Glycosylation of 3-Hydroxyflavone, 3-Methoxyflavone, Quercetin and Baicalein in Fungal Cultures of the Genus Isaria
Abstract
1. Introduction
2. Results and Discussion
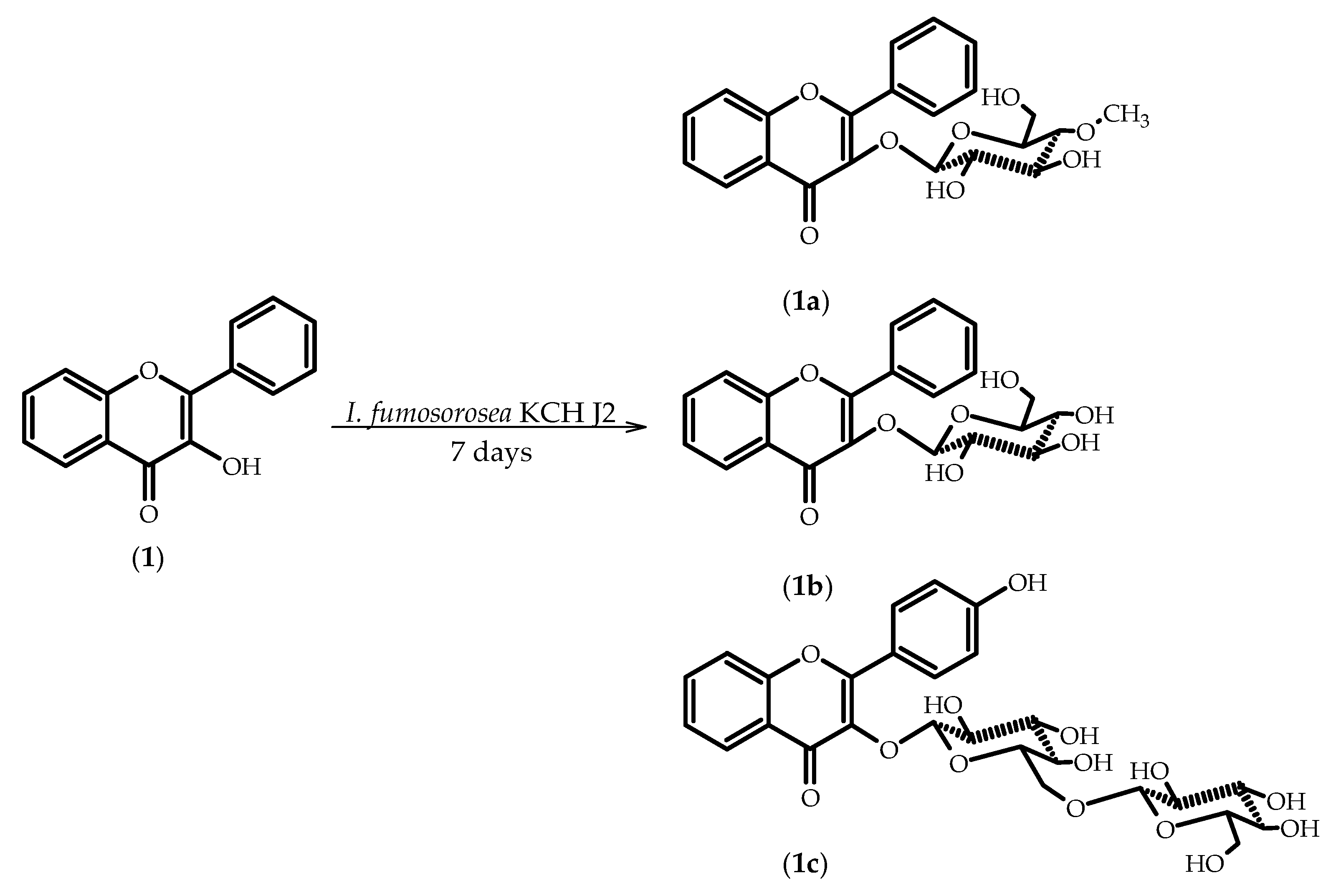
2.1. Biotransformations of 3-Hydroxyflavone (1)
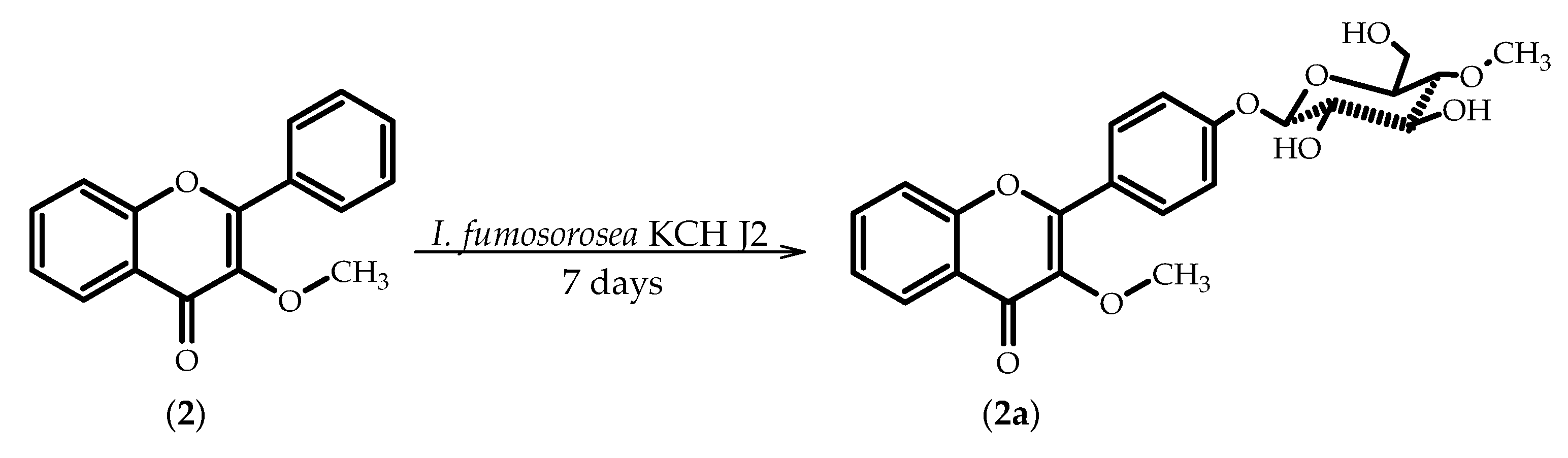
2.2. Biotransformations of 3-Methoxyflavone (2)
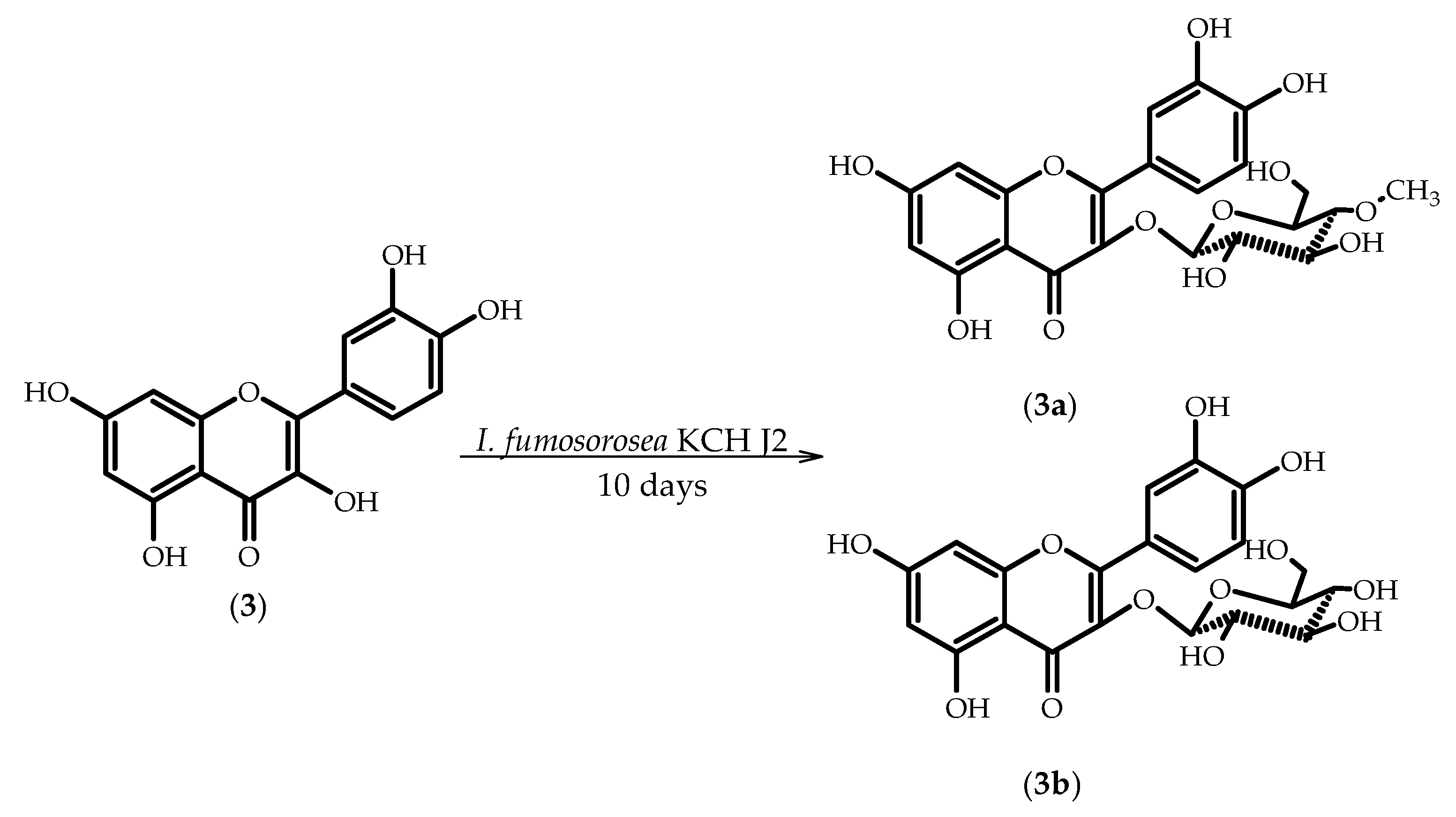
2.3. Biotransformations of 3,3′,4′,5,7-Pentahydroxyflavone (Quercetin) (3)
2.4. Biotransformations of 5,6,7-Trihydroxyflavone (Baicalein) (4)
3. Materials and Methods
3.1. Chemicals
3.1.1. 3-Hydroxylavone (1)
3.1.2. 3-Methoxyflavone (2)
3.1.3. 3,3′,4′,5,7-Pentahydroxyflavone (Quercetin) (3)
3.1.4. 5,6,7-Trihydroxyflavone (Baicalein) (4)
3.2. Microorganism
3.3. Analysis
3.4. Screening Procedure
3.5. Scale-Up Biotransformations
3.5.1. Flavone 3-O-β-d-(4′′-O-Methyl)-glucopyranoside (1a)
3.5.2. Flavone 3-O-β-d-Glucopyranoside (1b)
3.5.3. 3-O-[β-d-Glucopyranosyl-(1→6)-β-d-glucopyranosyl]-4’-hydroxyflavone (1c)
3.5.4. 3-Methoxyflavone 4′-O-β-d-(4′′-O-methyl)-glucopyranoside (2a)
3.5.5. 3′,4′,5,7-Tetrahydroxyflavone 3-O-β-d-(4′′-O-methyl)-glucopyranoside (3a)
3.5.6. 3′,4′,5,7-Tetrahydroxyflavone 3-O-β-d-glucopyranoside (isoquercetin) (3b)
3.5.7. 5,7-Dihydroxyflavone 6-O-β-d-(4′′-O-methyl)-glucopyranoside (4a)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guan, L.; Liu, B. Antidepressant-like effects and mechanisms of flavonoids and related analogues. Eur. J. Med. Chem. 2016, 121, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Seleem, D.; Pardi, V.; Mendonça, R. Review of flavonoids: A diverse group of natural compounds with anti-Candida albicans activity in vitro. Arch. Oral Biol. 2017, 76, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Sureda, A.; Morabito, S.; Sanches-silva, A.; Mocan, A.; Fazel, S.; Mohammad, S. Flavonoids and platelet aggregation: A brief review. Eur. J. Pharmacol. 2017, 807, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Burkard, M.; Leischner, C.; Lauer, U.M.; Busch, C.; Venturelli, S.; Frank, J. Dietary flavonoids and modulation of natural killer cells: Implications in malignant and viral diseases. J. Nutr. Biochem. 2017, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Q.; Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Pinta, D.; Majki, T.; Bekvalac, K.; Or, D.; Mimica-duki, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods J. 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Petersen, B.; Egert, S.; Bosy-Westphal, A.; Müller, M.J.; Wolffram, S.; Hubbermann, E.M.; Rimbach, G.; Schwarz, K. Bioavailability of quercetin in humans and the influence of food matrix comparing quercetin capsules and different apple sources. Food Res. Int. 2016, 88, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Miltonprabu, S.; Tomczyk, M.; Skalicka-Woźniak, K.; Rastrelli, L.; Daglia, M.; Nabavi, S.F.; Alavian, S.M.; Nabavi, S.M. Hepatoprotective effect of quercetin: From chemistry to medicine. Food Chem. Toxicol. 2017, 108, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F. Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 2012, 143, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, F.; Blasina, F.; Dajas, F.; Andr, J.; Echeverry, C.; Martínez, M.; Rivera, F.; Vaamonde, L. Quercetin in brain diseases: Potential and limits. Neurochem. Int. 2015, 89, 140–148. [Google Scholar]
- Fazel, S.; Luigi, G.; Daglia, M.; Mohammad, S. Role of quercetin as an alternative for obesity treatment: You are what you eat! Food Chem. 2015, 179, 305–310. [Google Scholar]
- Carvalho, D.; Paulino, M.; Polticelli, F.; Arredondo, F.; Williams, R.J.; Abin-Carriquiry, J.A. Structural evidence of quercetin multi-target bioactivity: A reverse virtual screening strategy. Eur. J. Pharm. Sci. 2017, 106, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Andrea, G.D. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Birhman, K.; Raheja, I.; Sharma, S.K.; Kar, H.K. Quercetin: A wonder bioflavonoid with therapeutic potential in disease management. Asian Pac. J. Trop. Dis. 2016, 6, 248–252. [Google Scholar] [CrossRef]
- Suganthy, N.; Pandima, K.; Fazel, S.; Braidy, N.; Mohammad, S. Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. Biomed. Pharmacother. 2016, 84, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Cathie, X.C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Life Med. Sci. 2016, 61, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Bie, B.; Sun, J.; Guo, Y.; Li, J.; Jiang, W.; Yang, J.; Huang, C. Baicalein: A review of its anti-cancer effects and mechanisms in Hepatocellular Carcinoma. Biomed. Pharmacother. 2017, 93, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, R.D.; José, S.; Delarmelina, M.; Cacilda, K. Electrochemical quantification of the structure/antioxidant activity relationship of flavonoids. Electrochim. Acta 2015, 163, 161–166. [Google Scholar]
- Cos, P.; Calomme, M.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship of flavonoids as antioxidant and pro-oxidant compounds. Stud. Nat. Prod. Chem. 2000, 22, 307–340. [Google Scholar]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Li, K.; Fan, H.; Yin, P.; Yang, L.; Xue, Q.; Li, X.; Sun, L.; Liu, Y. Structure-activity relationship of eight high content flavonoids analyzed with a preliminary assign-score method and their contribution to antioxidant ability of flavonoids-rich extract from Scutellaria baicalensis shoots. Arab. J. Chem. 2018, 11, 159–170. [Google Scholar] [CrossRef]
- Tsuchiya, H. Structure-dependent membrane interaction of flavonoids associated with their bioactivity. Food Chem. 2010, 120, 1089–1096. [Google Scholar] [CrossRef]
- Jana, B.; Senapati, S.; Ghosh, D.; Bose, D.; Chattopadhyay, N. Spectroscopic exploration of mode of binding of ctDNA with 3-hydroxyflavone: A contrast to the mode of binding with flavonoids having additional hydroxyl groups. J. Phys. Chem. B 2012, 116, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Nayak, Y.; Venkatachalam, H.; Daroji, V.K.; Mathew, G.; Jayashree, B.S.; Unnikrishnan, M.K. Antidiabetic activity of 3-hydroxyflavone analogues in high fructose fed insulin resistant rats. EXCLI J. 2014, 13, 1055–1074. [Google Scholar] [PubMed]
- Kim, J.H.; Song, M.; Kang, G.H.; Lee, E.R.; Choi, H.Y.; Lee, C.; Kim, J.H.; Kim, Y.; Koo, B.N.; Cho, S.G. Combined treatment of 3-hydroxyflavone and imatinib mesylate increases apoptotic cell death of imatinib mesylate-resistant leukemia cells. Leuk. Res. 2012, 36, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Mahfoudi, R.; Djeridane, A.; Benarous, K.; Gaydou, E.M.; Yousfi, M. Structure-activity relationships and molecular docking of thirteen synthesized flavonoids as horseradish peroxidase inhibitors. Bioorg. Chem. 2017, 74, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Methoxylated flavones, a superior cancer chemopreventive flavonoid subclass? Semin. Cancer Biol. 2007, 17, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.P.; Parajuli, P.; Koirala, N.; Park, J.W.; Sohng, J.K. Probing 3-hydroxyflavone for in vitro glycorandomization of flavonols by YjiC. Appl. Environ. Microbiol. 2013, 79, 6833–6838. [Google Scholar] [CrossRef] [PubMed]
- Mughal, E.U.; Javid, A.; Sadiq, A.; Murtaza, S.; Zafar, M.N.; Khan, B.A.; Sumra, S.H.; Tahir, M.N.; Kanwal; Khan, K.M. Synthesis, structure-activity relationship and molecular docking studies of 3-O-flavonol glycosides as cholinesterase inhibitors. Bioorg. Med. Chem. 2018, 26, 3696–3706. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Zhang, T.; Ganesan, K.; Xu, B.; Sum, S.; Chung, M. Isoquercetin ameliorates hyperglycemia and regulates key enzymes of glucose metabolism via insulin signaling pathway in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2018, 829, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xu, H.; Zhu, G.; Liu, S. Isoquercetin ameliorated hypoxia/reoxygenation-induced H9C2 cardiomyocyte apoptosis via a mitochondrial-dependent pathway. Biomed. Pharmacother. 2017, 95, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Zhang, L.; Zhang, X.; Yu, S.; Liang, X.; Ding, F.; Wang, Z. Isoquercetin protects cortical neurons from oxygen–glucose deprivation–reperfusion induced injury via suppression of TLR4–NF-kB signal pathway. Neurochem. Int. 2013, 63, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, H.; Zhang, J.; Yan, M. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway. Chem. Biol. Interact. 2018, 284, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhang, C.; Chai, M.; An, Y. Isoquercetin ameliorates tunicamycin-induced apoptosis in rat dorsal root ganglion neurons via suppressing ROS-dependent endoplasmic reticulum stress. Biomed. Pharmacother. 2016, 80, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Hu, M. Commentary: Bioavailability of flavonoids and polyphenols: Call to Arms. Mol. Pharm. 2007, 4, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Bijsman, M.N.; van Gameren, Y.; Cnossen, E.P.; de Vries, J.H.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Chebil, L.; Humeau, C.; Anthoni, J.; Dehez, F.; Engasser, J.-M.; Ghoul, M. Solubility of flavonoids in organic solvents. J. Chem. Eng. Data 2007, 52, 1552–1556. [Google Scholar] [CrossRef]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Biotransformations of flavones and an isoflavone (daidzein) in cultures of entomopathogenic filamentous fungi. Molecules 2018, 23, 1356. [Google Scholar] [CrossRef] [PubMed]
- Dymarska, M.; Grzeszczuk, J.; Urbaniak, M.; Janeczko, T.; Stępień, Ł.; Kostrzewa-Susłow, E. Glycosylation of 6-methylflavone by the strain Isaria fumosorosea KCH J2. PLoS ONE 2017, 12, e0184885. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, E.; Hoc, N.; Sycz, J.; Urbaniak, M.; Dymarska, M.; Grzeszczuk, J.; Kostrzewa-Susłow, E.; Stępień, Ł.; Pląskowska, E.; Janeczko, T. Biotransformation of steroids by entomopathogenic strains of Isaria farinosa. Microb. Cell Fact. 2018, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Herath, W.; Mikell, J.R.; Hale, A.L.; Ferreira, D.; Khan, I.A. Microbial metabolism. Part 6. Metabolites of 3- and 7-hydroxyflavones. Chem. Pharm. Bull. 2006, 54, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, S.; Azami, S.; Kuzuyama, T. Microbial glucosylation of flavonols by Cunninghamella echinulata. J. Biosci. Bioeng. 2010, 110, 320–321. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa-Susłow, E.; Dymarska, M.; Janeczko, T. Microbial transformations of 3-methoxyflavone by strains of Aspergillus niger. Pol. J. Microbiol. 2014, 63, 111–114. [Google Scholar] [PubMed]
- Bartmańska, A.; Tronina, T.; Huszcza, E. Transformation of 8-prenylnaringenin by Absidia coerulea and Beauveria bassiana. Bioorg. Med. Chem. Lett. 2012, 22, 6451–6453. [Google Scholar] [CrossRef] [PubMed]
- Sordon, S.; Popłoński, J.; Tronina, T.; Huszcza, E. Microbial glycosylation of daidzein, genistein and biochanin A: Two new glucosides of biochanin A. Molecules 2017, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Tronina, T.; Huszcza, E.; Gabrielska, J. Bioactivity in vitro of quercetin glycoside obtained in Beauveria bassiana culture and its interaction with liposome membranes. Molecules 2017, 22, 1520. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, L.; Wang, C.; Wang, X.; Xu, Y.; Yu, H.; Wu, P.; Li, S. Methylglucosylation of aromatic amino and phenolic moieties of drug-like biosynthons by combinatorial biosynthesis. Proc. Natl. Acad. Sci. USA. 2018, 115, E4980–E4989. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Q.; Fan, N.; Yu, B.Y.; Wang, Q.Q.; Zhang, J. Biotransformation of quercetin by Gliocladium deliquescens NRRL 1086. Chin. J. Nat. Med. 2017, 15, 0615–0624. [Google Scholar] [CrossRef]
- Sordon, S.; Popłoński, J.; Huszcza, E.W.A. Microbial glycosylation of flavonoids. Pol. J. Microbiol. 2016, 65, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Penso, J.; Cordeiro, K.C.F.A.; Carla, R.M.; Patricia, F.; Castro, S.; Martins, D.R.; Lião, L.M. Vasorelaxant activity of 7-β-O-glycosides biosynthesized from flavonoids. Eur. J. Pharmacol. 2014, 733, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Shi, X.; Liu, T.L.; Liu, H.; Yuan, K. RP-HPLC simultaneous determination of three glycosylflavones in Abelmoschus esculentus L. Chin. J. Pharm. Anal. 2012, 32, 2194–2197. [Google Scholar]
- Lin, S.; Zhu, Q.; Wen, L.; Yang, B.; Jiang, G.; Gao, H.; Chen, F.; Jiang, Y. Production of quercetin, kaempferol and their glycosidic derivatives from the aqueous-organic extracted residue of litchi pericarp with Aspergillus awamori. Food Chem. 2014, 145, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Yadava, S.; Yadav, K.D.S. a-l-rhamnosidase selective for rutin to isoquercitrin transformation from Penicillium griseoroseum MTCC-9224. Bioorg. Chem. 2017, 70, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Melo Branco de Araújo, M.E.; Moreira Franco, Y.E.; Grando Alberto, T.; Alves Sobreiro, M.; Conrado, M.A.; Gonçalves Priolli, D.; Frankland Sawaya, A.C.H.; Ruiz, A.L.T.G.; de Carvalho, J.E.; de Oliveira Carvalho, P. Enzymatic de-glycosylation of rutin improves its antioxidant and antiproliferative activities. Food Chem. 2013, 141, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, G.; Yu, L.; Wu, F.; Guo, X. Enhancement of the selective enzymatic biotransformation of rutin to isoquercitrin using an ionic liquid as a co-solvent. Bioresour. Technol. 2013, 128, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Kajjout, M.; Rolando, C. Regiospecific synthesis of quercetin O-B-d-glucosylated and O-B-d-glucuronidated isomers. Tetrahedron 2011, 67, 4731–4741. [Google Scholar] [CrossRef]
- Kostrzewa-Susłow, E.; Dmochowska-Gładysz, J.; Oszmiański, J. Microbial transformation of baicalin and baicalein. J. Mol. Catal. B Enzym. 2007, 49, 113–117. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Thuan, N.H.; Park, J.W.; Sohng, J.K. Toward the production of flavone-7-O-B-d-glucopyranosides using Arabidopsis glycosyltransferase in Escherichia coli. Process Biochem. 2013, 48, 1744–1748. [Google Scholar] [CrossRef]
- Hirotani, M.; Kuroda, R.; Suzuki, H.; Yoshikawa, T. Cloning and expression of UDP-glucose: Favonoid 7-O-glucosyltransferase from hairy root cultures of Scutellaria baicalensis. Planta 2000, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.; Park, Y.; Park, H.; Moon, K.; Ha, K.; Baek, N.; Park, C.; Joo, M.; Cha, J. Synthesis and biological evaluation of a novel baicalein glycoside as an anti-inflammatory agent. Eur. J. Pharmacol. 2014, 744, 147–156. [Google Scholar]
Sample Availability: Samples of the compounds 1, 1a, 1b, 1c, 2, 2a, 3, 3a, 3b, 4, 4a are available from the authors. |
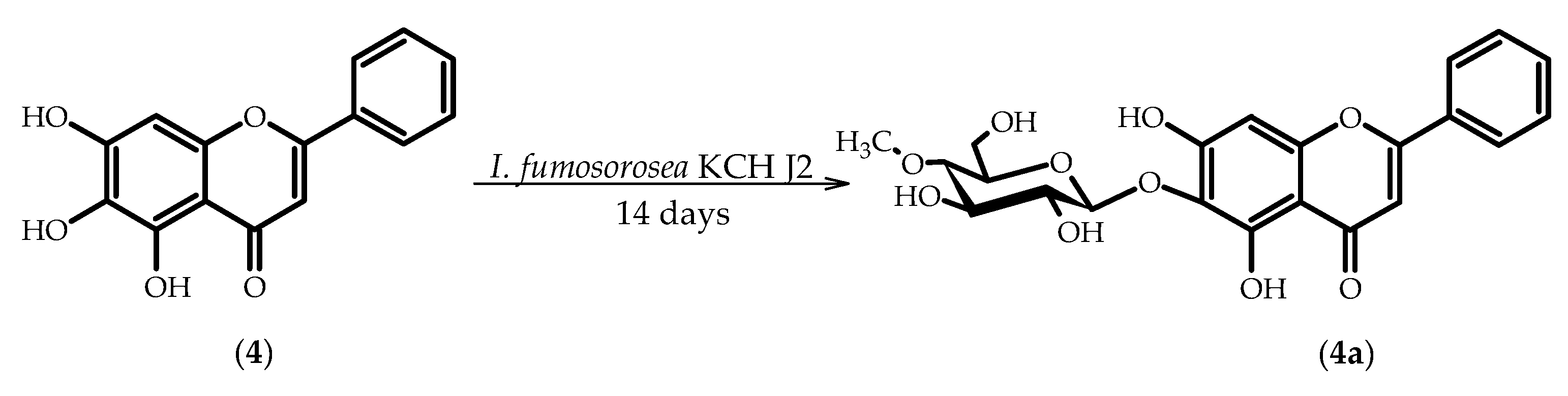
| Proton | Compound | |||||
|---|---|---|---|---|---|---|
| 1 | 1a | 1b | 1c | 2 | 2a | |
| H-3 | - | - | - | - | - | - |
| H-5 | 8.22 (dd) J5,6 = 7.9 Hz, J5,7 = 1.6 Hz | 8.23 (d) J5,6 = 8.0 Hz | 8.24 (d) J5,6 = 8.0 Hz | 8.22 (d) J5,6 = 8.0 Hz | 8.21 (dd) J5,6 = 8.1 Hz, J5,7 = 1.9 Hz | 8.19 (d) J5,6 = 7.7 Hz |
| H-6 | 7.55 (m) | 7.57 (t) J = 7.5 Hz | 7.58 (m) | 7.56 (t) J = 7.5 Hz | 7.53 (ddd) J6,5 = 8.0 Hz, J6,7 = 7.1 Hz, J = 0.9 Hz | 7.51 (t) J = 7.4 Hz |
| H-7 | 7.87 (ddd) J7,5 = 1.7 Hz, J7,6 = 7.0 Hz, J7,8 = 8.7 Hz | 7.90 (t) J7,8 = 7.7 Hz | 7.91 (t) J7,8 = 7.8 Hz | 7.88 (t) J7,8 = 7.6 Hz | 7.85 (ddd) J7,5 = 1.7 Hz, J7,6 = 7.1 Hz, J7,8 = 8.7 Hz | 7.83 (t) J = 7.8 Hz |
| H-8 | 7.81 (d) J8,7 = 8.3 Hz | 7.78 (d) J8,7 = 8.5 Hz | 7.79 (d) J8,7 = 8.4 Hz | 7.77 (d) J8,7 = 8.5 Hz | 7.74 (d) J8,7 = 8.3 Hz | 7.73 (d) J8,7 = 8.4 Hz |
| H-2′ | 8.37 (m) | 8.29 (dd) J = 5.9 Hz, J = 2.4 Hz | 8.29 (m) | 8.27 (d) J2’,3’ = 8.4 Hz | 8.17 (m) | 8.16 (d) J2’,3’ = 8.4 Hz |
| H-3′ | 7.63 (m) | 7.58 (m) | 7.58 (m) | 7.04 (d) J3’,2’ = 8.2 Hz | 7.62 (m) | 7.27 (d) J3’,2’ = 8.2 Hz |
| H-4′ | 7.55 (m) | 7.58 (m) | 7.58 (m) | - | 7.62 (m) | - |
| H-5′ | 7.63 (m) | 7.58 (m) | 7.58 (m) | 7.04 (d) J5’,6’ = 8.2 Hz | 7.62 (m) | 7.27 (d) J5’,6’ = 8.2 Hz |
| H-6′ | 8.37 (m) | 8.29 (dd) J = 5.9 Hz, J = 2.4 Hz | 8.29 (m) | 8.27 (d) J6’,5’ = 8.4 Hz | 8.17 (m) | 8.16 (d) J6’,5’ = 8.4 Hz |
| H-1′′ | - | 5.28 (d) J = 7.9 Hz | 5.29 (d) J = 7.7 Hz | 5.21 (d) J = 7.1 Hz | - | 5.13 (d) J = 7.4 Hz |
| H-2′′ | - | 3.44 (t) J = 8.5 Hz | 3.44 (t) J = 8.4 Hz | 3.48 (s) | - | 3.56 (m) |
| H-3′′ | - | 3.63 (t) J = 8.9 Hz | 3.50 (t) J = 9.1 Hz | 3.51 (d) J = 8.7 Hz | - | 3.71 (t) |
| H-4′′ | - | 3.15 (t) J = 9.3 Hz | 3.38 (t) J = 9.2 Hz | 3.41 (t) J = 9.6 Hz | - | 3.29 (t) |
| H-5′′ | - | 3.24 (m) | 3.29 (m) | 3.33–3.38 (m) | - | 3.56 (m) |
| H-6′′ | - | 3.58 (m) 3.52 (m) | 3.65 (d) J = 12.1 Hz 3.52 (m) | 3.81–3.85 (m) 3.98–4.02 (m) | - | 3.90 (m) 3.76 (m) |
| H-1′′′ | - | - | - | 4.34 (d) J = 7.9 Hz | - | - |
| H-2′′′ | - | - | - | 3.20 (m) | - | - |
| H-3′′′ | - | - | - | 3.43 (d) J = 8.4 Hz | - | - |
| H-4′′′ | - | - | - | 3.33–3.38 (m) | - | - |
| H-5′′′ | - | - | - | 3.30 (m) | - | - |
| H-6′′′ | - | - | - | 3.69 (m) 3.86–3.91 (m) | - | - |
| C-4′′-OCH3 | - | 3.55 (s) | - | - | - | 3.62 (s) |
| C3-OH | 8.26 (s) | - | - | - | - | |
| C3-OCH3 | - | - | - | - | 3.95 (s) | 3.93 (s) |
| Carbon | Compound | |||||
|---|---|---|---|---|---|---|
| 1 | 1a | 1b | 1c | 2 | 2a | |
| C-2 | 145.5 | 158.3 | 158.4 | 158.6 | 156.1 | 155.8 |
| C-3 | 139.8 | 138.4 | 138.5 | 137.9 | 142.2 | 141.6 |
| C-4 | 173.9 | 175.7 | 175.7 | 175.6 | 175.1 | 174.9 |
| C-4a | 122.0 | 124.5 | 124.6 | 126.2 | 125.1 | 125.1 |
| C-5 | 125.9 | 126.2 | 126.3 | 125.9 | 126.1 | 126.0 |
| C-6 | 125.4 | 126.0 | 126.1 | 124.5 | 125.7 | 125.6 |
| C-7 | 134.7 | 135.1 | 135.1 | 134.9 | 134.6 | 134.4 |
| C-8 | 119.3 | 119.3 | 119.3 | 119.1 | 119.2 | 119.1 |
| C-8a | 156.3 | 156.3 | 156.4 | 156.2 | 156.2 | 156.1 |
| C-1′ | 132.5 | 131.9 | 132.0 | 122.8 | 132.0 | 125.5 |
| C-2′ | 128.6 | 130.3 | 130.3 | 132.3 | 129.3 | 130.9 |
| C-3′ | 129.4 | 129.0 | 129.0 | 115.9 | 129.4 | 117.2 |
| C-4′ | 130.8 | 131.7 | 131.7 | 161.1 | 131.5 | 160.5 |
| C-5′ | 129.4 | 129.0 | 129.0 | 115.9 | 129.4 | 117.2 |
| C-6′ | 128.6 | 130.3 | 130.3 | 132.3 | 129.3 | 130.9 |
| C-1′′ | - | 104.5 | 104.9 | 105.6 | - | 101.2 |
| C-2′′ | - | 75.5 | 75.3 | 75.4 | - | 74.9 |
| C-3′′ | - | 79.9 | 78.1 | 78.3 | - | 78.0 |
| C-4′′ | - | 78.2 | 71.2 | 71.2 | - | 80.1 |
| C-5′′ | - | 77.2 | 77.9 | 77.6 | - | 77.2 |
| C-6′′ | - | 62.2 | 62.8 | 70.0 | - | 62.1 |
| C-1′′′ | - | - | - | 104.8 | - | - |
| C-2′′′ | - | - | - | 74.8 | - | - |
| C-3′′′ | - | - | - | 77.9 | - | - |
| C-4′′′ | - | - | - | 71.5 | - | - |
| C-5′′′ | - | - | - | 77.9 | - | - |
| C-6′′′ | - | - | - | 62.9 | - | - |
| C-4′′-OCH3 | - | 60.5 | - | - | - | 60.6 |
| C3-OCH3 | - | - | - | - | 60.2 | 60.0 |
| Proton | Compound | ||||
|---|---|---|---|---|---|
| 3 | 3a | 3b | 4 | 4a | |
| H-3 | - | - | - | 6.74 (s) | 6.79 (s) |
| H-5 | - | - | - | - | - |
| H-6 | 6.30 (d) J6,8 = 2.0 Hz | 6.32 (d) J6,8 = 2.0 Hz | 6.32 (d) J6,8 = 2.0 Hz | - | - |
| H-7 | - | - | - | ||
| H-8 | 6.56 (d) J8,6 = 2.1 Hz | 6.55 (d) J8,6 = 2.1 Hz | 6.55 (d) J8,6 = 2.1 Hz | 6.53 (s) | 6.55 (s) |
| H-2′ | 7.86 (d) J2’,6’ = 2.2 Hz | 8.04 (d) J2’,6’ =2.1 Hz | 8.04 (d) J2’,6’ = 2.1 Hz | 7.99 (m) | 8.01 (m) |
| H-3′ | - | - | - | 7.52 (m) | 7.53 (m) |
| H-4′ | - | - | - | 7.52 (m) | 7.53 (m) |
| H-5′ | 7.03 (d) J5’,6’ = 8.5 Hz | 7.00 (d) J5’,6’ = 8.4 Hz | 6.99 (d) J5’,6’ = 8.4 Hz | 7.52 (m) | 7.53 (m) |
| H-6′ | 7.74 (dd) J6’,2’ = 2.2 Hz, J6’,5’ = 8.5 Hz | 7.64 (dd) J6’,2’ = 2.2 Hz, J6’,5’ = 8.4 Hz | 7.62 (dd) J6’,2’ = 2.2 Hz, J6’,5’ = 8.4 Hz | 7.99 (m) | 8.01 (m) |
| H-1′′ | - | 5.27 (d) J = 7.8 Hz | 5.30 (d) J = 7.4 Hz | - | 4.63 (d) J = 7.4 Hz |
| H-2′′ | - | 3.50 (t) J = 8.1 Hz | 3.52 (m) | - | 3.47 (m) |
| H-3′′ | - | 3.63 (m) | 3.52 (m) | - | 3.47 (m) |
| H-4′′ | - | 3.18 (m) | 3.44 (t) J =8.8 Hz | - | 3.25 (m) |
| H-5′′ | - | 3.34 (m) | 3.37 (m) | - | 3.20 (ddd) J = 9.6, 3.6, 2.0 Hz |
| H-6′′ | - | 3.73 (d) J = 12.0 Hz 3.64 (m) | 3.78 (d) J = 10.7 Hz 3.64 (m) | - | 3.74 (d) J = 9.8 Hz 3.62 (m) |
| C-4′′-OCH3 | - | 3.57 (s) | - | - | 3.54 (s) |
| C3-OH | 8.05 (s) | - | - | - | - |
| C5-OH | 12.21 (s) | 12.34 | - | 12.78 (s) | 13.16 (s) |
| C6-OH | - | 8.46 (s) | - | ||
| C7-OH | 9.78 (s) | 9.82 (s) | - | 7.22 (s) | 9.51 (s) |
| C3′-OH | 8.38 (s) | 8.48 (s) | - | - | - |
| C4′-OH | 8.61 (s) | 8.52 (s) | - | - | - |
| Carbon | Compound | |||||
|---|---|---|---|---|---|---|
| 3 | 3a | 3b | 4 | 4a | ||
| C-2 | 146.9 | 158.8 | 158.8 | 164.3 | 164.7 | |
| C-3 | 136.8 | 135.6 | 135.6 | 105.6 | 105.8 | |
| C-4 | 176.6 | 179.2 | 179.2 | 183.4 | 183.5 | |
| C-4a | 104.1 | 105.5 | 105.4 | 105.6 | 105.8 | |
| C-5 | 162.3 | 158.0 | 158.0 | 147.9 | 154.8 | |
| C-6 | 99.2 | 94.7 | 94.7 | 130.3 | 130.4 | |
| C-7 | 165.0 | 165.3 | 165.4 | 154.4 | 158.9 | |
| C-8 | 94.5 | 99.8 | 99.7 | 94.6 | 95.0 | |
| C-8a | 157.8 | 162.9 | 162.9 | 151.6 | 155.0 | |
| C-1′ | 123.8 | 122.7 | 122.7 | 132.9 | 132.6 | |
| C-2′ | 115.8 | 118.0 | 117.9 | 127.1 | 127.2 | |
| C-3′ | 145.8 | 145.3 | 145.3 | 129.8 | 129.9 | |
| C-4′ | 148.4 | 149.3 | 149.3 | 132.3 | 132.6 | |
| C-5′ | 116.2 | 115.9 | 115.8 | 129.8 | 129.9 | |
| C-6′ | 121.5 | 122.8 | 122.6 | 127.1 | 127.2 | |
| C-1′′ | - | 104.8 | 105.0 | - | 107.3 | |
| C-2′′ | - | 75.7 | 75.5 | - | 75.5 | |
| C-3′′ | - | 78.2 | 78.1 | - | 77.8 | |
| C-4′′ | - | 79.8 | 70.9 | - | 79.7 | |
| C-5′′ | - | 77.0 | 77.8 | - | 77.8 | |
| C-6′′ | - | 62.3 | 62.7 | - | 61.8 | |
| C-4′′-OCH3 | - | 60.5 | - | - | 60.6 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Glycosylation of 3-Hydroxyflavone, 3-Methoxyflavone, Quercetin and Baicalein in Fungal Cultures of the Genus Isaria. Molecules 2018, 23, 2477. https://doi.org/10.3390/molecules23102477
Dymarska M, Janeczko T, Kostrzewa-Susłow E. Glycosylation of 3-Hydroxyflavone, 3-Methoxyflavone, Quercetin and Baicalein in Fungal Cultures of the Genus Isaria. Molecules. 2018; 23(10):2477. https://doi.org/10.3390/molecules23102477
Chicago/Turabian StyleDymarska, Monika, Tomasz Janeczko, and Edyta Kostrzewa-Susłow. 2018. "Glycosylation of 3-Hydroxyflavone, 3-Methoxyflavone, Quercetin and Baicalein in Fungal Cultures of the Genus Isaria" Molecules 23, no. 10: 2477. https://doi.org/10.3390/molecules23102477
APA StyleDymarska, M., Janeczko, T., & Kostrzewa-Susłow, E. (2018). Glycosylation of 3-Hydroxyflavone, 3-Methoxyflavone, Quercetin and Baicalein in Fungal Cultures of the Genus Isaria. Molecules, 23(10), 2477. https://doi.org/10.3390/molecules23102477





