A Novel Integrated Way for Deciphering the Glycan Code for the FimH Lectin
Abstract
1. Introduction
2. Results and Discussion
2.1. Experimentally Determined Binding Affinities Highlight a Higher Affinity of Fimh Towards Manα1,3Man
2.2. Computed Binding Affinities Concur with Experimental Data
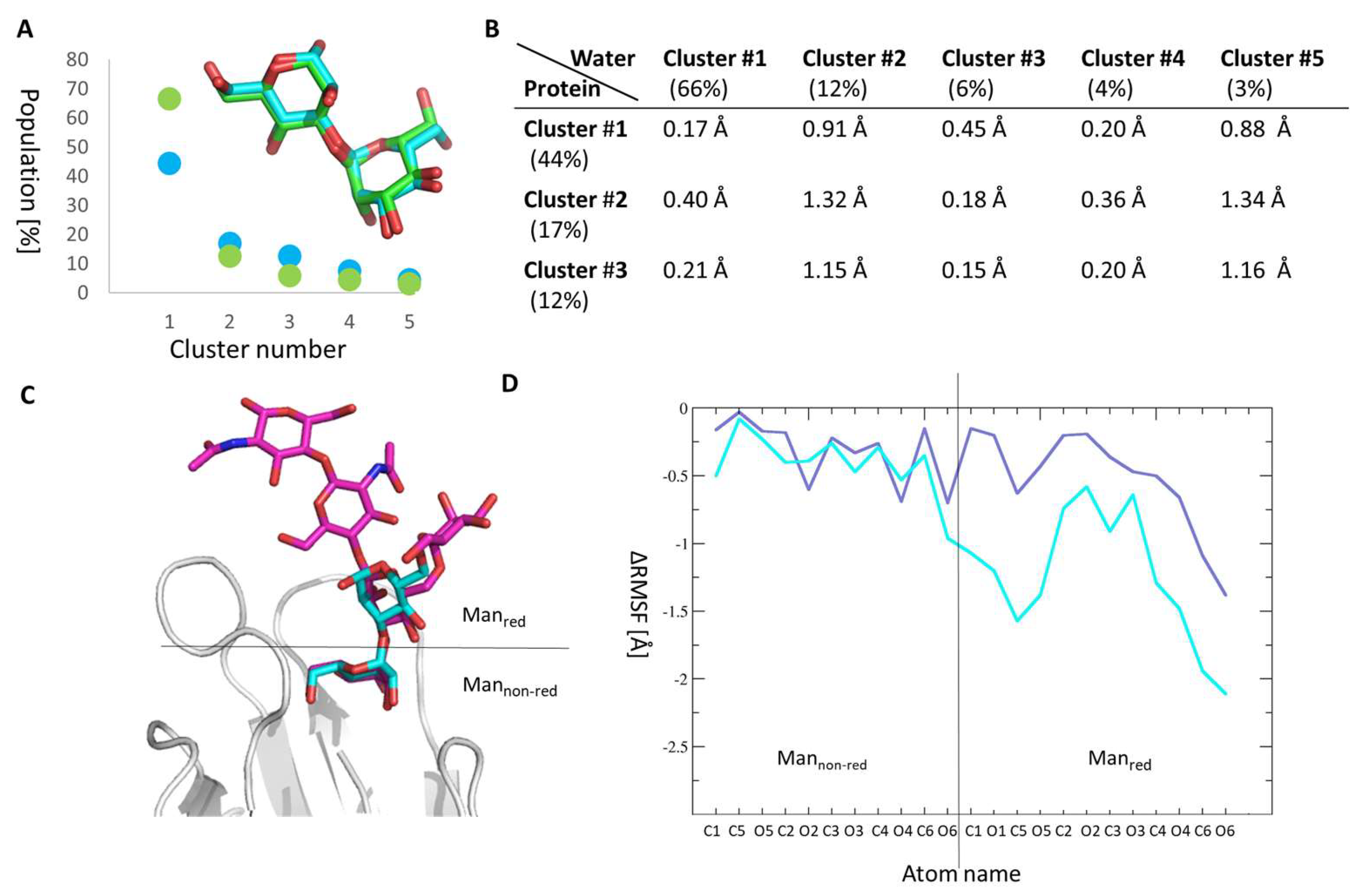
2.3. Molecular Details of Dimannose Binding to FimH
2.4. Manα1,3Man Finds a Stable Binding Position
2.5. Molecular Reason for the High Entropic Gain of Manα1,2Man upon Fimh Binding
3. Materials and Methods
3.1. Enzyme-Linked Lectinosorbent Assay
3.2. Isothermal Titration Calorimetry
3.3. Induced Fit Docking
3.4. Molecular Dynamics Simulation
3.5. Trajectory Analysis
3.6. Free Energy Calculations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krachler, A.M.; Orth, K. Targeting the bacteria-host interface: Strategies in anti-adhesion therapy. Virulence 2013, 4, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Ofek, I.; Mirelman, D.; Sharon, N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature 1977, 265, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Babu, J.P.; Abraham, S.N.; Dabbous, M.K.; Beachey, E.H. Interaction of a 60-kilodalton D-mannose-containing salivary glycoprotein with type 1 fimbriae of Escherichia coli. Infect. Immun. 1986, 54, 104–108. [Google Scholar] [PubMed]
- Old, D.C. Inhibition of the interaction between fimbrial haemagglutinins and erythrocytes by D-mannose and other carbohydrates. J. Gen. Microbiol. 1972, 71, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Sivignon, A.; Yamakawa, N.; Crepet, A.; Travelet, C.; Borsali, R.; Dumych, T.; Li, Z.; Bilyy, R.; Deniaud, D.; et al. Glycopolymers as Antiadhesives of E. coli Strains Inducing Inflammatory Bowel Diseases. Biomacromolecules 2015, 16, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Brument, S.; Sivignon, A.; Dumych, T.I.; Moreau, N.; Roos, G.; Guérardel, Y.; Chalopin, T.; Deniaud, D.; Bilyy, R.O.; Darfeuille-Michaud, A.; et al. Thiazolylaminomannosides As Potent Antiadhesives of Type 1 Piliated Escherichia coli Isolated from Crohn’s Disease Patients. J. Med. Chem. 2013, 56, 5395–5406. [Google Scholar] [CrossRef] [PubMed]
- Chalopin, T.; Alvarez Dorta, D.; Sivignon, A.; Caudan, M.; Dumych, T.I.; Bilyy, R.O.; Deniaud, D.; Barnich, N.; Bouckaert, J.; Gouin, S.G. Second Generation of Thiazolylmannosides, FimH Antagonists for E. coli-Induced Crohn’s Disease. Org. Biomol. Chem. 2016, 14, 3913–3925. [Google Scholar] [CrossRef] [PubMed]
- Budelier, M.M.; Cheng, W.W.L.; Bergdoll, L.; Chen, Z.W.; Janetka, J.W.; Abramson, J.; Krishnan, K.; Mydock-McGrane, L.; Covey, D.F.; Whitelegge, J.P.; et al. Photoaffinity labeling with cholesterol analogues precisely maps a cholesterol-binding site in voltage-dependent anion channel-1. J. Biol. Chem. 2017, 292, 9294–9304. [Google Scholar] [CrossRef] [PubMed]
- Krammer, E.-M.; Ruyck, J.; Roos, G.; Bouckaert, J.; Lensink, M. Targeting Dynamical Binding Processes in the Design of Non-Antibiotic Anti-Adhesives by Molecular Simulation—The Example of FimH. Molecules 2018, 23, 1641. [Google Scholar] [CrossRef] [PubMed]
- Leimbach, A.; Hacker, J.; Dobrindt, U. E. coli as an All-Rounder: The Thin Line Between Commensalism and Pathogenicity. In Between Pathogenicity and Commensalism; Springer: Berlin, Heidelberg, 2013; Volume 358, pp. 3–32. [Google Scholar]
- Baumgart, M.; Dogan, B.; Rishniw, M.; Weitzman, G.; Bosworth, B.; Yantiss, R.; Orsi, R.H.; Wiedmann, M.; McDonough, P.; Kim, S.G.; et al. Culture Independent Analysis of Ileal Mucosa Reveals a Selective Increase in Invasive Escherichia coli of Novel Phylogeny Relative to Depletion of Clostridiales in Crohn’s Disease Involving the Ileum. ISME J. 2007, 1, 403–418. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, E.M.; Longman, R.; Harbus, M.; Dannenberg, K.; Scherl, E.J. Crohn’s Disease: Evolution, Epigenetics, and the Emerging Role of Microbiome-Targeted Therapies. Curr. Gastroenterol. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sivignon, A.; Bouckaert, J.; Bernard, J.; Gouin, S.G.; Barnich, N. The Potential of FimH as a Novel Therapeutic Target for the Treatment of Crohn’s Disease. Expert Opin. Ther. Targets 2017, 21, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Le Trong, I.; Aprikian, P.; Kidd, B.A.; Forero-Shelton, M.; Tchesnokova, V.; Rajagopal, P.; Rodriguez, V.; Interlandi, G.; Klevit, R.; Vogel, V.; et al. Structural Basis for Mechanical Force Regulation of the Adhesin FimH via Finger Trap-like β Sheet Twisting. Cell 2010, 141, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Thompson, A.; Stojanoff, V.; Langermann, S.; Pinkner, J.; Hultgren, S.J.; Knight, S.D. X-ray structure of the FimC-FimH chaperone-adhesin complex from uropathogenic Escherichia coli. Science 1999, 285, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Barnich, N.; Carvalho, F.A.; Glasser, A.-L.; Darcha, C.; Jantscheff, P.; Allez, M.; Peeters, H.; Bommelaer, G.; Desreumaux, P.; Colombel, J.-F.; et al. CEACAM6 Acts as a Receptor for Adherent-invasive E. coli, Supporting Ileal Mucosa Colonization in Crohn Disease. J. Clin. Investig. 2007, 117, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Mo, W.J.; Sebbel, P.; Min, G.; Neubert, T.A.; Glockshuber, R.; Wu, X.R.; Sun, T.T.; Kong, X.P. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: Evidence from in vitro FimH binding. J. Cell Sci. 2001, 114, 4095–4103. [Google Scholar] [PubMed]
- Xie, B.; Zhou, G.; Chan, S.-Y.Y.; Shapiro, E.; Kong, X.-P.P.; Wu, X.-R.R.; Sun, T.-T.T.; Costello, C.E. Distinct glycan structures of uroplakins Ia and Ib: Structural basis for the selective binding of FimH adhesin to uroplakin Ia. J. Biol. Chem. 2006, 281, 14644–14653. [Google Scholar] [CrossRef] [PubMed]
- Thaysen-Andersen, M.; Venkatakrishnan, V.; Loke, I.; Laurini, C.; Diestel, S.; Parker, B.L.; Packer, N.H. Human Neutrophils Secrete Bioactive Paucimannosidic Proteins from Azurophilic Granules into Pathogen-Infected Sputum. J. Biol. Chem. 2015, 290, 8789–8802. [Google Scholar] [CrossRef] [PubMed]
- Dumych, T.; Yamakawa, N.; Sivignon, A.; Garenaux, E.; Robakiewicz, S.; Coddeville, B.; Bongiovanni, A.; Bray, F.; Barnich, N.; Szunerits, S.; et al. Oligomannose-Rich Membranes of Dying Intestinal Epithelial Cells Promote Host Colonization by Adherent-Invasive E. coli. Front. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bilyy, R.O.; Shkandina, T.; Tomin, A.; Muñoz, L.E.; Franz, S.; Antonyuk, V.; Kit, Y.Y.; Zirngibl, M.; Fürnrohr, B.G.; Janko, C.; et al. Macrophages discriminate glycosylation patterns of apoptotic cell-derived microparticles. J. Biol. Chem. 2012. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, I.J.; Mizuochi, T.; Hounsell, E.F.; Stoll, M.S.; Childs, R.A.; Feizi, T. New Type of Adhesive Specificity Revealed by Oligosaccharide Probes in Escherichia coli from Patients with Urinary Tract Infection. Lancet 1988, 332, 1327–1330. [Google Scholar] [CrossRef]
- Knight, S.D.; Bouckaert, J. Structure, function, and assembly of type 1 fimbriae. Top. Curr. Chem. 2009, 288, 67–107. [Google Scholar] [PubMed]
- Bouckaert, J.; Mackenzie, J.; de Paz, J.L.J.L.; Chipwaza, B.; Choudhury, D.; Zavialov, A.; Mannerstedt, K.; Anderson, J.; Piérard, D.; Wyns, L.; et al. The affinity of the FimH Fimbrial Adhesin is Receptor-Driven and Quasi-Independent of Escherichia coli Pathotypes. Mol. Microbiol. 2006, 61, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Gouin, S.G.; Roos, G.; Bouckaert, J. Discovery and Application of FimH Antagonists. In Carbohydrates as Drugs; Seeberger, P., Rademacher, C., Eds.; Springer International Publishing: New York, NY, USA, 2014; Volume 5, pp. 123–168. [Google Scholar]
- Prien, J.M.; Ashline, D.J.; Lapadula, A.J.; Zhang, H.; Reinhold, V.N. The high mannose glycans from bovine ribonuclease B isomer characterization by ion trap MS. J. Am. Soc. Mass Spectrom. 2009, 20, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Hung, C.S.; Pinkner, J.S.; Walker, J.N.; Cusumano, C.K.; Li, Z.; Bouckaert, J.; Gordon, J.I.; Hultgren, S.J. Positive Selection Identifies an in vivo Role for FimH During Urinary Tract Infection in Addition to Mannose Binding. Proc. Natl. Acad. Sci. USA 2009, 106, 22439–22444. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, J.; Berglund, J.; Schembri, M.; De Genst, E.; Cools, L.; Wuhrer, M.; Hung, C.-S.; Pinkner, J.; Slättegård, R.; Zavialov, A.; et al. Receptor Binding Studies Disclose a Novel Class of High-Affinity Inhibitors of the Escherichia coli FimH Adhesin. Mol. Microbiol. 2004, 55, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Roos, G.; Wellens, A.; Touaibia, M.; Yamakawa, N.; Geerlings, P.; Roy, R.; Wyns, L.; Bouckaert, J. Validation of Reactivity Descriptors to Assess the Aromatic Stacking within the Tyrosine Gate of FimH. ACS Med. Chem. Lett. 2013, 4, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, S.; Krammer, E.-M.; Roos, G.; Zalewski, A.; Preston, R.; Eid, S.; Zihlmann, P.; Prévost, M.; Lensink, M.F.; Thompson, A.; et al. Mutation of Tyr137 of the Universal Escherichia coli Fimbrial Adhesin FimH Relaxes the Tyrosine Gate Prior to Mannose Binding. IUCrJ 2017, 4, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Sager, C.P.; Fiege, B.; Zihlmann, P.; Vannam, R.; Rabbani, S.; Jakob, R.P.; Preston, R.C.; Zalewski, A.; Maier, T.; Peczuh, M.W.; et al. The Price of Flexibility—A Case Study on Septanoses as Pyranose Mimetics. Chem. Sci. 2018, 9, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Wellens, A.; Lahmann, M.; Touaibia, M.; Vaucher, J.; Oscarson, S.; Roy, R.; Remaut, H.; Bouckaert, J. The Tyrosine Gate as a Potential Entropic Lever in the Receptor-Binding Site of the Bacterial Adhesin FimH. Biochemistry 2012, 51, 4790–4799. [Google Scholar] [CrossRef] [PubMed]
- Durka, M.; Buffet, K.; Iehl, J.; Holler, M.; Nierengarten, J.-F.; Taganna, J.; Bouckaert, J.; Vincent, S.P. The functional valency of dodecamannosylated fullerenes with Escherichia coli FimH—Towards novel bacterial antiadhesives. Chem. Commun. 2011, 47, 1321–1323. [Google Scholar] [CrossRef] [PubMed]
- Fiege, B.; Rabbani, S.; Preston, R.C.; Jakob, R.P.; Zihlmann, P.; Schwardt, O.; Jiang, X.; Maier, T.; Ernst, B. The Tyrosine Gate of the Bacterial Lectin FimH: A Conformational Analysis by NMR Spectroscopy and X-ray Crystallography. ChemBioChem 2015, 16, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Kleeb, S.; Lemme, K.; Rabbani, S.; Scharenberg, M.; Zalewski, A.; Schädler, F.; Schwardt, O.; Ernst, B. FimH Antagonists: Structure-Activity and Structure-Property Relationships for Biphenyl α-D-Mannopyranosides. ChemMedChem. 2012, 7, 1404–1422. [Google Scholar] [CrossRef] [PubMed]
- Wellens, A.; Garofalo, C.; Nguyen, H.; Van Gerven, N.; Slättegård, R.; Hernalsteens, J.-P.; Wyns, L.; Oscarson, S.; De Greve, H.; Hultgren, S.; et al. Intervening with Urinary Tract Infections Using Anti-Adhesives Based on the Crystal Structure of the FimH–Oligomannose-3 Complex. PLoS ONE 2008, 3, e2040. [Google Scholar] [CrossRef]
- Lonardi, E.; Moonens, K.; Buts, L.; de Boer, A.; Olsson, J.; Weiss, M.; Fabre, E.; Guérardel, Y.; Deelder, A.; Oscarson, S.; et al. Structural Sampling of Glycan Interaction Profiles Reveals Mucosal Receptors for Fimbrial Adhesins of Enterotoxigenic Escherichia coli. Biology 2013, 2, 894–917. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-S.; Bouckaert, J.; Hung, D.; Pinkner, J.; Widberg, C.; DeFusco, A.; Auguste, C.G.; Strouse, R.; Langermann, S.; Waksman, G.; et al. Structural Basis of Tropism of Escherichia coli to the Bladder During Urinary Tract Infection. Mol. Microbiol. 2002, 44, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Touaibia, M.; Krammer, E.-M.; Shiao, T.; Yamakawa, N.; Wang, Q.; Glinschert, A.; Papadopoulos, A.; Mousavifar, L.; Maes, E.; Oscarson, S.; et al. Sites for Dynamic Protein-Carbohydrate Interactions of O- and C-Linked Mannosides on the E. coli FimH Adhesin. Molecules 2017, 22, 1101. [Google Scholar] [CrossRef] [PubMed]
- de Ruyck, J.; Lensink, M.F.; Bouckaert, J. Structures of C -mannosylated Anti-Adhesives Bound to the Type 1 Fimbrial FimH Adhesin. IUCrJ 2016, 3, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Kisiela, D.I.; Avagyan, H.; Friend, D.; Jalan, A.; Gupta, S.; Interlandi, G.; Liu, Y.; Tchesnokova, V.; Rodriguez, V.B.; Sumida, J.P.; et al. Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic E. coli. PLOS Pathog. 2015, 11, e1004857. [Google Scholar] [CrossRef] [PubMed]
- Tomašić, T.; Rabbani, S.; Gobec, M.; Raščan, I.M.; Podlipnik, Č.; Ernst, B.; Anderluh, M. Branched α-d-mannopyranosides: A New Class of Potent FimH Antagonists. Med. Chem. Commun. 2014, 5, 1247–1253. [Google Scholar] [CrossRef]
- Hoque, M.M.; Suzuki, K.; Tsunoda, M.; Jiang, J.; Zhang, F.; Takahashi, A.; Ohbayashi, N.; Zhang, X.; Tanaka, H.; Omura, S.; et al. Structural insights into the specific anti-HIV property of actinohivin: Structure of its complex with the α(1-2)mannobiose moiety of gp120. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Loris, R.; Van Walle, I.; De Greve, H.; Beeckmans, S.; Deboeck, F.; Wyns, L.; Bouckaert, J. Structural basis of oligomannose recognition by the Pterocarpus angolensis seed lectin. J. Mol. Biol. 2004, 335, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Moothoo, D.N.; Canan, B.; Field, R.A.; Naismith, J.H. Man α -2 Man α -OMe-concanavalin A complex reveals a balance of forces involved in carbohydrate recognition. Glycobiology 1999, 9, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, H.; Taylor, M.E.; Razi, N.; McBride, R.; Knirel, Y.A.; Graham, S.A.; Drickamer, K.; Weis, W.I. Structural basis for langerin recognition of diverse pathogen and mammalian glycans through a single binding site. J. Mol. Biol. 2011, 405, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Lutteke, T.; Frank, M.; von der Lieth, C.-W. Carbohydrate Structure Suite (CSS): Analysis of carbohydrate 3D structures derived from the PDB. Nucleic Acids Res. 2004, 33, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed]
- MacKerell, A.D.; Bashford, D.; Bellott, M.; Dunbrack, R.L.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef] [PubMed]
- MacKerell, A.D.; Feig, M.; Brooks, C.L. Extending the treatment of backbone energetics in protein force fields: Limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J. Comput. Chem. 2004, 25, 1400–1415. [Google Scholar] [CrossRef] [PubMed]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Guvench, O.; Mallajosyula, S.S.; Raman, E.P.; Hatcher, E.; Vanommeslaeghe, K.; Foster, T.J.; Jamison, F.W.; Mackerell, A.D. CHARMM additive all-atom force field for carbohydrate derivatives and its utility in polysaccharide and carbohydrate-protein modeling. J. Chem. Theory Comput. 2011, 7, 3162–3180. [Google Scholar] [CrossRef] [PubMed]
- Mallajosyula, S.S.; MacKerell, A.D. Influence of solvent and intramolecular hydrogen bonding on the conformational properties of O-linked glycopeptides. J. Phys. Chem. B 2011, 115, 11215–11229. [Google Scholar] [CrossRef] [PubMed]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993. [Google Scholar] [CrossRef]
- Tuckerman, M.; Berne, B.J.; Martyna, G.J. Reversible multiple time scale molecular dynamics. J. Chem. Phys. 1992. [Google Scholar] [CrossRef]
- Andersen, H.C. Rattle: A “velocity” version of the shake algorithm for molecular dynamics calculations. J. Comput. Phys. 1983, 52, 24–34. [Google Scholar] [CrossRef]
- Bas, D.C.; Rogers, D.M.; Jensen, J.H. Very fast prediction and rationalization of pKa values for protein-ligand complexes. Proteins 2008, 73, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; Van Der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Daura, X.; Gademann, K.; Jaun, B.; Seebach, D.; van Gunsteren, W.F.; Mark, A.E. Peptide Folding: When Simulation Meets Experiment. Angew. Chemie Int. Ed. 1999, 38, 236–240. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R.; Lynn, A. g_mmpbsa—A GROMACS tool for high-throughput MM-PBSA calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the performance of the MM/PBSA and MM/GBSA methods. 1. The accuracy of binding free energy calculations based on molecular dynamics simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |




| Ligand | Binding affinities | Thermodynamic parameters | |||||
|---|---|---|---|---|---|---|---|
| IC50 (ELLSA) [µM] | KD (ITC) [µM] | KD (SPR) [µM] | ΔG [kcal mol−1] | ΔH [kcal mol−1] | TΔS [kcal mol−1] | ||
| Manα1,2Man | 55.67 ± 28.8 | 0.942 ± 0.121 | 1.260 a | −8.14 | −4.26 ± 0.14 | 3.87 | |
| Manα1,3Man | 22.80 ± 4.75 | 0.298 ± 0.026 | 0.320 a | −8.81 | −8.23 ± 0.12 | 0.58 | |
| Man | 52.23 ± 21.71 | 1.672 ± 0.094 b | 2.300 c | −7.80 b | −13.64 ± 0.10 a | −5.84 b | |
| HM | 0.42 ± 0.05 | 0.007 ± 0.002 b | 0.005 c | −11.00 b | −13.64 ± 0.10 a | −2.65 b | |
| Energy Contributions | Manα1,2Man [kcal/mol] | Manα1,3Man [kcal/mol] | Man [kcal/mol] | HM [kcal/mol] |
|---|---|---|---|---|
| ΔEele | −156.6 ± 0.6 | −187.6 ± 1.1 | −153.2 ± 0.5 | −157.3 ± 0.7 |
| ΔEvdw | −34.5 ± 0.3 | −31.9 ± 0.3 | −17.1 ± 0.3 | −35.0 ± 0.3 |
| ΔEint | −191.1± 0.5 | −219.5 ± 1.1 | −170.3 ± 0.4 | −192.3 ± 0.6 |
| ΔGsolv POLAR | 98.3 ± 0.3 | 123.1 ± 0.8 | 79.8 ± 0.2 | 91.3 ± 0.3 |
| ΔGsolv UNPOLAR | −11.8 ± <0.1 | −11.4 ± <0.1 | −8.3 ± <0.1 | −11.6 ± <0.1 |
| ΔGsolv | 86.5 ± 0.3 | 111.7 ± 0.8 | 71.5 ± 0.2 | 79.7 ± 0.3 |
| ΔGbinding | −104.6 ± 0.4 | −107.7 ± 0.6 | −98.8 ± 0.3 | −112.6 ± 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumych, T.; Bridot, C.; Gouin, S.G.; Lensink, M.F.; Paryzhak, S.; Szunerits, S.; Blossey, R.; Bilyy, R.; Bouckaert, J.; Krammer, E.-M. A Novel Integrated Way for Deciphering the Glycan Code for the FimH Lectin. Molecules 2018, 23, 2794. https://doi.org/10.3390/molecules23112794
Dumych T, Bridot C, Gouin SG, Lensink MF, Paryzhak S, Szunerits S, Blossey R, Bilyy R, Bouckaert J, Krammer E-M. A Novel Integrated Way for Deciphering the Glycan Code for the FimH Lectin. Molecules. 2018; 23(11):2794. https://doi.org/10.3390/molecules23112794
Chicago/Turabian StyleDumych, Tetiana, Clarisse Bridot, Sébastien G. Gouin, Marc F. Lensink, Solomiya Paryzhak, Sabine Szunerits, Ralf Blossey, Rostyslav Bilyy, Julie Bouckaert, and Eva-Maria Krammer. 2018. "A Novel Integrated Way for Deciphering the Glycan Code for the FimH Lectin" Molecules 23, no. 11: 2794. https://doi.org/10.3390/molecules23112794
APA StyleDumych, T., Bridot, C., Gouin, S. G., Lensink, M. F., Paryzhak, S., Szunerits, S., Blossey, R., Bilyy, R., Bouckaert, J., & Krammer, E.-M. (2018). A Novel Integrated Way for Deciphering the Glycan Code for the FimH Lectin. Molecules, 23(11), 2794. https://doi.org/10.3390/molecules23112794









