Thermochemical Measurements of Alkali Cation Association to Hexatantalate
Abstract
1. Introduction
2. Results & Discussion
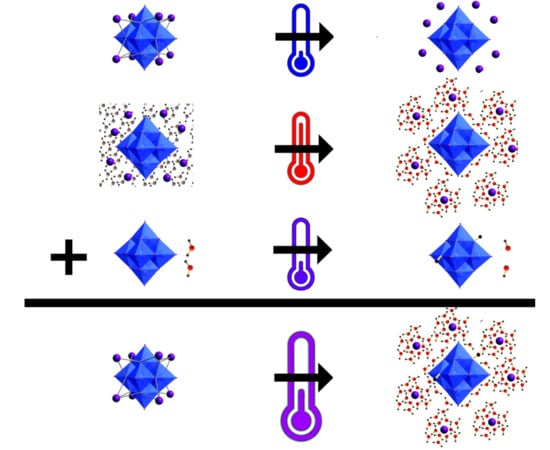
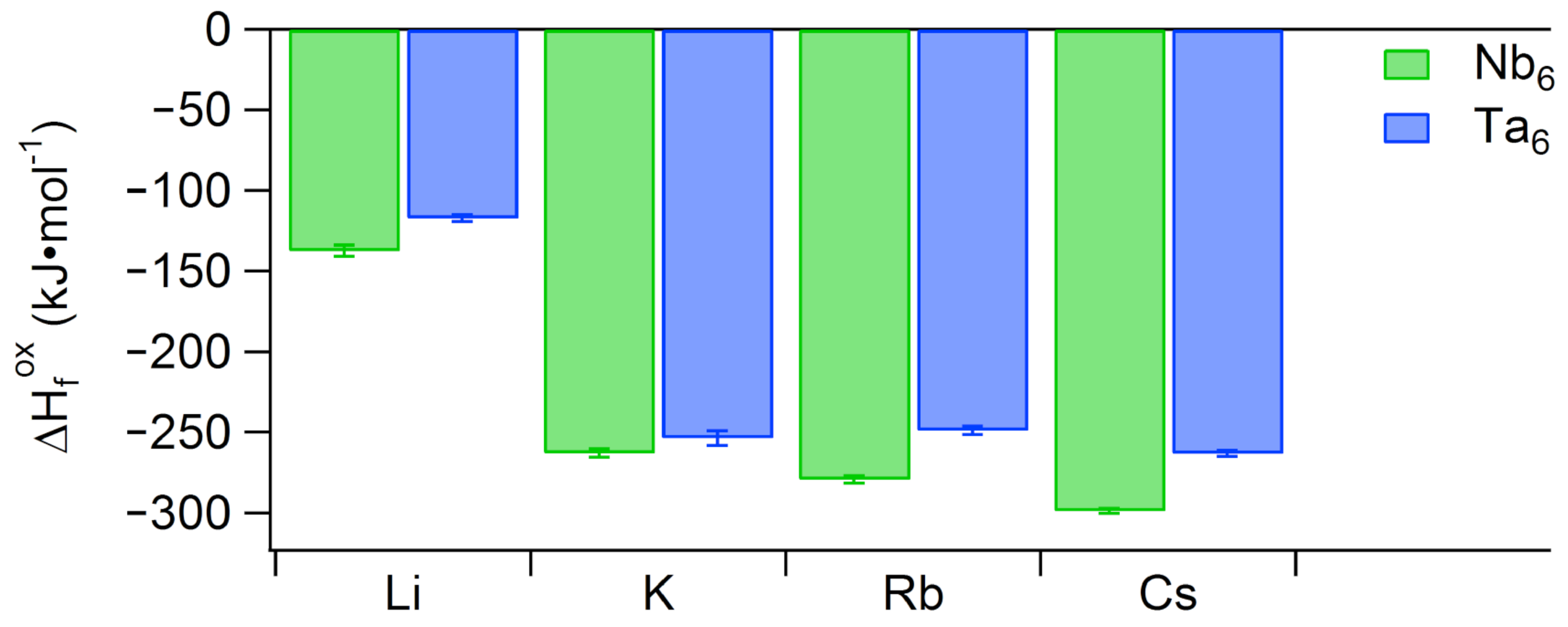
2.1. Solid-State Calorimetry of Ta Salts
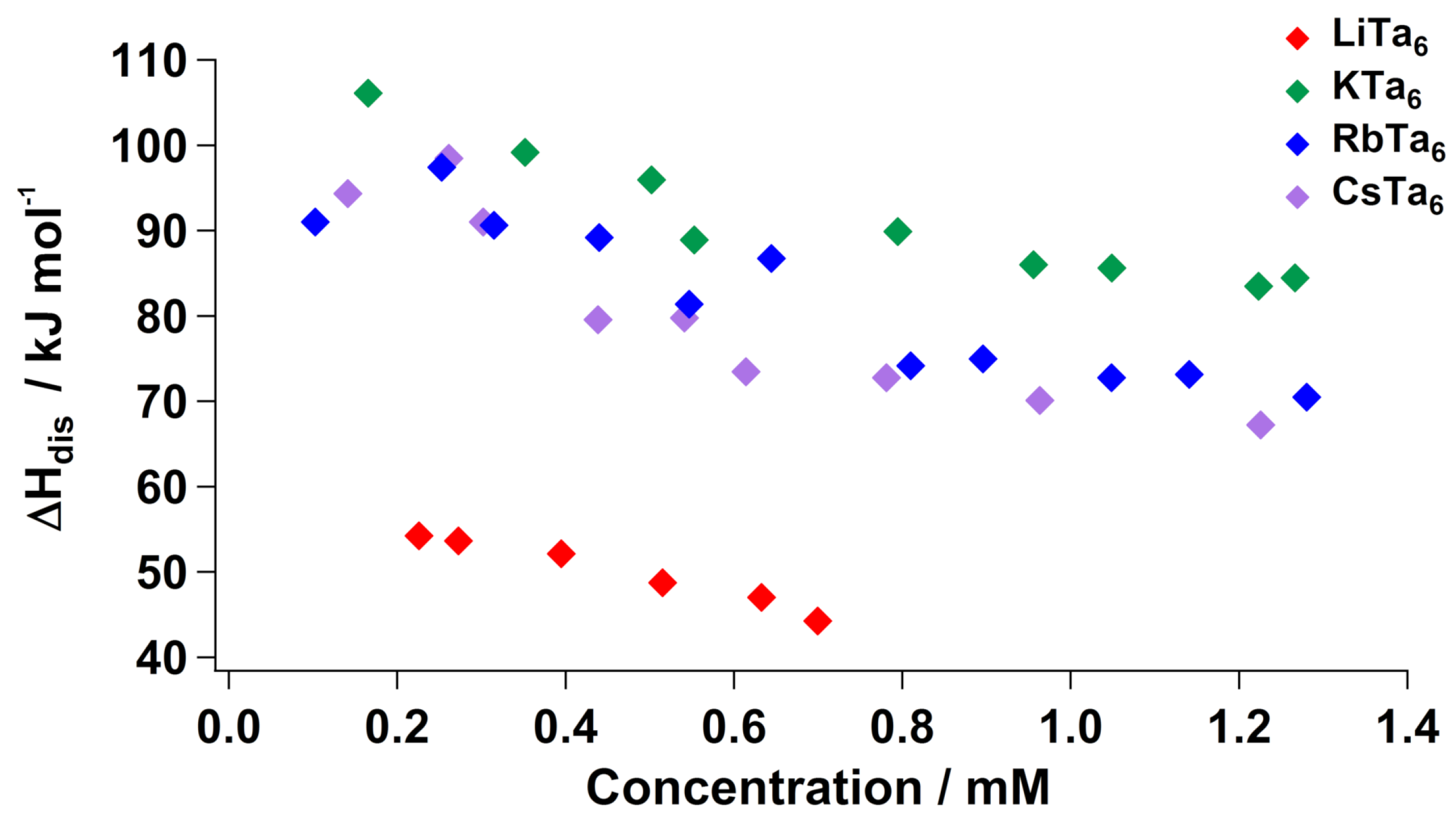
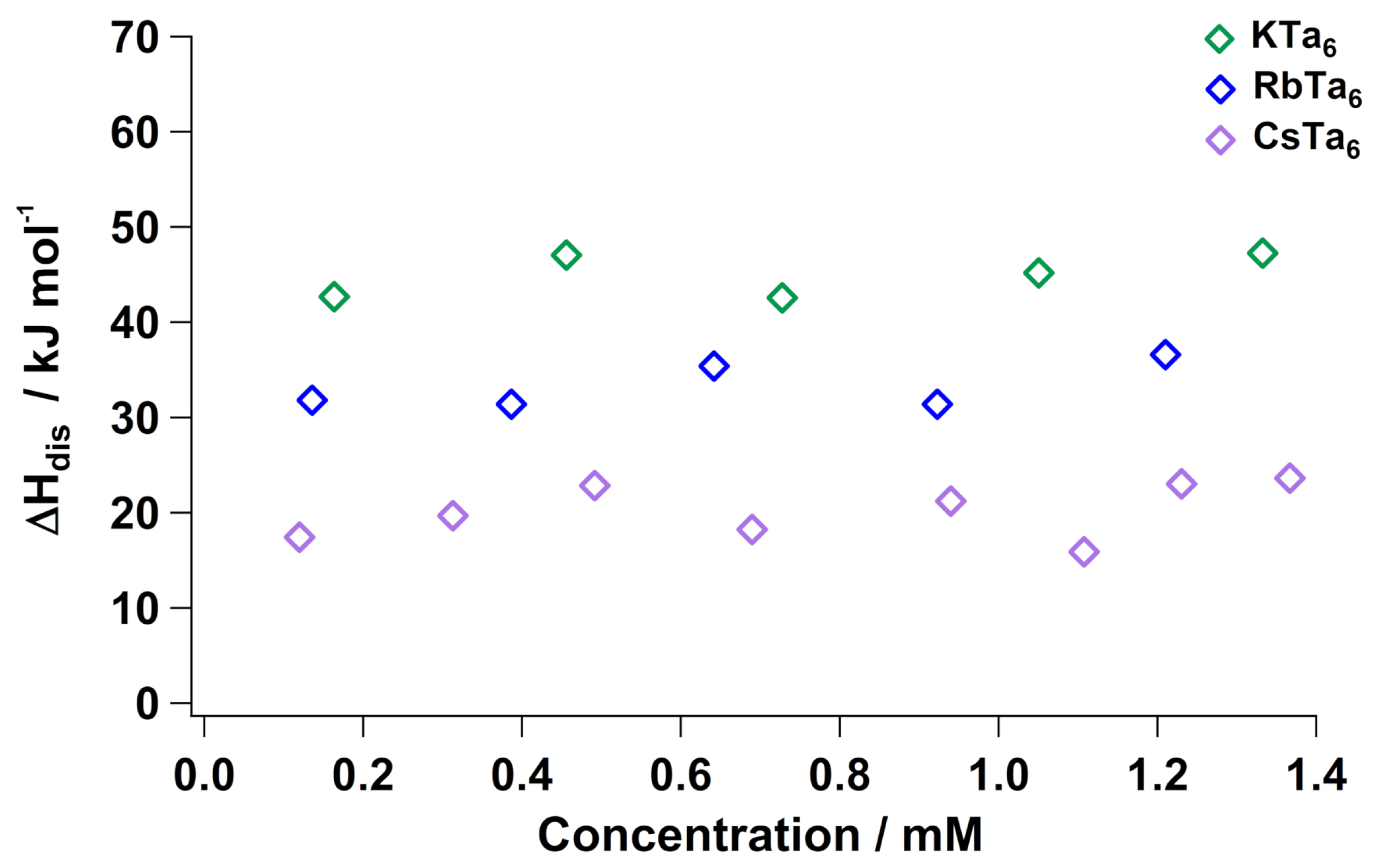
2.2. Dissolution Energy of Ta Salts
3. Materials and Methods
3.1. Determination of Drop Solution Enthalpies
3.2. Determination of Room Temperature Dissolution Enthalpies
3.3. Thermogravimetric Analysis
3.4. Syntheses
3.4.1. Alkali Salts
3.4.2. Tetramethylammonium Salt
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| POM | Polyoxometalate |
| Nb | Hexaniobate |
| Ta | Hexatantalate |
| TMA | Tetramethylammonium |
References
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Ayers, P.W. An elementary derivation of the hard/soft-acid/base principle. J. Chem. Phys. 2005, 122, 141102. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Evans, J.N. The Fukui function: a key concept linking frontier molecular orbital theory and the hard-soft-acid-base principle. J. Am. Chem. Soc. 1995, 117, 7756–7759. [Google Scholar] [CrossRef]
- Ayers, P.W.; Parr, R.G.; Pearson, R.G. Elucidating the hard/soft acid/base principle: A perspective based on half-reactions. J. Chem. Phys. 2006, 124, 194107. [Google Scholar] [CrossRef] [PubMed]
- Oncsik, T.; Trefalt, G.; Borkovec, M.; Szilagyi, I. Specific ion effects on particle aggregation induced by monovalent salts within the Hofmeister series. Langmuir 2015, 31, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Szwarc, M. Ions and Ion Pairs in Organic Reactions; John Wiley & Sons: Hoboken, NJ, USA, 1974; Volume 2. [Google Scholar]
- Linke, W.F. Solubilities, Inorganic and Metal Organic Compounds: A Compilation of Solubility Data from the Periodical Literature; Van Nostrand: Syracuse, NY, USA, 1965; Volume 2. [Google Scholar]
- Sures, D.J.; Serapian, S.A.; Kozma, K.; Molina, P.I.; Bo, C.; Nyman, M. Electronic and relativistic contributions to ion-pairing in polyoxometalate model systems. Phys. Chem. Chem. Phys. 2017, 19, 8715–8725. [Google Scholar] [CrossRef] [PubMed]
- Boehm, H. Acidic and basic properties of hydroxylated metal oxide surfaces. Discuss. Faraday Soc. 1971, 52, 264–275. [Google Scholar] [CrossRef]
- Connor, G.P.; Holland, P.L. Coordination chemistry insights into the role of alkali metal promoters in dinitrogen reduction. Catal. Today 2017, 286, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Bonzel, H. Alkali-promoted gas adsorption and surface reactions on metals. J. Vac. Sci. Technol. A Vac. Surf. Films 1984, 2, 866–872. [Google Scholar] [CrossRef]
- Mross, W. Alkali doping in heterogeneous catalysis. Catal. Rev. Sci. Eng. 1983, 25, 591–637. [Google Scholar] [CrossRef]
- Seidell, A. Solubilities of Inorganic and Organic Compounds C. 2; D. Van Nostrand Company: New York, NY, USA, 1919. [Google Scholar]
- Perry, D.L. Handbook of Inorganic Compounds; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Nyman, M.; Alam, T.M.; Bonhomme, F.; Rodriguez, M.A.; Frazer, C.S.; Welk, M.E. Solid-state structures and solution behavior of alkali salts of the [Nb6O19]8- Lindqvist ion. J. Clust. Sci. 2006, 17, 197–219. [Google Scholar] [CrossRef]
- Nyman, M. Polyoxoniobate chemistry in the 21st century. Dalton Trans. 2011, 40, 8049–8058. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Nyman, M.; Rodriguez, M.A. Soluble heteropolyniobates from the bottom of group IA. Angew. Chem. Int. Ed. 2011, 50, 12514–12517. [Google Scholar] [CrossRef] [PubMed]
- Sures, D.J.; Molina, P.I.; Miró, P.; Zakharov, L.N.; Nyman, M. Cesium salts of niobo-tungstate isopolyanions with intermediate group V–group VI character. New J. Chem. 2016, 40, 928–936. [Google Scholar] [CrossRef]
- Krtil, J. Exchange properties of ammonium salts of 12-heteropolyacids—II: Separation of rubidium and caesium on ammonium phosphotungstate. J. Inorg. Nucl. Chem. 1961, 19, 298–303. [Google Scholar] [CrossRef]
- Briand, L.E.; Baronetti, G.T.; Thomas, H.J. The state of the art on Wells–Dawson heteropoly-compounds: A review of their properties and applications. Appl. Catal. A Gen. 2003, 256, 37–50. [Google Scholar] [CrossRef]
- Ginsberg, A.P. Inorganic Syntheses; John Wiley & Sons: Hoboken, NJ, USA, 1990. [Google Scholar]
- Fullmer, L.B.; Mansergh, R.H.; Zakharov, L.N.; Keszler, D.A.; Nyman, M. Nb2O5 and Ta2O5 thin films from polyoxometalate precursors: A single proton makes a difference. Cryst. Growth Des. 2015, 15, 3885–3892. [Google Scholar] [CrossRef]
- Nyman, M.; Burns, P.C. A comprehensive comparison of transition-metal and actinyl polyoxometalates. Chem. Soc. Rev. 2012, 41, 7354–7367. [Google Scholar] [CrossRef] [PubMed]
- Fullmer, L.B.; Molina, P.I.; Antonio, M.R.; Nyman, M. Contrasting ion-association behaviour of Ta and Nb polyoxometalates. Dalton Trans. 2014, 43, 15295–15299. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, G.J.P.; Moncomble, A.; Cote, G.; Bélair, S.; Chagnes, A. Experimental and computational exploration of the UV-visible properties of hexaniobate and hexatantalate ions. RSC Adv. 2015, 5, 7619–7627. [Google Scholar] [CrossRef]
- Sures, D.J.; Sahu, S.K.; Molina, P.I.; Navrotsky, A.; Nyman, M. Distinctive interactions of cesium and hexaniobate in water. ChemistrySelect 2016, 1, 1858–1862. [Google Scholar] [CrossRef]
- Anderson, T.M.; Rodriguez, M.A.; Bonhomme, F.; Bixler, J.N.; Alam, T.M.; Nyman, M. An aqueous route to [Ta6O19]8- and solid-state studies of isostructural niobium and tantalum oxide complexes. Dalton Trans. 2007, 4517–4522. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.M.; Thoma, S.G.; Bonhomme, F.; Rodriguez, M.A.; Park, H.; Parise, J.B.; Alam, T.M.; Larentzos, J.P.; Nyman, M. Lithium Polyniobates. A Lindqvist-supported lithium- water adamantane cluster and conversion of hexaniobate to a discrete Keggin complex. Cryst. Growth Des. 2007, 7, 719–723. [Google Scholar] [CrossRef]
- Hou, Y.; Fast, D.B.; Ruther, R.E.; Amador, J.M.; Fullmer, L.B.; Decker, S.R.; Zakharov, L.N.; Dolgos, M.R.; Nyman, M. The atomic level journey from aqueous polyoxometalate to metal oxide. J. Solid State Chem. 2015, 221, 418–425. [Google Scholar] [CrossRef]
- Nyman, M.; Anderson, T.M.; Provencio, P.P. Comparison of aqueous and non-aqueous soft-chemical syntheses of lithium niobate and lithium tantalate powders. Cryst. Growth Des. 2008, 9, 1036–1040. [Google Scholar] [CrossRef]
- Deblonde, G.J.P.; Coelho-Diogo, C.; Chagnes, A.; Cote, G.; Smith, M.E.; Hanna, J.V.; Iuga, D.; Bonhomme, C. Multinuclear solid-state NMR investigation of hexaniobate and hexatantalate compounds. Inorg. Chem. 2016, 55, 5946–5956. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Ozawa, Y.; Yagasaki, A. Which is the most basic oxygen in [Ta6O19]8-?—Synthesis and structural characterization of [H2Ta6O19]6-. Inorg. Chem. Commun. 2011, 14, 115–117. [Google Scholar] [CrossRef]
- Matsumoto, M.; Ozawa, Y.; Yagasaki, A. Long hydrogen-bonded rod of molecular oxide: A hexatantalate tetramer. Inorg. Chem. 2012, 51, 5991–5993. [Google Scholar] [CrossRef] [PubMed]
- Robie, R.A.; Hemmingway, B.; Fisher, J.R. Thermodynamic Properties of Minerals and Related Substances at 298. 15 K and 1 Bar (105 Pascals) Pressure and at Higher Temperatures; U.S. Geological Survey: Reston, VA, USA, 1978.
- Zlotnik, S.; Sahu, S.K.; Navrotsky, A.; Vilarinho, P.M. Pyrochlore and perovskite potassium tantalate: Enthalpies of formation and phase transformation. Chem. Eur. J. 2015, 21, 5231–5237. [Google Scholar] [CrossRef] [PubMed]

| Species | # of Non-Hydrogen Atoms | Charge Density | Solubility Trend |
|---|---|---|---|
| [PO4]3- | 5 | 0.6 | Anomalous [13] |
| [CO3]2- | 4 | 0.5 | Anomalous [13] |
| [ClO4]- | 5 | 0.2 | Normal [14] |
| [Nb6O19]8- | 25 | 0.32 | Anomalous [15] |
| [Ta6O19]8- | 25 | 0.32 | Anomalous [16] |
| [SiNb12O40]16- | 53 | 0.30 | Anomalous [16] |
| [GaNb18O54]15- | 73 | 0.21 | Anomalous [17] |
| [Nb4W2O19]6- | 25 | 0.24 | Anomalous [18] |
| [Nb2W4O19]4- | 25 | 0.16 | Normal [18] |
| [W6O19]2- | 25 | 0.08 | Normal * |
| [SiW12O40]4- | 53 | 0.075 | Normal [19] |
| [P2W18O62]6- | 82 | 0.073 | Normal [20] |
| [PW12O40]3- | 53 | 0.057 | Normal [19] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sures, D.J.; Nagabhushana, G.P.; Navrotsky, A.; Nyman, M. Thermochemical Measurements of Alkali Cation Association to Hexatantalate. Molecules 2018, 23, 2441. https://doi.org/10.3390/molecules23102441
Sures DJ, Nagabhushana GP, Navrotsky A, Nyman M. Thermochemical Measurements of Alkali Cation Association to Hexatantalate. Molecules. 2018; 23(10):2441. https://doi.org/10.3390/molecules23102441
Chicago/Turabian StyleSures, Dylan J., G. P. Nagabhushana, Alexandra Navrotsky, and May Nyman. 2018. "Thermochemical Measurements of Alkali Cation Association to Hexatantalate" Molecules 23, no. 10: 2441. https://doi.org/10.3390/molecules23102441
APA StyleSures, D. J., Nagabhushana, G. P., Navrotsky, A., & Nyman, M. (2018). Thermochemical Measurements of Alkali Cation Association to Hexatantalate. Molecules, 23(10), 2441. https://doi.org/10.3390/molecules23102441