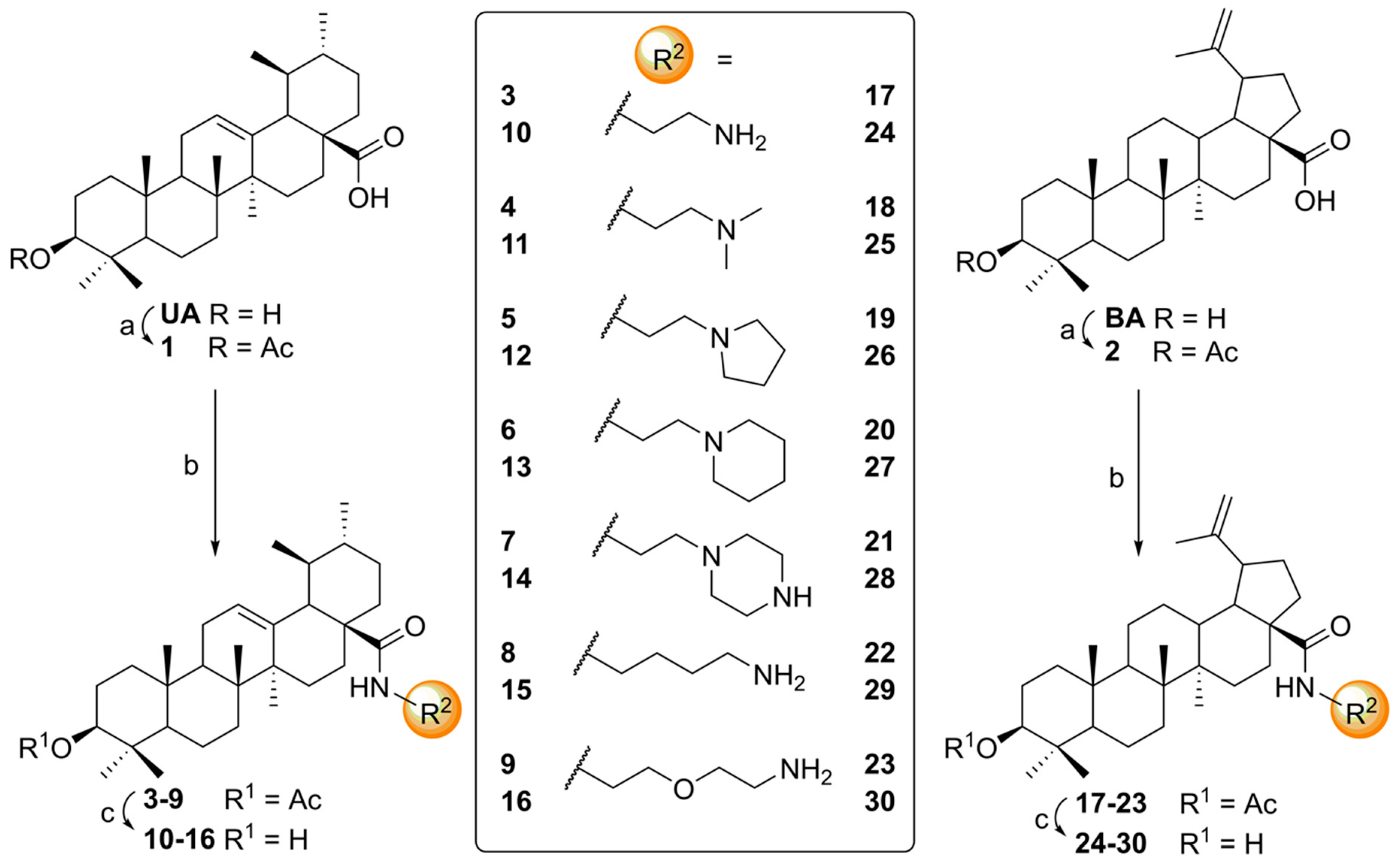
4.3. General Procedure C for Deacetylation of Triterpenoic Amides (10–16, 24–30)
To a solution of acetylated amides 3–9 or 17–23 (0.33 mmol) in methanol (10 mL) was added a solution of potassium hydroxide (1.65 mmol) in methanol (2 mL). The mixture was stirred at 25 °C for 2 or 3 days. After completion of the reaction (as indicated by TLC), aq. HCl was added until pH = 7. After usual work-up, the solvent was removed under reduced pressure, and the residue was subjected to column chromatography (silica gel, chloroform/methanol mixtures) yielding compounds 10–16 and 24–30 each as colorless solids.
(3β)-3-Acetyloxy-urs-12-en-28-oic acid (
1). Compound
1 was prepared according to general procedure A from ursolic acid. Yield: 96%; m.p. 287–290 °C (lit.: 289–290 °C [
15]).
(3β)-3-Acetyloxy-lup-20(29)en-28-oic acid (
2). Compound
2 was prepared according to general procedure A from betulinic acid. Yield: 93%; m.p. 281–284 °C (lit.: 280-282 °C [
16]).
(3β)-N-(2-Aminoethyl)-3-acetyloxy-urs-12-en-28-amide (
3). Compound
3 was prepared from
1 according to general procedure B using ethylenediamine. Column chromatography (SiO
2, CHCl
3/MeOH 9:1) gave
3 (yield: 80%); m.p. 202–205 °C (lit.: 140–142 °C [
17]); [δ]
D = +39.4° (
c 0.355, CHCl
3); R
f = 0.48 (CHCl
3/MeOH 9:1); IR (KBr): ν = 3413
br s, 2948
s, 1735
s, 1633
s, 1526
s, 1456
s, 1370
s, 1247
s, 1174
w, 1147
w, 1092
w, 1028
s, 1006
m, 986
m, 755
m cm
−1;
1H-NMR (500 MHz, CDCl
3):
δ = 6.88 (
t,
J = 5.3 Hz, 1H, NH), 5.34 (
t,
J = 3.3 Hz, 1H, 12-H), 4.49 (
dd,
J = 10.0, 5.9 Hz, 1H, 3-H), 3.62–3.54 (
m, 1H, 31-H
a), 3.38–3.30 (
m, 1H, 31-H
b), 3.13–3.01 (
m, 2H, 32-H
a, 32-H
b), 2.09–2.04 (
m, 1H, 18-H), 2.04 (
s, 3H, Ac), 2.03–1.87 (
m, 3H, 16-H
a, 11-H
a, 11-H
b), 1.82–1.22 (
m, 15H, 22-H
a, 16-H
b, 1-H
a, 15-H
a, 2-H
a, 2-H
b, 9-H, 22-H
b, 6-H
a, 21-H
a, 7-H
a, 19-H, 6-H
b, 7-H
b, 21-H
b), 1.08 (
s, 3H, 27-H), 1.07–0.95 (
m, 3H, 1-H
b, 15-H
b, 20-H), 0.96–0.92 (
m, 4H, 25-H, 20-H), 0.89–0.85 (
m, 6H, 23-H, 29-H), 0.85 (
s, 3H, 24-H), 0.84–0.80 (
m, 1H, 5-H), 0.74 (
s, 3H, 26-H) ppm;
13C-NMR (126 MHz, CDCl
3):
δ = 180.2 (C-28), 171.1 (Ac), 139.3 (C-13), 126.0 (C-12), 81.0 (C-3), 55.4 (C-5), 53.1 (C-18), 47.9 (C-17), 47.6 (C-9), 42.4 (C-14), 40.6 (C-32), 39.8 (C-19), 39.7 (C-8), 39.0 (C-20), 38.7 (C-31), 38.5 (C-1), 37.8 (C-4), 37.4 (C-22), 37.0 (C-10), 32.8 (C-7), 31.0 (C-21), 28.2 (C-23), 28.0 (C-15), 24.8 (C-16), 23.7 (C-2), 23.5 (C-11), 23.5 (C-27), 21.4 (Ac), 21.3 (C-30), 18.3 (C-6), 17.4 (C-29), 17.2 (C-26), 16.9 (C-24), 15.7 (C-25) ppm; MS (ESI, MeOH):
m/
z = 541 (100 %, [M + H]
+); analysis calcd for C
34H
56N
2O
3 (540.83): C 75.51, H 10.44, N 5.18; found: C 75.32, H 10.61, N 5.01.
(3β)-N-[2-(Dimethylamino)ethyl]-3-acetyloxy-urs-12-en-28-amide (4). Compound 4 was prepared from 1 according to general procedure B using N,N-dimethylethylenediamine. Column chromatography (SiO2, CHCl3/MeOH 95:5) gave 4 (yield: 88%); m.p. 121–124 °C; [α]D = +44.9° (c 0.300, CHCl3); Rf = 0.49 (CHCl3/MeOH 9:1); IR (KBr): ν = 3422br m, 2937m, 1734m, 1636m, 1522w, 1457m, 1384s, 1247m, 1028m cm−1; 1H-NMR (500 MHz, CDCl3): δ = 6.68 (t, J = 5.0 Hz, 1H, NH), 5.33 (t, J = 3.4 Hz, 1H, 12-H), 4.48 (dd, J = 10.5, 5.6 Hz, 1H, 3-H), 3.62–3.52 (m, 1H, 31-Ha), 3.29–3.21 (m, 1H, 31-Hb), 2.83 (t, J = 5.3 Hz, 2H, 32-H), 2.58 (s, 6H, 33-H, 33′-H), 2.03 (s, 3H, Ac), 2.02–1.86 (m, 4H, 16-Ha, 18-H, 11-Ha, 11-Hb), 1.83–1.76 (m, 1H, 22-Ha), 1.73–1.67 (m, 1H, 16-Hb), 1.67–1.57 (m, 4H, 1-Ha, 15-Ha, 2-Ha, 2-Hb), 1.57–1.26 (m, 9H, 9-H, 6-Ha, 7-Ha, 21-Ha, 22-Hb, 19-H, 6-Hb, 21-Hb, 7-Hb), 1.07 (s, 3H, 27-H), 1.06–0.94 (m, 3H, 1-Hb, 15-Hb, 20-H), 0.93 (d, J = 6.1 Hz, 3H, 30-H), 0.93 (s, 3H, 25-H), 0.87 (d, J = 6.5 Hz, 3H, 29-H), 0.85 (s, 3H, 23-H), 0.84 (s, 3H, 24-H), 0.83–0.80 (m, 1H, 5-H), 0.75 (s, 3H, 26-H) ppm; 13C-NMR (126 MHz, CDCl3): δ = 179.1 (C-28), 171.1 (Ac), 139.2 (C-13), 126.0 (C-12), 81.0 (C-3), 57.7 (C-32), 55.4 (C-5), 53.4 (C-18), 47.9 (C-17), 47.6 (C-9), 44.6 (C-33, C-33′), 42.4 (C-14), 39.8 (C-19), 39.7 (C-8), 39.0 (C-20), 38.4 (C-1), 37.8 (C-4), 37.4 (C-22), 37.0 (C-10), 35.9 (C-31), 32.8 (C-7), 31.0 (C-21), 28.2 (C-23), 28.0 (C-15), 24.8 (C-16), 23.7 (C-2), 23.5 (C-11), 23.4 (C-27), 21.4 (Ac), 21.3 (C-30), 18.3 (C-6), 17.3 (C-29), 17.1 (C-26), 16.9 (C-24), 15.7 (C-25) ppm; MS (ESI, MeOH): m/z = 569 (100%, [M + H]+); analysis calcd for C36H60N2O3 (568.89): C 76.01, H 10.63, N 4.92; found: C 75.87, H 10.84, N 4.69.
(3β)-N-(2-Pyrrolidin-1-ylethyl)-3-acetyloxy-urs-12-en-28-amide (5). Compound 5 was prepared from 1 according to general procedure B using 1-(2-aminoethyl)-pyrrolidine. Column chromatography (SiO2, CHCl3/MeOH 95:5) gave 5 (yield: 92%). m.p. 156–159 °C; [α]D = +45.9° (c 0.355, CHCl3); Rf = 0.44 (CHCl3/MeOH 9:1); IR (KBr): ν = 3404br s, 2948s, 1734s, 1651s, 1526s, 1456s, 1371s, 1246s, 1146w, 1091m, 1027s, 1006m, 985m, 753m cm−1; 1H-NMR (500 MHz, CDCl3): δ = 6.90 (dd, J = 11.5, 5.6 Hz, 1H, NH), 5.36 (dd, J = 7.1, 3.6 Hz, 1H, 12-H), 4.47 (dd, J = 10.5, 5.3 Hz, 1H, 3-H), 3.89–3.82 (m, 1H, 31-Ha), 3.82–3.73 (m, 2H, 33-Ha, 33′-Ha) 3.41–3.32 (m, 1H, 31-Hb), 3.30–3.21 (m, 2H, 32-H), 2.93–2.83 (m, 2H, 33-Hb, 33′-Hb), 2.23–2.06 (m, 4H, 34-H, 34′-H), 2.02 (s, 3H, Ac), 2.01–1.95 (m, 2H, 16-Ha, 18-H), 1.95–1.90 (m, 2H, 11-Ha, 11-Hb), 1.76–1.56 (m, 6H, 22-Ha, 16-Hb, 1-Ha, 15-Ha, 2-Ha, 2-Hb), 1.55–1.41 (m, 5H, 9-H, 6-Ha, 22-Hb, 7-Ha, 21-Ha), 1.41–1.21 (m, 4H, 19-H, 6-Hb, 7-Hb, 21-Hb), 1.06 (s, 3H, 27-H), 1.10–0.94 (m, 3H, 1-Hb, 15-Hb, 20-H), 0.92 (s, 3H, 25-H), 0.91 (d, J = 6.1 Hz, 3H, 30-H), 0.86 (d, J = 6.5 Hz, 3H, 29-H), 0.84 (s, 3H, 23-H), 0.83 (s, 3H, 24-H), 0.82–0.78 (m, 1H, 5-H), 0.71 (s, 3H, 26-H) ppm; 13C-NMR (126 MHz, CDCl3): δ = 179.8 (C-28), 171.1 (Ac), 138.9 (C-13), 126.0 (C-12), 80.9 (C-3), 55.3 (C-5), 55.0 (C-32), 54.8 (C-33, C-33′), 52.9 (C-18), 47.9 (C-17), 47.5 (C-9), 42.3 (C-14), 39.7 (C-19), 39.7 (C-8), 38.8 (C-20), 38.4 (C-1), 37.8 (C-4), 37.4 (C-22), 37.0 (C-10), 36.2 (C-31), 32.8 (C-7), 30.9 (C-21), 28.2 (C-23), 27.9 (C-15), 24.7 (C-16), 23.6 (C-2), 23.5 (C-27), 23.4 (C-11), 23.3 (C-34, C-34′), 21.4 (Ac), 21.3 (C-30), 18.3 (C-6), 17.2 (C-29), 17.1 (C-26), 16.8 (C-24), 15.6 (C-25) ppm; MS (ESI, MeOH): m/z = 595 (100%, [M + H]+); analysis calcd for C38H62N2O3 (594.93): C 76.72, H 10.50, N 4.71; found: C 76.60, H 10.72, N 4.59.
(3β)-N-(2-Piperidin-1-ylethyl)-3-acetyloxy-urs-12-en-28-amide (6). Compound 6 was prepared from 1 according to general procedure B using 1-(2-aminoethyl)-piperidine. Column chromatography (SiO2, CHCl3/MeOH 95:5) gave 6 (yield: 83%); m.p. 124–127 °C; [α]D = +34.6° (c 0.365, CHCl3); Rf = 0.26 (CHCl3/MeOH 95:5); IR (KBr): ν = 3424br s, 2936s, 2872m, 2854m, 1736s, 1638s, 1508m, 1456m, 1370m, 1246s, 1154w, 1128w, 1092w, 1028m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 6.56–6.51 (m, 1H, NH), 5.30 (t, J = 3.6 Hz, 1H, 12-H), 4.49 (dd, J = 10.4, 5.8 Hz, 1H, 3-H), 3.41–3.29 (m, 1H, 31-Ha), 3.24–3.12 (m, 1H, 31-Hb), 2.49–2.27 (m, 6H, 32-H, 33-H, 33′-H), 2.03 (s, 3H, Ac), 2.01–1.80 (m, 5H, 16-Ha, 11-Ha, 11-Hb, 18, 22-Ha), 1.78–1.22 (m, 20H, 16-Hb, 15-Ha, 1-Ha, 2-Ha, 2-Hb, 34-H, 34′-H, 9-H, 6-Ha, 21-Ha, 7-Ha, 35-H, 22-Hb, 19-H, 6-Hb, 21-Hb, 7-Hb), 1.08 (s, 3H, 27-H), 1.14–0.96 (m, 3H, 1-Hb, 15-Hb, 20-H), 0.95–0.93 (m, 3H, 30-H), 0.93 (s, 3H, 25-H), 0.88 (d, J = 6.5 Hz, 3H, 29-H), 0.86 (s, 3H, 23-H), 0.85 (s, 3H, 24-H), 0.84–0.79 (m, 1H, 5-H), 0.77 (s, 3H, 26-H) ppm; 13C-NMR (101 MHz, CDCl3): δ = 178.0 (C-28), 171.1 (Ac), 139.5 (C-13), 125.6 (C-12), 81.0 (C-3), 57.2 (C-32), 55.4 (C-5), 54.5 (C-33, C-33′), 54.0 (C-18), 47.9 (C-17), 47.6 (C-9), 42.5 (C-14), 39.9 (C-19), 39.7 (C-8), 39.2 (C-20), 38.4 (C-1), 37.8 (C-4), 37.5 (C-22), 37.0 (C-10), 36.0 (C-31), 32.9 (C-7), 31.1 (C-21), 28.2 (C-23), 28.0 (C-15), 26.2 (C-34, C-34′), 24.9 (C-16), 24.5 (C-35), 23.7 (C-2), 23.5 (C-11), 23.4 (C-27), 21.4 (Ac), 21.4 (C-30), 18.3 (C-6), 17.5 (C-29), 17.1 (C-26), 16.9 (C-24), 15.7 (C-25) ppm; MS (ESI, MeOH): m/z = 609 (100%, [M + H]+); analysis calcd for C39H64N2O3 (608.95): C 76.92, H 10.59, N 4.60; found: C 76.77, H 10.79, N 4.41.
(3β)-N-(2-Piperazin-1-ylethyl)-3-acetyloxy-urs-12-en-28-amide (
7). Compound
7 was prepared from
1 according to general procedure B using 1-(2-aminoethyl)-piperazine. Column chromatography (SiO
2, CHCl
3/MeOH 9:1) gave
7 (yield: 82%); m.p. 145–147 °C (lit.: 147–150 °C [
18]); [a]
D = +35.9° (
c 0.365, CHCl
3); R
f = 0.29 (CHCl
3/MeOH 9:1); IR (KBr): ν = 3441
s, 2947
m, 1734
m, 1636
m, 1458
w, 1370
w, 1247
m, 1027
w cm
−1;
1H-NMR (400 MHz, CDCl
3): δ = 6.42 (
t,
J = 4.6 Hz, 1H, N
H), 5.30 (
t,
J = 3.6 Hz, 1H, 12-H), 4.49 (
dd,
J = 10.4, 5.3 Hz, 1H, 3-H), 3.42–3.32 (
m, 1H, 31-H
a), 3.23–3.13 (
m, 1H, 31-H
b), 2.93 (
t,
J = 4.9 Hz, 4H, 34-H, 34′-H), 2.49–2.37 (
m, 6H, 32-H, 33-H, 33′-H), 2.04 (
s, 3H, Ac), 2.02–1.80 (
m, 5H, 16-H
a, 11-H
a, 11-H
b, 22-H
a, 18-H), 1.80–1.22 (
m, 14H, 16-H
b, 15-H
a, 1-H
a, 2-H
a, 2-H
b, 9-H, 6-H
a, 21-H
a, 7-H
a, 22-H
b, 19-H, 6-H
b, 21-H
b, 7-H
b), 1.08 (
s, 3H, 27-H), 1.07–0.94 (
m, 3H, 1-H
b, 15-H
b, 20-H), 0.96–0.94 (
m, 3H, 30-H), 0.93 (
s, 3H, 25-H), 0.88 (
d,
J = 6.5 Hz, 3H, 29-H), 0.86 (
s, 3H, 23-H), 0.85 (
s, 3H, 24-H), 0.84–0.78 (
m, 1H, 5-H), 0.77 (
s, 3H, 26-H) ppm;
13C-NMR (101 MHz, CDCl
3): δ = 178.0 (C-28), 171.1 (Ac), 139.7 (C-13), 125.5 (C-12), 81.0 (C-3), 57.1 (C-32), 55.4 (C-5), 54.1 (C-18), 53.9 (C-33), 47.9 (C-17), 47.6 (C-9), 46.1 (C-34), 42.6 (C-14), 39.9 (C-19), 39.7 (C-8), 39.3 (C-20), 38.4 (C-1), 37.8 (C-4), 37.5 (C-22), 37.0 (C-10), 35.8 (C-31), 32.8 (C-7), 31.1 (C-21), 28.2 (C-23), 28.0 (C-15), 25.0 (C-16), 23.7 (C-2), 23.6 (C-11), 23.4 (C-27), 21.4 (Ac), 21.3 (C-30), 18.3 (C-6), 17.5 (C-29), 17.1 (C-26), 16.9 (C-24), 15.7 (C-25) ppm; MS (ESI, MeOH):
m/
z = 610 (100 %, [M + H]
+); analysis calcd for C
38H
63N
3O
3 (609.94): C 74.83, H 10.41, N 6.89; found: C 74.57, H 10.69, N 6.64.
(3β)-N-(4-Aminobutyl)-3-acetyloxy-urs-12-en-28-amide (8). Compound 8 was prepared from 1 according to general procedure B using 1,4-diaminobutane. Column chromatography (SiO2, CHCl3/MeOH/NH4OH 90:10:1) gave 8 (yield: 79%); m.p. 117–120 °C; [α]D = +33.7° (c 0.305, CHCl3); Rf = 0.34 (CHCl3/MeOH/NH4OH 90:10:1); IR (KBr): ν = 3440s, 2928m, 1736w, 1636m, 1522w, 1458w, 1370w, 1246m, 1028w cm−1; 1H-NMR (400 MHz, CDCl3): δ = 6.03 (t, J = 5.5 Hz, 1H, NH), 5.30 (t, J = 3.6 Hz, 1H, 12-H), 4.53–4.46 (dd, J = 9.7, 6.2 Hz, 1H, 3-H), 3.41–3.30 (m, 1H, 31-Ha), 3.05–2.94 (m, 1H, 31-Hb), 2.78 (t, J = 6.2 Hz, 2H, 32-Ha, 32-Hb), 2.04 (s, 3H, Ac), 2.02–1.90 (m, 3H, 11-Ha, 11-Hb, 16-Ha), 1.90–1.80 (m, 2H, 18-H, 22-Ha), 1.76–1.20 (m, 18H, 16-Hb, 15-Ha, 1-Ha, 2-Ha, 2-Hb, 9-H, 6-Ha, 34-Ha, 34-Hb, 33-Ha, 33-Hb, 21-Ha, 7-Ha, 19-H, 22-Hb, 6-Hb, 21-Hb, 7-Hb), 1.09 (s, 3H, 27-H), 1.14–1.01 (m, 2H, 1-Hb, 15-Hb), 0.95 (s, 7H, 25-H, 30-H, 20-H), 0.89–0.86 (m, 3H, 29-H), 0.86 (s, 3H, 23-H), 0.85 (s, 3H, 24-H), 0.84–0.80 (m, 1H, 5-H), 0.77 (s, 3H, 26-H) ppm; 13C-NMR (101 MHz, CDCl3): δ = 178.2 (C-28), 171.1 (Ac), 140.2 (C-13), 125.6 (C-12), 81.0 (C-3), 55.4 (C-5), 54.1 (C-18), 47.8 (C-17), 47.6 (C-9), 42.7 (C-14), 41.8 (C-32), 39.9 (C-19), 39.7 (C-8), 39.4 (C-31), 39.3 (C-20), 38.5 (C-1), 37.8 (C-4), 37.4 (C-22), 37.0 (C-10), 32.8 (C-7), 31.2 (C-21), 30.8 (C-33), 28.2 (C-23), 28.0 (C-15), 26.9 (C-34), 25.0 (C-16), 23.7 (C-2), 23.6 (C-11), 23.4 (C-27), 21.5 (Ac), 21.4 (C-30), 18.3 (C-6), 17.4 (C-29), 17.1 (C-26), 16.9 (C-24), 15.7 (C-25) ppm; MS (ESI, MeOH): m/z = 569 (100%, [M + H]+); analysis calcd for C36H60N2O3 (568.89): C 76.01, H 10.63, N 4.92; found: C 75.84, H 10.91, N 4.63.
(3β)-N-[2-(2-Aminoethoxy)ethyl]-3-acetyloxy-urs-12-en-28-amide (9). Compound 9 was prepared from 1 according to general procedure B using 2,2′-oxybis(ethylamine). Column chromatography (SiO2, CHCl3/MeOH/NH4OH 90:10:0.1) gave 9 (yield: 78%); m.p. 91–94 °C; [α]D = +18.3° (c 0.310, CHCl3); Rf = 0.39 (CHCl3/MeOH 9:1); IR (KBr): ν = 3424br s, 2927s, 2871s, 1735s, 1640s, 1529m, 1455m, 1370m, 1247s, 1120m, 1027m cm−1; 1H-NMR (500 MHz, CDCl3): δ = 6.29 (t, J = 5.0 Hz, 1H, NH), 5.28 (t, J = 3.6 Hz, 1H, 12-H), 4.48 (dd, J = 10.9, 5.3 Hz, 1H, 3-H), 3.57–3.44 (m, 5H, 31-Ha, 32-H, 33-H), 3.30–3.22 (m, 1H, 31-Hb), 2.87 (t, J = 5.3 Hz, 2H, 34-H), 2.03 (s, 3H, Ac), 2.01–1.81 (m, 5H, 16-Ha, 11-Ha, 11-Hb, 18-H, 22-Ha), 1.78–1.22 (m, 14H, 16-Hb, 15-Ha, 1-Ha, 2-Ha, 2-Hb, 9-H, 6-Ha, 21-Ha, 7-Ha, 22-Hb, 19-H, 6-Hb, 21-Hb, 7-Hb), 1.08 (s, 3H, 27-H), 1.12–1.00 (m, 2H, 1-Hb, 15-Hb), 0.99–0.90 (m, 4H, 30-H, 20-H), 0.93 (s, 3H, 25-H), 0.86 (d, J = 6.6 Hz, 3H, 29-H), 0.85 (s, 3H, 23-H), 0.84 (s, 3H, 24-H), 0.83–0.80 (m, 1H, 5-H), 0.78 (s, 3H, 26-H) ppm; 13C-NMR (126 MHz, CDCl3): δ = 178.3 (C-28), 171.1 (Ac), 139.8 (C-13), 125.7 (C-12), 81.0 (C-3), 73.2 (C-33), 69.6 (C-32), 55.4 (C-5), 53.9 (C-18), 47.9 (C-17), 47.6 (C-9), 42.6 (C-14), 42.0 (C-34), 39.9 (C-19), 39.7 (C-8), 39.3 (C-31), 39.2 (C-20), 38.4 (C-1), 37.8 (C-4), 37.3 (C-22), 37.0 (C-10), 32.8 (C-7), 31.0 (C-21), 28.2 (C-23), 28.0 (C-15), 25.0 (C-16), 23.7 (C-2), 23.6 (C-11), 23.4 (C-27), 21.4 (Ac), 21.4 (C-30), 18.3 (C-6), 17.4 (C-29), 17.0 (C-26), 16.8 (C-24), 15.7 (C-25) ppm; MS (ESI, MeOH): m/z = 585 (100 %, [M + H]+); analysis calcd for C36H60N2O4 (584.89): C 73.93, H 10.34, N 4.79; found: C 73.77, H 10.51, N 4.56.
(3β)-N-(2-Aminoethyl)-3-hydroxy-urs-12-en-28-amide (
10). Compound
10 was prepared from
3 according to general procedure C. Column chromatography (SiO
2, CHCl
3/MeOH/NH
4OH 90:10:0.1) gave
10 (yield: 85%); m.p. 139–142 °C (lit.: 145–147 °C [
17]); [α]
D = +38.6° (
c 0.300, CHCl
3); R
f = 0.34 (CHCl
3/MeOH 9:1); IR (KBr): ν = 3425
br s, 2926
s, 1638
m, 1529
m, 1454
m, 1386
w, 1092
w, 1046
m, 755
m cm
−1;
1H-NMR (400 MHz, CDCl
3): δ = 6.36 (
t,
J = 5.4 Hz, 1H, N
H), 5.33 (
t,
J = 3.4 Hz, 1H, 12-H), 3.46–3.36 (
m, 1H, 31-H
a), 3.21 (
dd,
J = 11.1, 4.7 Hz, 1H, 3-H), 3.13–3.02 (
m, 1H, 31-H
b), 2.82 (
t,
J = 5.9 Hz, 2H, 32-H
a, 32-H
b), 2.05–1.82 (
m, 5H, 16-H
a, 11-H
a, 11-H
b, 18-H, 22-H
a), 1.77–1.23 (
m, 14H, 16-H
b, 15-H
a, 1-H
a, 2-H
a, 2-H
b, 9-H, 6-H
a, 21-H
a, 7-H
a, 22-H
b, 19-H, 6-H
b, 21-H
b, 7-H
b), 1.09 (
s, 3H, 27-H), 1.07–0.99 (
m, 2H, 15-H
b, 1-H
b), 0.98 (
s, 3H, 23-H), 0.96–0.93 (
m, 4H, 20-H, 30-H), 0.91 (
s, 3H, 25-H), 0.87 (
d,
J = 6.5 Hz, 3H, 29-H), 0.78 (
s, 6H, 24-H, 26-H), 0.74–0.69 (
m, 1H, 5-H) ppm;
13C-NMR (101 MHz, CDCl
3):
δ = 178.8 (C-28), 139.7 (C-13), 125.9 (C-12), 79.1 (C-3), 55.3 (C-5), 53.9 (C-18), 48.0 (C-17), 47.7 (C-9), 42.6 (C-14), 41.8 (C-31), 41.3 (C-32), 39.9 (C-19), 39.7 (C-8), 39.2 (C-20), 38.9 (C-4), 38.8 (C-1), 37.5 (C-22), 37.1 (C-10), 32.9 (C-7), 31.1 (C-21), 28.3 (C-23), 28.0 (C-15), 27.4 (C-2), 25.0 (C-16), 23.6 (C-11), 23.4 (C-27), 21.4 (C-30), 18.4 (C-6), 17.4 (C-29), 17.1 (C-26), 15.8 (C-24), 15.7 (C-25) ppm; MS (ESI, MeOH):
m/
z = 499 (100 %, [M + H]
+); analysis calcd for C
32H
54N
2O
2 (498.80): C 77.06, H 10.91, N 5.62; found: C 76.92, H 11.08, N 5.40.
(3β)-N-[2-(Dimethylamino)ethyl]-3-hydroxy-urs-12-en-28-amide (11). Compound 11 was prepared from 4 according to general procedure C. Column chromatography (SiO2, CHCl3/MeOH 95:5) gave 11 (yield: 86%). m.p. 270–273 °C (decomp.); [α]D = +38.5° (c 0.375, CHCl3); Rf = 0.44 (CHCl3/MeOH 9:1); IR (KBr): ν = 3402br s, 2924s, 2868s, 2684m, 1658s, 1518m, 1460s, 1386m, 1212w, 1138w, 1046m, 1028m, 994m cm−1; 1H-NMR (500 MHz, CDCl3): δ = 7.18 (dd, J = 5.6, 5.6 Hz, 1H, NH), 5.41 (t, J = 3.6 Hz, 1H, 12-H), 3.78–3.68 (m, 1H, 31-Ha), 3.59–3.49 (m, 1H, 31-Hb), 3.21 (dd, J = 11.0, 4.7 Hz, 1H, 3-H), 3.18–3.13 (m, 2H, 32-H), 2.84 (s, 6H, 33-H, 33′-H), 2.20 (d, J = 10.5 Hz, 1H, 18-H), 2.03 (ddd, J = 13.7, 13.7, 4.2 Hz, 1H, 16-Ha), 1.96–1.91 (m, 2H, 11-Ha, 11-Hb), 1.81–1.26 (m, 15H, 22-Ha, 16-Hb, 15-Ha, 1-Ha, 2-Ha, 2-Hb, 6-Ha, 9-H, 22-Hb, 7-Ha, 21-Ha, 19-H, 6-Hb, 7-Hb, 21-Hb), 1.08 (s, 3H, 27-H), 1.07–0.96 (m, 3H, 15-Hb, 20-H, 1-Hb), 0.98 (s, 3H, 23-H), 0.93 (d, J = 6.5 Hz, 3H, 30-H), 0.90 (s, 3H, 25-H), 0.88 (d, J = 6.5 Hz, 3H, 29-H), 0.77 (s, 3H, 24-H), 0.73 (s, 3H, 26-H), 0.72–0.69 (m, 1H, 5-H) ppm; 13C-NMR (126 MHz, CDCl3): δ = 179.6 (C-28), 138.8 (C-13), 126.2 (C-12), 79.1 (C-3), 58.2 (C-32), 55.3 (C-5), 52.7 (C-18), 48.0 (C-17), 47.7 (C-9), 44.2 (C-33), 43.9 (C-33′), 42.3 (C-14), 39.7 (C-8), 39.7 (C-19), 38.9 (C-1), 38.8 (C-20), 38.7 (C-4), 37.4 (C-22), 37.1 (C-10), 35.1 (C-31), 32.9 (C-7), 31.0 (C-21), 28.3 (C-23), 28.0 (C-15), 27.4 (C-2), 24.6 (C-16), 23.6 (C-27), 23.5 (C-11), 21.3 (C-30), 18.4 (C-6), 17.2 (C-29), 17.2 (C-26), 15.8 (C-24), 15.6 (C-25) ppm; MS (ESI, MeOH): m/z = 527 (100%, [M + H]+); analysis calcd for C34H58N2O2 (526.85): C 77.51, H 11.10, N 5.32; found: C 77.37, H 11.25, N 5.17.
(3β)-N-(2-Pyrrolidin-1-ylethyl)-3-hydroxy-urs-12-en-28-amide (12). Compound 12 was prepared from 5 according to general procedure C. Column chromatography (SiO2, CHCl3/MeOH 95:5) gave 12 (yield: 84%); m.p. 263–266 °C (decomp.); [α]D = +39.8° (c 0.445, MeOH); Rf = 0.40 (CHCl3/MeOH 9:1); IR (KBr): ν = 3420br s, 2926s, 2670s, 2616m, 2488m, 2360s, 2342m, 1636m, 1526m, 1456m, 1386m, 1278w, 1244w, 1092w, 1046m, 998m, 668m cm−1; 1H-NMR (500 MHz, CDCl3): δ = 7.06 (t, J = 5.5 Hz, 1H, NH), 5.40 (t, J = 3.5 Hz, 1H, 12-H), 3.89–3.77 (m, 2H, 33-Ha, 33′-Ha), 3.76–3.66 (m, 1H, 31-Ha), 3.61–3.51 (m, 1H, 31-Hb), 3.27–3.14 (m, 3H, 3-H, 32-H), 2.89–2.77 (m, 2H, 33-Hb, 33′-Hb), 2.26–2.17 (m, 2H, 34-Ha, 34′-Ha), 2.14 (d, J = 11.0 Hz, 1H, 18-H), 2.12–1.97 (m, 3H, 34-Hb, 34′-Hb, 16-Ha), 1.94 (dd, J = 8.8, 3.4 Hz, 2H, 11-Ha, 11-Hb), 1.81–1.22 (m, 15H, 22-Ha, 16-Hb, 15-Ha, 1-Ha, 2-Ha, 2-Hb, 6-Ha, 9-H, 22-Hb, 7-Ha, 21-Ha, 19-H, 6-Hb, 21-Hb, 7-Hb), 1.08 (s, 3H, 27-H), 1.07–0.96 (m, 3H, 15-Hb, 20-H, 1-Hb), 0.98 (s, 3H, 23-H), 0.94 (d, J = 6.3 Hz, 3H, 30-H), 0.91 (s, 3H, 25-H), 0.88 (d, J = 6.4 Hz, 3H, 29-H), 0.78 (s, 3H, 24-H), 0.73 (s, 3H, 26-H), 0.73–0.69 (m, 1H, 5-H) ppm; 13C-NMR (126 MHz, CDCl3): δ = 179.6 (C-28), 138.9 (C-13), 126.2 (C-12), 79.2 (C-3), 55.4 (C-32), 55.3 (C-5), 54.9 (C-33), 54.6 (C-33′), 52.9 (C-18), 47.9 (C-17), 47.7 (C-9), 42.4 (C-14), 39.8 (C-19), 39.7 (C-8), 38.9 (C-1), 38.9 (C-20), 38.7 (C-4), 37.4 (C-22), 37.1 (C-10), 36.1 (C-31), 32.9 (C-7), 31.0 (C-21), 28.3 (C-23), 28.0 (C-15), 27.4 (C-2), 24.7 (C-16), 23.6 (C-27), 23.5 (C-34, C-34′), 23.4 (C-11), 21.4 (C-30), 18.5 (C-6), 17.3 (C-29), 17.2 (C-26), 15.8 (C-24), 15.6 (C-25) ppm; MS (ESI, MeOH): m/z = 553 (100%, [M + H]+); analysis calcd for C36H60N2O2 (552.89): C 78.21, H 10.94, N 5.07; found: C78.02, H 11.09, N 4.83.
(3β)-N-(2-Piperidin-1-ylethyl)-3-hydroxy-urs-12-en-28-amide (13). Compound 13 was prepared from 6 according to general procedure C. Column chromatography (SiO2, CHCl3/MeOH 95:5) gave 13 (yield: 93%); m.p. 120–124 °C; [α]D = +40.5° (c 0.350, CHCl3); Rf = 0.23 (CHCl3/MeOH 95:5); IR (KBr): ν = 3416br s, 2934s, 2870m, 2854m, 1636s, 1512m, 1456m, 1378m, 1358w, 1304w, 1272w, 1256w, 1156w, 1130w, 1092w, 1046m, 998m, 754m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 6.62–6.54 (m, 1H, NH), 5.31 (t, J = 3.6 Hz, 1H, 12-H), 3.42–3.32 (m, 1H, 31-Ha), 3.25–3.16 (m, 2H, 3-H, 31-Hb), 2.50–2.35 (m, 6H, 32-H, 33-H, 33′-H), 2.02–1.80 (m, 5H, 16-Ha, 11-Ha, 11-Hb, 18-H, 22-Ha), 1.78–1.20 (m, 20H, 16-Hb, 15-Ha, 1-Ha, 34-H, 34′-H, 35-H, 9-H, 6-Ha, 21-Ha, 7-Ha, 2-Ha, 2-Hb, 22-Hb, 19-H, 6-Hb, 21-Hb, 7-Hb), 1.08 (s, 3H, 27-H), 1.07–0.97 (m, 3H, 15-Hb, 1-Hb, 20-H), 0.98 (s, 3H, 23-H), 0.96–0.93 (m, 3H, 30-H), 0.91 (s, 3H, 25-H), 0.87 (d, J = 6.4 Hz, 3H, 29-H), 0.77 (s, 6H, 24-H, 26-H), 0.74–0.69 (m, 1H, 5-H) ppm; 13C-NMR (101 MHz, CDCl3): δ = 178.0 (C-28), 139.4 (C-13), 125.8 (C-12), 79.1 (C-3), 57.2 (C-32), 55.3 (C-5), 54.4 (C-33, C-33′), 53.9 (C-18), 47.7 (C-17), 47.7 (C-9), 42.5 (C-14), 39.9 (C-19), 39.7 (C-8), 39.2 (C-20), 38.9 (C-1), 38.8 (C-4), 37.5 (C-22), 37.1 (C-10), 35.9 (C-31), 32.9 (C-7), 31.1 (C-21), 28.3 (C-23), 28.0 (C-15), 27.3 (C-35), 26.0 (C-34, C-34′), 24.9 (C-16), 24.4 (C-2), 23.5 (C-11), 23.4 (C-27), 21.4 (C-30), 18.4 (C-6), 17.4 (C-29), 17.1 (C-26), 15.8 (C-24), 15.6 (C-25) ppm; MS (ESI, MeOH): m/z = 567 (100%, [M + H]+); analysis calcd for C37H62N2O2 (566.92): C 78.39, H 11.02, N 4.94; found: C 78.11, H 11.19, N 4.80.
(3β)-N-(2-Piperazin-1-ylethyl)-3-hydroxy-urs-12-en-28-amide (
14). Compound
14 was prepared from
7 according to general procedure C. Column chromatography (SiO
2, CHCl
3/MeOH 9:1) gave
14 (yield: 88%); m.p. 214–217 °C (lit.: 217–220 °C [
18]); [α]
D = +32.2° (
c 0.335, CHCl
3); R
f = 0.20 (CHCl
3/MeOH 9:1); IR (KBr): ν = 3420
br s, 2928
s, 2870
m, 2496
w, 1630
s, 1528
m, 1458
m, 1384
s, 1178
w, 1138
w, 1092
w, 1046
m, 1030
m, 998
m cm
−1;
1H-NMR (400 MHz, CD
3OD):
δ = 5.37 (
t,
J = 3.6 Hz, 1H, 12-H), 3.42–3.31 (
m, 1H, 31-H
a), 3.28–3.18 (
m, 2H, 31-H
b, 3-H), 3.16 (
t,
J = 5.0 Hz, 4H, 34-H, 34‘-H), 2.77–2.61 (
m, 4H, 33-H, 33‘-H), 2.54 (
t,
J = 6.6 Hz, 2H, 32-H), 2.17–1.89 (
m, 4H, 18-H, 16-H
a, 11-H
a, 11-H
b), 1.86–1.30 (
m, 15H, 15-H
a, 22-H
a, 16-H
b, 1-H
a, 2-H
a, 2-H
b, 9-H, 6-H
a, 7-H
a, 22-H
b, 21-H
a, 19-H, 6-H
b, 21-H
b, 7-H
b), 1.16 (
s, 3H, 27-H), 1.15–1.02 (
m, 3H, 15-H
b, 1-H
b, 20-H), 1.02–0.97 (
m, 3H, 30-H), 1.01 (
s, 3H, 23-H), 0.99 (
s, 3H, 25-H), 0.95 (
d,
J = 6.4 Hz, 3H, 29-H), 0.84 (
s, 3H, 26-H), 0.81 (
s, 3H, 24-H), 0.80–0.76 (
m, 1H, 5-H) ppm;
13C-NMR (101 MHz, CD
3OD): δ = 180.3 (C-28), 140.1 (C-13), 127.0 (C-12), 79.6 (C-3), 57.8 (C-32), 56.7 (C-5), 54.3 (C-18), 51.8 (C-33, C-33‘), 49.0 (C-17), 48.9 (C-9), 45.3 (C-34, C-34‘), 43.4 (C-14), 40.9 (C-8), 40.9 (C-19), 40.3 (C-20), 39.9 (C-1), 39.8 (C-4), 38.8 (C-22), 38.1 (C-10), 37.4 (C-31), 34.1 (C-7), 31.9 (C-21), 29.0 (C-15), 28.8 (C-23), 27.9 (C-2), 25.3 (C-16), 24.5 (C-11), 24.0 (C-27), 21.6 (C-30), 19.4 (C-6), 18.0 (C-26), 17.7 (C-29), 16.4 (C-24), 16.1 (C-25) ppm; MS (ESI, MeOH):
m/
z = 568 (100%, [M + H]
+); analysis calcd for C
36H
61N
3O
2 (567.90): C 76.14, H 10.83, N 7.40; found: C 75.84, H 11.03, N 7.25.
(3β)-N-(4-Aminobutyl)-3-hydroxy-urs-12-en-28-amide (15). Compound 15 was prepared from 8 according to general procedure C. Column chromatography (SiO2, CHCl3/MeOH 9:1) gave 15 (yield: 87%); m.p. 177–180 °C; [α]D = +42.2° (c 0.315, DMSO); Rf = 0.33 (CHCl3/MeOH 88:12); IR (KBr): ν = 3424br m, 2927m, 1629w, 1534w, 1384s, 1029w cm−1; 1H-NMR (400 MHz, DMSO-d6): δ = 7.79–7.54 (m, 2H, NH2), 7.17 (t, J = 5.7 Hz, 1H, NH), 5.19 (t, J = 3.6 Hz, 1H, 12-H), 4.28 (s, 1H, OH), 3.07–2.90 (m, 3H, 3-H, 31-H), 2.76 (t, J = 7.4 Hz, 2H, 32-H), 2.15 (d, J = 10.9 Hz, 1H, 18-H), 1.97–1.65 (m, 4H, 16-Ha, 11-Ha, 11-Hb, 15-Ha), 1.65–1.17 (m, 18H, 16-Hb, 22-Ha, 1-Ha, 34-Ha, 34-Hb, 6-Ha, 9-H, 2-Ha, 2-Hb, 7-Ha, 22-Hb, 21-Ha, 33-Ha, 33-Hb, 19-H, 6-Hb, 21-Hb, 7-Hb), 1.03 (s, 3H, 27-H), 0.95–0.88 (m, 6H, 15-Hb, 1-Hb, 30-H, 20-H), 0.89 (s, 3H, 23-H), 0.85 (s, 3H, 25-H), 0.82 (d, J = 6.4 Hz, 3H, 29-H), 0.67 (s, 6H, 24-H, 26-H), 0.67–0.64 (m, 1H, 5-H) ppm; 13C-NMR (101 MHz, DMSO-d6): δ = 176.2 (C-28), 138.4 (C-13), 124.5 (C-12), 76.8 (C-3), 54.8 (C-5), 51.9 (C-18), 47.0 (C-9), 46.5 (C-17), 41.6 (C-14), 39.1 (C-8), 38.8 (C-19), 38.7 (C-32), 38.5 (C-20), 38.4 (C-4), 38.2 (C-1), 38.0 (C-31), 37.1 (C-22), 36.5 (C-10), 32.7 (C-7), 30.4 (C-21), 28.3 (C-23), 27.4 (C-15), 27.0 (C-2), 26.0 (C-33), 24.6 (C-34), 23.5 (C-16), 23.3 (C-27), 22.9 (C-11), 21.1 (C-30), 18.0 (C-6), 17.1 (C-29), 16.8 (C-26), 16.1 (C-24), 15.2 (C-25) ppm; MS (ESI, MeOH): m/z = 527 (100 %, [M + H]+), 1053 (18 %, [2M + H]+); analysis calcd for C34H58N2O2 (526.85): C 77.51, H 11.10, N 5.32; found: C 77.39, H 11.30, N 5.16.
(3β)-N-[2-(2-Aminoethoxy)ethyl]-3-acetyloxy-urs-12-en-28-amide (16). Compound 16 was prepared from 9 according to general procedure C. Column chromatography (SiO2, CHCl3/MeOH 9:1) gave 16 (yield: 86%). m.p. 126–129 °C; [α]D = +34.3° (c 0.305, CHCl3); Rf = 0.20 (CHCl3/MeOH 9:1); IR (KBr): ν = 3426br s, 2926s, 2870m, 1636m, 1534m, 1534m, 1456w, 1384w, 1118w, 1044w, 1030w cm−1; 1H-NMR (500 MHz, CD3OD): δ = 5.33 (t, J = 3.7 Hz, 1H, 12-H), 3.62 (dd, J = 5.7, 4.7 Hz, 2H, 33-H), 3.52 (t, J = 5.9 Hz, 2H, 32-H), 3.44–3.37 (m, 1H, 31-Ha), 3.34–3.24 (m, 1H, 31-Hb), 3.16 (dd, J = 11.3, 4.7 Hz, 1H, 3-H), 3.03 (dd, J = 5.7, 4.6 Hz, 2H, 34-H), 2.16–1.91 (m, 4H, 18-H, 16-Ha, 11-Ha, 11-Hb), 1.84–1.27 (m, 15H, 15-Ha, 22-Ha, 1-Ha, 16-Hb, 2-Ha, 2-Hb, 9-H, 6-Ha, 7-Ha, 22-Hb, 21-Ha, 19-H, 6-Hb, 21-Hb, 7-Hb), 1.13 (s, 3H, 27-H), 1.11–0.98 (m, 3H, 15-Hb, 1-Hb, 20-H), 0.98–0.95 (m, 3H, 30-H), 0.98 (s, 3H, 23-H), 0.96 (s, 3H, 25-H), 0.91 (d, J = 6.4 Hz, 3H, 29-H), 0.81 (s, 3H, 26-H), 0.78 (s, 3H, 24-H), 0.77–0.73 (m, 1H, 5-H) ppm; 13C-NMR (126 MHz, CD3OD): δ = 180.6 (C-28), 140.0 (C-13), 127.1 (C-12), 79.6 (C-3), 70.8 (C-32), 68.9 (C-33), 56.7 (C-5), 54.2 (C-18), 49.2 (C-17), 48.9 (C-9), 43.3 (C-14), 40.9 (C-8), 40.9 (C-34), 40.8 (C-19), 40.3 (C-20), 40.2 (C-31), 40.0 (C-1), 39.8 (C-4), 38.8 (C-22), 38.1 (C-10), 34.2 (C-7), 31.9 (C-21), 29.0 (C-15), 28.8 (C-23), 27.9 (C-2), 25.3 (C-16), 24.4 (C-11), 24.0 (C-27), 21.6 (C-30), 19.4 (C-6), 17.9 (C-29), 17.7 (C-26), 16.4 (C-24), 16.1 (C-25) ppm; MS (ESI, MeOH): m/z = 543 (100%, [M + H]+); analysis calcd for C34H58N2O3 (542.85): C 75.23, H 10.77, N 5.16; found: C 75.02, H 10.98, N 5.02.
(3β)-N-(2-Aminoethyl)-3-acetyloxy-lup-20(29)-en-28-amide (17). Compound 17 was prepared from 2 according to general procedure B using ethylenediamine. Column chromatography (SiO2, CHCl3/MeOH 9:1) gave 17 (yield: 83%); m.p. 152–154 °C; [α]D = +8.4° (c 0.330, CHCl3); Rf = 0.38 (CHCl3/MeOH 9:1); IR (KBr): ν = 3442br s, 2946s, 1734m, 1638m, 1522m, 1452m, 1376m, 1248s, 1030m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 6.39 (t, J = 5.5 Hz, 1H, NH), 4.73–4.70 (m, 1H, 29-Ha), 4.60–4.57 (m, 1H, 29-Hb), 4.45 (dd, J = 10.0, 6.2 Hz, 1H, 3-H), 3.40–3.33 (m, 2H, 31-H), 3.09 (ddd, J = 11.0, 11.0, 4.0 Hz, 1H, 19-H), 2.90 (t, J = 5.9 Hz, 2H, 32-H), 2.42 (ddd, J = 12.7, 12.7, 3.4 Hz, 1H, 13-H), 2.03 (s, 3H, Ac), 2.01–1.68 (m, 4H, 16-Ha, 21-Ha, 22-Ha,12-Ha), 1.67 (s, 3H, 30-Ha), 1.66–1.07 (m, 16H, 22-Hb, 2-Ha, 2-Hb, 18-H, 16-Hb, 15-Ha, 6-Ha, 1-Ha, 11-Ha, 6-Hb, 21-Hb, 7-Ha, 7-Hb, 9-H, 11-Hb, 15-Hb), 1.05–0.88 (m, 2H, 12-Hb, 1-Hb), 0.95 (s, 3H, 27-H), 0.92 (s, 3H, 26-H), 0.83 (s, 6H, 25-H, 23-H), 0.82 (s, 3H, 24-H), 0.80–0.74 (m, 1H, 5-H) ppm; 13C-NMR (101 MHz, CDCl3): δ = 177.2 (C-28), 171.1 (Ac), 150.9 (C-20), 109.6 (C-29), 81.1 (C-3), 55.9 (C-17), 55.6 (C-5), 50.7 (C-9), 50.3 (C-18), 47.0 (C-19), 42.6 (C-14), 41.5 (C-32), 40.9 (C-8), 40.8 (C-31), 38.6 (C-1, C-22), 37.9 (C-4), 37.9 (C-13), 37.3 (C-10), 34.5 (C-7), 33.8 (C-16), 31.1 (C-21), 29.6 (C-15), 28.1 (C-23), 25.7 (C-12), 23.8 (C-2), 21.5 (Ac), 21.1 (C-11), 19.6 (C-30), 18.3 (C-6), 16.6 (C-24), 16.4 (C-25), 16.3 (C-26), 14.8 (C-27) ppm; MS (ESI, MeOH): m/z = 541 (100 %, [M + H]+); analysis calcd for C34H56N2O3 (540.83): C 75.51, H 10.44, N 5.18; found: C 75.35, H 10.67, N 5.02.
(3β)-N-[2-(Dimethylamino)ethyl]-3-acetyloxy-lup-20(29)-en-28-amide (18). Compound 18 was prepared from 2 according to general procedure B using N,N-dimethylethylenediamine. Column chromatography (SiO2, CHCl3/MeOH 95:5) gave 18 (yield: 94%); m.p. 108–110 °C; [α]D = +16.4° (c 0.320, CHCl3); Rf = 0.51 (CHCl3/MeOH 9:1); IR (KBr): ν = 3420br s, 2945s, 2869m, 1736s, 1641m, 1456s, 1375m, 1246s, 1195w, 1029m, 979m cm−1; 1H-NMR (500 MHz, CDCl3): δ = 6.24 (t, J = 4.7 Hz, 1H, NH), 4.74–4.72 (m, 1H, 29-Ha), 4.60–4.58 (m, 1H, 29-Hb), 4.46 (dd, J = 10.4, 5.9 Hz, 1H, 3-H), 3.40–3.24 (m, 2H, 31-H), 3.11 (ddd, J = 11.0, 11.0, 4.2 Hz, 1H, 19-H), 2.47–2.38 (m, 3H, 32-H + 13-H), 2.26 (s, 6H, 33-H, 33′-H), 2.03 (s, 3H, Ac), 2.03–1.89 (m, 2H, 16-Ha, 21-Ha), 1.80–1.74 (m, 1H, 22-Ha), 1.73–1.63 (m, 2H, 12-Ha, 22-Hb), 1.68 (s, 3H, 30-H), 1.63–1.11 (m, 15H, 2-Ha, 2-Hb, 18-H, 16-Hb, 15-Ha, 6-Ha, 11-Ha, 1-Ha, 6-Hb, 7-Ha, 7-Hb, 21-Hb, 9-H, 11-Hb, 15-Hb), 1.05–0.94 (m, 2H, 12-Hb, 1-Hb), 0.96 (s, 3H, 27-H), 0.94 (s, 3H, 26-H), 0.83 (s, 6H, 23-H, 25-H), 0.82 (s, 3H, 24-H), 0.80–0.76 (m, 1H, 5-H) ppm; 13C-NMR (126 MHz, CDCl3): δ = 176.5 (C-28), 171.1 (Ac), 151.2 (C-20), 109.5 (C-29), 81.1 (C-3), 58.3 (C-32), 55.9 (C-17), 55.6 (C-5), 50.7 (C-9), 50.2 (C-18), 47.1 (C-19), 45.3 (C-33, C-33′), 42.7 (C-14), 40.9 (C-8), 38.6 (C-22), 38.6 (C-1), 38.0 (C-13), 38.0 (C-4), 37.3 (C-10), 36.6 (C-31), 34.5 (C-7), 33.8 (C-16), 31.1 (C-21), 29.6 (C-15), 28.1 (C-23), 25.8 (C-12), 23.9 (C-2), 21.5 (Ac), 21.1 (C-11), 19.6 (C-30), 18.4 (C-6), 16.6 (C-24), 16.4 (C-25), 16.3 (C-26), 14.8 (C-27) ppm; MS (ESI, MeOH): m/z = 569 (100%, [M + H]+); analysis calcd for C26H60N2O3 (568.89): C 76.01, H 10.63, N 4.92; found: C 75.77, H 10.84, N 4.63.
(3β)-N-(2-Pyrrolidin-1-ylethyl)-3-acetyloxy-lup-20(29)-en-28-amide (19). Compound 19 was prepared from 2 according to general procedure B using 1-(2-aminoethyl)-pyrrolidine. Column chromatography (SiO2, CHCl3/MeOH 95:5) gave 19 (yield: 86%); m.p. 143–145 °C; [α]D = +11.7° (c 0.330, CHCl3); Rf = 0.53 (CHCl3/MeOH 9:1); IR (KBr): ν =3422br m, 2946s, 1734m, 1640m, 1451m, 1384s, 1247s, 1029m, 979m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 7.23 (t, J = 5.5 Hz, 1H, NH), 4.72–4.70 (m, 1H, 29-Ha), 4.58–4.56 (m, 1H, 29-Hb), 4.44 (dd, J = 10.8, 5.5 Hz, 1H, 3-H), 3.68–3.56 (m, 2H, 31-H), 3.47–3.27 (m, 4H, 33-H, 33′-H), 3.24 (t, J = 6.1 Hz, 2H, 32-H), 3.05 (ddd, J = 10.9, 10.9, 4.2 Hz, 1H, 19-H), 2.39 (ddd, J = 12.8, 12.8, 3.5 Hz, 1H, 13-H), 2.13–2.04 (m, 5H, 34-H, 34′-H, 16-Ha), 2.02 (s, 3H, Ac), 1.89–1.76 (m, 2H, 21-Ha, 22-Ha), 1.66 (s, 3H, 30-H), 1.71–1.11 (m, 17H, 12-Ha, 1-Ha, 2-Ha, 2-Hb, 18-H, 16-Hb, 6-Ha, 22-Hb, 11-Ha, 21-Hb, 7-Ha, 7-Hb, 15-Ha, 6-Hb, 9-H, 11-Hb, 15-Hb), 1.03–0.91 (m, 2H, 12-Hb, 1-Hb), 0.93 (s, 3H, 27-H), 0.89 (s, 3H, 26-H), 0.82 (s, 6H, 23-H, 25-H), 0.81 (s, 3H, 24-H), 0.78–0.75 (m, 1H, 5-H) ppm; 13C-NMR (101 MHz, CDCl3): δ = 177.8 (C-28), 171.1 (Ac), 151.0 (C-20), 109.6 (C-29), 81.1 (C-3), 55.9 (C-17), 55.6 (C-5), 55.5 (C-32), 54.8 (C-33, C-33′), 50.6 (C-9), 50.3 (C-18), 47.0 (C-19), 42.6 (C-14), 40.9 (C-8), 38.5 (C-1), 38.2 (C-22), 37.9 (C-13), 37.9 (C-4), 37.3 (C-10), 36.3 (C-31), 34.5 (C-7), 33.2 (C-16), 31.0 (C-21), 29.6 (C-15), 28.1 (C-23), 25.7 (C-12), 23.8 (C-2), 23.4 (C-34, C-34′), 21.4 (Ac), 21.1 (C-11), 19.5 (C-30), 18.3 (C-6), 16.6 (C-24), 16.3 (C-25), 16.3 (C-26), 14.7 (C-27) ppm; MS (ESI, MeOH): m/z = 595 (100%, [M + H]+); analysis calcd for C38H62N2O3 (594.93): C 76.72, H 10.50, N 4.71; found: C 76.50, H 10.74, N 4.51.
(3β)-N-(2-Piperidin-1-ylethyl)-3-acetyloxy-lup-20(29)-en-28-amide (20). Compound 20 was prepared from 2 according to general procedure B using 1-(2-aminoethyl)-piperidine. Column chromatography (SiO2, CHCl3/MeOH 95:5) gave 20 (yield: 81%); m.p. 124–127 °C; [α]D = +14.1° (c 0.340, CHCl3); Rf = 0.25 (CHCl3/MeOH 95:5); IR (KBr): ν = 3424br s, 2942s, 2968m, 1736s, 1638s, 1508m, 1452m, 1376m, 1246s, 1154w, 1128w, 1028m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 6.52–6.39 (m, 1H, NH), 4.75–4.70 (m, 1H, 29-Ha), 4.61–4.56 (m, 1H, 29-Hb), 4.46 (dd, J = 9.8, 6.5 Hz, 1H, 3-H), 3.41–3.25 (m, 2H, 31-Ha, 31-Hb), 3.08 (ddd, J = 11.1, 10.9, 3.9 Hz, 1H, 19-H), 2.53–2.40 (m, 6H, 32-H, 33-H, 33′-H), 2.35 (ddd, J = 12.4, 12.3, 3.6 Hz, 1H, 13-H), 2.03 (s, 3H, Ac), 2.12–1.89 (m, 2H, 16-Ha, 21-Ha), 1.83–1.74 (m, 1H, 22-Ha), 1.68 (s, 3H, 30-H), 1.72–1.54 (m, 9H, 12-Ha, 1-Ha, 2-Ha, 2-Hb, 18-H, 34-H, 34′-H), 1.55–1.15 (m, 13H, 16-Hb, 15-Ha, 6-Ha, 35-H, 11-Ha, 22-Hb, 21-Hb, 6-Hb, 7-Ha, 7-Hb, 9-H, 11-Hb), 1.15–1.09 (m, 1H, 15-Hb), 1.08–0.93 (m, 2H, 12-Hb, 1-Hb), 0.96 (s, 3H, 27-H), 0.92 (s, 3H, 26-H), 0.83 (s, 6H, 25-H, 23-H), 0.82 (s, 3H, 24-H), 0.80–0.74 (m, 1H, 5-H) ppm; 13C-NMR (101 MHz, CDCl3): δ = 176.3 (C-28), 171.1 (Ac), 151.1 (C-20), 109.5 (C-29), 81.1 (C-3), 57.1 (C-32), 56.0 (C-17), 55.6 (C-5), 54.3 (C-33, C-33′), 50.6 (C-9), 50.0 (C-18), 47.2 (C-19), 42.7 (C-14), 40.9 (C-8), 38.5 (C-1), 38.5 (C-22), 38.1 (C-13), 37.9 (C-4), 37.3 (C-10), 35.7 (C-31), 34.5 (C-7), 33.8 (C-16), 31.1 (C-21), 29.6 (C-15), 28.1 (C-23), 26.1 (C-34, C-34′), 25.8 (C-12), 24.4 (C-35), 23.8 (C-2), 21.4 (Ac), 21.1 (C-11), 19.6 (C-30), 18.4 (C-6), 16.6 (C-24), 16.4 (C-25), 16.3 (C-26), 14.8 (C-27) ppm; MS (ESI, MeOH): m/z = 609 (100%, [M + H]+); analysis calcd for C39H64N2O3 (608.95): C 76.92, H 10.59, N 4.60; found:
(3β)-N-(2-Piperazin-1-ylethyl)-3-acetyloxy-lup-20(29)-en-28-amide (21). Compound 21 was prepared from 2 according to general procedure B using 1-(2-aminoethyl)-piperazine. Column chromatography (SiO2, CHCl3/MeOH/NH4OH 90:11:0.5) gave 21 (yield: 84%); m.p. 107–110 °C; [α]D = +13.7° (c 0.335, CHCl3); Rf = 0.44 (CHCl3/MeOH/NH4OH 90:10:1);IR (KBr): ν = 3422br s, 2944s, 2870m, 1734s, 1638s, 1522m, 1452m, 1374m, 1318w, 1248s, cm−1; 1194w, 1138w, 1028m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 6.22 (t, J = 4.8 Hz, 1H, NH), 4.75–4.70 (m, 1H, 29-Ha), 4.61–4.58 (m, 1H, 29-Hb), 4.46 (dd, J = 9.7, 6.5 Hz, 1H, 3-H), 3.41–3.23 (m, 2H, 31-H), 3.08 (ddd, J = 11.1, 11.1, 3.8 Hz, 1H, 19-H), 2.92 (t, J = 4.9 Hz, 4H, 34-H, 34′-H), 2.55–2.41 (m, 6H, 32-H, 33-H, 33′-H), 2.36 (ddd, J = 12.4, 12.4, 3.6 Hz, 1H, 13-H), 2.03 (s, 3H, Ac), 2.05–1.89 (m, 2H, 16-Ha, 21-Ha), 1.68 (s, 3H, 30-H), 1.81–1.53 (m, 6H, 22-Ha, 12-Ha, 1-Ha, 18-H, 2-Ha, 2-Hb), 1.52–1.19 (m, 11H, 16-Hb, 15-Ha, 6-Ha, 11-Ha, 22-Hb, 6-Hb, 21-Hb, 7-Ha, 7-Hb, 9-H, 11-Hb), 1.20–0.90 (m, 3H, 15-Hb, 12-Hb, 1-Hb), 0.96 (s, 3H, 27-H), 0.91 (s, 3H, 26-H), 0.83 (s, 6H, 23-H, 25-H), 0.82 (s, 3H, 24-H), 0.80–0.74 (m, 1H, 5-H) ppm; 13C-NMR (101 MHz, CDCl3): δ = 176.3 (C-28), 171.1 (Ac), 151.0 (C-20), 109.6 (C-29), 81.1 (C-3), 57.1 (C-32), 56.0 (C-17), 55.6 (C-5), 53.7 (C-33, C-33′), 50.6 (C-9), 50.0 (C-18), 47.2 (C-19), 46.1 (C-34, C-34′), 42.7 (C-14), 40.9 (C-8), 38.5 (C-1, C-22), 38.1 (C-13), 37.9 (C-4), 37.3 (C-10), 35.6 (C-31), 34.5 (C-7), 33.8 (C-16), 31.1 (C-21), 29.6 (C-15), 28.1 (C-23), 25.7 (C-12), 23.8 (C-2), 21.5 (Ac), 21.1 (C-11), 19.6 (C-30), 18.3 (C-6), 16.6 (C-24), 16.4 (C-25), 16.3 (C-26), 14.8 (C-27) ppm; MS (ESI, MeOH): m/z = 610 (100%, [M + H]+); analysis calcd for C38H63N3O3 (609.94): C 74.83, H 10.41, N 6.89; found: C 74.65, H 10.69, N 6.64.
(3β)-N-(4-Aminobutyl)-3-acetyloxy-lup-20(29)-en-28-amide (22). Compound 22 was prepared from 2 according to general procedure B using 1,4-diaminobutane. Column chromatography (SiO2, CHCl3/MeOH/NH4OH 90:11:0.5) gave 22 (yield: 84%); m.p. 133–135 °C; [α]D = +7.4° (c 0.350, CHCl3); Rf = 0.33 (CHCl3/MeOH/NH4OH 90:10:1); IR (KBr): ν = 3422br s, 2944s, 2868m, 1736s, 1638s, 1522m, 1452m, 1374m, 1248s, 1028m, 980m, 754m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 5.94 (t, J = 5.8 Hz, 1H, NH), 4.75–4.69 (m, 1H, 29-Ha), 4.61–4.55 (m, 1H, 29-Hb), 4.45 (dd, J = 8.7, 7.6 Hz, 1H, 3-H), 3.35–3.15 (m, 2H, 31-Ha, 31-Hb), 3.11 (ddd, J = 11.0, 4.0 Hz, 4.0 Hz, 1H, 19-H), 2.84–2.73 (m, 2H, 32-Ha, 32-Hb), 2.45 (ddd, J = 12.4, 3.6 Hz, 3.6 Hz, 1H, 13-H), 2.03 (s, 3H, Ac), 2.00–1.80 (m, 2H, 21-Ha, 16-Ha), 1.67 (s, 3H, 30-H), 1.77–1.18 (m, 21H, 22-Ha, 12-Ha, 1-Ha, 2-Ha, 2-Hb, 34-Ha, 34-Hb, 18-H, 33-Ha, 33-Hb, 16-Hb, 6-Ha, 15-Ha, 11-Ha, 22-Hb, 6-Hb, 21-Hb, 7-Ha, 7-Hb, 11-Hb, 9-H), 1.16–1.08 (m, 1H, 15-Hb), 1.04–0.90 (m, 2H, 12-Hb, 1-Hb), 0.95 (s, 3H, 27-H), 0.92 (s, 3H, 26-H), 0.84 (s, 3H, 25-H), 0.83 (s, 3H, 23-H), 0.82 (s, 3H, 24-H), 0.80–0.74 (m, 1H, 5-H) ppm; 13C-NMR (101 MHz, CDCl3): δ = 176.4 (C-28), 171.1 (Ac), 151.1 (C-20), 109.5 (C-29), 81.1 (C-3), 55.8 (C-17), 55.6 (C-5), 50.7 (C-9), 50.3 (C-18), 46.9 (C-19), 42.6 (C-14), 41.4 (C-32), 40.9 (C-8), 39.1 (C-31), 38.6 (C-22), 38.5 (C-1), 38.0 (C-4), 37.9 (C-13), 37.3 (C-10), 34.5 (C-7), 33.9 (C-16), 31.1 (C-21), 29.9 (C-33), 29.6 (C-15), 28.1 (C-23), 27.3 (C-34), 25.8 (C-12), 23.9 (C-2), 21.5 (Ac), 21.1 (C-11), 19.6 (C-30), 18.3 (C-6), 16.6 (C-24), 16.4 (C-25), 16.3 (C-26), 14.8 (C-27) ppm; MS (ESI, MeOH): m/z = 569 (100%, [M + H]+); analysis calcd for C36H60N2O3 (568.89): C 76.01, H 10.63, N 4.92; found: C75.81, H 10.77, N 4.75.
(3β)-N-[2-(2-Aminoethoxy)ethyl]-3-acetyloxy-lup-20(29)-en-28-amide (23). Compound 23 was prepared from 2 according to general procedure B using 2,2′-oxybis(ethylamine). Column chromatography (SiO2, CHCl3/MeOH 9:1) gave 23 (yield: 81%); m.p. 109–112 °C; [α]D = +38.4° (c 0.325, CHCl3); Rf = 0.58 (CHCl3/MeOH/NH4OH 90:10:1); IR (KBr): ν = 3448br s, 2944m, 1734m, 1637m, 1527w, 1375w, 1248m, 1029w cm−1; 1H-NMR (500 MHz, CDCl3): δ = 6.09 (t, J = 5.4 Hz, 1H, NH), 4.74–4.70 (m, 1H, 29-Ha), 4.60–4.56 (m, 1H, 29-Hb), 4.46 (dd, J = 10.6, 5.8 Hz, 1H, 3-H), 3.58–3.44 (m, 5H, 32-H, 33-H, 31-Ha), 3.43–3.35 (m, 1H, 31-Hb), 3.10 (ddd, J = 11.1, 11.0, 4.2 Hz, 1H, 19-H), 2.88 (t, J = 5.2 Hz, 2H, 34-H), 2.43 (ddd, J = 12.9, 11.5, 3.7 Hz, 1H, 13-H), 2.03 (s, 3H, Ac), 2.00–1.88 (m, 2H, 16-Ha, 21-Ha), 1.78–1.72 (m, 1H, 22-Ha), 1.67 (s, 3H, 30-H), 1.72–1.17 (m, 16H, 12-Ha, 1-Ha, 2-Ha, 2-Hb, 18-H, 16-Hb, 6-Ha, 15-Ha, 22-Hb, 11-Ha, 6-Hb, 21-Hb, 7-Ha, 7-Hb, 9-H, 11-Hb), 1.16–1.09 (m, 1H, 15-Hb), 1.05–0.95 (m, 2H, 12-Hb, 1-Hb), 0.95 (s, 3H, 27-H), 0.93 (s, 3H, 26-H), 0.83 (s, 3H, 25-H), 0.83 (s, 3H, 23-H), 0.82 (s, 3H, 24-H), 0.80–0.74 (m, 1H, 5-H) ppm; 13C-NMR (126 MHz, CDCl3): δ = 176.4 (C-28), 171.1 (Ac), 151.0 (C-20), 109.5 (C-29), 81.1 (C-3), 72.8 (C-33), 70.1 (C-32), 55.9 (C-17), 55.6 (C-5), 50.7 (C-9), 50.2 (C-18), 47.0 (C-19), 42.6 (C-14), 41.8 (C-34), 40.9 (C-8), 39.1 (C-31), 38.6 (C-1), 38.5 (C-22), 37.9 (C-4), 37.9 (C-13), 37.3 (C-10), 34.5 (C-7), 33.9 (C-16), 31.0 (C-21), 29.5 (C-15), 28.1 (C-23), 25.7 (C-12), 23.9 (C-2), 21.4 (Ac), 21.1 (C-11), 19.6 (C-30), 18.4 (C-6), 16.6 (C-24), 16.3 (C-25), 16.3 (C-26), 14.8 (C-27) ppm; MS (ESI, MeOH): m/z = 585 (100 %, [M + H]+), 607 (47 %, [M + Na]+); analysis calcd for C36H60N2O4 (584.89): C 73.93, H 10.34, N 4.79; found: C 73.69, H 10.54, N 4.56.
(3β)-N-(2-Aminoethyl)-3-hydroxy-lup-20(29)-en-28-amide (24). Compound 24 was prepared from 17 according to general procedure C. Column chromatography (SiO2, CHCl3/MeOH 9:1) gave 24 (yield: 86%); m.p. 218–220 °C; [α]D = +4.5° (c 0.300, DMSO); Rf = 0.28 (CHCl3/MeOH 9:1);IR (KBr): ν = 3424br s, 2941m, 1636m, 1449m, 1044m, 879w cm−1; 1H-NMR (400 MHz, DMSO-d6): δ = 7.53 (t, J = 5.5 Hz, 1H, NH), 4.67–4.63 (m, 1H, 29-Ha), 4.54–4.51 (m, 1H, 29-Hb), 3.15–2.92 (m, 4H, 32-Ha, 19-H, 32-Hb, 3-H), 2.60–2.51 (m, 3H, 13-H, 31-Ha, 31-Hb), 2.16–2.09 (m, 1H, 16-Ha), 1.82–1.65 (m, 2H, 22-Ha, 21-Ha), 1.62 (s, 3H, 30-H), 1.61–0.92 (m, 17H, 12-Ha, 1-Ha, 2-Ha, 2-Hb, 6-Ha, 18-H, 16-Hb, 11-Ha, 22-Hb, 15-Ha, 6-Hb, 7-Ha, 7-Hb, 21-Hb, 9-H, 11-Hb, 15-Hb), 0.92–0.78 (m, 2H, 12-Hb, 1-Hb), 0.91 (s, 3H, 27-H), 0.87 (s, 3H, 23-H), 0.84 (s, 3H, 26-H), 0.76 (s, 3H, 25-H), 0.65 (s, 3H, 24-H), 0.64–0.60 (m, 1H, 5-H) ppm; 13C-NMR (101 MHz, DMSO-d6): δ = 175.6 (C-28), 150.9 (C-20), 109.1 (C-29), 76.8 (C-3), 54.9 (C-5), 54.9 (C-17), 50.1 (C-9), 49.7 (C-18), 46.2 (C-19), 41.9 (C-14), 41.8 (C-32), 41.4 (C-31), 40.3 (C-8), 38.5 (C-4), 38.3 (C-1), 37.7 (C-22), 36.7 (C-10), 36.6 (C-13), 34.0 (C-7), 32.4 (C-16), 30.3 (C-21), 28.9 (C-15), 28.1 (C-23), 27.1 (C-2), 25.2 (C-12), 20.6 (C-11), 19.0 (C-30), 17.9 (C-6), 15.9 (C-25), 15.8 (C-26), 15.7 (C-24), 14.3 (C-27) ppm; MS (ESI, MeOH): m/z = 499 (100 %, [M + H]+); analysis calcd for C32H54N2O2 (498.80): C 77.06, H 10.91, N 5.62; found: C 76.81, H 11.07, N 5.55.
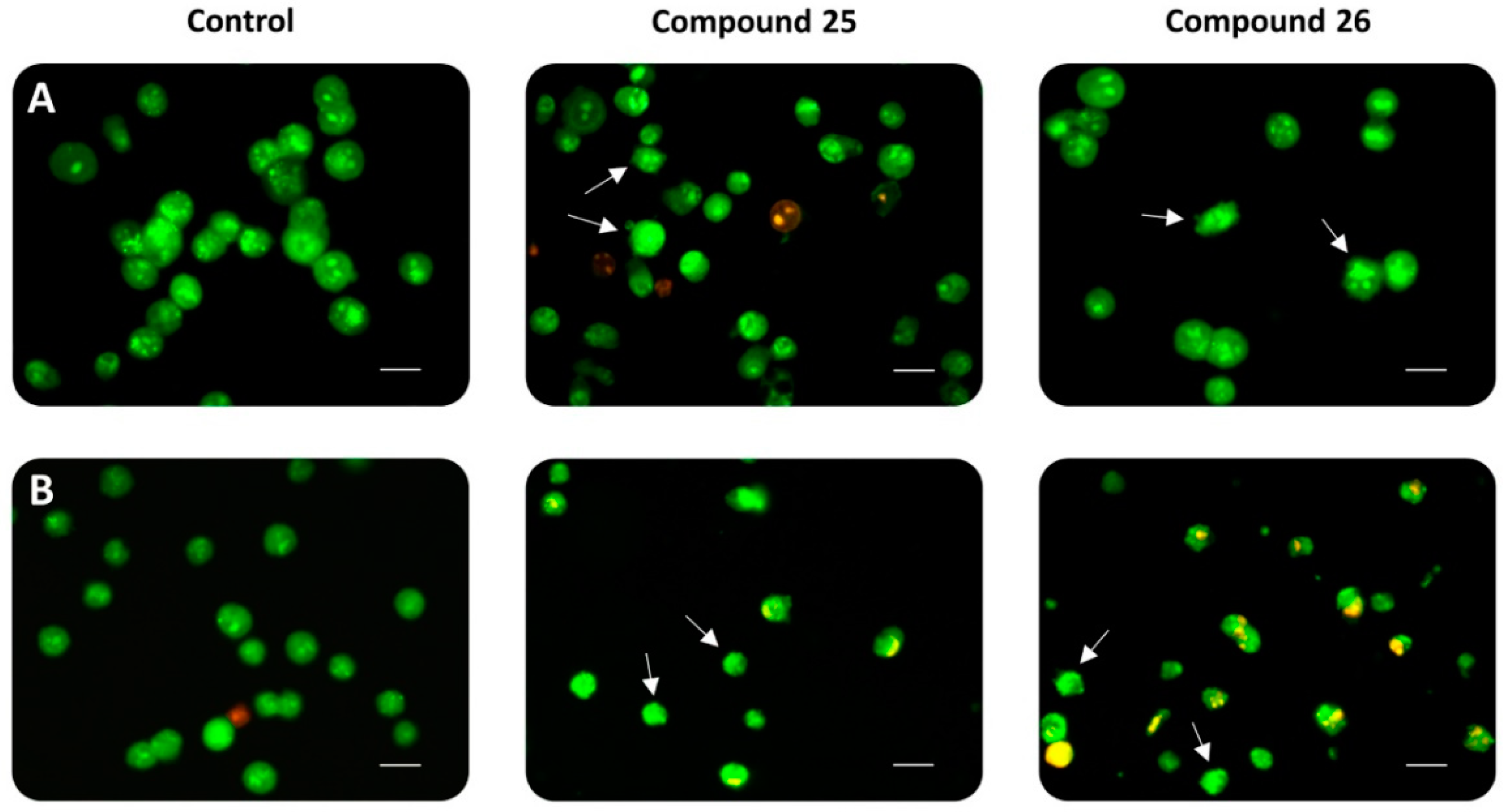
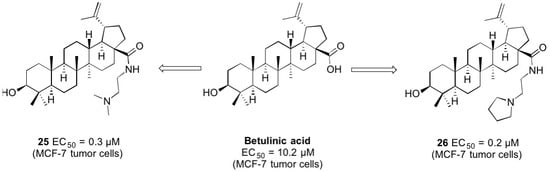
(3β)-N-[2-(Dimethylamino)ethyl]-3-hydroxy-lup-20(29)-en-28-amide (25). Compound 25 was prepared from 18 according to general procedure C. Column chromatography (SiO2, CHCl3/MeOH 95:5) gave 25 (yield: 89%); m.p. 118–120 °C; [α]D = −4.4° (c 0.330, MeOH); Rf = 0.43 (CHCl3/MeOH 9:1); IR (KBr): ν = 3408br s, 2944s, 2866s, 1638s, 1528m, 1464s, 1376m, 1246m, 1194m, 1044m, 880m cm−1; 1H-NMR (500 MHz, CDCl3): δ = 6.26 (t, J = 4.9 Hz, 1H, NH), 4.73–4.71 (m, 1H, 29-Ha), 4.58–4.56 (m, 1H, 29-Hb), 3.37–3.22 (m, 2H, 31-H), 3.16 (dd, J = 11.0, 5.2 Hz, 1H, 3-H), 3.10 (ddd, J = 11.1, 11.1, 4.2 Hz, 1H, 19-H), 2.46–2.37 (m, 3H, 13-H, 32-H), 2.22 (s, 6H, 33-H, 33′-H), 2.06–1.89 (m, 2H, 16-Ha, 21-Ha), 1.79–1.72 (m, 1H, 22-Ha), 1.67 (s, 3H, 30-H), 1.72– 1.16 (m, 16H, 12-Ha, 1-Ha, 2-Ha, 2-Hb, 18-H, 6-Ha, 16-Hb, 15-Ha, 11-Ha, 22-Hb, 6-Hb, 7-Ha, 7-Hb, 21-Hb, 9-H, 11-Hb), 1.15–1.10 (m, 1H, 15-Hb), 1.04–0.96 (m, 1H, 12-Hb), 0.95 (s, 3H, 27-H), 0.95 (s, 3H, 23-H), 0.93 (s, 3H, 26-H), 0.91–0.81 (m, 1H, 1-Hb), 0.80 (s, 3H, 25-H), 0.74 (s, 3H, 24-H), 0.69–0.64 (m, 1H, 5-H) ppm; 13C-NMR (126 MHz, CDCl3): δ = 176.4 (C-28), 151.2 (C-20), 109.4 (C-29), 79.1 (C-3), 58.3 (C-32), 55.9 (C-17), 55.5 (C-5), 50.8 (C-9), 50.2 (C-18), 47.0 (C-19), 45.3 (C-33, C-33′), 42.6 (C-14), 40.9 (C-8), 39.0 (C-4), 38.9 (C-1), 38.6 (C-22), 38.0 (C-13), 37.4 (C-10), 36.7 (C-31), 34.6 (C-7), 33.8 (C-16), 31.1 (C-21), 29.6 (C-15), 28.1 (C-23), 27.6 (C-2), 25.8 (C-12), 21.1 (C-11), 19.6 (C-30), 18.5 (C-6), 16.3 (C-26), 16.2 (C-25), 15.5 (C-24), 14.8 (C-27) ppm; MS (ESI, MeOH): m/z = 527 (100%, [M + H]+); analysis calcd for C34H58N2O2 (526.85): C 77.51, H 11.10, N 5.32; found: C 77.40, H 11.22, N 5.18.
(3β)-N-(2-Pyrrolidin-1-ylethyl)-3-hydroxy-lup-20(29)-en-28-amide (26). Compound 26 was prepared from 19 according to general procedure C. Column chromatography (SiO2, CHCl3/MeOH 95:5) gave 26 (yield: 80%); m.p. 253–256 °C (decomp.); [α]D = −14.7° (c 0.320, MeOH); Rf = 0.40 (CHCl3/MeOH 9:1); IR (KBr): ν = 3426br s, 2942s, 2866s, 2696m, 2620m, 2500m, 1638s, 1544m, 1450m, 1376m, 1246w, 1196w, 1046m, 880m cm−1; 1H-NMR (500 MHz, CDCl3): δ = 7.54 (t, J = 5.7 Hz, 1H, NH), 4.73–4.71 (m, 1H, 29-Ha), 4.59–4.57 (m, 1H, 29-Hb), 3.91–3.79 (m, 2H, 33-Ha, 33′-Ha), 3.78–3.61 (m, 2H, 31-H), 3.24–3.15 (m, 3H, 32-H, 3-H), 3.07 (ddd, J = 10.9, 10.9, 4.2 Hz, 1H, 19-H), 2.89–2.78 (m, 2H, 33-Hb, 33′-Hb), 2.42 (ddd, J = 12.6, 12.6, 3.6 Hz, 1H, 13-H), 2.31–2.18 (m, 3H, 16-Ha, 34-Ha, 34′-Ha), 2.15–2.05 (m, 2H, 34-Hb, 34′-Hb), 1.96–1.78 (m, 2H, 22-Ha, 21-Ha), 1.67 (s, 3H, 30-H), 1.73–1.14 (m, 17H, 12-Ha, 1-Ha, 2-Ha, 2-Hb, 18-H, 16-Hb, 6-Ha, 22-Hb, 11-Ha, 6-Hb, 21-Hb, 7-Ha, 7-Hb, 15-Ha, 9-H, 11-Hb, 15-Hb), 1.01–0.92 (m, 1H, 12-Hb), 0.96 (s, 6H, 23-H, 27-H), 0.91 (s, 3H, 26-H), 0.89–0.81 (m, 1H, 1-Hb), 0.81 (s, 3H, 25-H), 0.75 (s, 3H, 24-H), 0.70–0.65 (m, 1H, 5-H) ppm; 13C-NMR (126 MHz, CDCl3): δ = 177.9 (C-28), 151.1 (C-20), 109.5 (C-29), 79.1 (C-3), 56.6 (C-32), 56.1 (C-17), 55.5 (C-5), 54.8 (C-33, C-33′), 50.8 (C-9), 50.4 (C-18), 47.0 (C-19), 42.6 (C-14), 40.9 (C-8), 39.0 (C-4), 38.9 (C-1), 38.2 (C-22), 37.9 (C-13), 37.4 (C-10), 35.7 (C-31), 34.6 (C-7), 33.2 (C-16), 31.1 (C-21), 29.7 (C-15), 28.1 (C-23), 27.6 (C-2), 25.8 (C-12), 23.5 (C34, C-34′), 21.1 (C-11), 19.6 (C-30), 18.5 (C-6), 16.4 (C-26), 16.3 (C-25), 15.5 (C-24), 14.8 (C-27) ppm; MS (ESI, MeOH): m/z = 553 (100%, [M + H]+); analysis calcd for C36H60N2O2 (552.89): C 78.21, H 10.94, N 5.07; found: C 78.00, H 11-09, N 4.81.
(3β)-N-(2-Piperidin-1-ylethyl)-3-hydroxy-lup-20(29)-en-28-amide (27). Compound 27 was prepared from 20 according to general procedure C. Column chromatography (SiO2, CHCl3/MeOH 95:5) gave 27 (yield: 83%); m.p. 141–144 °C (decomp.); [α]D = +4.9° (c 0.315, CHCl3); Rf = 0.21 (CHCl3/MeOH 95:5); IR (KBr): ν = 3424br s, 2940s, 2866m, 2364w, 1638s, 1508m, 1452m, 1376m, 1248w, 1194w, 1128w, 1046m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 6.78–6.57 (m, 1H, NH), 4.76–4.69 (m, 1H, 29-Ha), 4.61–4.56 (m, 1H, 29-Hb), 3.48–3.31 (m, 2H, 31-H), 3.17 (dd, J = 11.1, 5.0 Hz, 1H, 3-H), 3.08 (ddd, J = 11.0, 10.8, 3.9 Hz, 1H, 19-H), 2.65–2.46 (m, 6H, 32-H, 33-H, 33′-H), 2.37 (ddd, J = 12.4, 12.3, 3.6 Hz, 1H, 13-H), 2.15–2.08 (m, 1H, 16-Ha), 2.00–1.88 (m, 1H, 21-Ha), 1.85–1.76 (m, 1H, 22-Ha), 1.68 (s, 3H, 30-H), 1.73–1.08 (m, 23H, 12-Ha, 35-H, 1-Ha, 18-H, 34-H, 34′-H, 6-Ha, 2-Ha, 2-Hb, 16-Hb, 15-Ha, 11-Ha, 22-Hb, 21-Hb, 6-Hb, 7-Ha, 7-Hb, 9-H, 11-Hb, 15-Hb), 1.08–0.95 (m, 1H, 12-Hb), 0.96 (s, 3H, 27-H), 0.95 (s, 3H, 23-H), 0.91 (s, 3H, 26-H), 0.91–0.82 (m, 1H, 1-Hb), 0.80 (s, 3H, 25-H), 0.74 (s, 3H, 24-H), 0.70–0.63 (m, 1H, 5-H) ppm; 13C-NMR (101 MHz, CDCl3): δ = 176.6 (C-28), 151.1 (C-20), 109.5 (C-29), 79.1 (C-3), 57.3 (C-32), 56.1 (C-17), 55.5 (C-5), 54.3 (C-33, C-33′), 50.7 (C-9), 50.1 (C-18), 47.1 (C-19), 42.7 (C-14), 40.9 (C-8), 39.0 (C-4), 38.9 (C-1), 38.4 (C-22), 38.1 (C-13), 37.4 (C-10), 35.5 (C-31), 34.6 (C-7), 33.6 (C-16), 31.1 (C-21), 29.6 (C-15), 28.1 (C-23), 27.6 (C-34, C-34′), 25.8 (C-12), 25.5 (C-35), 24.0 (C-2), 21.1 (C-11), 19.6 (C-30), 18.5 (C-6), 16.3 (C-26), 16.2 (C-25), 15.5 (C-24), 14.8 (C-27) ppm; MS (ESI, MeOH): m/z = 567 (100%, [M + H]+); analysis calcd for C37H62N2O2 (566.92): C 78.39, H 11.02, N 4.94; found: C 78.16, H 11.20, N 4.71.
(3β)-N-(2-Piperazin-1-ylethyl)-3-hydroxy-lup-20(29)-en-28-amide (28). Compound 28 was prepared from 21 according to general procedure C. Column chromatography (SiO2, CHCl3/MeOH/NH4OH 90:10:0.5) gave 28 (yield: 90%); m.p. 146–148 °C (decomp.); [α]D = +6.5° (c 0.380, CHCl3); Rf = 0.30 (CHCl3/MeOH/NH4OH 90:10:1); IR (KBr): ν = 3422br s, 3072w, 2942s, 2868m, 1638s, 1510m, 1452m, 1376m, 1320w, 1248w, 1194w, 1138w, 1046w, 754m cm−1; 1H-NMR (400 MHz, CDCl3): δ = 6.19 (t, J = 4.9 Hz, 1H, NH), 4.77–4.70 (m, 1H, 29-Ha), 4.63–4.55 (m, 1H, 29-Hb), 3.42–3.28 (m, 2H, 31-H), 3.17 (dd, J = 11.1, 5.0 Hz, 1H, 3-H), 3.09 (ddd, J = 10.8, 10.3, 3.7 Hz, 1H, 19-H), 2.97 (t, J = 4.9 Hz, 4H, 34-H, 34′-H), 2.58–2.42 (m, 6H, 32-H, 33-H, 33′-H), 2.37 (ddd, J = 12.4, 12.3, 3.7 Hz, 1H, 13-H), 2.06–1.89 (m, 2H, 16-Ha, 21-Ha), 1.68 (s, 3H, 30-H), 1.81–1.08 (m, 18H, 22-Ha, 12-Ha, 1-Ha, 18-H, 2-Ha, 2-Hb, 6-Ha, 16-Hb, 15-Ha, 11-Ha, 22-Hb, 6-Hb, 21-Hb, 7-Ha, 7-Hb, 9-H, 11-Hb, 15-Hb), 1.07–0.95 (m, 1H, 12-Hb), 0.97 (s, 3H, 27-H), 0.95 (s, 3H, 23-H), 0.92 (s, 3H, 26-H), 0.96–0.83 (m, 1H, 1-Hb), 0.80 (s, 3H, 25-H), 0.75 (s, 3H, 24-H), 0.70–0.64 (m, 1H, 5-H) ppm; 13C-NMR (101 MHz, CDCl3): δ = 176.3 (C-28), 151.0 (C-20), 109.6 (C-29), 79.1 (C-3), 57.1 (C-32), 56.0 (C-17), 55.5 (C-5), 53.6 (C-33, C-33′), 50.7 (C-9), 50.1 (C-18), 47.2 (C-19), 46.0 (C-34, C-34′), 42.7 (C-14), 40.9 (C-8), 39.0 (C-4), 38.9 (C-1), 38.5 (C-22), 38.1 (C-13), 37.4 (C-10), 35.6 (C-31), 34.6 (C-7), 33.9 (C-16), 31.1 (C-21), 29.6 (C-15), 28.2 (C-23), 27.6 (C-2), 25.8 (C-12), 21.1 (C-11), 19.6 (C-30), 18.5 (C-6), 16.4 (C-26), 16.3 (C-25), 15.5 (C-24), 14.8 (C-27) ppm; MS (ESI, MeOH): m/z = 568 (100%, [M + H]+); analysis calcd for C36H61N3O2 (567.90): C 76.14, H 10.83, N 7.40; found: C 75.96, H 11.01, N 7.27.
(3β)-N-(4-Aminobutyl)-3-hydroxy-lup-20(29)-en-28-amide (29). Compound 29 was prepared from 22 according to general procedure C. Column chromatography (SiO2, CHCl3/MeOH 9:1) gave 29 (yield: 85%); m.p. 130–133 °C; [α]D = +4.8° (c 0.380, DMSO); Rf = 0.31 (CHCl3/MeOH 88:12);IR (KBr): ν = 3448br s, 2941s, 2867m, 1636m, 1534m, 1452m, 1384w, 1195w, 1045w cm−1; 1H-NMR (400 MHz, DMSO-d6): δ = 7.55 (t, J = 5.8 Hz, 1H, NH), 4.67–4.62 (m, 1H, 29-Ha), 4.55–4.50 (m, 1H, 29-Hb), 3.12–2.88 (m, 4H, 31-Ha, 19-H, 3-H, 31-Hb), 2.61–2.51 (m, 3H, 13-H, 32-Ha, 32-Hb), 2.18–2.09 (m, 1H, 16-Ha), 1.81–1.64 (m, 2H, 22-Ha, 21-Ha), 1.62 (s, 3H, 30-H), 1.61–1.51 (m, 2H, 1-Ha, 12-Ha), 1.49–0.98 (m, 19H, 2-Ha, 2-Hb, 6-Ha, 18-H, 34-Ha, 34-Hb, 11-Ha, 16-Hb, 15a, 22-Hb, 6-Hb, 33-Ha, 33-Hb, 7-Ha, 7-Hb, 9-H, 21-Hb, 11-Hb, 15-Hb), 0.97–0.78 (m, 2H, 1-Hb, 12-Hb), 0.90 (s, 3H, 27-H), 0.86 (s, 3H, 23-H), 0.84 (s, 3H, 26-H), 0.76 (s, 3H, 25-H), 0.65 (s, 3H, 24-H), 0.64–0.59 (m, 1H, 5-H) ppm; 13C-NMR (101 MHz, DMSO-d6): δ = 175.2 (C-28), 150.9 (C-20), 109.1 (C-29), 76.8 (C-3), 55.0 (C-5), 54.8 (C-17), 50.1 (C-9), 49.7 (C-18), 46.1 (C-19), 41.9 (C-14), 41.2 (C-32), 40.3 (C-8), 38.5 (C-4), 38.3 (C-31), 38.2 (C-1), 37.7 (C-22), 36.7 (C-10), 36.6 (C-13), 34.0 (C-7), 32.4 (C-16), 30.4 (C-21, C33), 28.9 (C-15), 28.1 (C-23), 27.2 (C-2), 26.7 (C-34), 25.2 (C-12), 20.6 (C-11), 19.0 (C-30), 18.0 (C-6), 16.0 (C-25), 15.8 (C-26), 15.7 (C-24), 14.3 (C-27) ppm; MS (ESI, MeOH): m/z = 527 (100 %, [M + H]+), 1053 (22 %, [2M + H]+); analysis calcd for C34H58N2O2 (526.45): C 77.51, H 11.10, N 5.32; found: C 77.38, H 11.30, N 5.13.
(3β)-N-[2-(2-Aminoethoxy)ethyl]-3-hydroxy-lup-20(29)-en-28-amide (30). Compound 30 was prepared from 23 according to general procedure C. Column chromatography (SiO2, CHCl3/MeOH 9:1) gave 30 (yield: 91%); m.p. 182–183 °C; [α]D = −1.1° (c 0.315, MeOH); Rf = 0.45 (CHCl3/MeOH/NH4OH 90:10:1); IR (KBr): ν = 3424br s, 2942s, 2868m, 1636s, 1534m, 1450w, 1384w, 1318w, 1278w, 1248w, 1196w, 1108m, 1044m cm−1; 1H-NMR (500 MHz, CD3OD): δ = 4.72–4.68 (m, 1H, 29-Ha), 4.61–4.57 (m, 1H, 29-Hb), 3.70–3.63 (m, 2H, 33-H), 3.57–3.51 (m, 2H, 32-H), 3.47–3.31 (m, 2H, 31-H), 3.15–3.05 (m, 4H, 3-H, 19-H, 34-H), 2.56 (ddd, J = 12.8, 12.4, 3.6 Hz, 1H, 13-H), 2.12 (ddd, J = 13.1, 3.3, 3.2 Hz, 1H, 16-Ha), 1.93–1.78 (m, 2H, 21-Ha, 22-Ha), 1.69 (s, 3H, 30-H), 1.76–1.63 (m, 2H, 12-Ha, 1-Ha), 1.65–1.10 (m, 15H, 2-Ha, 2-Hb, 18-H, 16-Hb, 6-Ha, 15-Ha, 22-Hb, 11-Ha, 6-Hb, 7-Ha, 7-Hb, 21-Hb, 9-H, 11-Hb, 15-Hb), 1.09–0.96 (m, 1H, 12-Hb), 1.00 (s, 3H, 27-H), 0.97 (s, 3H, 26-H), 0.95 (s, 3H, 23-H), 0.95–0.88 (m, 1H, 1-Hb), 0.86 (s, 3H, 25-H), 0.75 (s, 3H, 24-H), 0.73–0.69 (m, 1H, 5-H) ppm; 13C-NMR (126 MHz, CD3OD): δ = 179.6 (C-28), 152.3 (C-20), 110.0 (C-29), 79.6 (C-3), 71.4 (C-32), 67.8 (C-33), 57.1 (C-17), 56.9 (C-5), 52.1 (C-9), 51.4 (C-18), 48.2 (C-19), 43.5 (C-14), 42.0 (C-8), 40.7 (C-34), 40.1 (C-1), 39.9 (C-4), 39.7 (C-31), 39.3 (C-22), 39.0 (C-13), 38.3 (C-10), 35.6 (C-7), 34.1 (C-16), 31.9 (C-21), 30.6 (C-15), 28.6 (C-23), 28.0 (C-2), 27.0 (C-12), 22.2 (C-11), 19.6 (C-30), 19.5 (C-6), 16.9 (C-24), 16.8 (C-25), 16.1 (C-26), 15.1 (C-27) ppm; MS (ESI, MeOH): m/z = 543 (100 %, [M + H]+), 1085 (10 %, [2M + H]+); analysis calcd for C34H58N2O3 (542.58): C 75.23, H 10.77, N 5.16; found: C 75.11, H 10.94, N 4.97.