Acetylene in Organic Synthesis: Recent Progress and New Uses
Abstract
1. Introduction
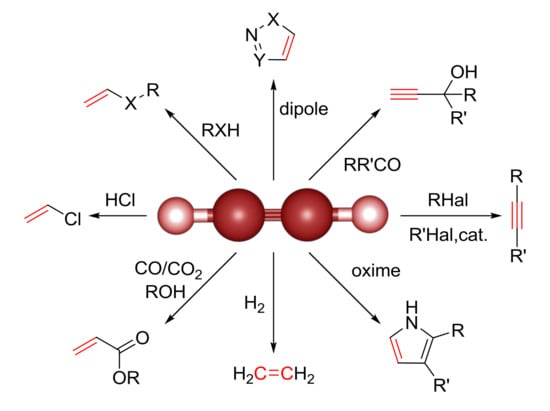
2. Acetylene in Organic Synthesis
2.1. Functionalization of Triple Bond
2.1.1. Synthesis of Vinyl Ethers
2.1.2. Synthesis of S-, Se- and Te-Vinyl Derivatives
2.1.3. Synthesis of N-Vinyl Derivatives
2.1.4. Synthesis of C-Vinyl Derivatives
2.2. Synthesis of Acrylic Acid Derivatives
2.3. Preparation of Substituted Alkynes
2.3.1. Cross-Coupling Reactions
2.3.2. Favorskii Reaction
2.4. Synthesis of Heterocycles
2.4.1. Cycloaddition Reactions
2.4.2. Trofimov Reaction
2.4.3. Other Reactions for Accessing Heterocyclic Molecules
2.5. Applications in Medicinal Chemistry and Drug Discovery
2.6. Organometallic Complexes of Acetylene
 (R = H, Me, Ph) reaction with acetylene [186] The reaction afforded four-membered iridacycles with IrC(=CH2)N moiety.
(R = H, Me, Ph) reaction with acetylene [186] The reaction afforded four-membered iridacycles with IrC(=CH2)N moiety. 2.7. Miscellaneous Synthetic Transformations
2.8. Selective Semi-Hydrogenation of Acetylene
2.8.1. Bimetallic Pd-based Catalysts: Pd and Ga/In
2.8.2. Bimetallic Pd-based Catalysts: Pd and Zn/Co
2.8.3. Pd-Based Bimetallic Catalysts: Pd-Cu/Ag/Au
2.8.4. Pd-Free Monometallic Catalysts: Mo
2.8.5. Pd-Free Monometallic Catalysts: Au
2.8.6. Pd-Free Monometallic Catalysts: In
2.8.7. Pd-Free Bimetallic Catalysts: Ni/Zn
2.8.8. Pd-Free Bimetallic Catalysts: Fe/Mo
2.8.9. Pd-Free Bimetallic Catalysts: Pt/Sn
2.8.10. Pd-Free Bimetallic Catalysts: Cu/Au
2.8.11. Non-Metallic Catalysts
2.9. Synthesis of Vinyl Chloride
2.10. Emerging Fundamental Concepts for Alkynes Transformations
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hay, A.S. Oxidative coupling of acetylenes. II. J. Org. Chem. 1962, 27, 3320–3321. [Google Scholar] [CrossRef]
- Larock, R.C.; Yum, E.K. Synthesis of indoles via palladium-catalyzed heteroannulation of internal alkynes. J. Am. Chem. Soc. 1991, 113, 6689–6690. [Google Scholar] [CrossRef]
- Lin, V.S.; DiMagno, S.G.; Therien, M.J. Highly conjugated, acetylenyl bridged porphyrins: New models for light-harvesting antenna systems. Science 1994, 264, 1105. [Google Scholar] [CrossRef] [PubMed]
- Siemsen, P.; Livingston Robert, C.; Diederich, F. Acetylenic coupling: A powerful tool in molecular construction. Angew. Chem. Int. Ed. 2000, 39, 2632–2657. [Google Scholar] [CrossRef]
- Sonogashira, K. Development of Pd-Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. J. Organomet. Chem. 2002, 653, 46–49. [Google Scholar] [CrossRef]
- Winterfeldt, E. Additions to the activated CC triple bond. Angew. Chem. Int. Ed. 1967, 6, 423–434. [Google Scholar] [CrossRef]
- Teles, J.H.; Brode, S.; Chabanas, M. Cationic gold(I) complexes: Highly efficient catalysts for the addition of alcohols to alkynes. Angew. Chem. Int. Ed. 1998, 37, 1415–1418. [Google Scholar] [CrossRef]
- Studt, F.; Abild-Pedersen, F.; Bligaard, T.; Sørensen, R.Z.; Christensen, C.H.; Nørskov, J.K. Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. Science 2008, 320, 1320. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, R.; Kitaura, R.; Kitagawa, S.; Kubota, Y.; Belosludov, R.V.; Kobayashi, T.C.; Sakamoto, H.; Chiba, T.; Takata, M.; Kawazoe, Y.; et al. Highly controlled acetylene accommodation in a metal-organic microporous material. Nature 2005, 436, 238. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xiang, S.; Qian, G. Metal-organic frameworks with functional pores for recognition of small molecules. Acc. Chem. Res. 2010, 43, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Getman, R.B.; Bae, Y.-S.; Wilmer, C.E.; Snurr, R.Q. Review and analysis of molecular simulations of methane, hydrogen, and acetylene storage in metal-organic frameworks. Chem. Rev. 2012, 112, 703–723. [Google Scholar] [CrossRef] [PubMed]
- Diederich, F.; Stang, P.J.; Tykwinski, R.R. Acetylene Chemistry: Chemistry, Biology, and Material Science; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; p. 508. [Google Scholar]
- Trofimov, B.A.; Gusarova, N.K. Acetylene: New prospects of classical reactions. Russ. Chem. Rev. 2007, 76, 507. [Google Scholar] [CrossRef]
- Trotuş, I.-T.; Zimmermann, T.; Schüth, F. Catalytic reactions of acetylene: A feedstock for the chemical industry revisited. Chem. Rev. 2014, 114, 1761–1782. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R.J. Acetylene-Based Chemical from Coal and Other Natural Resources; Dekker: New York, NY, USA, 1982; p. 232. [Google Scholar]
- Pässler, P.; Hefner, W.; Buckl, K.; Meinass, H.; Meiswinkel, A.; Wernicke, H.J.; Ebersberg, G.; Müller, R.; Bässler, J.; Behringer, H.; et al. Acetylene; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; Volume 1. [Google Scholar]
- Williams, A.; Smith, D.B. Combustion and oxidation of acetylene. Chem. Rev. 1970, 70, 267–293. [Google Scholar] [CrossRef]
- Weman, K. Gas welding. Welding Processes Handbook, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 13–18. [Google Scholar]
- Morehead, J.T.; de Chalmot, G. The manufacture of calcium carbide. J. Am. Chem. Soc. 1896, 18, 311–331. [Google Scholar] [CrossRef]
- Ledovskaya, M.S.; Voronin, V.V.; Rodygin, K.S. Methods for the synthesis of O-, S- and N-Vinyl derivatives. Russ. Chem. Rev. 2018, 87, 167–191. [Google Scholar] [CrossRef]
- Maki, Y.; Mori, H.; Endo, T. Xanthate-mediated controlled radical polymerization of N-Vinylindole derivatives. Macromolecules 2007, 40, 6119–6130. [Google Scholar] [CrossRef]
- Bühler, V. Polyvinylpyrrolidone Excipients for Pharmaceuticals: Povidone, Crospovidone and Copovidone; Springer: Berlin, Germany, 2005. [Google Scholar]
- Allsopp, M.W.; Vianello, G. Poly(vinyl chloride). In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; Volume 28, pp. 441–468. [Google Scholar]
- Zhang, J.; Liu, N.; Li, W.; Dai, B. Progress on cleaner production of vinyl chloride monomers over non-mercury catalysts. Front. Chem. Sci. Eng. 2011, 5, 514–520. [Google Scholar] [CrossRef]
- Ohara, T.; Sato, T.; Shimizu, N.; Prescher, G.; Schwind, H.; Weiberg, O.; Marten, K. Acrylic acid and derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; Volume 1, pp. 347–364. [Google Scholar]
- Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann Wolfgang, A.; Kühn Fritz, E. Transformation of carbon dioxide with homogeneous transition-metal catalysts: A molecular solution to a global challenge? Angew. Chem. Int. Ed. 2011, 50, 8510–8537. [Google Scholar] [CrossRef] [PubMed]
- Herth, G.; Schornick, G.; Fredric, L.B. Polyacrylamides and Poly(acrylic acids); Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; Volume 28. [Google Scholar]
- Hou, J.; Xie, J.H.; Zhou, Q.L. Palladium-catalyzed hydrocarboxylation of alkynes with formic acid. Angew. Chem. Int. Ed. 2015, 54, 6302–6305. [Google Scholar] [CrossRef] [PubMed]
- Osswald, J.; Kovnir, K.; Armbrüster, M.; Giedigkeit, R.; Jentoft, R.E.; Wild, U.; Grin, Y.; Schlögl, R. Palladium-gallium intermetallic compounds for the selective hydrogenation of acetylene: Part II: Surface characterization and catalytic performance. J. Catal. 2008, 258, 219–227. [Google Scholar] [CrossRef]
- Osswald, J.; Giedigkeit, R.; Jentoft, R.E.; Armbrüster, M.; Girgsdies, F.; Kovnir, K.; Ressler, T.; Grin, Y.; Schlögl, R. Palladium-gallium intermetallic compounds for the selective hydrogenation of acetylene: Part I: Preparation and structural investigation under reaction conditions. J. Catal. 2008, 258, 210–218. [Google Scholar] [CrossRef]
- Khan, N.A.; Shaikhutdinov, S.; Freund, H.J. Acetylene and ethylene hydrogenation on alumina supported Pd-Ag model catalysts. Catal. Lett. 2006, 108, 159–164. [Google Scholar] [CrossRef]
- Borodziński, A.; Bond, G.C. Selective hydrogenation of ethyne in ethene-rich streams on palladium catalysts, part 2: Steady-state kinetics and effects of palladium particle size, carbon monoxide, and promoters. Catal. Rev. 2008, 50, 379–469. [Google Scholar] [CrossRef]
- Han, Y.; Peng, D.; Xu, Z.; Wan, H.; Zheng, S.; Zhu, D. Tio2 supported Pd@Ag as highly selective catalysts for hydrogenation of acetylene in excess ethylene. Chem. Commun. 2013, 49, 8350–8352. [Google Scholar] [CrossRef] [PubMed]
- Sobenina, L.N.; Tomilin, D.N.; Petrova, O.V.; Mikhaleva, A.I.; Trofimov, B.A. Synthesis of secondary propargyl alcohols from aromatic and heteroaromatic aldehydes and acetylene in the system KOH-H2O-DMSO. Russ. J. Org. Chem. 2013, 49, 356–359. [Google Scholar] [CrossRef]
- Kutepow, N.; Viehe, H.G. Chemistry of Acetylenes; Marcel Dekker Inc.: New York, NY, USA, 1969. [Google Scholar]
- Vasilevsky, S.F.; Klyatskaya, S.V.; Elguero, J. One-pot synthesis of monosubstituted aryl(hetaryl)acetylenes by direct introduction of the C≡CH residue into arenes and hetarenes. Tetrahedron 2004, 60, 6685–6688. [Google Scholar] [CrossRef]
- Fu, R.; Li, Z. Direct synthesis of symmetric diarylethynes from calcium carbide and arylboronic acids/esters. Eur. J. Org. Chem. 2017, 2017, 6648–6651. [Google Scholar] [CrossRef]
- Turberg, M.; Ardila-Fierro, K.J.; Bolm, C.; Hernández, J.G. Altering copper-catalyzed A3 couplings by mechanochemistry: One-pot synthesis of 1,4-diamino-2-butynes from aldehydes, amines, and calcium carbide. Angew. Chem. Int. Ed. 2018, 57, 10718–10722. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, B.A. Acetylene and its derivatives in reactions with nucleophiles: Recent advances and current trends. Curr. Org. Chem. 2002, 6, 1121–1162. [Google Scholar] [CrossRef]
- Kaewchangwat, N.; Sukato, R.; Vchirawongkwin, V.; Vilaivan, T.; Sukwattanasinitt, M.; Wacharasindhu, S. Direct synthesis of aryl substituted pyrroles from calcium carbide: An underestimated chemical feedstock. Green Chem. 2015, 17, 460–465. [Google Scholar] [CrossRef]
- Ledovskaya, M.S.; Rodygin, K.S.; Ananikov, V.P. Calcium-mediated one-pot preparation of isoxazoles with deuterium incorporation. Org. Chem. Front. 2018, 5, 226–231. [Google Scholar] [CrossRef]
- Matake, R.; Niwa, Y.; Matsubara, H. Phase-vanishing method with acetylene evolution and its utilization in several organic syntheses. Org. Lett. 2015, 17, 2354–2357. [Google Scholar] [CrossRef] [PubMed]
- Voronin, V.V.; Ledovskaya, M.S.; Gordeev, E.G.; Rodygin, K.S.; Ananikov, V.P. [3 + 2]-cycloaddition of in situ generated nitrile imines and acetylene for assembling of 1,3-disubstituted pyrazoles with quantitative deuterium labeling. J. Org. Chem. 2018, 83, 3819–3828. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yan, B.; Yang, G.; Chen, Y. Green synthesis of 1-monosubstituted 1,2,3-triazoles via ‘click chemistry’ in water. Heterocycl. Commun. 2013, 19, 397. [Google Scholar] [CrossRef]
- Schmidt, C.D.; Kaschel, J.; Schneider, T.F.; Kratzert, D.; Stalke, D.; Werz, D.B. Donor-substituted nitrocyclopropanes: Immediate ring-enlargement to cyclic nitronates. Org. Lett. 2013, 15, 6098–6101. [Google Scholar] [CrossRef] [PubMed]
- Garve, L.K.B.; Barkawitz, P.; Jones, P.G.; Werz, D.B. Ring-opening 1,3-dichlorination of donor–acceptor cyclopropanes by iodobenzene dichloride. Org. Lett. 2014, 16, 5804–5807. [Google Scholar] [CrossRef] [PubMed]
- Cavitt, M.A.; France, S. Aluminum(III)-catalyzed, formal homo-nazarov-type ring-opening cyclizations toward the synthesis of functionalized tetrahydroindolizines. Synthesis 2016, 48, 1910–1919. [Google Scholar] [CrossRef]
- Jiménez-Moreno, E.; Guo, Z.; Oliveira Bruno, L.; Albuquerque Inês, S.; Kitowski, A.; Guerreiro, A.; Boutureira, O.; Rodrigues, T.; Jiménez-Osés, G.; Bernardes Gonçalo, J.L. Vinyl ether/tetrazine pair for the traceless release of alcohols in cells. Angew. Chem. 2016, 129, 249–253. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, K.; Tan, B.; Gu, Y. Ruthenium complexes immobilized on functionalized knitted hypercrosslinked polymers as efficient and recyclable catalysts for organic transformations. Adv. Synth. Catal. 2017, 359, 78–88. [Google Scholar] [CrossRef]
- Wallbaum, J.; Garve, L.K.B.; Jones, P.G.; Werz, D.B. Ring-opening 1,3-halochalcogenation of cyclopropane dicarboxylates. Org. Lett. 2017, 19, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Leger, P.R.; Murphy, R.A.; Pushkarskaya, E.; Sarpong, R. Synthetic efforts toward the lycopodium alkaloids inspires a hydrogen iodide mediated method for the hydroamination and hydroetherification of olefins. Chem. Eur. J. 2015, 21, 4377–4383. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-H.; Pronin, S.V.; Antonova-Koch, Y.; Meister, S.; Winzeler, E.A.; Shenvi, R.A. Synthesis of (+)-7,20-diisocyanoadociane and liver-stage antiplasmodial activity of the isocyanoterpene class. J. Am. Chem. Soc. 2016, 138, 7268–7271. [Google Scholar] [CrossRef] [PubMed]
- Koh Ming, J.; Khan, R.K.M.; Torker, S.; Hoveyda Amir, H. Broadly applicable Z- and diastereoselective ring-opening/cross-metathesis catalyzed by a dithiolate ru complex. Angew. Chem. Int. Ed. 2014, 53, 1968–1972. [Google Scholar]
- Torker, S.; Koh, M.J.; Khan, R.K.M.; Hoveyda, A.H. Regarding a persisting puzzle in olefin metathesis with ru complexes: Why are transformations of alkenes with a small substituent Z-selective? Organometallics 2016, 35, 543–562. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Kita, Y.; Ueda, H.; Imaoka, H.; Chiba, K.; Yasuda, M.; Baba, A. Coupling reaction of enol derivatives with silyl ketene acetals catalyzed by gallium trihalides. Chem. Eur. J. 2016, 22, 11837–11845. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Miyata, Y.; Akimoto, R.; Fujii, Y.; Kuniyasu, H.; Kambe, N. Diarylrhodates as promising active catalysts for the arylation of vinyl ethers with grignard reagents. J. Am. Chem. Soc. 2014, 136, 9260–9263. [Google Scholar] [CrossRef] [PubMed]
- Favorskii, A.E. Action of potassium hydroxide on mixtures of ketones and phenylacetylene. Zhurnal Russkago Fiziko-Khimicheskago Obshchestva 1905, 37, 643–645. [Google Scholar]
- Favorskii, A.E. Action de la potasse caustique sur les mélanges des cétones avec le phénylacétylène. Bulletin de la Société Chimique de France 1907, 2, 1087–1088. [Google Scholar]
- Rodygin, K.S.; Werner, G.; Kucherov, F.A.; Ananikov, V.P. Calcium carbide: A unique reagent for organic synthesis and nanotechnology. Chem. Asian J. 2016, 11, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Ban, M.; Yamamoto, T.; Otsuka, S. Vinylation of phenols in aqueous solution. (the researches of vinylation reaction. X.). Nippon kagaku Zassi 1956, 77, 176–182. [Google Scholar] [CrossRef]
- Potapov, V.A.; Panov, V.A.; Musalov, M.V.; Zhivet’eva, S.A.; Musalova, M.V.; Khabibulina, A.G.; Amosova, S.V. Efficient synthetic methods for unsaturated 3,4,5-trimethoxybenzyl sulfides and ethers. Russ. J. Org. Chem. 2016, 52, 1571–1575. [Google Scholar] [CrossRef]
- Orlov, A.V.; Komissarova, N.G.; Shitikova, O.V.; Spirikhin, L.V.; Yunusov, M.S. Intramolecular cyclization involving O-vinyl or O-vinylketoxime function in a number of pentacyclic triterpenoids in superbasic medium. Russ. Chem. Bull. 2013, 62, 687–691. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Oparina, L.A.; Tarasova, O.A.; Artem’ev, A.V.; Kobychev, V.B.; Gatilov, Y.V.; Albanov, A.I.; Gusarova, N.K. Tuneable superbase-catalyzed vinylation of α-hydroxyalkylferrocenes with alkynes. Tetrahedron 2014, 70, 5954–5960. [Google Scholar] [CrossRef]
- Schobert, H. Production of acetylene and acetylene-based chemicals from coal. Chem. Rev. 2014, 114, 1743–1760. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.I.; Shkapenko, G. Vinylation: Kinetics and mechanism of the methoxide-catalyzed addition of methanol to phenylacetylene1. J. Am. Chem. Soc. 1955, 77, 5038–5041. [Google Scholar] [CrossRef]
- Matake, R.; Adachi, Y.; Matsubara, H. Synthesis of vinyl ethers of alcohols using calcium carbide under superbasic catalytic conditions (KOH/DMSO). Green Chem. 2016, 18, 2614–2618. [Google Scholar] [CrossRef]
- Teong, S.P.; Chua, A.Y.H.; Deng, S.; Li, X.; Zhang, Y. Direct vinylation of natural alcohols and derivatives with calcium carbide. Green Chem. 2017, 19, 1659–1662. [Google Scholar] [CrossRef]
- Rattanangkool, E.; Vilaivan, T.; Sukwattanasinitt, M.; Wacharasindhu, S. An atom-economic approach for vinylation of indoles and phenols using calcium carbide as acetylene surrogate. Eur. J. Org. Chem. 2016, 2016, 4347–4353. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Werner, I.; Ananikov, V.P. A green and sustainable route to carbohydrate vinyl ethers for accessing bioinspired materials with a unique microspherical morphology. ChemSusChem 2017, 11, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.; Rodygin, K.S.; Kostin, A.A.; Gordeev, E.G.; Kashin, A.S.; Ananikov, V.P. A solid acetylene reagent with enhanced reactivity: Fluoride-mediated functionalization of alcohols and phenols. Green Chem. 2017, 19, 3032–3041. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Ananikov, V.P. Transition-metal-catalyzed C−S, C−Se, and C−Te bond formation via cross-coupling and atom-economic addition reactions. Chem. Rev. 2011, 111, 1596–1636. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Beletskaya, I.P.; Yus, M. Transition-metal-catalyzed addition of heteroatom−hydrogen bonds to alkynes. Chem. Rev. 2004, 104, 3079–3160. [Google Scholar] [CrossRef] [PubMed]
- Paulmier, C. Selenium Reagents and Intermediates in Organic Synthesis; Pergamon Press: Oxford, UK, 1986. [Google Scholar]
- Marcantoni, E.; Massaccesi, M.; Petrini, M.; Bartoli, G.; Bellucci, M.C.; Bosco, M.; Sambri, L. A novel route to the vinyl sulfide nine-membered macrocycle moiety of griseoviridin. J. Org. Chem. 2000, 65, 4553–4559. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pineiro, R.; Dai, S.; Alvarez-Puebla, R.; Wigginton, J.; Al-Hourani, B.J.; Fenniri, H. Synthesis of sulfur-containing aryl and heteroaryl vinyls via suzuki–miyaura cross-coupling for the preparation of sers-active polymers. Tetrahedron Lett. 2009, 50, 5467–5469. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, K.; Abiko, Y.; Mori, H. RAFT polymerization of S-vinyl sulfide derivatives and synthesis of block copolymers having two distinct optoelectronic functionalities. Macromolecules 2013, 46, 5998–6012. [Google Scholar] [CrossRef]
- Silveira, C.C.; Martins, G.M.; Mendes, S.R. Regio- and stereoselective synthesis of (Z)-2-arylsulfanyl allylic alcohols using anhydrous CeCl3 as catalyst under solvent free conditions. Tetrahedron Lett. 2013, 54, 5492–5495. [Google Scholar] [CrossRef]
- Liu, J.-G.; Ueda, M. High refractive index polymers: Fundamental research and practical applications. J. Mater. Chem. 2009, 19, 8907–8919. [Google Scholar] [CrossRef]
- Huang, H.M.; Chang, C.Y.; Liu, I.C.; Tsai, H.C.; Lai, M.K.; Tsiang Raymond, C.C. Synthesis of gold nanocomposite via chemisorption of gold nanoparticles with poly(p-methylstyrene) containing multiple bonding groups on the chain side. J. Polym. Sci. A Polym. Chem. 2005, 43, 4710–4720. [Google Scholar] [CrossRef]
- Gusarova, N.K.; Chernysheva, N.A.; Yas’ko, S.V.; Trofimov, B.A. Highly efficient atom economical “green chemistry” synthesis of vinyl sulfides from thiols and acetylene in water. Russ. Chem. Bull. 2013, 62, 438–440. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Ananikov, V.P. An efficient metal-free pathway to vinyl thioesters with calcium carbide as the acetylene source. Green Chem. 2016, 18, 482–486. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Kostin, A.A.; Ananikov, V.P. Calcium carbide as a convenient acetylene source in the synthesis of unsaturated sulfides, promising functionalized monomers. Mendeleev Commun. 2015, 25, 415–416. [Google Scholar] [CrossRef]
- Potapov, V.A.; Malinovich, D.A.; Amosova, S.V.; Bhasin, K.K. Synthesis of 2-(vinylselanyl)pyridine. Russ. J. Org. Chem. 2015, 51, 443–444. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalova, M.V.; Ishigeev, R.S.; Musalov, M.V.; Panov, V.A.; Khabibulina, A.G.; Amosova, S.V.; Bhasin, K.K. Efficient and selective syntheses of novel unsaturated chalcogen-containing pyridine derivatives. Tetrahedron Lett. 2016, 57, 5341–5343. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Gyrdymova, Y.V.; Zarubaev, V.V. Synthesis of vinyl thioethers and bis-thioethenes from calcium carbide and disulfides. Mendeleev Commun. 2017, 27, 476–478. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Lan, J.; You, J.; Chen, L. Synthesis and biological applications of imidazolium-based polymerized ionic liquid as a gene delivery vector. Chem. Biol. Drug. Des. 2009, 74, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, Y.; Shirota, Y.; Mikawa, H. Synthesis of polymers with polar side groups. III. Tricyanovinylation of poly(N-Vinylindole) and N-Vinylindole-fumaronitrile copolymer, and dielectric properties of these polymers. Polym. J. 1974, 6, 364. [Google Scholar] [CrossRef]
- Hoegl, H. On photoelectric effects in polymers and their sensitization by dopants1. J. Phys. Chem. 1965, 69, 755–766. [Google Scholar] [CrossRef]
- Grazulevicius, J.V.; Strohriegl, P.; Pielichowski, J.; Pielichowski, K. Carbazole-containing polymers: Synthesis, properties and applications. Prog. Polym. Sci. 2003, 28, 1297–1353. [Google Scholar] [CrossRef]
- Shmidt, E.Y.; Protsuk, N.I.; Vasil’tsov, A.M.; Ivanov, A.V.; Mikhaleva, A.I.; Trofimov, B.A. Improved method for the synthesis of 1-vinylindole. Chem. Heterocycl. Compd. 2013, 49, 404–407. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Bogachenkov, A.S.; Ananikov, V.P. Vinylation of a secondary amine core with calcium carbide for efficient post-modification and access to polymeric materials. Molecules 2018, 23, 648. [Google Scholar] [CrossRef] [PubMed]
- Wünsch, J.R. Polystyrene: Synthesis, Production and Applications; Rapra Technology Limited: Croydon, UK, 2000; p. 171. [Google Scholar]
- Xue, F.; Deng, H.; Xue, C.; Mohamed, D.K.B.; Tang, K.Y.; Wu, J. Reaction discovery using acetylene gas as the chemical feedstock accelerated by the “stop-flow” micro-tubing reactor system. Chem. Sci. 2017, 8, 3623–3627. [Google Scholar] [CrossRef] [PubMed]
- Luong, T.; Chen, S.; Qu, K.; McInturff, E.L.; Krische, M.J. Ruthenium(0)-catalyzed C–C coupling of alkynes and 3-hydroxy-2-oxindoles: Direct C–H vinylation of alcohols. Org. Lett. 2017, 19, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Bertleff, W.; Roeper, M.; Sava, X. Carbonylation. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Reppe, W. Carbonylierung i. Über die umsetzung von acetylen mit kohlenoxyd und verbindungen mit reaktionsfähigen wasserstoffatomen synthesen α,β-ungesättigter carbonsäuren und ihrer derivate. Liebigs Ann. 1953, 582, 1–37. [Google Scholar] [CrossRef]
- Xie, H.; Lin, T.; Shi, L.; Meng, X. Acetylene carbonylation over Ni-containing catalysts: Role of surface structure and active site distribution. RSC Adv. 2016, 6, 97285–97292. [Google Scholar] [CrossRef]
- Lin, T.J.; Meng, X.; Shi, L. Catalytic hydrocarboxylation of acetylene to acrylic acid using Ni2O3 and cupric bromide as combined catalysts. J. Mol. Catal. A Chem. 2015, 396, 77–83. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.J.; Meng, X.; Shi, L. Ni-exchanged Y-zeolite: An efficient heterogeneous catalyst for acetylene hydrocarboxylation. Appl. Catal. A General 2014, 485, 163–171. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Q.; Jackstell, R.; Beller, M. Carbonylations of alkenes with co surrogates. Angew. Chem. Int. Ed. 2014, 53, 6310–6320. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Nájera, C. Chemicals from alkynes with palladium catalysts. Chem. Rev. 2014, 114, 1783–1826. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, H.; Wang, T.; Li, C.; Yan, L.; Jiang, M.; Liu, J.; Sun, X.; Jiang, Z.; Ding, Y. The 2V-P,N polymer supported palladium catalyst for methoxycarbonylation of acetylene. J. Mol. Catal. A Chem. 2016, 414, 37–46. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Wang, W.; Du, H.; Wang, T.; Yan, L.; Hu, X.; Ding, Y. Multifunctional single-site catalysts for alkoxycarbonylation of terminal alkynes. ChemSusChem 2016, 9, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Hosseini, A.; Pilevar, A.; Hogan, E.; Mogwitz, B.; Schulze, A.S.; Schreiner, P.R. Calcium carbide catalytically activated with tetra-n-butyl ammonium fluoride for sonogashira cross coupling reactions. Org. Biomol. Chem. 2017, 15, 6800–6807. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Picken, S.J.; Dingemans, T.J. Synthesis and properties of aligned all-aromatic liquid crystal networks. High Perform. Polym. 2014, 26, 381–391. [Google Scholar] [CrossRef]
- Kozhemyakin, Y.; Kretzschmar, A.; Krämer, M.; Rominger, F.; Dreuw, A.; Bunz Uwe, H.F. Synthesis and properties of functional twisted tolanes. Chem. Eur. J. 2017, 23, 9908–9918. [Google Scholar] [CrossRef] [PubMed]
- Kitching Matthew, O.; Dixon Olivia, E.; Baumann, M.; Baxendale Ian, R. Flow-assisted synthesis: A key fragment of SR 142948A. Eur. J. Org. Chem. 2017, 2017, 6540–6553. [Google Scholar] [CrossRef]
- Potapov, V.; Musalov, M.; Panov, V.A.; Musalova, M.; Amosova, S. Allylation of acetylene under atmospheric pressure. Russ. J. Org. Chem. 2013, 2013, 1834–1835. [Google Scholar] [CrossRef]
- Boronat, M.; Laursen, S.; Leyva-Pérez, A.; Oliver-Meseguer, J.; Combita, D.; Corma, A. Partially oxidized gold nanoparticles: A catalytic base-free system for the aerobic homocoupling of alkynes. J. Catal. 2014, 315, 6–14. [Google Scholar] [CrossRef]
- Favorskii, A.E.; Skosarevskii, M.P. O reaktsii poroshkovatogo edkogo kali na smes fenilatsetilena s atsetonom. Zh. Russ. Khim. Ob–va. 1900, 32, 652. [Google Scholar]
- Tomilin, D.N.; Petrova, O.V.; Sobenina, L.N.; Mikhaleva, A.I.; Trofimov, B.A. A convenient synthesis of hetarylethynyl ketones from hetarylcarbaldehydes and acetylene. Chem. Heterocycl. Compd. 2013, 49, 341–344. [Google Scholar] [CrossRef]
- Shmidt, E.Y.; Bidusenko, I.A.; Protsuk, N.I.; Mikhaleva, A.I.; Trofimov, B.A. Improved synthesis of tertiary propargyl alcohols by the favorskii reaction of alkyl aryl (hetaryl) ketones with acetylene. Russ. J. Org. Chem. 2013, 49, 8–11. [Google Scholar] [CrossRef]
- Schmidt, E.Y.; Cherimichkina, N.A.; Bidusenko, I.A.; Protzuk, N.I.; Trofimov, B.A. Alkynylation of aldehydes and ketones using the Bu4NOH/H2O/DMSO catalytic composition: A wide-scope methodology. Eur. J. Org. Chem. 2014, 2014, 4663–4670. [Google Scholar] [CrossRef]
- Valova, T.M.; Gorelik, A.M.; Barachevsky, V.A. Synthesis of photochromic optically transparent polycarbonate glasses and study of their properties. Russ. J. Appl. Chem. 2017, 90, 501–506. [Google Scholar] [CrossRef]
- Deng, Q.; Shen, R.; Ding, R.; Zhang, L. Generation of ethynyl-grignard reagent in a falling film microreactor: An expeditious flow synthesis of propargylic alcohols and analogues. Adv. Synth. Catal. 2014, 356, 2931–2936. [Google Scholar] [CrossRef]
- Hylse, O.; Maier, L.; Kučera, R.; Perečko, T.; Svobodová, A.; Kubala, L.; Paruch, K.; Švenda, J. A concise synthesis of forskolin. Angew. Chem. 2017, 129, 12760–12763. [Google Scholar] [CrossRef]
- Hosseini, A.; Seidel, D.; Miska, A.; Schreiner, P.R. Fluoride-assisted activation of calcium carbide: A simple method for the ethynylation of aldehydes and ketones. Org. Lett. 2015, 17, 2808–2811. [Google Scholar] [CrossRef] [PubMed]
- Sum, Y.N.; Yu, D.; Zhang, Y. Synthesis of acetylenic alcohols with calcium carbide as the acetylene source. Green Chem. 2013, 15, 2718–2721. [Google Scholar] [CrossRef]
- Clarke, P.A.; Santos, S.; Martin, W.H.C. Combining pot, atom and step economy (pase) in organic synthesis. Synthesis of tetrahydropyran-4-ones. Green Chem. 2007, 9, 438–440. [Google Scholar] [CrossRef]
- Diaz Velazquez, H.; Ruiz Garcia, Y.; Vandichel, M.; Madder, A.; Verpoort, F. Water-soluble NHC-cu catalysts: Applications in click chemistry, bioconjugation and mechanistic analysis. Org. Biomol. Chem. 2014, 12, 9350–9356. [Google Scholar] [CrossRef] [PubMed]
- Brahma, K.; Achari, B.; Chowdhury, C. Facile synthesis of [1,2,3]-triazole-fused isoindolines, tetrahydroisoquinolines, benzoazepines and benzoazocines by palladium-copper catalysed heterocyclisation. Synthesis 2013, 45, 545–555. [Google Scholar]
- Sharma, S.; Saquib, M.; Verma, S.; Mishra, N.N.; Shukla, P.K.; Srivastava, R.; Prabhakar, Y.S.; Shaw, A.K. Synthesis of 2,3,6-trideoxy sugar triazole hybrids as potential new broad spectrum antimicrobial agents. Eur. J. Med. Chem. 2014, 83, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Avula Vijay Kumar, R.; Vallela, S.; Anireddy Jaya, S.; Chamarthi Naga, R. Copper-catalyzed synthesis of N-alkylated 2-(4-substituted-1H-1,2,3-triazol-1-yl)-1H-indole-3-carbaldehyde by step-wise and one-pot three-component huisgen’s 1,3-dipolar cycloaddition reaction. J. Heterocycl. Chem. 2017, 54, 3071–3076. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, W.; Chen, Y.; Gao, B.; Wu, W.; Jiang, H. Calcium carbide as the acetylide source: Transition-metal-free synthesis of substituted pyrazoles via [1,5]-sigmatropic rearrangements. Green Chem. 2016, 18, 6445–6449. [Google Scholar] [CrossRef]
- Albertin, G.; Antoniutti, S.; Bortoluzzi, M.; Botter, A.; Castro, J. Reactivity with alkene and alkyne of pentamethylcyclopentadienyl half-sandwich diazoalkane complexes of ruthenium. J. Organomet. Chem. 2016, 822, 259–268. [Google Scholar] [CrossRef]
- Demina, O.V.; Khodonov, A.A.; Sinauridze, E.I.; Shvets, V.I.; Varfolomeev, S.D. 5-substituted pyridylisoxazoles as effective inhibitors of platelet aggregation. Russ. Chem. Bull. 2014, 63, 2092–2113. [Google Scholar] [CrossRef]
- Canlas, G.M.R.; Gilbertson, S.R. [4 + 2 + 2] cycloaddition catalyzed by a new cationic rhodium-bisphosphine monooxide complex. Chem. Comm. 2014, 50, 5007–5010. [Google Scholar] [CrossRef] [PubMed]
- More, A.A.; Ramana, C.V. Total synthesis of the putative structure of xylarinol B. Chem. Asian J. 2014, 9, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, M.; Böhnke, J.; Braunschweig, H.; Celik Mehmet, A.; Claes, C.; Ewing William, C.; Krummenacher, I.; Lubitz, K.; Schneider, C. Neutral diboron analogues of archetypal aromatic species by spontaneous cycloaddition. Angew. Chem. Int. Ed. 2016, 55, 11271–11275. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Schäfer, J.; Dewhurst Rian, D.; Ewing William, C.; Krahfuß, M.; Kuntze-Fechner Maximilian, W.; Wehner, M.; Lambert, C.; Braunschweig, H. Regioselective catalytic and stepwise routes to bulky, functional-group-appended, and luminescent 1,2-azaborinines. Chem. Eur. J. 2016, 22, 8603–8609. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Guo, J.-D.; Sasamori, T.; Karatsu, Y.; Furukawa, Y.; Ferao, A.E.; Nagase, S.; Tokitoh, N. Reaction of a stable digermyne with acetylenes: Synthesis of a 1,2-digermabenzene and a 1,4-digermabarrelene. Bull. Chem. Soc. Jpn. 2016, 89, 1375–1384. [Google Scholar] [CrossRef]
- Lee, V.Y.; Gapurenko, O.A.; Minkin, V.I.; Horiguchi, S.; Sekiguchi, A. [2 + 2] cycloaddition of the schrock titanium silylidene and acetylene. Russ. Chem. Bull. 2016, 65, 1139–1141. [Google Scholar] [CrossRef]
- Trofimov, B.A. Preparation of pyrroles from ketoximes and acetylenes. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: Cambridge, MA, USA, 1990; Volume 51, pp. 177–301. [Google Scholar]
- Trofimov, B.A.; Korostova, S.E.; Balabanova, L.N.; Mikhaleva, A.l. Pyrroles from ketoximes and acetylene. 7. 3-alkyl(phenyl)-2-phenylpyrroles and their N-Vinyl derivatives. Zh. Org. Khim. 1978, 14, 2182–2184. [Google Scholar]
- Petrova, O.V.; Sobenina, L.N.; Mikhaleva, A.I. 3-Alkyl-2-phenyl-1-vinylpyrroles from ketoximes and acetylene: An improved synthesis by the trofimov reaction. Chem. Heterocycl. Compd. 2013, 48, 1628–1633. [Google Scholar] [CrossRef]
- Schmidt, E.Y.; Zorina, N.V.; Ivanova, E.V.; Tatarinova, I.V.; Ushakov, I.A.; Mikhaleva, A.I.; Trofimov, B.A. One-pot synthesis of 3-(E)-styrylpyrroles from (E)-styrylmethyl ketoximes and acetylene. Mendeleev Commun. 2013, 23, 340–341. [Google Scholar] [CrossRef]
- Sobenina, L.N.; Stepanova, Z.V.; Petrova, O.V.; Ma, J.S.; Yang, G.; Tatarinova, A.A.; Mikhaleva, A.I.; Trofimov, B.A. Synthesis of 3-[5-(biphenyl-4-yl)pyrrol-2-yl]-1-phenylprop-2-yn-1-ones by palladium-free cross-coupling between pyrroles and haloalkynes on aluminum oxide. Russ. Chem. Bull. 2013, 62, 88–92. [Google Scholar] [CrossRef]
- Petrova, O.V.; Sobenina, L.N.; Ushakov, I.A.; Budaev, A.B.; Ivanov, A.V.; Samsonov, V.A.; Tikhonov, A.Y.; Trofimov, B.A. Multi-channel annulation of acetylene with 3-methyl-7,8-dihydrocinnolin-5(6H)-one oxime in the KOH/DMSO superbasic system. Mendeleev Commun. 2017, 27, 344–345. [Google Scholar] [CrossRef]
- Shabalin, D.A.; Dvorko, M.Y.; Schmidt, E.Y.; Protsuk, N.I.; Trofimov, B.A. Synthesis of 5-hydroxy-δ1-pyrrolines from aryl isoalkyl ketoximes and acetylene in a tuned superbase medium. Tetrahedron Lett. 2016, 57, 3156–3159. [Google Scholar] [CrossRef]
- Shabalin, D.A.; Dvorko, M.Y.; Schmidt, E.Y.; Ushakov, I.A.; Trofimov, B.A. Synthesis of 5-hydroxy-δ1-pyrrolines from sec-alkyl aryl ketoximes and acetylene. Tetrahedron 2016, 72, 6661–6667. [Google Scholar] [CrossRef]
- Shabalin, D.A.; Glotova, T.E.; Schmidt, E.Y.; Ushakov, I.A.; Mikhaleva, A.b.I.; Trofimov, B.A. Synthesis of 3,3-dimethyl-2-phenyl-3H-pyrrole from isopropyl phenyl ketoxime and acetylene: A side formation of 4,4-dimethyl-5-phenyl-1-vinyl-2-pyrrolidinone as clue to the reaction mechanism. Mendeleev Commun. 2014, 24, 100–101. [Google Scholar] [CrossRef]
- Shabalin, D.A.; Glotova, T.E.; Ushakov, I.A.; Dvorko, M.Y.; Vashchenko, A.V.; Smirnov, V.I.; Schmidt, E.Y.; Mikhaleva, A.b.I.; Trofimov, B.A. 2-(2-Ethynyl-1-aziranyl)-3,4-dihydro-2H-pyrrole: A one-pot assembly from isopropyl phenyl ketoxime and acetylene during the synthesis of 3H-pyrrole. Mendeleev Commun. 2014, 24, 368–369. [Google Scholar] [CrossRef]
- Schmidt, E.Y.; Bidusenko, I.A.; Cherimichkina, N.A.; Ushakov, I.A.; Trofimov, B.A. Polycyclic bridgehead acetals with enol functionality: One-pot assembly from aliphatic ketones and acetylene in KOH/DMSO suspension. Tetrahedron 2016, 72, 4510–4517. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Schmidt, E.Y.; Ushakov, I.A.; Mikhaleva, A.I.; Zorina, N.V.; Protsuk, N.I.; Senotrusova, E.Y.; Skital’tseva, E.V.; Kazheva, O.N.; Alexandrov, G.G.; et al. One-pot assembly of 7-methylene-6,8-dioxabicyclo[3.2.1]octanes, congeners of frontalin, from ketones and acetylene. Eur. J. Org. Chem. 2009, 2009, 5142–5145. [Google Scholar] [CrossRef]
- Schmidt, E.Y.; Bidusenko, I.A.; Ushakov, I.A.; Vashchenko, A.V.; Trofimov, B.A. Decorated cyclopentadienes from acetylene and ketones in just two steps. Org. Lett. 2017, 19, 3127–3130. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.Y.; Bidusenko, I.A.; Protsuk, N.I.; Ushakov, I.A.; Trofimov, B.A. Superbase-promoted selective cascade cyclization reaction of 1,5-diketones with acetylenes to methylene-6,8-dioxabicyclo[3.2.1]octanes. Eur. J. Org. Chem. 2013, 2013, 2453–2460. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Schmidt, E.Y.; Bidusenko, I.A.; Cherimichkina, N.A.; Ushakov, I.A. Assemblage of 1-hydroxy-2-cyclopentene and 7-methylene-6,8-dioxabicyclo[3.2.1]octane from acetylene and 4-acetylpyridine molecules in a suspension of KOH-DMSO. Russ. Chem. Bull. 2014, 63, 2402–2404. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Schmidt, E.Y.; Bidusenko, I.A.; Ushakov, I.A.; Cherimichkina, N.A.; Protsuk, N.I. Diastereoselective self-organization of acetylene and acetophenone molecules into 1-benzoyl-3-hydroxy-cyclopentene in the presence of potassium hydroxide. Russ. J. Org. Chem. 2014, 50, 1207–1209. [Google Scholar] [CrossRef]
- Fu, R.; Li, Z. Direct synthesis of 2-methylbenzofurans from calcium carbide and salicylaldehyde p-tosylhydrazones. Org. Lett. 2018, 20, 2342–2345. [Google Scholar] [CrossRef] [PubMed]
- Fakharian, M.; Keivanloo, A.; Nabid Mohammad, R. Using calcium carbide as an acetylene source for cascade synthesis of pyrrolo[2,3-b]quinoxalines via copper-free sonogashira coupling reaction. Helv. Chim. Acta 2018, 101, e1800004. [Google Scholar] [CrossRef]
- Shukys, J.G.; Township, C.; County, M. Trifluoroethyl Vinyl Ether Compositions and Methods for Preparing and Using the Same. US Patent 2830007A, 4 August 1958. [Google Scholar]
- Townsend, P.W.; Park, F. Method of Preparing 2,2,2-Trifluoroethyl Vinyl Ether. US Patent 2870218A, 20 January 1959. [Google Scholar]
- Campbell, K.N.; Campbell, B.K.; Eby, L.T. The preparation of acetylenic carbinols. J. Am. Chem. Soc. 1938, 60, 2882–2884. [Google Scholar] [CrossRef]
- Isler, O.; Wiss, O. Chemistry and biochemistry of the K vitamins. In Vitamins & Hormones; Harris, R.S., Marrian, G.F., Thimann, K.V., Eds.; Academic Press: Cambridge, MA, USA, 1959; Volume 17, pp. 53–90. [Google Scholar]
- McLamore, W.M.; P’An, S.Y.; Bavley, A. Hypnotics and anticonvulsants. Ii. Halogenated tertiary acetylenic carbinols. J. Org. Chem. 1955, 20, 109–117. [Google Scholar] [CrossRef]
- Grimme, W.; Emde, U.-M.; Emde, H. Allophanates of alpha-ethynylcarbinols. US Patent 2822379A, 4 February 1958. [Google Scholar]
- Elks, J. The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies; Springer: Berlin, Germany, 2014. [Google Scholar]
- Morton, I.K.; Hall, J.M. Concise Dictionary of Pharmacological Agents: Properties and Synonyms; Springer: Berlin, Germany, 2012; p. 342. [Google Scholar]
- Moutou, J.-L.; Mouton, F.; Pellegrino, G.; Dillenschneider, J.-M.; Lafay, J. A Process for Introducing a Double Bond into Position 15,16 of a Steroid. WO2011098439 (A2), 18 August 2011. [Google Scholar]
- Inhoffen Hans, H.; Logemann, W.; Hohlweg, W.; Serini, A. Untersuchungen in der sexualhormon-reihe. Ber. Dtsch. Chem. Ges. A/B 1971, 71, 1024–1032. [Google Scholar] [CrossRef]
- Ercoli, A. 3-Cyclopentyl and Cyclopentenyl Ethers of Estrone and Derivatives there of. US Patent 3159543A, 1 December 1961. [Google Scholar]
- Tietze, L.F.; Krimmelbein, I.K. Enantioselective total synthesis of the oral contraceptive desogestrel by a double heck reaction. Chem. Eur. J. 2008, 14, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Wuts, P.G.M.; Anderson, A.M.; Ashford, S.W.; Goble, M.P.; White, M.J.; Beck, D.; Gilbert, I.; Hrab, R.E. A chemobiological synthesis of eplerenone. Synlett 2008, 2008, 418–422. [Google Scholar] [CrossRef]
- Kuhnz, W.; Fritzemeier, K.H.; Hegele-Hartung, C.; Krattenmacher, R. Comparative progestational activity of norgestimate, levonorgestrel-oxime and levonorgestrel in the rat and binding of these compounds to the progesterone receptor. Contraception 1995, 51, 131–139. [Google Scholar] [CrossRef]
- Blickenstaff, R.T.; Ghosh, A.C.; Wolf, G.C. Total Synthesis of Steroids; Academic Press: New York, NY, USA; London, UK, 1974; p. 328. [Google Scholar]
- Ren, T. Diruthenium σ-alkynyl compounds: A new class of conjugated organometallics. Organometallics 2005, 24, 4854–4870. [Google Scholar] [CrossRef]
- Buschbeck, R.; Low, P.J.; Lang, H. Homoleptic transition metal acetylides. Coord. Chem. Rev. 2011, 255, 241–272. [Google Scholar] [CrossRef]
- Long, N.J.; Williams, C.K. Metal alkynyl σ complexes: Synthesis and materials. Angew. Chem. Int. Ed. 2003, 42, 2586–2617. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.R.; Lalinde, E.; Teresa Moreno, M. An overview of the chemistry of homo and heteropolynuclear platinum complexes containing bridging acetylide (μ-C≡CR) ligands. Coord. Chem. Rev. 2010, 254, 832–875. [Google Scholar] [CrossRef]
- Green, K.A.; Cifuentes, M.P.; Samoc, M.; Humphrey, M.G. Metal alkynyl complexes as switchable nlo systems. Coord. Chem. Rev. 2011, 255, 2530–2541. [Google Scholar] [CrossRef]
- Grelaud, G.; Cifuentes, M.P.; Paul, F.; Humphrey, M.G. Group 8 metal alkynyl complexes for nonlinear optics. J. Organomet. Chem. 2014, 751, 181–200. [Google Scholar] [CrossRef]
- Mei, J.; Ogawa, K.; Kim, Y.-G.; Heston, N.C.; Arenas, D.J.; Nasrollahi, Z.; McCarley, T.D.; Tanner, D.B.; Reynolds, J.R.; Schanze, K.S. Low-band-gap platinum acetylide polymers as active materials for organic solar cells. ACS Appl. Mater. Interfaces 2009, 1, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.-Y.; Ho, C.-L. Organometallic photovoltaics: A new and versatile approach for harvesting solar energy using conjugated polymetallaynes. Acc. Chem. Res. 2010, 43, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Lüning, A.; Schur, J.; Hamel, L.; Ott, I.; Klein, A. Strong cytotoxicity of organometallic platinum complexes with alkynyl ligands. Organometallics 2013, 32, 3662–3672. [Google Scholar] [CrossRef]
- Meyer, A.; Bagowski, C.P.; Kokoschka, M.; Stefanopoulou, M.; Alborzinia, H.; Can, S.; Vlecken, D.H.; Sheldrick, W.S.; Wölfl, S.; Ott, I. On the biological properties of alkynyl phosphine gold(I) complexes. Angew. Chem. Int. Ed. 2012, 51, 8895–8899. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.S.M.; Law, Y.C.; Kui Steven, C.F.; Zhu, N.; Leung King, H.; Phillips David, L.; Che, C.M. Ligand-to-ligand charge-transfer transitions of platinum(II) complexes with arylacetylide ligands with different chain lengths: Spectroscopic characterization, effect of molecular conformations, and density functional theory calculations. Chem. Eur. J. 2010, 16, 6540–6554. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.M.-C.; Yam, V.W.-W. Self-assembly of luminescent alkynylplatinum(II) terpyridyl complexes: Modulation of photophysical properties through aggregation behavior. Acc. Chem. Res. 2011, 44, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, V.; Funaioli, T.; Leoni, P.; Marchetti, F.; Marchetti, L.; Pasquali, M. Synthesis and characterization of non-bridging mono- and bis-σ-η1-alkynyl derivatives of the phosphido-bridged hexaplatinum core [Pt6(μ-PBut2)4(CO)4]2+. Dalton Trans. 2016, 45, 6878–6892. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Lamacchia, V.; Antonucci, D.; Papadia, P.; Pacifico, C.; Natile, G.; Fanizzi, F.P. Insertion of alkynes into Pt-X bonds of square planar [PtX2(N^N)] (X = Cl, Br, I) complexes. Dalton Trans. 2014, 43, 8826–8834. [Google Scholar] [CrossRef] [PubMed]
- Mitchenko, S.A.; Khazipov, O.V.; Krasnyakova, T.V. New stereoselective Csp2 - Csp3 coupling: Catalytic iodomethylation of acetylene with methyl iodide into E-1-iodopropene. Kinet. Catal. 2014, 55, 304–310. [Google Scholar] [CrossRef]
- Campos, J. Dihydrogen and acetylene activation by a gold(I)/platinum(0) transition metal only frustrated lewis pair. J. Am. Chem. Soc. 2017, 139, 2944–2947. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.; Miranda, M.; Chittineni, N.P.B.; Pinion, T.A.; Perez, R. Mechanistic studies on platinum(II) catalyzed hydroarylation of alkynes. Organometallics 2014, 33, 3040–3050. [Google Scholar] [CrossRef]
- Ortega-Moreno, L.; Peloso, R.; López-Serrano, J.; Iglesias-Sigüenza, J.; Maya, C.; Carmona, E. A cationic unsaturated platinum(II) complex that promotes the tautomerization of acetylene to vinylidene. Angew. Chem. Int. Ed. 2017, 56, 2772–2775. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, C.; Hernández Yohar, A.; López-Serrano, J.; Paneque, M.; Petronilho, A.; Poveda Manuel, L.; Salazar, V.; Vattier, F.; Álvarez, E.; Maya, C.; et al. Reactivity studies of iridium pyridylidenes [Tpme2Ir(C6H5)2(C(CH)3C(R)NH] (R=H, Me, Ph). Chem. Eur. J. 2013, 19, 4003–4020. [Google Scholar] [CrossRef] [PubMed]
- Esteruelas, M.A.; Oñate, E.; Palacios, A.U. Selective synthesis and photophysical properties of phosphorescent heteroleptic iridium(III) complexes with two different bidentate groups and two different monodentate ligands. Organometallics 2017, 36, 1743–1755. [Google Scholar] [CrossRef]
- Espada, M.A.F.; Poveda, M.L.; Carmona, E. Reactivity of a cationic (C5Me5)IrIII-cyclometalated phosphine complex with alkynes. Organometallics 2014, 33, 7164–7175. [Google Scholar] [CrossRef]
- Komine, N.; Kuramoto, A.; Nakanishi, K.; Hirano, M.; Komiya, S. Alkene and alkyne insertion into hydrogen-transition metal bonds catalyzed by palladium(0) complex. Top. Catal. 2014, 57, 960–966. [Google Scholar] [CrossRef]
- Helmdach, K.; Ludwig, S.; Villinger, A.; Hollmann, D.; Kosters, J.; Seidel, W.W. Synthesis and activation potential of an open shell diphosphine. Chem. Commun. 2017, 53, 5894–5897. [Google Scholar] [CrossRef] [PubMed]
- Helmdach, K.; Dork, S.; Villinger, A.; Seidel, W.W. Sterically encumbered metalla-diphosphines: Unlocking alkyne rotation by ptii coordination. Dalton Trans. 2017, 46, 11140–11144. [Google Scholar] [CrossRef] [PubMed]
- Ananikov, V.P.; Beletskaya, I.P. Alkyne and alkene insertion into metal–heteroatom and metal–hydrogen bonds: The key stages of hydrofunctionalization process. In Hydrofunctionalization; Ananikov, V.P., Tanaka, M., Eds.; Springer: Berlin, Heidelberg, 2013; pp. 1–19. [Google Scholar]
- Musalova, M.V.; Potapov, V.A.; Musalov, M.V.; Amosova, S.V. Stereoselective synthesis of (e)-(2-bromovinyl)tellurium tribromide. Russ. J. Org. Chem. 2013, 49, 1397–1398. [Google Scholar] [CrossRef]
- Zeni, G.; Lüdtke, D.S.; Panatieri, R.B.; Braga, A.L. Vinylic tellurides: From preparation to their applicability in organic synthesis. Chem. Rev. 2006, 106, 1032–1076. [Google Scholar] [CrossRef] [PubMed]
- Mitchenko, S.A.; Khazipov, O.V.; Krasnyakova, T.V. Platinum-catalyzed addition of iodomethane to acetylene. Russ. Chem. Bull. 2013, 62, 984–988. [Google Scholar] [CrossRef]
- Yoshimura, A.; Saga, Y.; Sato, Y.; Ogawa, A.; Chen, T.; Han, L.-B. An efficient base-catalyzed double addition of H-phosphine oxides to alkynes. Tetrahedron Lett. 2016, 57, 3382–3384. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, Y.; Yao, Y.; Yang, W.; Tao, Y. Synthesis of (4E,6Z,10Z)-hexadeca-4,6,10-trien-1-ol and (4E,6E,10Z)-hexadeca-4,6,10-trien-1-ol, the pheromone components of cocoa pod borer moth conopomorpha cramerella. RSC Adv. 2017, 7, 35575–35580. [Google Scholar] [CrossRef]
- Beevor, P.S.; Cork, A.; Hall, D.R.; Nesbitt, B.F.; Day, R.K.; Mumford, J.D. Components of female sex pheromone of cocoa pod borer moth,conopomorpha cramerella. J. Chem. Ecol. 1986, 12, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lim Yu, N.; Lee, C.; Jang, H.Y.; Lee Bun, Y. 1,5,7-Triazabicyclo[4.4.0]dec-1-ene-mediated acetylene dicarboxylation and alkyne carboxylation using carbon dioxide. Eur. J. Org. Chem. 2013, 2013, 1867–1871. [Google Scholar] [CrossRef]
- Yu, D.; Sum Yin, N.; Ean Amanda Chng, C.; Chin Mei, P.; Zhang, Y. Acetylide ion (C22−) as a synthon to link electrophiles and nucleophiles: A simple method for enaminone synthesis. Angew. Chem. Int. Ed. 2013, 52, 5125–5128. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Nakamoto, M.; Sekiguchi, A. A diels–alder super diene breaking benzene into C2H2 and C4H4 units. Nat. Commun. 2014, 5, 3018. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Lough, A.J.; Gossage, R.A.; Foucher, D.A. Pd-catalysed reactions of alkynes with model distannanes and poly[di-(n-butyl)]stannane. Dalton Trans. 2013, 42, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.A.; Martín, S.E. Syntheses and applications of organostannanes bonded to elements of groups XIV, XV, and XVI. Coord. Chem. Rev. 2006, 250, 575–601. [Google Scholar] [CrossRef]
- Sachtler, W.M.H. Handbook of Heterogeneous Catalysis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Leviness, S.; Nair, V.; Weiss, A.H.; Schay, Z.; Guczi, L. Acetylene hydrogenation selectivity control on PdCu/Al2O3 catalysts. J. Mol. Catal. 1984, 25, 131–140. [Google Scholar] [CrossRef]
- Wehrli, J.T.; Thomas, D.J.; Wainwright, M.S.; Trimm, D.L.; Cant, N.W. Selective hydrogenation of propyne over an ion-exchanged copper on silica catalyst. Appl. Catal. 1990, 66, 199–208. [Google Scholar] [CrossRef]
- Vilé, G.; Baudouin, D.; Remediakis Ioannis, N.; Copéret, C.; López, N.; Pérez-Ramírez, J. Silver nanoparticles for olefin production: New insights into the mechanistic description of propyne hydrogenation. ChemCatChem 2013, 5, 3750–3759. [Google Scholar] [CrossRef]
- Gieshoff, T.N.; Villa, M.; Welther, A.; Plois, M.; Chakraborty, U.; Wolf, R.; Jacobi von Wangelin, A. Iron-catalyzed olefin hydrogenation at 1 bar H2 with a FeCl3-LiAlH4 catalyst. Green Chem. 2015, 17, 1408–1413. [Google Scholar] [CrossRef]
- Trimm, D.L.; Cant, N.W.; Liu, I.O.Y. The selective hydrogenation of acetylene in the presence of carbon monoxide over Ni and Ni-Zn supported on MgAl2O4. Catal. Today 2011, 178, 181–186. [Google Scholar] [CrossRef]
- Trimm, D.L.; Liu, I.O.Y.; Cant, N.W. The selective hydrogenation of acetylene over a Ni/SiO2 catalyst in the presence and absence of carbon monoxide. Appl. Catal. A General 2010, 374, 58–64. [Google Scholar] [CrossRef]
- Segura, Y.; López, N.; Pérez-Ramírez, J. Origin of the superior hydrogenation selectivity of gold nanoparticles in alkyne + alkene mixtures: Triple-versus double-bond activation. J. Catal. 2007, 247, 383–386. [Google Scholar] [CrossRef]
- Nikolaev, S.A.; Smirnov, V.V.; Vasil’kov, A.Y.; Podshibikhin, V.L. Synergism of the catalytic effect of nanosized gold-nickel catalysts in the reaction of selective acetylene hydrogenation to ethylene. Kinet. Catal. 2010, 51, 375–379. [Google Scholar] [CrossRef]
- Nikolaev, S.A.; Smirnov, V.V. Synergistic and size effects in selective hydrogenation of alkynes on gold nanocomposites. Catal. Today 2009, 147, S336–S341. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Lee, J.W.; Hong, C.-Y.; Mou, C.-Y.; Jang, B.W.L. Selective hydrogenation of acetylene in excess ethylene over SiO2 supported Au–Ag bimetallic catalyst. Appl. Catal. A General 2012, 439, 8–14. [Google Scholar] [CrossRef]
- Bridier, B.; López, N.; Pérez-Ramírez, J. Partial hydrogenation of propyne over copper-based catalysts and comparison with nickel-based analogues. J. Catal. 2010, 269, 80–92. [Google Scholar] [CrossRef]
- Bridier, B.; Pérez-Ramírez, J. Cooperative effects in ternary Cu−Ni−Fe catalysts lead to enhanced alkene selectivity in alkyne hydrogenation. J. Am. Chem. Soc. 2010, 132, 4321–4327. [Google Scholar] [CrossRef] [PubMed]
- Vilé, G.; Bridier, B.; Wichert, J.; Pérez-Ramírez, J. Ceria in hydrogenation catalysis: High selectivity in the conversion of alkynes to olefins. Angew. Chem. Int. Ed. 2012, 51, 8620–8623. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.; Vilé, G.; Fernández-Torre, D.; Pérez, R.; Pérez-Ramírez, J.; Ganduglia-Pirovano, M.V. Molecular-level understanding of CeO2 as a catalyst for partial alkyne hydrogenation. J. Phys. Chem. C 2014, 118, 5352–5360. [Google Scholar] [CrossRef]
- Johnson, M.M.; Walker, D.W.; Nowack, G.P. Selective Hydrogenation Catalyst. US Patent 4404124, 13 September 1983. [Google Scholar]
- Than, C.N.; Didillion, B.; Sarrazin, P.; Cameron, C. Selective Hydrogenation Catalyst and A Process Using That Catalyst. US Patent 6054409 (A), 24 April 2000. [Google Scholar]
- Gabasch, H.; Hayek, K.; Klötzer, B.; Knop-Gericke, A.; Schlögl, R. Carbon incorporation in pd(111) by adsorption and dehydrogenation of ethene. J. Phys. Chem. B 2006, 110, 4947–4952. [Google Scholar] [CrossRef] [PubMed]
- García-Mota, M.; Bridier, B.; Pérez-Ramírez, J.; López, N. Interplay between carbon monoxide, hydrides, and carbides in selective alkyne hydrogenation on palladium. J. Catal. 2010, 273, 92–102. [Google Scholar] [CrossRef]
- Teschner, D.; Borsodi, J.; Wootsch, A.; Révay, Z.; Hävecker, M.; Knop-Gericke, A.; Jackson, S.D.; Schlögl, R. The roles of subsurface carbon and hydrogen in palladium-catalyzed alkyne hydrogenation. Science 2008, 320, 86. [Google Scholar] [CrossRef] [PubMed]
- Sá, J.; Arteaga, G.D.; Daley, R.A.; Bernardi, J.; Anderson, J.A. Factors influencing hydride formation in a Pd/TiO2 catalyst. J. Phys. Chem. B 2006, 110, 17090–17095. [Google Scholar] [CrossRef] [PubMed]
- Armbrüster, M.; Behrens, M.; Cinquini, F.; Föttinger, K.; Grin, Y.; Haghofer, A.; Klötzer, B.; Knop-Gericke, A.; Lorenz, H.; Ota, A.; et al. How to control the selectivity of palladium-based catalysts in hydrogenation reactions: The role of subsurface chemistry. Chem. Catal. Chem. 2012, 4, 1048–1063. [Google Scholar] [CrossRef]
- Ludwig, W.; Savara, A.; Dostert, K.-H.; Schauermann, S. Olefin hydrogenation on pd model supported catalysts: New mechanistic insights. J. Catal. 2011, 284, 148–156. [Google Scholar] [CrossRef]
- Nikolaev, S.A.; Zanaveskin, L.N.; Smirnov, V.V.; Averyanov, V.A.; Zanaveskin, K.L. Catalytic hydrogenation of alkyne and alkadiene impurities from alkenes. Practical and theoretical aspects. Russ. Chem. Rev. 2009, 78, 231. [Google Scholar] [CrossRef]
- Kovnir, K.; Armbrüster, M.; Teschner, D.; Venkov, T.V.; Jentoft, F.C.; Knop-Gericke, A.; Grin, Y.; Schlögl, R. A new approach to well-defined, stable and site-isolated catalysts. Sci. Technol. Adv. Mater. 2007, 8, 420–427. [Google Scholar] [CrossRef]
- Vilé, G.; Almora-Barrios, N.; Mitchell, S.; López, N.; Pérez-Ramírez, J. From the lindlar catalyst to supported ligand-modified palladium nanoparticles: Selectivity patterns and accessibility constraints in the continuous-flow three-phase hydrogenation of acetylenic compounds. Chem. Eur. J. 2014, 20, 5926–5937. [Google Scholar] [CrossRef] [PubMed]
- Witte, P.T.; Berben, P.H.; Boland, S.; Boymans, E.H.; Vogt, D.; Geus, J.W.; Donkervoort, J.G. Basf nanoselect™ technology: Innovative supported Pd- and Pt-based catalysts for selective hydrogenation reactions. Top. Catal. 2012, 55, 505–511. [Google Scholar] [CrossRef]
- Mori, A.; Mizusaki, T.; Miyakawa, Y.; Ohashi, E.; Haga, T.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Chemoselective hydrogenation method catalyzed by Pd/C using diphenylsulfide as a reasonable catalyst poison. Tetrahedron 2006, 62, 11925–11932. [Google Scholar] [CrossRef]
- Mori, A.; Mizusaki, T.; Kawase, M.; Maegawa, T.; Monguchi, Y.; Takao, S.; Takagi, Y.; Sajiki, H. Novel palladium-on-carbon/diphenyl sulfide complex for chemoselective hydrogenation: Preparation, characterization, and application. Adv. Synth. Catal. 2008, 350, 406–410. [Google Scholar] [CrossRef]
- McKenna, F.M.; Mantarosie, L.; Wells, R.P.K.; Hardacre, C.; Anderson, J.A. Selective hydrogenation of acetylene in ethylene rich feed streams at high pressure over ligand modified Pd/TiO2. Catal. Sci. Technol. 2012, 2, 632–638. [Google Scholar] [CrossRef]
- McKenna, F.-M.; Wells, R.P.K.; Anderson, J.A. Enhanced selectivity in acetylene hydrogenation by ligand modified Pd/TiO2 catalysts. Chem. Commun. 2011, 47, 2351–2353. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-J.; Moon, S.H. Modified Pd catalysts for the selective hydrogenation of acetylene. Catal. Today 2012, 185, 2–16. [Google Scholar] [CrossRef]
- Tauster, S.J.; Fung, S.C. Strong metal-support interactions: Occurrence among the binary oxides of groups IIA-VB. J. Catal. 1978, 55, 29–35. [Google Scholar] [CrossRef]
- Fleisch, T.H.; Hicks, R.F.; Bell, A.T. An xps study of metal-support interactions on PdSiO2 and PdLa2O3. J. Catal. 1984, 87, 398–413. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition. Sci. Rep. 2013, 3, 1775. [Google Scholar] [CrossRef]
- Yang, M.; Li, S.; Wang, Y.; Herron, J.A.; Xu, Y.; Allard, L.F.; Lee, S.; Huang, J.; Mavrikakis, M.; Flytzani-Stephanopoulos, M. Catalytically active Au-O(OH)x-species stabilized by alkali ions on zeolites and mesoporous oxides. Science 2014, 346, 1498. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Lu, N.; Xie, Z.; Wang, J.; Kim Moon, J.; Xia, Y. Synthesis of Pd-Rh core–frame concave nanocubes and their conversion to rh cubic nanoframes by selective etching of the pd cores. Angew. Chem. 2012, 124, 10412–10416. [Google Scholar] [CrossRef]
- Shao, M.; He, G.; Peles, A.; Odell, J.H.; Zeng, J.; Su, D.; Tao, J.; Yu, T.; Zhu, Y.; Xia, Y. Manipulating the oxygen reduction activity of platinum shells with shape-controlled palladium nanocrystal cores. Chem. Comm. 2013, 49, 9030–9032. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xie, S.; Liu, M.; Peng, H.-C.; Lu, N.; Wang, J.; Kim, M.J.; Xia, Y. On the role of surface diffusion in determining the shape or morphology of noble-metal nanocrystals. Proc. Natl. Acad. Sci. USA 2013, 110, 6669. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Choi, S.-I.; Lu, N.; Roling, L.T.; Herron, J.A.; Zhang, L.; Park, J.; Wang, J.; Kim, M.J.; Xie, Z.; et al. Atomic layer-by-layer deposition of Pt on Pd nanocubes for catalysts with enhanced activity and durability toward oxygen reduction. Nano Lett. 2014, 14, 3570–3576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Roling, L.T.; Wang, X.; Vara, M.; Chi, M.; Liu, J.; Choi, S.-I.; Park, J.; Herron, J.A.; Xie, Z.; et al. Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 2015, 349, 412. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Narayan, T.C.; Koh, A.L.; Dionne, J.A. In situ detection of hydrogen-induced phase transitions in individual palladium nanocrystals. Nat. Mater. 2014, 13, 1143. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.; Schmidt, M.; McGlacken, G.P.; O’Dwyer, C.; Holmes, J.D. Stability, oxidation, and shape evolution of PVP-capped Pd nanocrystals. J. Phys. Chem. C 2014, 118, 6522–6530. [Google Scholar] [CrossRef]
- Wu, J.; Helveg, S.; Ullmann, S.; Peng, Z.; Bell, A.T. Growth of encapsulating carbon on supported Pt nanoparticles studied by in situ tem. J. Catal. 2016, 338, 295–304. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, B.; Luo, J.; Zhang, L.; Chen, C.M.; Su Dang, S. Correlation between microstructure evolution of a well-defined cubic palladium catalyst and selectivity during acetylene hydrogenation. ChemCatChem 2017, 9, 3435–3439. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, C.; Lee, J.H.; Kim, J.; Lee, H.; Moon, S.H. Performance of shape-controlled pd nanoparticles in the selective hydrogenation of acetylene. J. Catal. 2013, 306, 146–154. [Google Scholar] [CrossRef]
- He, Y.; Fan, J.; Feng, J.; Luo, C.; Yang, P.; Li, D. Pd nanoparticles on hydrotalcite as an efficient catalyst for partial hydrogenation of acetylene: Effect of support acidic and basic properties. J. Catal. 2015, 331, 118–127. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Podyacheva, O.Y.; Richards, R.M. Influence of carbon nanomaterials on the properties of Pd/C catalysts in selective hydrogenation of acetylene. Mater. Res. Bull. 2017, 88, 78–84. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, J.; Wu, B.; Dai, W.; Chen, Z.; Ma, M. An alumina-coated, egg-shell Pd/α-Al2O3@SiC catalyst with enhanced ethylene selectivity in the selective hydrogenation of acetylene. RSC Adv. 2016, 6, 57174–57182. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Q.; Hou, N.; Fu, Y.; Wen, L.; Chen, J. Development of novel monolithic catalyst with porous hollow silica nanoparticles for selective hydrogenation reactions. Catal. Today 2013, 216, 205–210. [Google Scholar] [CrossRef]
- Song, H.; Yan, N.; Fei, Z.; Kilpin, K.J.; Scopelliti, R.; Li, X.; Dyson, P.J. Evaluation of ionic liquid soluble imidazolium tetrachloropalladate pre-catalysts in suzuki coupling reactions. Catal. Today 2012, 183, 172–177. [Google Scholar] [CrossRef]
- Yuan, X.; Sun, G.; Asakura, H.; Tanaka, T.; Chen, X.; Yuan, Y.; Laurenczy, G.; Kou, Y.; Dyson Paul, J.; Yan, N. Development of palladium surface-enriched heteronuclear Au–Pd nanoparticle dehalogenation catalysts in an ionic liquid. Chem. Eur. J. 2013, 19, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Fonseca, G.S.; Umpierre, A.P.; Fichtner, P.F.P.; Teixeira, S.R. Transition-metal nanoparticles in imidazolium ionic liquids: Recycable catalysts for biphasic hydrogenation reactions. J. Am. Chem. Soc. 2002, 124, 4228–4229. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jiang, T.; Han, B.; Gao, H.; Chang, Y.; Zhao, G.; Wu, W. Hydrogenation of olefins using ligand-stabilized palladium nanoparticles in an ionic liquid. Chem. Commun. 2003, 1654–1655. [Google Scholar] [CrossRef]
- Ruta, M.; Laurenczy, G.; Dyson, P.J.; Kiwi-Minsker, L. Pd nanoparticles in a supported ionic liquid phase: Highly stable catalysts for selective acetylene hydrogenation under continuous-flow conditions. J. Phys. Chem. C 2008, 112, 17814–17819. [Google Scholar] [CrossRef]
- Zhou, T.; Jang, K.; Jang, B.W.L. Ionic liquid and plasma effects on sio2 supported pd for selective hydrogenation of acetylene. Catal. Today 2013, 211, 147–155. [Google Scholar] [CrossRef]
- Xie, Y.B.; Liu, C.J. Stability of ionic liquids under the influence of glow discharge plasmas. Plasma Processes Polym. 2008, 5, 239–245. [Google Scholar] [CrossRef]
- Riyapan, S.; Boonyongmaneerat, Y.; Mekasuwandumrong, O.; Praserthdam, P.; Panpranot, J. Effect of surface Ti3+ on the sol-gel derived TiO2 in the selective acetylene hydrogenation on Pd/TiO2 catalysts. Catal. Today 2015, 245, 134–138. [Google Scholar] [CrossRef]
- Riyapan, S.; Boonyongmaneerat, Y.; Mekasuwandumrong, O.; Yoshida, H.; Fujita, S.-I.; Arai, M.; Panpranot, J. Improved catalytic performance of Pd/TiO2 in the selective hydrogenation of acetylene by using h2-treated sol-gel tio2. J. Mol. Catal. A Chem. 2014, 383–384, 182–187. [Google Scholar] [CrossRef]
- McCue, A.J.; McKenna, F.-M.; Anderson, J.A. Triphenylphosphine: A ligand for heterogeneous catalysis too? Selectivity enhancement in acetylene hydrogenation over modified Pd/TiO2 catalyst. Catal. Sci. Technol. 2015, 5, 2449–2459. [Google Scholar] [CrossRef]
- Kim, E.; Shin, E.W.; Bark, C.W.; Chang, I.; Yoon, W.J.; Kim, W.-J. Pd catalyst promoted by two metal oxides with different reducibilities: Properties and performance in the selective hydrogenation of acetylene. Appl. Catal. A General 2014, 471, 80–83. [Google Scholar] [CrossRef]
- Hou, R.; Wang, T.; Lan, X. Enhanced selectivity in the hydrogenation of acetylene due to the addition of a liquid phase as a selective solvent. Ind. Eng. Chem. Res. 2013, 52, 13305–13312. [Google Scholar] [CrossRef]
- Smirnova, N.S.; Mironenko, O.O.; Shlyapin, D.A.; Kochubey, D.I.; Tsyrulnikov, P.G. Exafs study of model Pd/Ga2O3 catalysts for the liquid-phase hydrogenation of acetylene before and after the reaction. Bull. Russ. Acad. Sci. Phys. 2013, 77, 1151–1153. [Google Scholar] [CrossRef]
- Smirnova, N.S.; Shlyapin, D.A.; Shitova, N.B.; Kochubey, D.I.; Tsyrul’nikov, P.G. Exafs study of Pd/Sibunit and Pd-Ga/Sibunit catalysts for liquid-phase hydrogenation of acetylene to ethylene. J. Mol. Catal. A Chem. 2015, 403, 10–14. [Google Scholar] [CrossRef]
- Afonasenko, T.N.; Smirnova, N.S.; Temerev, V.L.; Leont’eva, N.N.; Gulyaeva, T.I.; Tsyrul’nikov, P.G. Pd/Ga2O3-AL2O3 catalysts for the selective liquid-phase hydrogenation of acetylene to ethylene. Kinet. Catal. 2016, 57, 490–496. [Google Scholar] [CrossRef]
- He, Y.; Liang, L.; Liu, Y.; Feng, J.; Ma, C.; Li, D. Partial hydrogenation of acetylene using highly stable dispersed bimetallic Pd-Ga/MgO-AL2O3 catalyst. J. Catal. 2014, 309, 166–173. [Google Scholar] [CrossRef]
- Zimmermann René, R.; Hahn, T.; Reschetilowski, W.; Armbrüster, M. Kinetic parameters for the selective hydrogenation of acetylene on GaPd2 and GaPd. Chem. Phys. Chem. 2017, 18, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Siebert, M.; Zimmermann René, R.; Armbrüster, M.; Dittmeyer, R. Inkjet printing of GaPd2 into micro-channels for the selective hydrogenation of acetylene. Chem. Catal. Chem. 2017, 9, 3733–3742. [Google Scholar] [CrossRef]
- Jin, Q.; He, Y.; Miao, M.; Guan, C.; Du, Y.; Feng, J.; Li, D. Highly selective and stable pdni catalyst derived from layered double hydroxides for partial hydrogenation of acetylene. Appl. Catal. A General 2015, 500, 3–11. [Google Scholar] [CrossRef]
- Ding, L.; Yi, H.; Zhang, W.; You, R.; Cao, T.; Yang, J.; Lu, J.; Huang, W. Activating edge sites on pd catalysts for selective hydrogenation of acetylene via selective Ga2O3 decoration. ACS Catal. 2016, 6, 3700–3707. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, X.; Wang, A.; Miao, S.; Liu, X.; Pan, X.; Su, Y.; Li, L.; Tan, Y.; Zhang, T. Pd/ZnO catalysts with different origins for high chemoselectivity in acetylene semi-hydrogenation. Chin. J. Catal. 2016, 37, 692–699. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, X.; Li, L.; Liu, X.; Huang, Y.; Pan, X.; Wang, A.; Li, J.; Zhang, T. PdZn intermetallic nanostructure with Pd-Zn-Pd ensembles for highly active and chemoselective semi-hydrogenation of acetylene. ACS Catal. 2016, 6, 1054–1061. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, F.; Lu, H.; Hong, X.; Jiang, H.; Wu, Y.; Li, Y. Hollow Zn/Co ZIF particles derived from core-shell ZIF-67@ZIF-8 as selective catalyst for the semi-hydrogenation of acetylene. Angew. Chem. Int. Ed. 2015, 54, 10889–10893. [Google Scholar] [CrossRef] [PubMed]
- Menezes, W.G.; Altmann, L.; Zielasek, V.; Thiel, K.; Bäumer, M. Bimetallic Co-Pd catalysts: Study of preparation methods and their influence on the selective hydrogenation of acetylene. J. Catal. 2013, 300, 125–135. [Google Scholar] [CrossRef]
- Pei, G.X.; Liu, X.Y.; Wang, A.; Li, L.; Huang, Y.; Zhang, T.; Lee, J.W.; Jang, B.W.L.; Mou, C.-Y. Promotional effect of Pd single atoms on Au nanoparticles supported on silica for the selective hydrogenation of acetylene in excess ethylene. New J. Chem. 2014, 38, 2043–2051. [Google Scholar] [CrossRef]
- Pongthawornsakun, B.; Mekasuwandumrong, O.; Santos Aires, F.J.C.; Büchel, R.; Baiker, A.; Pratsinis, S.E.; Panpranot, J. Variability of particle configurations achievable by 2-nozzle flame syntheses of the Au-Pd-TiO2 system and their catalytic behaviors in the selective hydrogenation of acetylene. Appl. Catal. A General 2018, 549, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Diao, W.; Williams, C.T.; Monnier, J.R. Selective hydrogenation of acetylene in excess ethylene using Ag- and Au-Pd/SiO2 bimetallic catalysts prepared by electroless deposition. Appl. Catal. A General 2014, 469, 419–426. [Google Scholar] [CrossRef]
- Komeili, S.; Ravanchi, M.T.; Taeb, A. The influence of alumina phases on the performance of the Pd–Ag/Al2O3 catalyst in tail-end selective hydrogenation of acetylene. Appl. Catal. A General 2015, 502, 287–296. [Google Scholar] [CrossRef]
- Liu, Y.N.; Feng, J.T.; He, Y.F.; Sun, J.H.; Li, D.Q. Partial hydrogenation of acetylene over a niti-layered double hydroxide supported pdag catalyst. Catal. Sci. Technol. 2015, 5, 1231–1240. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Y.; Jing, F.; Luo, J.; Huang, Q.; Chu, W. Layered double hydroxides derived ZnO-Al2O3 supported Pd-Ag catalysts for selective hydrogenation of acetylene. Chin. J. Chem. 2017, 35, 1009–1015. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; He, Y.; Feng, J.; Wu, T.; Li, D. Highly efficient PdAg catalyst using a reducible Mg-Ti mixed oxide for selective hydrogenation of acetylene: Role of acidic and basic sites. J. Catal. 2017, 348, 135–145. [Google Scholar] [CrossRef]
- Pei, G.X.; Liu, X.Y.; Wang, A.; Lee, A.F.; Isaacs, M.A.; Li, L.; Pan, X.; Yang, X.; Wang, X.; Tai, Z.; et al. Ag alloyed Pd single-atom catalysts for efficient selective hydrogenation of acetylene to ethylene in excess ethylene. ACS Catal. 2015, 5, 3717–3725. [Google Scholar] [CrossRef]
- McCue, A.J.; McRitchie, C.J.; Shepherd, A.M.; Anderson, J.A. Cu/Al2O3 catalysts modified with Pd for selective acetylene hydrogenation. J. Catal. 2014, 319, 127–135. [Google Scholar] [CrossRef]
- Kruppe, C.M.; Krooswyk, J.D.; Trenary, M. Selective hydrogenation of acetylene to ethylene in the presence of a carbonaceous surface layer on a Pd/Cu(111) single-atom alloy. ACS Catal. 2017, 7, 8042–8049. [Google Scholar] [CrossRef]
- Pei, G.X.; Liu, X.Y.; Yang, X.; Zhang, L.; Wang, A.; Li, L.; Wang, H.; Wang, X.; Zhang, T. Performance of Cu-alloyed Pd single-atom catalyst for semihydrogenation of acetylene under simulated front-end conditions. ACS Catal. 2017, 7, 1491–1500. [Google Scholar] [CrossRef]
- McCue, A.J.; Shepherd, A.M.; Anderson, J.A. Optimisation of preparation method for Pd doped Cu/Al2O3 catalysts for selective acetylene hydrogenation. Catal. Sci. Technol. 2015, 5, 2880–2890. [Google Scholar] [CrossRef]
- Pei, G.; Liu, X.; Chai, M.; Wang, A.; Zhang, T. Isolation of Pd atoms by Cu for semi-hydrogenation of acetylene: Effects of cu loading. Chin. J. Catal. 2017, 38, 1540–1548. [Google Scholar] [CrossRef]
- Kuznetsov, D.A.; Bazhenova, T.A.; Fedyanin, I.V.; Martynenko, V.M.; Shestakov, A.F.; Petrova, G.N.; Komarova, N.Y.S. Tri-, tetra-, and hexanuclear mixed-valence molybdenum clusters: Structural diversity and catalysis of acetylene hydrogenation. Dalton Trans. 2016, 45, 16309–16316. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wheeler, J.; Jang, B.; Lin, W.-Y.; Zhao, B. Stable Au catalysts for selective hydrogenation of acetylene in ethylene. Appl. Catal. A General 2014, 487, 36–44. [Google Scholar] [CrossRef]
- Albani, D.; Capdevila-Cortada, M.; Vilé, G.; Mitchell, S.; Martin, O.; López, N.; Pérez-Ramírez, J. Semihydrogenation of acetylene on indium oxide: Proposed single-ensemble catalysis. Angew. Chem. 2017, 129, 10895–10900. [Google Scholar] [CrossRef]
- Spanjers, C.S.; Held, J.T.; Jones, M.J.; Stanley, D.D.; Sim, R.S.; Janik, M.J.; Rioux, R.M. Zinc inclusion to heterogeneous nickel catalysts reduces oligomerization during the semi-hydrogenation of acetylene. J. Catal. 2014, 316, 164–173. [Google Scholar] [CrossRef]
- Pickett, C.J. The chatt cycle and the mechanism of enzymic reduction of molecular nitrogen. J. Biol. Inorg. Chem. 1996, 1, 601–606. [Google Scholar] [CrossRef]
- Lee, S.C.; Holm, R.H. The clusters of nitrogenase: Synthetic methodology in the construction of weak-field clusters. Chem. Rev. 2004, 104, 1135–1158. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.C.; Dean, D.R.; Hu, Y.; Ribbe, M.W. Formation and insertion of the nitrogenase iron−molybdenum cofactor. Chem. Rev. 2004, 104, 1159–1174. [Google Scholar] [CrossRef] [PubMed]
- Crossland, J.L.; Tyler, D.R. Iron–dinitrogen coordination chemistry: Dinitrogen activation and reactivity. Coord. Chem. Rev. 2010, 254, 1883–1894. [Google Scholar] [CrossRef]
- Burgess, B.K.; Lowe, D.J. Mechanism of molybdenum nitrogenase. Chem. Rev. 1996, 96, 2983–3012. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.-P.; Quadrelli, E.A. Mechanistic aspects of dinitrogen cleavage and hydrogenation to produce ammonia in catalysis and organometallic chemistry: Relevance of metal hydride bonds and dihydrogen. Chem. Soc. Rev. 2014, 43, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Barrière, F. Modeling of the molybdenum center in the nitrogenase femo-cofactor. Coord. Chem. Rev. 2003, 236, 71–89. [Google Scholar] [CrossRef]
- Yoshimoto, K.; Yatabe, T.; Matsumoto, T.; Tran, V.-H.; Robertson, A.; Nakai, H.; Asazawa, K.; Tanaka, H.; Ogo, S. Inorganic clusters with a [Fe2MoOS3] core - a functional model for acetylene reduction by nitrogenases. Dalton Trans. 2016, 45, 14620–14627. [Google Scholar] [CrossRef] [PubMed]
- Tejeda-Serrano, M.; Cabrero-Antonino, J.R.; Mainar-Ruiz, V.; López-Haro, M.; Hernández-Garrido, J.C.; Calvino, J.J.; Leyva-Pérez, A.; Corma, A. Synthesis of supported planar iron oxide nanoparticles and their chemo- and stereoselectivity for hydrogenation of alkynes. ACS Catal. 2017, 7, 3721–3729. [Google Scholar] [CrossRef]
- Altmann, L.; Wang, X.; Stöver, J.; Klink, M.; Zielasek, V.; Thiel, K.; Kolny-Olesiak, J.; Al-Shamery, K.; Borchert, H.; Parisi, J.; et al. Impact of organic ligands on the structure and hydrogenation performance of colloidally prepared bimetallic ptsn nanoparticles. ChemCatChem 2013, 5, 1803–1810. [Google Scholar] [CrossRef]
- Lee Jonathan, W.; Liu, X.; Mou, C.Y. Selective hydrogenation of acetylene over SBA-15 supported Au-Cu bimetallic catalysts. J. Chin. Chem. Soc. 2013, 60, 907–914. [Google Scholar]
- Primo, A.; Neatu, F.; Florea, M.; Parvulescu, V.; Garcia, H. Graphenes in the absence of metals as carbocatalysts for selective acetylene hydrogenation and alkene hydrogenation. Nat. Commun. 2014, 5, 5291. [Google Scholar] [CrossRef] [PubMed]
- Bychko, I.B.; Abakumov, A.A.; Lemesh, N.V.; Strizhak, P.E. Catalytic activity of multiwalled carbon nanotubes in acetylene hydrogenation. Chem. Catal. Chem. 2017, 9, 4470–4474. [Google Scholar] [CrossRef]
- Hutchings, G.J. Vapor phase hydrochlorination of acetylene: Correlation of catalytic activity of supported metal chloride catalysts. J. Catal. 1985, 96, 292–295. [Google Scholar] [CrossRef]
- Conte, M.; Carley, A.F.; Hutchings, G.J. Reactivation of a carbon-supported gold catalyst for the hydrochlorination of acetylene. Catal. Lett. 2008, 124, 165–167. [Google Scholar] [CrossRef]
- Conte, M.; Davies, C.J.; Morgan, D.J.; Davies, T.E.; Elias, D.J.; Carley, A.F.; Johnston, P.; Hutchings, G.J. Aqua regia activated Au/C catalysts for the hydrochlorination of acetylene. J. Catal. 2013, 297, 128–136. [Google Scholar] [CrossRef]
- Conte, M.; Davies, C.J.; Morgan, D.J.; Davies, T.E.; Carley, A.F.; Johnston, P.; Hutchings, G.J. Modifications of the metal and support during the deactivation and regeneration of au/c catalysts for the hydrochlorination of acetylene. Catal. Sci. Technol. 2013, 3, 128–134. [Google Scholar] [CrossRef]
- Conte, M.; Davies, C.J.; Morgan, D.J.; Carley, A.F.; Johnston, P.; Hutchings, G.J. Characterization of Au3+ species in Au/C catalysts for the hydrochlorination reaction of acetylene. Catal. Lett. 2014, 144, 1–8. [Google Scholar] [CrossRef]
- Chao, S.; Guan, Q.; Li, W. Study of the active site for acetylene hydrochlorination in AuCl3/C catalysts. J. Catal. 2015, 330, 273–279. [Google Scholar] [CrossRef]
- Li, X.; Zhu, M.; Dai, B. AuCl3 on polypyrrole-modified carbon nanotubes as acetylene hydrochlorination catalysts. Appl. Catal. B: Environmental 2013, 142–143, 234–240. [Google Scholar] [CrossRef]
- li, X.; Pan, X.; Bao, X. Nitrogen Doped Carbon Catalyzing Acetylene Conversion to Vinyl Chloride. J. Energy Chem. 2014, 23, 131–135. [Google Scholar]
- Zhou, K.; Si, J.; Jia, J.; Huang, J.; Zhou, J.; Luo, G.; Wei, F. Reactivity enhancement of N-CNTs in green catalysis of C2H2 hydrochlorination by a cu catalyst. RSC Adv. 2014, 4, 7766–7769. [Google Scholar] [CrossRef]
- Li, X.; Pan, X.; Yu, L.; Ren, P.; Wu, X.; Sun, L.; Jiao, F.; Bao, X. Silicon carbide-derived carbon nanocomposite as a substitute for mercury in the catalytic hydrochlorination of acetylene. Nat. Commun. 2014, 5, 3688. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Chen, K.; Wang, Y.; Kang, L.; Zhu, M. Boron and nitrogen doping in graphene for the catalysis of acetylene hydrochlorination. ACS Catal. 2015, 5, 2541–2547. [Google Scholar] [CrossRef]
- Li, J.; Hu, J.; Li, G. Au(III)/N-containing ligand complex: A novel and efficient catalyst in carbonylation of alkyl nitrite. Catal. Comm. 2011, 12, 1401–1404. [Google Scholar] [CrossRef]
- Huang, C.; Zhu, M.; Kang, L.; Dai, B. A novel high-stability Au(III)/schiff-based catalyst for acetylene hydrochlorination reaction. Catal. Commun. 2014, 54, 61–65. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, B.; Wang, X.; Li, W.; Han, Y.; Gu, J.; Zhang, J. Non-mercury catalytic acetylene hydrochlorination over bimetallic Au-Co(III)/SAC catalysts for vinyl chloride monomer production. Green Chem. 2013, 15, 829–836. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Xu, J.; Ni, J.; Zhang, T.; Xu, X.; Li, X. Activated-carbon-supported gold-cesium(I) as highly effective catalysts for hydrochlorination of acetylene to vinyl chloride. Chem. Plus. Chem. 2014, 80, 196–201. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, T.; Di, X.; Xu, J.; Xu, J.; Feng, F.; Ni, J.; Li, X. Nitrogen-modified activated carbon supported bimetallic gold-cesium(I) as highly active and stable catalyst for the hydrochlorination of acetylene. RSC Adv. 2015, 5, 6925–6931. [Google Scholar] [CrossRef]
- Zhao, J.; Gu, S.; Xu, X.; Zhang, T.; Di, X.; Pan, Z.; Li, X. Promotional effect of copper(II) on an activated carbon supported low content bimetallic gold-cesium(I) catalyst in acetylene hydrochlorination. RSC Adv. 2015, 5, 101427–101436. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, B.; Li, W.; Wang, X.; Zhang, J.; Zhu, M.; Gu, J. Non-mercury catalytic acetylene hydrochlorination over spherical activated-carbon-supported Au-Co(III)-Cu(II) catalysts. J. Catal. 2014, 316, 141–148. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Wang, J.; Tang, X.; Zhao, Y.; Yang, D.; Jia, F.; Hao, T. Hydrochlorination of acetylene to vinyl chloride over Pd supported on zeolite y. React. Kinet. Mech. Catal. 2013, 110, 187–194. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Wang, J. Catalytic properties of Pd/HY catalysts modified with NH4F for acetylene hydrochlorination. Catal. Comm. 2015, 65, 41–45. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Wang, J. Non-mercury catalytic acetylene hydrochlorination over a NH4F-urea-modified Pd/HY catalyst for vinyl chloride monomer production. New J. Chem. 2016, 40, 3019–3023. [Google Scholar] [CrossRef]
- Zhu, M.; Kang, L.; Su, Y.; Zhang, S.; Dai, B. MClx (M = Hg, Au, Ru; x = 2, 3) catalyzed hydrochlorination of acetylene—A density functional theory study. Can. J. Chem. 2013, 91, 120–125. [Google Scholar] [CrossRef]
- Zhang, J.; Sheng, W.; Guo, C.; Li, W. Acetylene hydrochlorination over bimetallic Ru-based catalysts. RSC Adv. 2013, 3, 21062–21068. [Google Scholar] [CrossRef]
- Li, G.; Li, W.; Zhang, H.; Pu, Y.; Sun, M.; Zhang, J. Non-mercury catalytic acetylene hydrochlorination over ru catalysts enhanced by carbon nanotubes. RSC Adv. 2015, 5, 9002–9008. [Google Scholar] [CrossRef]
- Jin, Y.; Li, G.; Zhang, J.; Pu, Y.; Li, W. Effects of potassium additive on the activity of Ru catalyst for acetylene hydrochlorination. RSC Adv. 2015, 5, 37774–37779. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, J.; Zhang, T.; Di, X.; Gu, S.; Ni, J.; Li, X. Ultra-low Ru-promoted CuCl2 as highly active catalyst for the hydrochlorination of acetylene. RSC Adv. 2015, 5, 38159–38163. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, J.; Pu, Y.; Li, W. Effects of nitrogen-dopants on Ru-supported catalysts for acetylene hydrochlorination. RSC Adv. 2016, 6, 18026–18032. [Google Scholar] [CrossRef]
- Xu, N.; Zhu, M.; Zhang, J.; Zhang, H.; Dai, B. Nitrogen functional groups on an activated carbon surface to effect the ruthenium catalysts in acetylene hydrochlorination. RSC Adv. 2015, 5, 86172–86178. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Y.; Li, W.; Han, Y.; Zhang, J. Improvement of imidazolium-based ionic liquids on the activity of ruthenium catalyst for acetylene hydrochlorination. Mo. Catal. 2017, 443, 220–227. [Google Scholar] [CrossRef]
- Ananikov, V.P.; Khemchyan, L.L.; Yu, V.I.; Bukhtiyarov, V.I.; Sorokin, A.M.; Prosvirin, I.P.; Vatsadze, S.Z.; Medved’ko, A.V.; Nuriev, V.N.; Dilman, A.D.; et al. Development of new methods in modern selective organic synthesis: Preparation of functionalized molecules with atomic precision. Russ. Chem. Rev. 2014, 83, 885. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Gold, B. “Two functional groups in one package”: Using both alkyne π-bonds in cascade transformations. J. Org. Chem. 2013, 78, 7777–7784. [Google Scholar] [CrossRef] [PubMed]
- Alabugin, I.V.; Gonzalez-Rodriguez, E. Alkyne origami: Folding oligoalkynes into polyaromatics. Acc. Chem. Res. 2018, 51, 1206–1219. [Google Scholar] [CrossRef] [PubMed]
- Byers, P.M.; Alabugin, I.V. Polyaromatic ribbons from oligo-alkynes via selective radical cascade: Stitching aromatic rings with polyacetylene bridges. J. Am. Chem. Soc. 2012, 134, 9609–9614. [Google Scholar] [CrossRef] [PubMed]
- Byers, P.M.; Rashid, J.I.; Mohamed, R.K.; Alabugin, I.V. Polyaromatic ribbon/benzofuran fusion via consecutive endo cyclizations of enediynes. Org. Lett. 2012, 14, 6032–6035. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, D.; Protti, S.; Fagnoni, M. Carbon–carbon bond forming reactions via photogenerated intermediates. Chem. Rev. 2016, 116, 9850–9913. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Naredla, R.R.; Thompson, S.K.; Hoye, T.R. The pentadehydro-diels–alder reaction. Nature 2016, 532, 484. [Google Scholar] [CrossRef] [PubMed]
- Roland, C.D.; Li, H.; Abboud, K.A.; Wagener, K.B.; Veige, A.S. Cyclic polymers from alkynes. Nat. Chem. 2016, 8, 791. [Google Scholar] [CrossRef] [PubMed]
- Garcia Bernal, J.M.; Tirado, M.M.; Freire, J.J.; Garcia de la Torre, J. Monte carlo calculation of hydrodynamic properties of linear and cyclic polymers in good solvents. Macromolecules 1991, 24, 593–598. [Google Scholar] [CrossRef]
- Bannister, D.J.; Semlyen, J.A. Studies of cyclic and linear poly(dimethyl siloxanes): 6. Effect of heat. Polymer 1981, 22, 377–381. [Google Scholar] [CrossRef]
- Orrah, D.J.; Semlyen, J.A.; Ross-Murphy, S.B. Studies of cyclic and linear poly(dimethylsiloxanes): 28. Viscosities and densities of ring and chain poly(dimethylsiloxane) blends. Polymer 1988, 29, 1455–1458. [Google Scholar] [CrossRef]
- Clarson, S.J.; Dodgson, K.; Semlyen, J.A. Studies of cyclic and linear poly(dimethylsiloxanes): 19. Glass transition temperatures and crystallization behaviour. Polymer 1985, 26, 930–934. [Google Scholar] [CrossRef]
- Griffiths, P.C.; Stilbs, P.; Yu, G.E.; Booth, C. Role of molecular architecture in polymer diffusion: A PGSE-NMR study of linear and cyclic poly(ethylene oxide). J. Phys. Chem. 1995, 99, 16752–16756. [Google Scholar] [CrossRef]
- Patel, A.; Cosgrove, T.; Semlyen, J.A. Studies of cyclic and linear poly(dimethylsiloxanes): 30. Adsorption studies on silica in solution. Polymer 1991, 32, 1313–1317. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. Alkynes as a versatile platform for construction of chemical molecular complexity and realization of molecular 3D printing. Russ. Chem. Rev. 2016, 85, 226. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

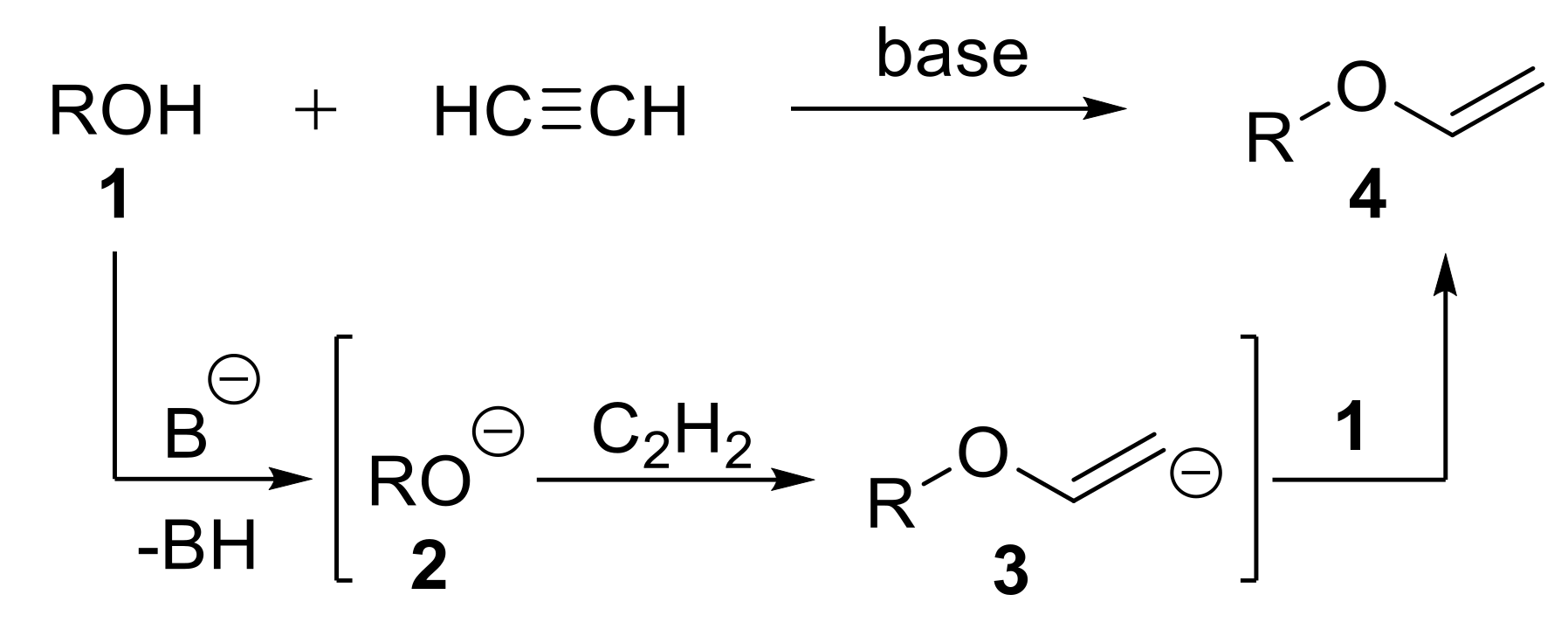
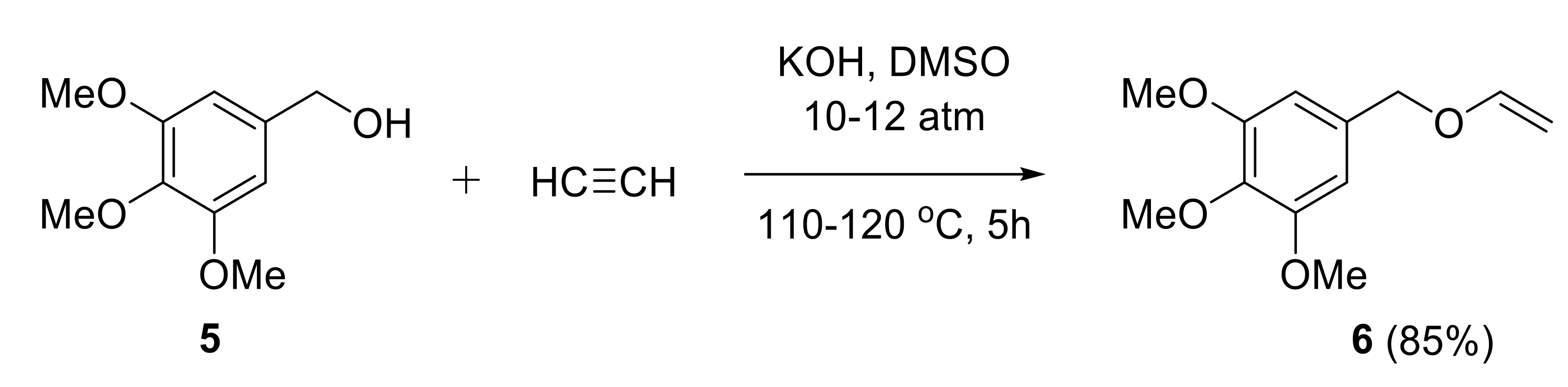

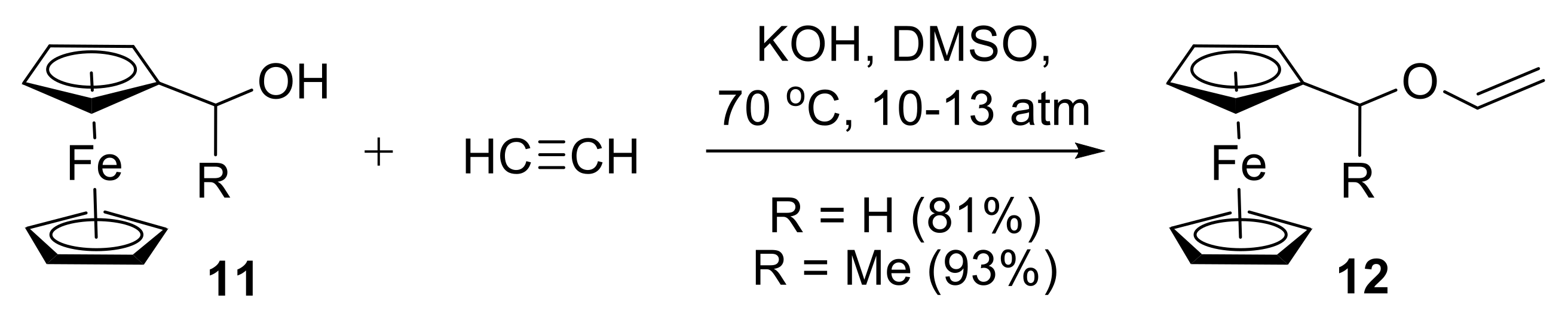

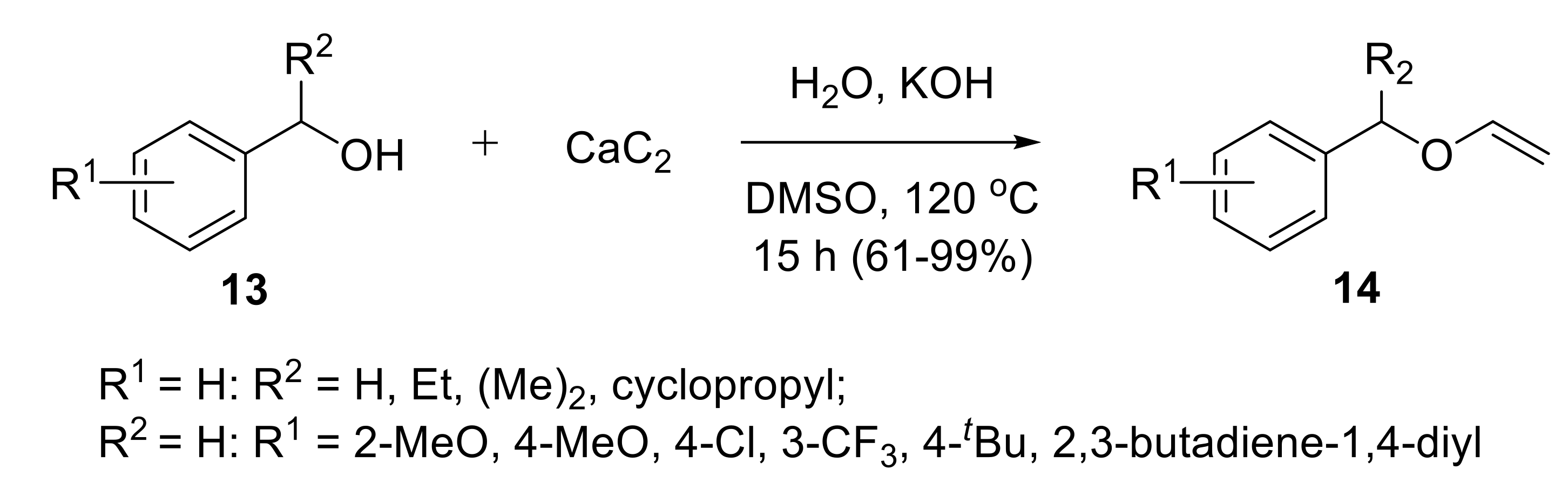

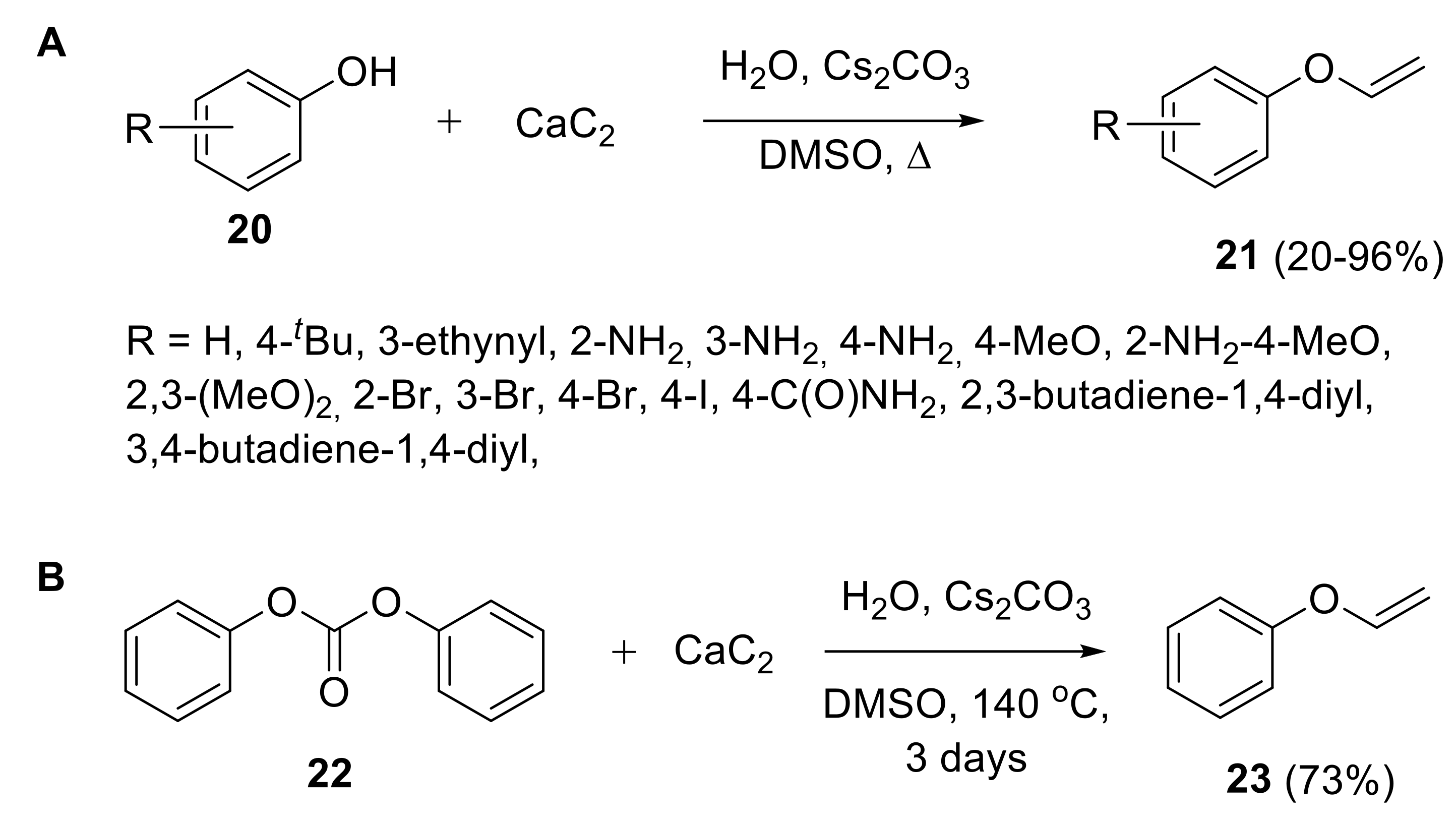
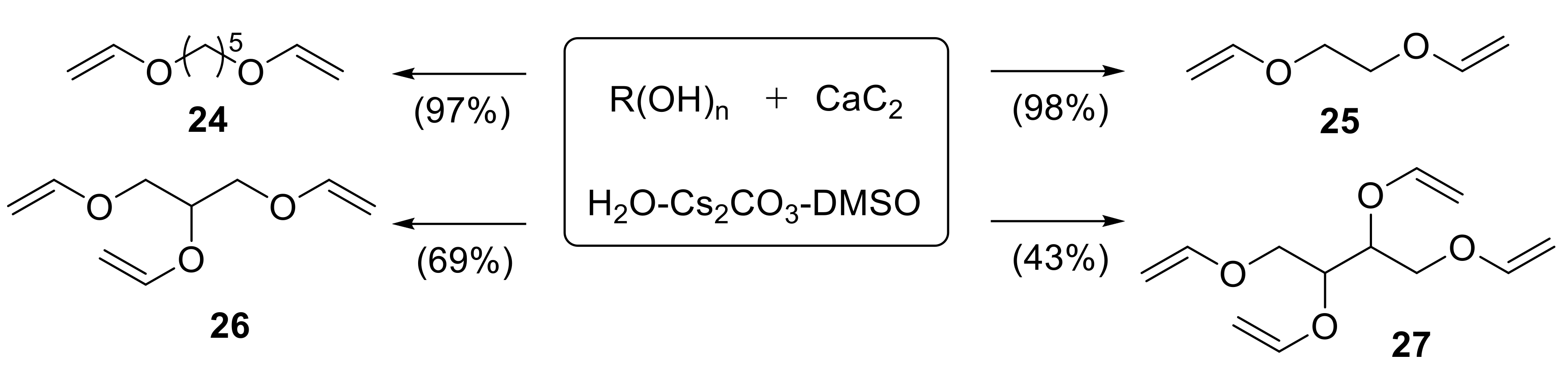
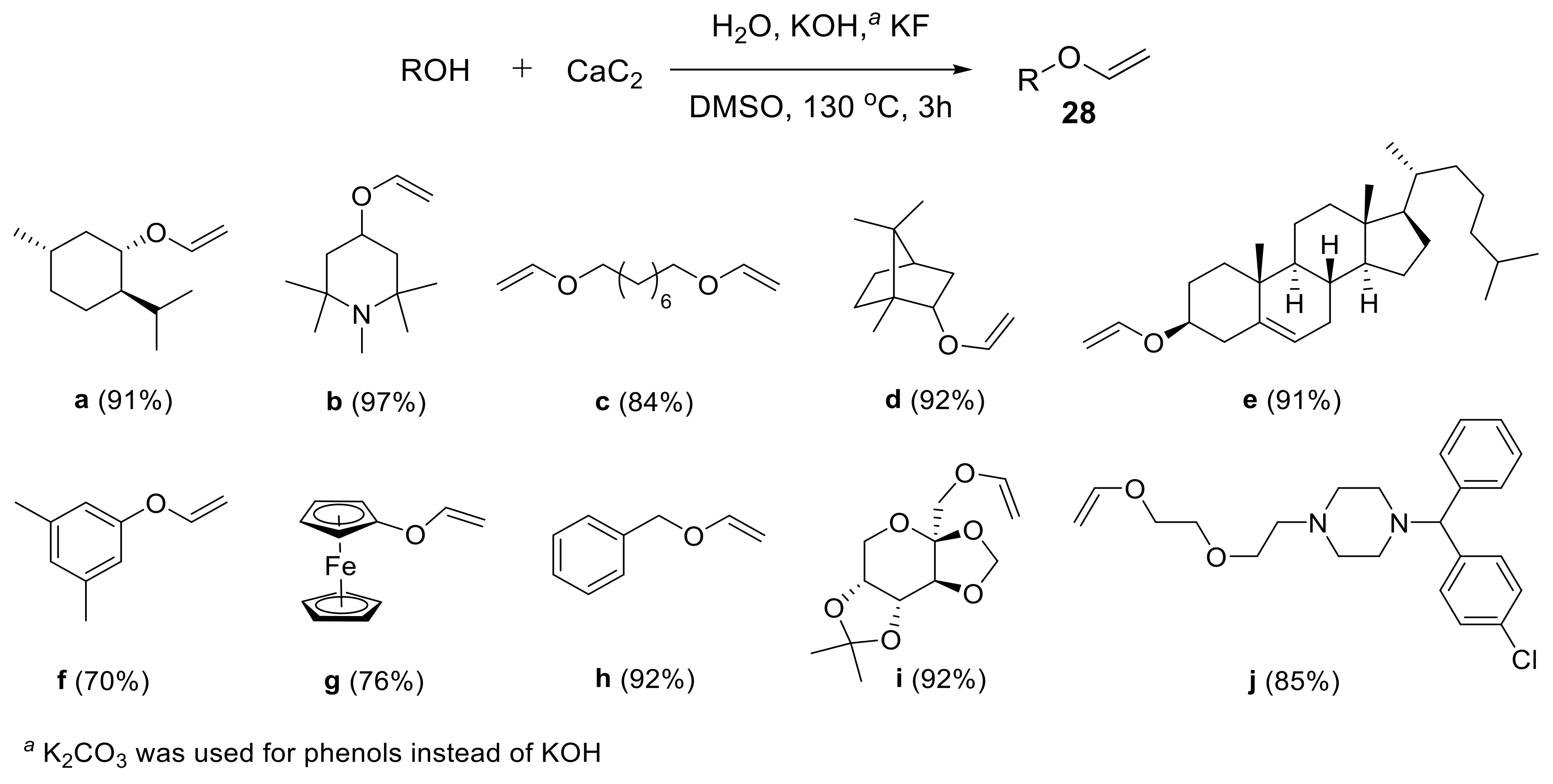

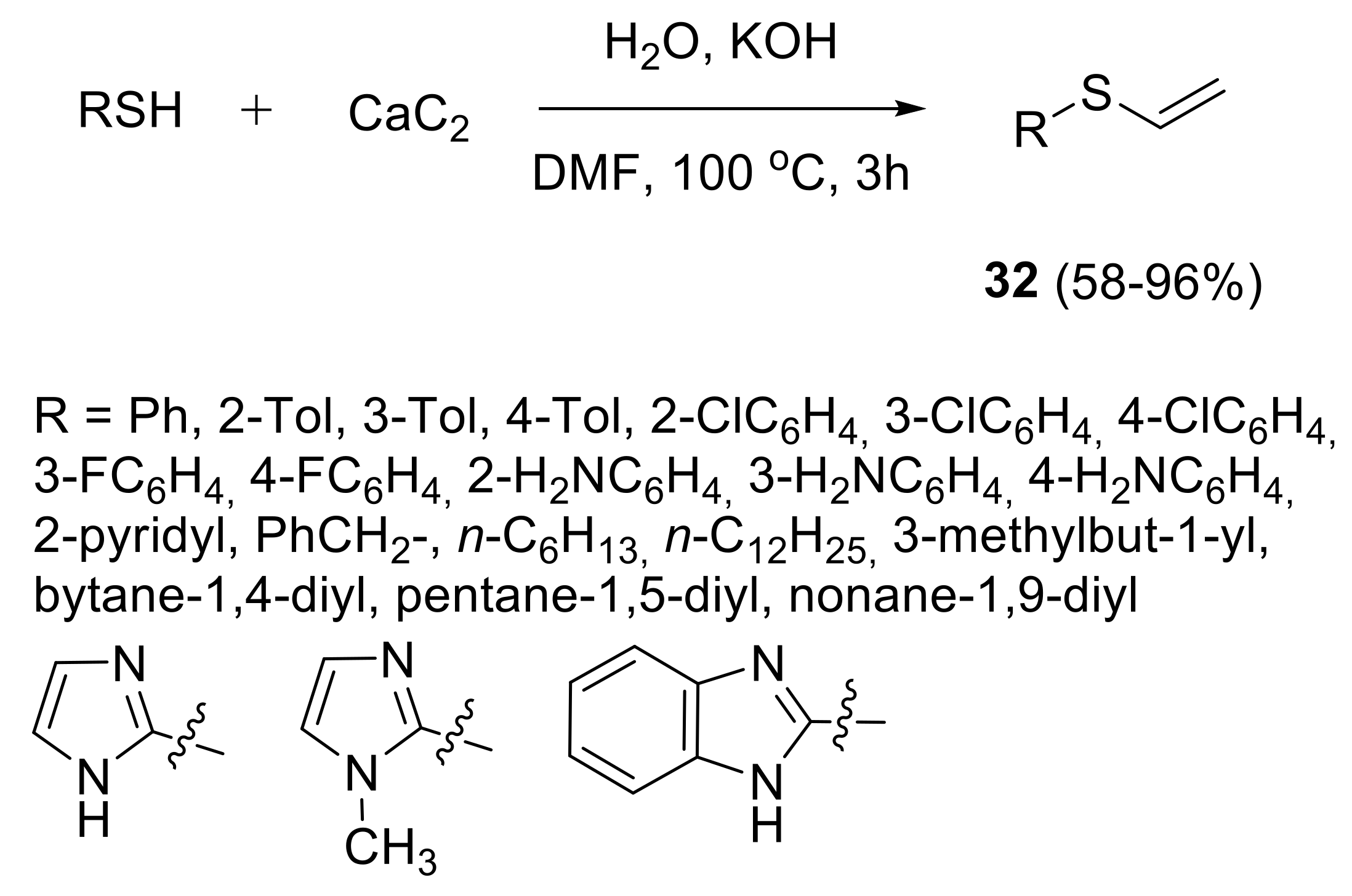
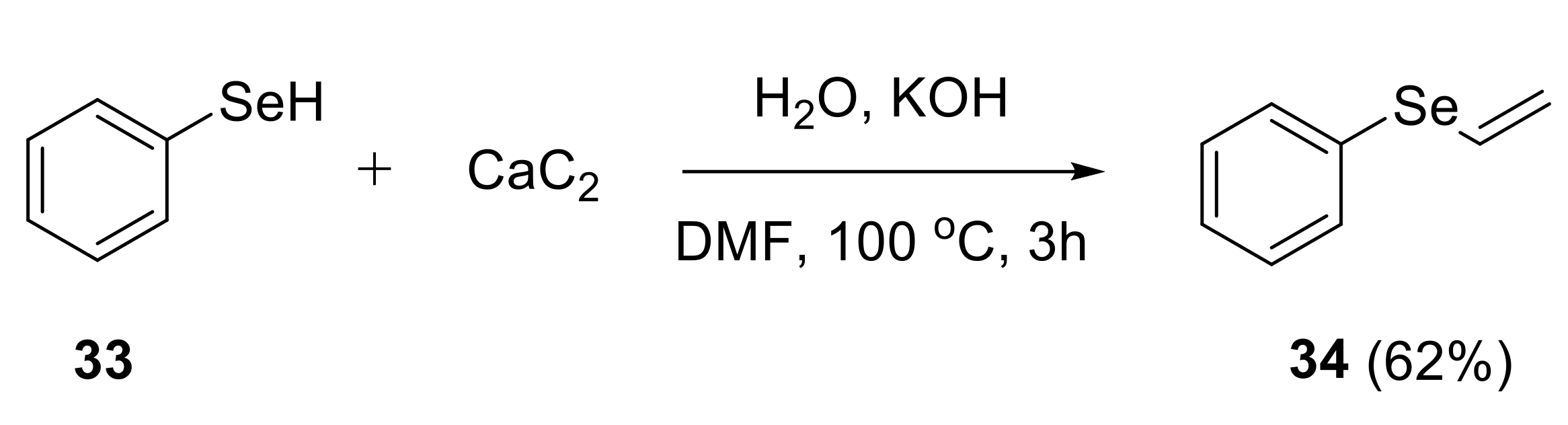

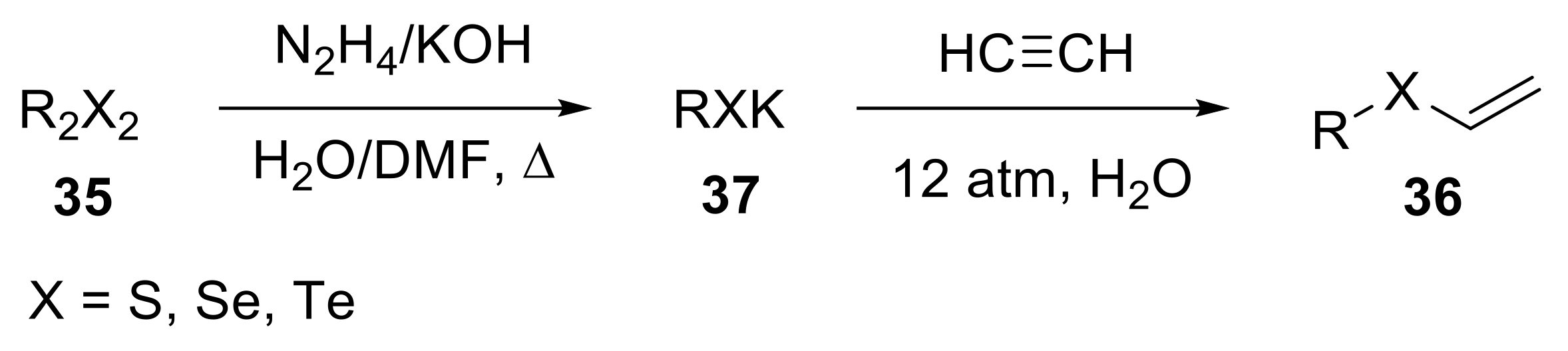
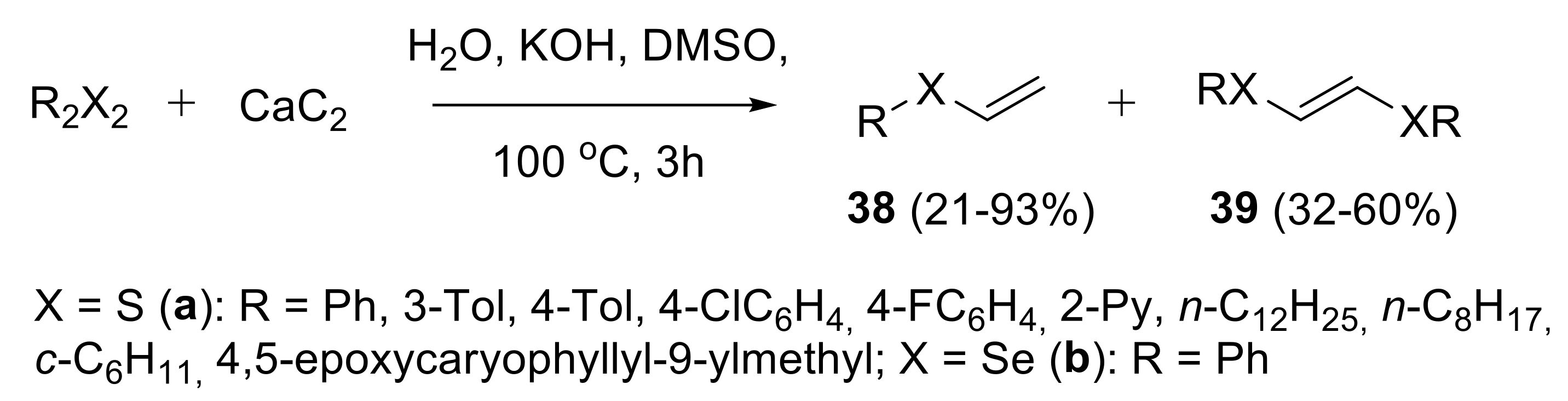
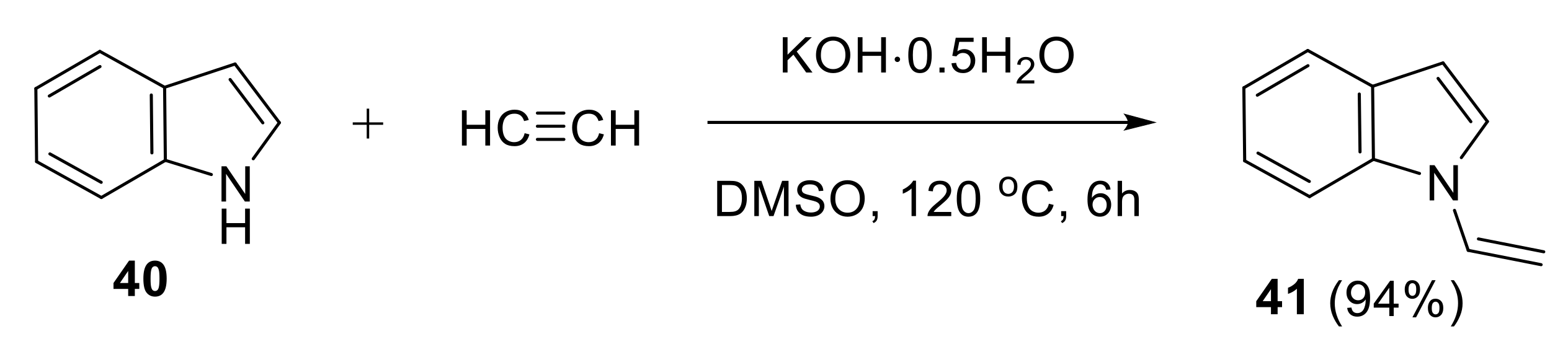
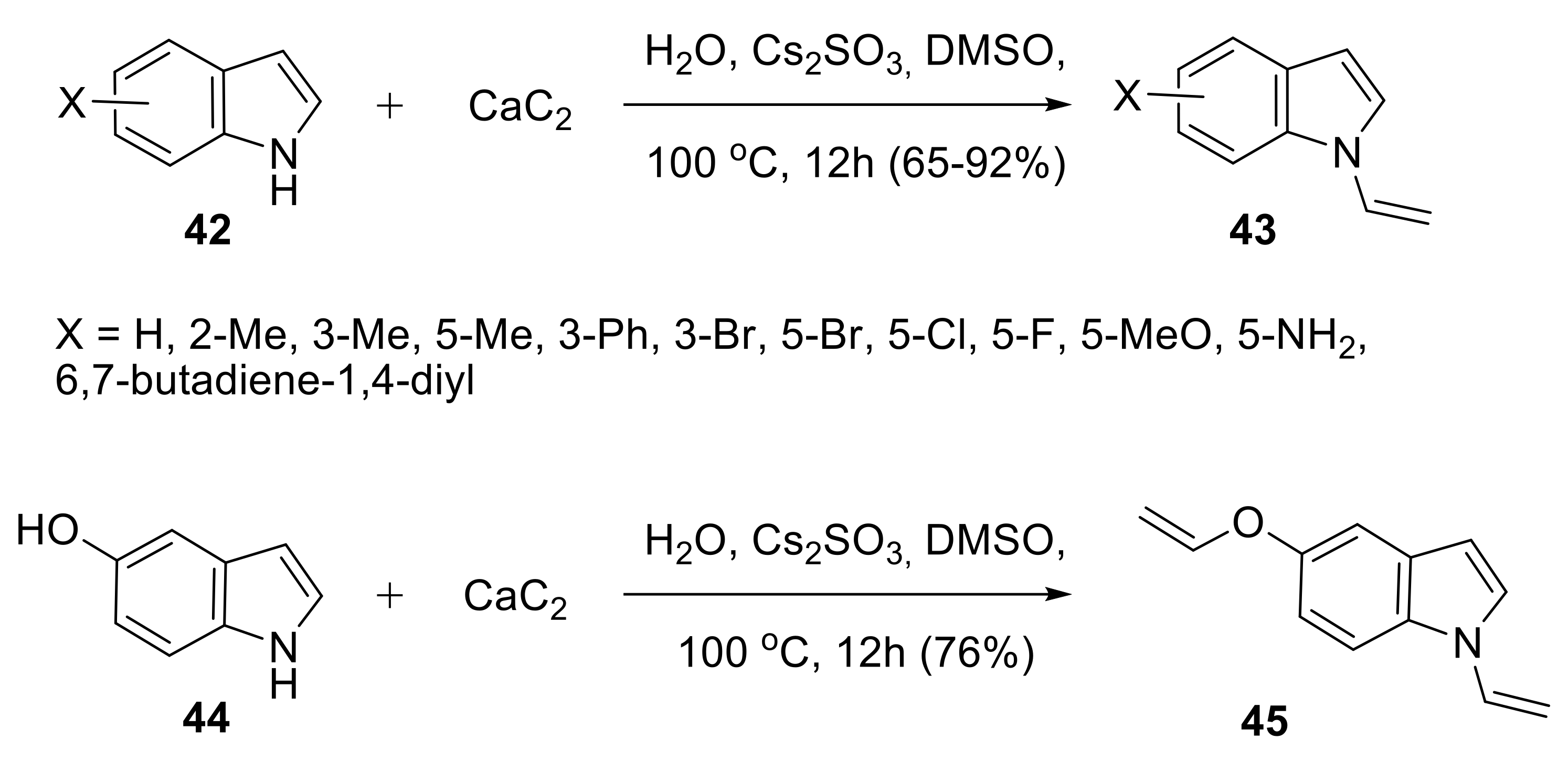
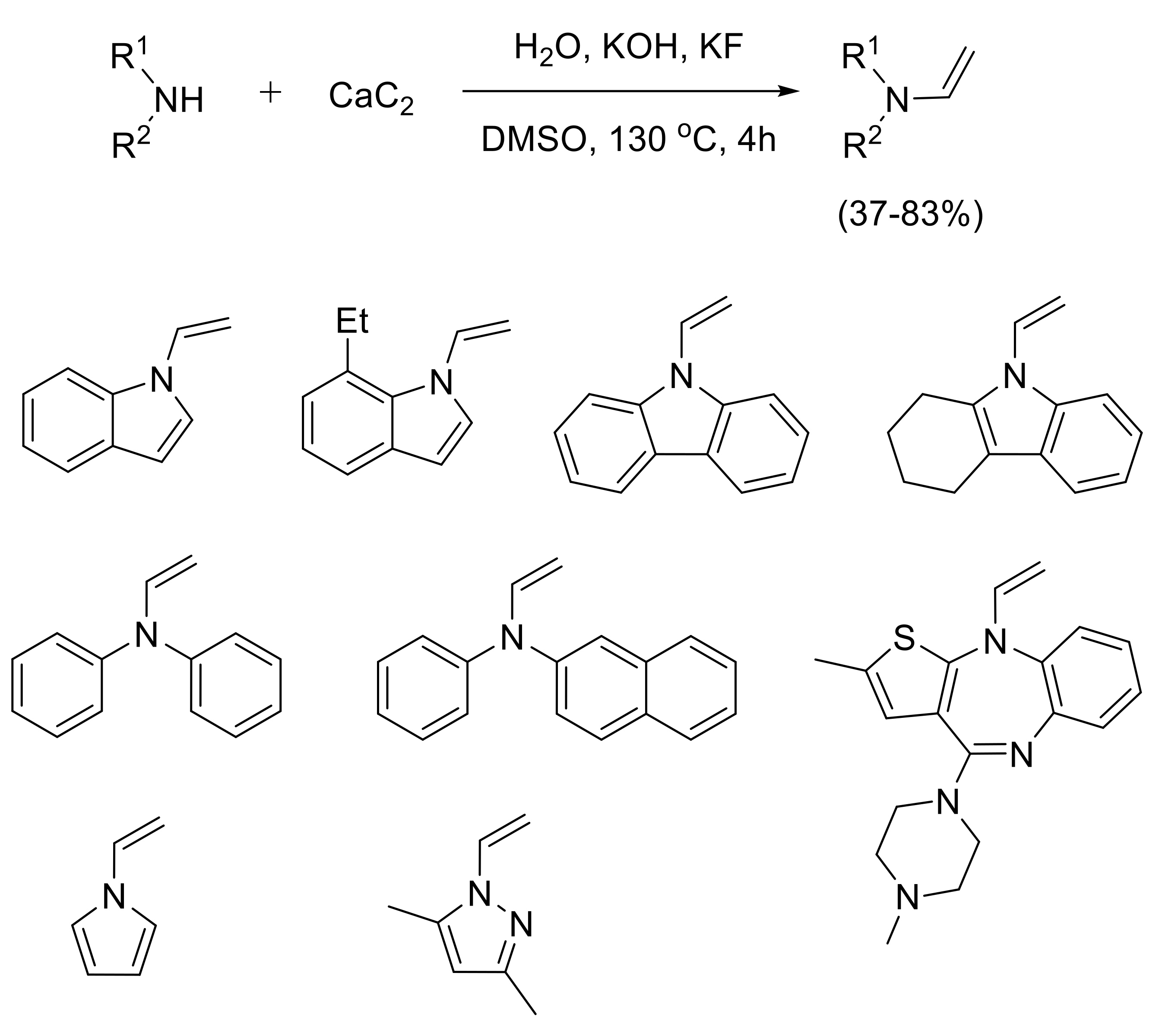

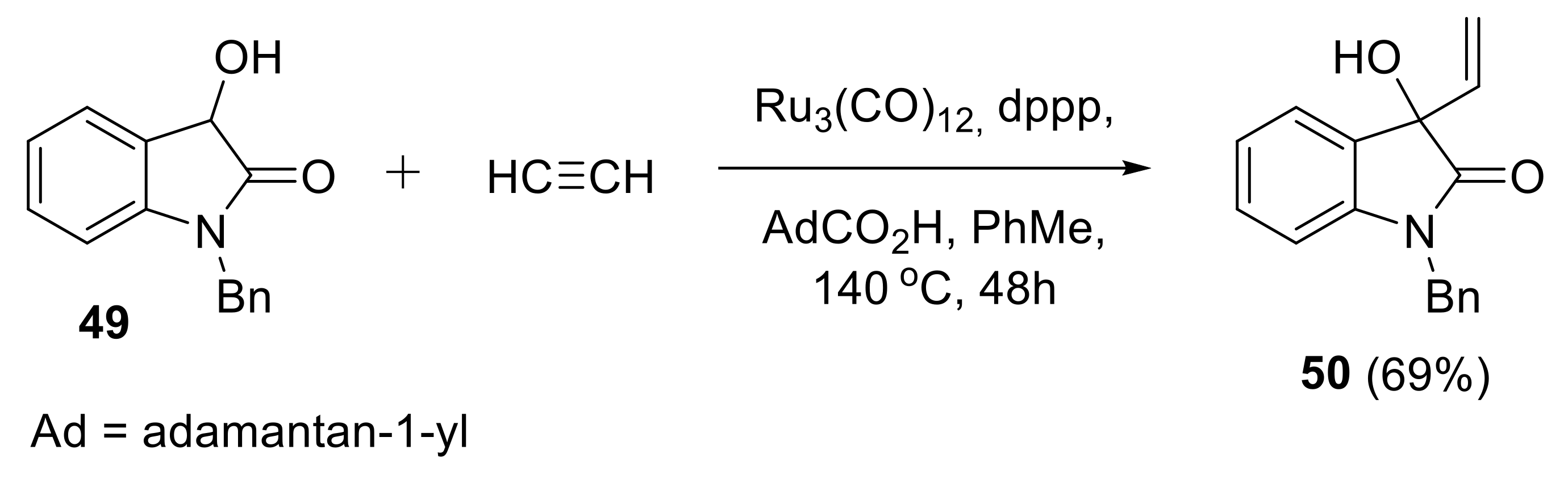
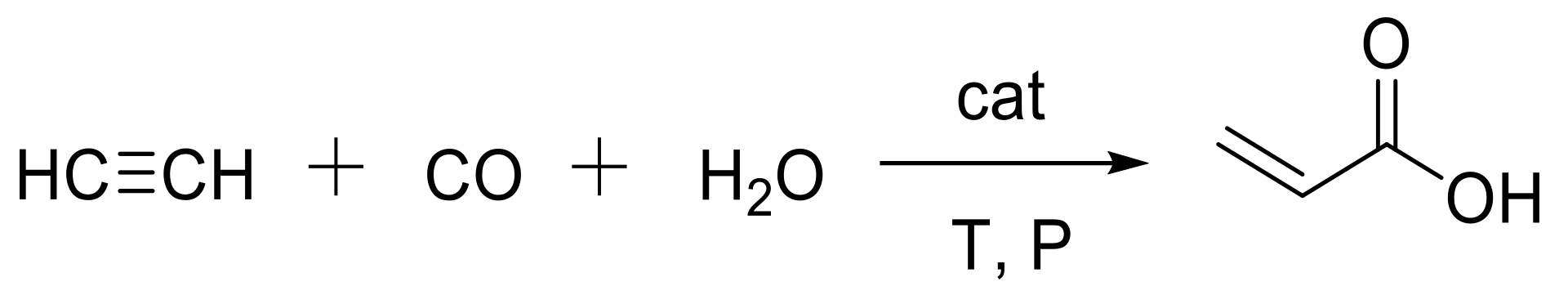
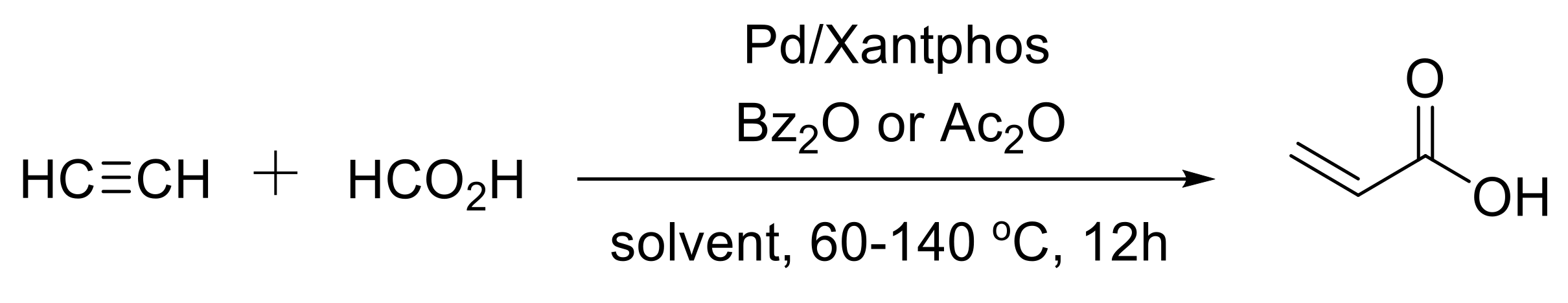
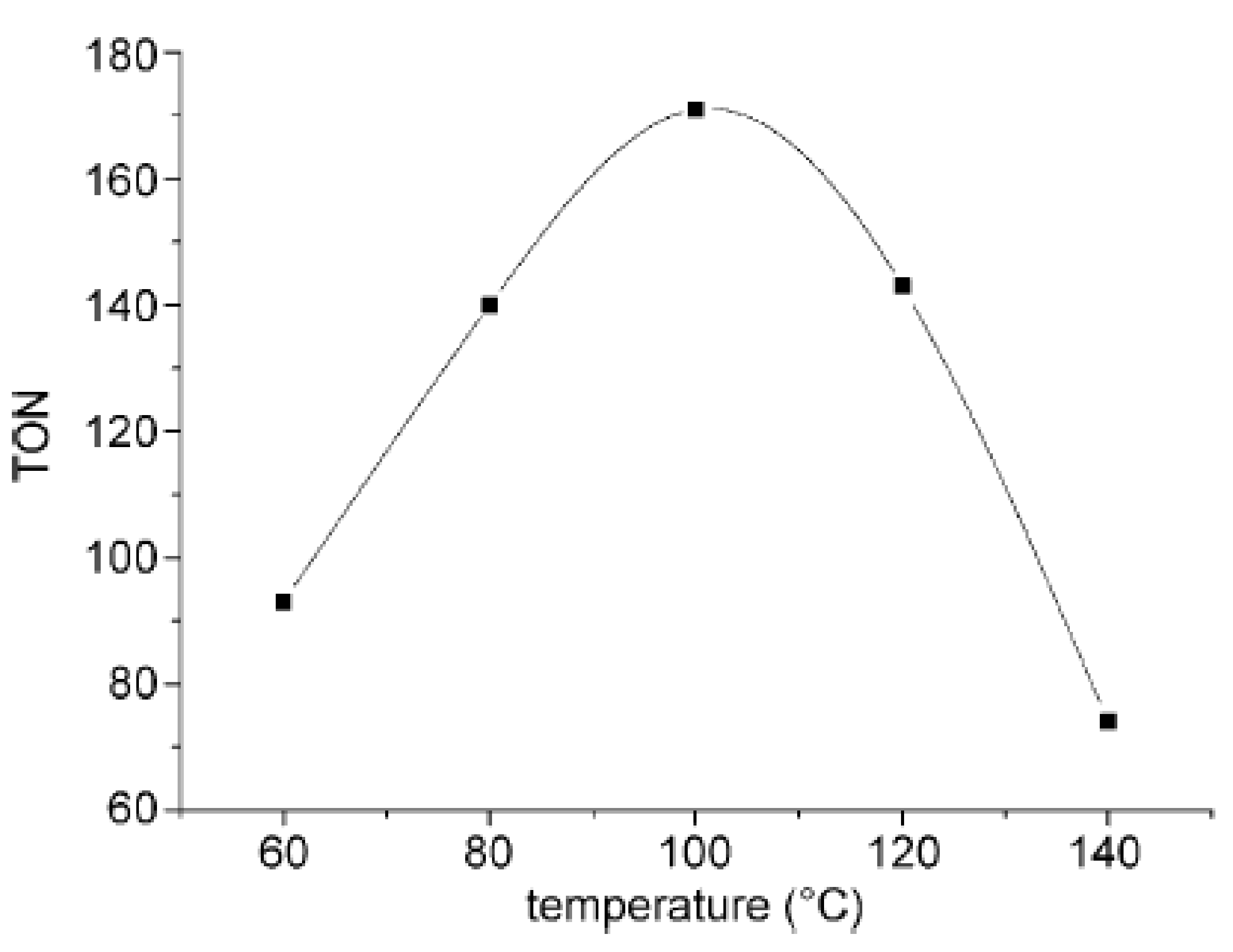
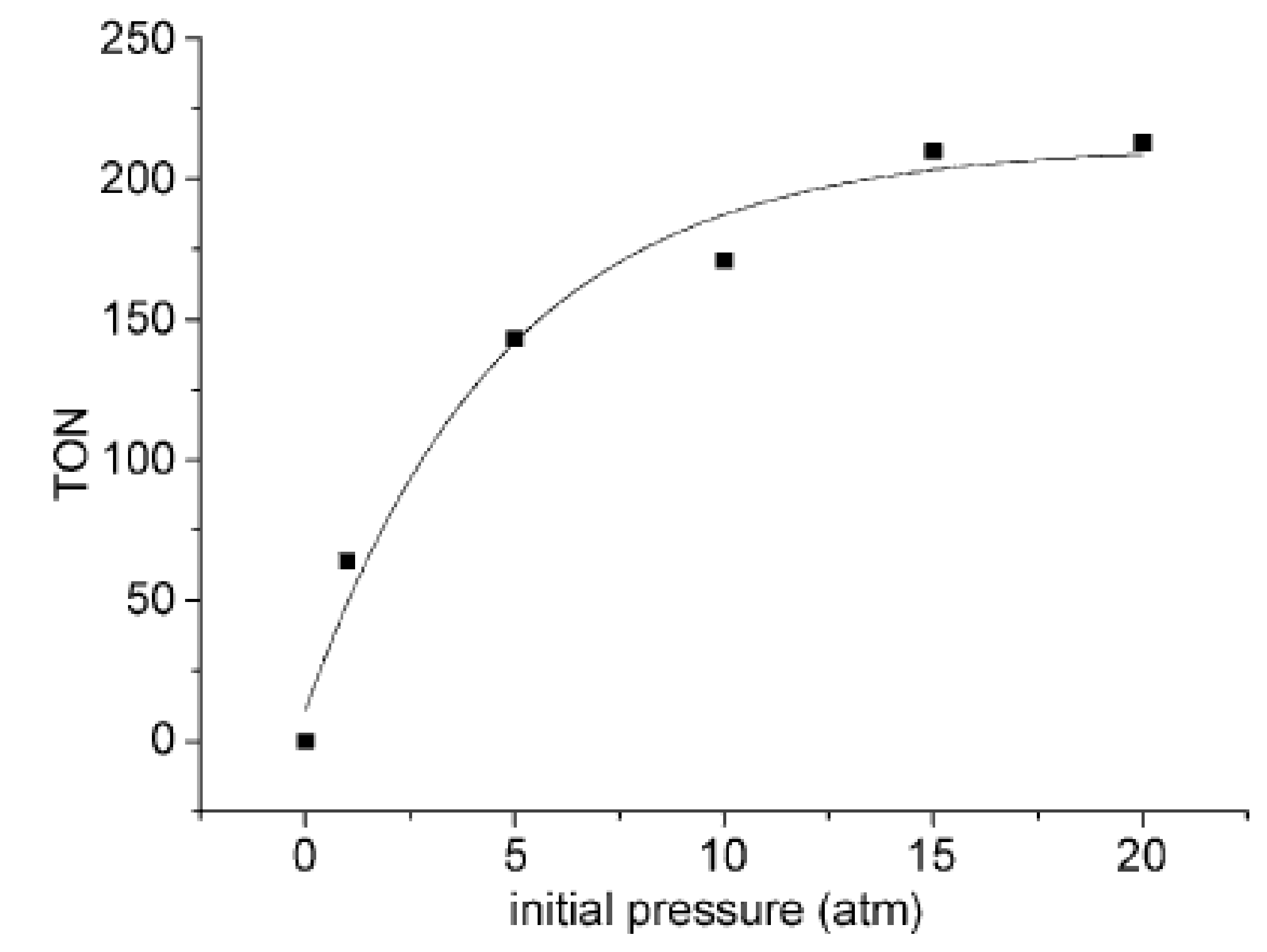
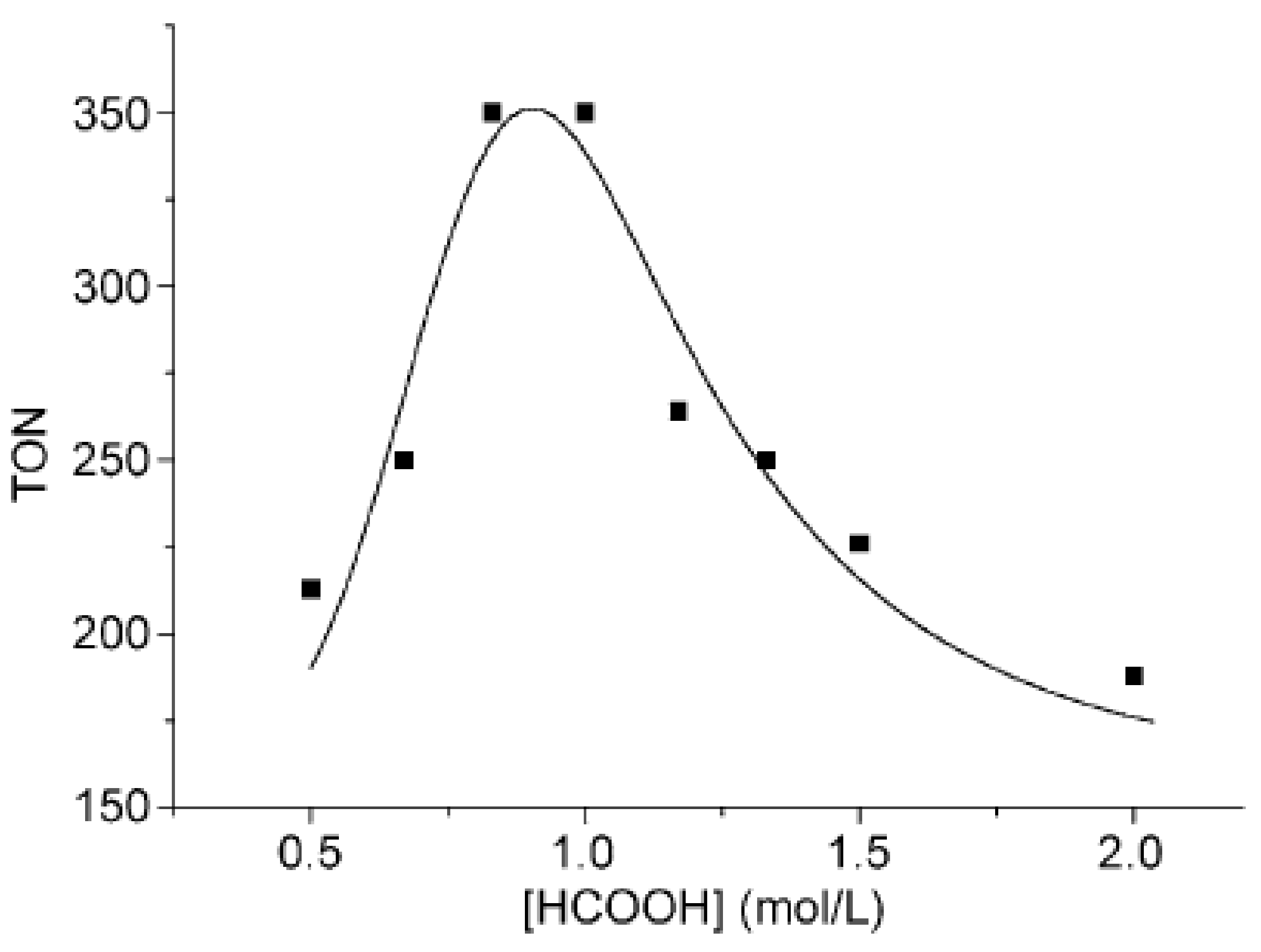
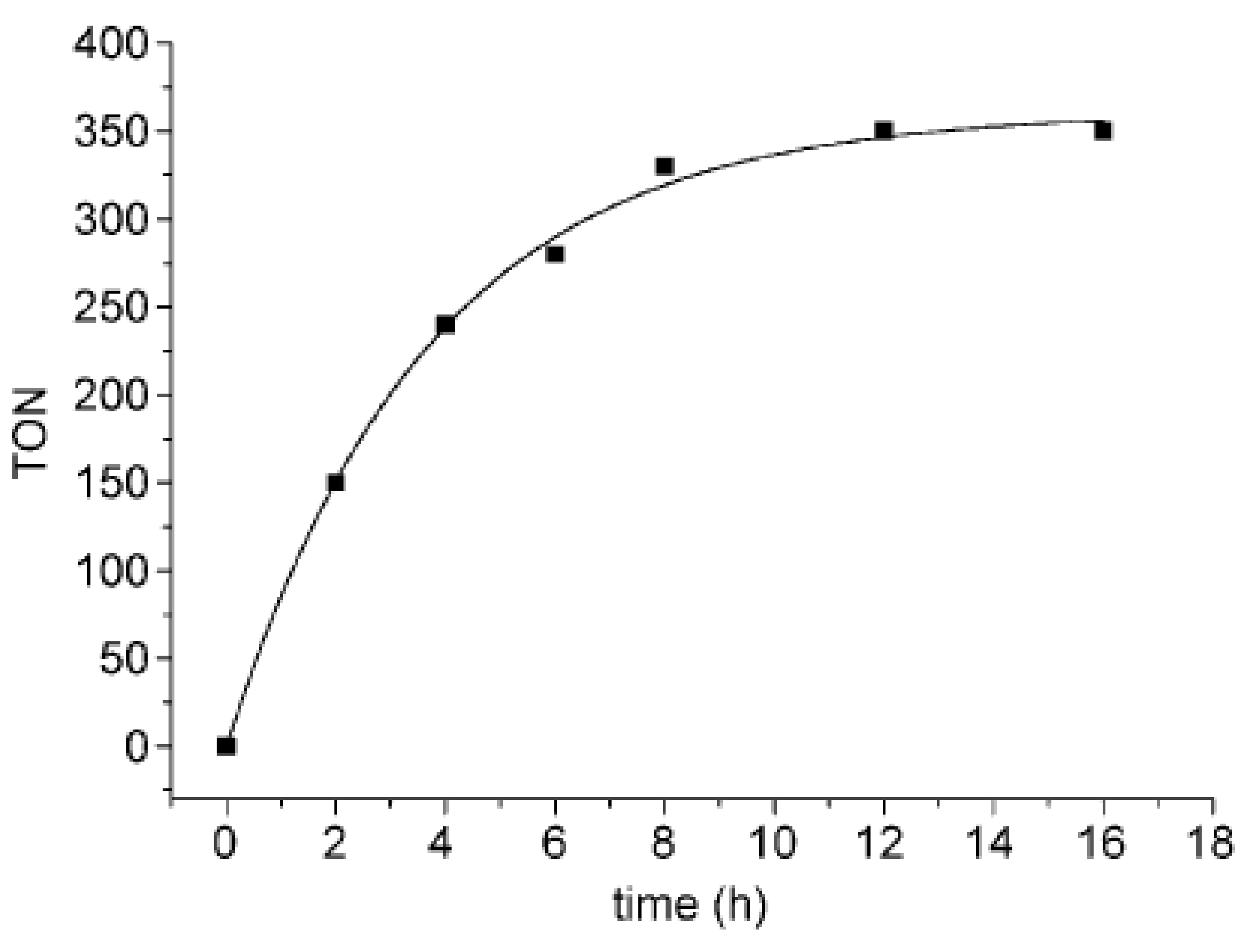
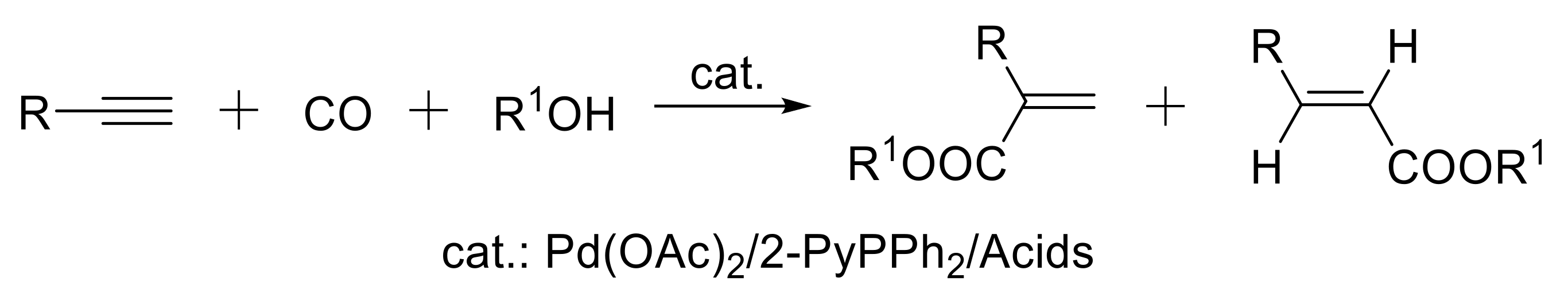
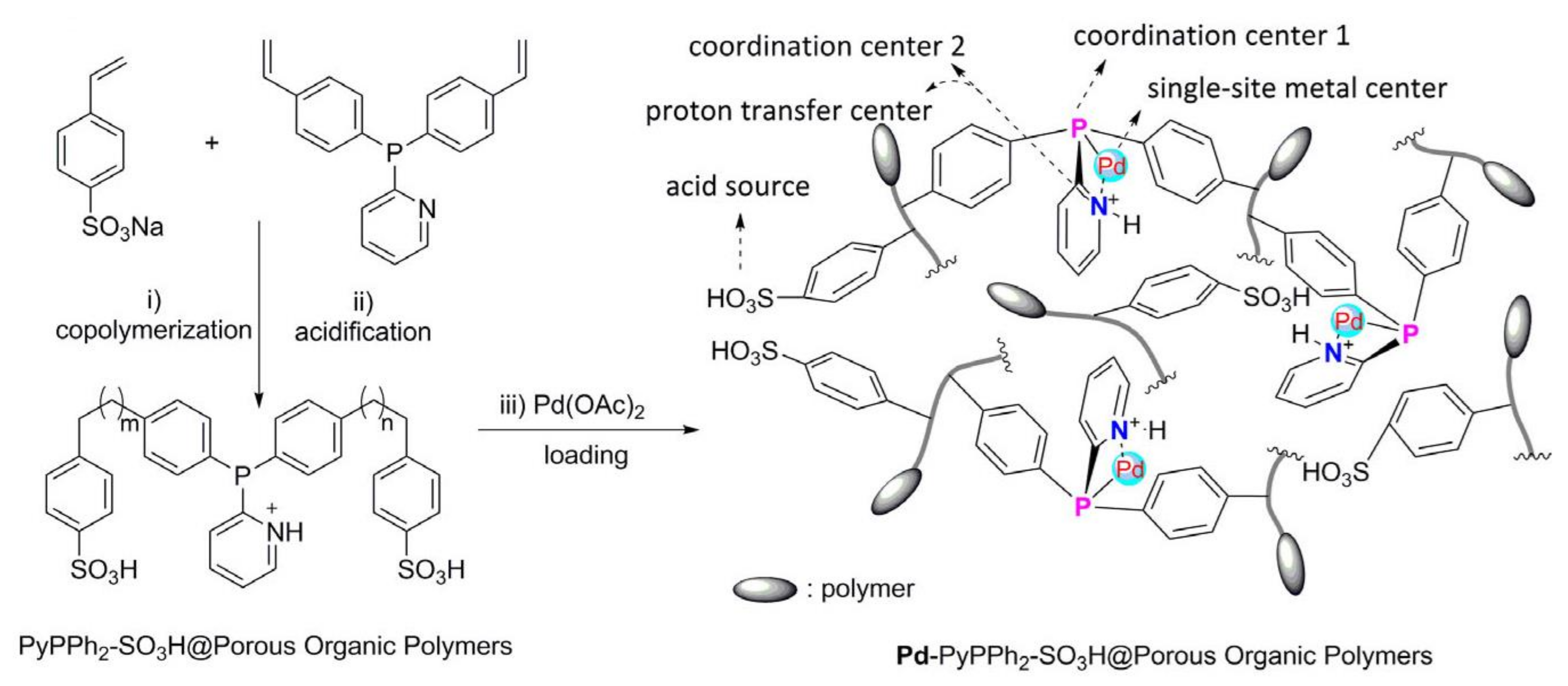
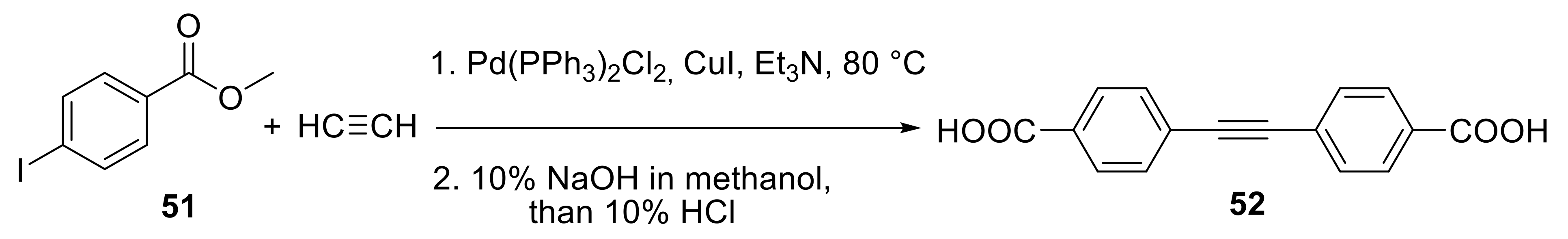
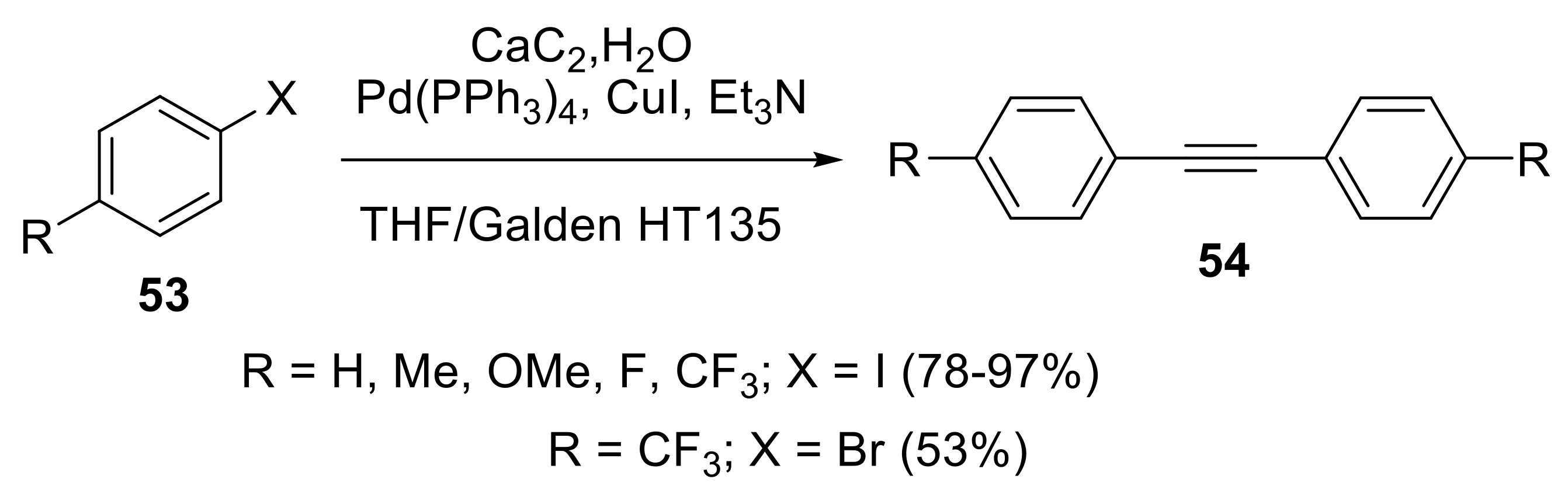
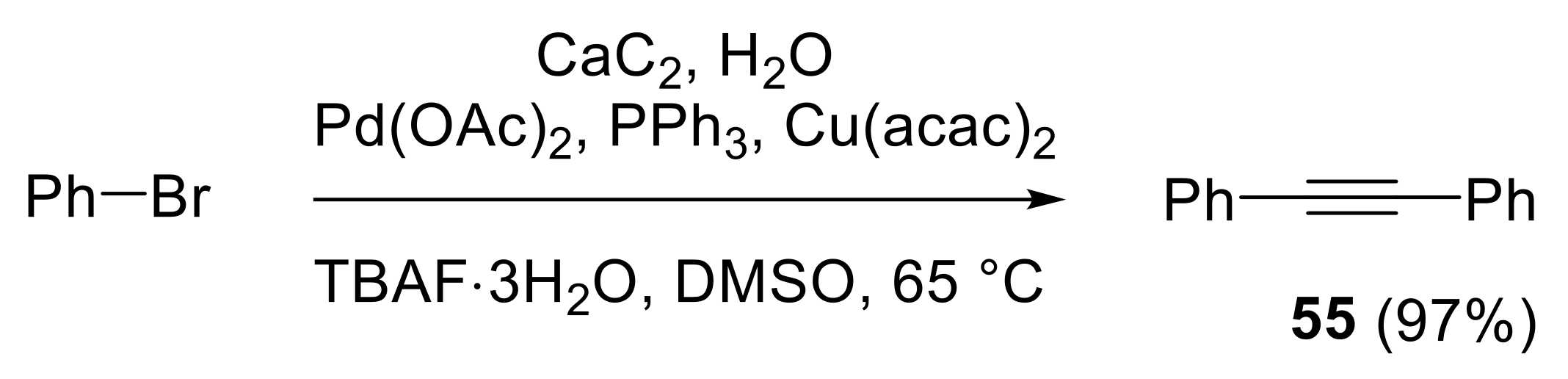
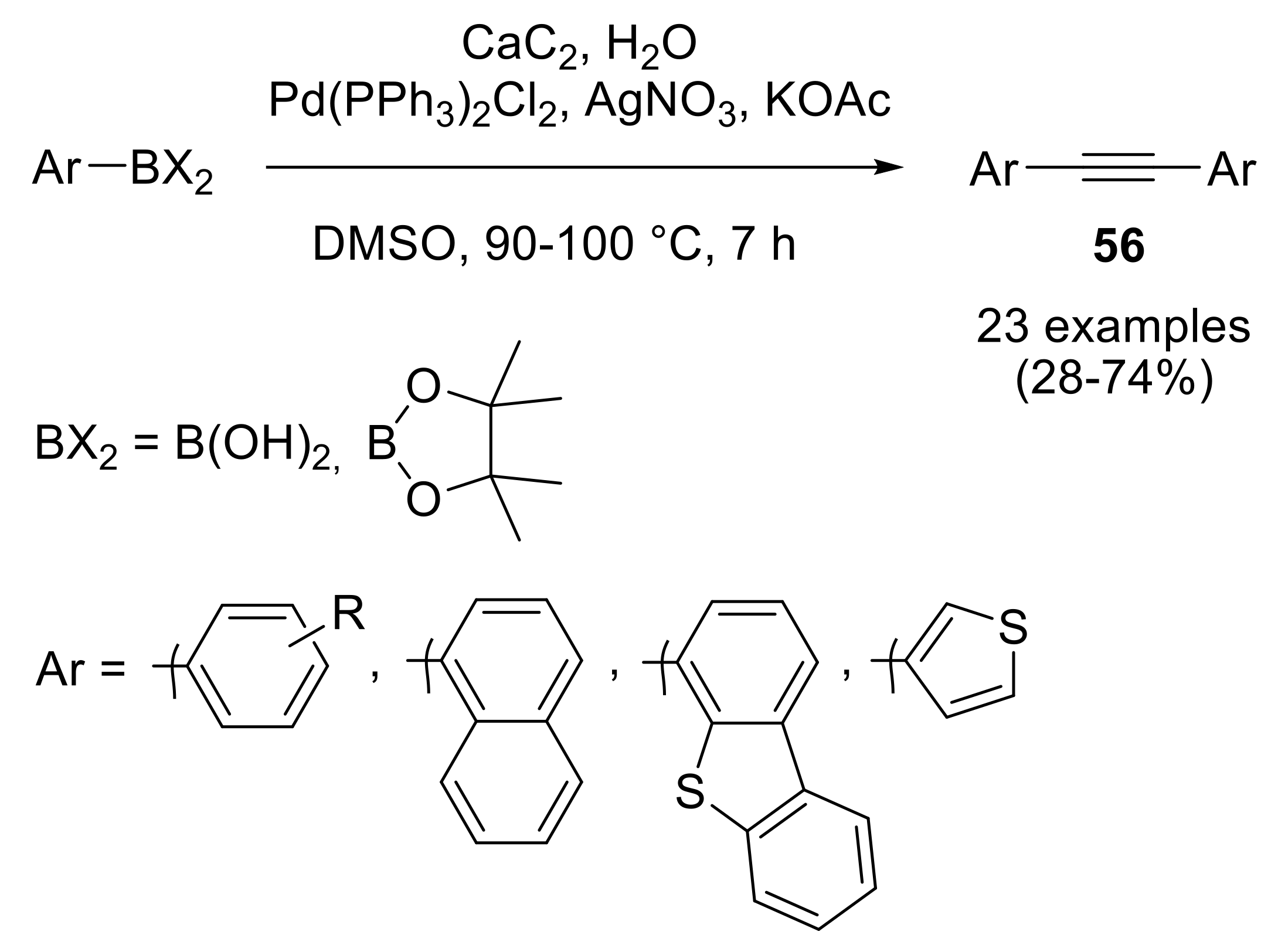
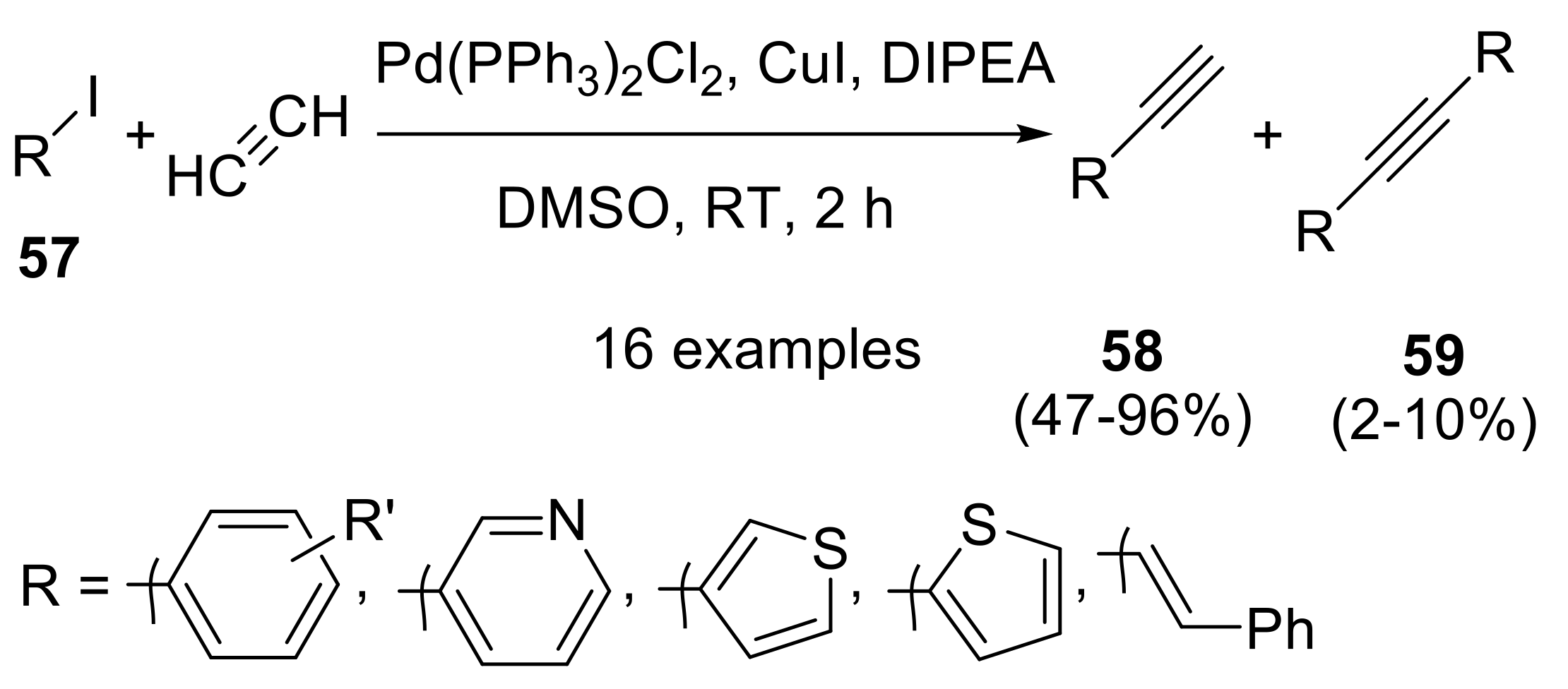
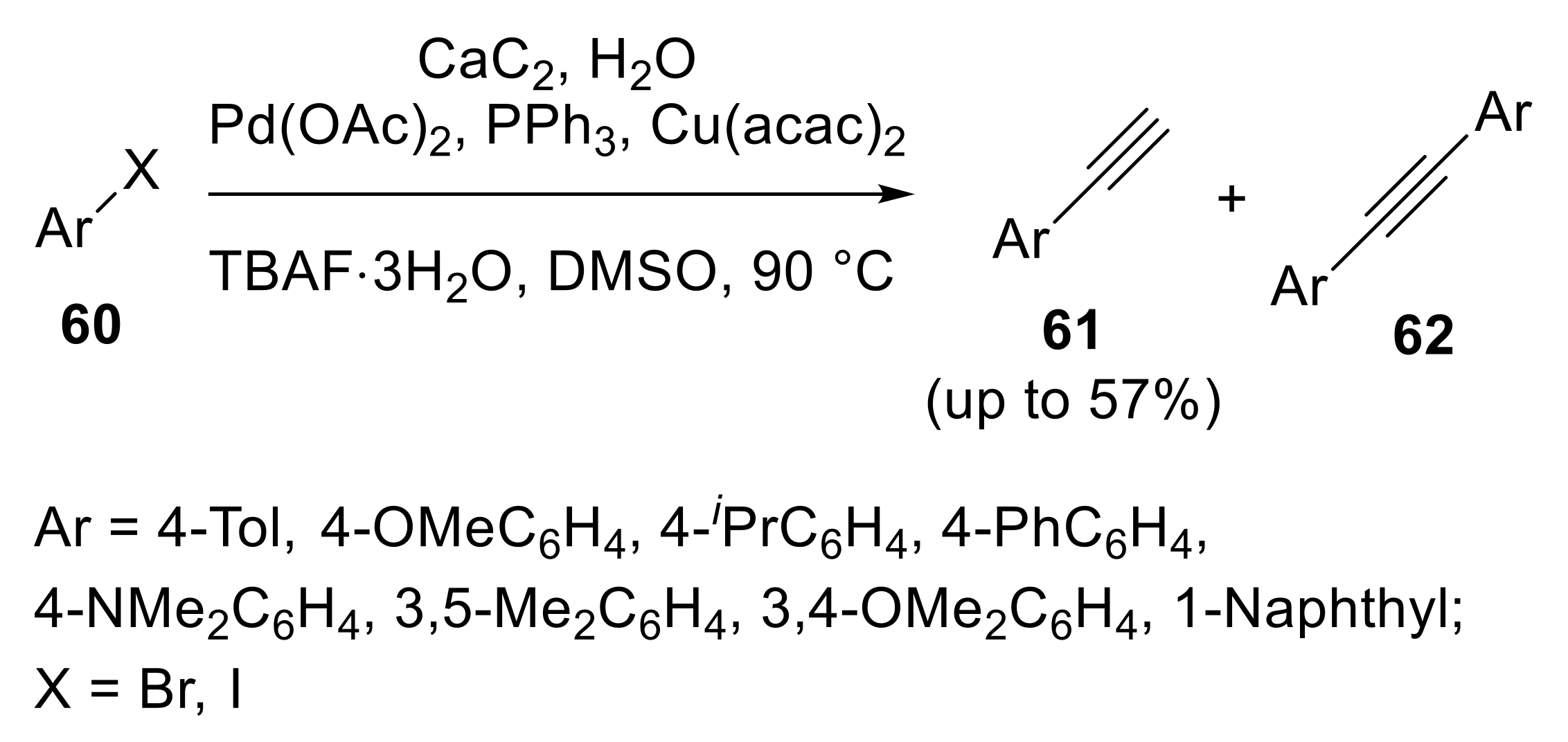
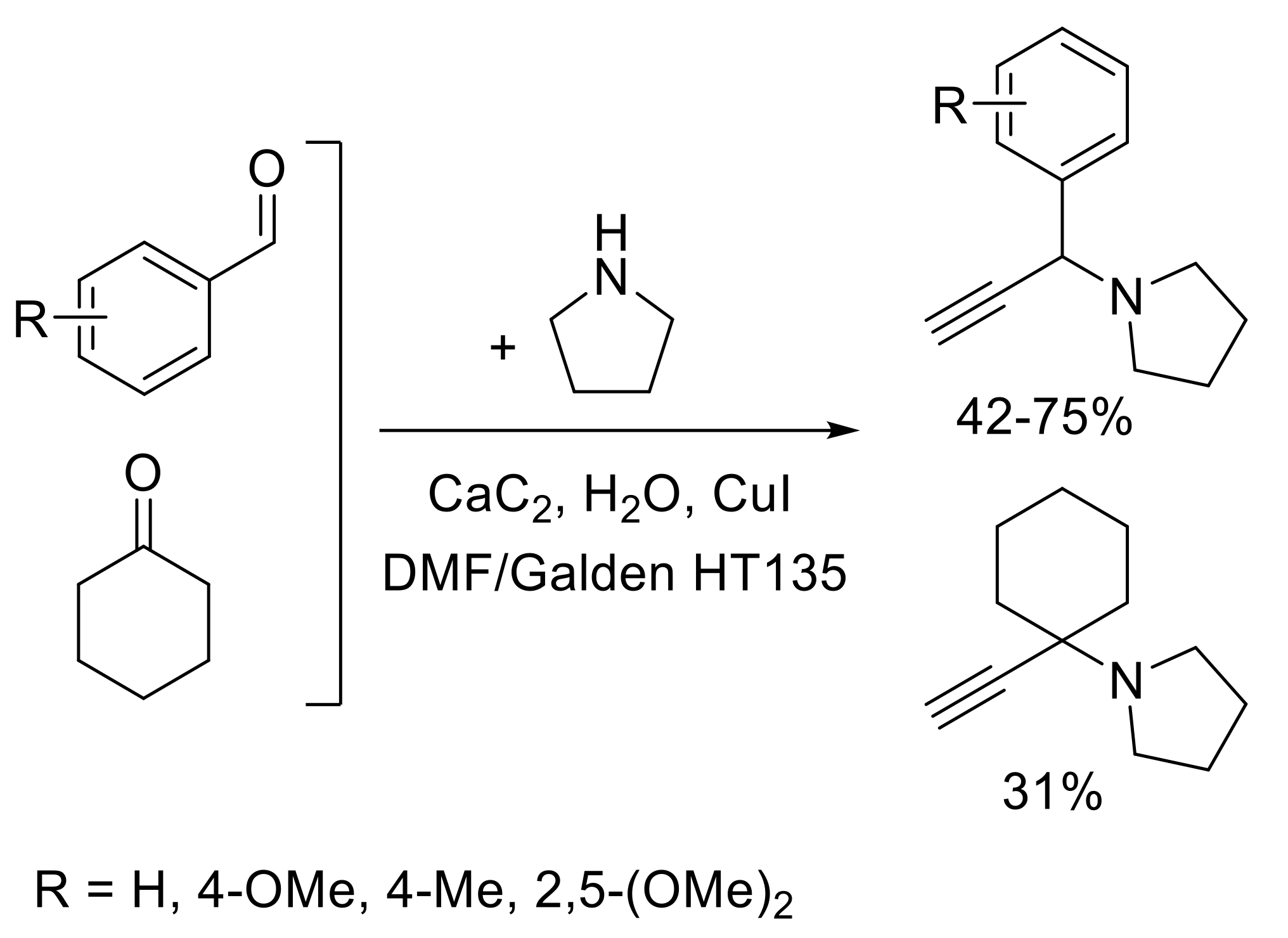
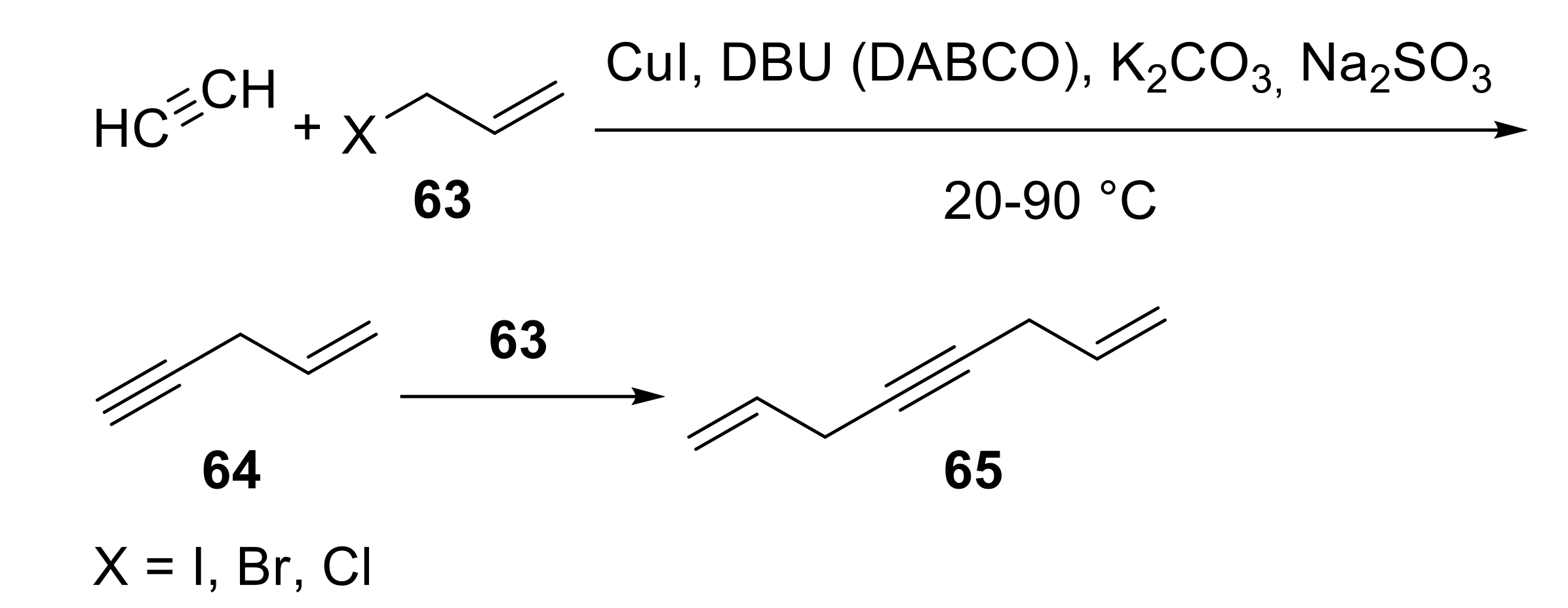
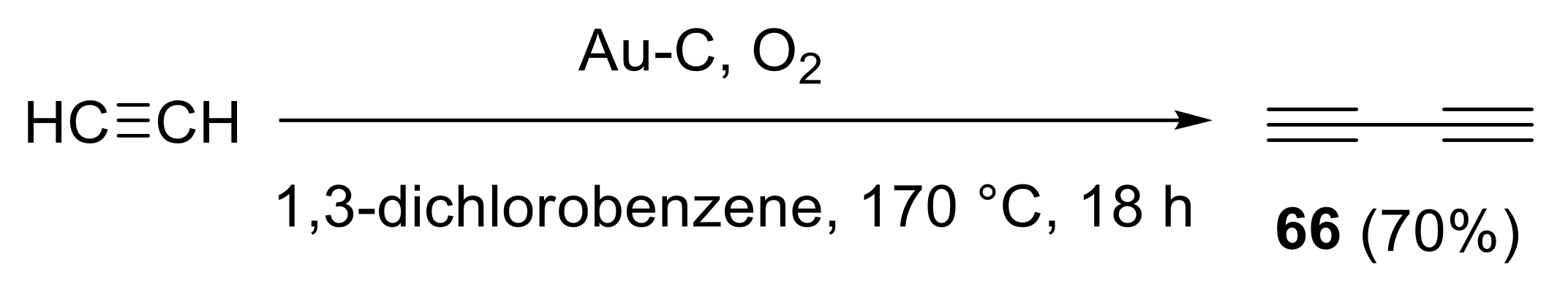
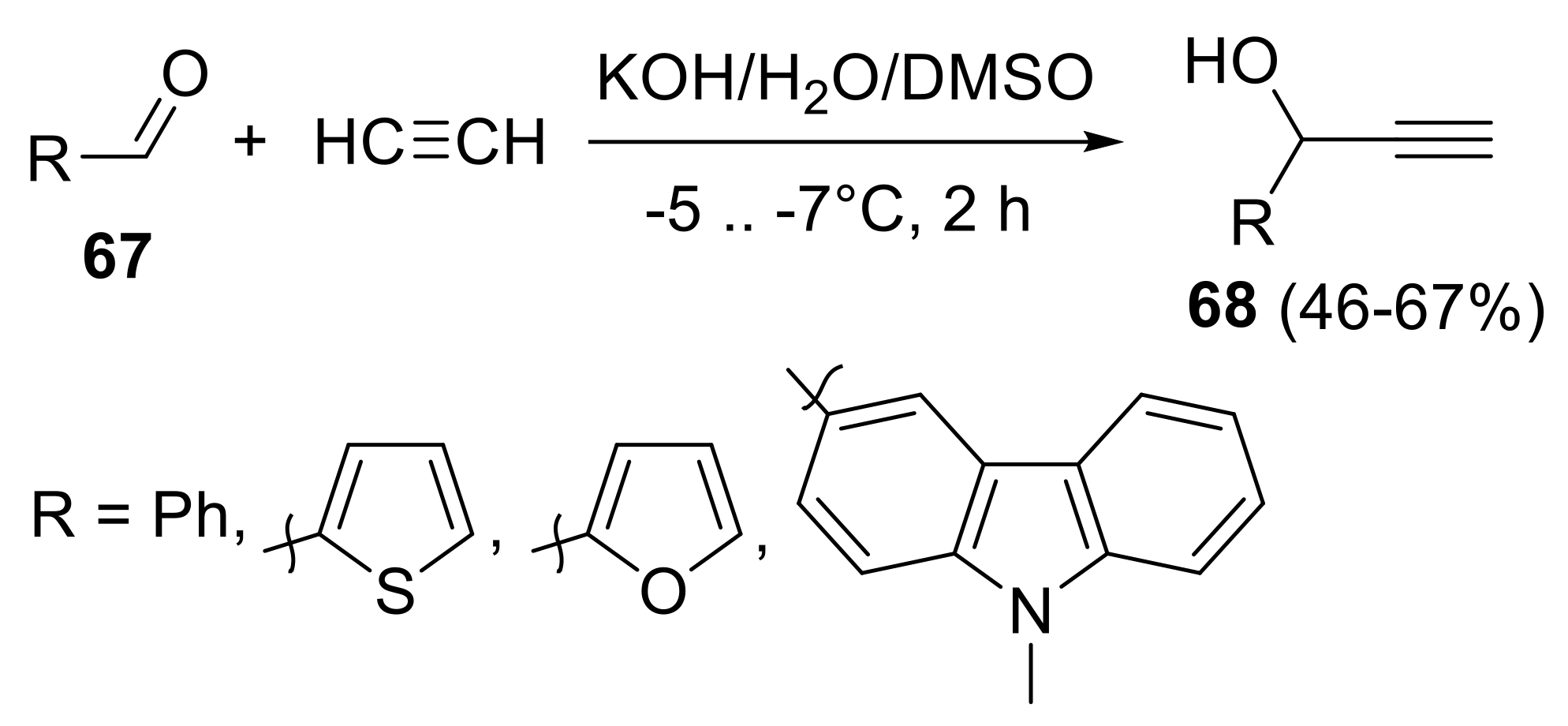
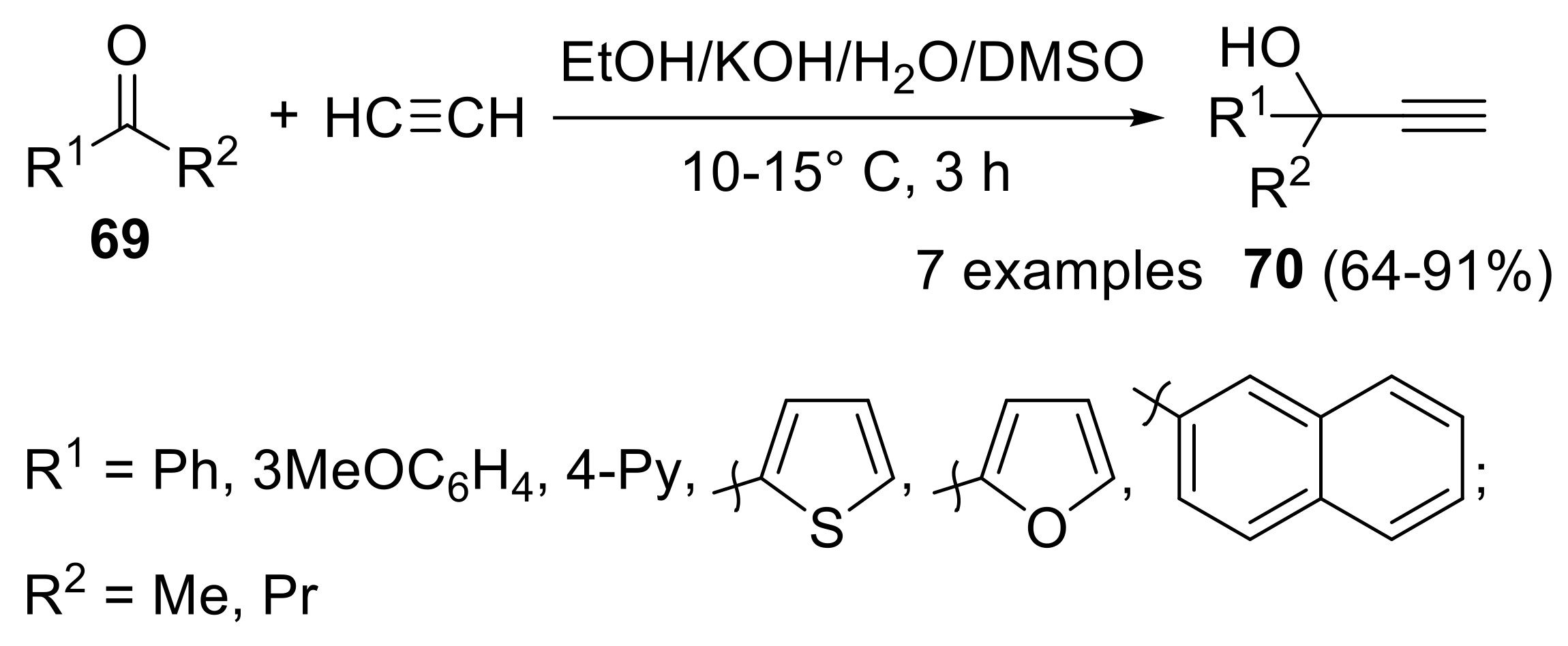
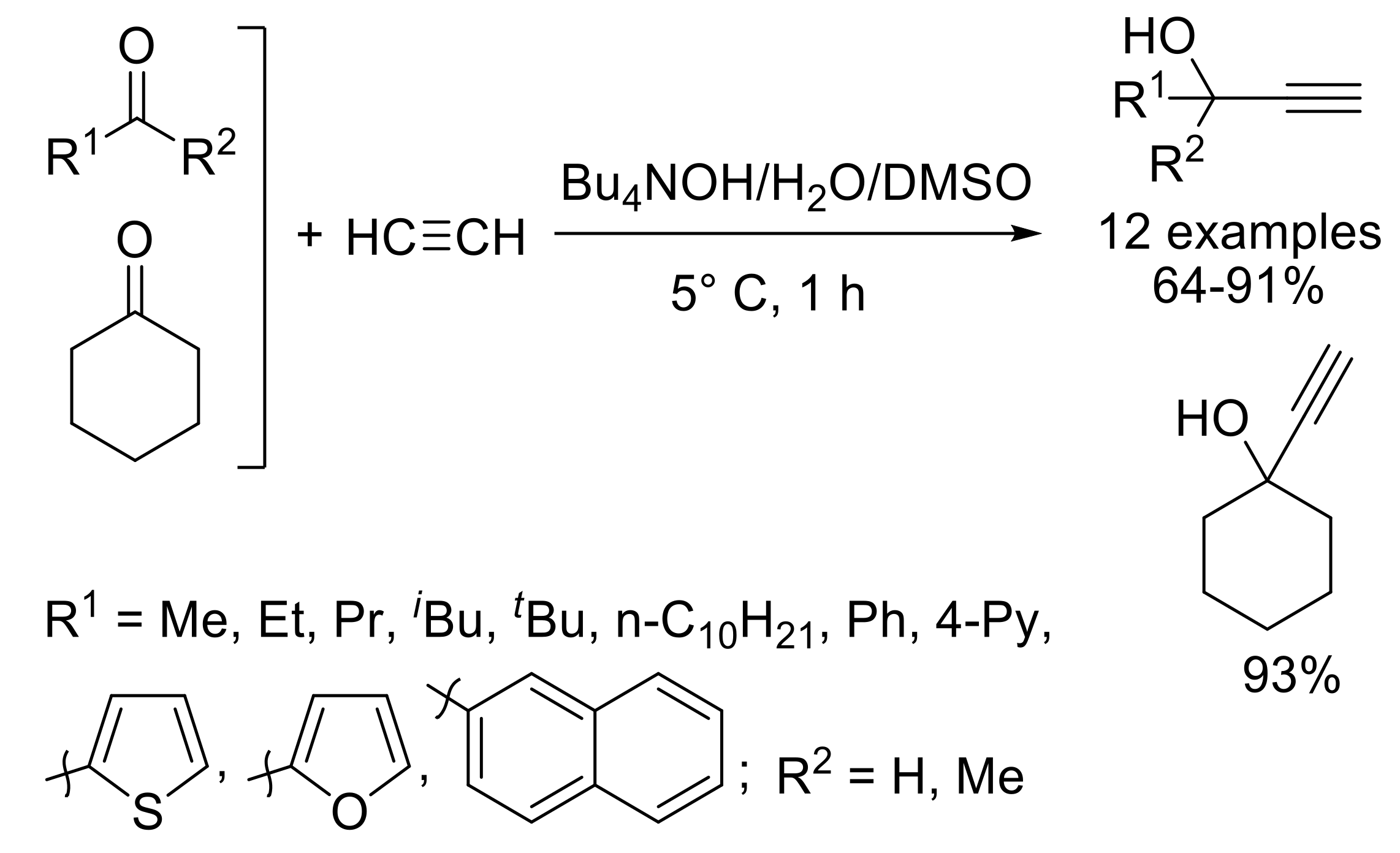
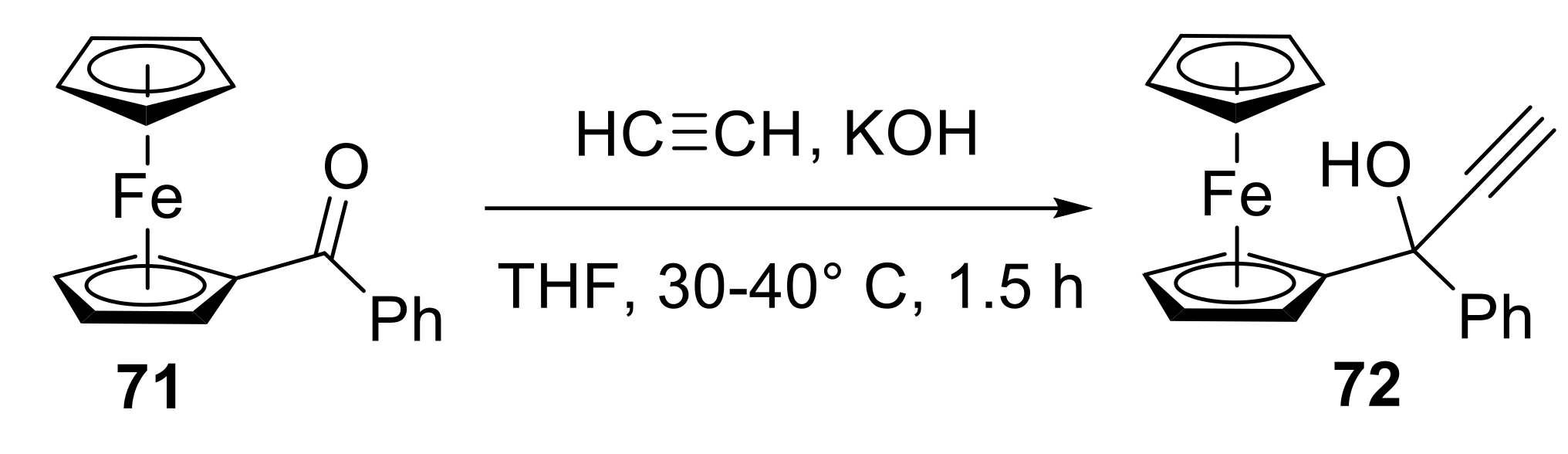
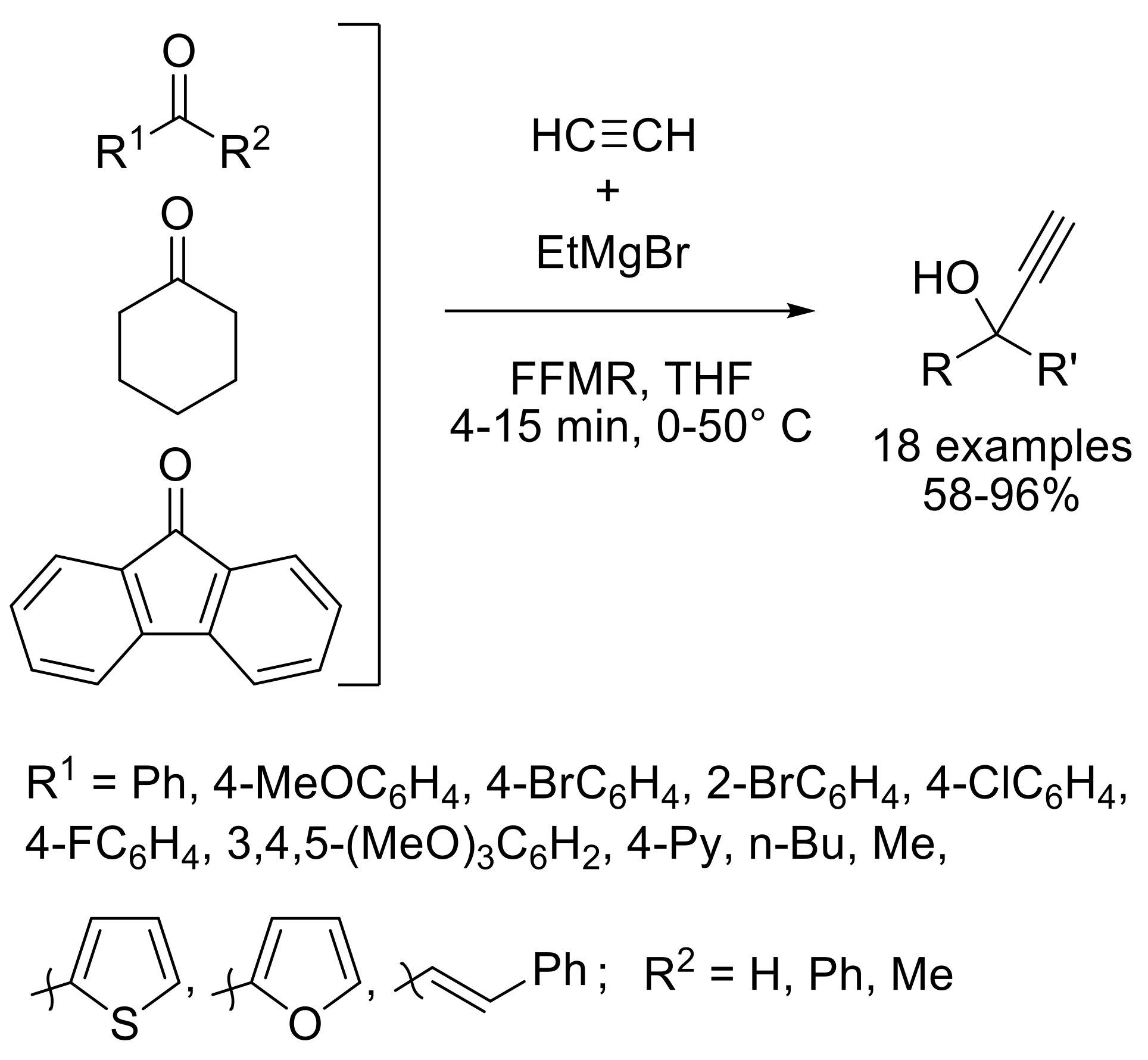
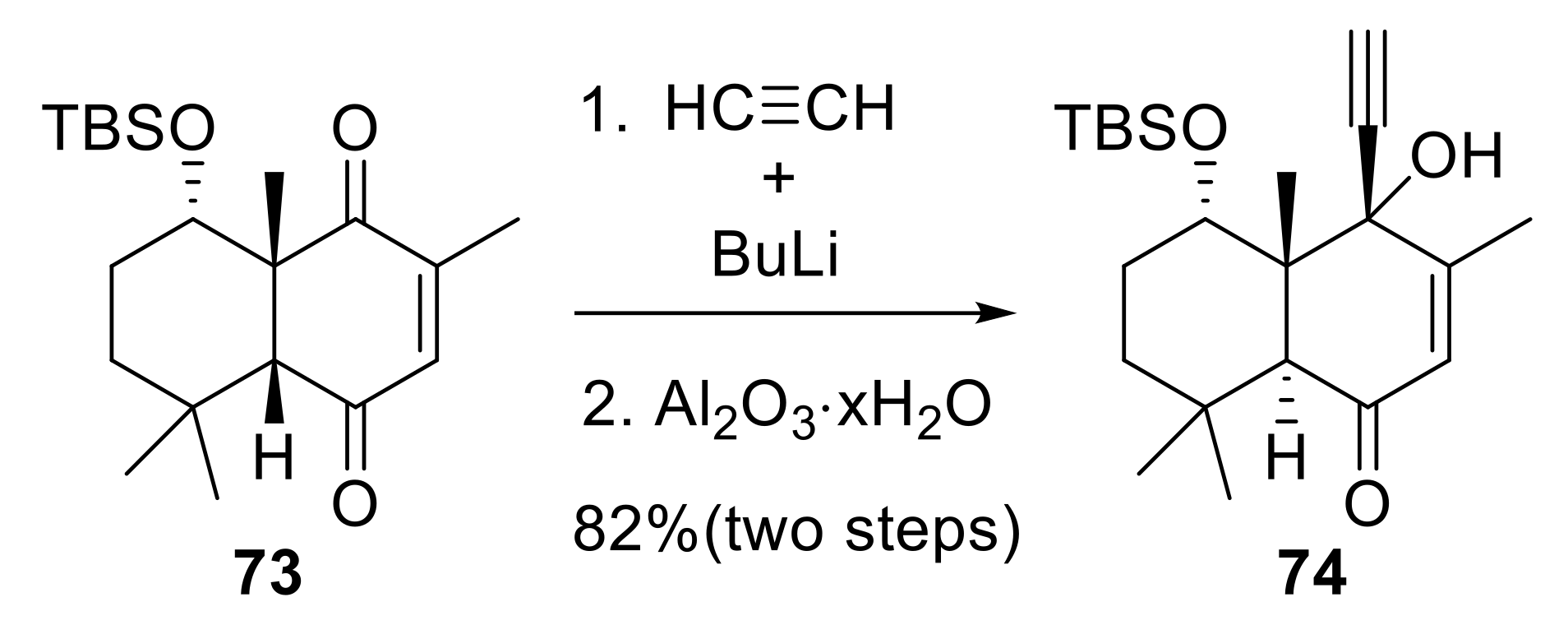
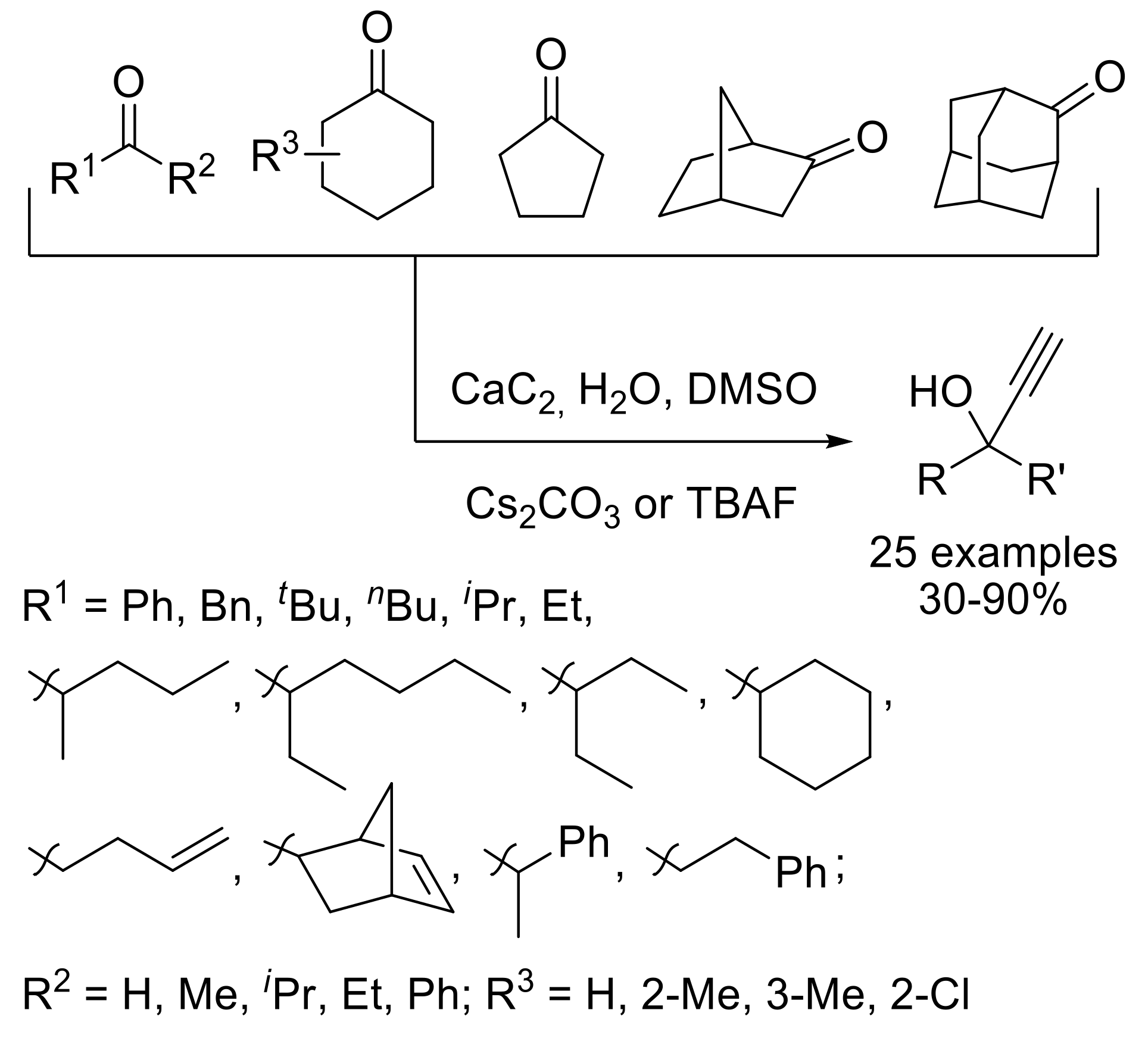
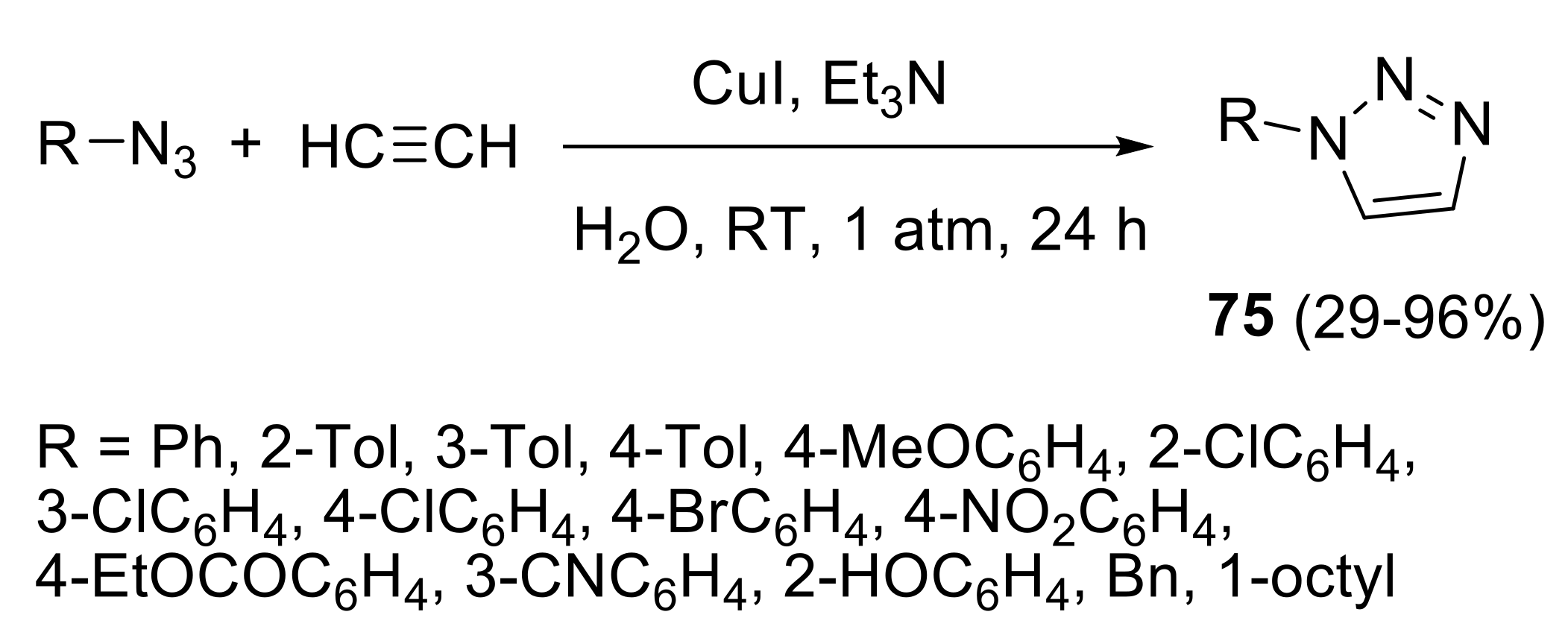
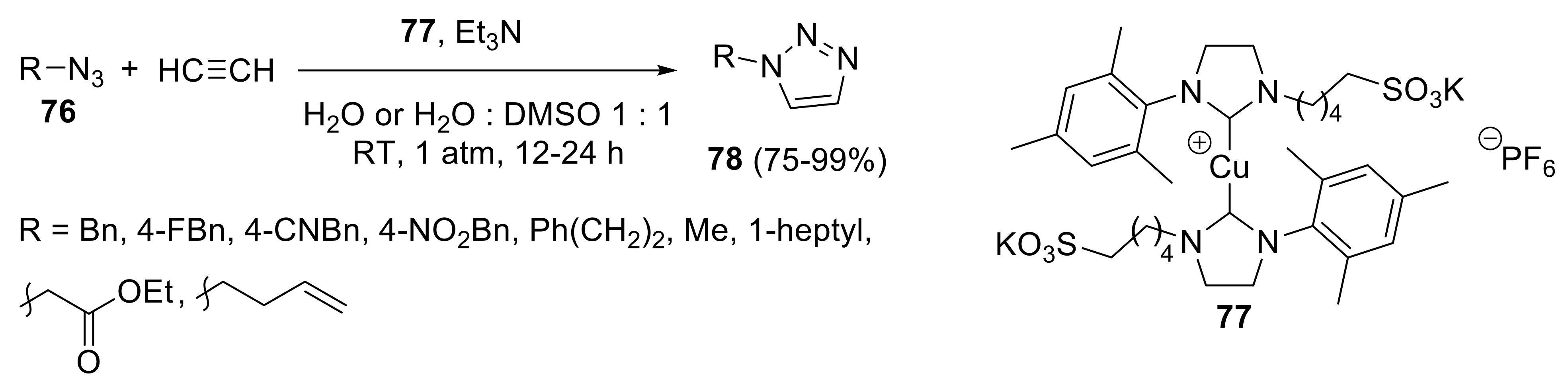
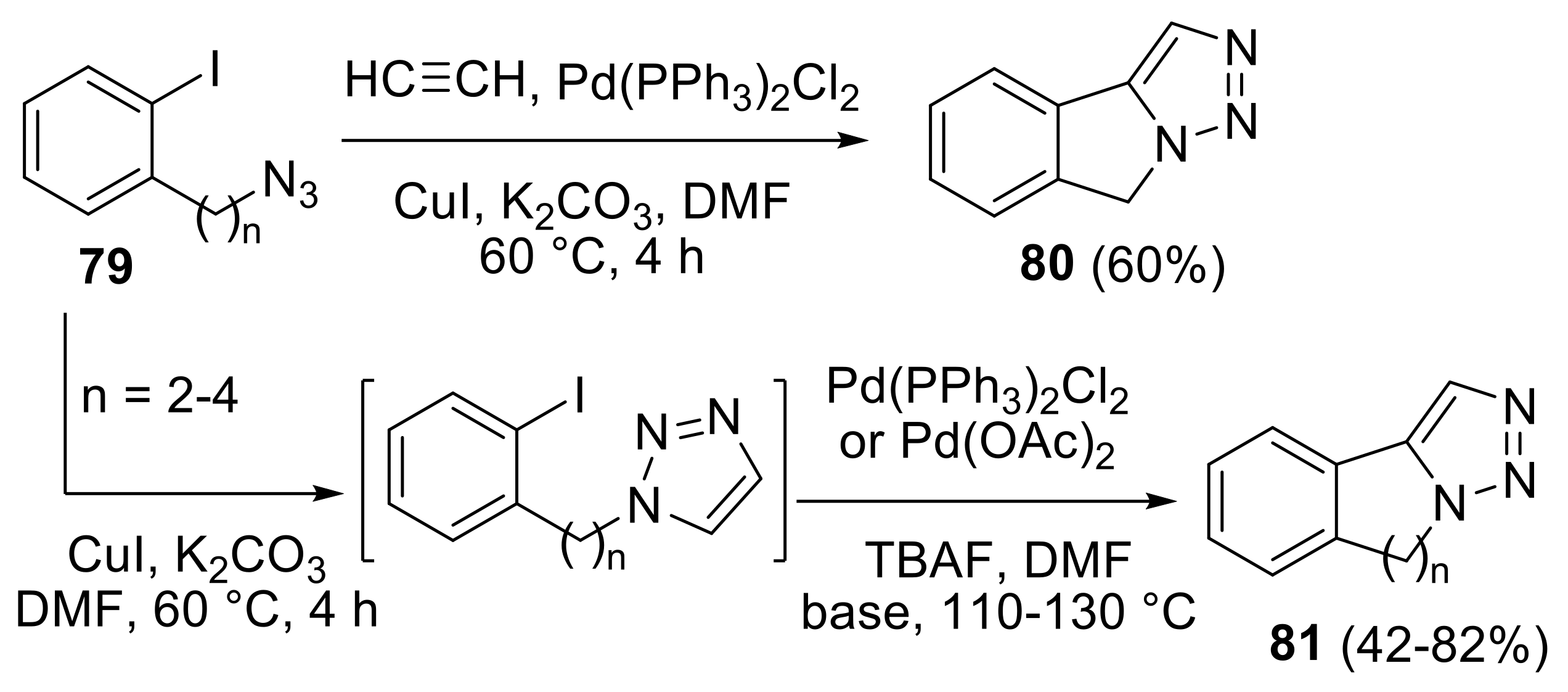
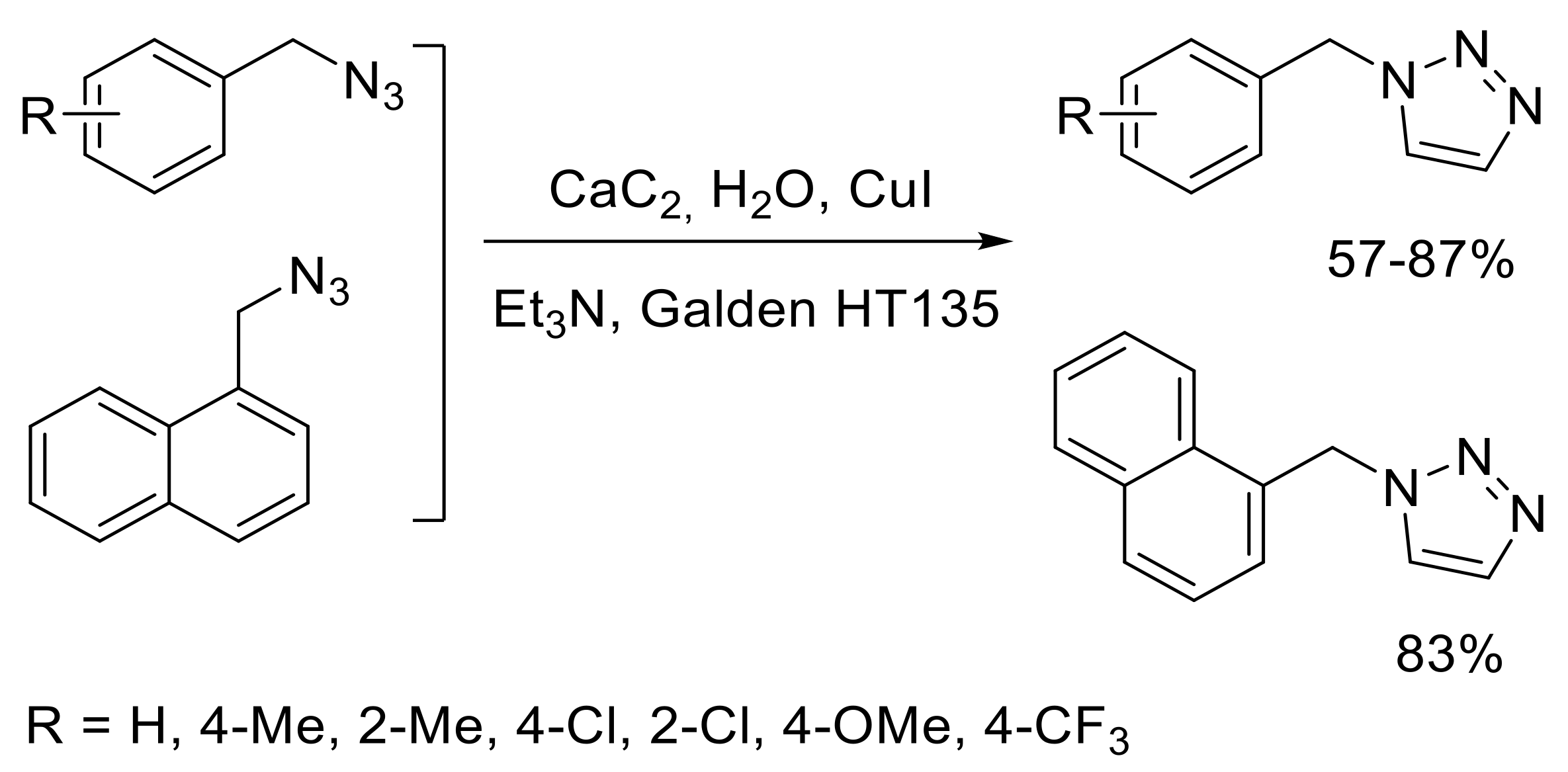
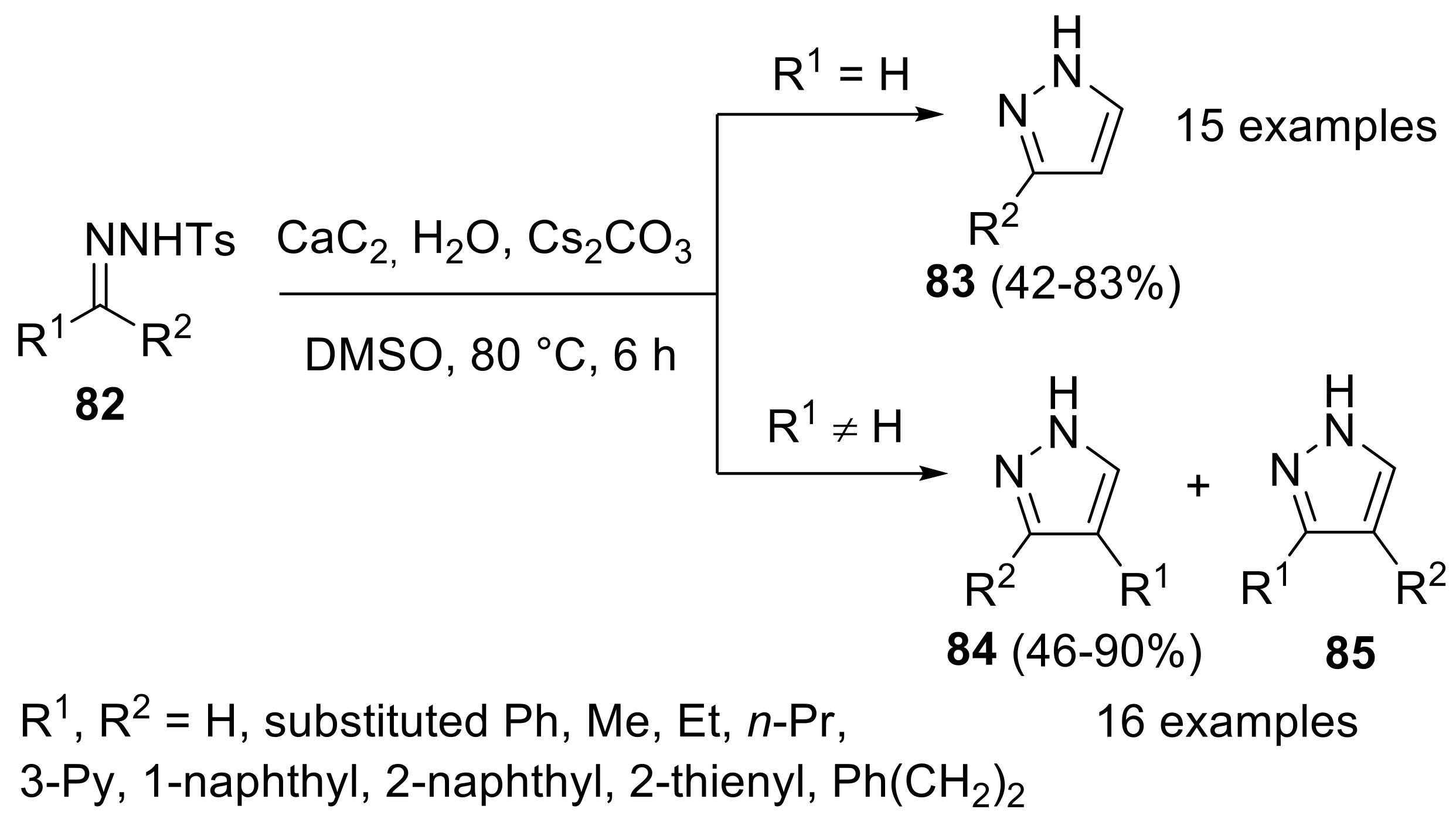
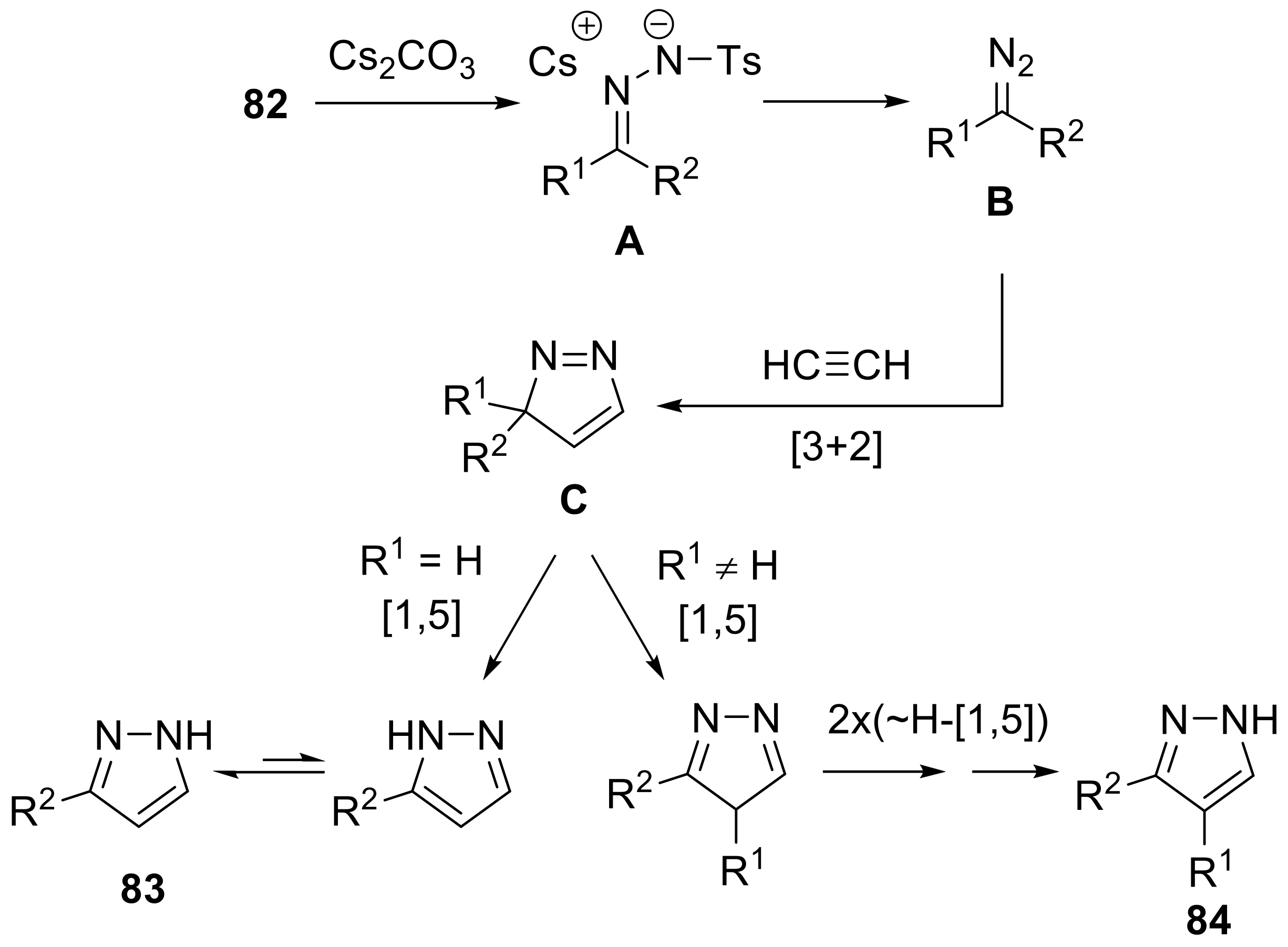
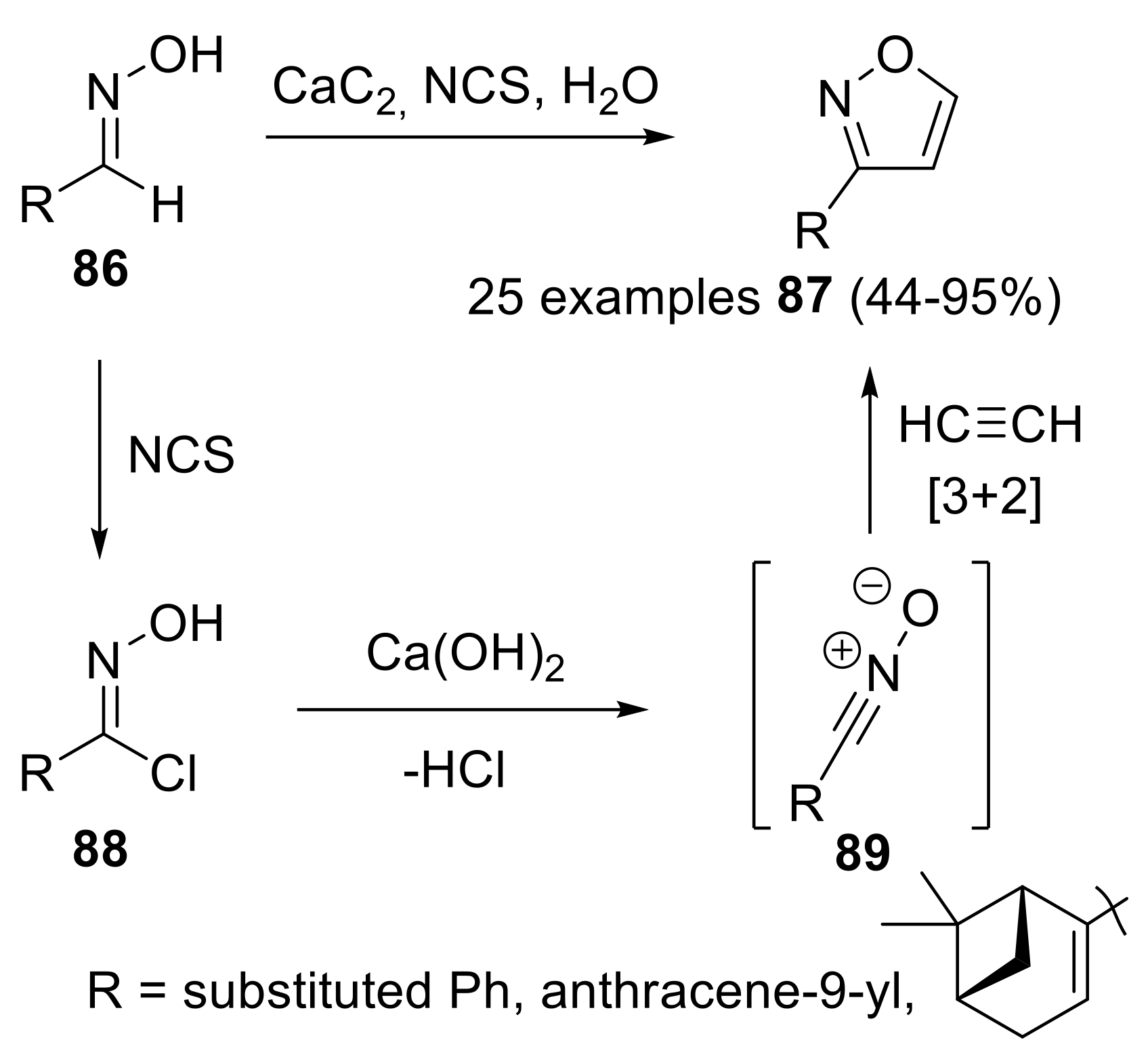

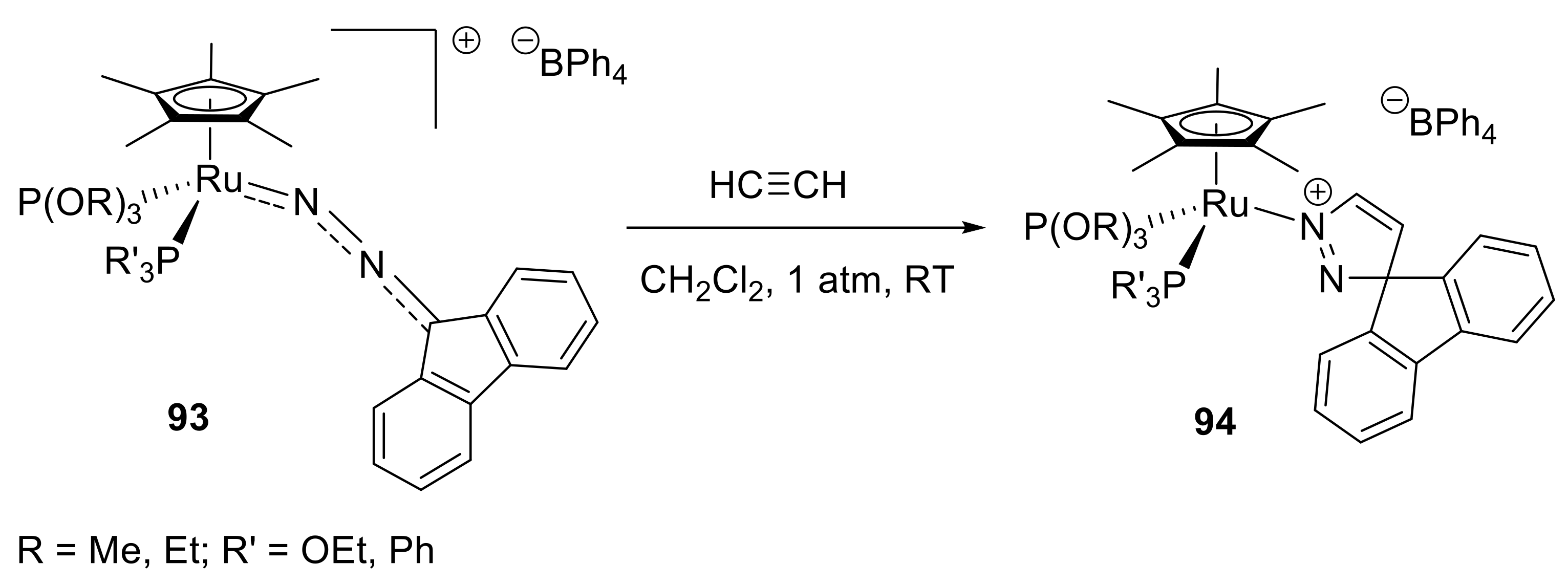
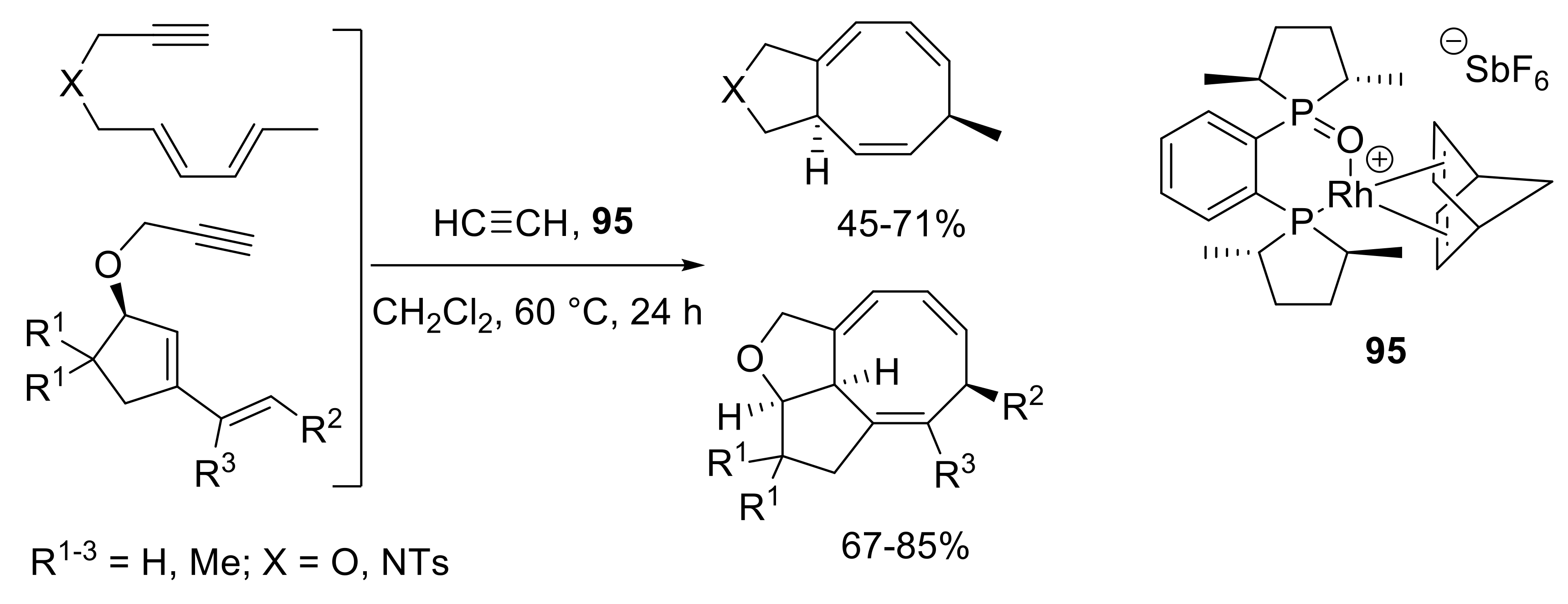
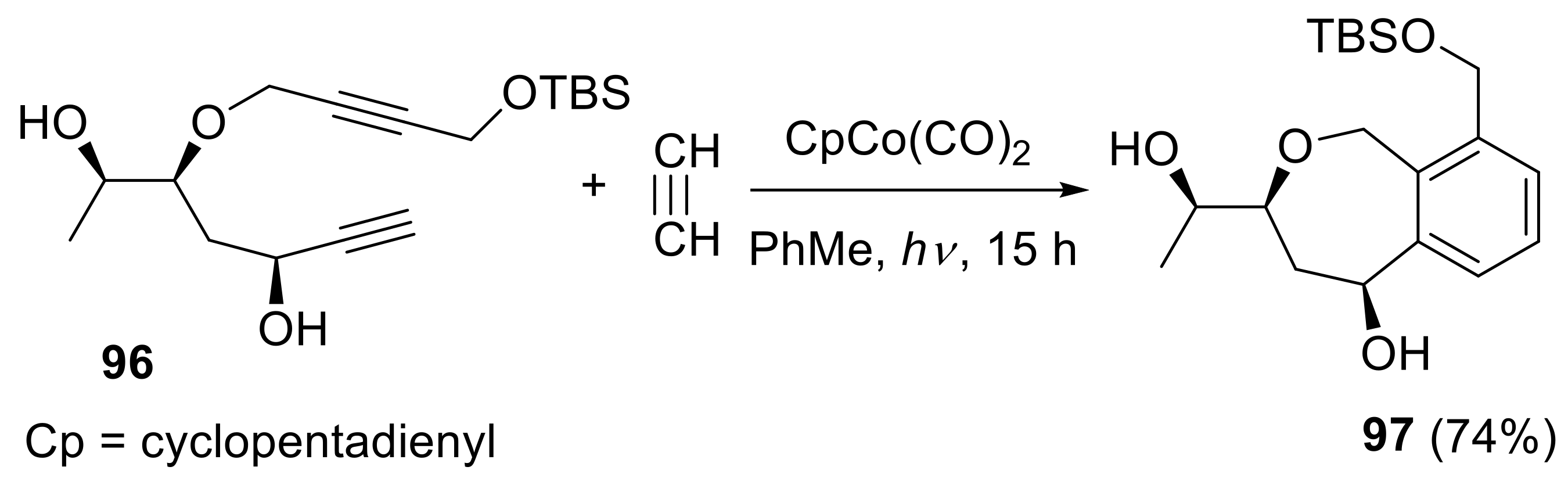
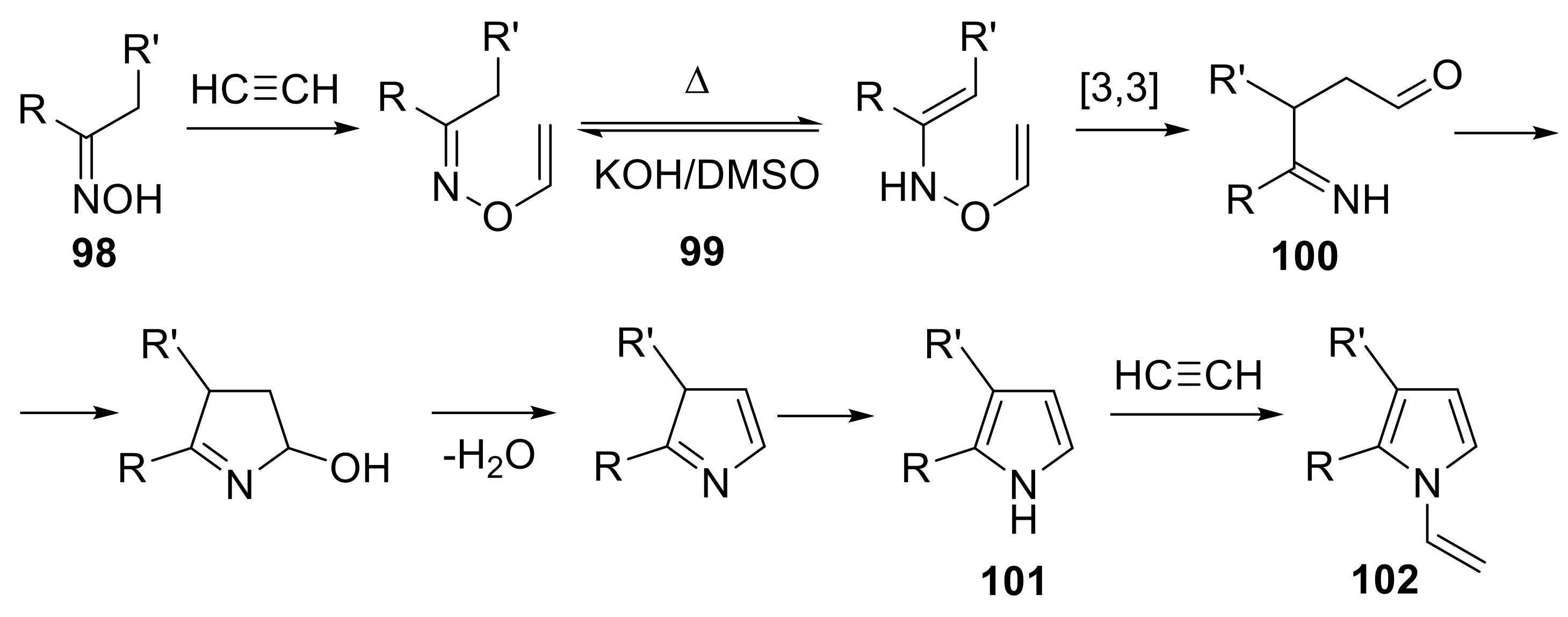
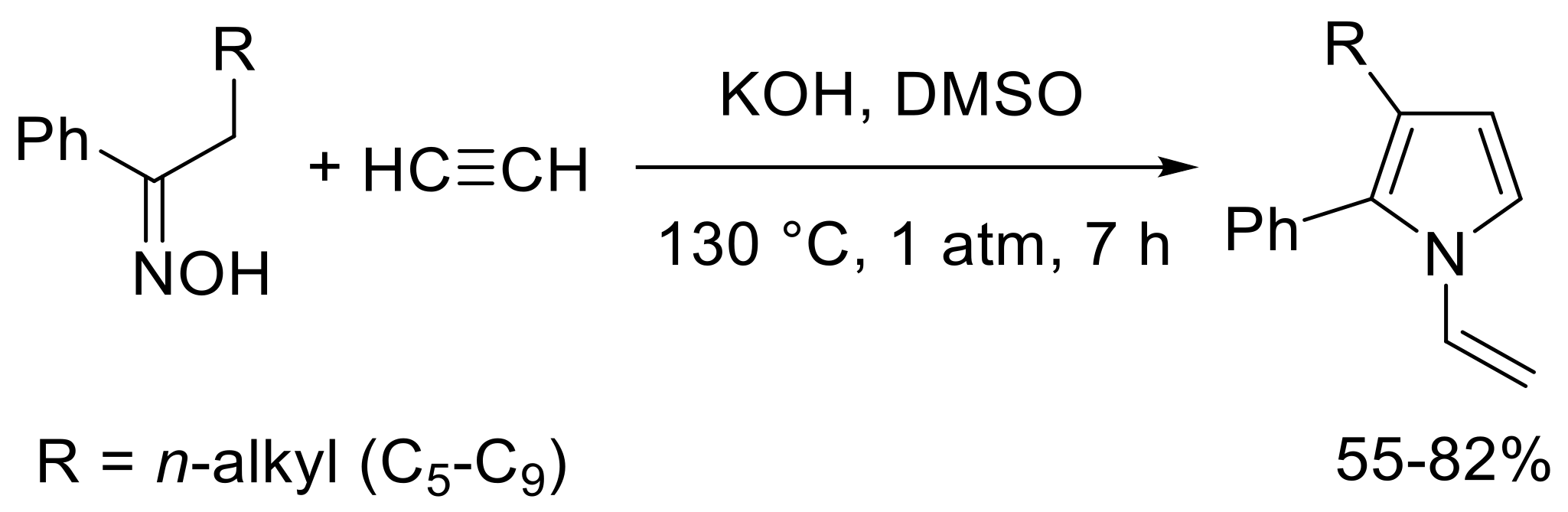
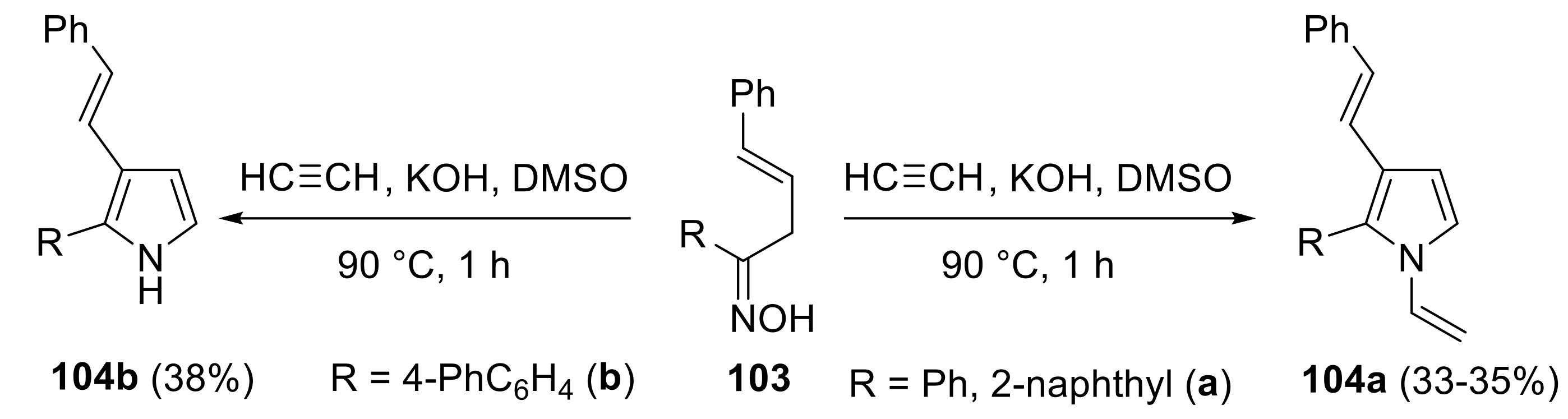
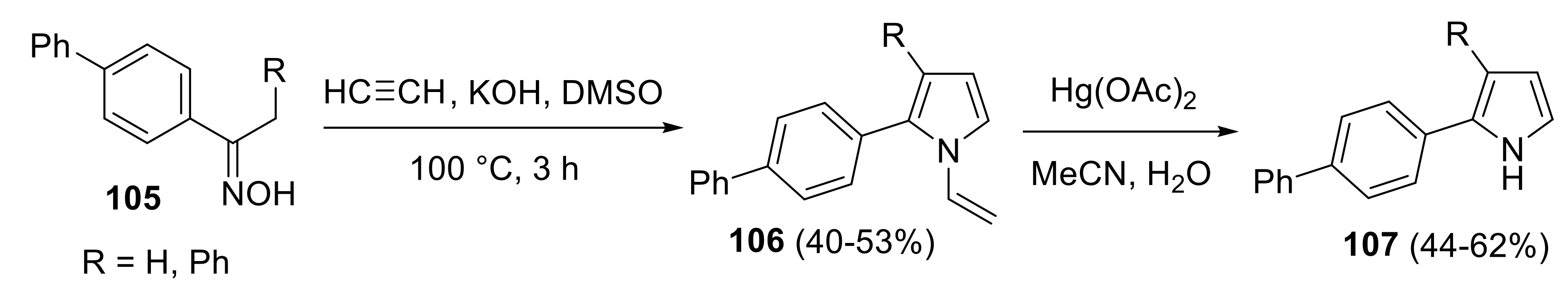
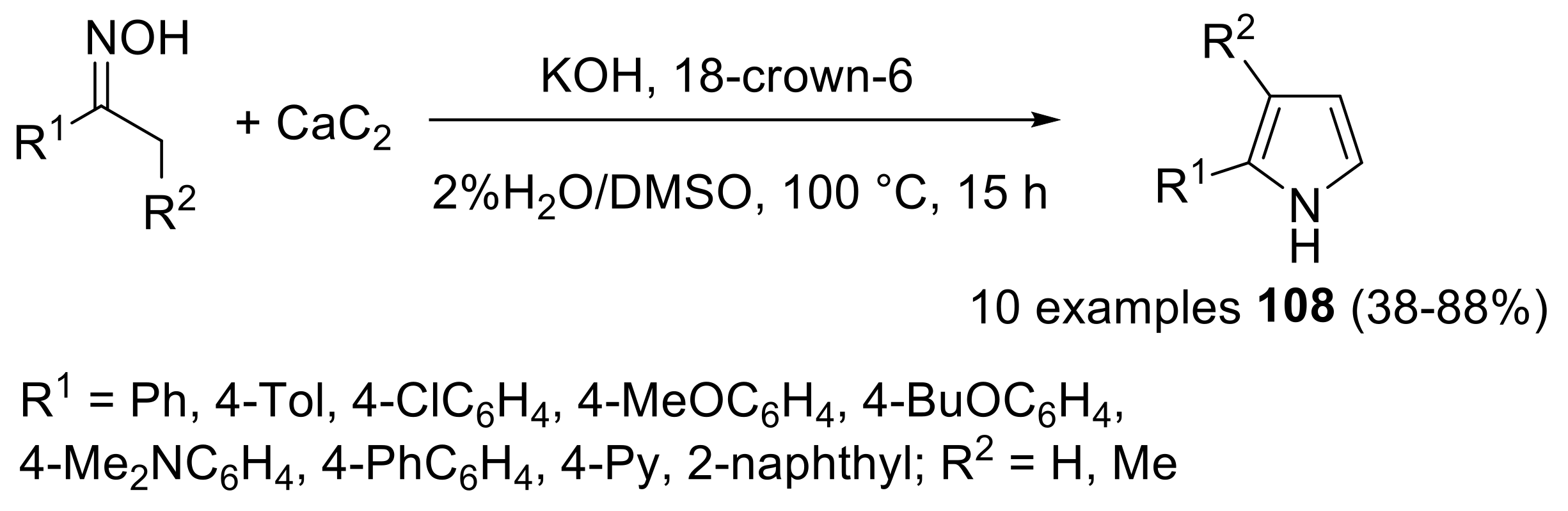
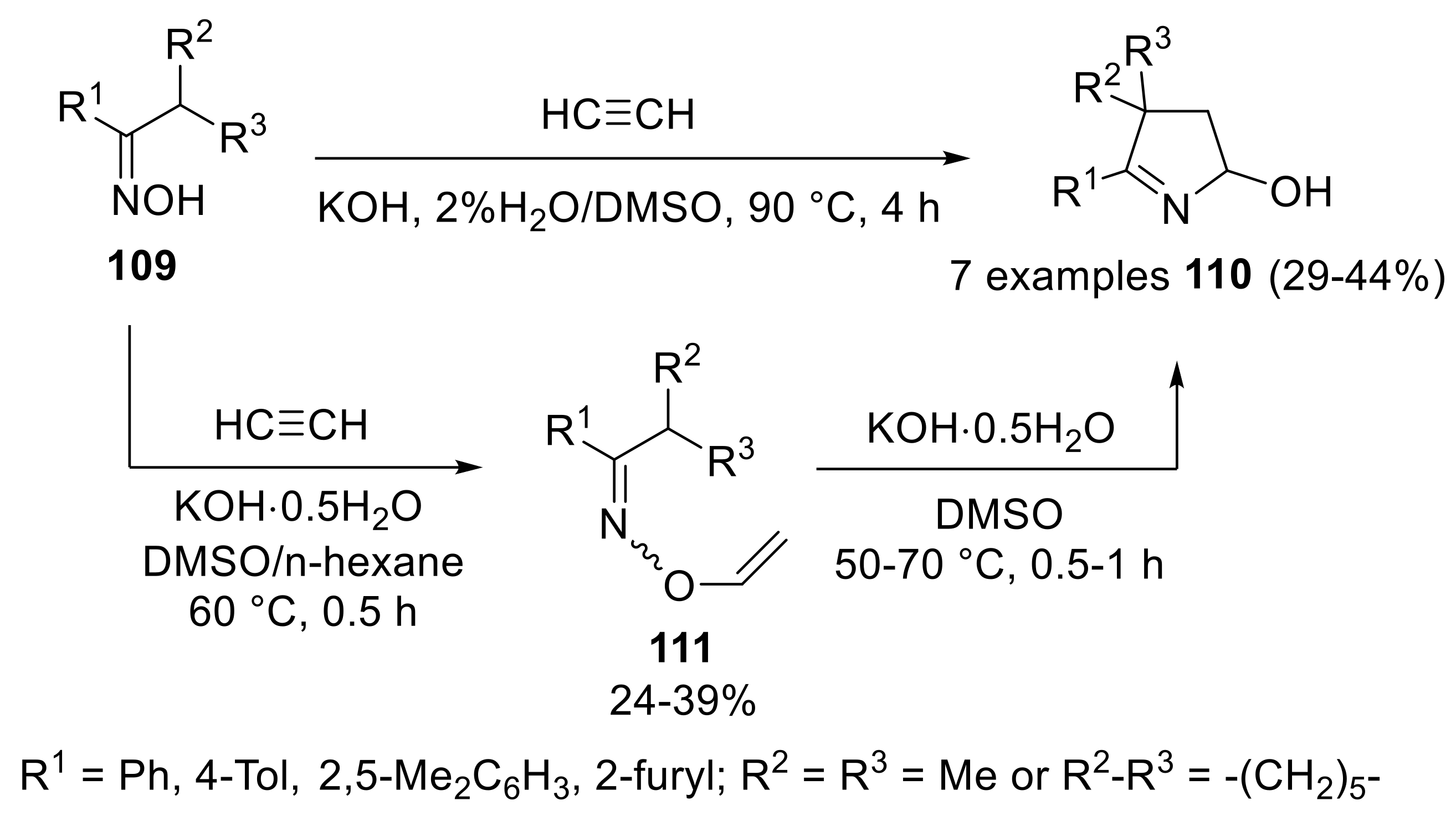
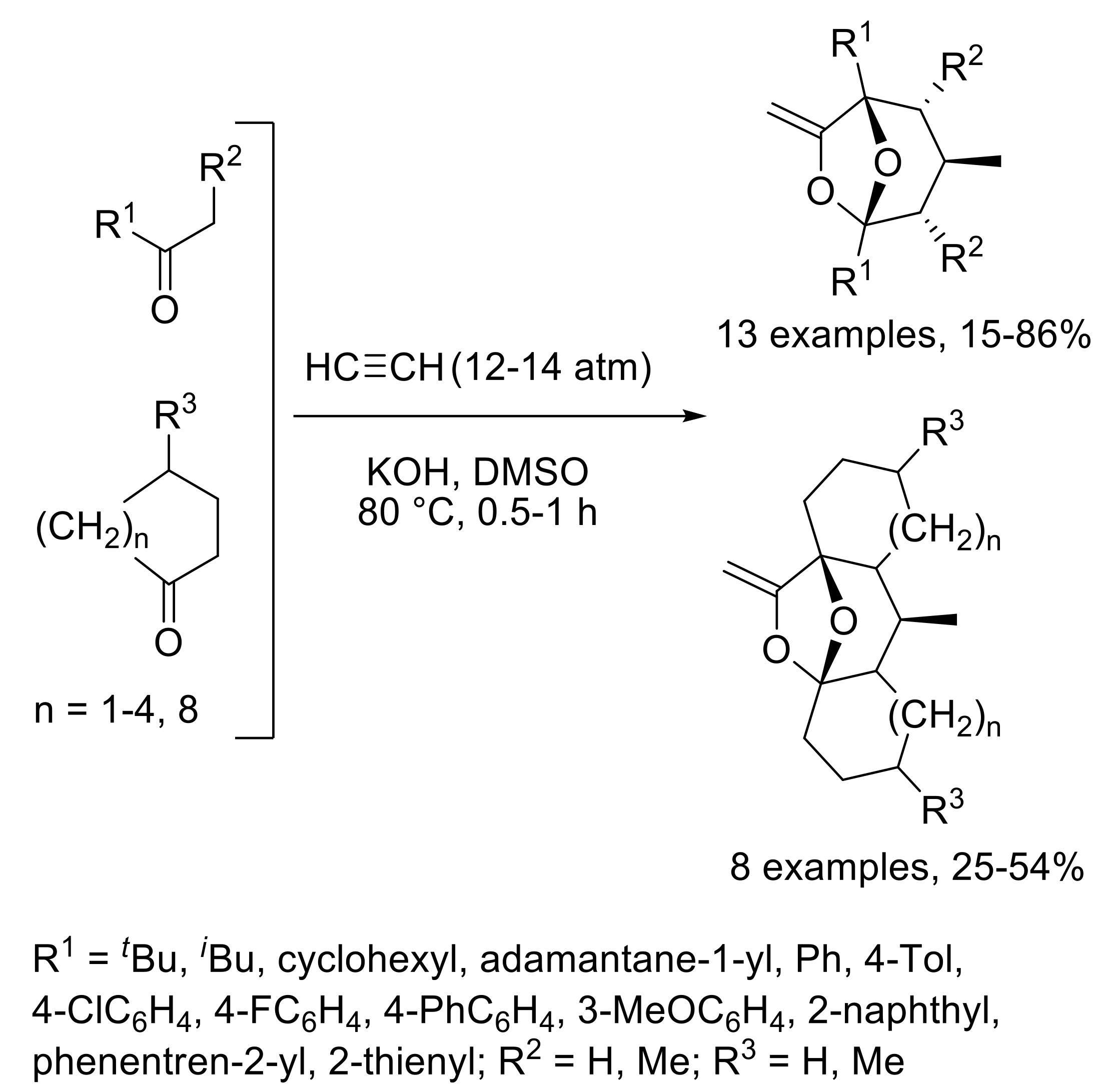
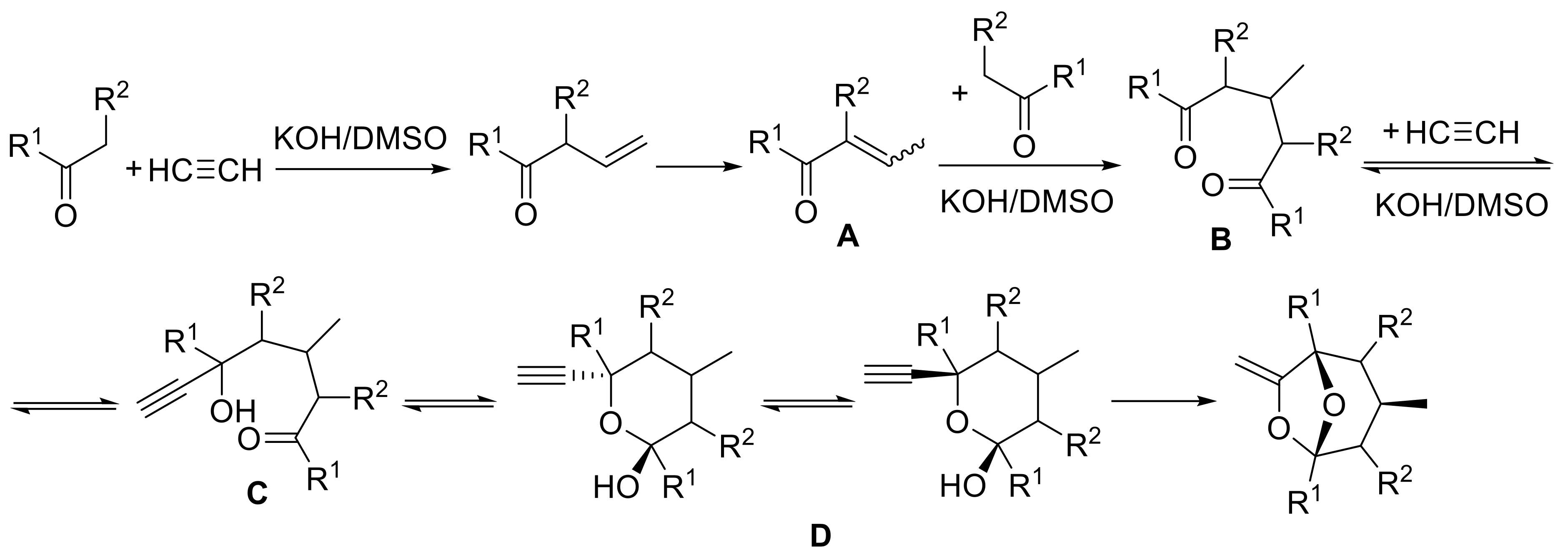
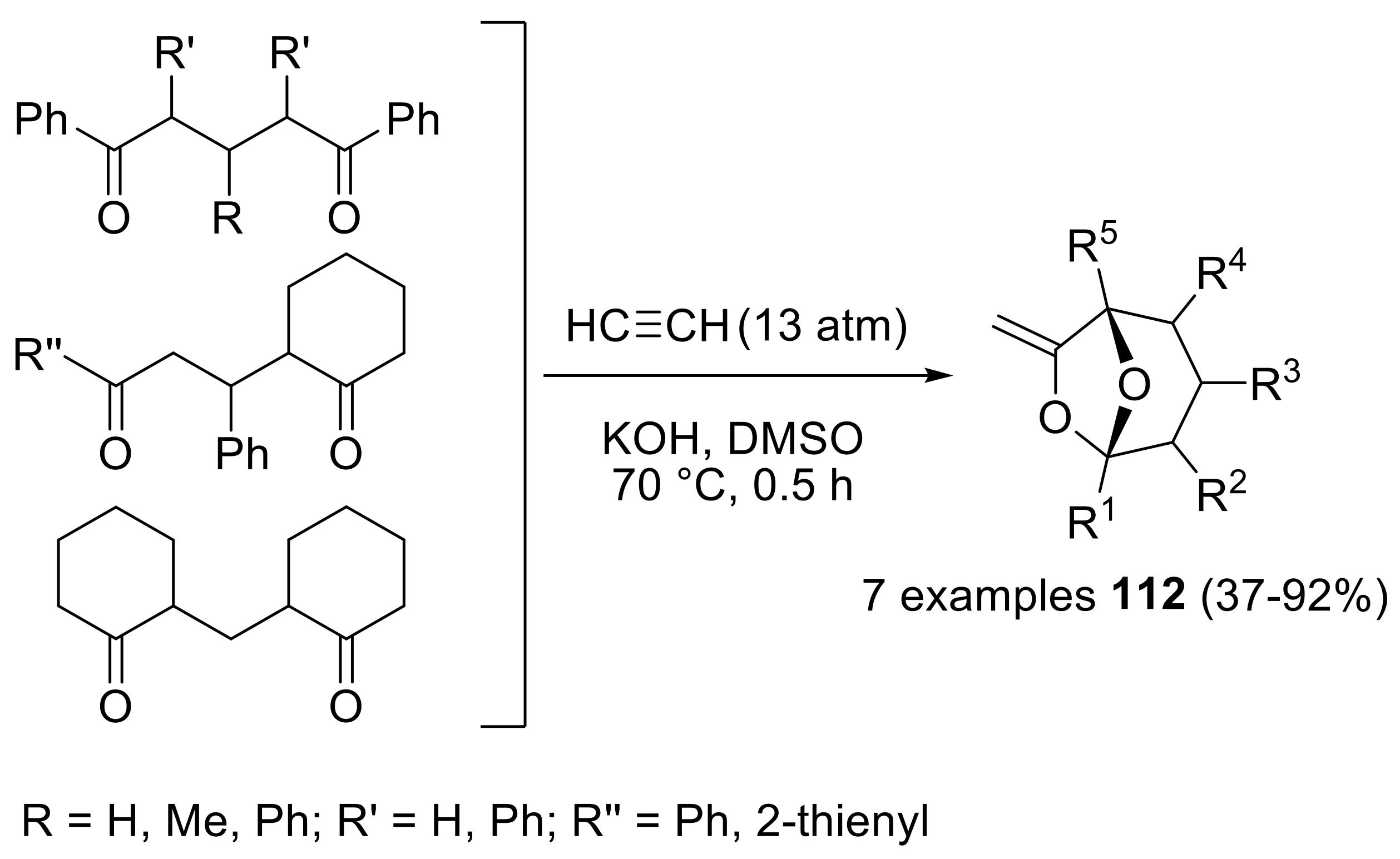
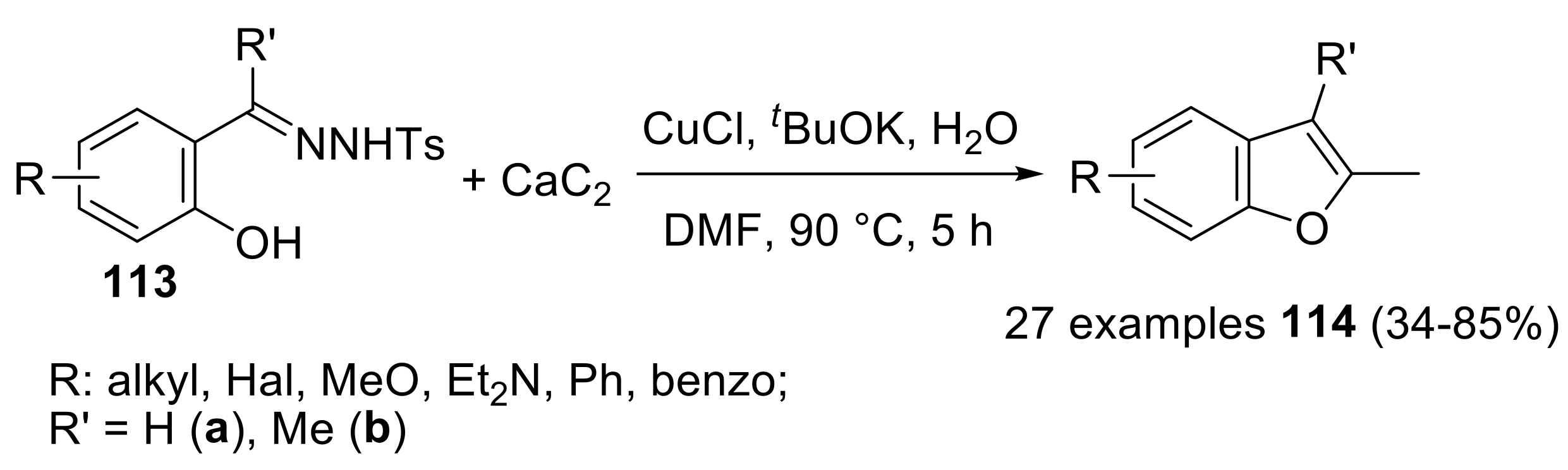
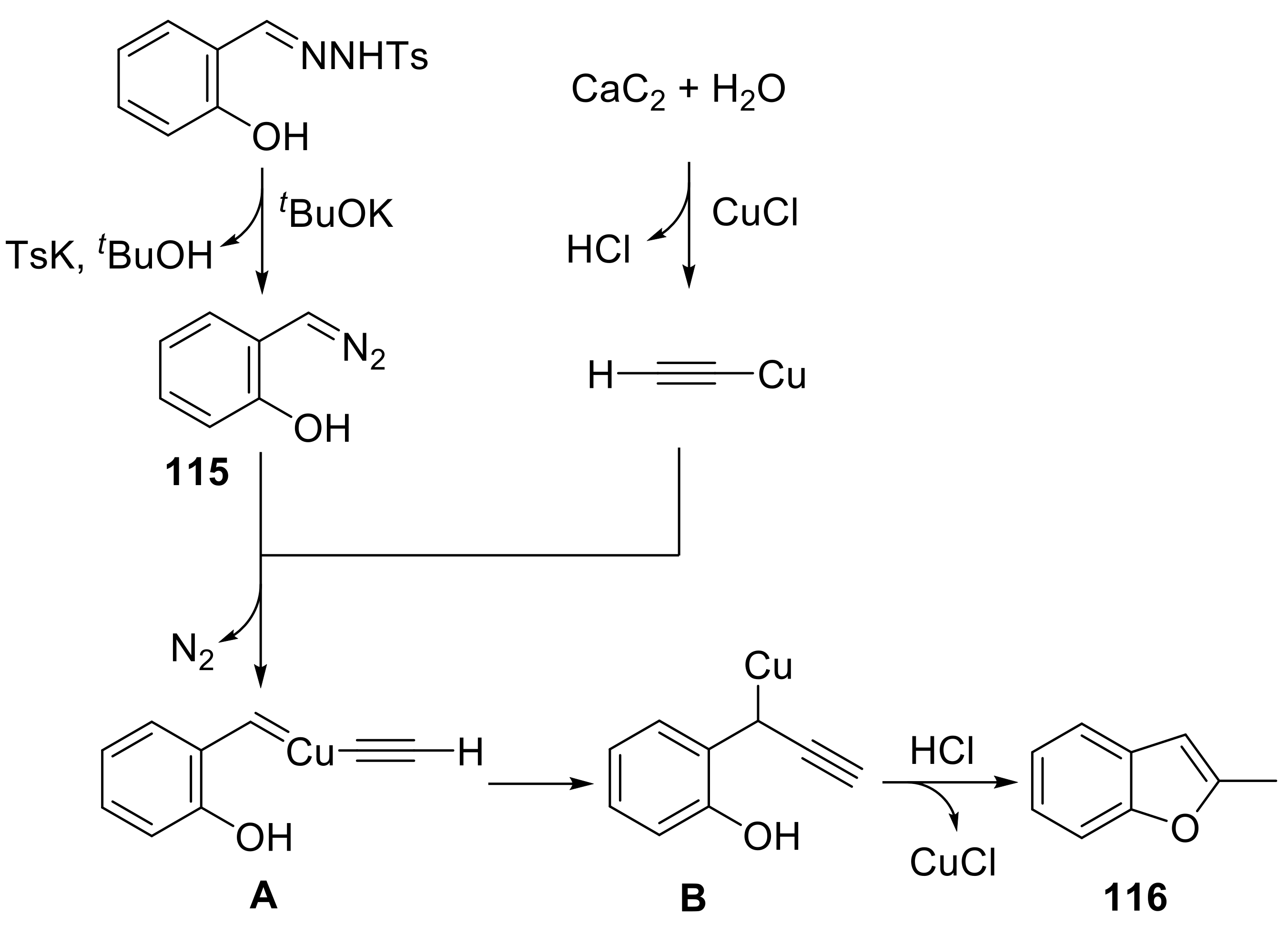
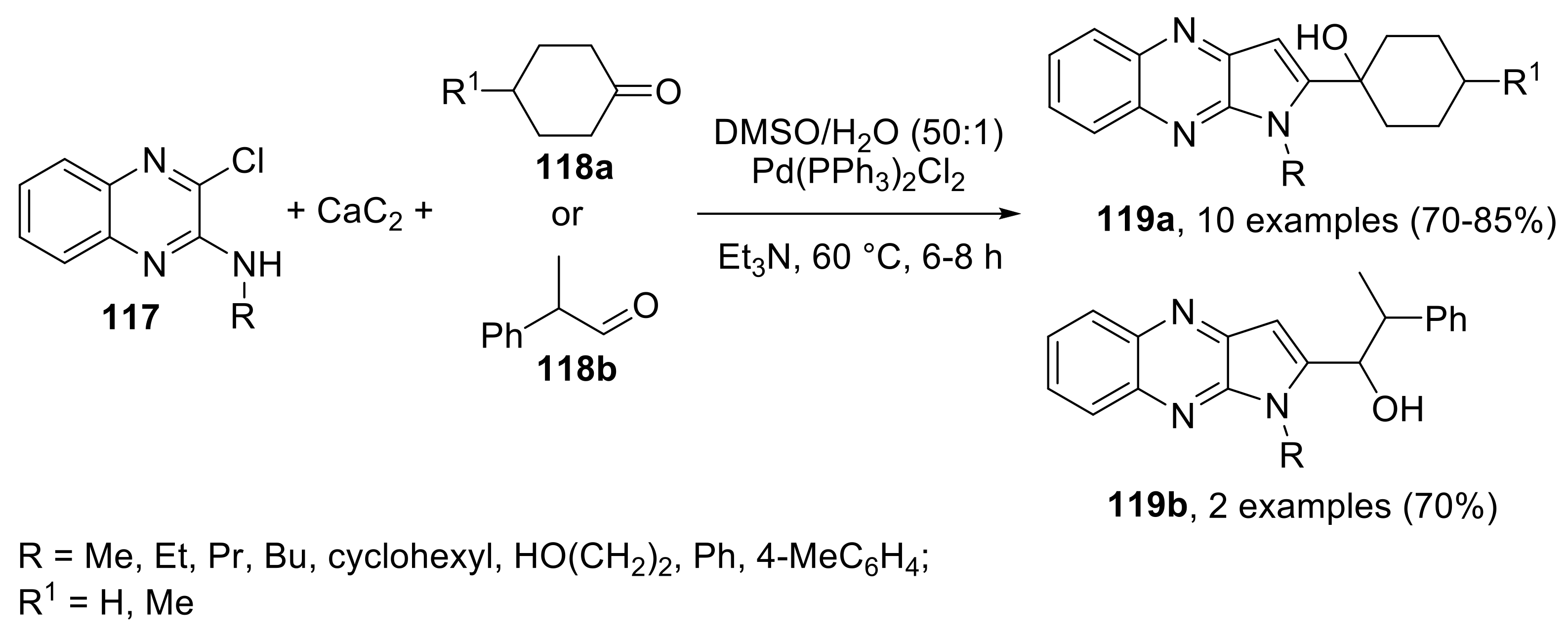
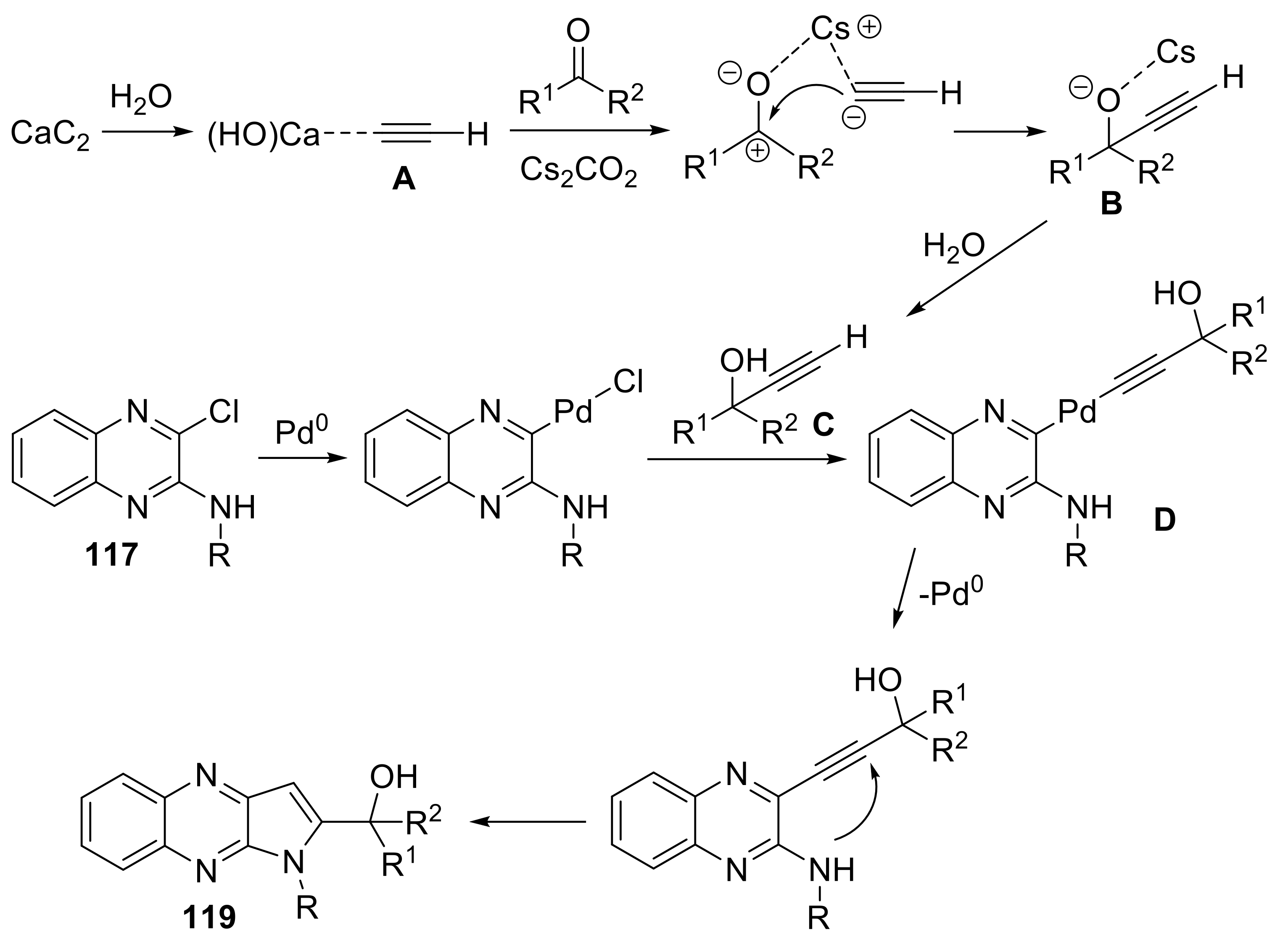
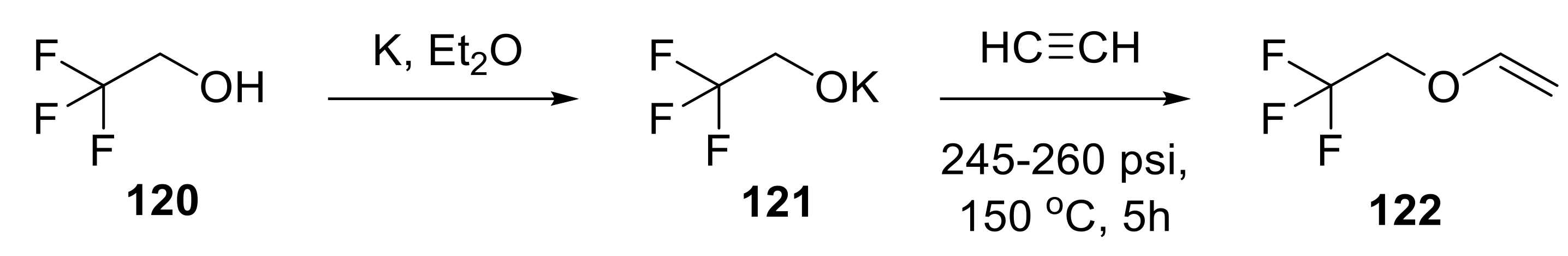
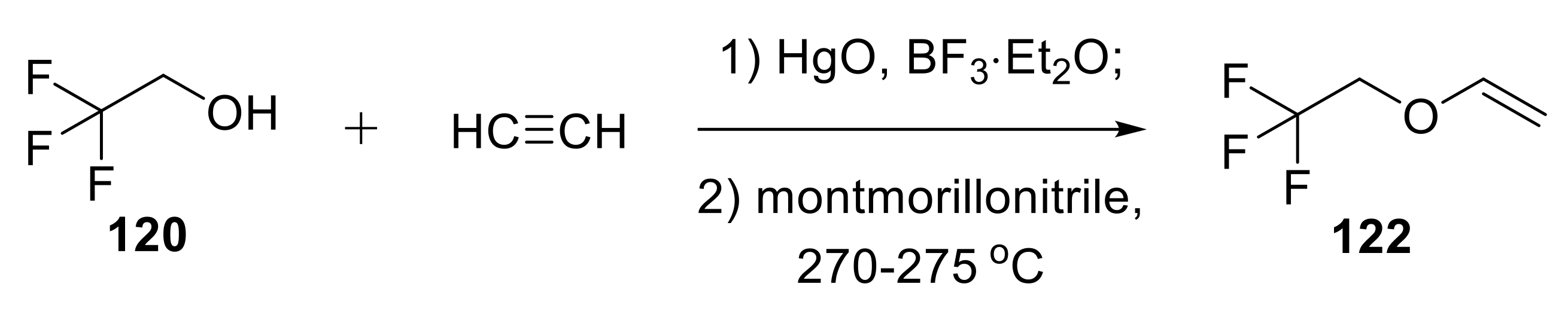
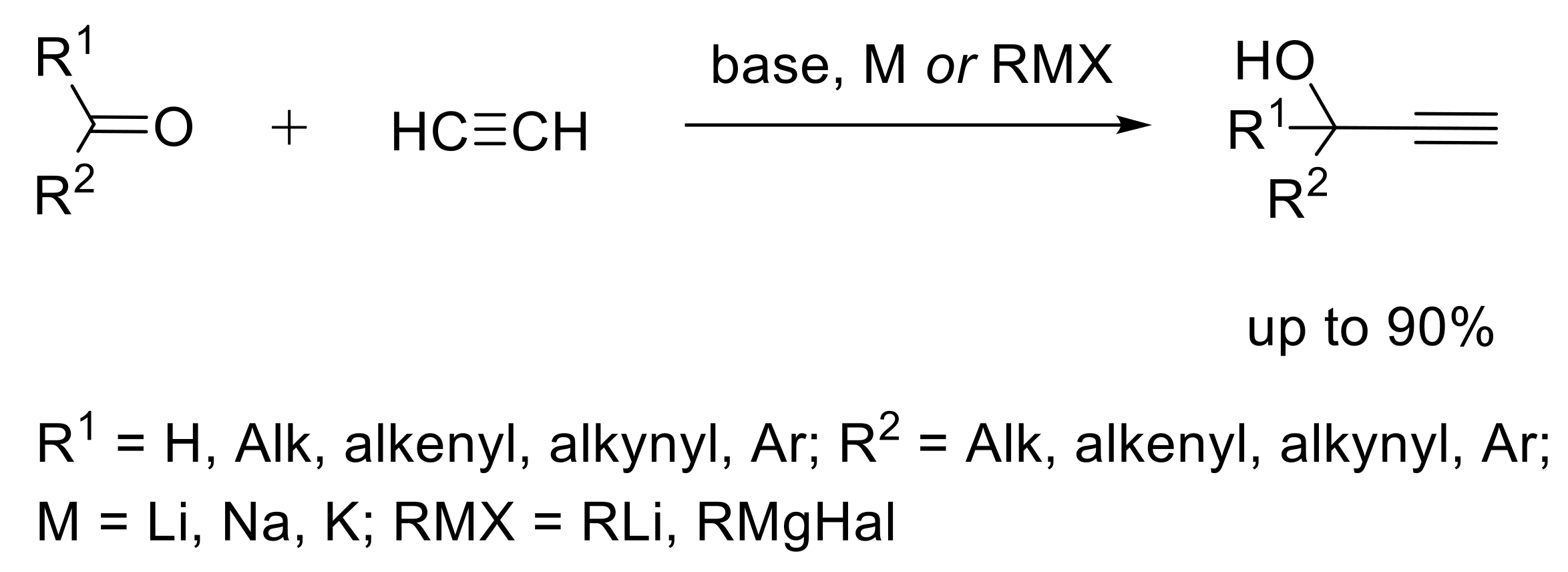
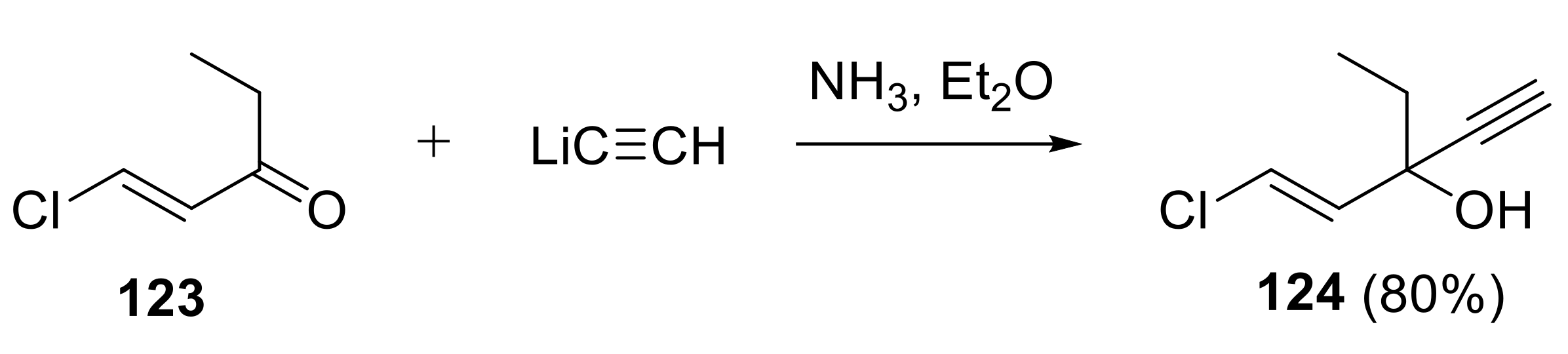
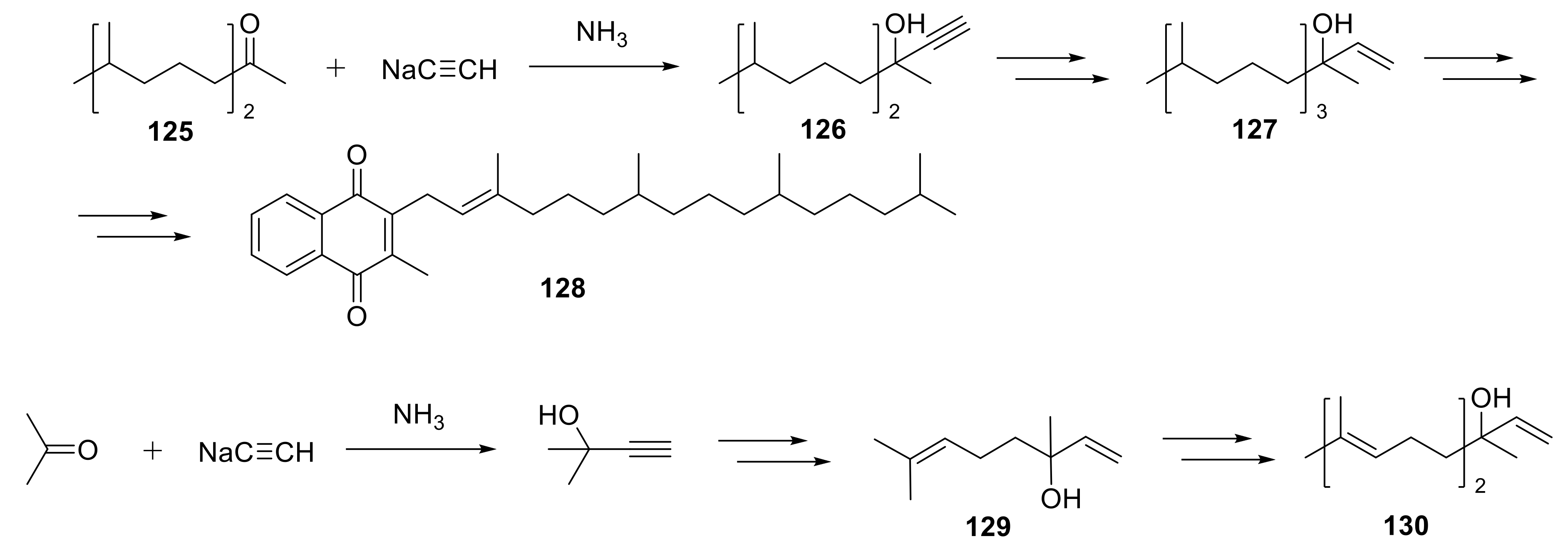
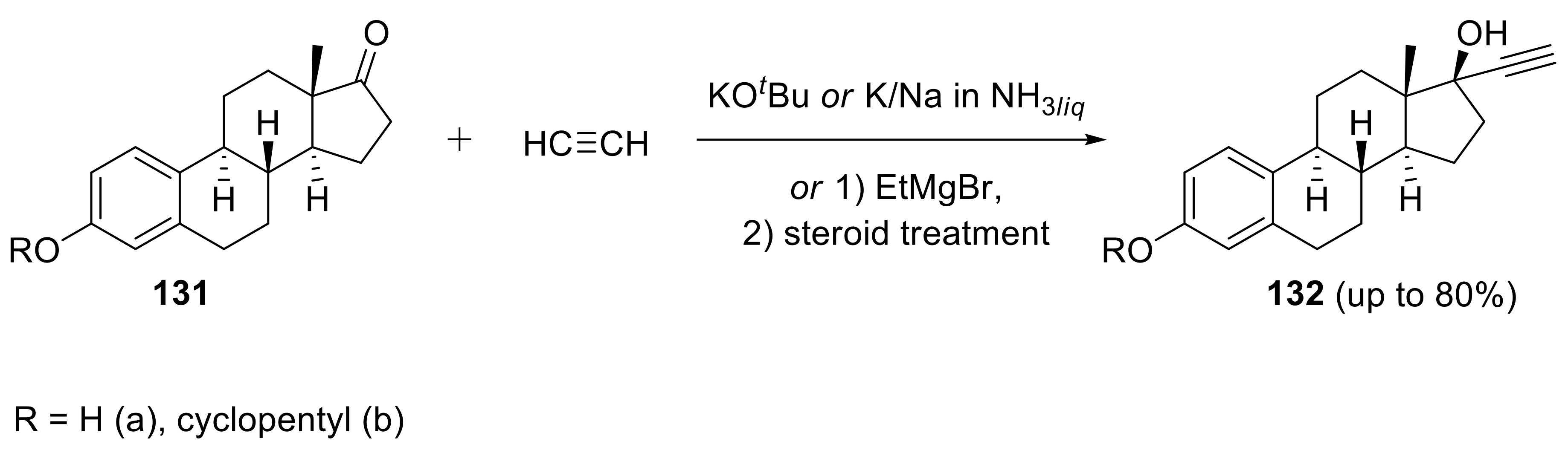
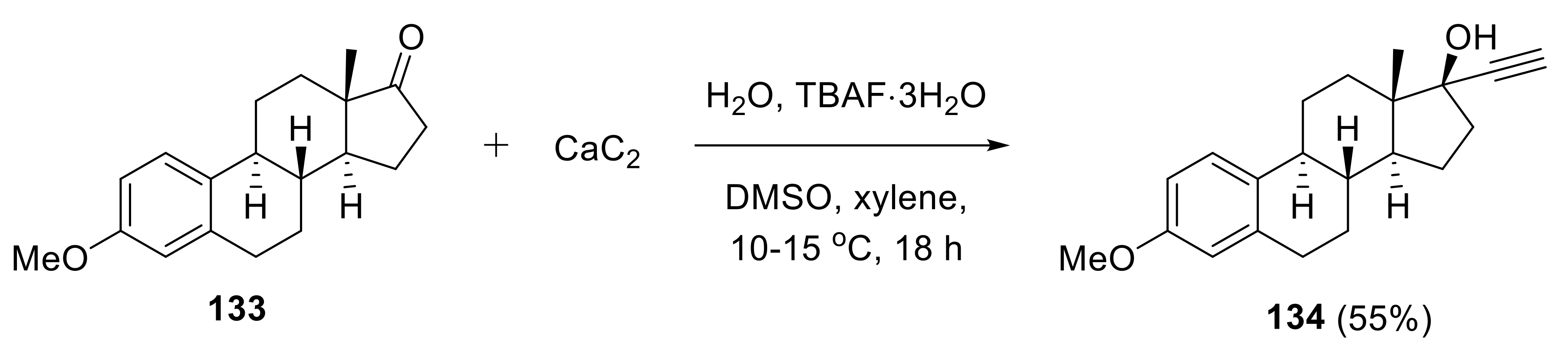
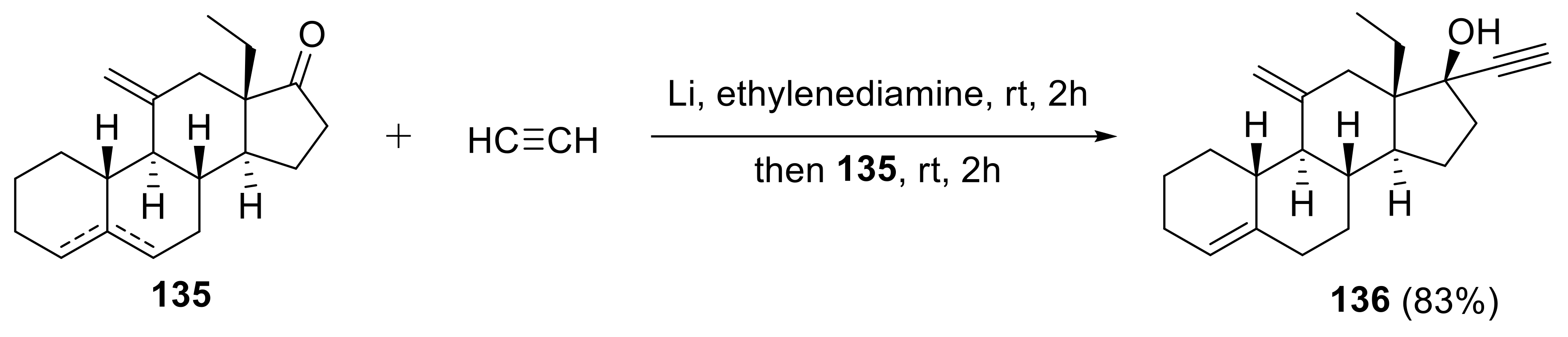
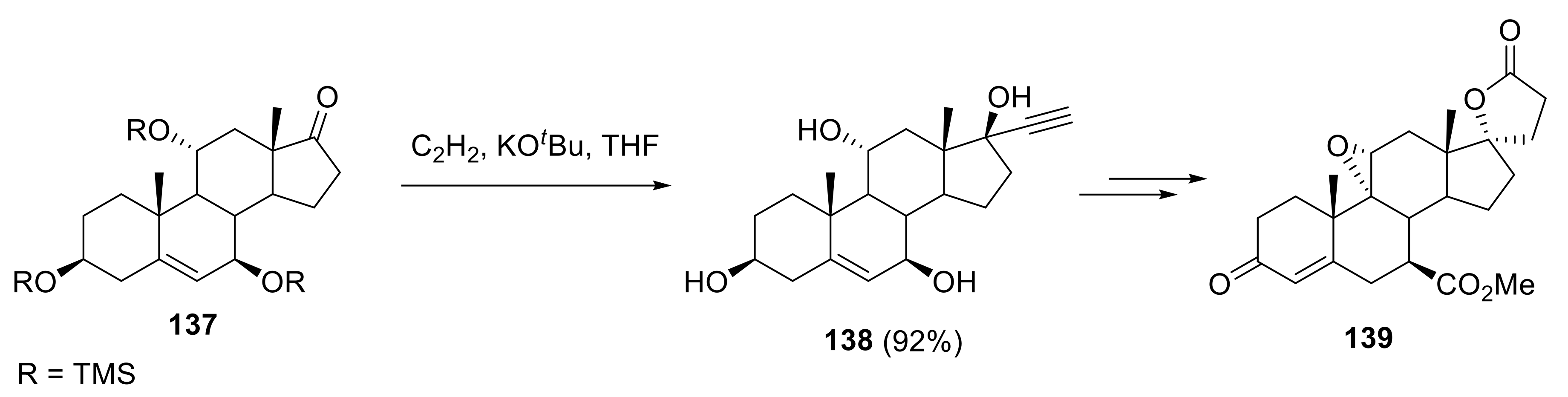
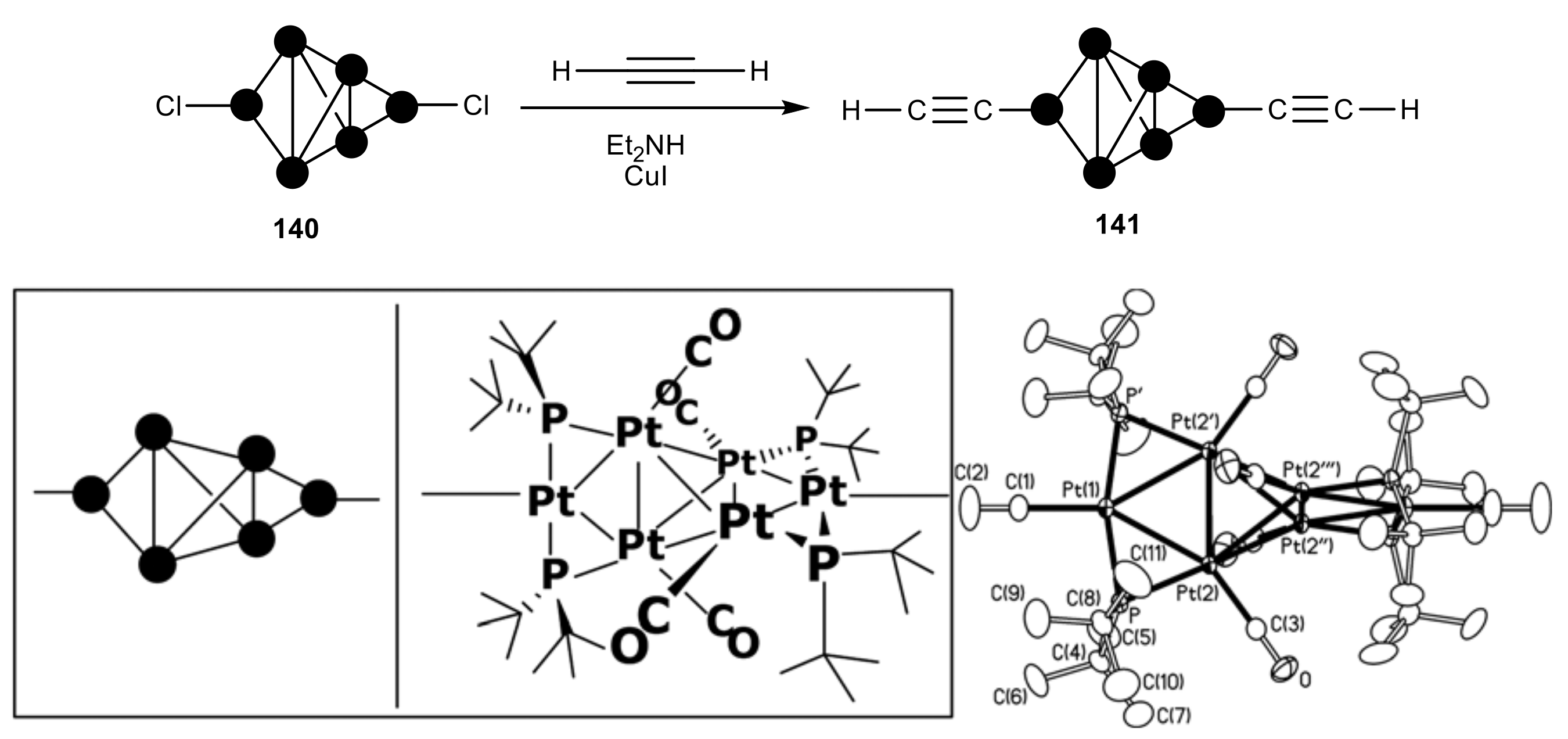
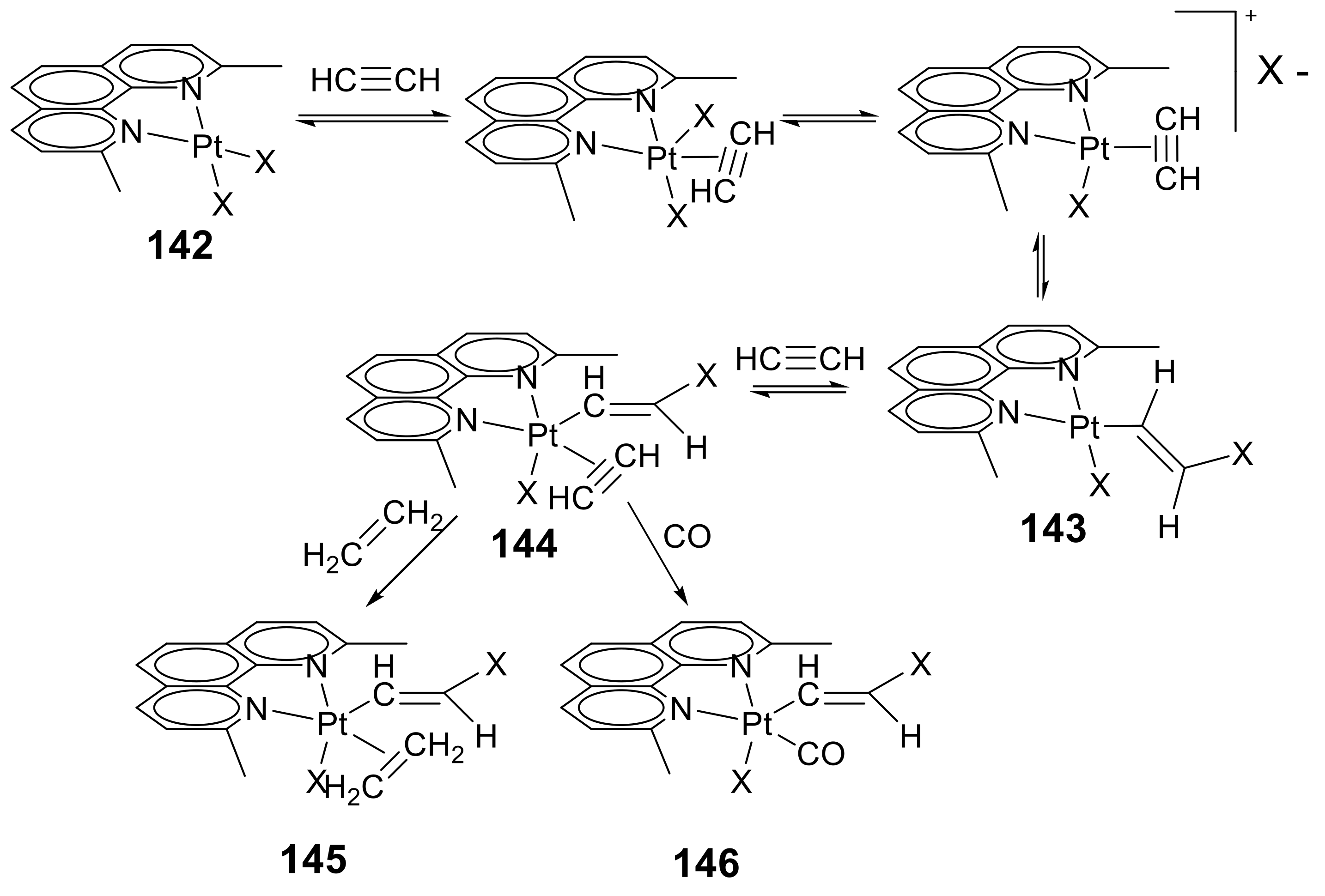

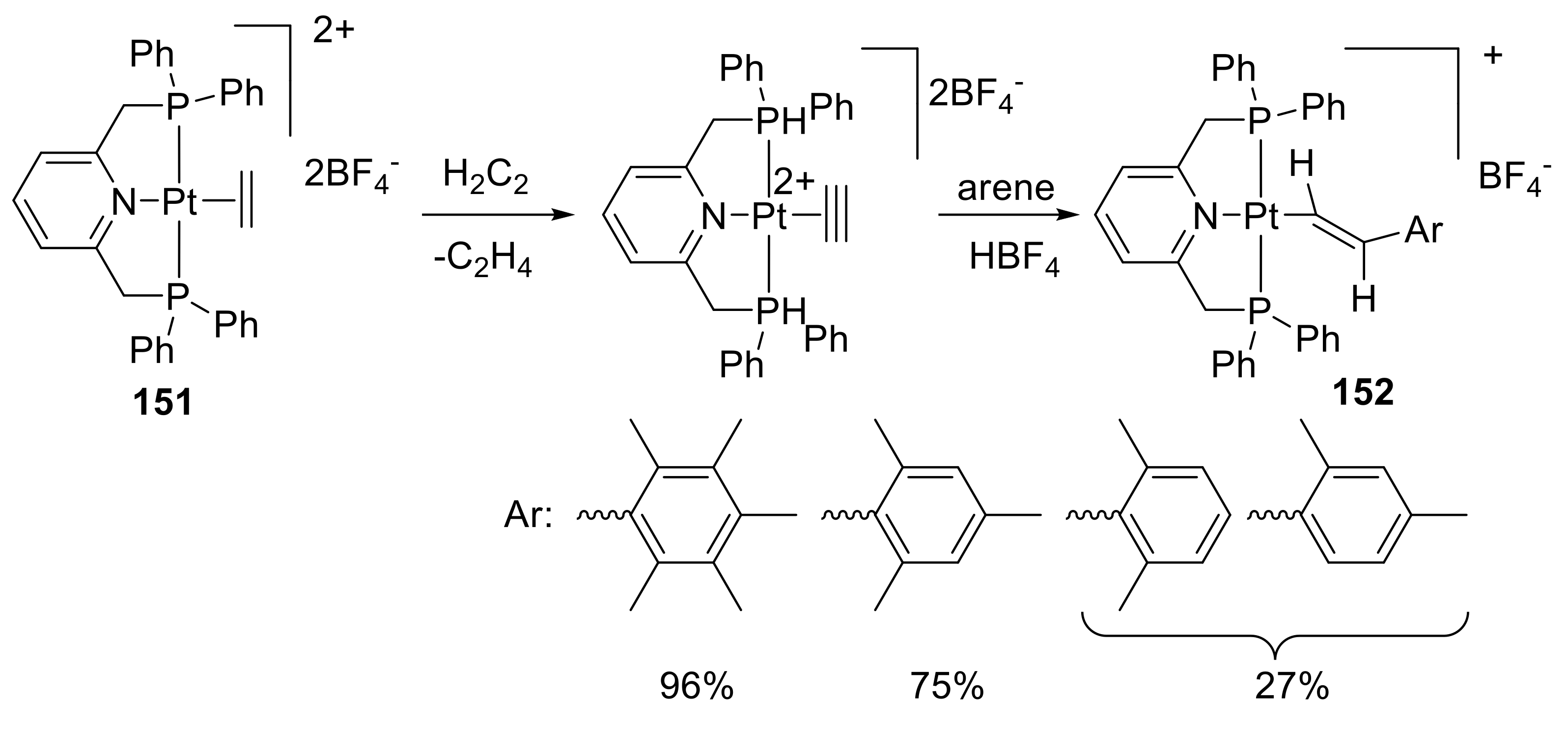
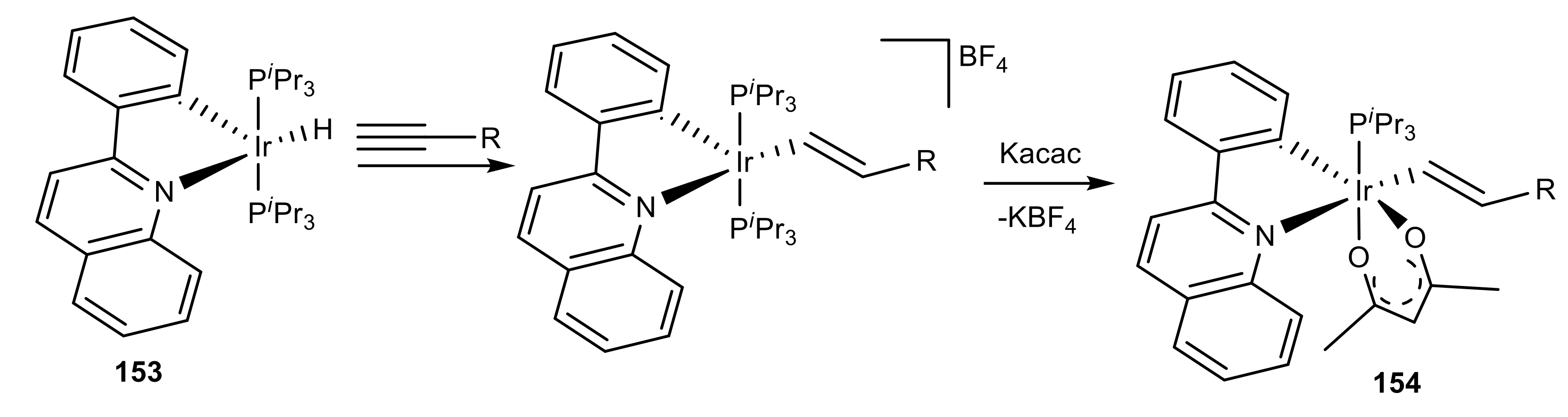
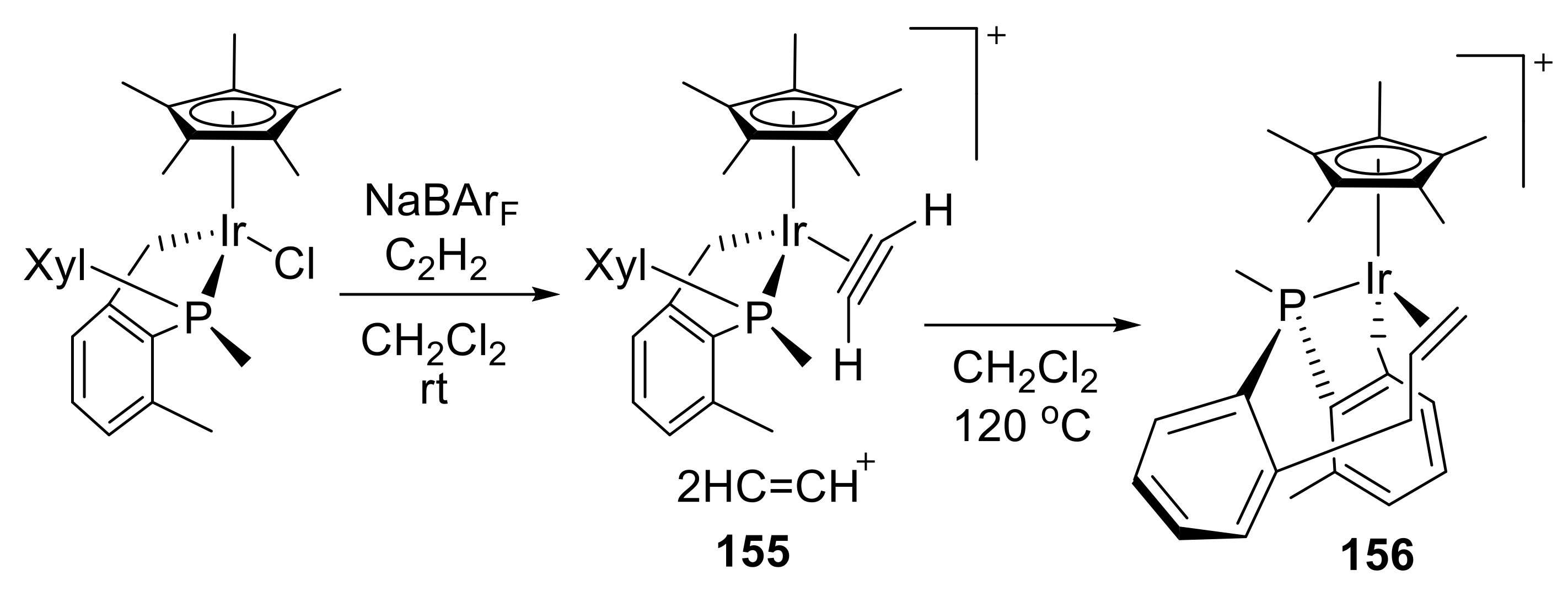

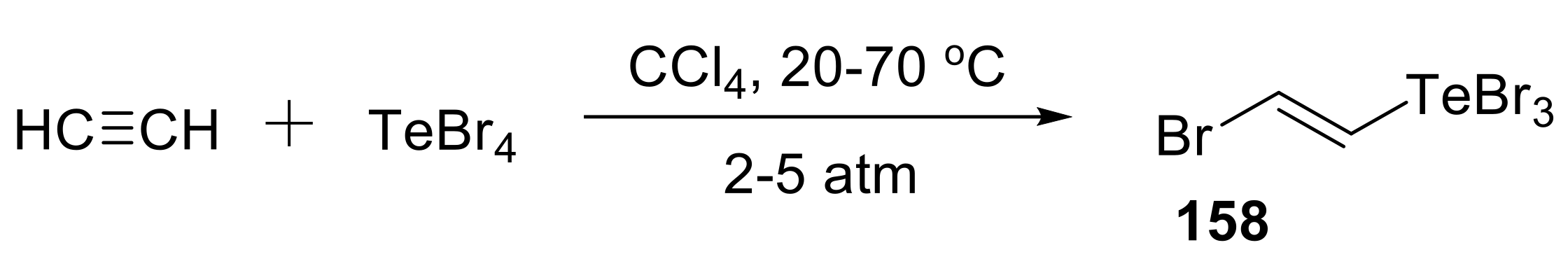
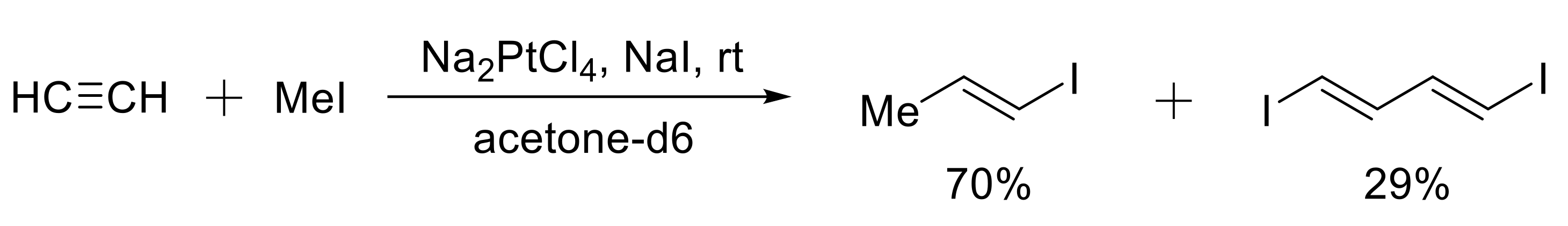
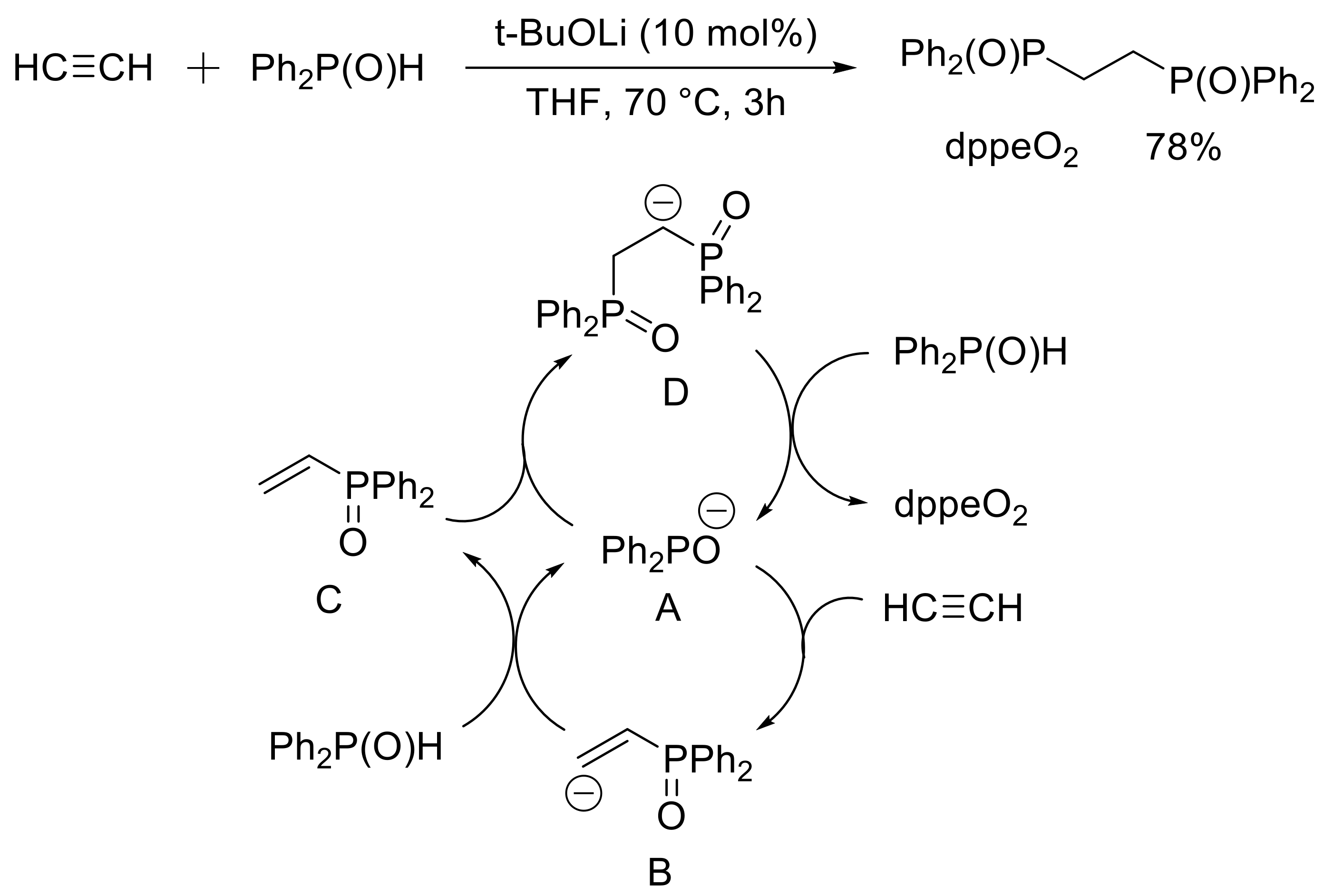

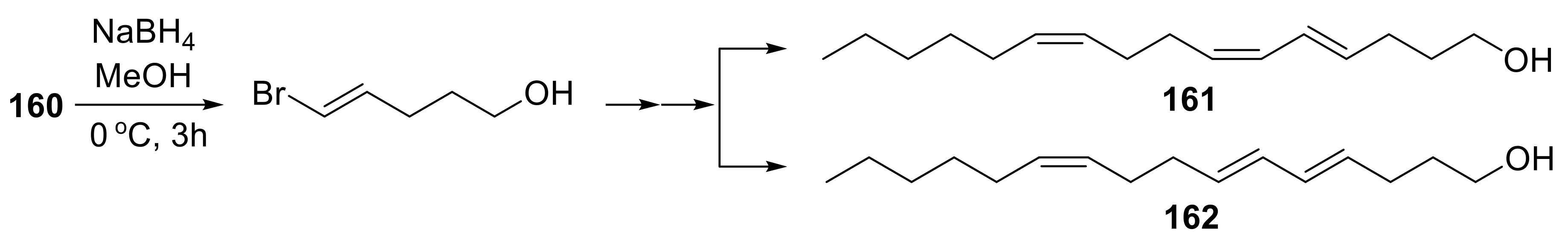
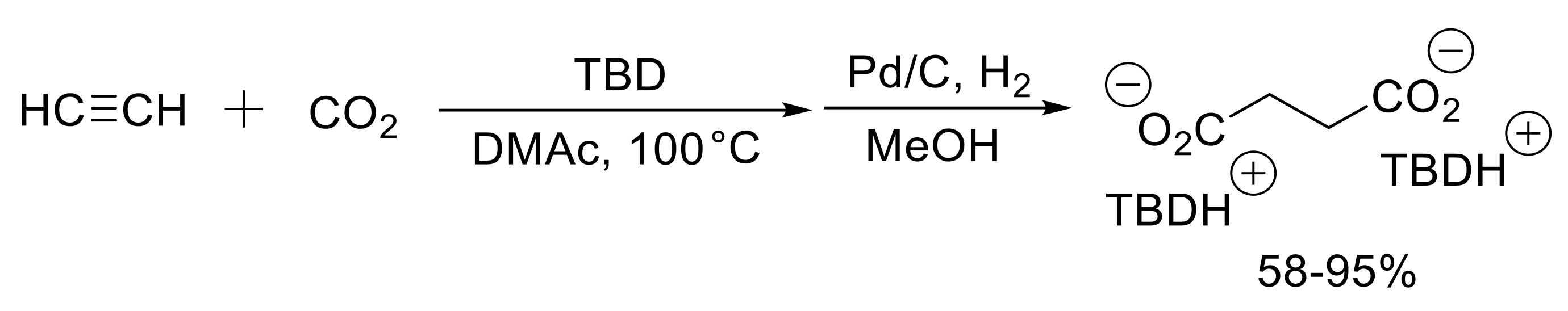
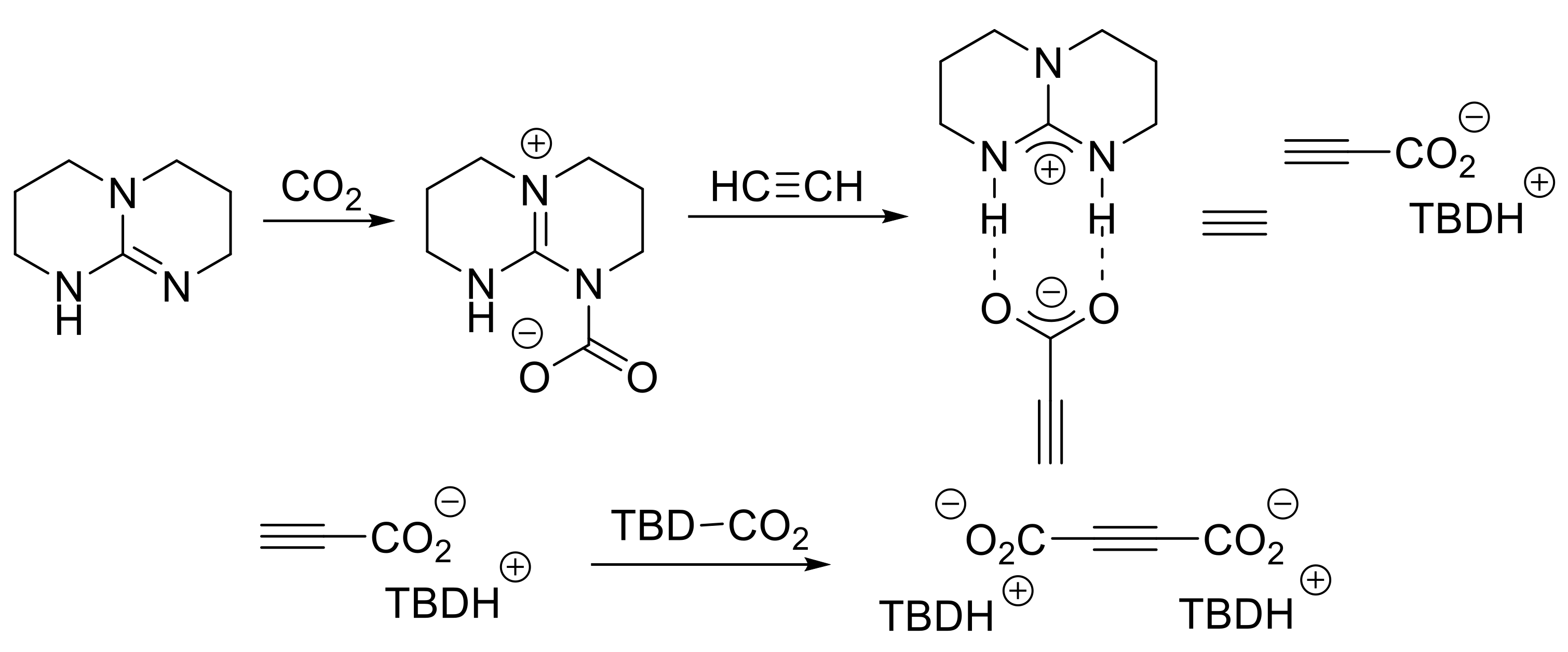


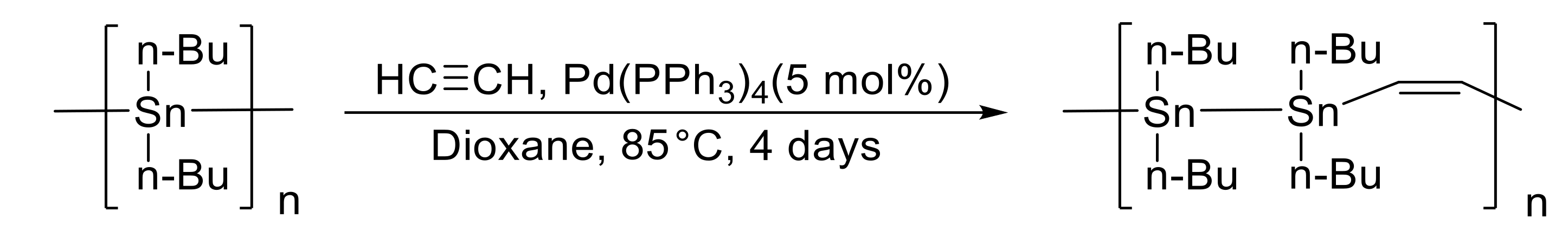

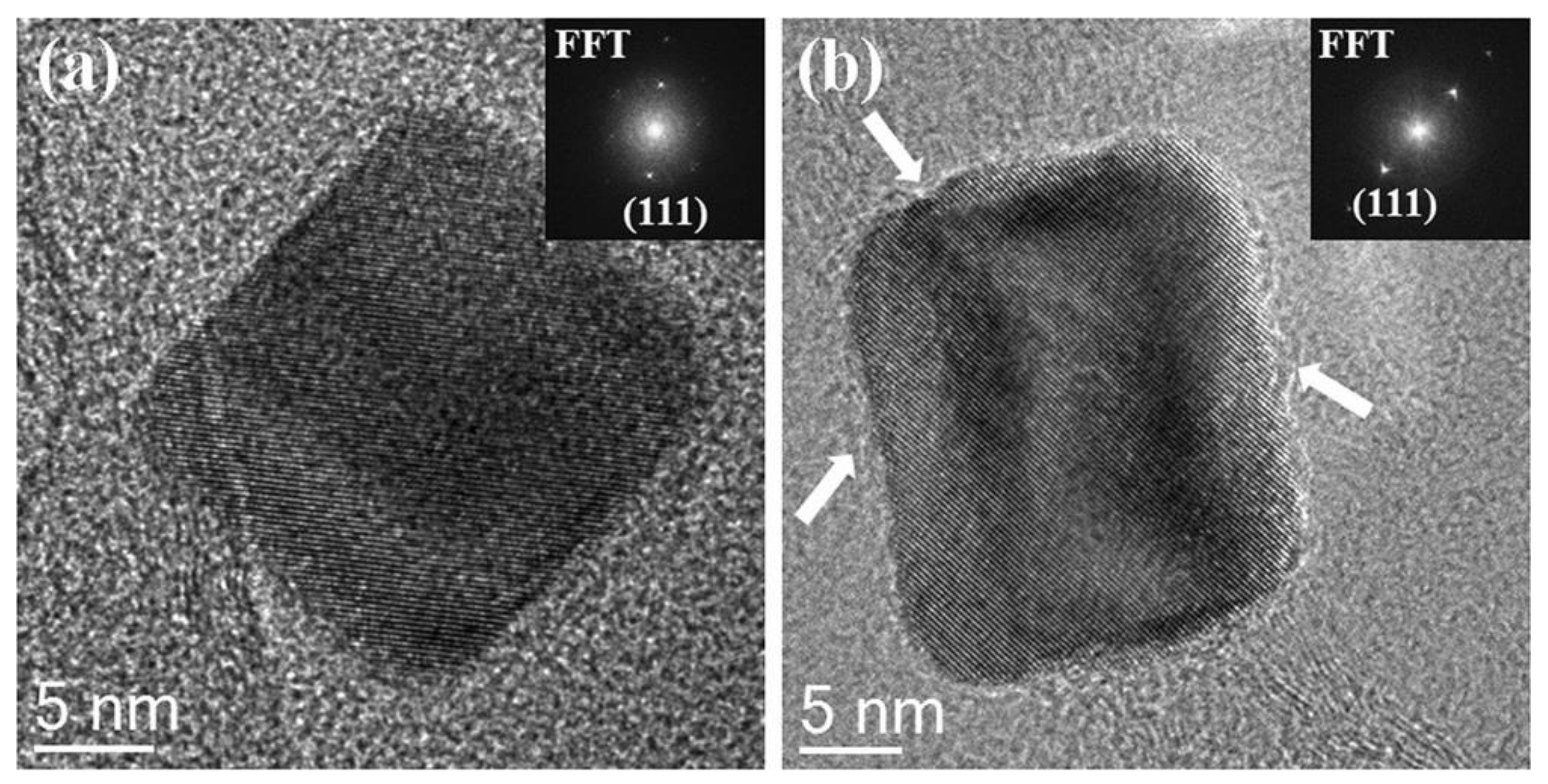
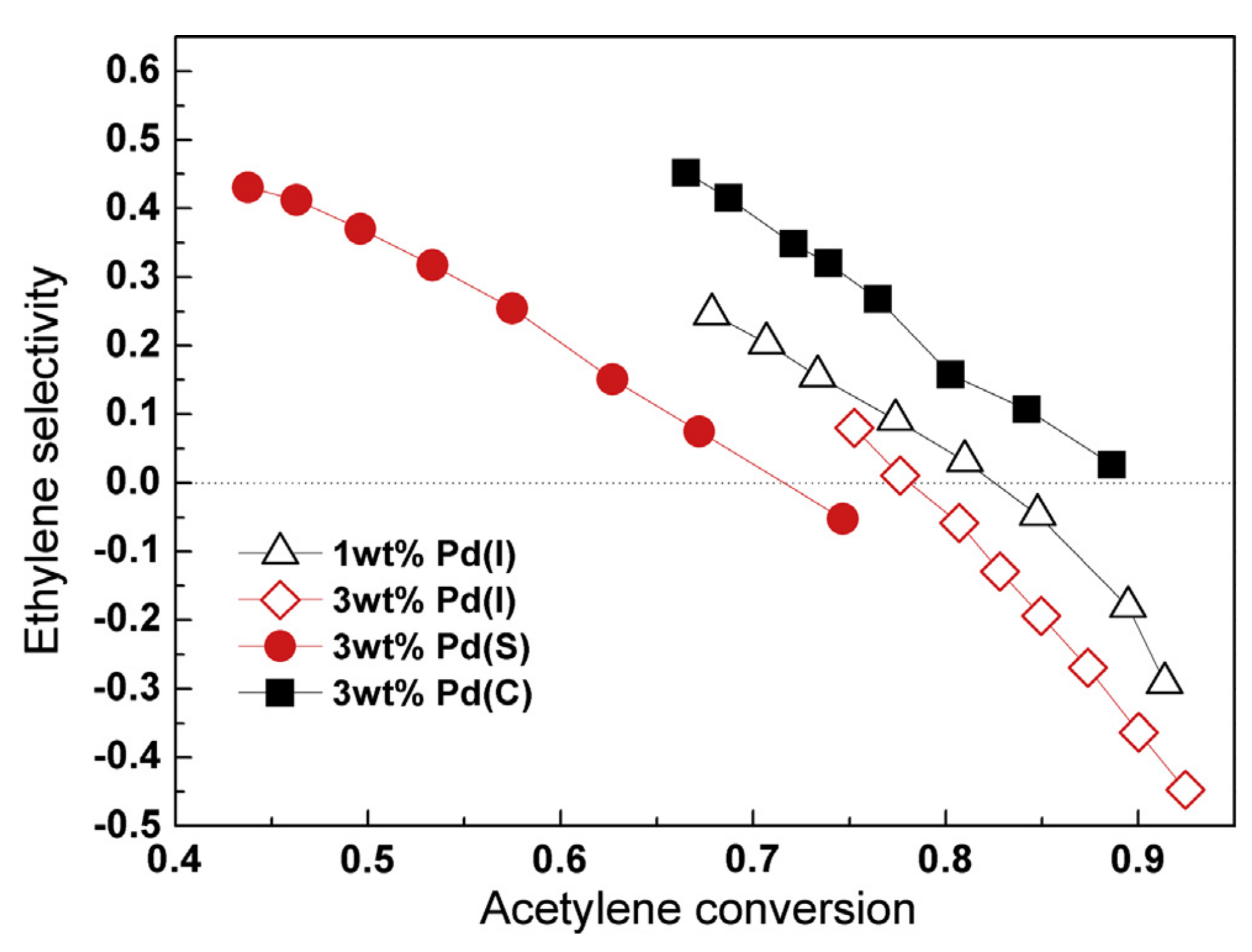
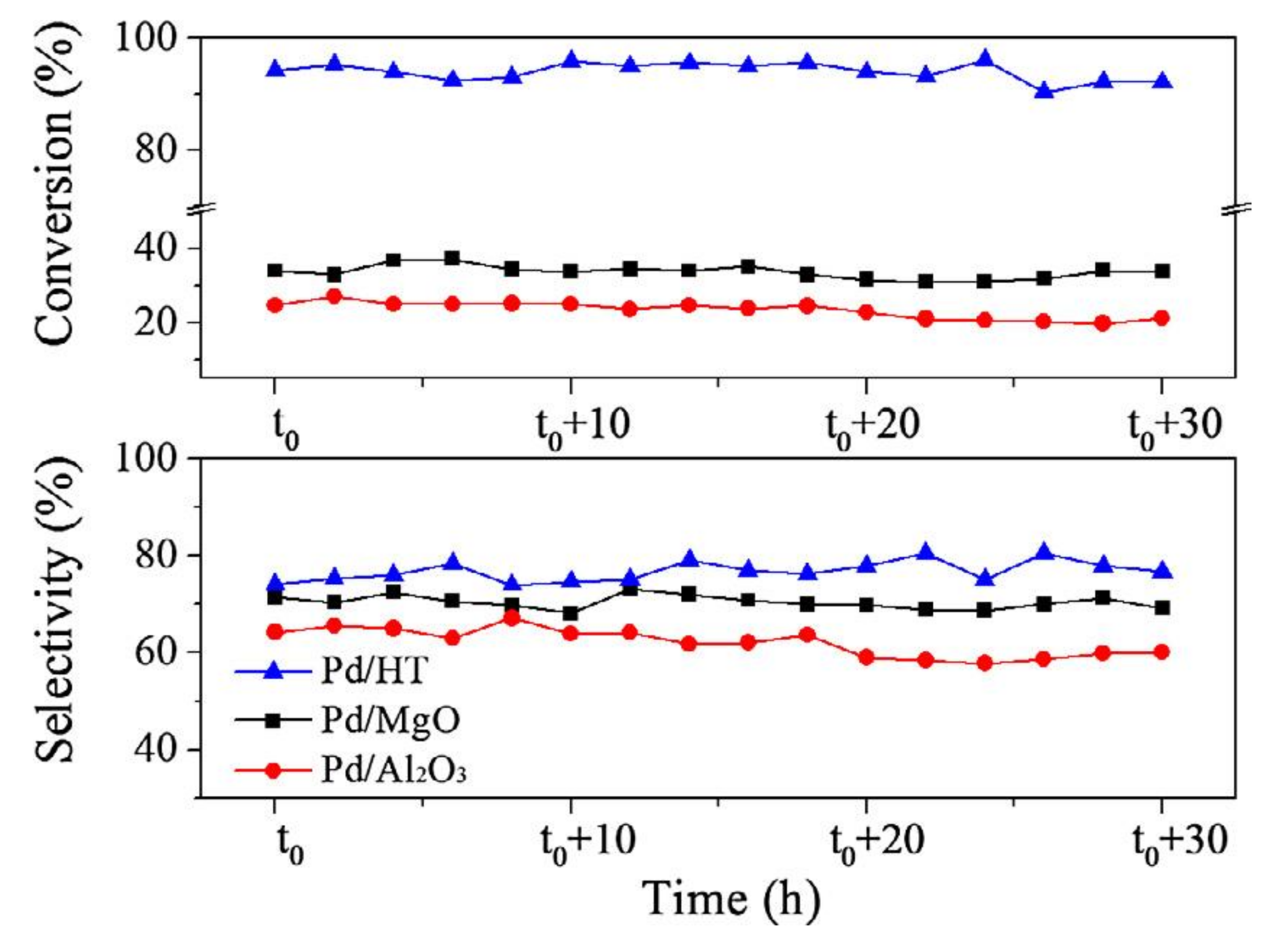
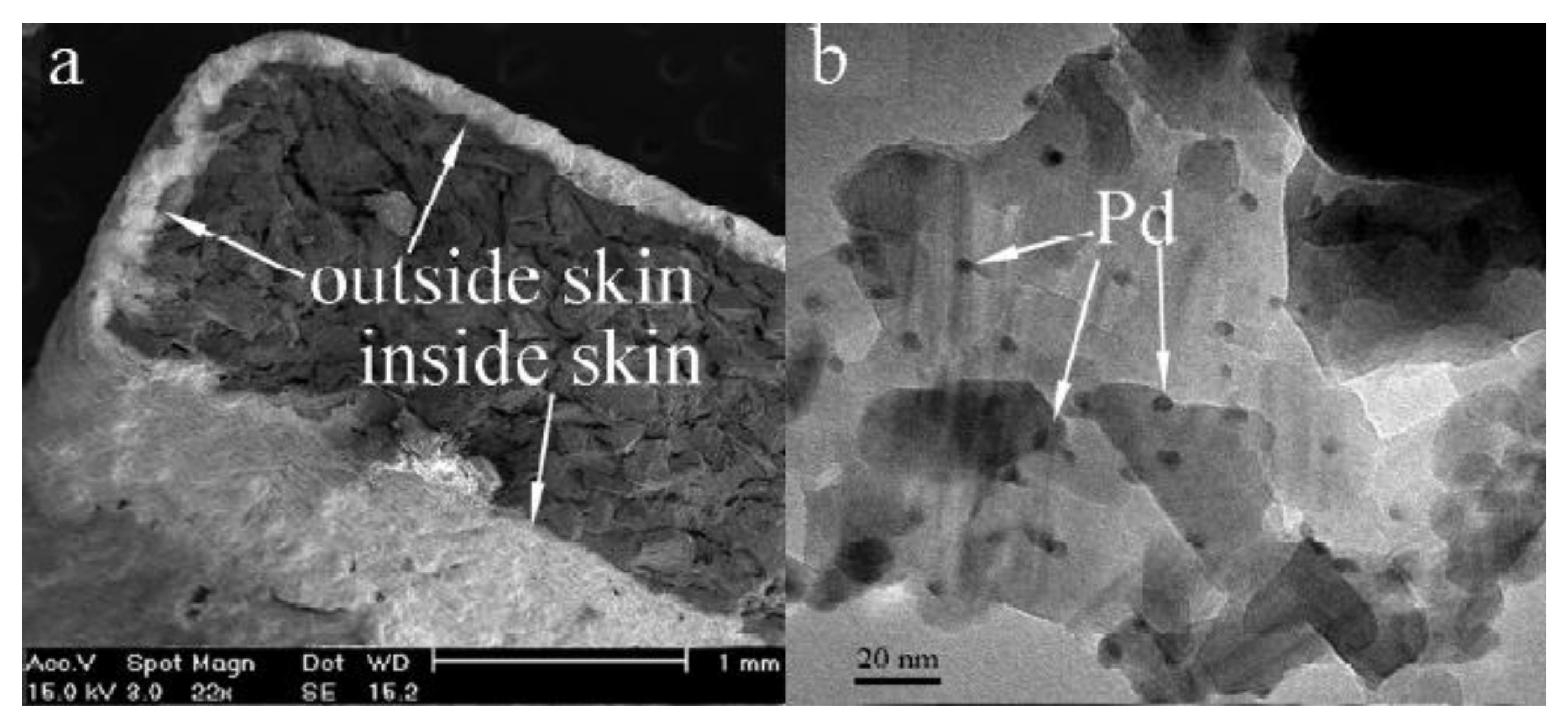
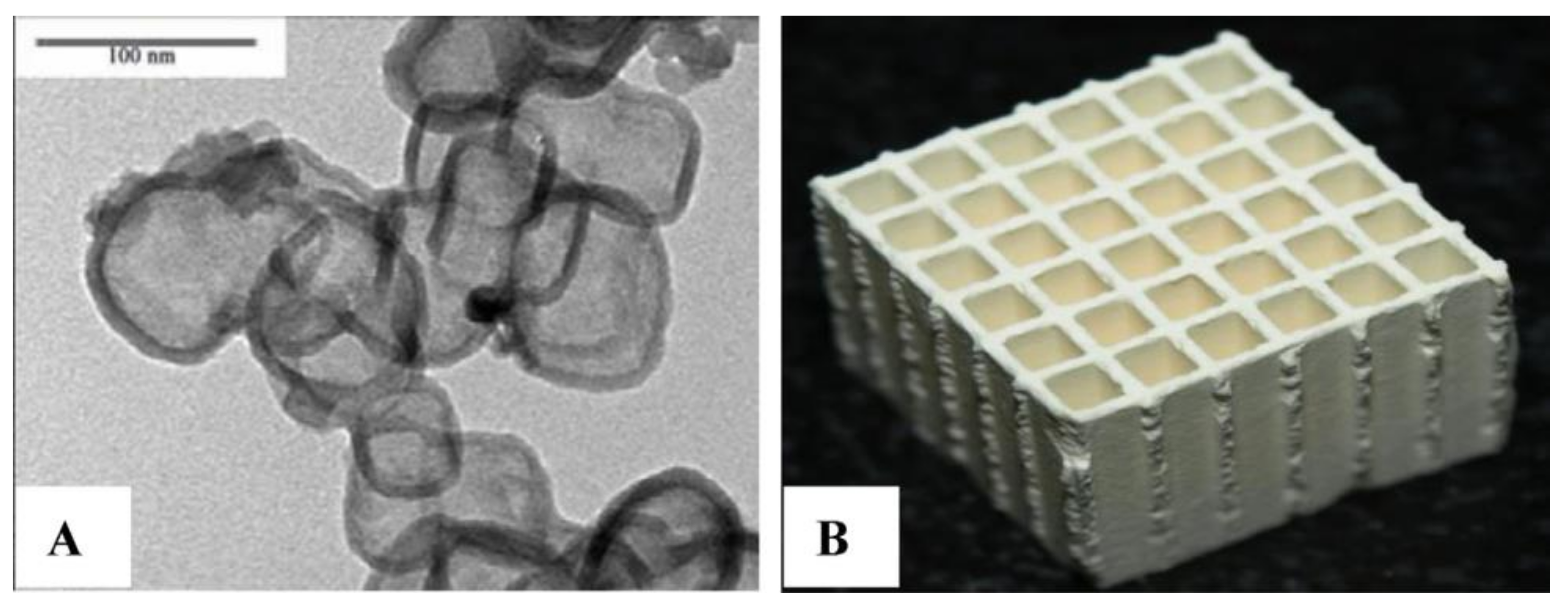
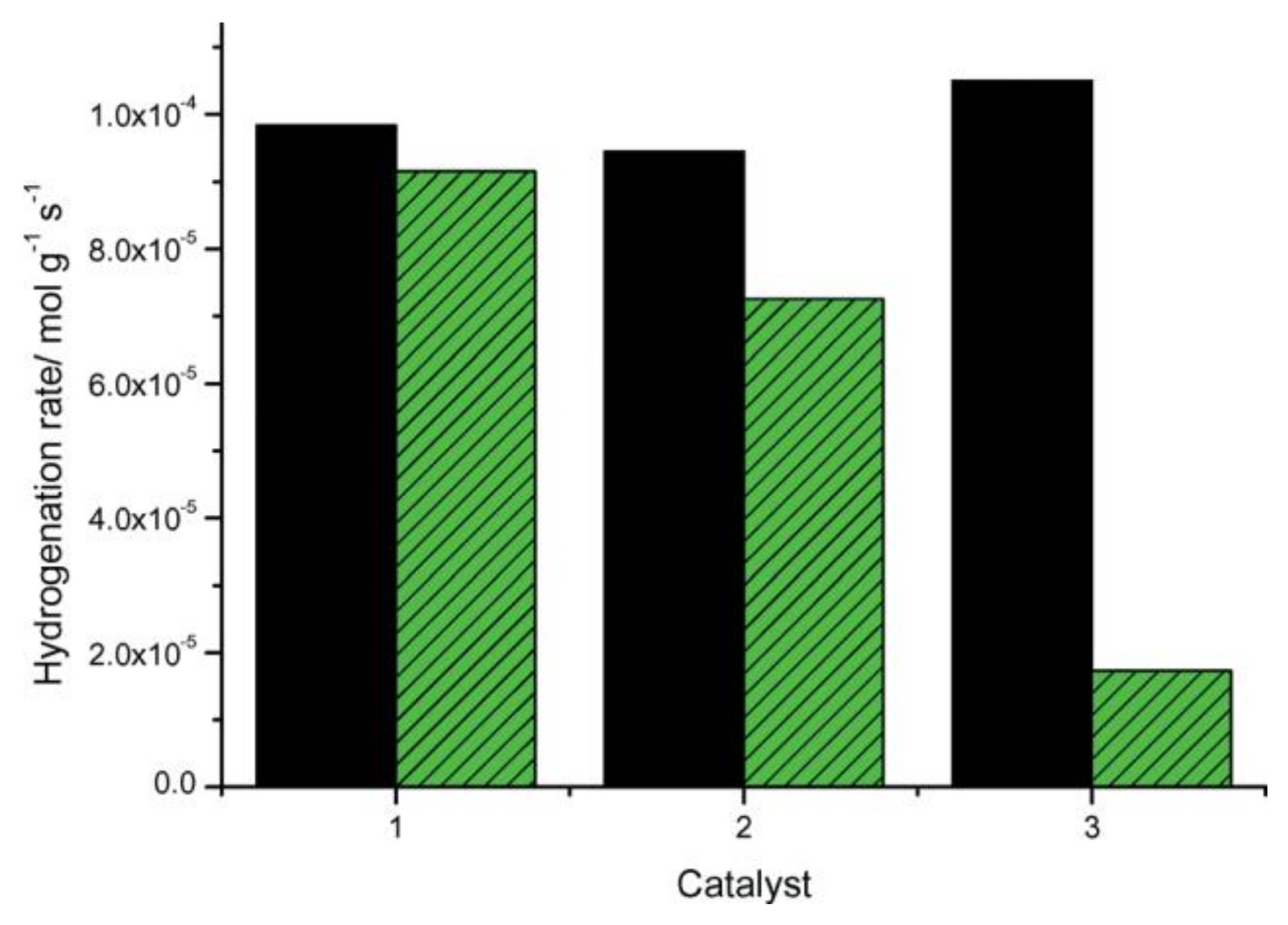
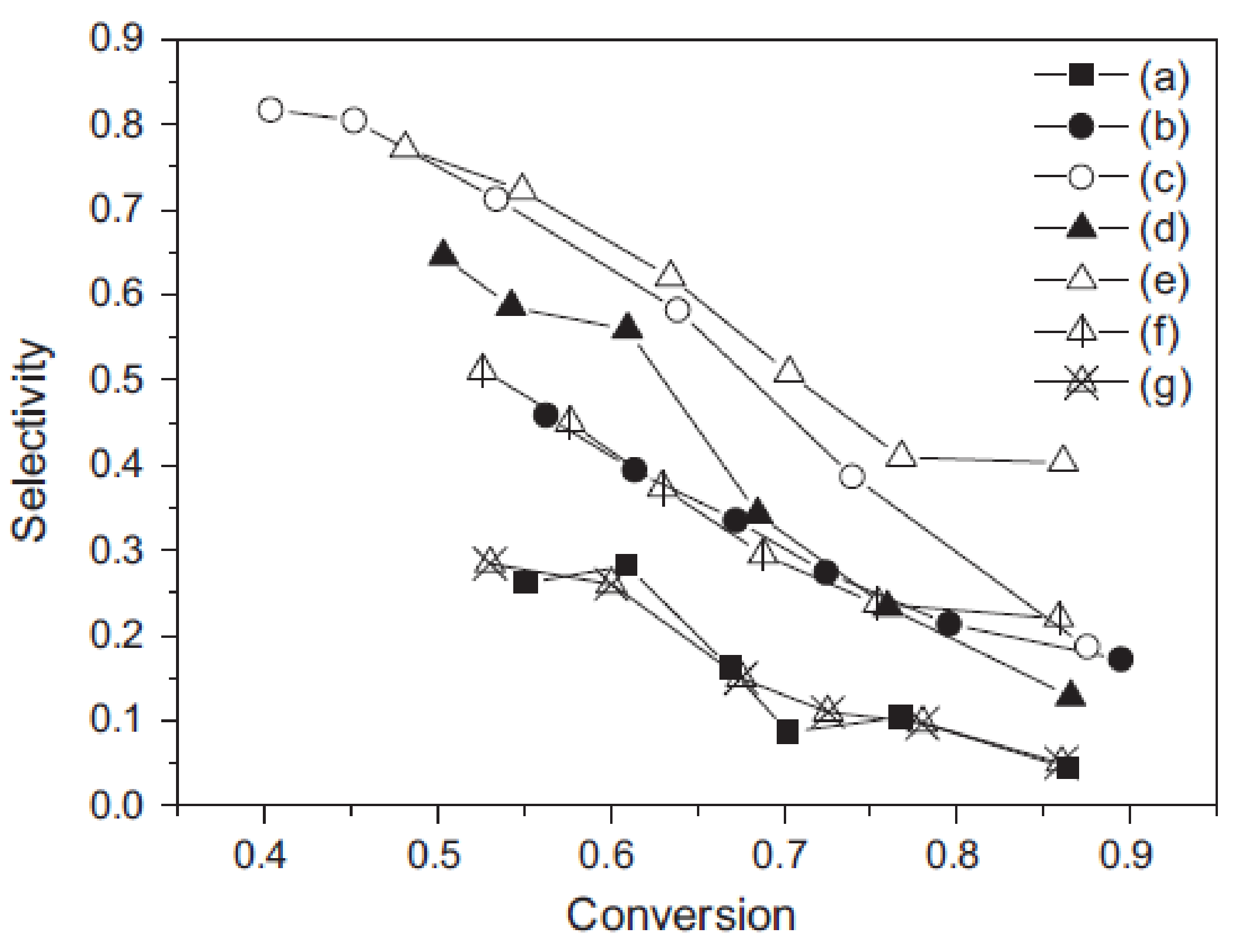

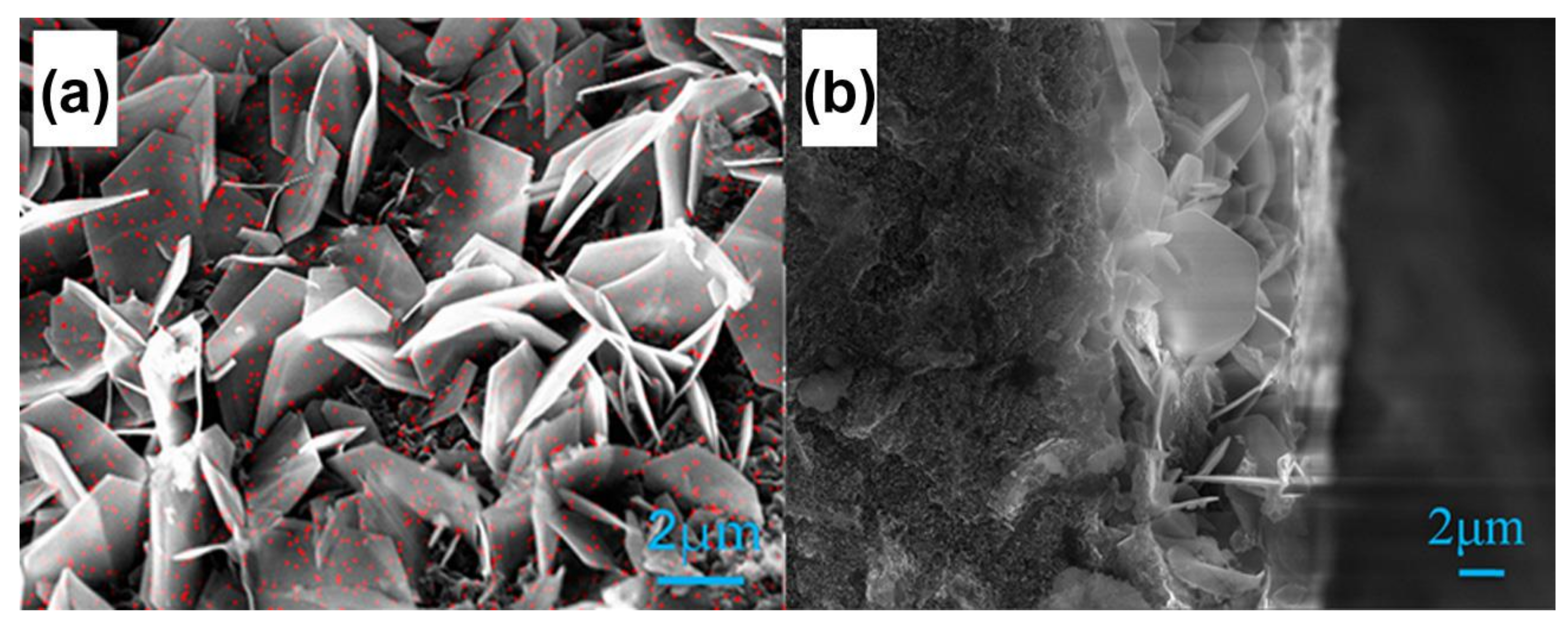
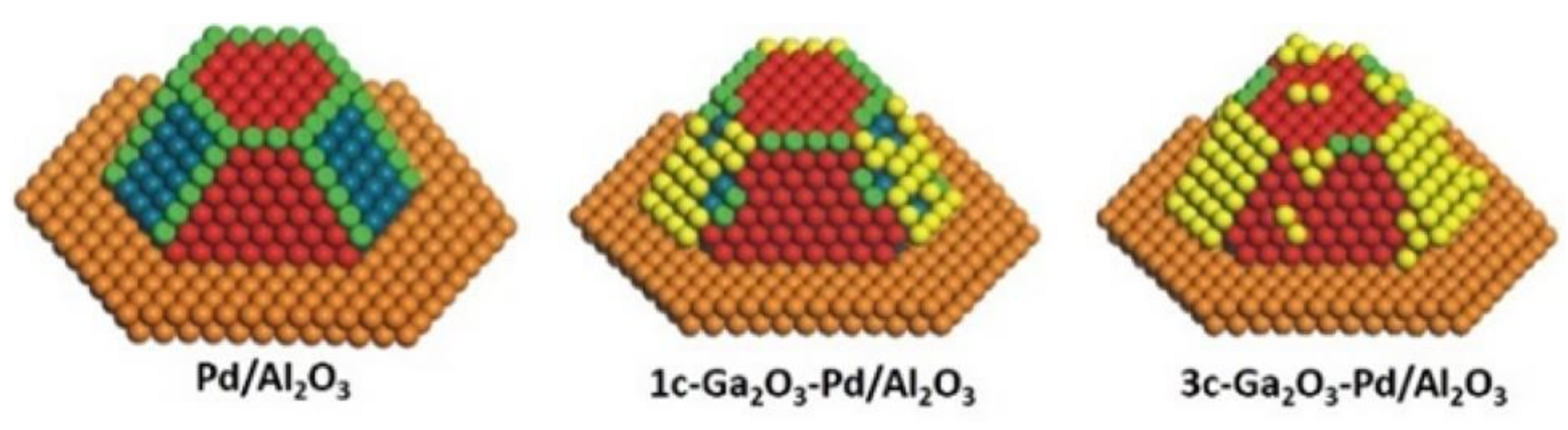
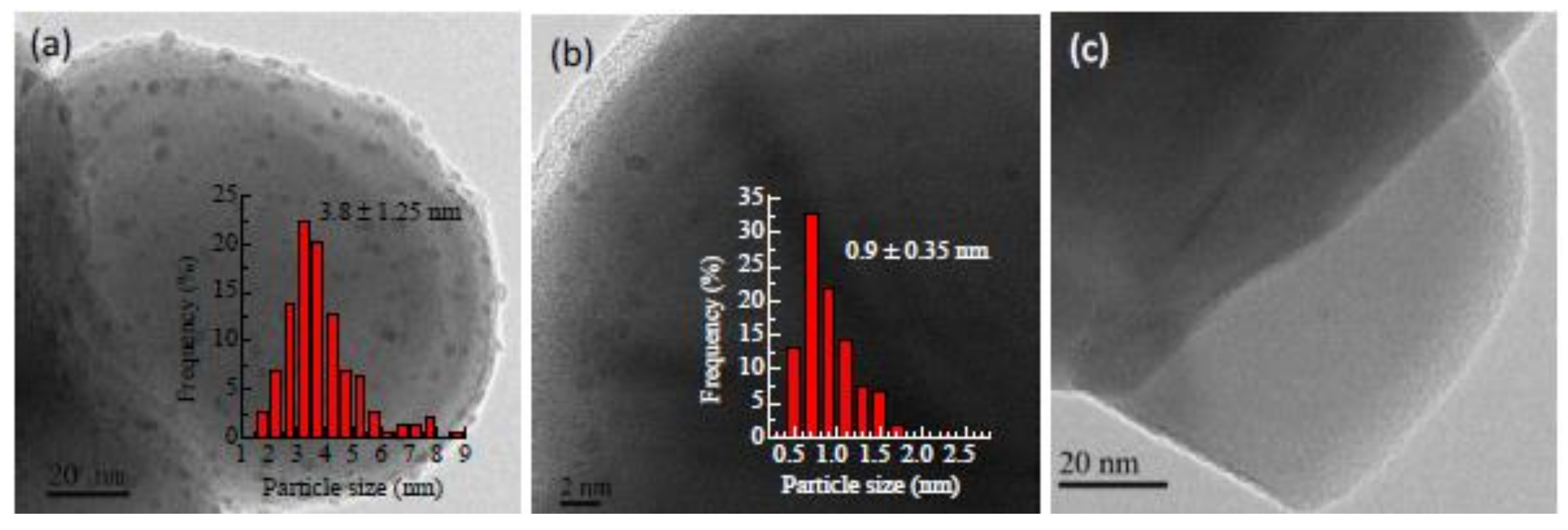
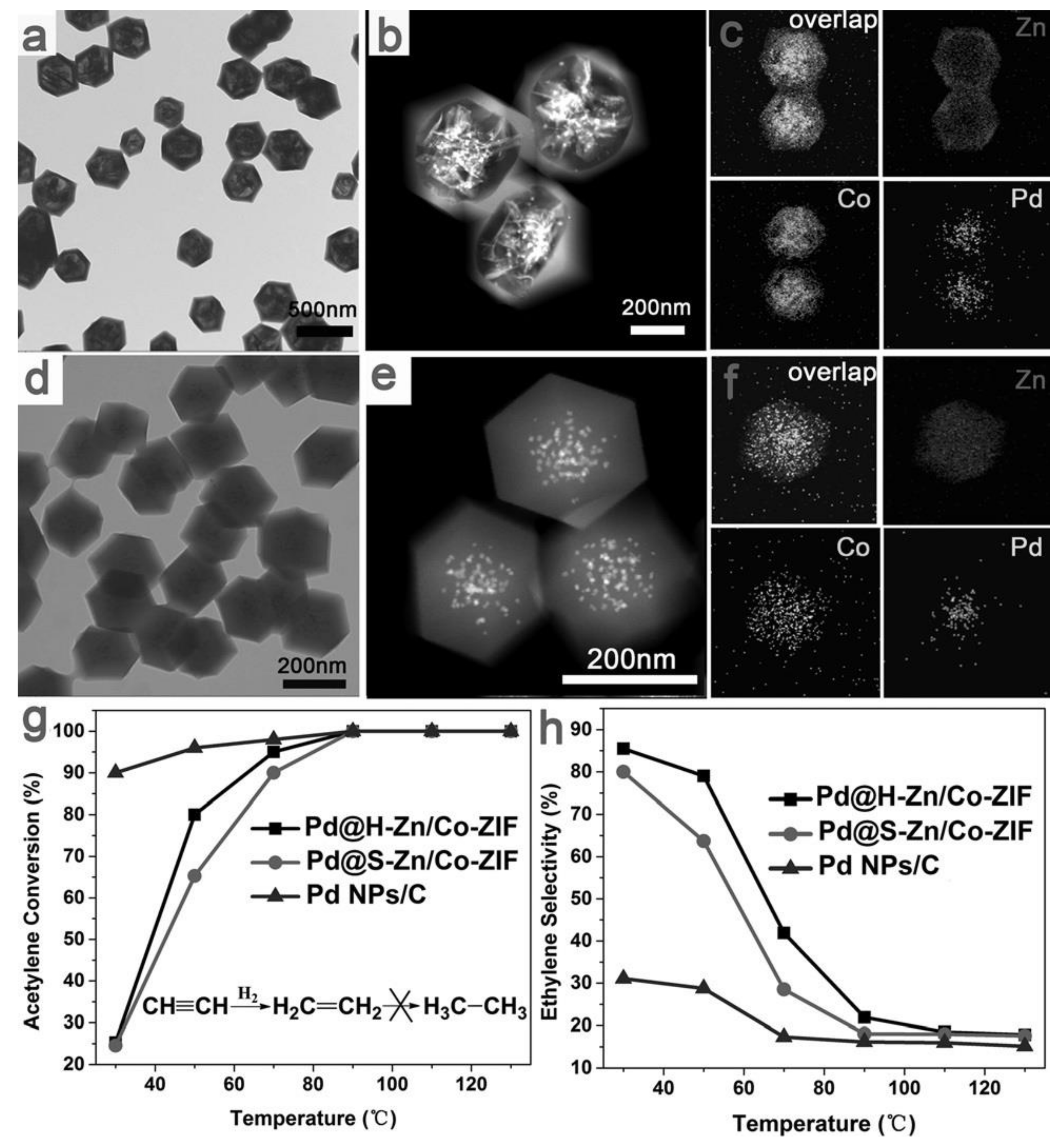
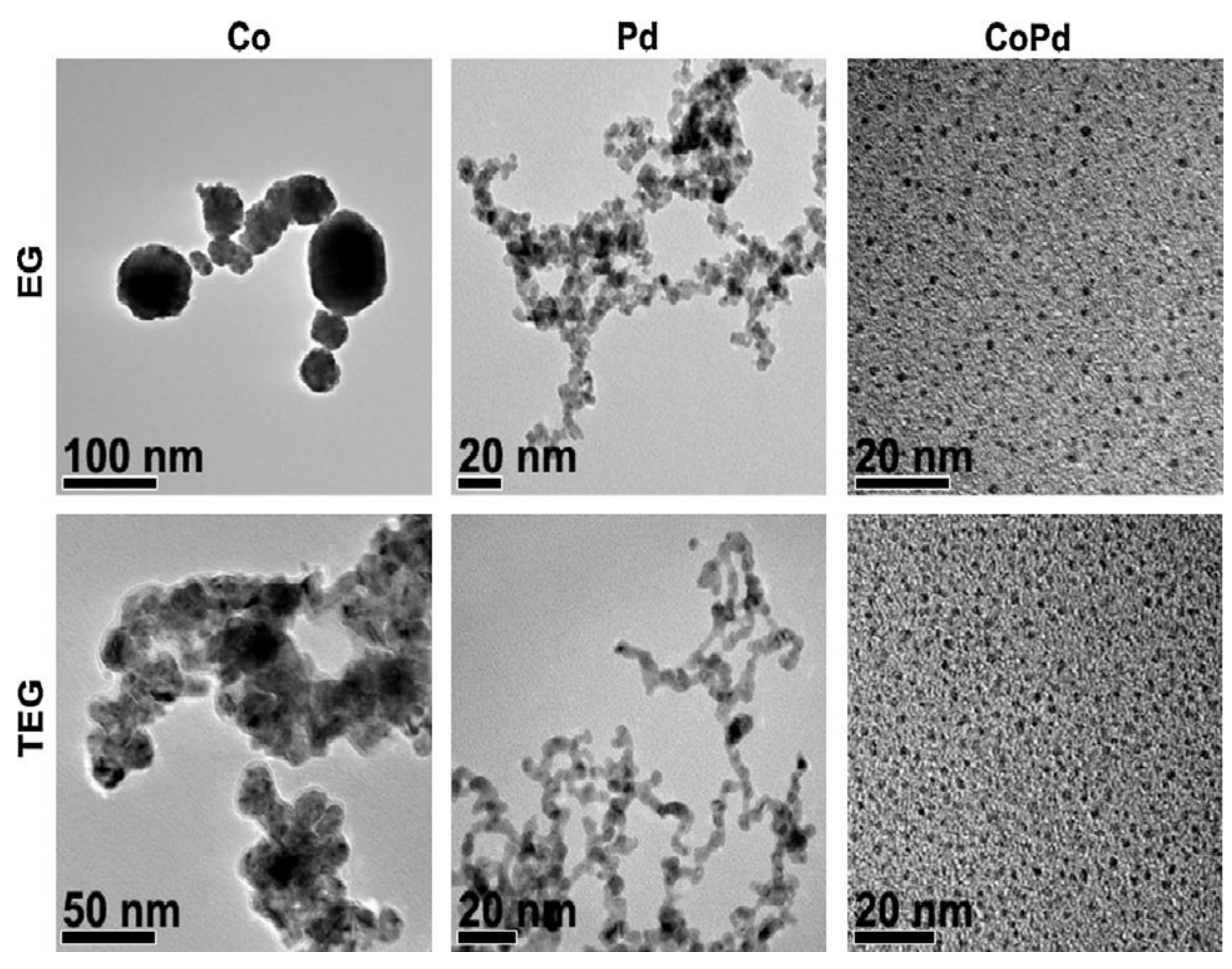
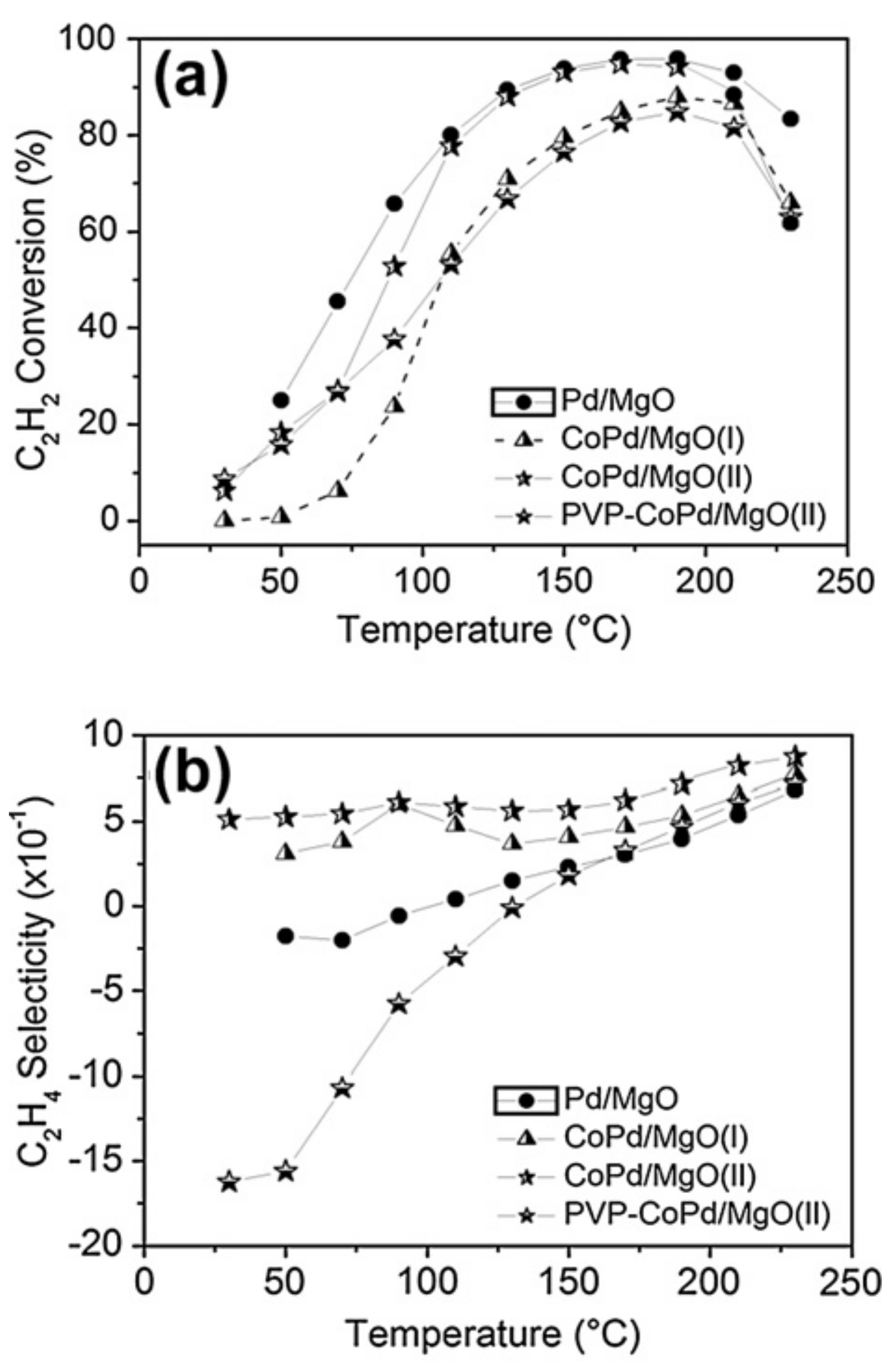
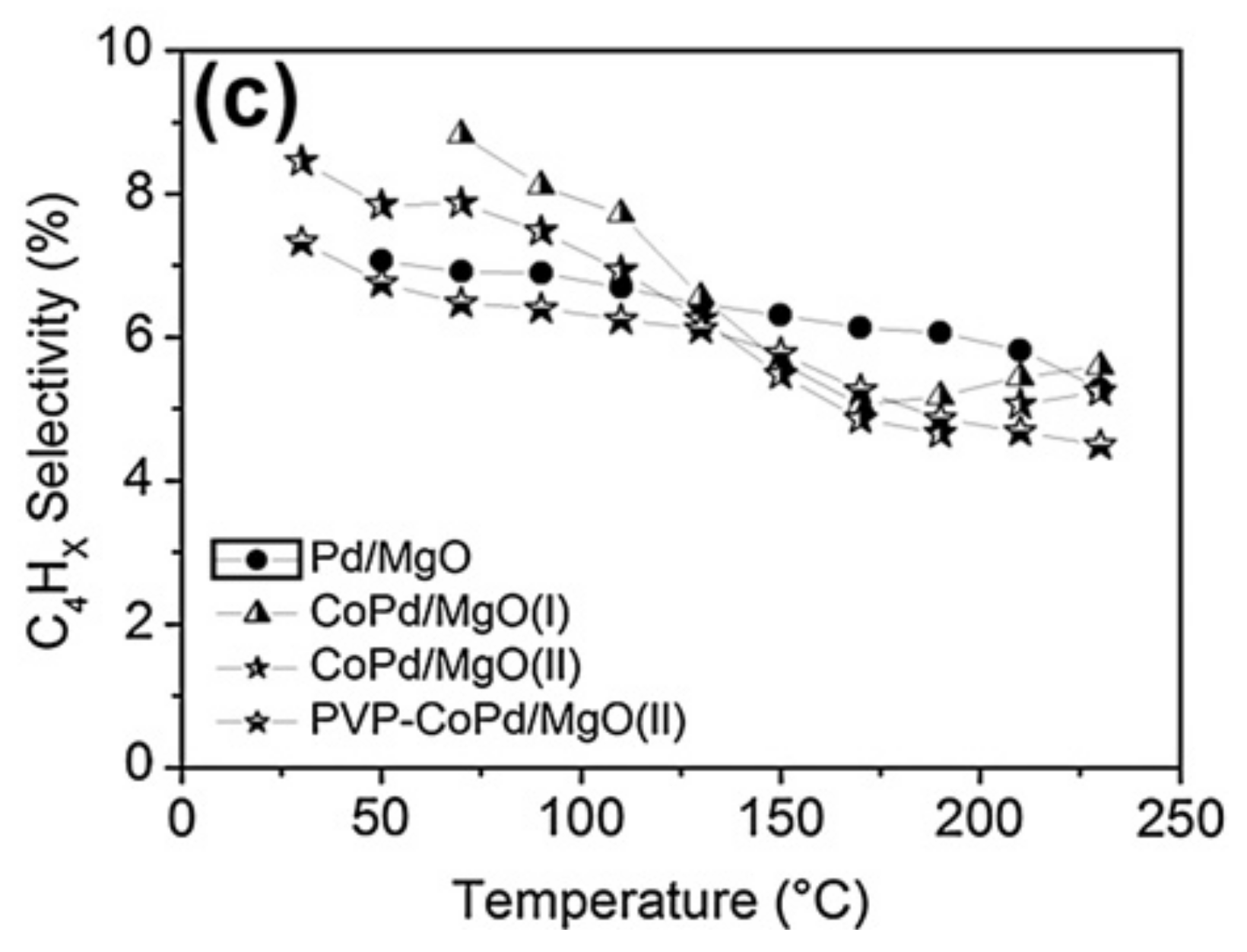
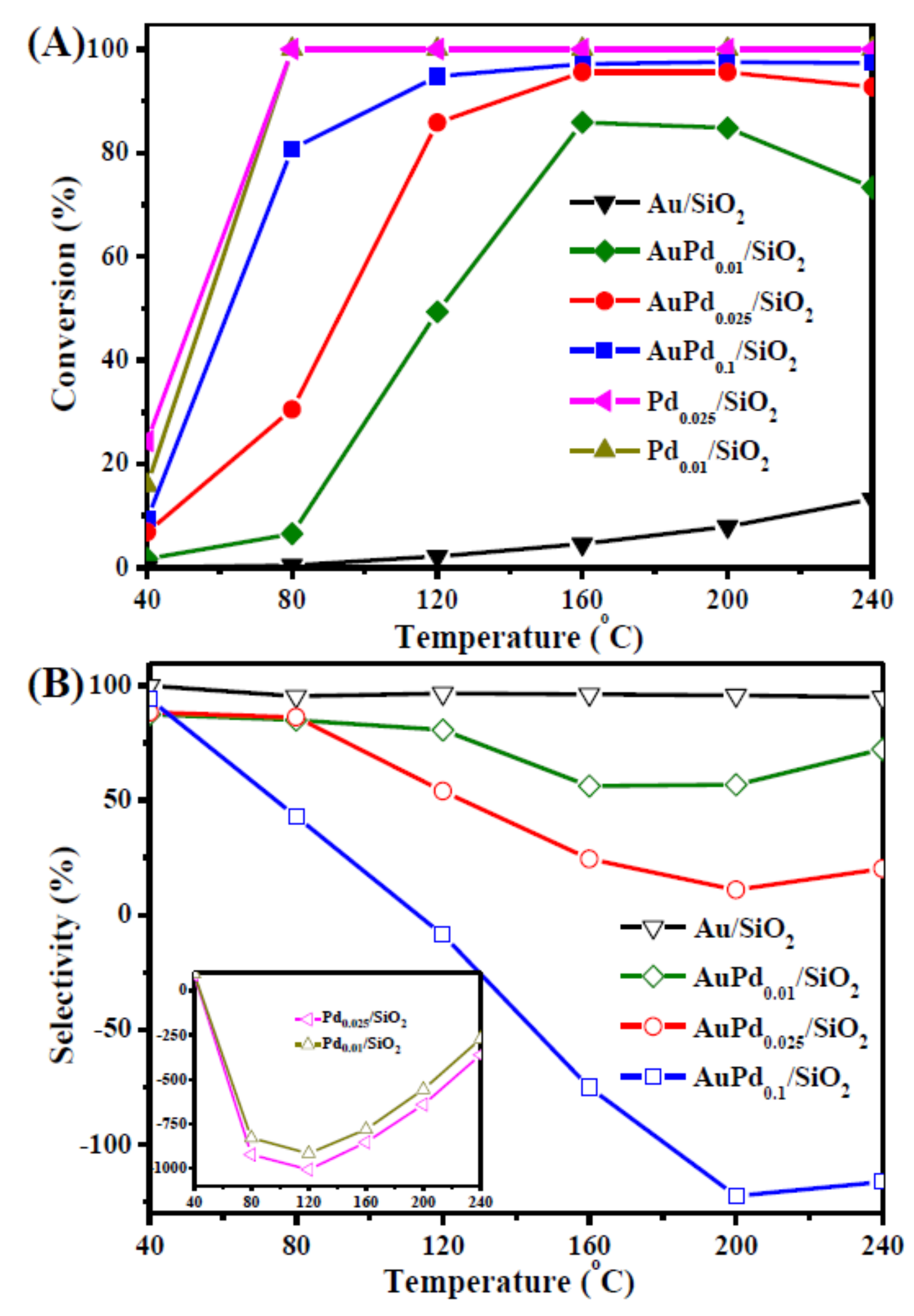
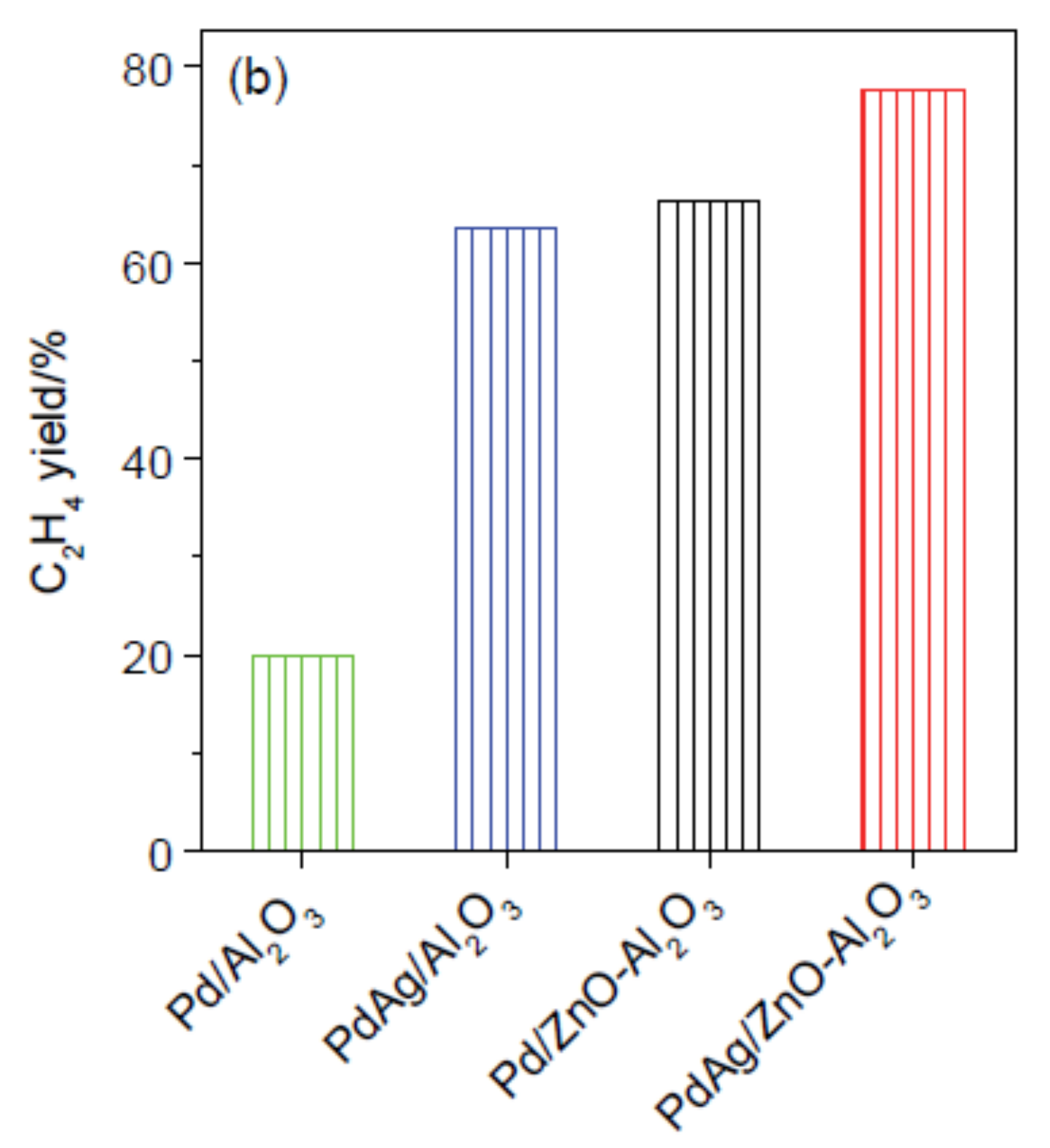
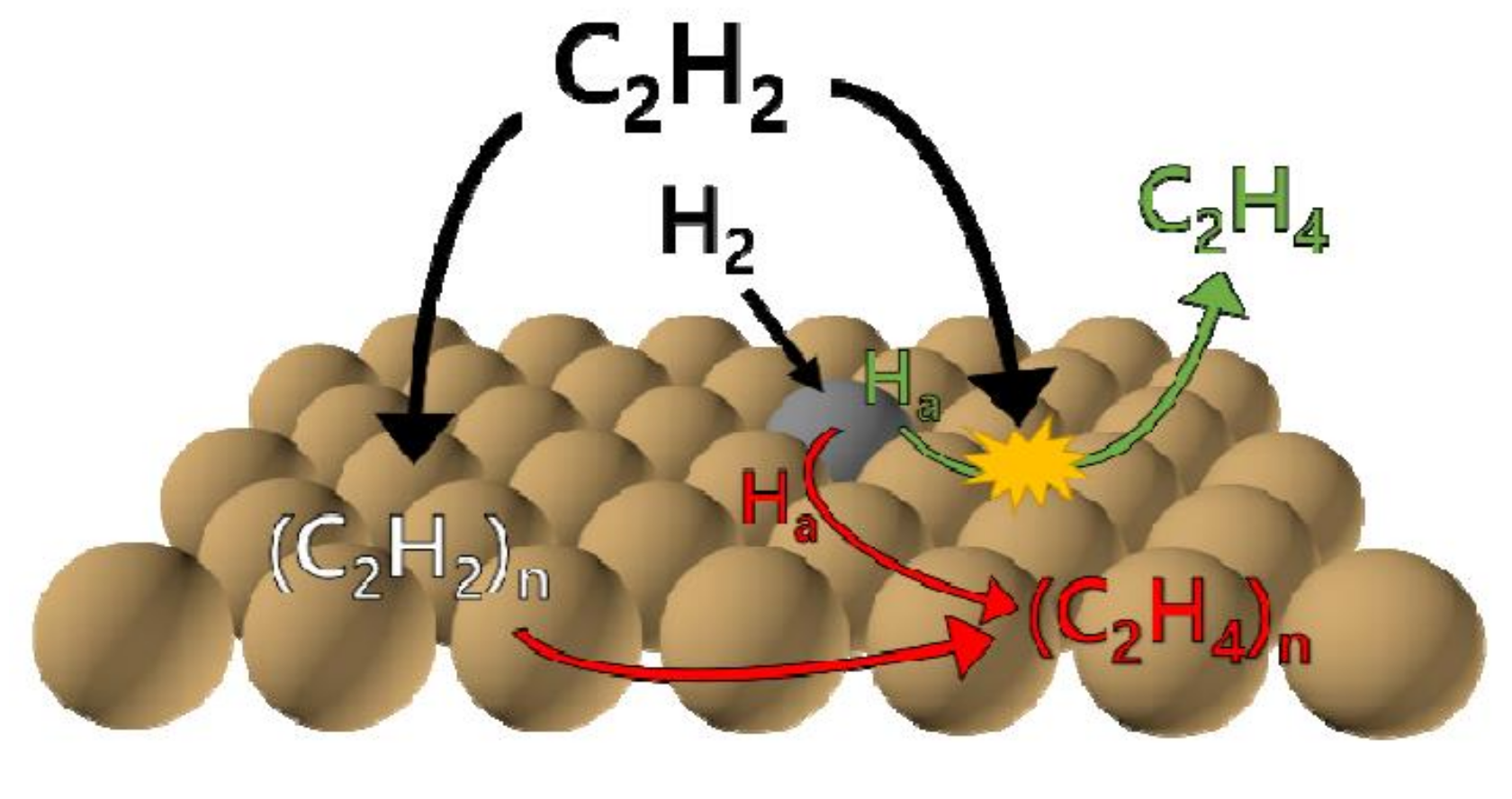
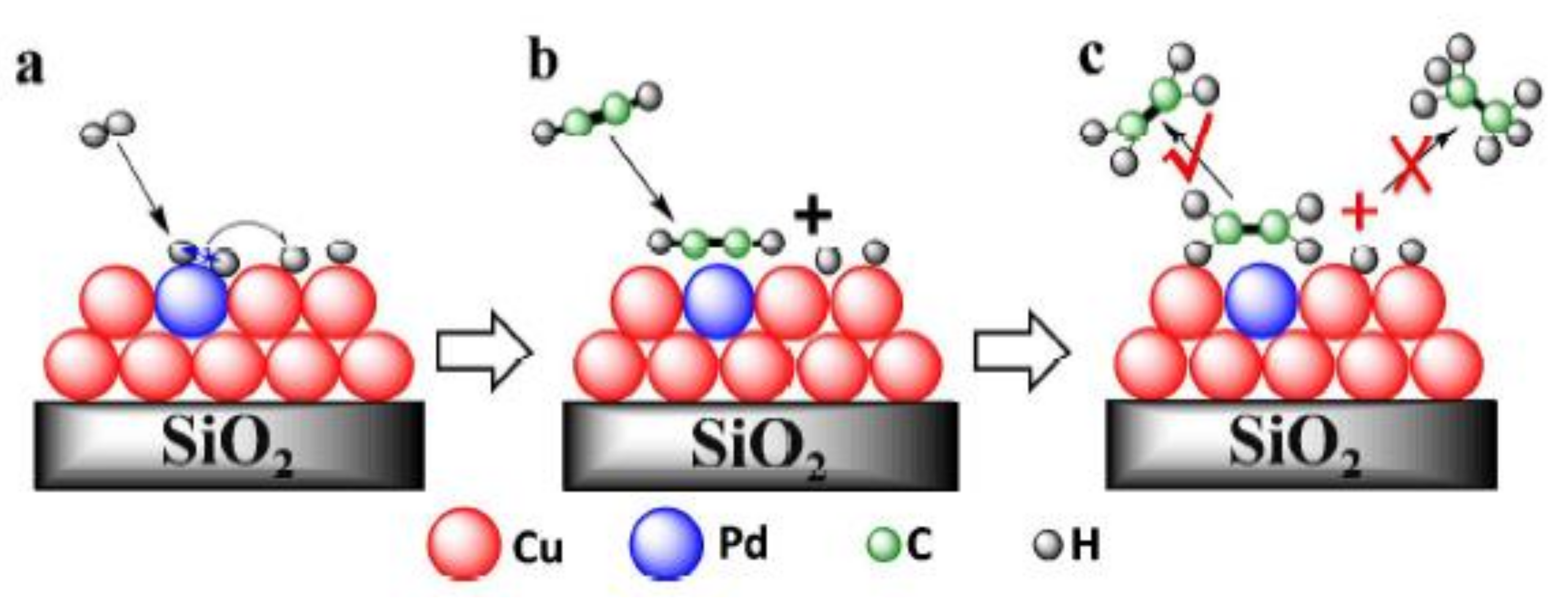
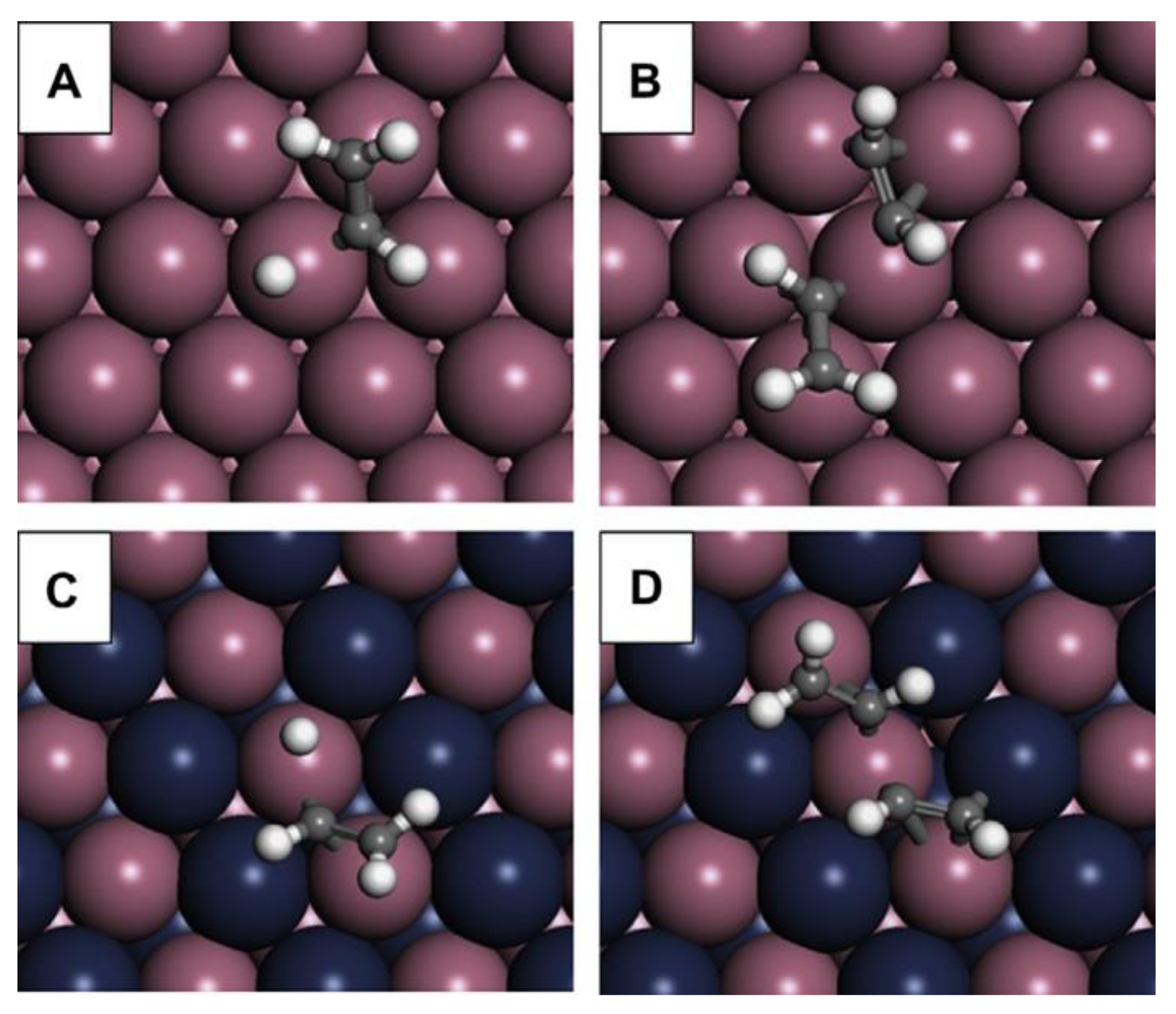
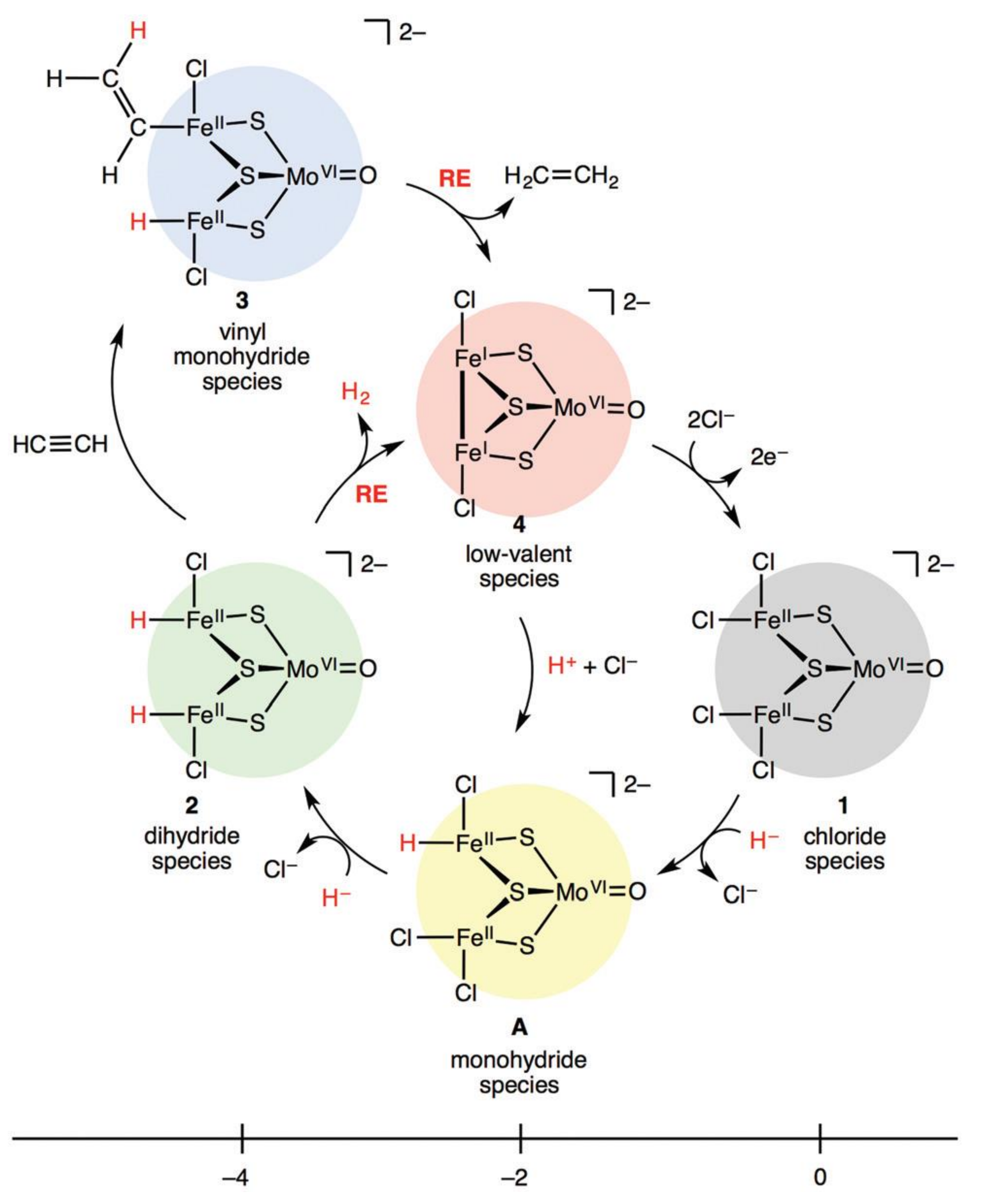
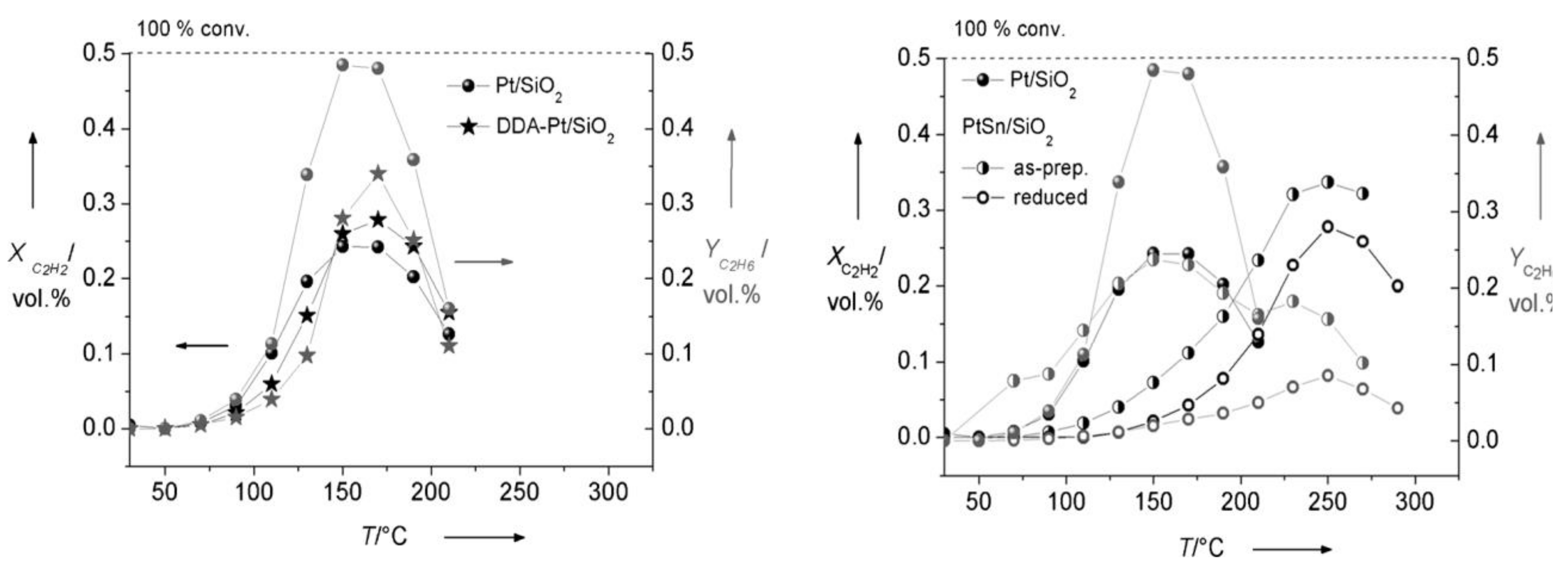
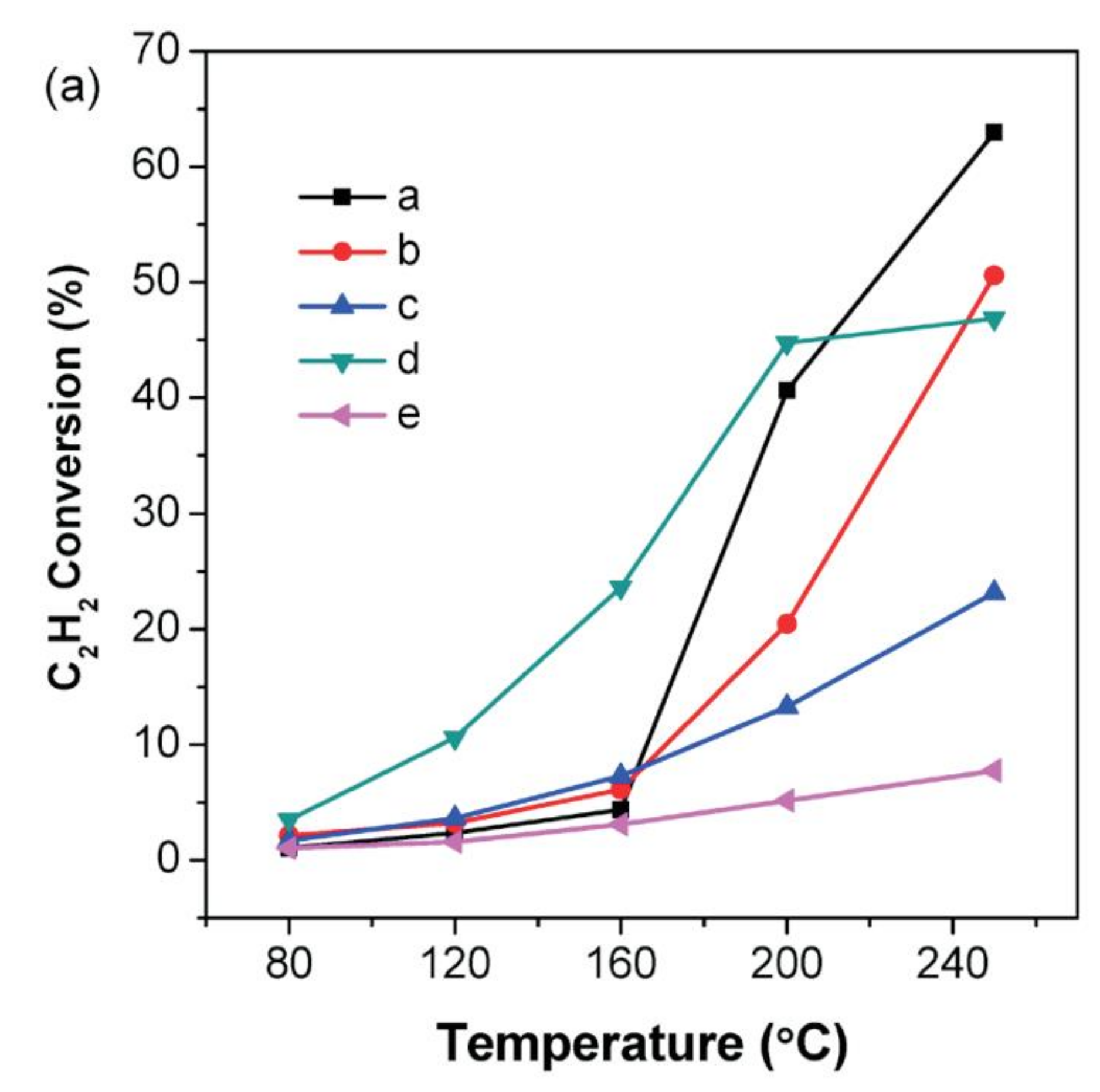
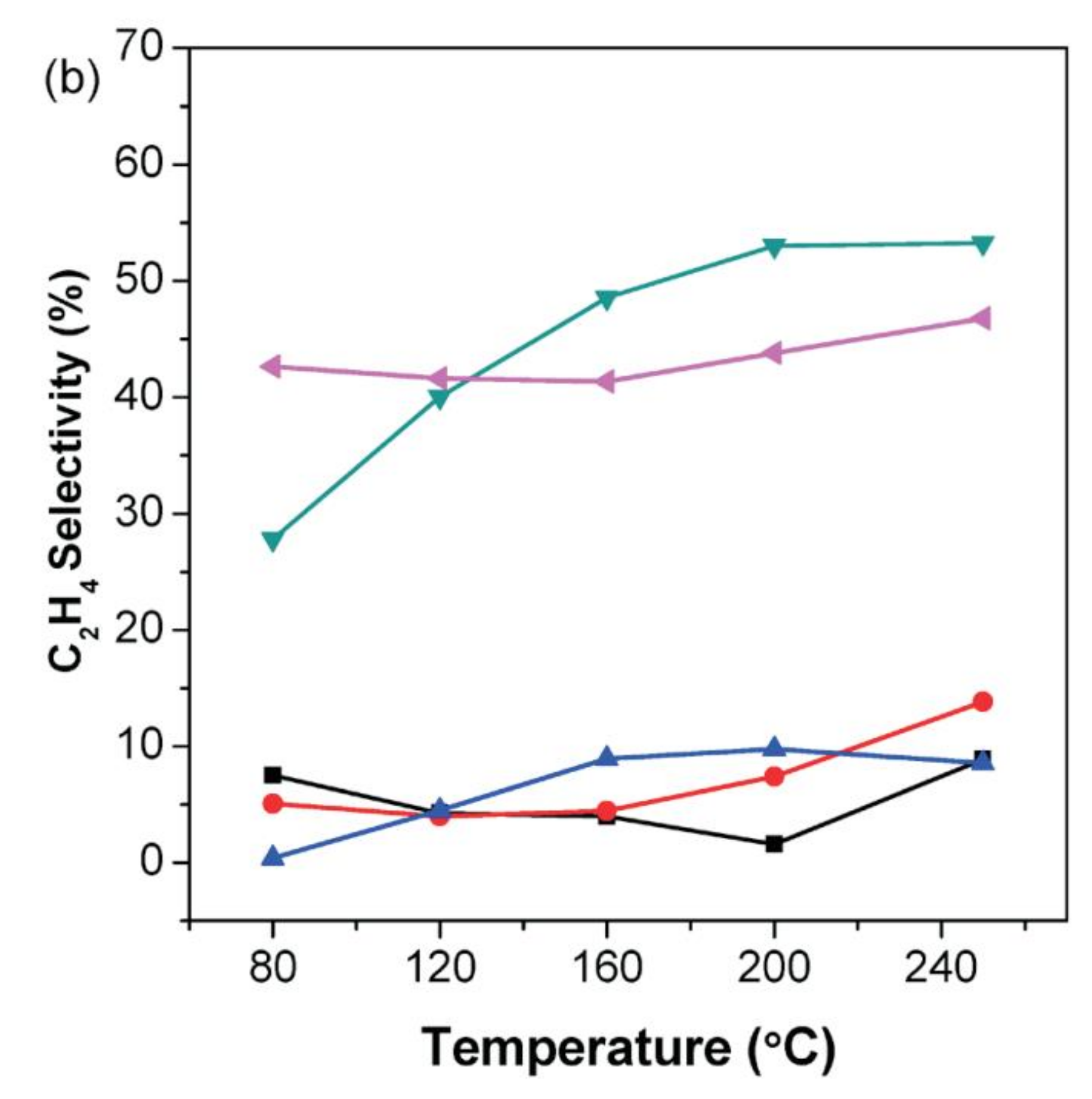
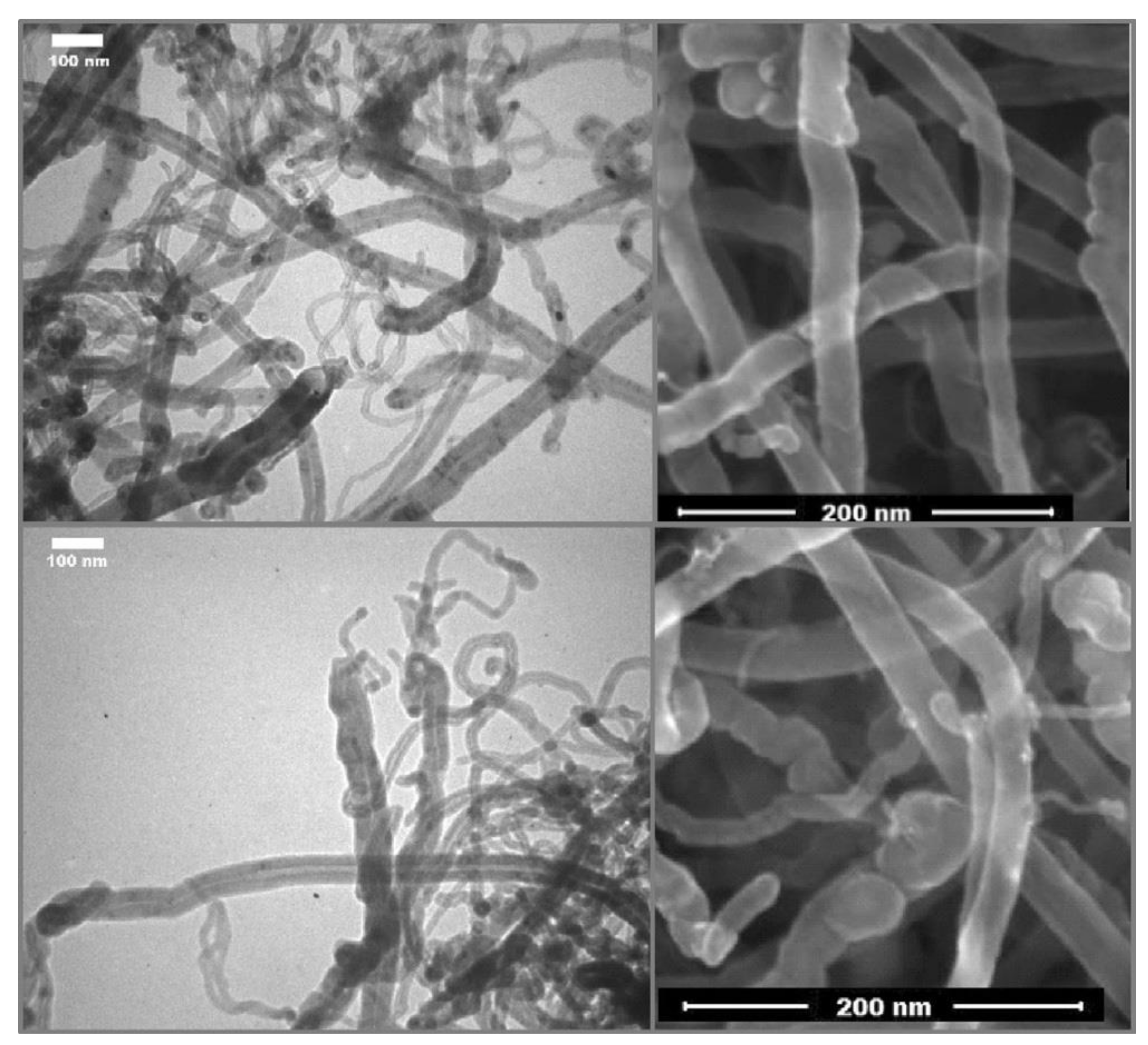
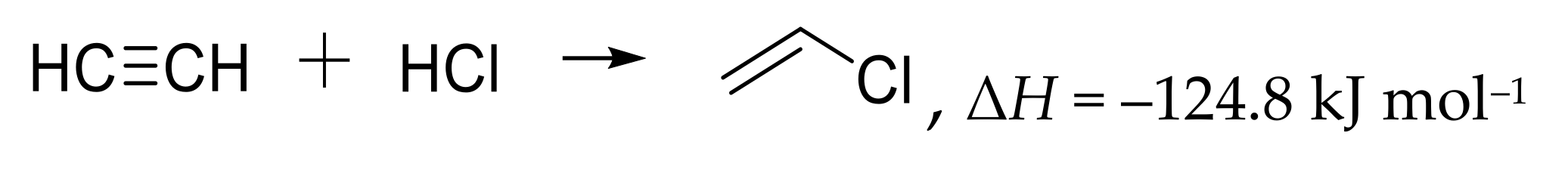
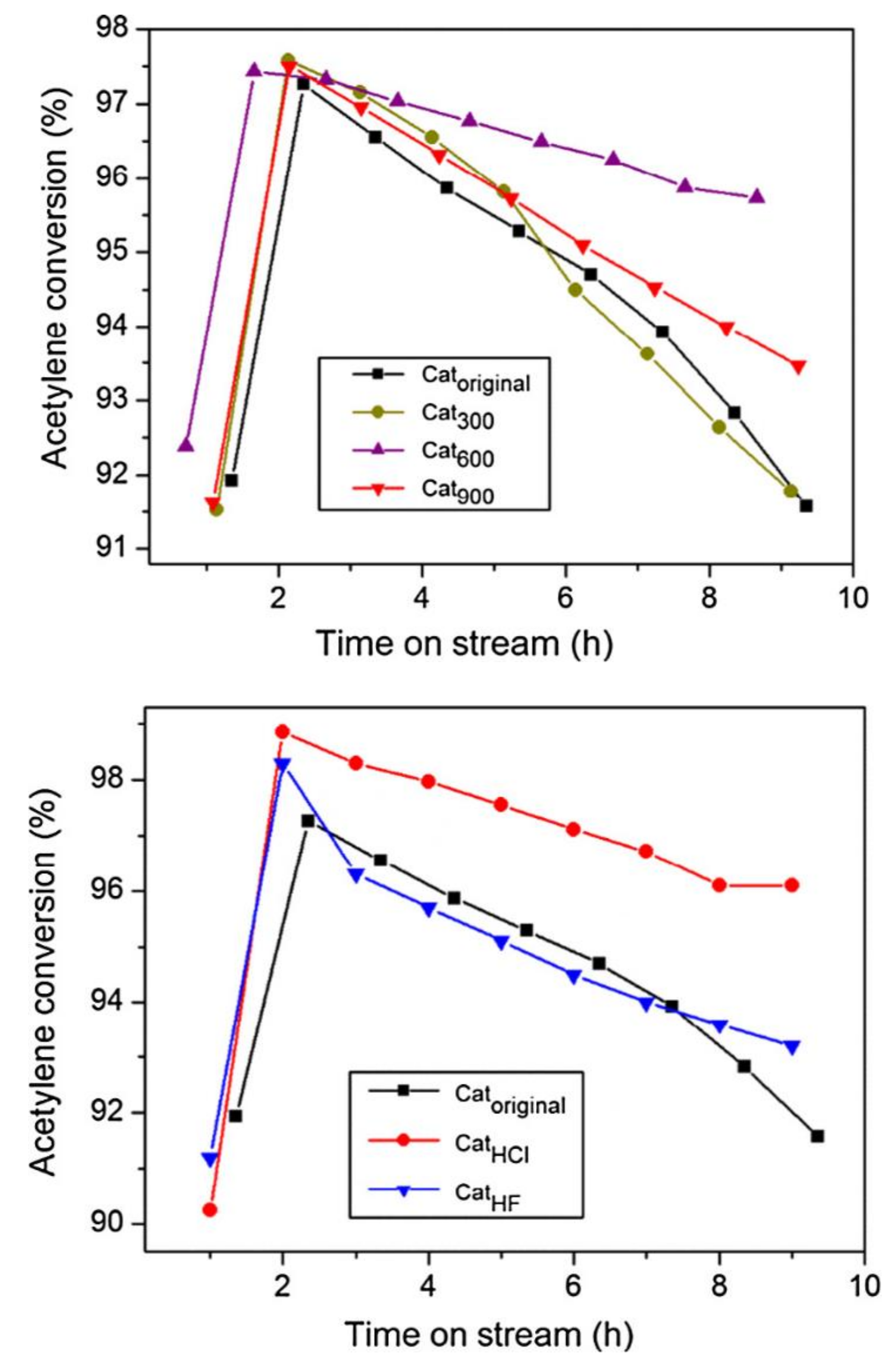
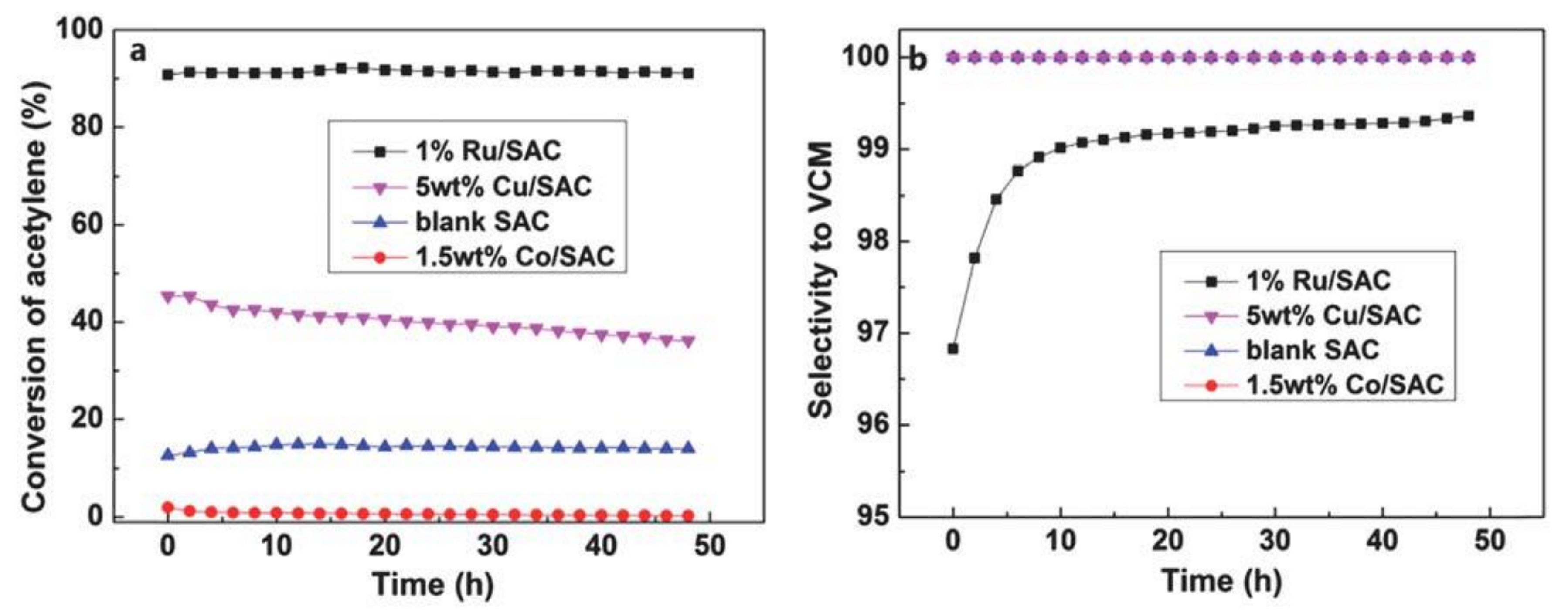
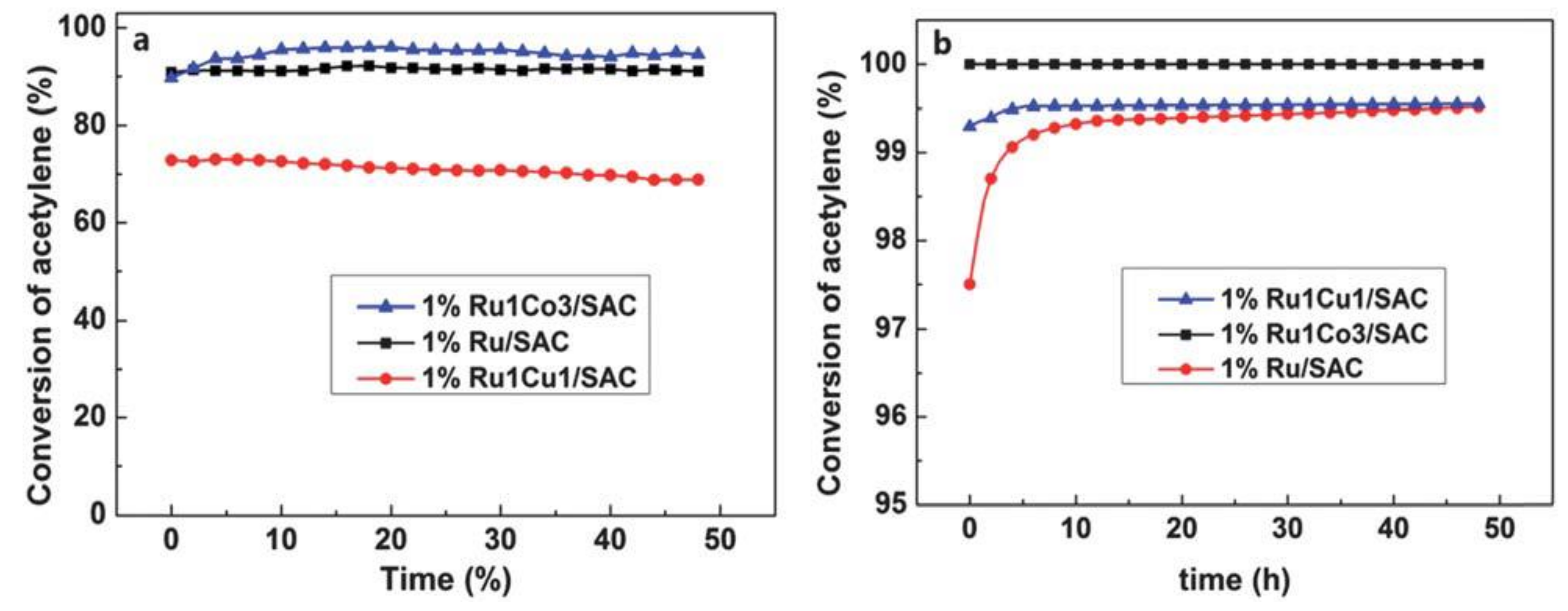
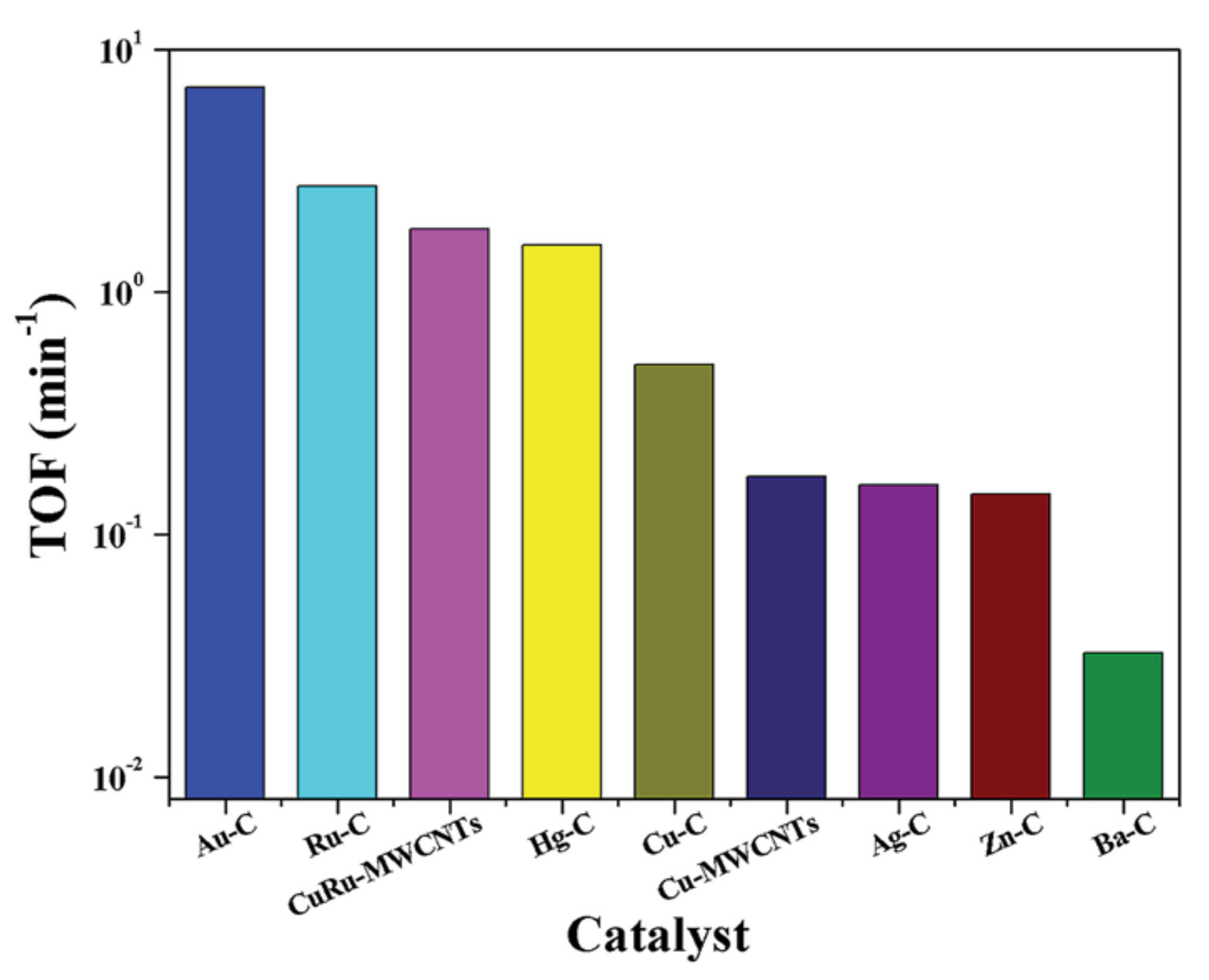
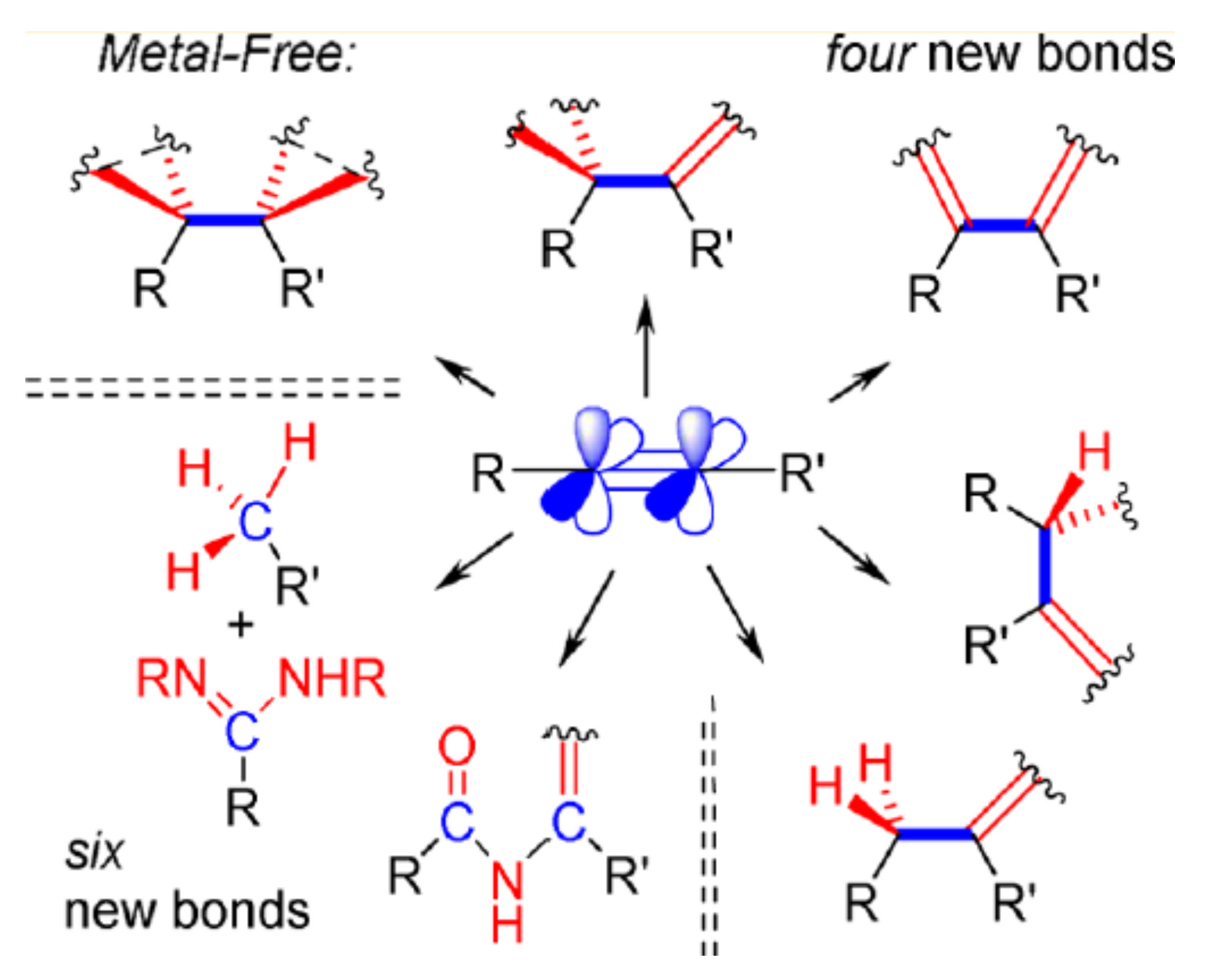
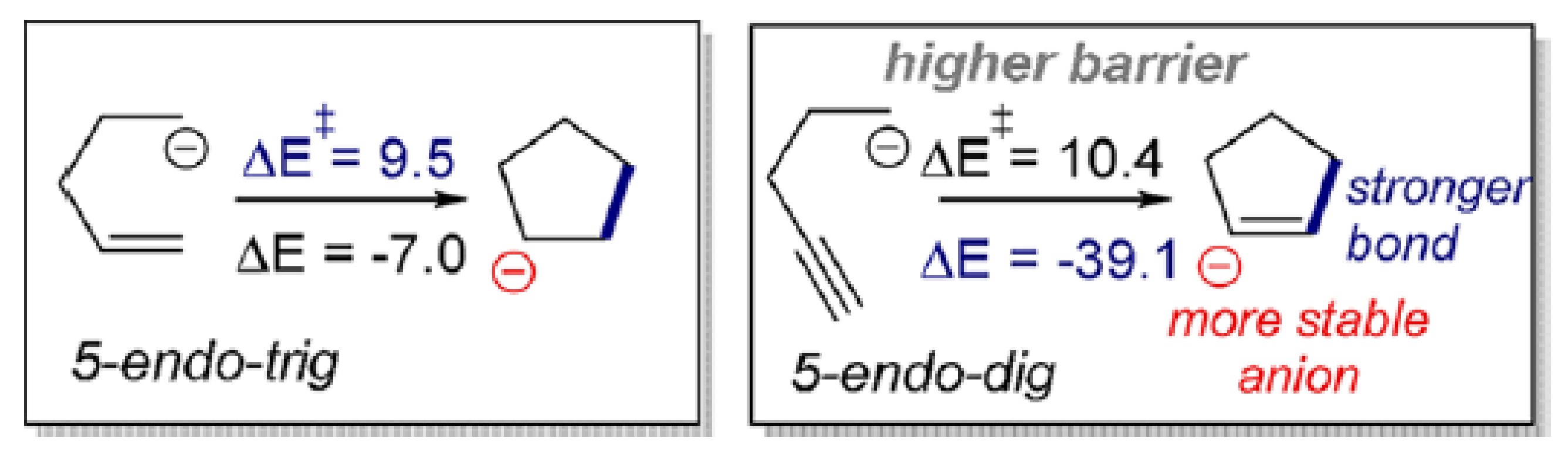
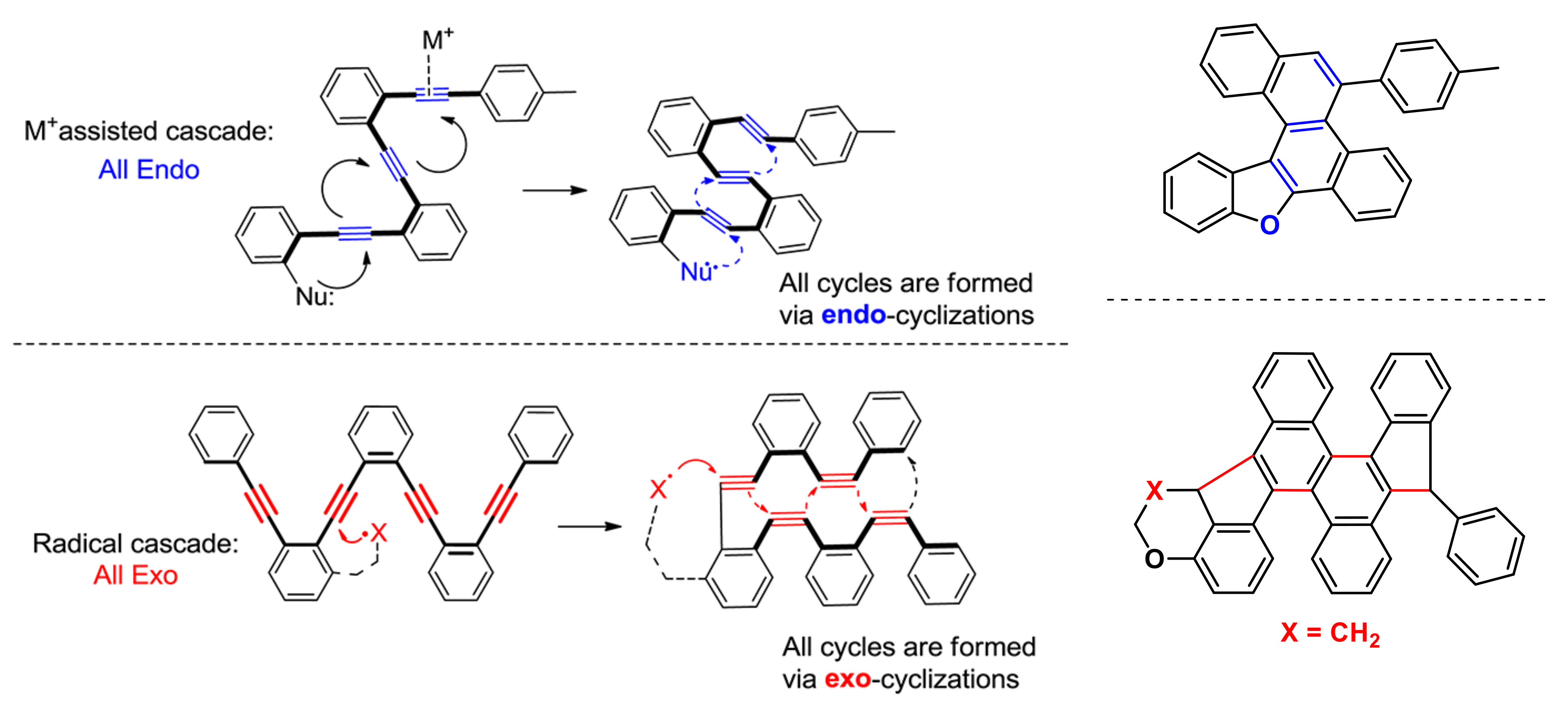
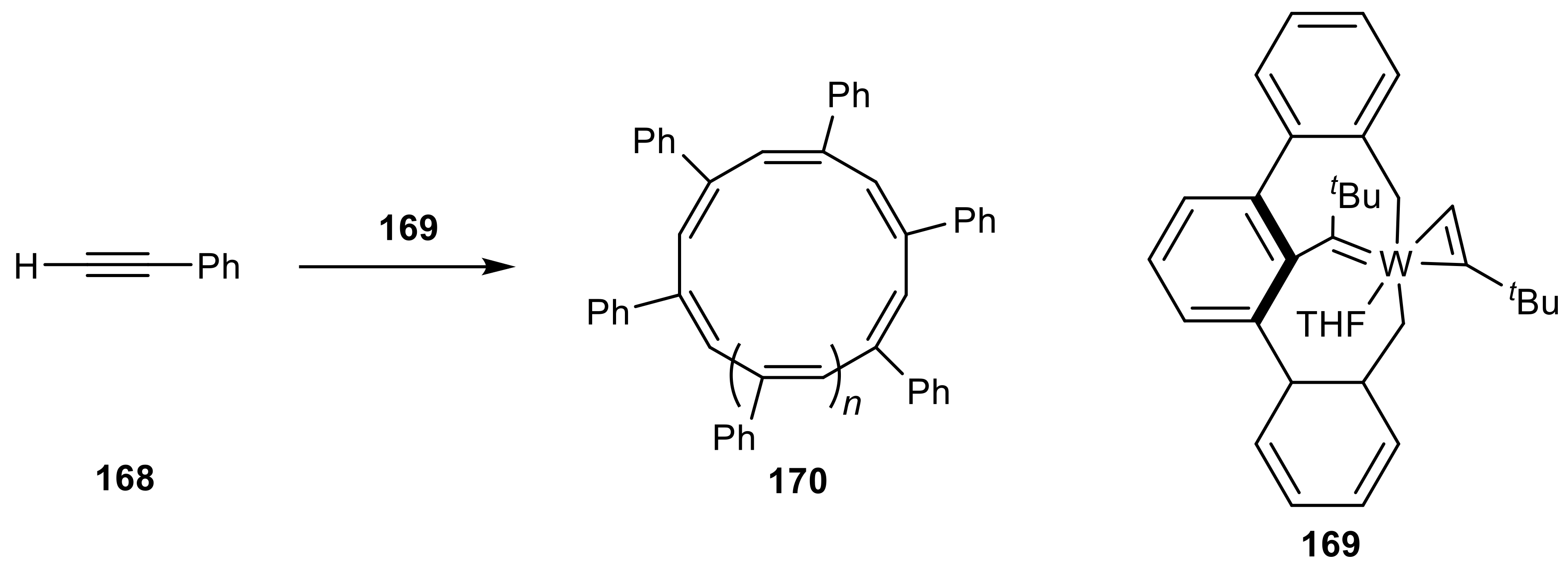
| Oligomers/FID Signal | Methane/FID Signal | |
|---|---|---|
| Acetylene only | ||
| Pd/TiO2 | 248,173 | 61,404 |
| Pd/TiO2 + 3‰ CO | 296,540 | 103,000 |
| PPh3 2.5 Pd/TiO2 | 315,656 | - |
| Ethylene only | ||
| Pd/TiO2 | 170 | 3445 |
| Pd/TiO2 + 3‰ CO | 799 | 3392 |
| PPh3 2.5 Pd/TiO2 | 290 | 3100 |
| Mixed acetylene/ethylene reaction | ||
| Pd/TiO2 | 52,415 | 14,763 |
| Pd/TiO2 + 3‰ CO | 174,911 | - |
| PPh3 2.5 Pd/TiO2 | 4969 | 9393 |
| PPh3 10 Pd/TiO2 | 4332 | 6262 |
| Catalyst | Temperature (K) | Acetylene Conversion (%) | Ethylene Selectivity (%) | Ethane Selectivity (%) | Oligomer Selectivity (%) |
|---|---|---|---|---|---|
| 10% Cu/Al2O3 | 323 | 9.59 | 53.2 | 9.9 | 37.0 |
| 373 | 60.71 | 60.5 | 8.6 | 30.8 | |
| 423 | 99.20 | 71.5 | 8.0 | 20.5 | |
| 1.67% Pd/Al2O3 | 323 | 85.17 | 0.0 | 93.1 | 6.9 |
| Catalyst | Conversion (%) | Product Distribution (%) | |||||
|---|---|---|---|---|---|---|---|
| C2H2 | C2H4 | C2H4 | C2H2 | C2H6 | C4 | C4+ | |
| Gr | 81.0 | 14.0 | 92.2 | 3.2 | 1.1 | 3.5 | — |
| Gr * | 99.0 | 21.0 | 91.6 | 0.9 | 1.2 | 6.3 | — |
| GO | 22.5 | 0 | 48.1 | 5.0 | 0.3 | 46.6 | — |
| rGO | 87.5 | 4.7 | 55.1 | 1.2 | 1.3 | 42.4 | — |
| (N)Gr | 26.7 | 6.7 | 62.5 | 7.2 | 0.7 | 11.5 | 25.2 |
| (P)Gr | 14.6 | 2.7 | 72.7 | 8.8 | 0.7 | 17.7 | 0.1 |
| (S)Gr | 24.7 | 6.4 | 54.1 | 6.3 | 0.4 | 0.0 | 39.1 |
| Building Block Molecule | Number of Low-Reactive Substituents at the C Atom | Number of Activated Substituents at the C Atom | Number of Atom-Economical Steps |
|---|---|---|---|
 | 4 | 0 | 0 |
 | 2 | 0 | 1 |
 | 0 | 1 | 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voronin, V.V.; Ledovskaya, M.S.; Bogachenkov, A.S.; Rodygin, K.S.; Ananikov, V.P. Acetylene in Organic Synthesis: Recent Progress and New Uses. Molecules 2018, 23, 2442. https://doi.org/10.3390/molecules23102442
Voronin VV, Ledovskaya MS, Bogachenkov AS, Rodygin KS, Ananikov VP. Acetylene in Organic Synthesis: Recent Progress and New Uses. Molecules. 2018; 23(10):2442. https://doi.org/10.3390/molecules23102442
Chicago/Turabian StyleVoronin, Vladimir V., Maria S. Ledovskaya, Alexander S. Bogachenkov, Konstantin S. Rodygin, and Valentine P. Ananikov. 2018. "Acetylene in Organic Synthesis: Recent Progress and New Uses" Molecules 23, no. 10: 2442. https://doi.org/10.3390/molecules23102442
APA StyleVoronin, V. V., Ledovskaya, M. S., Bogachenkov, A. S., Rodygin, K. S., & Ananikov, V. P. (2018). Acetylene in Organic Synthesis: Recent Progress and New Uses. Molecules, 23(10), 2442. https://doi.org/10.3390/molecules23102442