An Anti-Inflammatory Azaphenothiazine Inhibits Interferon β Expression and CXCL10 Production in KERTr Cells
Abstract
1. Introduction
2. Results
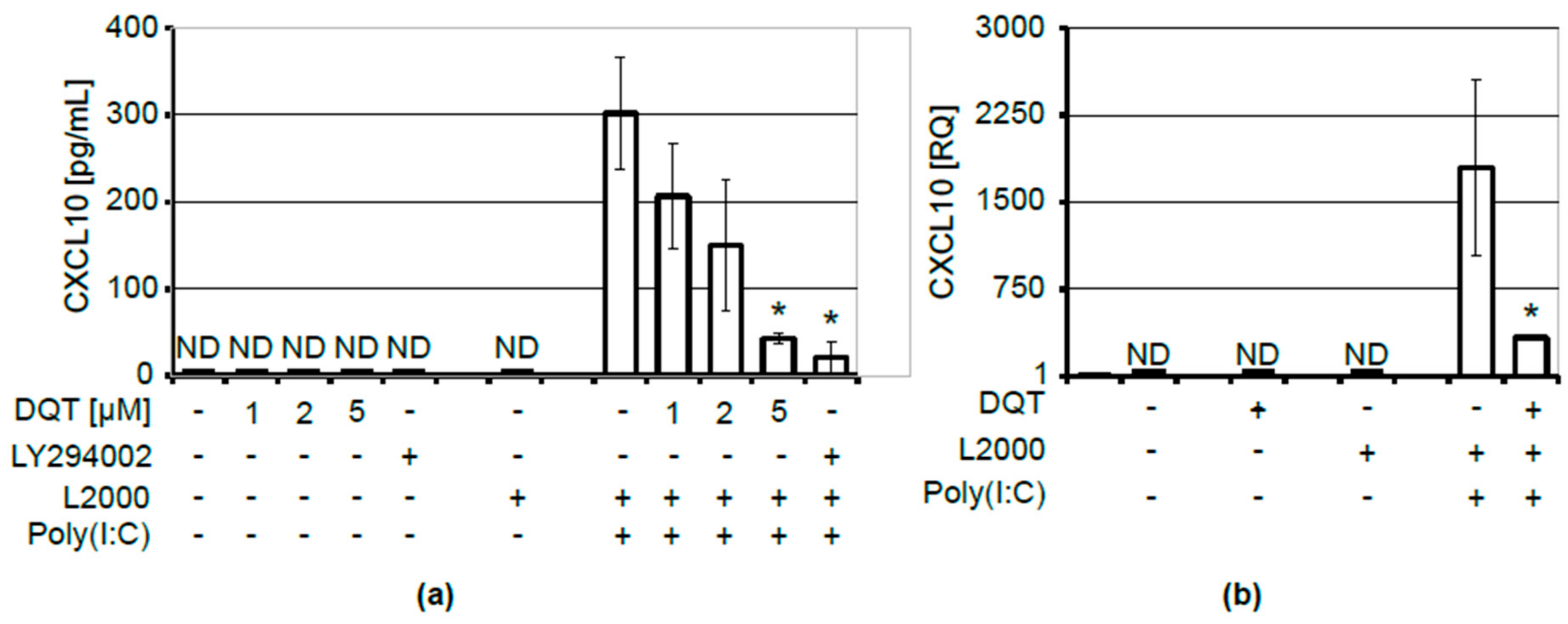
2.1. DQT Compound Inhibits CXCL10 Release upon Induction with Poly(I:C)
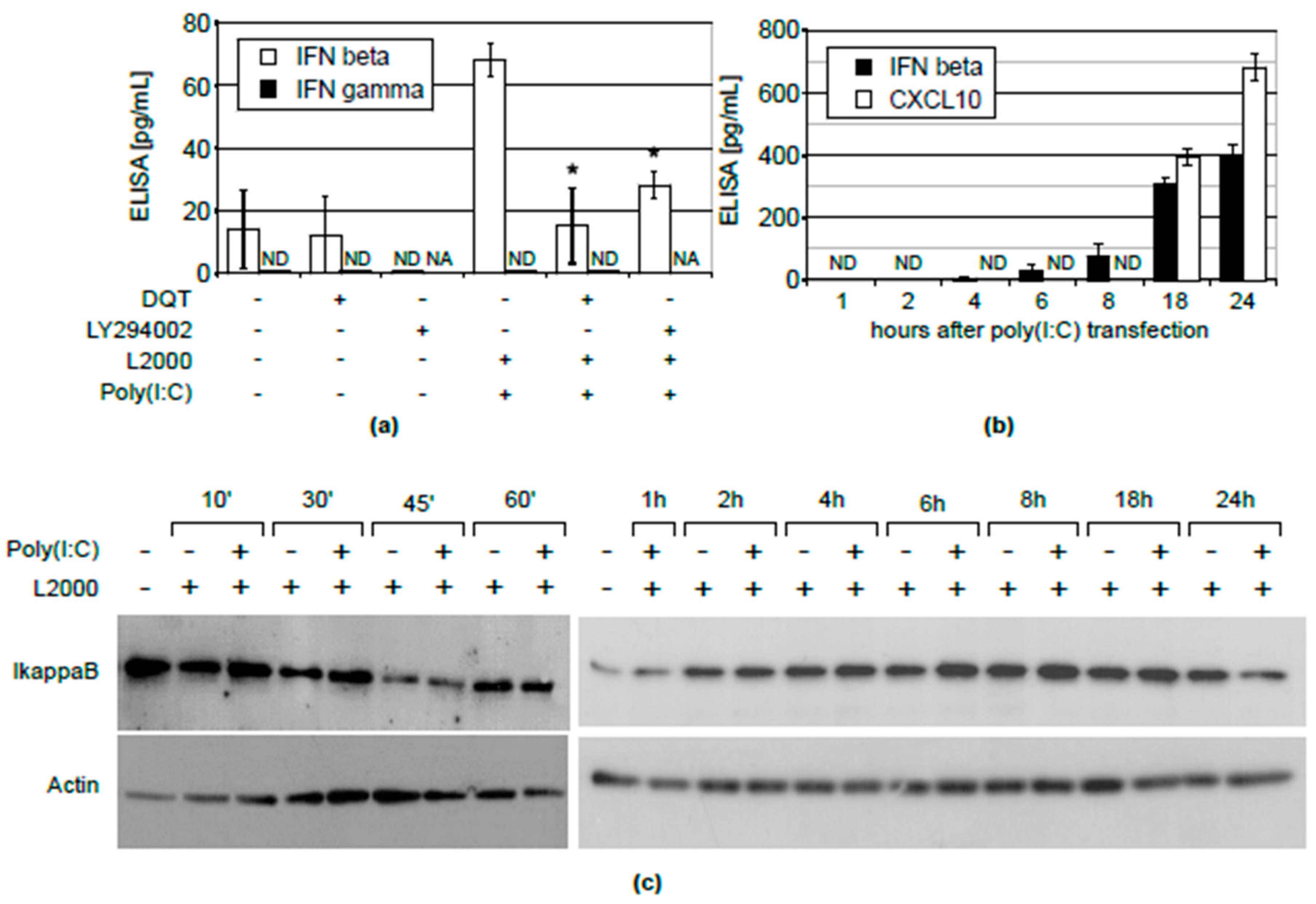
2.2. Inhibition of CXCL10 Release by DQT Is Associated with Its Effect on IFNβ, But Not IFNγ Expression or NFĸB Activation
3. Discussion
4. Materials and Methods
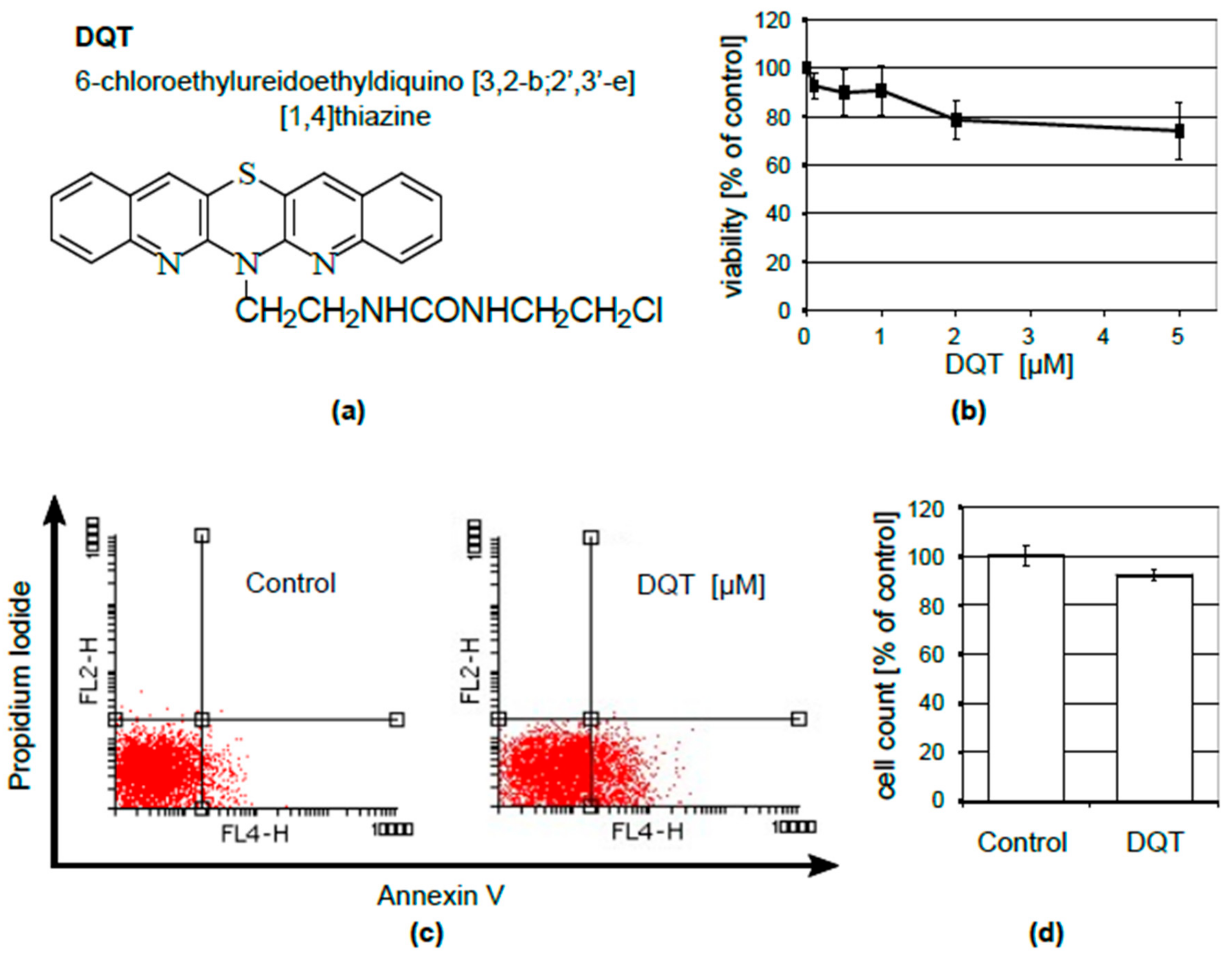
4.1. DQT
4.2. Cell Culture and Treatment
4.3. Cell Viability Assay
4.4. Flow Cytometry
4.5. Cell Counting
4.6. Western Blotting
4.7. Quantitative Polymerase Chain Reaction (Real-Time RT-PCR)
4.8. ELISA Assays
4.9. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaszczyszyn, A.; Gąsiorowski, K.; Świątek, P.; Malinka, W.; Cieślik-Boczula, K.; Petrus, J.; Czarnik-Matusewicz, B. Chemical structure of phenothiazines and their biological activity. Pharmacol. Rep. 2012, 64, 16–23. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, M. Synthesis, properties and reactions of phenothiazines. In Phenothiazines and 1,4-Benzothiazines—Chemical and Biological Aspects; Elsevier: Amsterdam, The Netherlands, 1988; pp. 1–166. [Google Scholar]
- Pluta, K.; Jeleń, M.; Morak-Młodawska, B.; Zimecki, M.; Artym, J.; Kocięba, M.; Zaczyńska, E. Azaphenothiazines—Promising phenothiazine derivatives. An insight into nomenclature, synthesis, structure elucidation and biological properties. Eur. J. Med. Chem. 2017, 138, 774–806. [Google Scholar] [CrossRef] [PubMed]
- Zimecki, M.; Artym, J.; Kocięba, M.; Pluta, K.; Morak-Młodawska, B.; Jeleń, M. The immunosuppressive activities of newly synthesized azaphenothiazines in human and mouse models. Cell. Mol. Biol. Lett. 2009, 14, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Pluta, K.; Jeleń, M.; Morak-Młodawska, B.; Zimecki, M.; Artym, J.; Kocieba, M. Anticancer activity of newly synthesized azaphenothiazines from NCI’s anticancer screening bank. Pharmacol. Rep. 2010, 62, 319–332. [Google Scholar] [CrossRef]
- Jeleń, M.; Pluta, K.; Zimecki, M.; Morak-Młodawska, B.; Artym, J.; Kocięba, M. Synthesis and selected immunological properties of substituted quino[3,2-b]benzo[1,4]thiazines. Eur. J. Med. Chem. 2013, 63, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Jeleń, M.; Pluta, K.; Zimecki, M.; Morak-Młodawska, B.; Artym, J.; Kocięba, M. 6-Substituted 9-fluoroquino[3,2-b]benzo[1,4]thiazines display strong antiproliferative and antitumor properties. Eur. J. Med. Chem. 2015, 89, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Morak-Młodawska, B.; Pluta, K.; Zimecki, M.; Jeleń, M.; Artym, J.; Kocięba, M. Synthesis and selected immunological properties of 10-substituted 1,8-diazaphenothiazines. Med. Chem. Res. 2015, 24, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Artym, J.; Kochanowska, I.E.; Kocięba, M.; Zaczyńska, E.; Zimecki, M.; Jeleń, M.; Morak-Młodawska, B.; Pluta, K. Selected azaphenothiazines inhibit delayed type hypersensitivity and carrageenan reaction in mice. Int. Immunopharmacol. 2016, 40, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Artym, J.; Kocięba, M.; Zaczyńska, E.; Zimecki, M.; Jeleń, M.; Morak-Młodawska, B.; Pluta, K.; Kaleta-Kuratewicz, K.; Madej, J.P.; Kuropka, P.; et al. Topically applied azaphenothiazines inhibit contact sensitivity to oxazolone in mice. Histol. Histopathol. 2018, 33, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Artym, J.; Kocięba, M.; Zaczyńska, E.; Kochanowska, I.; Zimecki, M.; Kałas, W.; Fiedorowicz, A.; Pawlak, A.; Strządała, L.; Jeleń, M.; et al. Topically applied azaphenothiazines inhibit experimental psoriasis in mice. Int. Immunopharmacol. 2018, 59, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.-F.; Kim, D.-H.; Yoon, Y.-S.; Jin, D.; Huang, X.-Z.; Li, J.-H.; Deung, Y.-K.; Lee, K.-J. Essential involvement of cross-talk between IFN-gamma and TNF-alpha in CXCL10 production in human THP-1 monocytes. J. Cell. Physiol. 2009, 220, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P.; Ghiri, E.; Crescioli, C.; Romagnani, P.; Vitti, P.; Serio, M.; Ferrannini, E. Interferon-alpha, -beta and -gamma induce CXCL9 and CXCL10 secretion by human thyrocytes: Modulation by peroxisome proliferator-activated receptor-gamma agonists. Cytokine 2010, 50, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Qi, J.; Wang, L.; Zhang, M.; Wang, P.; Gao, C. LY294002 inhibits TLR3/4-mediated IFN-β production via inhibition of IRF3 activation with a PI3K-independent mechanism. FEBS Lett. 2012, 586, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Ruffilli, I.; Colaci, M.; Antonelli, A.; Ferri, C.; Fallahi, P. CXCL10 in psoriasis. Adv. Med. Sci. 2015, 60, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, H.; Söderström, I.; Rocklöv, J.; Hallmans, G.; Lejon, K.; Rantapää Dahlqvist, S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheumatol. 2010, 62, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Cossu, M.; van Bon, L.; Preti, C.; Rossato, M.; Beretta, L.; Radstake, T.R. Earliest Phase of Systemic Sclerosis Typified by Increased Levels of Inflammatory Proteins in the Serum. Arthritis Rheumatol. 2017, 69, 2359–2369. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.; Grützkau, A.; Klotsche, J.; Enghard, P.; Flechsig, A.; Keller, J.; Riemekasten, G.; Radbruch, A.; Burmester, G.-R.; Dörner, T.; et al. Are interferon-related biomarkers advantageous for monitoring disease activity in systemic lupus erythematosus? A longitudinal benchmark study. Rheumatology 2017, 56, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Ruffilli, I. Sjögren’s syndrome and chemokines. Clin. Ther 2014, 165, e464–e469. [Google Scholar]
- De Paepe, B.; Creus, K.K.; De Bleecker, J.L. Role of cytokines and chemokines in idiopathic inflammatory myopathies. Curr. Opin. Rheumatol. 2009, 21, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Stoof, T.J.; Flier, J.; Sampat, S.; Nieboer, C.; Tensen, C.P.; Boorsma, D.M. The antipsoriatic drug dimethylfumarate strongly suppresses chemokine production in human keratinocytes and peripheral blood mononuclear cells. Br. J. Dermatol. 2001, 144, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Oslund, K.L.; Zhou, X.; Lee, B.; Zhu, L.; Duong, T.; Shih, R.; Baumgarth, N.; Hung, L.-Y.; Wu, R.; Chen, Y. Synergistic up-regulation of CXCL10 by virus and IFN γ in human airway epithelial cells. PLoS ONE 2014, 9, e100978. [Google Scholar] [CrossRef] [PubMed]
- Shultz, D.B.; Rani, M.R.S.; Fuller, J.D.; Ransohoff, R.M.; Stark, G.R. Roles of IKK-beta, IRF1, and p65 in the activation of chemokine genes by interferon-gamma. J. Interferon Cytokine Res. 2009, 29, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Van der Fits, L.; van der Wel, L.I.; Laman, J.D.; Prens, E.P.; Verschuren, M.C.M. In psoriasis lesional skin the type I interferon signaling pathway is activated, whereas interferon-alpha sensitivity is unaltered. J. Investig. Dermatol. 2004, 122, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Assassi, S. The Role of Type 1 Interferon in Systemic Sclerosis. Front. Immunol. 2013, 4, 266. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A.; Hsu, H.-C.; Mountz, J.D. Role of production of type I interferons by B cells in the mechanisms and pathogenesis of systemic lupus erythematosus. Discov. Med. 2018, 25, 21–29. [Google Scholar] [PubMed]
- Li, H.; Ice, J.A.; Lessard, C.J.; Sivils, K.L. Interferons in Sjögren’s Syndrome: Genes, Mechanisms, and Effects. Front. Immunol. 2013, 4, 290. [Google Scholar] [CrossRef] [PubMed]
- Paty, D.W.; Li, D.K. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology 1993, 43, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Amschler, K.; Meyersburg, D.; Kitze, B.; Schön, M.P.; Mössner, R. Onset of psoriasis upon interferon beta treatment in a multiple sclerosis patient. Eur. J. Dermatol. 2016, 26, 211–212. [Google Scholar] [CrossRef] [PubMed]
- La Mantia, L.; Capsoni, F. Psoriasis during interferon beta treatment for multiple sclerosis. Neurol. Sci. 2010, 31, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Kolb-Mäurer, A.; Goebeler, M.; Mäurer, M. Cutaneous Adverse Events Associated with Interferon-β Treatment of Multiple Sclerosis. Int. J. Mol. Sci. 2015, 16, 14951–14960. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds DQT are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzadala, L.; Fiedorowicz, A.; Wysokinska, E.; Ziolo, E.; Grudzień, M.; Jelen, M.; Pluta, K.; Morak-Mlodawska, B.; Zimecki, M.; Kalas, W. An Anti-Inflammatory Azaphenothiazine Inhibits Interferon β Expression and CXCL10 Production in KERTr Cells. Molecules 2018, 23, 2443. https://doi.org/10.3390/molecules23102443
Strzadala L, Fiedorowicz A, Wysokinska E, Ziolo E, Grudzień M, Jelen M, Pluta K, Morak-Mlodawska B, Zimecki M, Kalas W. An Anti-Inflammatory Azaphenothiazine Inhibits Interferon β Expression and CXCL10 Production in KERTr Cells. Molecules. 2018; 23(10):2443. https://doi.org/10.3390/molecules23102443
Chicago/Turabian StyleStrzadala, Leon, Anna Fiedorowicz, Edyta Wysokinska, Ewa Ziolo, Małgorzata Grudzień, Malgorzata Jelen, Krystian Pluta, Beata Morak-Mlodawska, Michal Zimecki, and Wojciech Kalas. 2018. "An Anti-Inflammatory Azaphenothiazine Inhibits Interferon β Expression and CXCL10 Production in KERTr Cells" Molecules 23, no. 10: 2443. https://doi.org/10.3390/molecules23102443
APA StyleStrzadala, L., Fiedorowicz, A., Wysokinska, E., Ziolo, E., Grudzień, M., Jelen, M., Pluta, K., Morak-Mlodawska, B., Zimecki, M., & Kalas, W. (2018). An Anti-Inflammatory Azaphenothiazine Inhibits Interferon β Expression and CXCL10 Production in KERTr Cells. Molecules, 23(10), 2443. https://doi.org/10.3390/molecules23102443





