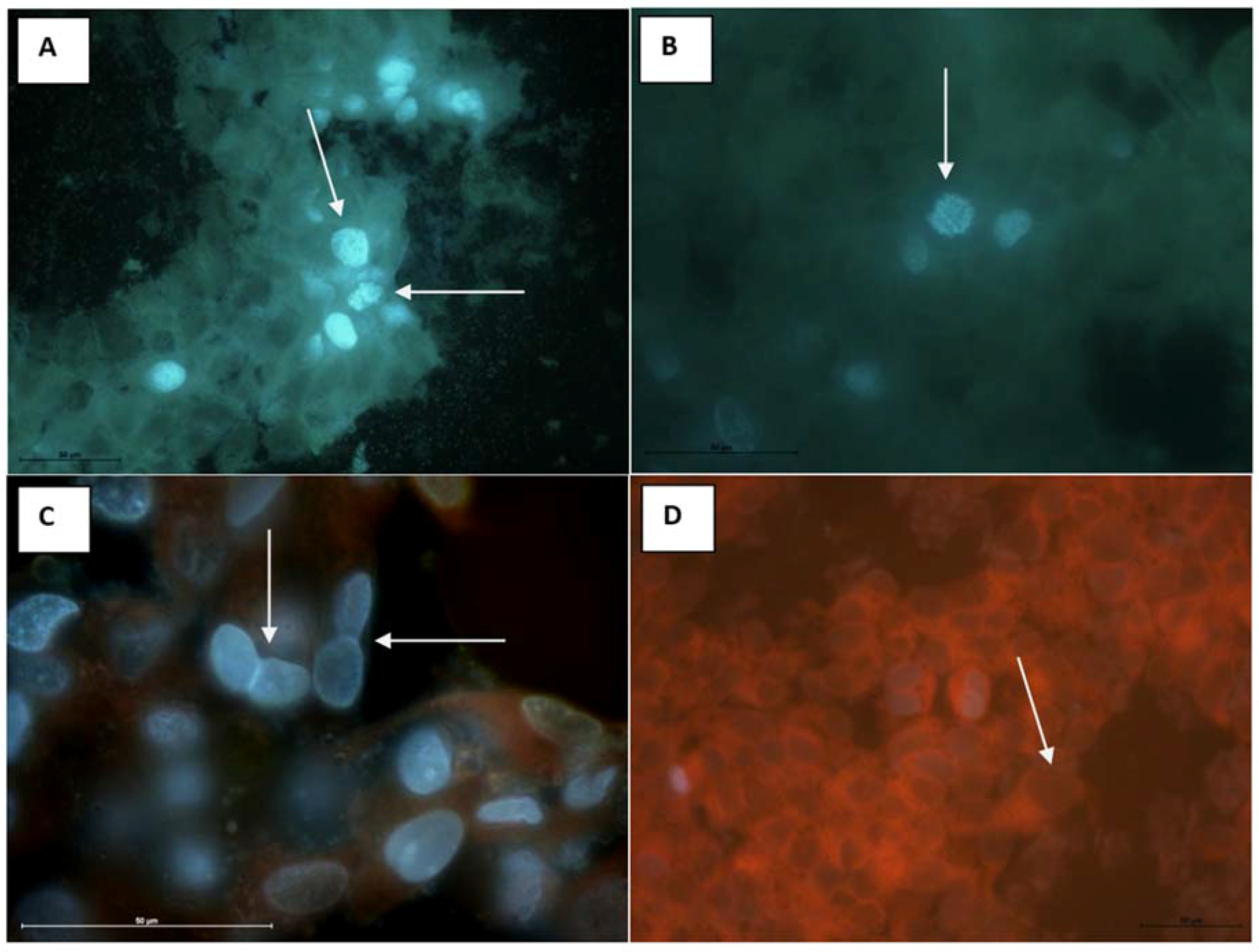
2.9. Antitumor Properties on MCF-7 (Human Breast Adenocarcinoma Cell Line)
3. Materials and Methods
3.1. Materials
3.2. Elderberry Supplement
3.3. High-Performance Liquid Chromatography/Mass Spectrometry (HPLC/MS) Methods
3.4. Liposome Preparation
3.5. Affinity of Elderberry Extract for Cell-Mimic Membranes
3.6. 1H-NMR Measurements
3.7. Antioxidant Activity
3.8. Free-Radical Scavenging Assay
3.9. Cyclooxygenase Activity
3.10. Effectiveness of Microencapsulation of Elderberry Extract (EE) in Liposomes and Vesicle Size Determination
3.11. Fluorescence Quenching of Human Serum Albumin
3.12. Biological Studies
3.12.1. Cytotoxic Properties as Tested on BHK-21and RK-13 Cell Lines
3.12.2. Cytological Investigations as Tested on BHK-21and RK-13 Cell Lines and CEF Cell Cultures
3.12.3. Antitumor Properties on MCF-7 (Human Breast Adenocarcinoma Cell Line)
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loi, M.C.; Poli, F.; Sacchetti, G.; Selenu, M.B.; Ballero, M. Ethnopharmacology of Ogliastra (Villagrande Strisaili, Sardinia, Italy). Fitoterapia 2004, 75, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Uncini Manganelli, R.E.; Zaccaro, L.; Tomei, P.E. Antiviral activity in vitro of Urtica dioica L., Parietaria diffusa M. et K. and Sambucus nigra L. J. Ethnopharmacol. 2005, 98, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Konczak, I.; Zhang, W. Anthocyanins-more than nature’s colours. J. Biomed. Biotechnol. 2004, 5, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.D.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; de la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-Chemistry. Foodomics and Health Effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, J.E.; Cameron, M.; Chrubasik, S. A Systematic Review on the Sambuci fructus effect and efficacy profiles. Phytother. Res. 2010, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska-Pawlęga, B.; Dziubińska, H.; Krol, E.; Trębacz, K.; Jarosz-Wilkołazka, A.; Paduch, R.; Gawron, A.; Gruszecki, W.I. Characteristics of quercetin interactions with liposomal and vacuolar membranes. Biochim. Biophys. Acta Biomembr. 2014, 1838, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Cayahana, Y.; Gordon, M.H. Interaction of anthocyanins with human albumin: Influence of pH and chemical structure on binding. Food Chem. 2013, 141, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Muellera, D.; Junga, K.; Wintera, M.; Rogollb, D.; Melcherb, R.; Kulozikc, U.; Schwarzd, K.; Richling, E. Encapsulation of anthocyanins from bilberries—Effects on bioavailability and intestinal accessibility in humans. Food Chem. 2018, 15, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Patrasa, A.; Bruntona, N.P.; O’Donnell, C.O.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.; Ghorbani, M.M.; Assadpoor, E. Spray-Drying Microencapsulation of Anthocyanins by Natural Biopolymers: A Review. Drying Technol. 2014, 32, 509–518. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Başer, K.H. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 15, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidative properties of extracts). LWT-Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Olejnik, A.; Olkowicz, M.; Kowalska, K.; Rychlik, J.; Dembczyński, R.; Myszka, K.; Juzwa, W.; Białas, W.; Moyer, M.P. Gastrointestinal digested Sambucus nigra L. fruit extract protects in vitro cultured human colon cells against oxidative stress. Food Chem. 2016, 197, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Kšonžeková, P.; Mariychuk, R.; Eliašová, A.; Mudroňová, D.; Csank, T.; Király, J.; Marcinčáková, D.; Pistl, J.; Tkáčiková, L. In vitro study of biological activities of anthocyanin-rich berry extracts on porcineintestinal epithelial cells. J. Sci. Food Agric. 2015, 96, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Kaack, K.; Frett, X.C.; Christensen, L.P.; Landbo, A.-K.; Meyer, A.S. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of juice. Eur. Food Res. Technol. 2008, 226, 843–855. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of ribes, aronia, and sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Miculic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of anthocyanin profile of four elderberry species and interspecific hybrids. J. Agric. Food Chem. 2014, 62, 5573–5580. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Dudra, A.; Gabrielska, J. Interaction between mimic lipid membranes and acylated and nonacylated cyanidin and its bioactivity. J. Agric. Food Chem. 2016, 64, 7414–7422. [Google Scholar] [CrossRef] [PubMed]
- Williamson, P.; Mattocksa, K.; Schlegelb, R.A. Merocyanine 540, a fluorescent probe sensitive to lipid packing. Biochim. Biophys. Acta 1983, 732, 387–393. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Londoño-Londoño, J.; Jemioła-Rzemińska, M.; Strzałka, K.; Villena, F.; Avello, M.; Suwalsky, M. Structural effects of the Solanum steroids solasodine, diosgenin and solanine on human erythrocytes and molecular models of eukaryotic membranes. Biochim. Biophys. Acta 2014, 1838, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Dudra, A.; Gabrielska, J. Activity of blackcurrant and chokeberry extracts and two major cyanidin glycosides against lipid membrane oxidation and their binding properties to albumin. Acta Pol. Pharm. 2017, 74, 676–687. [Google Scholar]
- Kaiser, R.D.; London, E. Location of diphenylhexatriene (DPH) and its derivatives within membranes: Comparison of different fluorescence quenching analyses of membrane depth. Biochemistry 1998, 37, 8180–8190. [Google Scholar] [CrossRef] [PubMed]
- Tammela, P.; Laitinen, L.; Galkin, A.; Wennberg, T.; Heczko, R.; Vuorela, H.; Slotte, J.P.; Vuorela, P. Permeability characteristics and membrane affinity of flavonoids and alkyl gallates in Caco-2 cells and in phospholipid vesicles. Arch. Biochem. Biophys. 2004, 425, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Inbar, M. Fluidity of membrane lipids: A single cell analysis of mouse normal lymphocytes and malignant lymphoma cells. FEBS Lett. 1976, 67, 180–185. [Google Scholar] [CrossRef]
- Kojima, K. Molecular aspects of the plasma membrane in tumor cells. Nagoya J. Med. Sci. 1993, 56, 1–18. [Google Scholar] [PubMed]
- Sok, M.; Šentjurc, M.; Schara, M. Membrane fluidity characteristics of human lung cancer. Cancer Lett. 1999, 139, 215–220. [Google Scholar] [CrossRef]
- Inbar, M.; Shinitzky, M. Cholesterol as a bioregulator in the development and inhibition of leukemia. Proc. Natl. Acad. Sci. USA 1974, 71, 4229–4231. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, I.; Iwaizumi, M. A role of the cancer cell membrane fluidity in the cancer metastases: An ESR study. Tohoku J. Exp. Med. 1989, 157, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Rybczynska, M.; Spitaler, M.; Knebel, N.G.; Boeck, G.; Grunicke, H.; Hofmann, J. Effects of miltefosine on various biochemical parameters in a panel of tumor cell lines with different sensitivities. Biochem. Pharmacol. 2001, 62, 765–772. [Google Scholar] [CrossRef]
- Berra, B.; Bordoni, A.; Rapelli, S.; Biagi, P.L.; Pezzotta, S.; Malgrassi, L.; Montorfano, G.; Hrelia, S. Altered membrane lipid composition in a human meningosarcoma. Int. J. Clin. Lab. Res. 1994, 24, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Structure-dependent membrane interaction of flavonoids associated with their bioactivity. Food Chem. 2010, 120, 1089–1096. [Google Scholar] [CrossRef]
- Strugała, P.; Tronina, T.; Huszcza, E.; Gabrielska, J. Bioactivity in vitro of quercetin glycoside obtained in beauveria bassiana culture and its interaction with liposome membranes. Molecules 2017, 22, 1520. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Cyboran-Mikołajczyk, S.; Dudra, A.; Mizgier, P.; Kucharska, A.Z.; Olejniczak, T.; Gabrielska, J. Biological activity of Japanese quince extract and its interaction with lipids, erythrocyte membrane and human albumin. J. Membr. Biol. 2016, 249, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Gładkowski, W.; Kucharska, A.Z.; Sokół-Łętowska, A.; Gabrielska, J. Antioxidant activity and anti-inflammatory effect of fruit extracts from blackcurrant, chokeberry, hawthorn, and rosehip, and their mixture with linseed oil on a model lipid membrane. Eur. J. Lipid Sci. Technol. 2016, 118, 461–474. [Google Scholar] [CrossRef]
- Bratu, M.M.; Doroftei, E.; Negreanu-Pirjol, T.A.; Hostina, C.A.; Porta, S. Determination of antioxidant activity and toxicity of Sambucus nigra fruit extract using alternative methods. Food Technol. Biotechnol. 2012, 50, 177–182. [Google Scholar]
- Barak, V.; Birkenfeld, S.; Halperin, T.; Kalickman, I. The effect of herbal remedies on the production of human inflammatory and anti-inflammatory cytokines. Isr. Med. Assoc. J. 2002, 4, 919–922. [Google Scholar] [PubMed]
- Farrell, N.J.; Norris, G.H.; Ryan, J.; Porter, C.M.; Jiang, C.; Blesso, C.N. Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. Br. J. Nutr. 2015, 114, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Fredes, C. The encapsulation of anthocyanins from berry-type fruits trends in foods. Molecules 2015, 20, 5875–5888. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Oehme, A.; Valotis, A.; Krammer, G.; Zimmermann, I.; Schreier, P. Preparation and characterization of shellac-coated anthocyanin pectin beads as dietary colonic delivery system. Mol. Nutr. Food Res. 2011, 55, S75–S85. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, U.S. Liposomes in drug delivery: Progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Nii, T.; Ishii, F. Encapsulation efficiency of water-soluble and insoluble drugs in liposomes prepared by the microencapsulation vesicle method. Int. J. Pharm. 2005, 14, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Bryła, A.; Lewandowicz, G.; Juzwa, W. Encapsulation of elderberry extract into phospholipid nanoparticles. J. Food Eng. 2015, 167, 189–195. [Google Scholar] [CrossRef]
- Yang, S.; Liu, W.; Liu, C.; Liu, W.; Tong, G.; Zenh, H.; Zhou, W. Characterization and bioavailability of vitamin C nanparticles prepared by film evaporation—Dynamic high pressure microfluidization. J. Disper. Sci. Technol. 2011, 33, 1608–1614. [Google Scholar] [CrossRef]
- Siddiqi, M.; Nusrat, S.; Alam, P.; Malik, S.; Chaturvedi, S.K.; Ajmal, M.R.; Abdelhameed, A.S.; Khan, R.H. Investigating the site selective binding of busulfan to human serum albumin: Biophysical and molecular docking approaches. Int. J. Biol. Macromol. 2018, 107, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Quenching of fluorescence. In Principles of Fluorescence Spectroscopy, 3rd ed.; Lakowicz, J.R., Ed.; Springer: New York, NY, USA, 2006; pp. 278–285. ISBN 13: 978-0387-31278-1. [Google Scholar]
- Trnková, L.; Boušová, I.; Staňková, V.; Dršata, J. Study on the interaction of catechins with human serum albumin using spectroscopic and electrophoretic techniques. J. Mol. Struct. 2011, 985, 243–250. [Google Scholar] [CrossRef]
- Dai, J.; Zou, T.; Wang, L.; Zhang, Y.; Liu, Y. Investigation of the interaction between quercetin and human serum albumin by multiple spectra, electrochemical impedance spectra and molecular modeling. Luminescence 2014, 29, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Feroz, S.R.; Mohamad, S.B.; Bakri, Z.S.D.; Malek, S.N.A.; Tayyab, S. Probing the interaction of a therapeutic flavonoid, pinostrobin with human serum albumin: Multiple spectroscopic and molecular modeling investigations. PLoS ONE 2013, 8, e76067. [Google Scholar] [CrossRef] [PubMed]
- Klotz, I.M. Physiochemical aspects of drug-protein interactions: A general perspective. Ann. N. Y. Acad. Sci. 1973, 226, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Guo, R. Interactions between flavonoids and hemoglobin in lecithin liposomes. Int. J. Biol. Macromol. 2007, 40, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Remila, S.; Atmani-Kilani, D.; Delemasure, S.; Connat, J.-L.; Azib, L.; Richard, T.; Atmani, D. Antioxidant, cytoprotective, anti-inflammatory and anticancer activities of Pistacia lentiscus (Anacardiaceae) leaf and fruit extracts. Eur. J. Integr. Med. 2015, 7, 274–286. [Google Scholar] [CrossRef]
- Olsson, M.E.; Gustavsson, K.E.; Andersson, S.; Nilsson, A.; Duan, R.D. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J. Agric. Food Chem. 2004, 52, 7264–7271. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.N.; Chu, S.C.; Chiou, H.L.; Chiang, C.L.; Yang, S.F.; Hsieh, Y.S. Cyanidin 3-glucoside and peonidin 3-glucoside inhibit tumor cell growth and induce apoptosis in vitro and suppress tumor growth in vivo. Nutr. Cancer 2005, 53, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lee, W.S.; Kim, G.S.; Park, O.J. Anthocyanins are novel AMPKα1 stimulators that suppress tumor growth by inhibiting mTOR phosphorylation. Oncol. Rep. 2010, 24, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, J.; Tang, X.; Liu, Y.; Yu, X.; Wang, Z.; Liu, W. Anthocyanins inhibit trastuzumab-resistant breast cancer in vitro and in vivo. Mol. Med. Rep. 2016, 13, 4007–4013. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa E induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Hakimuddin, F.; Paliyath, G.; Meckling, K. Selective cytotoxicity of a red grape wine flavonoid fraction against MCF-7 cells. Breast Cancer Res. Treat. 2004, 85, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Thole, J.M.; Kraft, T.F.; Sueiro, L.A.; Kang, Y.H.; Gills, J.J.; Cuendet, M.; Pezzuto, J.M.; Seigler, D.S.; Lila, M.A. A comparative evaluation of the anticancer properties of European and American elderberry fruits. J. Med. Food 2006, 9, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.Z.; Szumny, A.; Sokół-Łętowska, A.; Piórecki, N.; Klymenko, S.V. Iridoids and anthocyanins in cornelian cherry (Cornus mas L.) cultivars. J. Food Compos. Anal. 2015, 40, 95–102. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Wińska, K.; Szumny, A.; Nawirska-Olszańska, A.; Mizgier, P.; Wyspiańska, D. Composition and antioxidant activity of red fruit liqueurs. Food Chem. 2014, 157, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, B.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Jang, M.S.; Pezzuto, J.M. Assessment of cyclooxygenase inhibitors using in vitro assay systems. Method Cell Sci. 1997, 19, 25–31. [Google Scholar] [CrossRef]
- Bykowska, A.; Starosta, R.; Brzuszkiewicz, A.; Bażanów, B.; Florek, M.; Jackulak, N.; Król, J.; Grzesiak, J.; Kaliński, K.; Jeżowska-Bojczuk, M. Synthesis, properties and biological activity of a novel phosphines ligand derived from ciprofloxacin. Polyhedron 2013, 60, 23–29. [Google Scholar] [CrossRef]
- Rubin, H. Chick embryo cells. In Tissue Culture Methods and Application; Kruse, P.F., Patterson, M.K., Eds.; Academic Press: New York, NY, USA, 1973; pp. 119–123. ISBN 978-0-12-427150-0. [Google Scholar]
Sample Availability: Samples of the compounds elderberry extract is available from the authors. |
| Phenolic Compounds | Content (mg/g) | Rt (min) | [MS] (m/z) | Ion Mode | MS/MS Fragments (m/z) |
|---|---|---|---|---|---|
| Cyanidin 3-sambubioside-5-glucoside | 0.744 | 4.242 | 743 | + | 287 |
| Cyanidin 3,5-diglucoside | 0.602 | 7.08 | 611 | + | 287 |
| Cyanidin 3-O-glucoside | 120.230 | 7.93 | 449 | + | 287 |
| Cyanidin 3-O-rutinoside | 1.465 | 9.13 | 595 | + | 287 |
| Peonidin 3-O-glucoside | 13.263 | 10.27 | 463 | + | 301 |
| Quercetin 3-O-glucoside | 2.703 | 13.07 | 463 | − | 301 |
| Quercetin | 1.334 | 20.80 | 301 | − | |
| Protocatechuic acid | 13.966 | 2.73 | 153 | − | 109 |
| Hydroxybenzoic acid derivative | 0.293 | 6.12 | not identified | − | |
| Hydroxybenzoic acid derivative | 0.307 | 9.52 | not identified | - | |
| Total | 160.95 |
| Elderberry Extract/Concentration (µg/mL) | MC540 Intensity Change from Control (%) | PNA Anisotropy Change from Control (%) |
|---|---|---|
| 2 | −5.55 ± 1.96 * | 0.015 ± 2.2 |
| 5 | −16.70 ± 1.88 * | 1.38 ± 3.97 |
| 10 | −30.93 ± 2.64 * | 3.77 ± 2.57 |
| 15 | −40.95 ± 0.16 * | 4.53 ± 3.03 |
| 20 | −45.58 ± 3.12 * | 4.41 ± 0.68 |
| Liposome Composition | Parameter | |||||
|---|---|---|---|---|---|---|
| ν-N+-(CH3)3 Out (ppm) | ν-N+-(CH3)3 In (ppm) | Δδ (ppm) | ν-CH2 (ppm) | ν-CH3 (ppm) | IOut/IIn | |
| DPPC | 0.05222 | 0.03545 | 0.1810 | 0.11679 | 0.05212 | 1.1545 |
| DPPC + EE | 0.05312 | 0.03966 | 0.1870 | 0.12218 | 0.07459 | 1.2221 |
| DPPC + Cy 3-gluc | 0.05438 | 0.03568 | 0.1950 | 0.11674 | 0.05621 | 1.3222 |
| Parameters/Compound | Elderberry (µg/mL) | Cyanidin 3-O-glucoside (µg/mL) |
|---|---|---|
| IC50AAPH | 2.57 ± 0.31 | 0.72 ± 0.09 |
| EC50DPPH | 3.54 ± 0.16 | 4.25 ± 0.84 |
| IC50COX-1 | 65.26 ± 4.53 | 6.86 ± 0.35 |
| IC50COX-2 | 46.58 ± 5.22 | 7.21 ± 0.28 |
| Sample | Encapsulation Efficiency (%) | Mean Diameter (nm) |
|---|---|---|
| Liposomes (LMV) | - | 4500 ± 227 |
| LMV + EE (0.5 mg/mL) | 36.4 ± 1.2 | 5120 ± 1167 |
| LMV + EE (1.25 mg/mL) | 43.0 ± 3.0 | 9732 ± 718 |
| Liposomes (SV) | - | 181 ± 44 |
| SV + EE (0.5 mg/mL) | 16.4 ± 1.0 | 398 ± 44 |
| SV + EE (1.25 mg/mL) | 13.3 ± 1.6 | 2150 ± 289 |
| E-SUV | - | 132 ± 6 |
| E-SUV + EE (0.5 mg/mL) | 23.5 ± 0.7 | 93 ± 5 |
| E-SUV + EE (1.25 mg/mL) | 13.5 ± 0.7 | 7761 ± 167 |
| Compound | T (K) | Ksv (mL/g) | Kb (mL/g) | n | ∆G (kJ/g·mL−1) | ∆H (kJ/g mL−1) | ∆S (J/(g mL−1·K)) |
|---|---|---|---|---|---|---|---|
| Elderberry | 300 | 59.709 × 103 | 13.273 × 103 | 0.858 | −10.286 | −47.494 | −123.189 |
| 305 | 58.558 × 103 | 10.365 × 103 | 0.846 | −10.183 | |||
| 310 | 51.353 × 103 | 5.137 × 103 | 0.770 | −9.564 | |||
| 315 | 49.311 × 103 | 1.557 × 103 | 0.644 | −8.360 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strugała, P.; Loi, S.; Bażanów, B.; Kuropka, P.; Kucharska, A.Z.; Włoch, A.; Gabrielska, J. A Comprehensive Study on the Biological Activity of Elderberry Extract and Cyanidin 3-O-Glucoside and Their Interactions with Membranes and Human Serum Albumin. Molecules 2018, 23, 2566. https://doi.org/10.3390/molecules23102566
Strugała P, Loi S, Bażanów B, Kuropka P, Kucharska AZ, Włoch A, Gabrielska J. A Comprehensive Study on the Biological Activity of Elderberry Extract and Cyanidin 3-O-Glucoside and Their Interactions with Membranes and Human Serum Albumin. Molecules. 2018; 23(10):2566. https://doi.org/10.3390/molecules23102566
Chicago/Turabian StyleStrugała, Paulina, Sabrina Loi, Barbara Bażanów, Piotr Kuropka, Alicja Z. Kucharska, Aleksandra Włoch, and Janina Gabrielska. 2018. "A Comprehensive Study on the Biological Activity of Elderberry Extract and Cyanidin 3-O-Glucoside and Their Interactions with Membranes and Human Serum Albumin" Molecules 23, no. 10: 2566. https://doi.org/10.3390/molecules23102566
APA StyleStrugała, P., Loi, S., Bażanów, B., Kuropka, P., Kucharska, A. Z., Włoch, A., & Gabrielska, J. (2018). A Comprehensive Study on the Biological Activity of Elderberry Extract and Cyanidin 3-O-Glucoside and Their Interactions with Membranes and Human Serum Albumin. Molecules, 23(10), 2566. https://doi.org/10.3390/molecules23102566