In Vitro and in Vivo Imaging of Nitroxyl with Copper Fluorescent Probe in Living Cells and Zebrafish
Abstract
1. Introduction
2. Results
2.1. Design Strategy, Synthesis, and Properties of AbTCA and Cu(II)-AbTCA
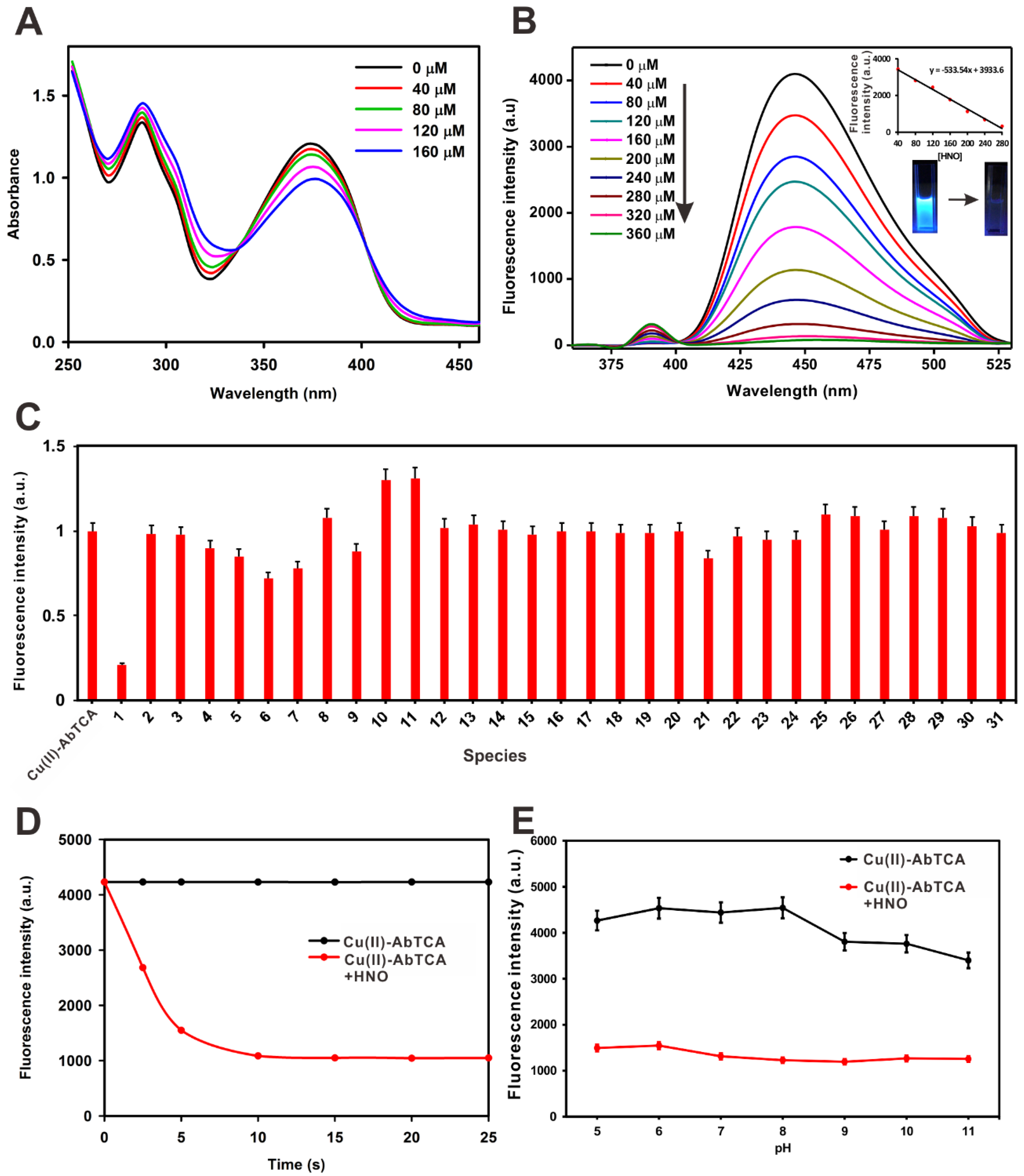
2.2. Assessment of UV-Visible and Fluorescence Response to HNO
2.3. Selectivity Studies
2.4. Reaction Time Effects on HNO Sensing
2.5. Effects of pH Studies
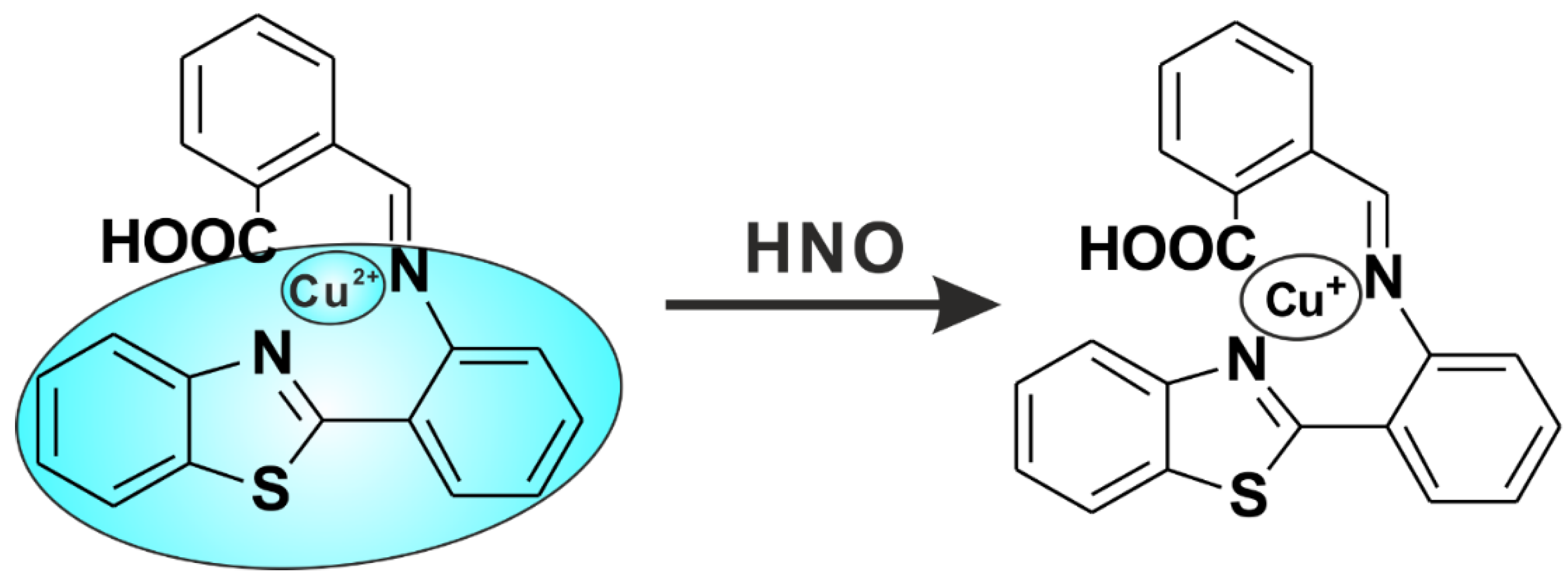
2.6. Mechanism of Cu(II)-Abtca in Sensing HNO
2.7. Detection of Nitroxyl Release from DNIC Complex, [PPN][Fe(NO)4] and Thiophenol
2.8. Cytotoxicity
2.9. Confocal Imaging
2.9.1. Detection of HNO Using EAHY-44926 Cells
2.9.2. Detection of HNO Using RAW 264.7 Cells
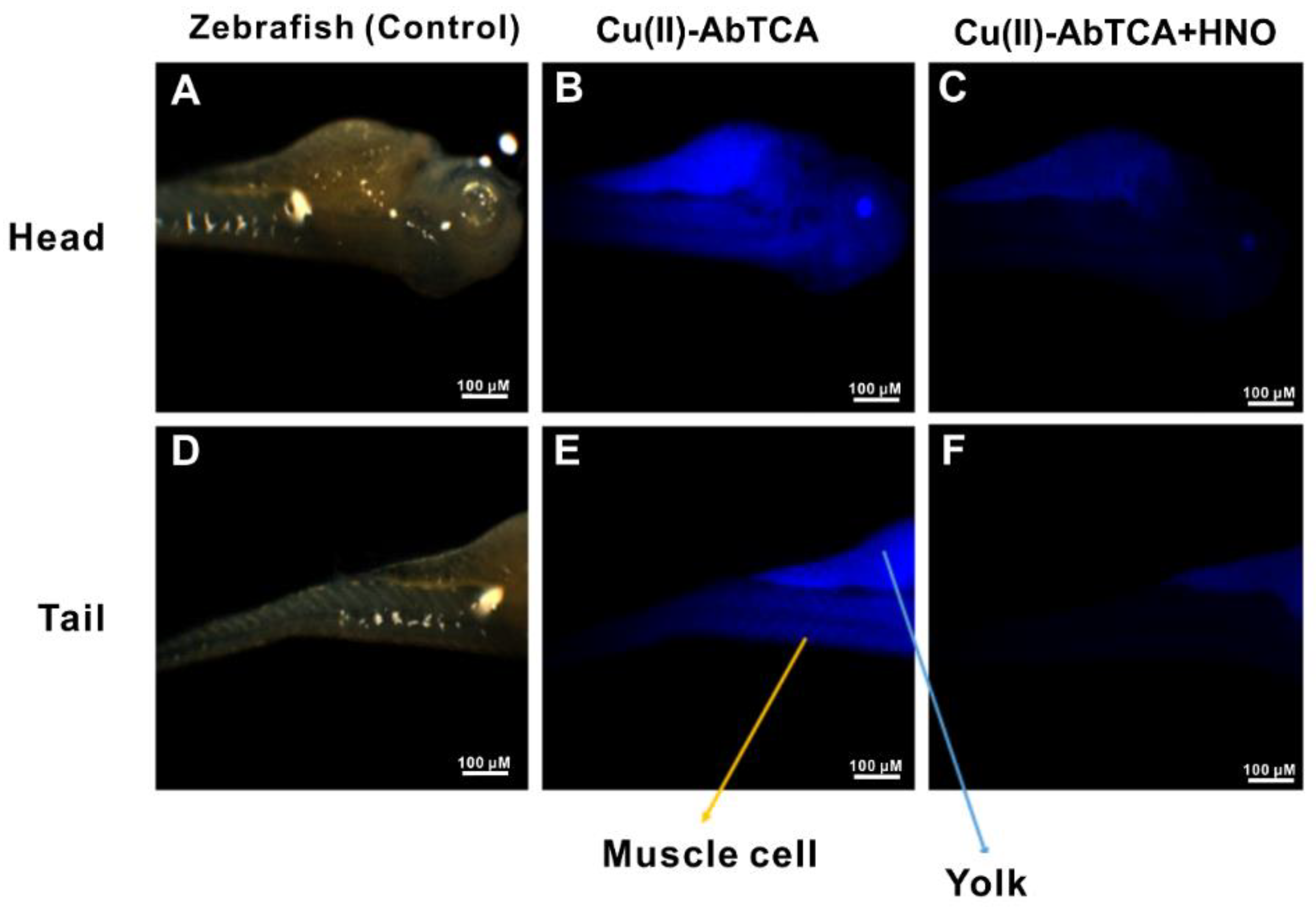
2.10. In Vivo Imaging of HNO in Zebrafish
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instrumentation
3.3. Chemistry
3.3.1. Synthesis of 2-((2-(benzothiazole-2yl)benzylidene)amino)benzoic acid, AbTCA
3.3.2. Synthesis of Cu(II)-AbTCA
3.4. Fluorimetry Analysis
3.5. Determination of the Detection Limit
3.6. Cell Culture and Cytotoxicity
3.6.1. Confocal Microscopy
3.6.2. HNO imaging Studies
3.6.3. Zebrafish Imaging Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Miranda, K.M. The chemistry of nitroxyl (HNO) and implications in biology. Coordin. Chem. Rev. 2005, 249, 433–455. [Google Scholar] [CrossRef]
- Wink, D.A.; Miranda, K.M.; Katori, T.; Mancardi, D.; Thomas, D.D.; Ridnour, L.; Espey, M.G.; Feelisch, M.; Colton, C.A.; Fukuto, J.M. Orthogonal properties of the redox siblings nitroxyl and nitric oxide in the cardiovascular system: A novel redox paradigm. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2264–H2276. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Mahapatro, S.N.; Broene, R.D.; Guy, J.K. Oxidation and reduction of hemoproteins by trioxodinitrate(II). The role of nitrosyl hydride and nitrite. J. Am. Chem. Soc. 1988, 110, 593–599. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Chiang, K.; Hszieh, R.; Wong, P.; Chaudhuri, G. The pharmacological activity of nitroxyl: A potent vasodilator with activity similar to nitric oxide and/or endothelium-derived relaxing factor. J. Pharmacol. Exp. Ther. 1992, 263, 546–551. [Google Scholar] [PubMed]
- Ellis, A.; Li, C.G.; Rand, M.J. Differential actions of L-cysteine on responses to nitric oxide, nitroxyl anions and EDRF in the rat aorta. Br. J. Pharmacol. 2000, 129, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Wanstall, J.C.; Jeffery, T.K.; Gambino, A.; Lovren, F.; Triggle, C.R. Vascular smooth muscle relaxation mediated by nitric oxide donors: A comparison with acetylcholine, nitric oxide and nitroxyl ion. Br. J. Pharmacol. 2001, 134, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.L.; Irvine, J.C.; Tare, M.; Apostolopoulos, J.; Favaloro, J.L.; Triggle, C.R.; Kemp-Harper, B.K. A role for nitroxyl (HNO) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. Br. J. Pharmacol. 2009, 157, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Irvine, J.C.; Favaloro, J.L.; Kemp-Harper, B.K. NO-activates soluble guanylate cyclase and Kv channels to vasodilate resistance arteries. Hypertension 2003, 41, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Choi, Y.B.; Rayudu, P.V.; Das, P.; Asaad, W.; Arnelle, D.R.; Stamler, J.S.; Lipton, S.A. Attenuation of NMDA receptor activity and neurotoxicity by nitroxyl anion, NO. Neuron 1999, 24, 461–469. [Google Scholar] [CrossRef]
- Ma, X.L.; Gao, F.; Liu, G.L.; Lopez, B.L.; Christopher, T.A.; Fukuto, J.M.; Wink, D.A.; Feelisch, M. Opposite effects of nitric oxide and nitroxyl on postischemic myocardial injury. Proc. Natl. Acad. Sci. USA 1999, 96, 14617–14622. [Google Scholar] [CrossRef] [PubMed]
- Dobmeier, K.P.; Riccio, D.A.; Schoenfisch, M.H. Xerogel optical sensor films for quantitative detection of nitroxyl. Anal. Chem. 2008, 80, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.S.; Hyun, J.; Fukuto, J.M.; Shirota, F.N.; DeMaster, E.G.; Shoeman, D.W.; Nagasawa, H.T. Reaction between S-nitrosothiols and thiols: Generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry 1998, 37, 5362–5371. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Zink, C.N.; King, S.B. Rapid and selective nitroxyl (HNO) trapping by phosphines: Kinetics and new aqueous ligations for HNO detection and quantitation. J. Am. Chem. Soc. 2011, 133, 11675–11685. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.R.; Tu, C.; Silverman, D.N.; Toscano, J.P. Detection of nitroxyl (HNO) by membrane inlet mass spectrometry. Free Radic. Biol. Med. 2011, 50, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Malinski, T.; Taha, Z. Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature 1992, 358, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Yoshimura, T. Bioimaging of nitric oxide. Chem. Rev. 2002, 102, 1235–1270. [Google Scholar] [CrossRef] [PubMed]
- Ridnour, L.A.; Sim, J.E.; Hayward, M.A.; Wink, D.A.; Martin, S.M.; Buettner, G.R.; Spitz, D.R. A spectrophotometric method for the direct detection and quantitation of nitric oxide, nitrite, and nitrate in cell culture media. Anal. Biochem. 2000, 281, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 2013, 42, 622–661. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Zheng, K.; Zhu, S. FRET-based small-molecule fluorescent probes: Rational design and bioimaging applications. Acc. Chem. Res. 2013, 46, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Lin, W.; Cheng, D.; Chen, H.; Liu, Y.; Liu, K. A two-photon fluorescent turn-on probe for nitroxyl (HNO) and its bioimaging application in living tissues. Chem. Commun. 2015, 51, 5754–5757. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Klorig, E.B.; Wright, M.W.; King, S.B. Reductive phosphine-mediated ligation of nitroxyl (HNO). Org. Lett. 2009, 11, 2719–2721. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Chen, H.; Zhu, S.; He, L. A unique family of rigid analogues of the GFP chromophore with tunable two-photon action cross-sections for biological imaging. Angew. Chem. Int. Ed. 2013, 52, 10018–10022. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.R.; Toscano, J.P. Detection of nitroxyl (HNO) by a prefluorescent probe. J. Phys. Org. Chem. 2011, 24, 993–998. [Google Scholar] [CrossRef]
- Rosenthal, J.; Lippard, S.J. Direct detection of nitroxyl in aqueous solution using a tripodal copper(II) BODIPY complex. J. Am. Chem. Soc. 2010, 132, 5536–5537. [Google Scholar] [CrossRef] [PubMed]
- Royzen, M.; Wilson, J.J.; Lippard, S.J. Physical and structural properties of [Cu(BOT1)Cl]Cl, a fluorescent imaging probe for HNO. J. Inorg. Biochem. 2013, 118, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, K.; Li, J.Y.; Fang, Y.; Zhao, T.C.; Yao, C. Visualization of nitroxyl in living cells by a chelated copper(II) coumarin complex. Org. Lett. 2011, 13, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Apfel, U.P.; Buccella, D.; Wilson, J.J.; Lippard, S.J. Detection of nitric oxide and nitroxyl with benzoresorufin-based fluorescent sensors. Inorg. Chem. 2013, 52, 3285–3294. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, A.T.; Johnstone, T.C.; Deliz Liang, A.; Lippard, S.J.; Rivera-Fuentes, P. A fast and selective near-infrared fluorescent sensor for multicolor imaging of biological nitroxyl (HNO). J. Am. Chem. Soc. 2014, 136, 4697–4705. [Google Scholar] [CrossRef] [PubMed]
- Shoman, M.E.; Aly, O.M. Nitroxyl (HNO): A reduced form of nitric oxide with distinct chemical, pharmacological, and therapeutic properties. Oxid. Med. Cell. Longev. 2016, 2016, 4867124. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.E.; Sies, H. Reversible conversion of nitroxyl anion to nitric oxide by superoxide dismutase. Proc. Natl. Acad. Sci. USA 1991, 88, 10860–10864. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yao, Y.W.; Li, J.Y.; Yao, C.; Lin, B.P. Nitroxyl induced fluorescence enhancement via reduction of a copper(II) coumarin-ester complex: Its application for bioimaging in vivo. Sens. Actuat. B-Chem. 2012, 174, 414–420. [Google Scholar] [CrossRef]
- Liu, C.Y.; Cao, Z.M.; Wang, Z.H.; Jia, P.; Liu, J.; Wang, Z.K.; Han, B.J.; Huang, X.; Li, X.; Zhu, B.C. A highly sensitive and reductant-resistant fluorescent chemodosimeter for the rapid detection of nitroxyl. Sens. Actuat. B-Chem. 2015, 220, 727–733. [Google Scholar] [CrossRef]
- Dedeoglu, B.; Monari, A.; Etienne, T.; Aviyente, V.; Özen, A.S. Detection of nitroaromatic explosives based on fluorescence quenching of silafluorene- and silole-containing polymers: A time-dependent density functional theory study. J. Phys. Chem. C 2014, 118, 23946–23953. [Google Scholar] [CrossRef]
- Demirel, G.B.; Daglar, B.; Bayindir, M. Extremely fast and highly selective detection of nitroaromatic explosive vapours using fluorescent polymer thin films. Chem. Commun. 2013, 49, 6140–6142. [Google Scholar] [CrossRef] [PubMed]
- Venkatramaiah, N.; Kumar, S.; Patil, S. Fluoranthene based fluorescent chemosensors for detection of explosive nitroaromatics. Chem. Commun. 2012, 48, 5007–5009. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Chai, Q.; Liang, X.M.; Li, M.Q.; Wang, Z.Q.; Fu, Y. A highly selective and sensitive fluorescent turn-off probe for Cu2+ based on a guanidine derivative. Molecules 2017, 22, 1741. [Google Scholar] [CrossRef] [PubMed]
- Hyman, L.M.; Franz, K.J. Probing oxidative stress: Small molecule fluorescent sensors of metal ions, reactive oxygen species, and thiols. Coord. Chem. Rev. 2012, 256, 2333–2356. [Google Scholar] [CrossRef] [PubMed]
- Loas, A.; Radford, R.J.; Deliz Liang, A.; Lippard, S.J. Solid-phase synthesis provides a modular, lysine-based platform for fluorescent discrimination of nitroxyl and biological thiols. Chem. Sci. 2015, 6, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, M.S.; Guilbault, G.G. Fiber-optic sensor for the determination of glucose using micellar enhanced chemiluminescence of the peroxyoxalate reaction. Anal. Chem. 1988, 60, 2671–2674. [Google Scholar] [CrossRef] [PubMed]
- Ke, I.S.; Myahkostupov, M.; Castellano, F.N.; Gabbai, F.P. Stibonium ions for the fluorescence turn-on sensing of F- in drinking water at parts per million concentrations. J. Am. Chem. Soc. 2012, 134, 15309–15311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Xiao, N.; Li, Y. Simple quinoline-based “turn-on” fluorescent sensor for imaging copper (II) in living cells. Can. J. Chem. 2014, 92, 1092–1097. [Google Scholar] [CrossRef]
- Lin, Z.S.; Chiou, T.W.; Liu, K.Y.; Hsieh, C.C.; Yu, J.S.; Liaw, W.F. A dinitrosyliron complex within the homoleptic Fe(NO)4 anion: NO as nitroxyl and nitrosyl ligands within a single structure. Inorg. Chem. 2012, 51, 10092–10094. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Oushiki, D.; Kojima, H.; Terai, T.; Arita, M.; Hanaoka, K.; Urano, Y.; Nagano, T. Development and application of a near-infrared fluorescence probe for oxidative stress based on differential reactivity of linked cyanine dyes. J. Am. Chem. Soc. 2010, 132, 2795–2801. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palanisamy, S.; Wang, Y.-L.; Chen, Y.-J.; Chen, C.-Y.; Tsai, F.-T.; Liaw, W.-F.; Wang, Y.-M. In Vitro and in Vivo Imaging of Nitroxyl with Copper Fluorescent Probe in Living Cells and Zebrafish. Molecules 2018, 23, 2551. https://doi.org/10.3390/molecules23102551
Palanisamy S, Wang Y-L, Chen Y-J, Chen C-Y, Tsai F-T, Liaw W-F, Wang Y-M. In Vitro and in Vivo Imaging of Nitroxyl with Copper Fluorescent Probe in Living Cells and Zebrafish. Molecules. 2018; 23(10):2551. https://doi.org/10.3390/molecules23102551
Chicago/Turabian StylePalanisamy, Sathyadevi, Yu-Liang Wang, Yu-Jen Chen, Chiao-Yun Chen, Fu-Te Tsai, Wen-Feng Liaw, and Yun-Ming Wang. 2018. "In Vitro and in Vivo Imaging of Nitroxyl with Copper Fluorescent Probe in Living Cells and Zebrafish" Molecules 23, no. 10: 2551. https://doi.org/10.3390/molecules23102551
APA StylePalanisamy, S., Wang, Y.-L., Chen, Y.-J., Chen, C.-Y., Tsai, F.-T., Liaw, W.-F., & Wang, Y.-M. (2018). In Vitro and in Vivo Imaging of Nitroxyl with Copper Fluorescent Probe in Living Cells and Zebrafish. Molecules, 23(10), 2551. https://doi.org/10.3390/molecules23102551



