The authors wish to make the following corrections to their paper [1]. The authors are sorry to report that the absolute configurations of the 1,2-diols (1–5d) as well as the acetates (1–5e) obtained in this paper are inverted. In order to establish the absolute configuration of these compounds, specific rotation values should be compared with those described in [2] for (R)-1d, (R)-4d and (R)-5d, instead of the value provided in [3] for (R)-ethyl 2-benzyl-2,3-dihydroxypropanoate. This compound is the benzylic analogue of 1,2-diols 1–5d. Compound 6d (ethyl 2-hydroxy-2-hydroxymethyl-4-phenylbutanoate) presents the chiral quaternary center bound to an aliphatic carbon instead of an aromatic one. Thus, specific rotation values cannot be compared with the ones given in [2], and only the relative configurations of 6d and 6e are indicated. Consequently, the authors wish to make the following corrections to the paper:
In the enzymatic kinetic resolutions of 1,2-diols containing the chiral center bound to an aromatic carbon atom, (S)-1,2-diols (S)-1–5d and (R)-acetates (R)-1–5e are obtained. With regard to substrate 6d, the 1,2-diol achieved was (−)-6d, whereas the acetate obtained was (+)-6e.
Thus, we replace all over the manuscript (R)-1–5d with (S)-1–5d, (S)-1–5e with (R)-1–5e, (R)-6d with (−)-6d and (S)-6e with (+)-6e. In page 4, replace “(±)-1e” with “(±)-1d”, “(S)-acetates 2–6e” with “(R)-acetates 2–5e”.
In page 6 we change “The absolute configuration of the 1,2-diols (R)-1–6d and the acetates (S)-1–6e” with “The absolute configuration of the 1,2-diols (S)-1–5d and the acetates (R)-1–5e”, and “(R)-ethyl 2-benzyl-2,3-dihydroxypropanoate [23].” to “(R)-ethyl 2,3-dihydroxy-2-phenylpropanoate [(R)-1d], (R)-ethyl 2,3-dihydroxy-2-(4-methoxyphenyl)propanoate [(R)-4d], and (R)-ethyl 2,3-dihydroxy-2-(tiophen-2-yl)propanoate, [(R)-5d] in reference [26].”
The scheme of Table 2 is to be replaced:
Table 2. Lipase-catalysed acylation of rac-ethyl 2,3-dihydroxy-2-phenylprpanoate (1d) at different reaction conditions.
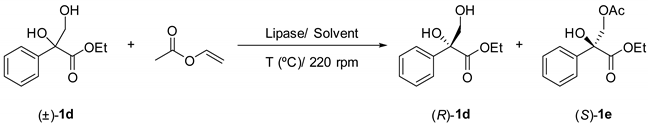 with
with
 with
withTable 2. Lipase-catalysed acylation of rac-ethyl 2,3-dihydroxy-2-phenylpropanoate (1d) at different reaction conditions.
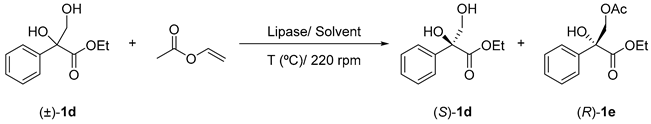 with
with
 with
withThe scheme of Table 3 is also to be replaced:
Table 3. PSL-C catalysed kinetic resolution of racemic diols 2–6d in tert-butyl methyl ether (TBME) at 30 °C using vinyl acetate as the acyl donor.
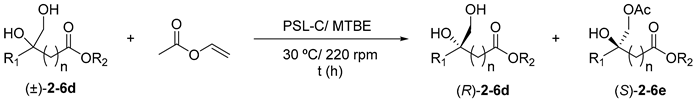 with
with
 with
withTable 3. PSL-C catalysed kinetic resolution of racemic diols 2–6d in tert-butyl methyl ether (TBME) at 30 °C using vinyl acetate as the acyl donor.
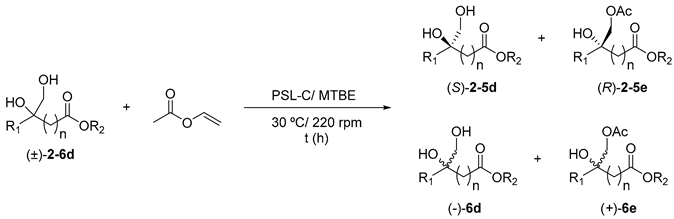
The author would like to apologize for any inconvenience caused to the readers. The manuscript will be updated and the original will remain online on the article webpage, with a reference to this Correction.
References
- de Gonzalo, G. Lipase Catalysed Kinetic Resolution of Racemic 1,2-Diols Containing a Chiral Quaternary Center. Molecules 2018, 23, 1585. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Peña, A.; Monge, D.; Martín-Zamora, E.; Álvarez, E.; Fernández, R.; Lassaletta, J.M. Asymmetric Formal Carbonyl-Ene Reactions of Formaldehyde tert-Butyl Hydrazone with α-Keto Esters: Dual Activation by Bis-urea Catalysts. J. Am. Chem. Soc. 2012, 134, 12912–12915. [Google Scholar] [CrossRef] [PubMed]
- Jew, S.; Roh, E.; Baek, E.; Mireille, L.; Kim, H.; Jeong, B.; Park, M.; Park, H. Asymmetric synthesis of (R)-(+)-etomoxir via enzymatic resolution. Tetrahedron Asymmetry 2000, 11, 3395–3401. [Google Scholar] [CrossRef]
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).