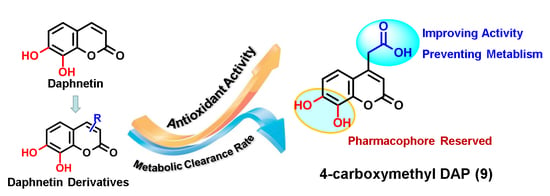
Synthesis and Structure-Activity Relationship of Daphnetin Derivatives as Potent Antioxidant Agents
Abstract
:1. Introduction
2. Results and Discussion
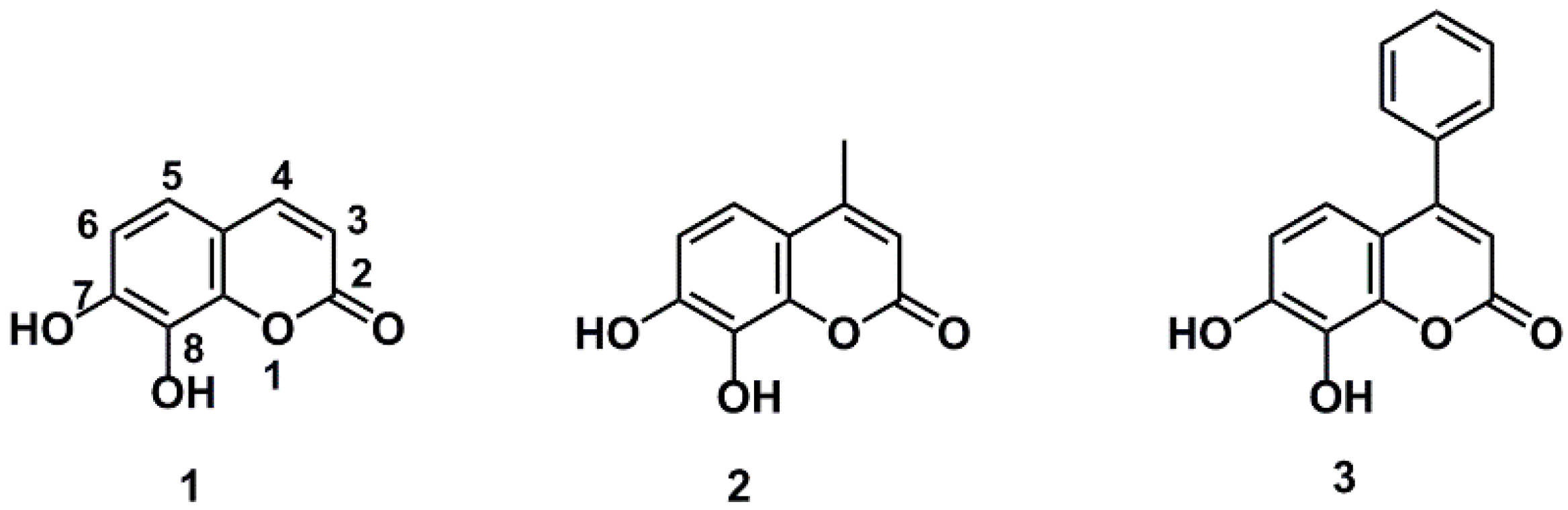
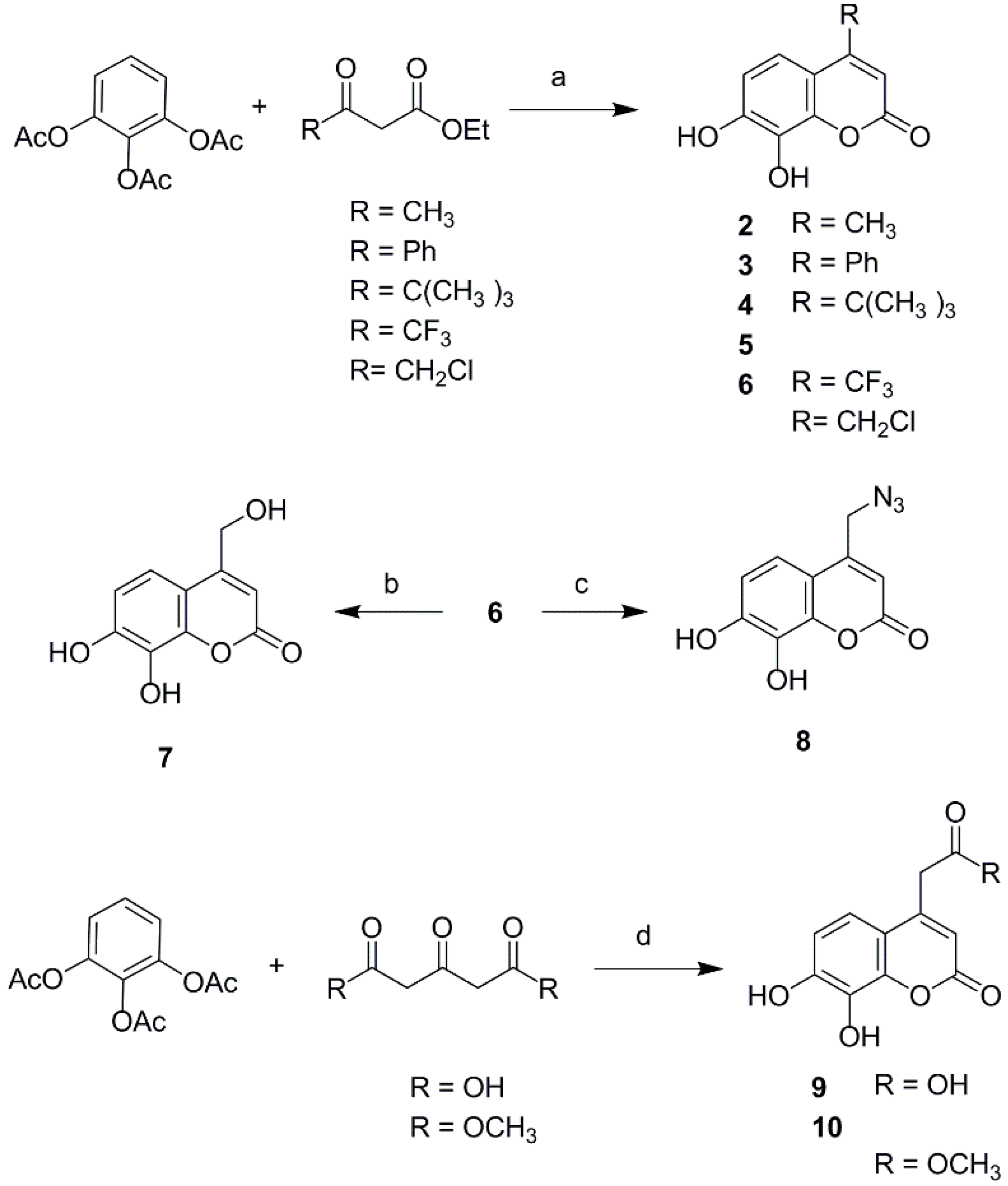
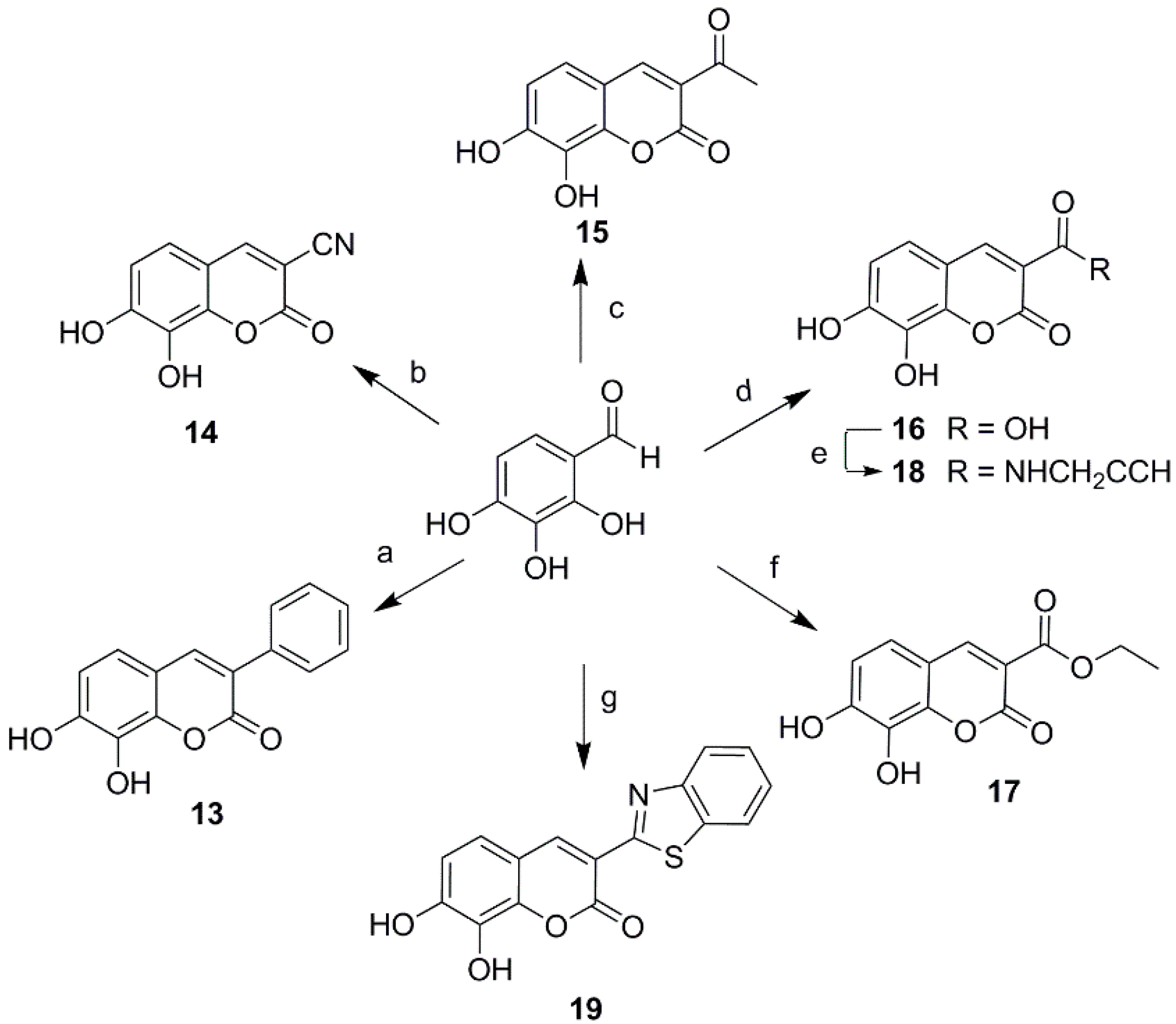
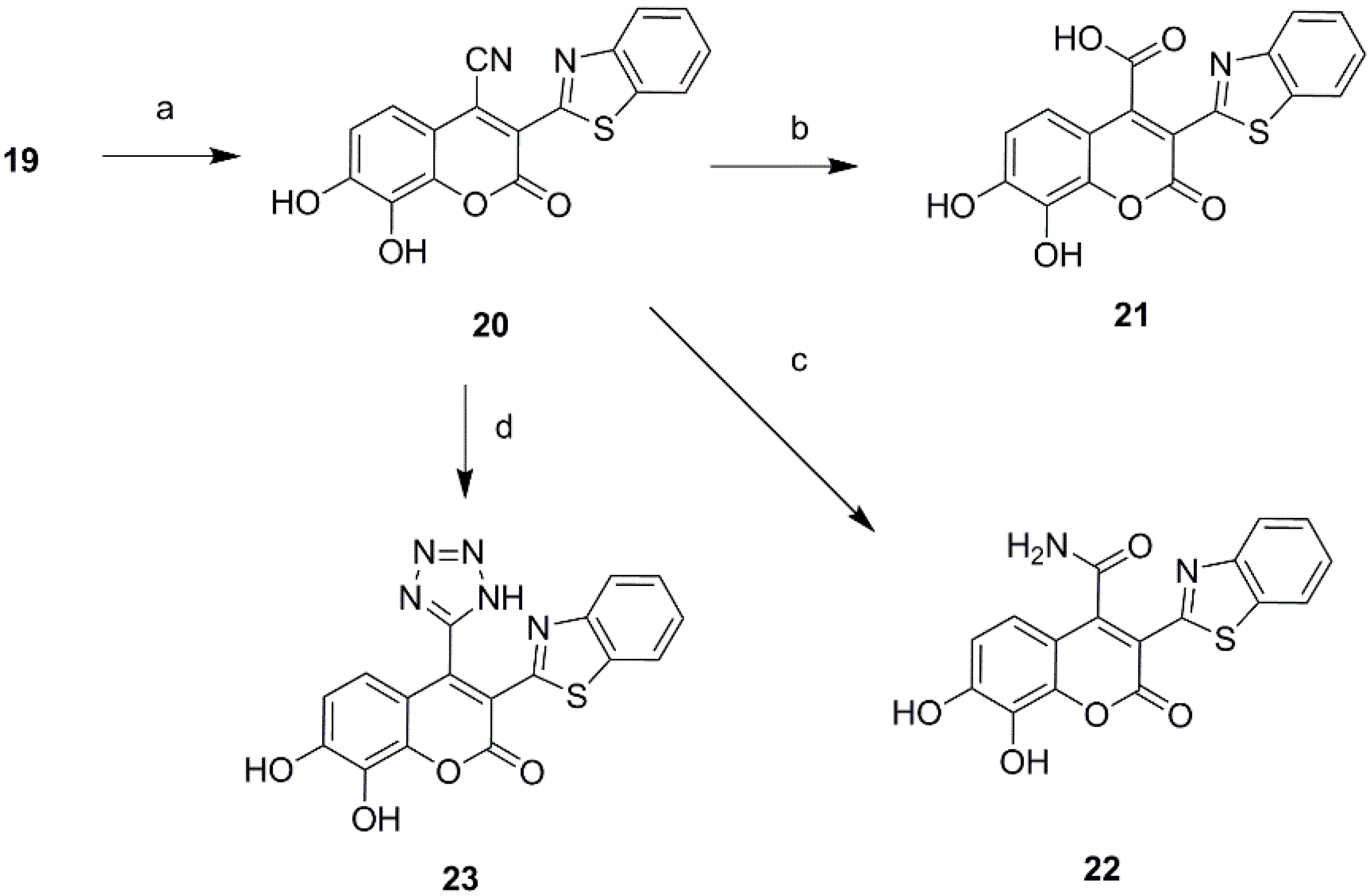
2.1. Chemistry
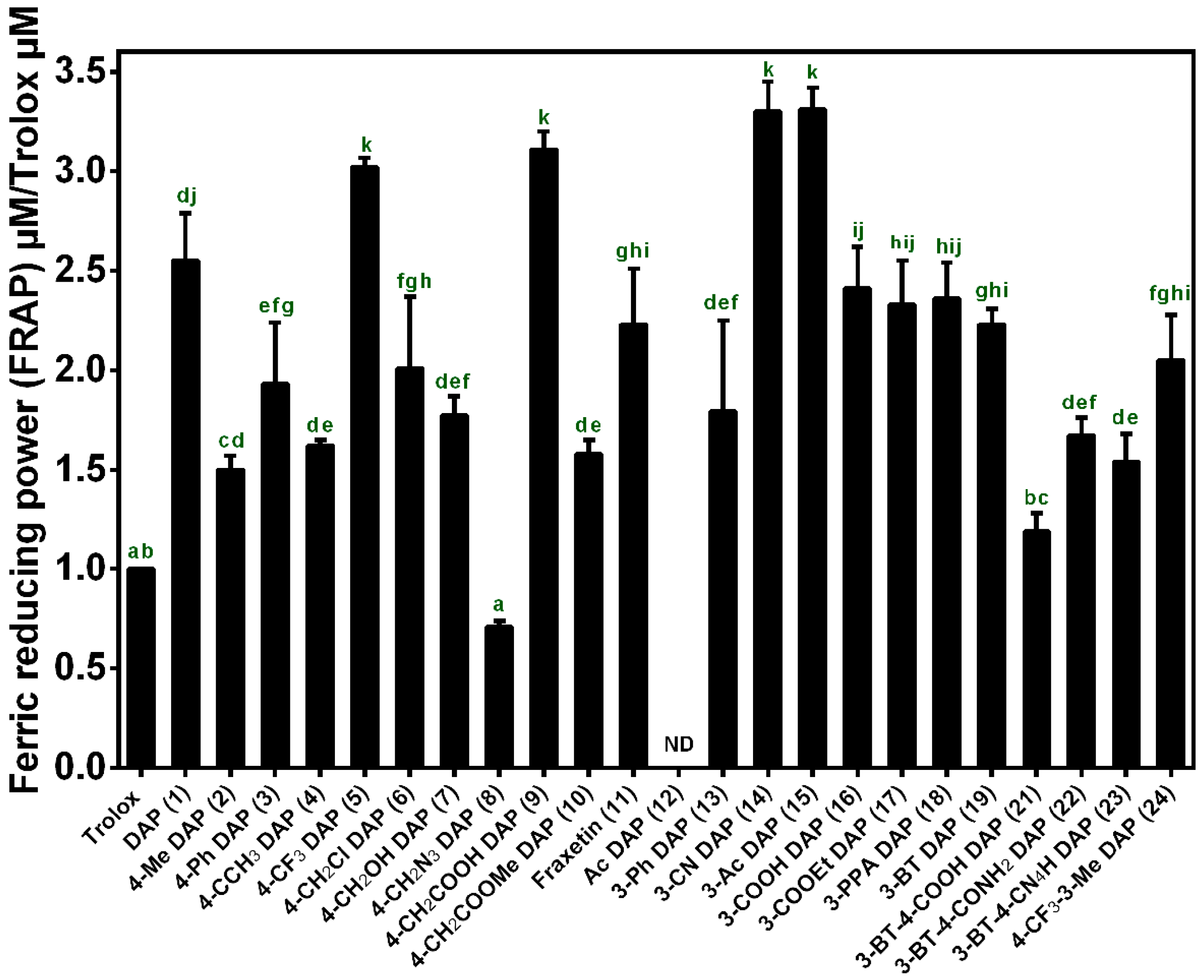
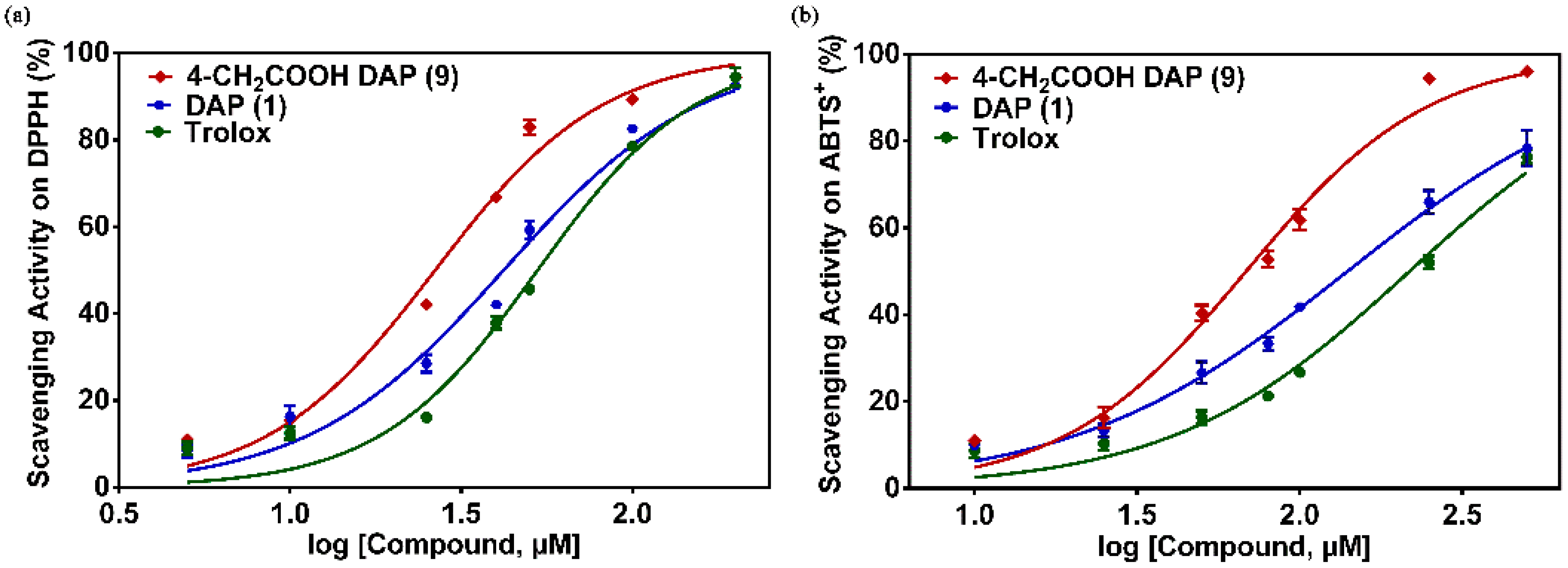
2.2. Antioxidant Activity Assay In Vitro
3. Materials and Methods
3.1. Materials and Measurements
3.2. Synthesis of 4-Substituted Daphnetin Derivatives
3.3. Synthesis of 3-Substituted Daphnetin Derivatives
3.4. Antioxidant Activity Assay In Vitro
3.4.1. DPPH Assay
3.4.2. ABTS+ Assay
3.4.3. FRAP Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef]
- Niki, E. Free radicals, antioxidants, and cancer. In Food Factors for Cancer Prevention; Ohigashi, H., Osawa, T., Terao, J., Watanabe, S., Yoshikawa, T., Eds.; Springer: Tokyo, Japan, 1997; pp. 55–57. [Google Scholar]
- Poulson, H.E.; Prieme, H.; Loft, S. Role of oxidative DNA damage in cancer initiation and promotion. Eur. J. Cancer Prev. 1998, 7, 9–16. [Google Scholar]
- Chung, K.T.; Wong, T.Y.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Nakatani, N. Phenolic antioxidants from herbs and spices. Biofactors 2000, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, X.; Xu, W.; Farzaneh, F.; Xu, R. The structure and pharmacological functions of coumarins and their derivatives. Curr. Med. Chem. 2009, 16, 4236–4260. [Google Scholar] [CrossRef] [PubMed]
- Riveiro, M.E.; De Kimpe, N.; Moglioni, A.; Vazquez, R.; Monczor, F.; Shayo, C.; Davio, C. Coumarins: Old compounds with novel promising therapeutic perspectives. Curr. Med. Chem. 2010, 17, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Santana, L.; Uriarte, E.; Delogu, G.; Corda, M.; Fadda, M.B.; Era, B.; Fais, A. New halogenated phenylcoumarins as tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 3342–3345. [Google Scholar] [CrossRef] [PubMed]
- Galkin, A.; Fallarero, A.; Vuorela, P.M. Coumarins permeability in Caco-2 cell model. J. Pharm. Pharmacol. 2009, 61, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Shan, J.; Di, L.; Jiang, L.; Xu, H. Therapeutic effects of daphnetin on adjuvantinduced arthritic rats. J. Ethnopharmacol. 2008, 120, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, X.J.; Ma, J.W.; Zheng, R.L. DNA damage in healthy term neonate. Early Hum. Dev. 2004, 77, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.A.; Zheng, R.L. Protection against damaged DNA in the single cell by polyphenols. Pharmazie 2002, 57, 852–854. [Google Scholar] [PubMed]
- Van der Waterbeemd, H. Quantitative Approaches to Structure Activity Relationships. In the Practice of Medicinal Chemistry; Wermuth, C.G., Ed.; Academic Press: London, UK, 1996; p. 367. [Google Scholar]
- Wang, P.; Xia, Y.L.; Zou, L.W.; Qian, X.K.; Dou, T.Y.; Jin, Q.; Li, S.Y.; Yu, Y.; Wang, D.D.; Luo, Q.; et al. An optimized two-photon fluorescent probe for Biological Sensing and Imaging of Catechol-O-methyltransferase. Chemistry 2017, 23, 10800–10807. [Google Scholar] [CrossRef] [PubMed]
- Signore, G.; Nifosì, R.; Albertazzi, L.; Storti, B.; Bizzarri, R. Polarity-sensitive coumarins tailored to live cell imaging. J. Am. Chem. Soc. 2010, 132, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Liyana-Pathirana, C.M.; Wall, D.S. Antioxidant activity of white and black sesame seeds and their hull fractions. Food Chem. 2006, 99, 478–483. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, J.A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (abts) method to measure antioxidant capacity of Selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Koleva, I.I.; van Breek, T.A.; Linssen, J.P.H.; Groot, A.D.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, J.; Li, Y.; Ma, T.; Sang, P.; Wang, W.; Gao, C. Variation in nutritional compositions, antioxidant activity and microstructure of Lycopus lucidus Turcz. root at different harvest times. Food Chem. 2015, 183, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.Y.; Wu, Y.J.; Wang, Y.H.; Zuo, Y.X. Metabolism and pharmacokinetics of daphnetin (article in Chinese). Yao Xue Xue Bao. 1983, 18, 496–500. [Google Scholar] [PubMed]
- Tsai, T.H.; Huang, C.T.; Shum, A.Y.; Chen, C.F. Simultaneous blood and biliary sampling of esculetin by microdialysis in the rat. Life Sci. 1999, 65, 1647–1655. [Google Scholar] [CrossRef]
- Xia, Y.L.; Liang, S.C.; Zhu, L.L.; Ge, G.B.; He, G.Y.; Ning, J.; Lv, X.; Ma, X.C.; Yang, L.; Yang, S.L. Identification and characterization of human UDP-glucuronosyltransfezzrases responsible for the glucuronidation of fraxetin. Drug Metab. Pharmacokinet. 2014, 29, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.C.; Xia, Y.L.; Hou, J.; Ge, G.B.; Zhang, J.W.; He, Y.Q.; Wang, J.Y.; Qi, X.Y.; Yang, L. Methylation, glucuronidation, and sulfonation of daphnetin in human hepatic preparations In vitro: Metabolic profiling, pathway comparison, and bioactivity analysis. J. Pharm. Sci. 2016, 105, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.L.; Dou, T.Y.; Liu, Y.; Wang, P.; Ge, G.B.; Yang, L. In vitro evaluation of the effect of C-4 substitution on methylation of 7,8-dihydroxycoumarin: Metabolic profile and catalytic kinetics. R. Soc. Open Sci. 2018, 5, 171–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xia, Y.L.; Yu, Y.; Lu, J.X.; Zou, L.W.; Feng, L.; Ge, G.B.; Yang, L. Design, synthesis and biological evaluation of esculetin derivatives as anti-tumour agents. RSC Adv. 2015, 5, 53477–53483. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2–10, 12–24 are available from the authors. |
| Compound | DPPH (EC50/μM) | ABTS+ (EC50/μM) |
|---|---|---|
| Trolox | 53.16 ± 2.02 ab | 241.33 ± 2.52 k |
| DAP (1) | 46.20 ± 1.45 ab | 159.34 ± 4.63 fghi |
| 4-Me DAP (2) | 85.63 ± 2.13 c | 224.50 ± 1.94 ijk |
| 4-Ph DAP (3) | 55.60 ± 0.78 ab | 198.33 ± 28.18 ijk |
| 4-CCH3 DAP (4) | 65.50 ± 2.84 bc | 234.68 ± 2.03 k |
| 4-CF3 DAP (5) | 45.69 ± 1.26 ab | 75.23 ± 1.58 ab |
| 4-CH2Cl DAP (6) | 46.57 ± 2.57 ab | 107.39 ± 6.72 abcde |
| 4-CH2OH DAP (7) | 61.99 ± 1.43 bc | 184.94 ± 2.04 hij |
| 4-CH2N3 DAP (8) | 62.17 ± 3.73 bc | 238.31 ± 4.47 k |
| 4-CH2COOH DAP (9) | 31.38 ± 0.68 a | 72.31 ± 2.70 a |
| 4-CH2COOMe DAP (10) | 50.20 ± 1.12 ab | 222.70 ± 1.84 jk |
| Fraxetin (11) | 45.59 ± 1.48 ab | 173.07 ± 4.83 ghi |
| Ac DAP (12) | 698.04 ± 5.22 f | 4562.49 ± 50.1 l |
| 3-Ph DAP (13) | 43.67 ± 2.08 ab | 108.01 ± 1.42 abcde |
| 3-CN DAP (14) | 47.02 ± 2.05 ab | 95.80 ± 4.18 abcd |
| 3-Ac DAP (15) | 50.62 ± 0.61 ab | 80.52 ± 0.61 abc |
| 3-COOH DAP (16) | 37.67 ± 1.16 ab | 145.67 ± 5.77 efgh |
| 3-COOEt DAP (17) | 43.98 ± 0.89 ab | 138.88 ± 0.14 defg |
| 3-PPA DAP (18) | 55.65 ± 1.48 ab | 91.25 ± 2.29 abcd |
| 3-BT DAP (19) | 71.31 ± 2.40 bc | 86.57 ± 3.17 abcd |
| 3-BT-4-COOH DAP (21) | 130.50 ± 3.54 d | 108.33 ± 6.35 abcde |
| 3-BT-4-CONH2 DAP (22) | 66.82 ± 1.38 bc | 122.34 ± 4.41 bcdef |
| 3-BT-4-CN4H DAP (23) | 167.85 ± 11.63 e | 121.72 ± 0.22 cdef |
| 4-CF3-3-Me DAP (24) | 53.76 ± 2.25 ab | 107.21 ± 6.23 abcde |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Chen, C.; Liu, Y.; Ge, G.; Dou, T.; Wang, P. Synthesis and Structure-Activity Relationship of Daphnetin Derivatives as Potent Antioxidant Agents. Molecules 2018, 23, 2476. https://doi.org/10.3390/molecules23102476
Xia Y, Chen C, Liu Y, Ge G, Dou T, Wang P. Synthesis and Structure-Activity Relationship of Daphnetin Derivatives as Potent Antioxidant Agents. Molecules. 2018; 23(10):2476. https://doi.org/10.3390/molecules23102476
Chicago/Turabian StyleXia, Yangliu, Chen Chen, Yong Liu, Guangbo Ge, Tongyi Dou, and Ping Wang. 2018. "Synthesis and Structure-Activity Relationship of Daphnetin Derivatives as Potent Antioxidant Agents" Molecules 23, no. 10: 2476. https://doi.org/10.3390/molecules23102476
APA StyleXia, Y., Chen, C., Liu, Y., Ge, G., Dou, T., & Wang, P. (2018). Synthesis and Structure-Activity Relationship of Daphnetin Derivatives as Potent Antioxidant Agents. Molecules, 23(10), 2476. https://doi.org/10.3390/molecules23102476