Biosynthesis of Grandione: An Example of Tandem Hetero Diels-Alder/Retro-Claisen Rearrangement Reaction?
Abstract
1. Introduction
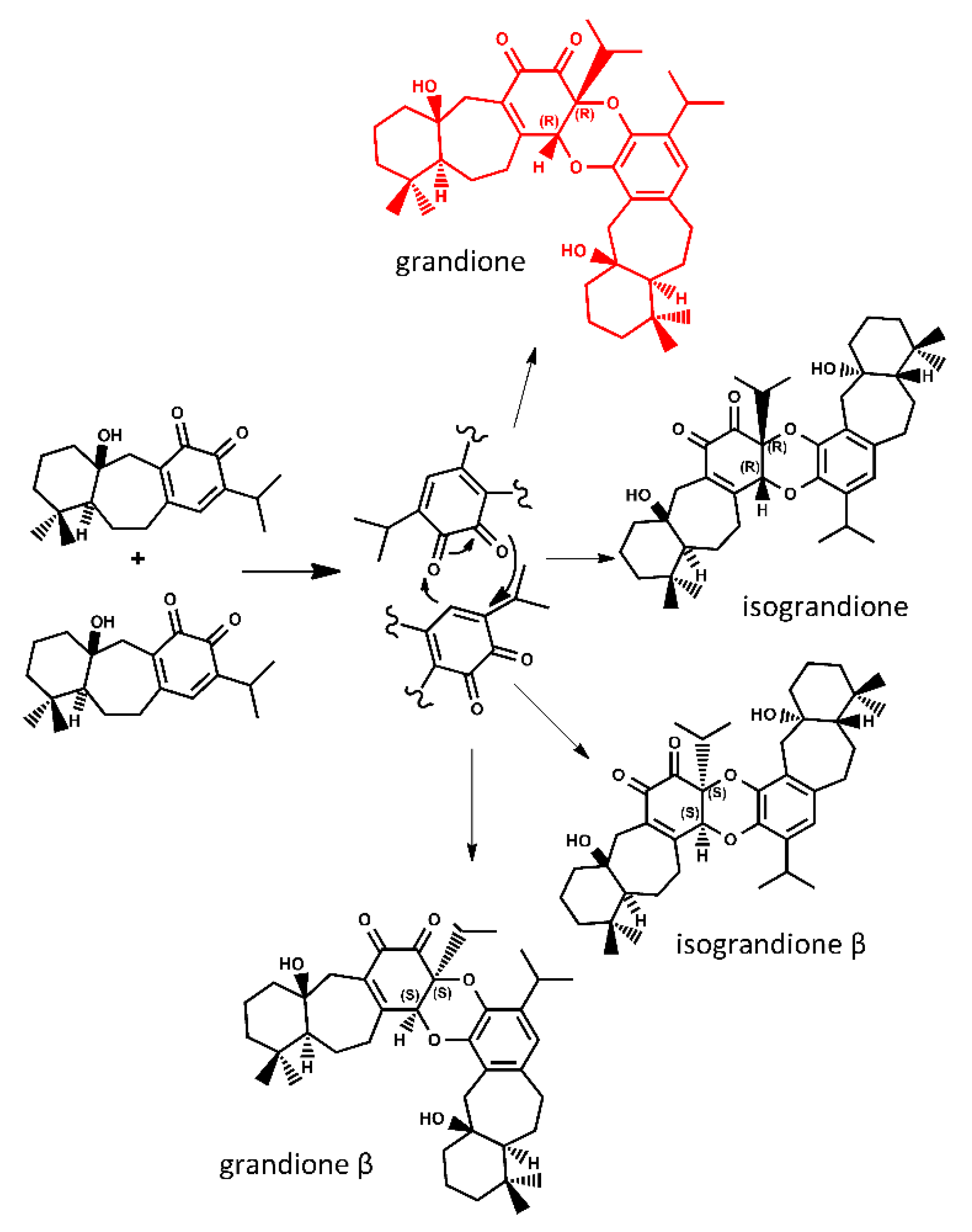
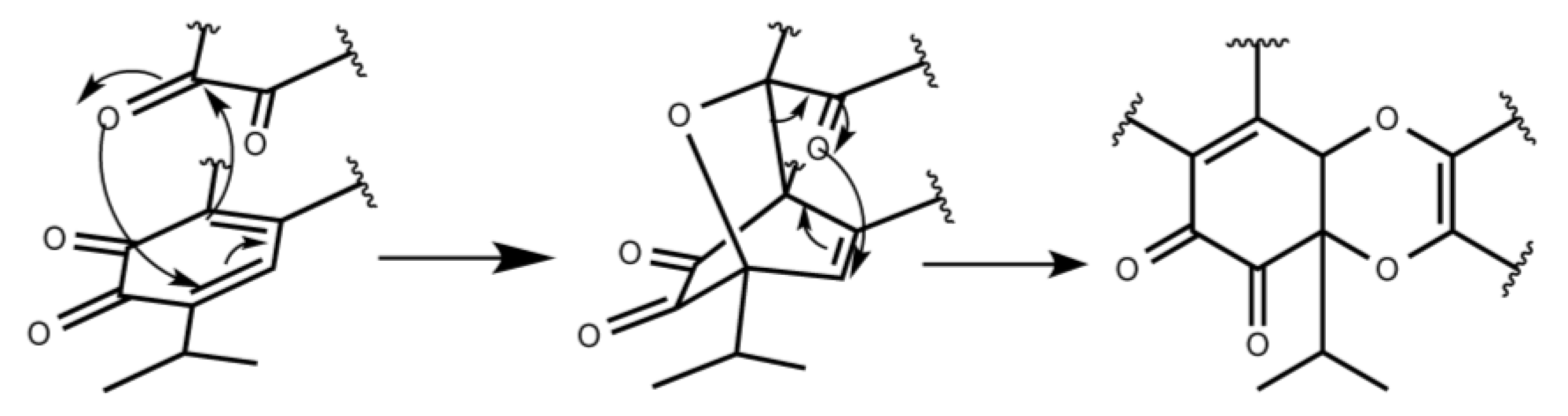
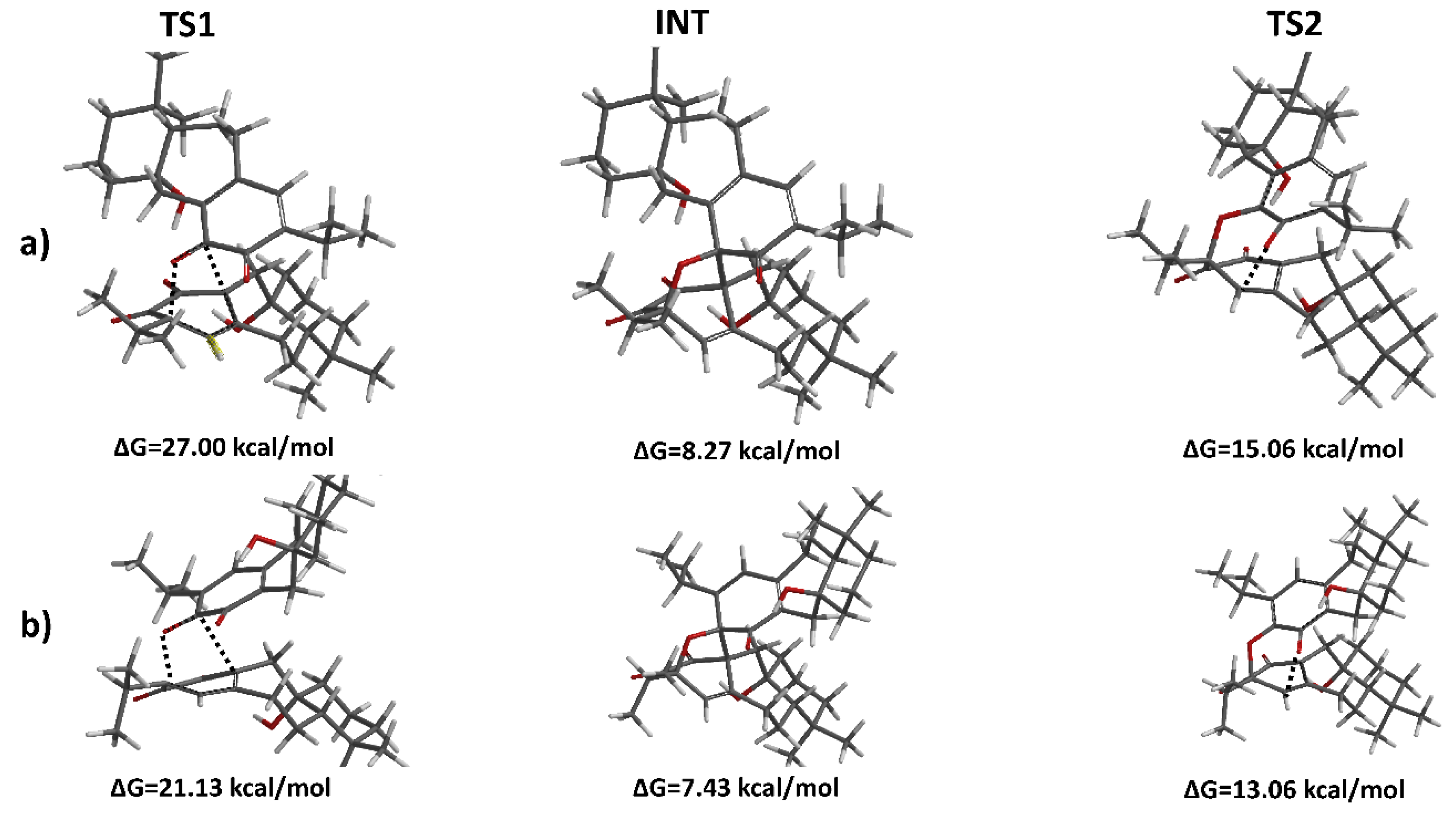
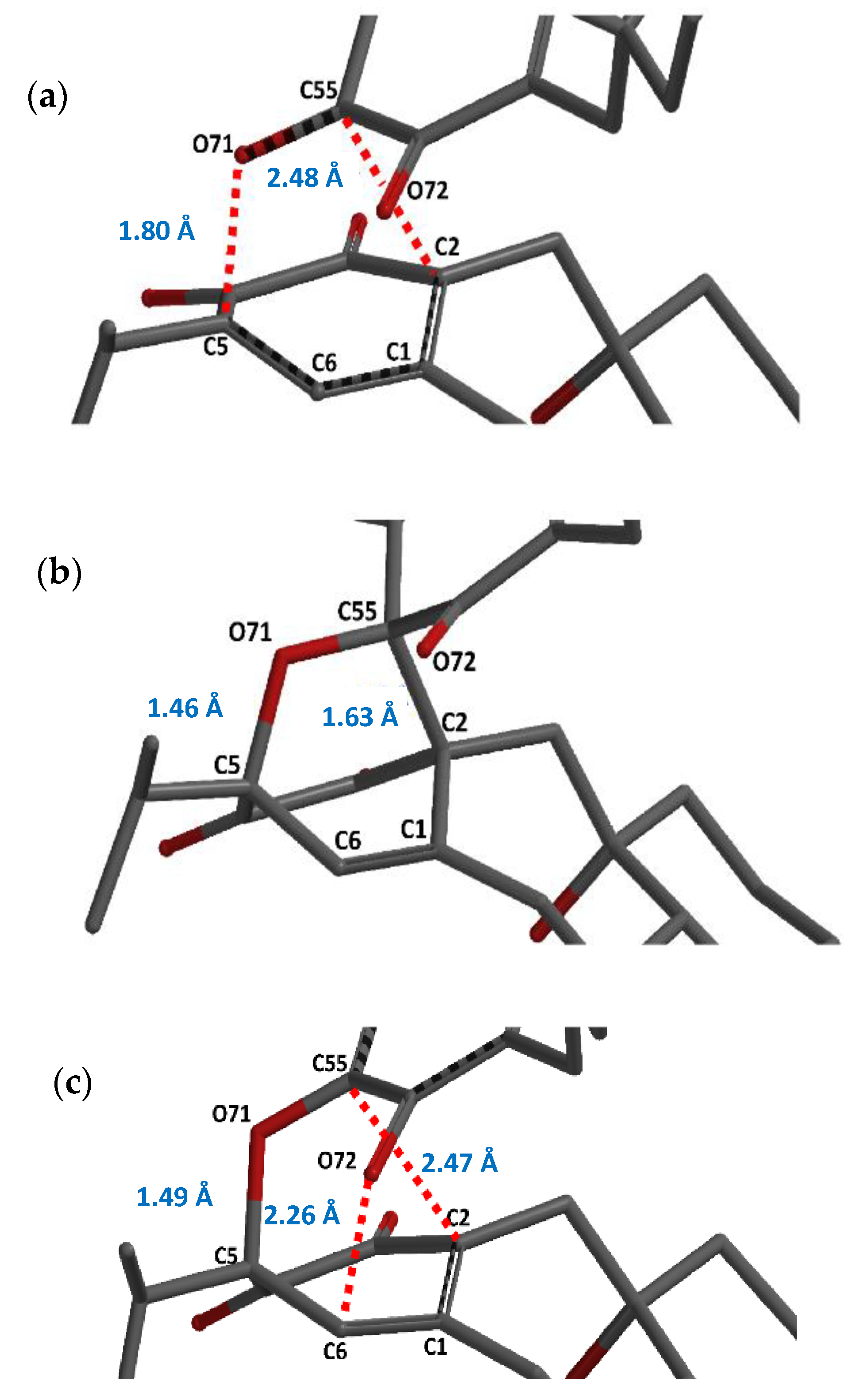
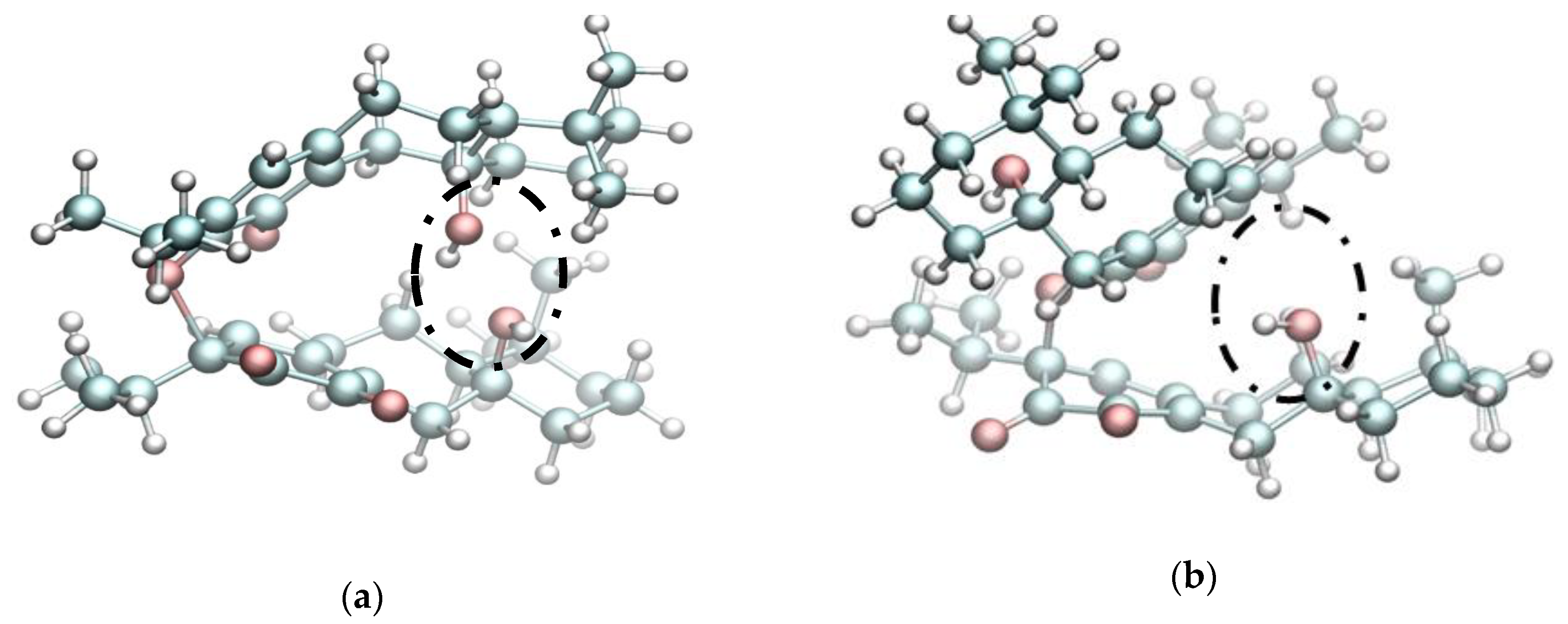
2. Results and Discussion
Tandem Reaction Analysis of Gradione
3. Materials and Methods
Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oikawa, H. Diels-Alderases. In Comprehensive Natural Products II Chemistry and Biology; Mander, A., Lui, H.W., Eds.; Elsevier: Oxford, UK, 2010; Volume 8, pp. 277–314. ISBN 978-008045382-8. [Google Scholar]
- Auclair, K.; Sutherland, A.; Kennedy, J.; Witter, D.J.; Van den Heever, J.P.; Hutchinson, C.R.; Vederas, J.C.J. Lovastatin Nonaketide Synthase catalyzes an intramolecular Diels-Alder reaction of a substrate analogue. J. Am. Chem. Soc. 2000, 122, 11519–11520. [Google Scholar] [CrossRef]
- Ose, T.; Watanabe, K.; Mie, T.; Honma, M.; Watanabe, H.; Yao, M.; Oikawa, H.; Tanaka, I. Insight into a natural Diels-Alder reaction from the structure of macrophomate synthase. Nature 2003, 422, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.M.; Li, J.W.H.; Choi, J.W.; Zhou, H.; Lee, K.K.M.; Moorthie, V.A.; Xie, X.; Kealey, J.T.; Da Silva, N.A.; Vederas, J.C.; et al. Complete reconstitution of a highly reducing iterative polyketide synthase. Science 2009, 326, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.R.; Illarionov, B.; Joshi, M.; Cushman, M.; Lee, C.Y.; Eisenreich, W.; Fischer, M.; Bacher, A. Mechanistic insights on Riboflavin Synthase inspired by selective binding of the 6,7-dimethyl-8-ribityllumazine exomethylene anion A. J. Am. Chem. Soc. 2010, 132, 2983–2990. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ruszczycky, M.W.; Choi, S.; Liu, Y.; Liu, H. Enzyme-catalysed [4+2] cycloaddition is a key step in the biosynthesis of Spinosyn A. Nature 2011, 473, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Fage, C.D.; Isiorho, E.A.; Liu, Y.; Wagner, D.T.; Liu, H.; Keatinge-Clay, A.T. The structure of SpnF, a standalone enzyme that catalyzes [4+2] cycloaddition. Nat. Chem. Biol. 2015, 11, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Hashimoto, J.; Teruya, K.; Hirano, T.; Shin-ya, K.; Ikeda, H.; Liu, H.; Nishiyama, M.; Kuzuyama, T. Biosynthesis of Versipelostatin: Identification of an enzyme-catalyzed [4+2]-cycloaddition required for macrocyclization of spirotetronate-containing polyketides. J. Am. Chem. Soc. 2015, 137, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Sun, P.; Yan, Y.; Wu, Z.; Zheng, Q.; Zhou, S.; Zhang, H.; Yu, F.; Jia, X.; Chen, D.; et al. An enzymatic [4+2] cyclization cascade creates the pentacyclic core of pyrroindomycins. Nat. Chem. Biol. 2015, 11, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Townsend, C.A. A “Diels-Alderase” at Last. ChemBioChem 2011, 12, 2267–2269. [Google Scholar] [CrossRef] [PubMed]
- Kelly, W.L. Intramolecular cyclizations of polyketide biosynthesis: Mining for a “Diels-Alderase”? Org. Biomol. Chem. 2008, 6, 4483–4493. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ruszczycky, M.W.; Liu, H.W. Current developments and challenges in the search for a naturally selected Diels-Alderase. Curr. Opin. Chem. Biol. 2012, 16, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.J.; Lees, N.R.; Han, L.C.; van der Kamp, M.W.; Mulholland, A.J.; Stach, J.E.M.; Willis, C.L.; Race, P.R. The catalytic mechanism of a natural Diels-Alderase revealed in molecular detail. J. Am. Chem. Soc. 2016, 138, 6095–6098. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, H.; Tokiwano, T. Enzymatic catalysis of the Diels-Alder reaction in the biosynthesis of natural products. Nat. Prod. Rep. 2004, 21, 321–352. [Google Scholar] [CrossRef] [PubMed]
- Singleton, D.A.; Schulmeier, B.E.; Hang, C.; Thomas, A.A.; Leung, S.-W.; Merrigan, S.R. Isotope effects and the distinction between synchronous, asynchronous, and stepwise Diels-Alder reactions. Tetrahedron 2001, 57, 5149–5160. [Google Scholar] [CrossRef]
- Jasiński, R. A reexamination of the molecular mechanism of the Diels-Alder reaction between tetrafluoroethene and cyclopentadiene. React. Kinet. Mech. Cat. 2016, 119, 49–57. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, F.; Wu, R.; Hess, A.B. Biosynthesis of Spinosyn A: A [4+2] or [6+4] cycloaddition? ACS Catal. 2018, 8, 2353–2358. [Google Scholar] [CrossRef]
- Maiga-Wandiam, B.; Corbu, A.; Massiot, G.; Sautel, F.; Yu, P.; Lin, B.; Houk, K.N.; Cossy, J. Intramolecular Diels-Alder approaches to the decalin core of Verongidolide: The origin of the exo-selectivity, a DFT analysis. J. Org. Chem. 2018, 83, 5975–5985. [Google Scholar] [CrossRef] [PubMed]
- Kokkonda, P.; Brown, K.R.; Seguin, T.J.; Wheeler, S.E.; Vaddypally, S.; Zdilla, M.J.; Andrade, R.B. Biomimetic total syntheses of (−)-Leucoridines A and C through the dimerization of (−)-Dihydrovalparicine. Angew. Chem. 2015, 54, 12632–12635. [Google Scholar] [CrossRef] [PubMed]
- Quesadas-Rojas, M.; Mena-Rejón, G.J.; Cáceres-Castillo, D.; Cuevas, G.; Quijano-Quiñones, R.F. Biogenesis of triterpene dimers from orthoquinones related to quinonemethides: Theoretical study on the reaction mechanism. Molecules 2016, 21, 1551. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.L. A new theory of the origin and nature of life. Science 1942, 96, 14. [Google Scholar] [CrossRef] [PubMed]
- Negrón-Mendoza, A.; Alfonso, L. Herrera: A Mexican pioneer in the study of chemical evolution. J. Biol. Phys. 1994, 20, 11–15. [Google Scholar] [CrossRef]
- Galii, B.; Gasparrini, F.; Lanzotti, V.; Misiti, D.; Riccio, R.; Villani, C.; He, G.; Ma, Z.; Yin, W. Grandione, a new heptacyclic dimeric diterpene from Torreya grandis Fort. Tetrahedron 1999, 55, 11385–11394. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Takahashi, Y.; Satake, Y.; Fukaya, H.; Takeya, K.; Aiyama, R.; Matsuzaki, T.; Hashimoto, S.; Shiina, T.; Kurihara, T. Biomimetic synthesis of grandione from Demethylsalvicanol via hetero-Diels-Alder type dimerization and structure revision of Grandione. Tetrahedron Lett. 2005, 46, 7885–7887. [Google Scholar] [CrossRef]
- Majetich, G.; Zou, G. Total Synthesis of (−)-Barbatusol, (+)-Demethylsalvicanol, (−)-Brussonol, and (+)-Grandione. Org. Lett. 2008, 10, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Oppolzer, W.; Francotte, E.; Bättig, K. Total synthesis of (±)-Lysergic acid by an intramolecular imino-Diels-Alder reaction. Preliminary communication. Helv. Chim. Acta 1981, 64, 478–481. [Google Scholar] [CrossRef]
- Ismail, Z.M.; Hoffmann, H.M.R. New dihydropyrans: Lewis acid catalyzed cycloadditions of α,β-unsaturated acyl cyanides to simple, unactivated olefins and dienes: A readily accessible route to derivatives of rose oxide. Angew. Chem. Int. Ed. 1982, 21, 859–860. [Google Scholar] [CrossRef]
- Boeckman, R.K.; Flann, C.F.M.; Poss, K.M. Synthetic and mechanistic studies of the retro-Claisen rearrangement: An example of cation acceleration of a [3,3]-sigmatropic rearrangement. J. Am. Chem. Soc. 1985, 107, 4359–4362. [Google Scholar] [CrossRef]
- Hanessian, S.; Compain, P. Lewis acid promoted cyclocondensations of α-ketophosphonoenoates with dienes—From Diels-Alder to hetero Diels-Alder reactions. Tetrahedron 2002, 58, 6521–6529. [Google Scholar] [CrossRef]
- Wu, H.J.; Chern, J.-H. Synthesis of 4-oxo- and 4-anti-formyl-8,10,12,13-tetraoxapentacyclo-[5.5.1.02,6.03,11.05,9]tridecanes. Tetrahedron 1997, 53, 17653–17668. [Google Scholar] [CrossRef]
- Arimori, S.; Kouno, T.; Okauchi, T.; Minami, T. The first synthesis of Phosphonoacrolein. Application to Diels-Alder reaction as heterodiene. J. Org. Chem. 2002, 67, 7303–7308. [Google Scholar] [CrossRef] [PubMed]
- Çelebi-Ölçüm, N.; Ess, D.H.; Aviyente, V.; Houk, K.N. Lewis acid catalysis alters the shapes and products of bis-pericyclic Diels-Alder transition states. J. Am. Chem. Soc. 2007, 129, 4528–4529. [Google Scholar] [CrossRef] [PubMed]
- Desimoni, G.; Faita, G.; Toscanini, M.; Boiocchi, M. Peri- and enantioselectivity of thermal, scandium-, and [Pybox/Scandium]-catalyzed Diels-Alder and hetero-Diels-Alder reactions of methyl (E)-2-oxo-4-aryl-butenoates with cyclopentadiene. Chem.-Eur. J. 2007, 13, 9478–9485. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, Q.; Goeke, A. Organocatalytic multicomponent α-methylenation/Diels-Alder reactions: A versatile route to substituted cyclohexenecarbaldehyde derivatives. Chem.-Eur. J. 2008, 14, 5335–5345. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comp. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Jasiński, R. One-step versus two-step mechanism of Diels-Alder reaction of 1-chloro-1-nitroethene with cyclopentadiene and furan. J. Mol. Graph. Model. 2017, 75, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Tantillo, D.J. Theoretical studies on synthetic and biosynthetic oxidopyrylium-alkene cycloadditions: Pericyclic pathways to Intricarene. J. Org. Chem. 2008, 73, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Weinhold, F. Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure. J. Mol. Struct. 1988, 169, 41–62. [Google Scholar] [CrossRef]
- Foster, J.P.; Weinhold, F. Natural hybrid orbitals. J. Am. Chem. Soc. 1980, 102, 7211–7218. [Google Scholar] [CrossRef]
- Carpenter, J.E. Extension of Lewis Structure Concepts to Open-Shell and Excited-State Molecular Species. Ph.D. Thesis, University of Wisconsin, Madison, WI, USA, 1987. [Google Scholar]
- Reed, A.E.; Weinhold, F. Natural bond orbital analysis of near-Hartree-Fock water dimer. J. Chem. Phys. 1983, 78, 4066–4073. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural localized molecular orbitals. J. Chem. Phys. 1985, 83, 1736–1740. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A. Understanding the mechanism of polar Diels-Alder reactions. Org. Biomol. Chem. 2009, 7, 3576–3583. [Google Scholar] [CrossRef] [PubMed]
- Neier, R.; Banach, E. Applications of Tandem Diels-Alder/sigmatropic rearrangement reactions to natural product synthesis. Curr. Org. Chem. 2016, 20, 2326–2357. [Google Scholar]
- Spartan016; Wavefunction Inc.: Irvine, CA, USA, 2017.
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 5, 799–805. [Google Scholar] [CrossRef]
- Andzelm, J.; Kölmel, C.; Klamt, A. Incorporation of solvent effects into density functional calculations of molecular energies and geometries. J. Chem. Phys. 1995, 103, 9312–9320. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, S.; Clemente, F.; Houk, K. Sources of error in DFT computations of C–C bond formation thermochemistries: π→σ transformations and error cancellation by DFT methods. Angew. Chem. Int. Ed. 2008, 47, 7746–7749. [Google Scholar] [CrossRef] [PubMed]
- Hohenstein, E.G.; Chill, S.T.; Sherrill, C.D. Assessment of the performance of the M05−2X and M06−2X exchange-correlation functionals for noncovalent interactions in biomolecules. J. Chem. Theory Comput. 2008, 4, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.; Brinck, T. Stepwise Diels-Alder: More than just an oddity? A computational mechanistic study. J. Org. Chem. 2012, 77, 6563–6573. [Google Scholar] [CrossRef] [PubMed]
- Tajabadi, J.; Bakavoli, M.; Gholizadeh, M.; Eshghi, H. A mechanistic insight into the effect of piperidine as an organocatalyst on the [3+2] cycloaddition reaction of benzalacetone with phenyl azide from a computational study. Org. Biomol. Chem. 2016, 14, 7324–7333. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Zou, L.; Cao, Y.; Houk, K.N. Computational methods to calculate accurate activation and reaction energies of 1,3-dipolar cycloadditions of 24 1,3-Dipoles. J. Phys. Chem. A 2012, 115, 13906–13920. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ayala, P.Y.; Schlegel, H.B.; Frisch, M.J. Using redundant internal coordinates to optimize equilibrium geometries and transition states. J. Comput. Chem. 1996, 17, 49–56. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical reactions-the IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bickelhaupt, M.F.; Houk, K.N. Analyzing reaction rates with the distortion/interaction-activation strain model. Angew. Chem. Int. Ed. 2017, 56, 10070–10086. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Molecule | Exo | Endo | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ΔGreac | ΔHreac | TΔSreac | ΔGact | ΔHact | TΔSact | ΔGact | ΔHact | TΔSact | |
| Grandione | −19.07 | −36.31 | −17.24 | 37.31 | 20.85 | −16.45 | - | - | - |
| Grandione β | −19.52 | −38.51 | −18.99 | 40.98 | 22.43 | −18.56 | 44.26 | 25.79 | −18.47 |
| Isograndione | −20.29 | −38.54 | −18.24 | 37.74 | 22.13 | −15.61 | - | - | - |
| Isograndione β | −20.26 | −38.84 | −18.58 | 43.94 | 25.95 | −17.99 | 33.43 | 15.80 | −17.63 |
| Molecule | ΔGact(TS1) | ΔG(INT) | ΔGact(TS2) | ΔGreac |
|---|---|---|---|---|
| Grandione | 21.13 | 7.43 | 13.06 | −19.07 |
| Isograndione | 27.00 | 8.27 | 15.06 | −20.29 |
| Molecule | Exo | TS1 | ||
|---|---|---|---|---|
| ∆Estr (ζ) | ∆Eint (ζ) | ∆Estr (ζ) | ∆Eint (ζ) | |
| Grandione | 23.42 | −2.73 | 27.68 | −23.66 |
| Isograndione | 23.90 | −1.84 | 35.61 | −27.66 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quijano-Quiñones, R.F.; Castro-Segura, C.S.; Mena-Rejón, G.J.; Quesadas-Rojas, M.; Cáceres-Castillo, D. Biosynthesis of Grandione: An Example of Tandem Hetero Diels-Alder/Retro-Claisen Rearrangement Reaction? Molecules 2018, 23, 2505. https://doi.org/10.3390/molecules23102505
Quijano-Quiñones RF, Castro-Segura CS, Mena-Rejón GJ, Quesadas-Rojas M, Cáceres-Castillo D. Biosynthesis of Grandione: An Example of Tandem Hetero Diels-Alder/Retro-Claisen Rearrangement Reaction? Molecules. 2018; 23(10):2505. https://doi.org/10.3390/molecules23102505
Chicago/Turabian StyleQuijano-Quiñones, Ramiro F., Carolina S. Castro-Segura, Gonzalo J. Mena-Rejón, Mariana Quesadas-Rojas, and David Cáceres-Castillo. 2018. "Biosynthesis of Grandione: An Example of Tandem Hetero Diels-Alder/Retro-Claisen Rearrangement Reaction?" Molecules 23, no. 10: 2505. https://doi.org/10.3390/molecules23102505
APA StyleQuijano-Quiñones, R. F., Castro-Segura, C. S., Mena-Rejón, G. J., Quesadas-Rojas, M., & Cáceres-Castillo, D. (2018). Biosynthesis of Grandione: An Example of Tandem Hetero Diels-Alder/Retro-Claisen Rearrangement Reaction? Molecules, 23(10), 2505. https://doi.org/10.3390/molecules23102505





