Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy
Abstract
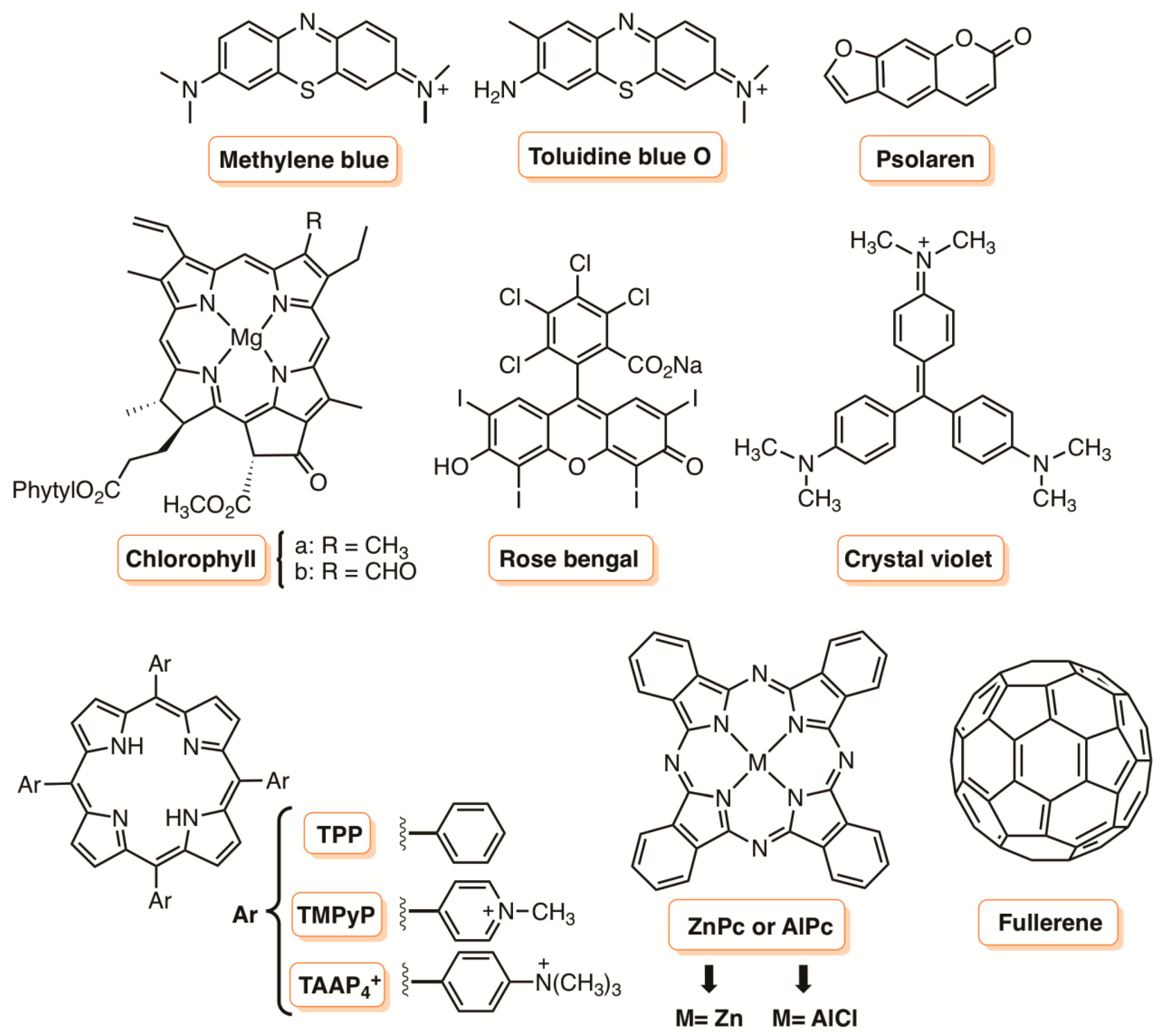
1. Introduction
2. Metal Nanoparticles
2.1. Gold Nanoparticles
2.2. Silver Nanoparticles
2.3. Platinum Nanoparticles
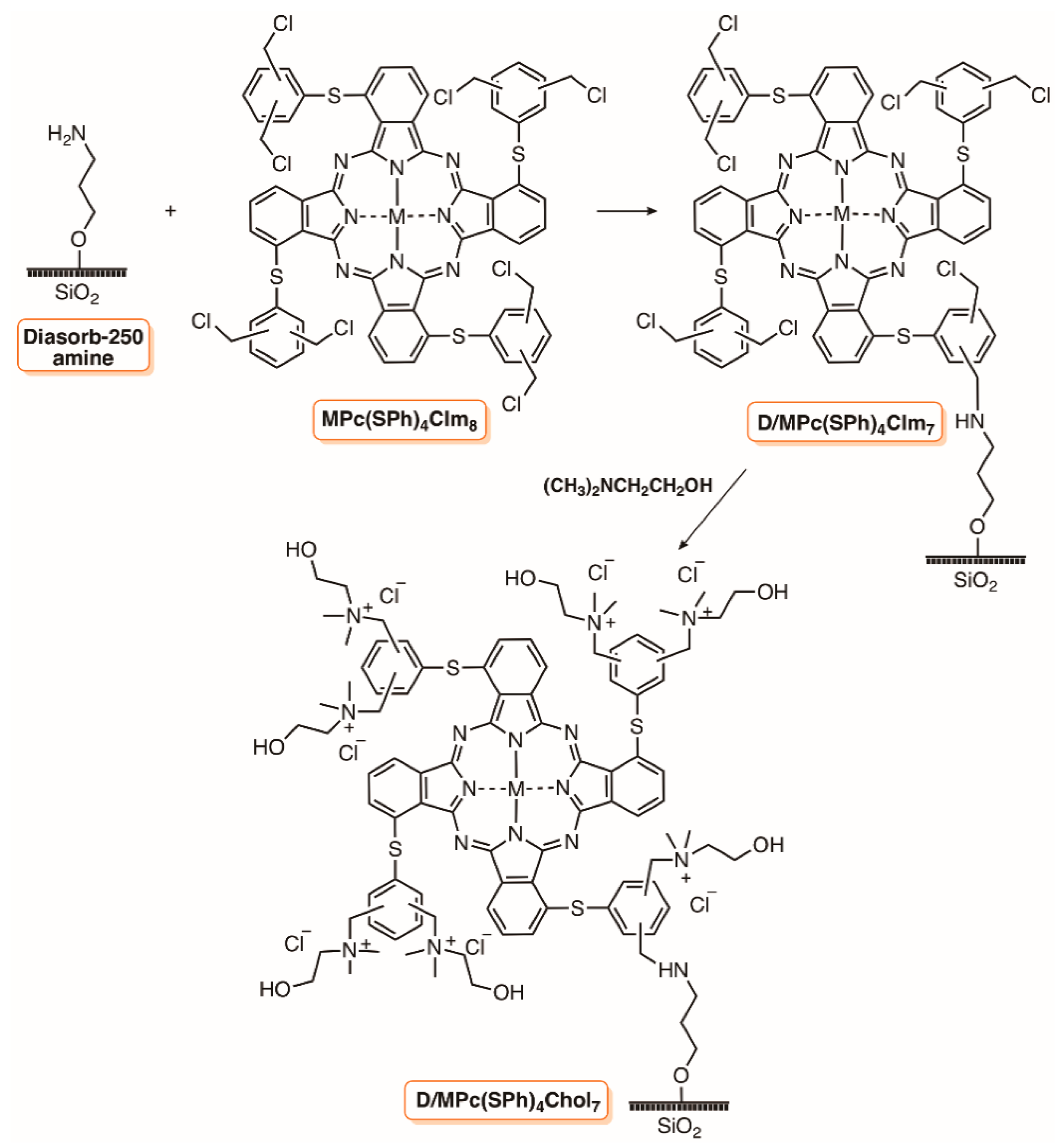
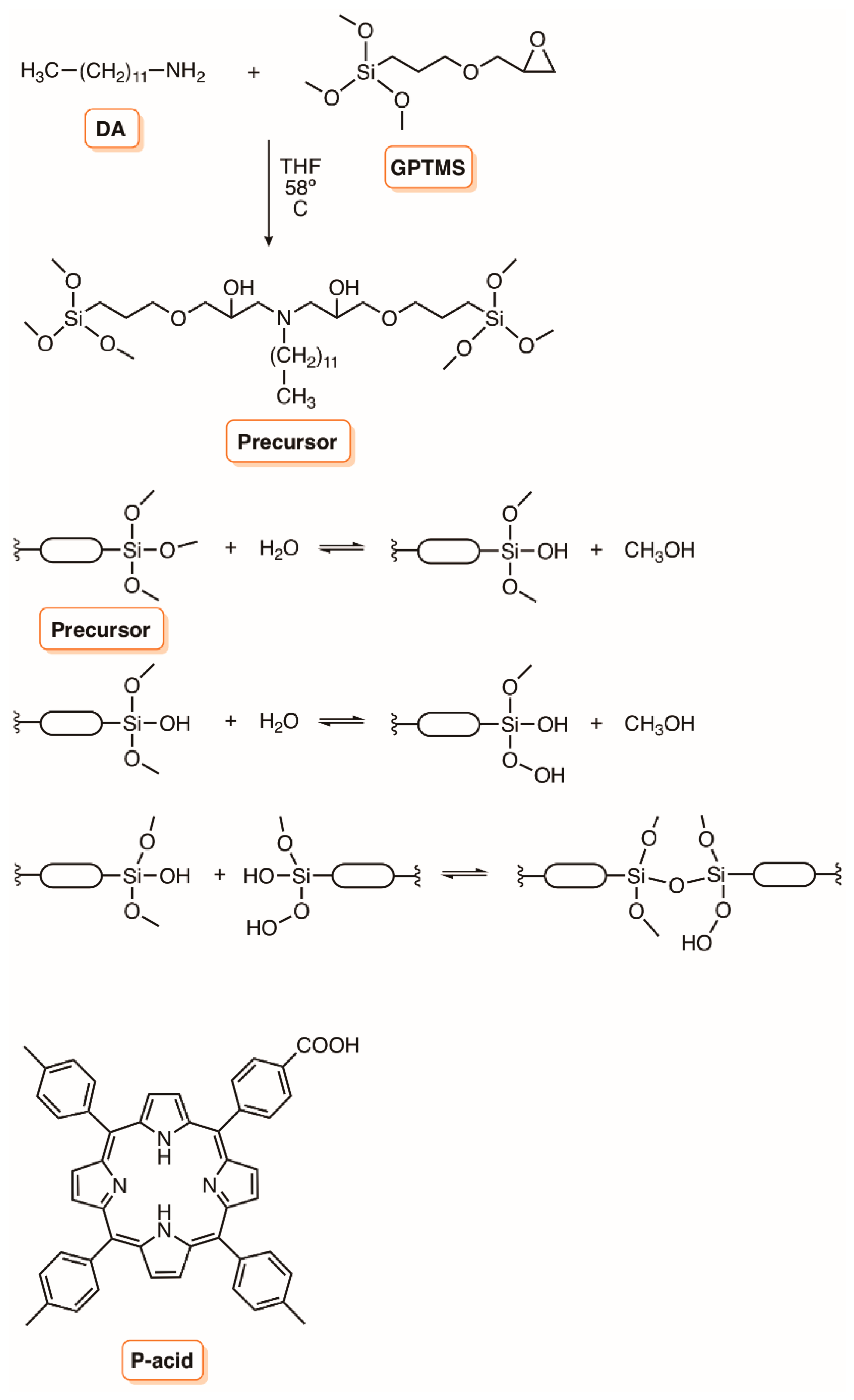
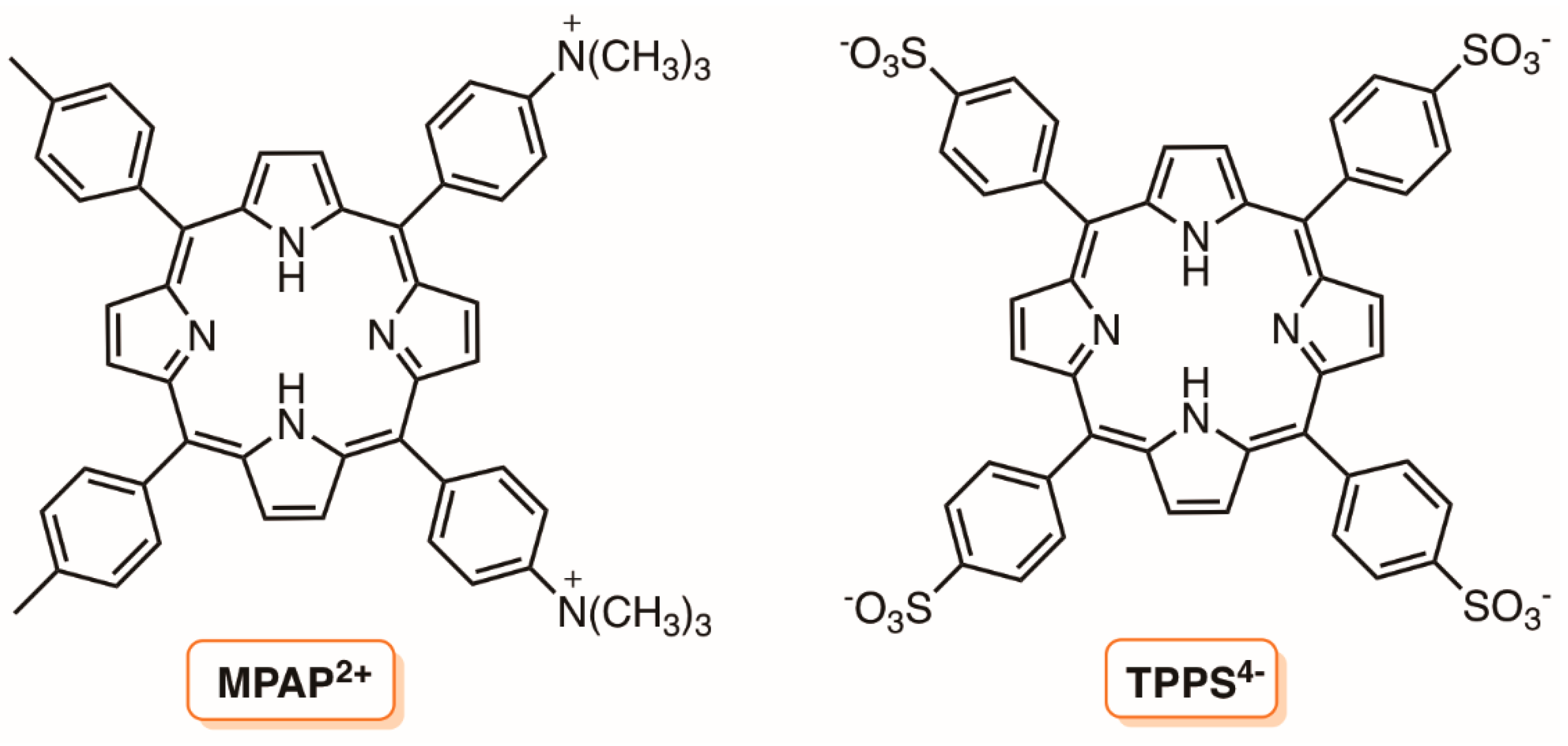
3. Silica Supports
4. Biopolymers
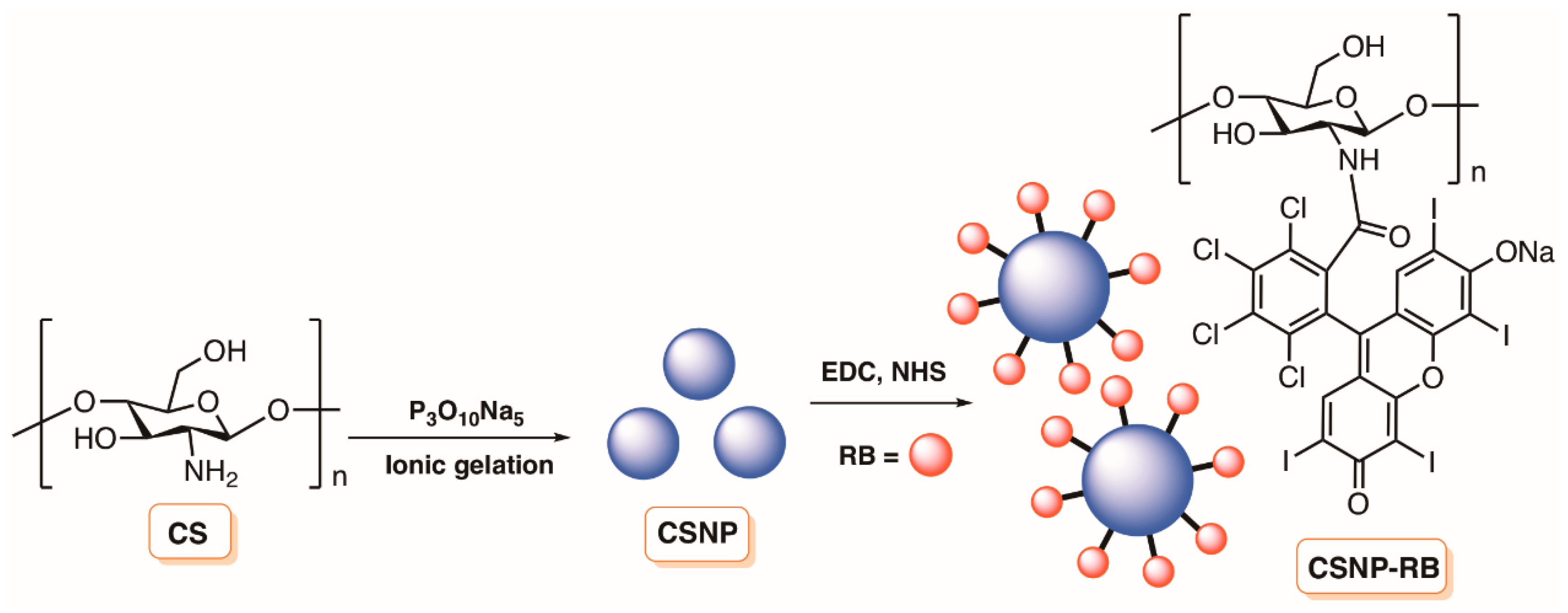
4.1. Chitosan
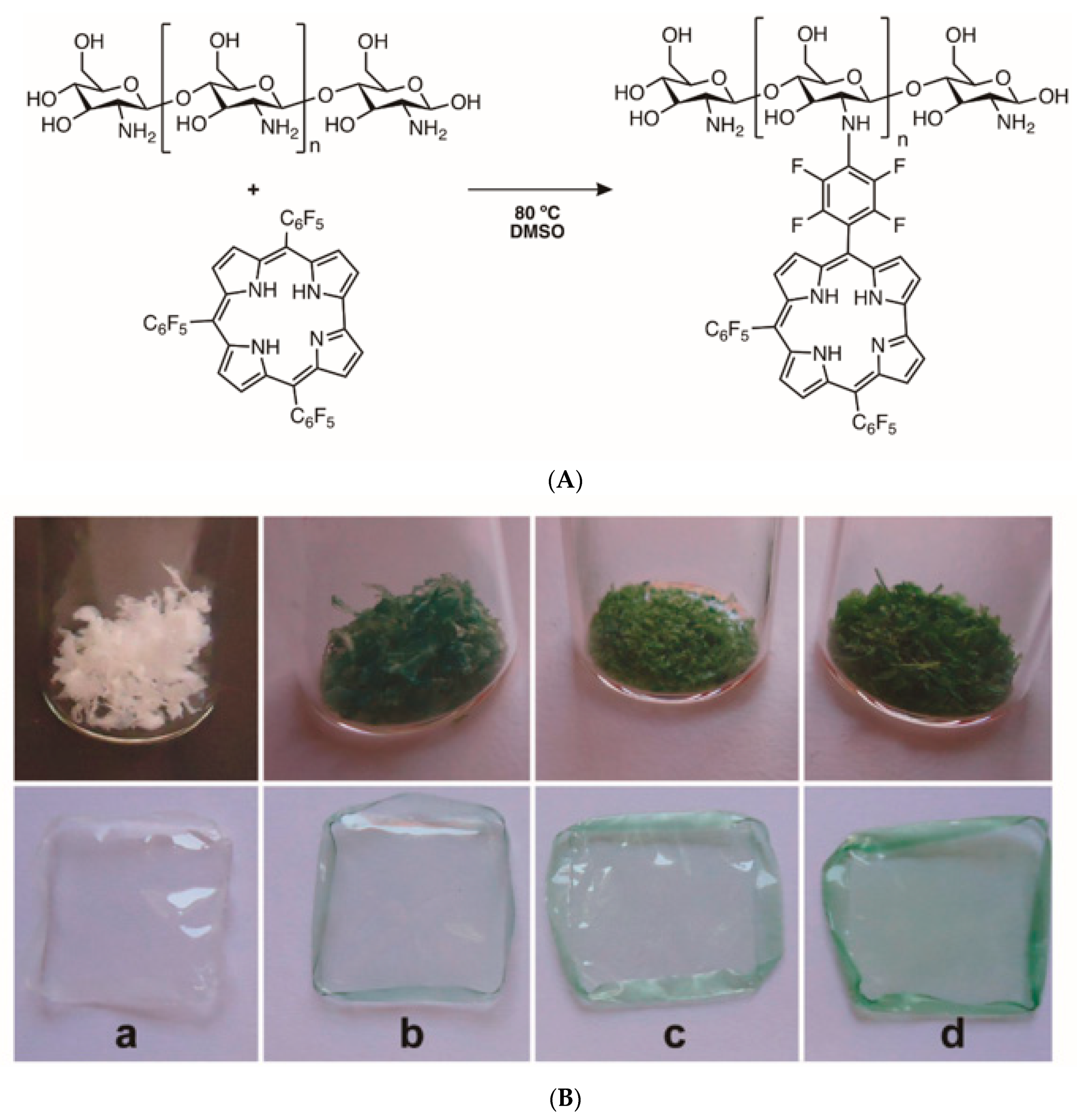
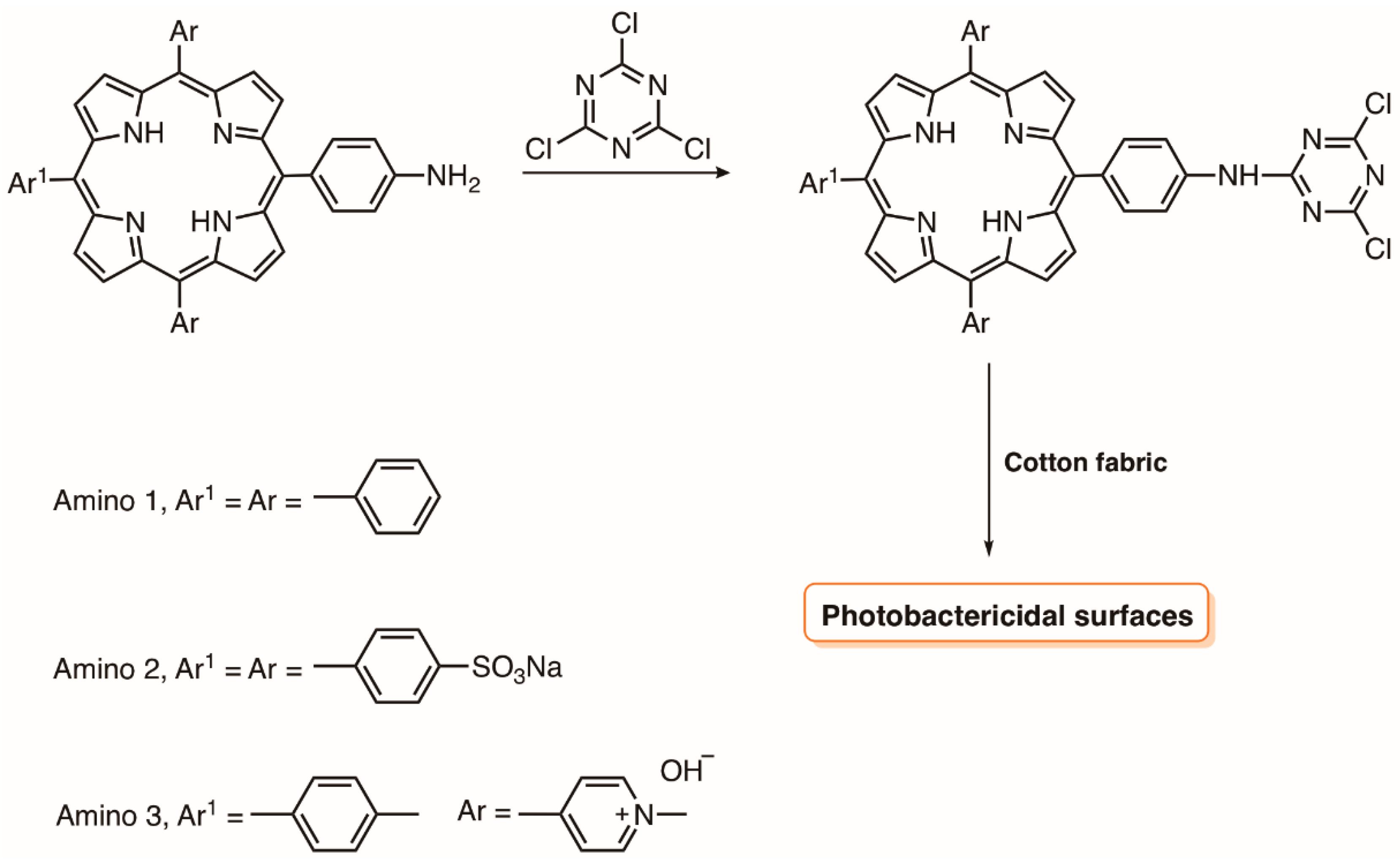
4.2. Cellulose
4.3. Superparamagnetic Iron Oxide Nanoparticles (SPION) Functionalized with Dextran
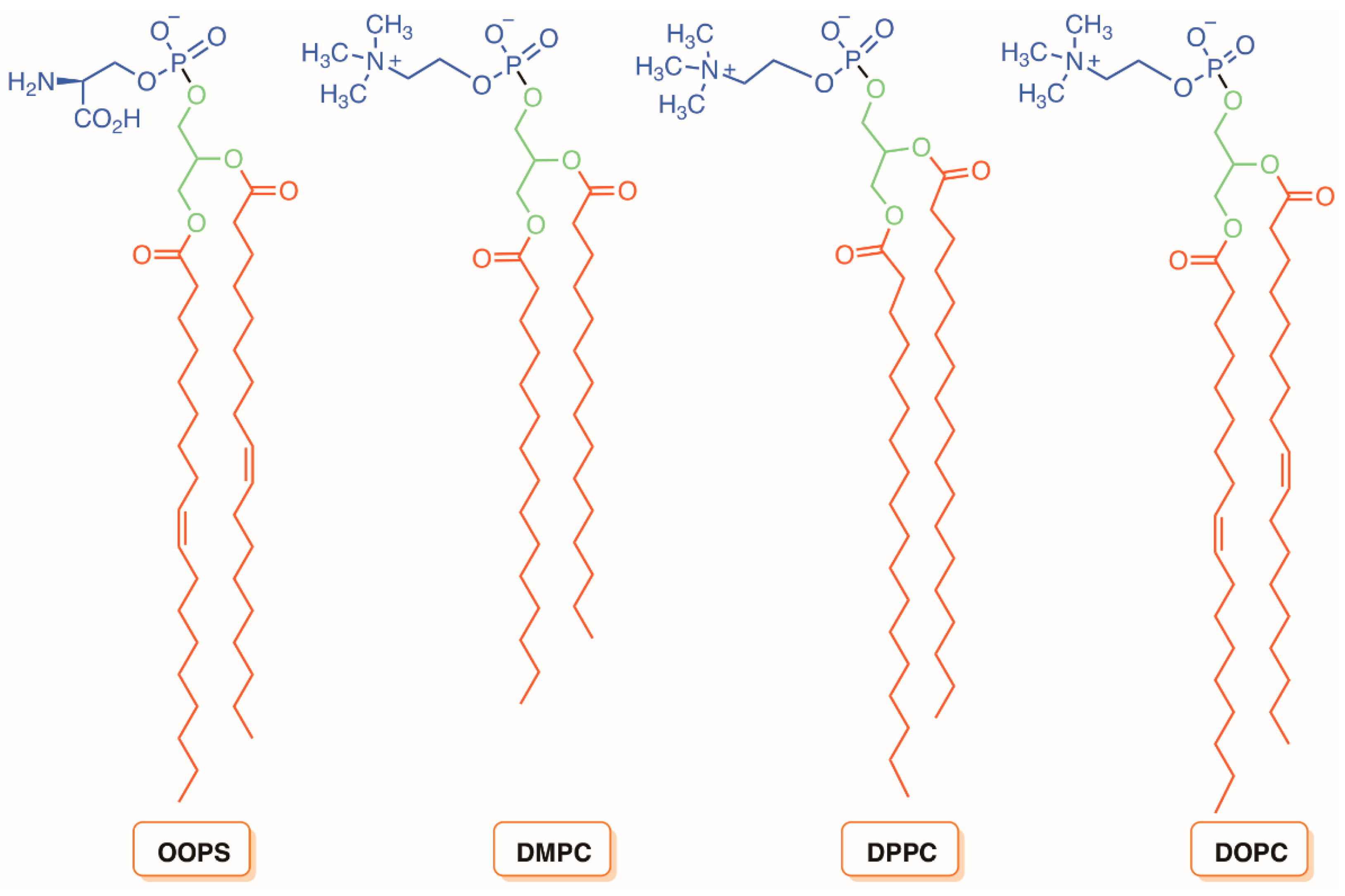
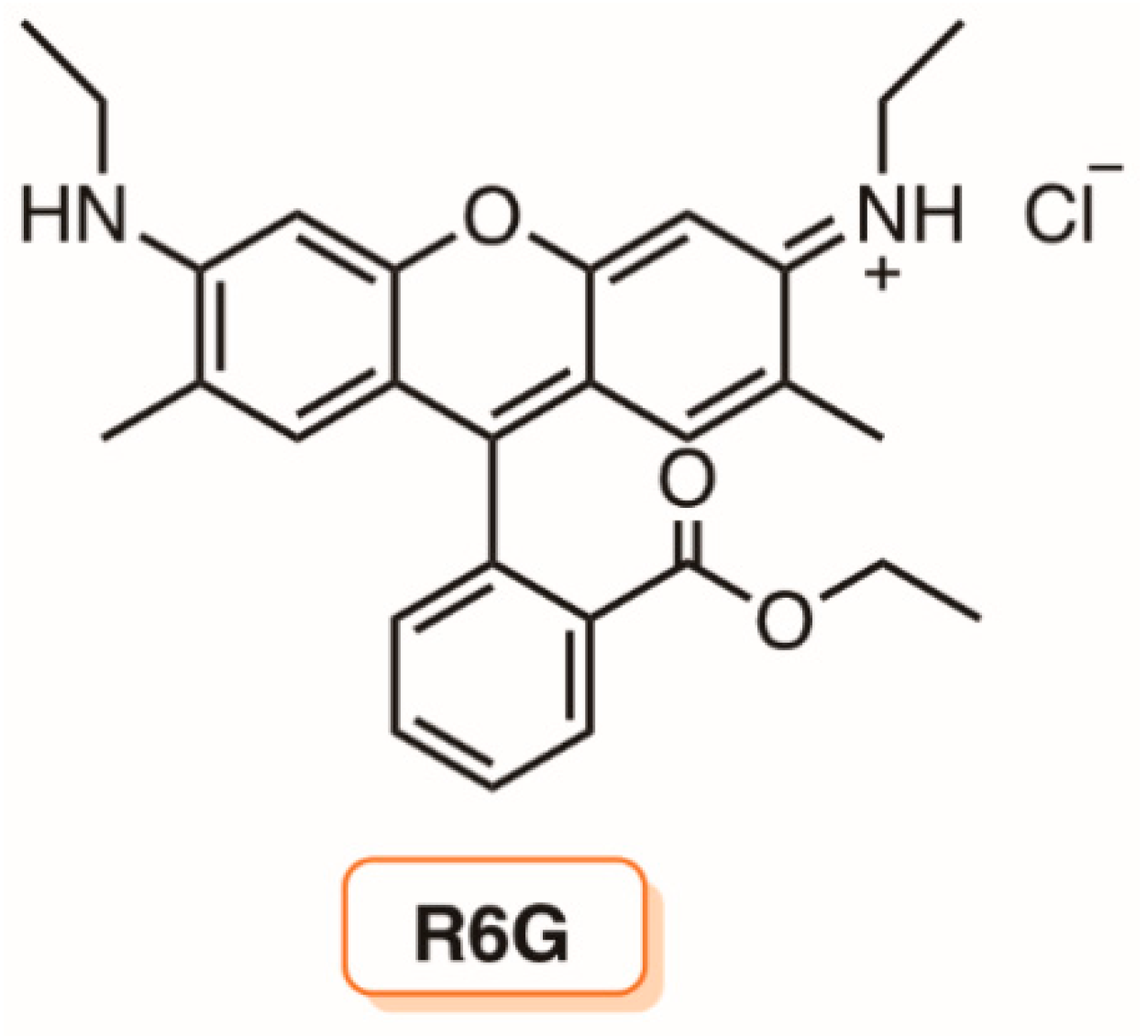
5. Liposomes
6. Hydrogel Materials
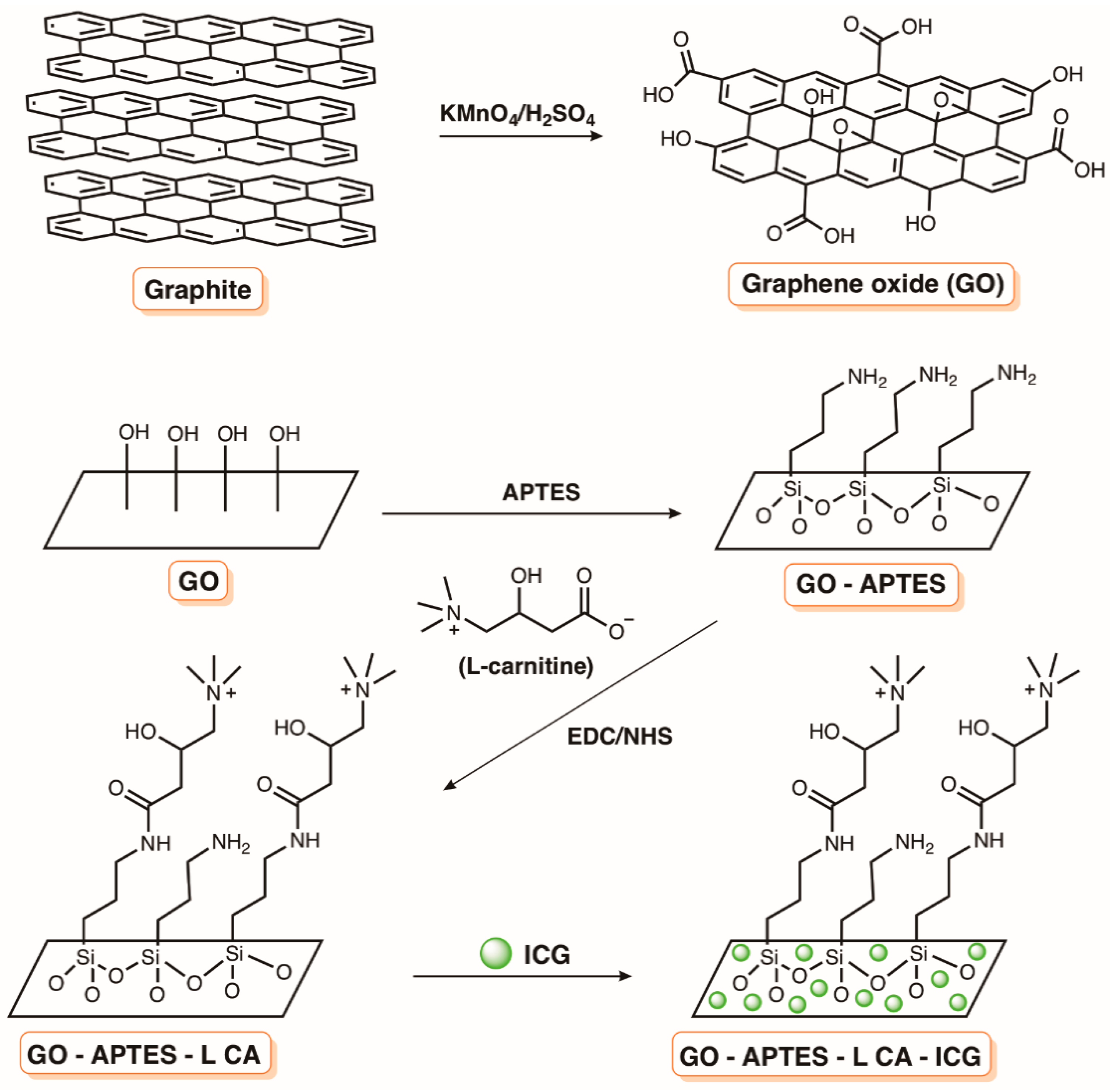
7. Supports Based on Carbon Nanomaterials
8. Final Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Dąbrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Yoshikawa, T.T. Antimicrobial resistance and aging: beginning of the end of the antibiotic era? J. Amer. Geriatr. Soc. 2002, 50, 226–229. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Crispim, C.A.; Gaylarde, P.M.; Gaylarde, C.C. Algal and cyanobacterial biofilms on calcareous historic buildings. Curr. Microbiol. 2003, 46, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Preuß, A.; Bornhütter, T.; Färber, A.; Schaller, C.; Röder, B. Photodynamic inactivation of biofilm building microorganisms by photoactive facade paints. J. Photochem. Photobiol. B Biol. 2016, 160, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Gorbushina, A.A.; Broughton, W.J. Microbiology of the atmosphere-rock interface: How biological interactions and physical stresses modulate a sophisticated microbial ecosystem. Annu. Rev. Microbiol. 2009, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Orell, A.; Fröls, S.; Albers, S.-V. Archaeal Biofilms: The great unexplored. Annu. Rev. Microbiol. 2013, 67, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Mehrad, B. Innate immunity to Aspergillus species. Clin. Microbiol. Rev. 2009, 22, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I. The current role of Aspergillus and Penicillium in human and animal health. J. Med. Vet. Mycol. 1994, 32, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Young, M.E.; Alakomi, H.L.; Fortune, I.; Gorbushina, A.A.; Krumbein, W.E.; Maxwell, I.; McCullagh, C.; Robertson, P.; Saarela, M.; Valero, J.; et al. Development of a biocidal treatment regime to inhibit biological growths on cultural heritage: BIODAM. Environ. Geol. 2008, 56, 631–641. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M. Antifungal photodynamic therapy. Microbiol. Res. 2008, 163, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Demidova, T.N.; Hamblin, M.R. Photodynamic therapy targeted to pathogens. Int. J. Immunopathol. Pharmacol. 2004, 17, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Prates, R. a.; Silva, E.G.; Yamada, A.M.; Suzuki, L.C.; Paula, C.R.; Ribeiro, M.S.; da Silva, E.G.; Yamada, A.M., Jr.; Suzuki, L.C.; Paula, C.R.; et al. Light parameters influence cell viability in antifungal photodynamic therapy in a fluence and rate fluence-dependent manner. Laser Phys. 2009, 19, 1038–1044. [Google Scholar] [CrossRef]
- Maisch, T. A new strategy to destroy antibiotic resistant microorganisms: Antimicrobial photodynamic treatment. Mini Rev. Med. Chem. 2009, 9, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Chabrier-Roselló, Y.; Foster, T.H.; Pérez-Nazario, N.; Mitra, S.; Haidaris, C.G. Sensitivity of Candida albicans germ tubes and biofilms to photofrin-mediated phototoxicity. Antimicrob. Agents Chemother. 2005, 49, 4288–4295. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.; Romeiro, R.L.; Costa, A.C.B.P.; MacHado, A.K.S.; Junqueira, J.C.; Jorge, A.O.C. Susceptibility of Candida albicans, Staphylococcus aureus, and Streptococcus mutans biofilms to photodynamic inactivation: An in vitro study. Lasers Med. Sci. 2011, 26, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Kashef, N.; Huang, Y.Y.; Hamblin, M.R. Advances in antimicrobial photodynamic inactivation at the nanoscale. Nanophotonics 2017, 6, 853–879. [Google Scholar] [CrossRef] [PubMed]
- Makky, A.; Michel, J.P.; Maillard, P.; Rosilio, V. Biomimetic liposomes and planar supported bilayers for the assessment of glycodendrimeric porphyrins interaction with an immobilized lectin. Biochim. Biophys. Acta Biomembr. 2011, 1808, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Van Hillegersberg, R.; Kort, W.J.; Wilson, J.H.P. Current status of photodynamic therapy in oncology. Drugs 1994, 48, 510–527. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Faustino, M.A.F.; Tomé, J.P.C. Photodynamic inactivation of bacteria: Finding the effective targets. Future Med. Chem. 2015, 7, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Esteves, A.C.; Cunha, A.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Almeida, A. Modifications in the protein profile os Escherichia coli and Staphylococcus warneri induced by photosensitization with cationic porphyrins. Photochem. Photobiol. Sci. 2015, 14, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Nadais, H.; Almeida, A. Potential applications of porphyrins in photodynamic inactivation beyond the medical scope. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 34–57. [Google Scholar] [CrossRef]
- Lopes, D.; Melo, T.; Santos, N.; Rosa, L.; Alves, E.; Gomes, M.C.; Cunha, Â.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Domingues, M.R.M.; Almeida, A. Evaluation of the interplay among the charge of porphyrinic photosensitizers, lipid oxidation and photoinactivation efficiency in Escherichia coli. J. Photochem. Photobiol. B Biol. 2014, 141, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Hackbarth, S.; Regensburger, J.; Felgenträger, A.; Bäumler, W.; Landthaler, M.; Röder, B. Photodynamic inactivation of multi-resistant bacteria (PIB)—A new approach to treat superficial infections in the 21st century. J. Dtsch. Dermatol. Ges. 2011, 9, 360–366. [Google Scholar] [CrossRef] [PubMed]
- St. Denis, T.G.; Dai, T.; Izikson, L.; Astrakas, C.; Anderson, R.R.; Hamblin, M.R.; Tegos, G.P. All you need is light: Antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence 2011, 2, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.; Cardote, T.; MGPMS, N.; Faustino, M.; Cunha, A.; Almeida, A.; Alves, E.J.C. Evaluation of meso-substituted cationic corroles as potential antibacterial agents. Ann. Braz. Acad. Sci. 2018, 90, 1175–1185. [Google Scholar]
- Pohl, J.; Saltsman, I.; Mahammed, A.; Gross, Z.; Röder, B. Inhibition of green algae growth by corrole-based photosensitizers. J. Appl. Microbiol. 2015, 118, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.F.B.; Pinto, R.J.B.; Vaz Serra, V.I.R.C.; Silvestre, A.J.D.; Trindade, T.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Daina, S.; Sadocco, P.; Freire, C.S.R. Fluorescent bioactive corrole grafted-chitosan films. Biomacromolecules 2016, 17, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, F.F.; Huang, Y.-Y.; Hamblin, M.R. antimicrobial photodynamic therapy to kill gram-negative bacteria. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 1–23. [Google Scholar] [CrossRef]
- Tavares, A.; Carvalho, C.M.B.; Faustino, M.A.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; Alves, E.; et al. Antimicrobial photodynamic therapy: Study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Faustino, M.A.F.; Cunha, Â.; Gomes, N.C.M.; Almeida, A. Evaluation of resistance development and viability recovery by a non-enveloped virus after repeated cycles of aPDT. Antiviral Res. 2011, 91, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Lauro, F.M.; Pretto, P.; Covolo, L.; Jori, G.; Bertoloni, G. Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene-polylysine conjugates. Photochem. Photobiol. Sci. 2002, 1, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.B.; Alves, E.; Costa, L.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Almeida, A.; Cunha, Â.; et al. Functional cationic nanomagnet—Porphyrin hybrids for the photoinactivation of microorganisms. ACS Nano 2010, 4, 7133–7140. [Google Scholar] [CrossRef] [PubMed]
- Magaraggia, M.; Jori, G.; Soncin, M.; Schofield, C.L.; Russell, D. A Porphyrin-silica microparticle conjugates as an efficient tool for the photosensitised disinfection of water contaminated by bacterial pathogens. Photochem. Photobiol. Sci. 2013, 12, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Hernández, M.E.; Manjón, F.; García-Fresnadillo, D.; Orellana, G. Solar water disinfection by singlet oxygen photogenerated with polymer-supported Ru(II) sensitizers. Sol. Energy 2006, 80, 1382–1387. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Spagnul, C.; Turner, L.C.; Boyle, R.W. Immobilized photosensitizers for antimicrobial applications. J. Photochem. Photobiol. B Biol. 2015, 150, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Gan, C.R.R.; Gao, J.; Loh, X.J. A Perspective on the Trends and Challenges Facing Porphyrin-Based Anti-Microbial Materials. Small 2016, 12, 3609–3644. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.A.; Seckler, M.M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; et al. Silver nanoparticles: Therapeutical uses, toxicity, and safety issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Jana, S.; Pal, T. Synthesis, characterization and catalytic application of silver nanoshell coated functionalized polystyrene beads. J. Nanosci. Nanotechnol. 2007, 7, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Sanvicens, N.; Marco, M.P. Multifunctional nanoparticles-properties and prospects for their use in human medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Park, J.E.; Osaka, T.; Park, S.G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Managa, M.; Antunes, E.; Nyokong, T. Conjugates of platinum nanoparticles with gallium tetra-(4-Carboxyphenyl) porphyrin and their use in photodynamic antimicrobial chemotherapy when in solution or embedded in electrospun fiber. Polyhedron 2014, 76, 94–101. [Google Scholar] [CrossRef]
- Sousa, F.L.; Almeida, A.; Girão, A.V.; Fateixa, S.; Trindade, T. Multiple emulsion templating of hybrid Ag/SiO2 capsules for antibacterial applications. Part. Part. Syst. Charact. 2014, 32, 561–566. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Almeida, A.; Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Trindade, T. Antifungal activity of transparent nanocomposite thin films of pullulan and silver against Aspergillus niger. Colloids Surfaces B Biointerfaces 2013, 103, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Fateixa, S.; Neves, M.C.; Almeida, A.; Oliveira, J.; Trindade, T. Anti-fungal activity of SiO2/Ag2S nanocomposites against Aspergillus niger. Colloids Surfaces B Biointerfaces 2009, 74, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767–2781. [Google Scholar] [CrossRef]
- Endo, M.; Wei, Z.; Wang, K.; Karabiyik, B.; Yoshiiri, K.; Rokicka, P.; Ohtani, B.; Markowska-Szczupak, A.; Kowalska, E. Noble metal-modified titania with visible-light activity for the decomposition of microorganisms. Beilstein J. Nanotechnol. 2018, 9, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Rajeshkumar, S.; Kumar, V. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour. Technol. 2017, 3, 516–527. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, G. Light-induced generation of singlet oxygen by naked gold nanoparticles and its implications to cancer cell phototherapy. Small 2013, 9, 4130–4134. [Google Scholar] [CrossRef] [PubMed]
- Sherwani, M.A.; Tufail, S.; Khan, A.A.; Owais, M. Gold Nanoparticle-Photosensitizer Conjugate Based Photodynamic Inactivation of Biofilm Producing Cells: Potential for Treatment of C. albicans Infection in BALB/c Mice. PLoS ONE 2015, 10, e0131684. [Google Scholar] [CrossRef] [PubMed]
- Saber, R.; Amini, S.M.; Kharrazi, S.; Fateh, M.; Hadizadeh, M. Effect of gold nanoparticles on photodynamic efficiency of 5-aminolevolenic acid photosensitiser in epidermal carcinoma cell line: An in vitro study. IET Nanobiotechnology 2013, 7, 151–156. [Google Scholar] [CrossRef]
- Huang, H.; Hasan, T. The “Nano” World in Photodynamic Therapy. Austin J. Nanomed. Nanotechnol. 2014, 2, 2–5. [Google Scholar]
- Perni, S.; Piccirillo, C.; Pratten, J.; Prokopovich, P.; Chrzanowski, W.; Parkin, I.P.; Wilson, M. The antimicrobial properties of light-activated polymers containing methylene blue and gold nanoparticles. Biomaterials 2009, 30, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Prokopovich, P.; Parkin, I.P.; Wilson, M.; Pratten, J. Prevention of biofilm accumulation on a light-activated antimicrobial catheter material. J. Mater. Chem. 2010, 20, 8668. [Google Scholar] [CrossRef]
- Noimark, S.; Allan, E.; Parkin, I.P. Light-activated antimicrobial surfaces with enhanced efficacy induced by a dark-activated mechanism. Chem. Sci. 2014, 5, 2216–2223. [Google Scholar] [CrossRef]
- Perni, S.; Piccirillo, C.; Kafizas, A.; Uppal, M.; Pratten, J.; Wilson, M.; Parkin, I.P. Antibacterial Activity of Light-Activated Silicone Containing Methylene Blue and Gold Nanoparticles of Different Sizes. J. Clust. Sci. 2010, 21, 427–438. [Google Scholar] [CrossRef]
- Page, K.; Correia, A.; Wilson, M.; Allan, E.; Parkin, I.P. Light-activated antibacterial screen protectors for mobile telephones and tablet computers. J. Photochem. Photobiol. A Chem. 2015, 296, 19–24. [Google Scholar] [CrossRef]
- Bovis, M.J.; Noimark, S.; Woodhams, J.H.; Kay, C.W.M.; Weiner, J.; Peveler, W.J.; Correia, A.; Wilson, M.; Allan, E.; Parkin, I.P.; et al. Photosensitisation studies of silicone polymer doped with methylene blue and nanogold for antimicrobial applications. RSC Adv. 2015, 5, 54830–54842. [Google Scholar] [CrossRef]
- Noimark, S.; Bovis, M.; MacRobert, A.J.; Correia, A.; Allan, E.; Wilson, M.; Parkin, I.P. Photobactericidal polymers; the incorporation of crystal violet and nanogold into medical grade silicone. RSC Adv. 2013, 3, 18383. [Google Scholar] [CrossRef]
- Noimark, S.; Dunnill, C.W.; Kay, C.W.M.; Perni, S.; Prokopovich, P.; Ismail, S.; Wilson, M.; Parkin, I.P. Incorporation of methylene blue and nanogold into polyvinyl chloride catheters; a new approach for light-activated disinfection of surfaces. J. Mater. Chem. 2012, 22, 15388. [Google Scholar] [CrossRef]
- Naik, A.J.T.; Ismail, S.; Kay, C.; Wilson, M.; Parkin, I.P. Antimicrobial activity of polyurethane embedded with methylene blue, toluidene blue and gold nanoparticles against Staphylococcus aureus; Illuminated with white light. Mater. Chem. Phys. 2011, 129, 446–450. [Google Scholar] [CrossRef]
- Walker, T.; Canales, M.; Noimark, S.; Page, K.; Parkin, I.; Faull, J.; Bhatti, M.; Ciric, L. A Light-Activated Antimicrobial Surface Is Active Against Bacterial, Viral and Fungal Organisms. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, T.J.; Wu, K.; Sehmi, S.K.; Noimark, S.; Peveler, W.J.; du Toit, H.; Voelcker, N.H.; Allan, E.; MacRobert, A.J.; Gavriilidis, A.; et al. Thiol-Capped Gold Nanoparticles Swell-Encapsulated into Polyurethane as Powerful Antibacterial Surfaces Under Dark and Light Conditions. Sci. Rep. 2016, 6, 39272. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.Q.; Dias, C.J.; Gamelas, S.; Fardilha, M.; Neves, M.G.P.M.S.; Faustino, M.A.F. An insight on the role of photosensitizer nanocarriers for Photodynamic Therapy. Ann. Braz. Acad. Sci. 2018, 90, 1101–1130. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.A.; Alsharnoubi, J.; Morsy, M. Photodynamic antibacterial enhanced effect of methylene blue-gold nanoparticles conjugate on Staphylococcal aureus isolated from impetigo lesions in vitro study. Photodiagn. Photodyn. Ther. 2015, 12, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Darabpour, E.; Kashef, N.; Amini, S.M.; Kharrazi, S.; Djavid, G.E. Fast and effective photodynamic inactivation of 4-day-old biofilm of methicillin-resistant Staphylococcus aureus using methylene blue-conjugated gold nanoparticles. J. Drug Deliv. Sci. Technol. 2017, 37, 134–140. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E-coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, Z.; Wu, H.; Pan, X.; Xie, X.; Wu, C. Antimicrobial activity and the mechanism of silver nanoparticle thermosensitive gel. Int. J. Nanomed. 2011, 6, 2873–2877. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.K.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surfaces B Biointerfaces 2010, 79, 340–344. [Google Scholar] [CrossRef] [PubMed]
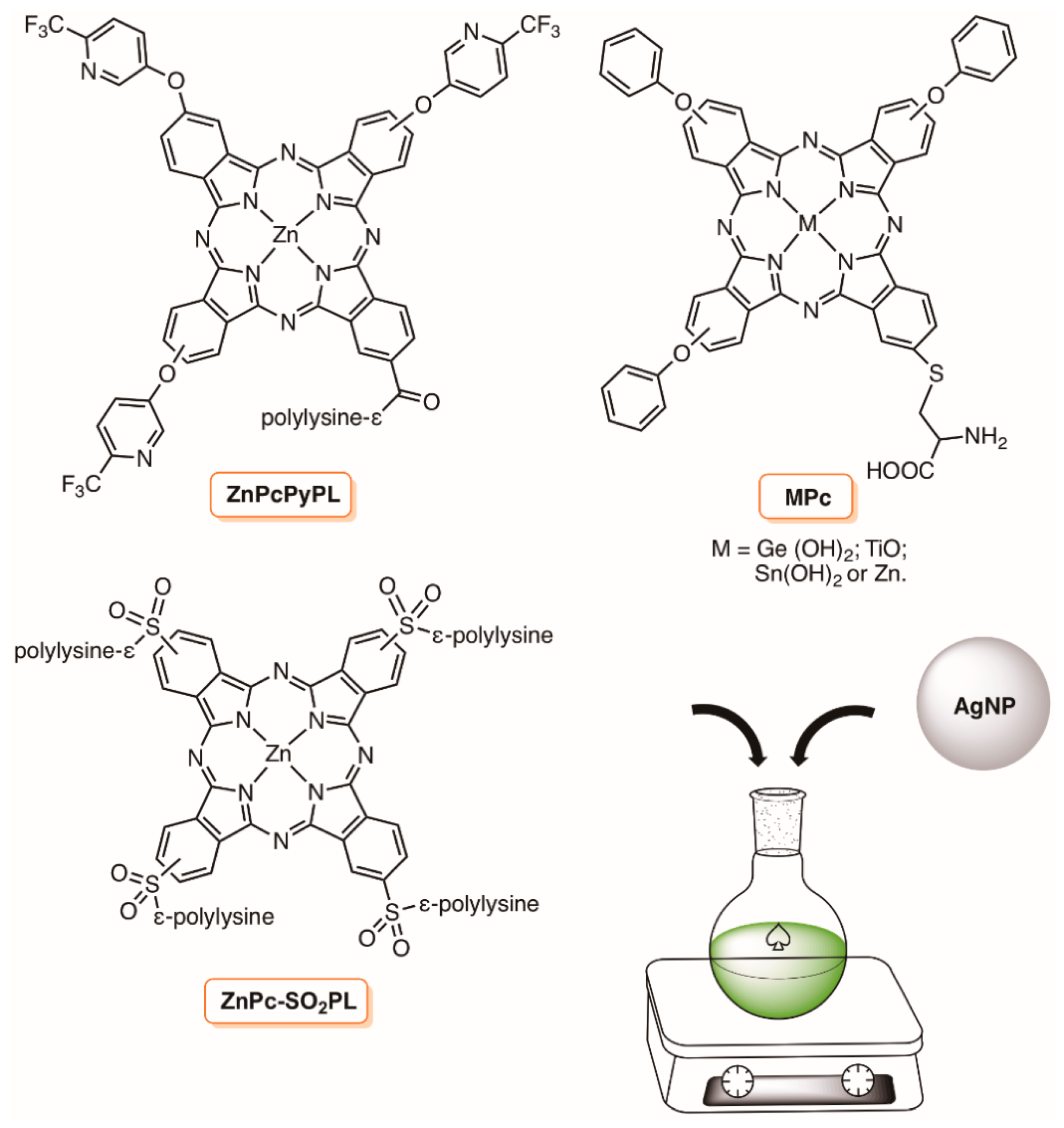
- Nombona, N.; Antunes, E.; Chidawanyika, W.; Kleyi, P.; Tshentu, Z.; Nyokong, T. Synthesis, photophysics and photochemistry of phthalocyanine-ε-polylysine conjugates in the presence of metal nanoparticles against Staphylococcus aureus. J. Photochem. Photobiol. A Chem. 2012, 233, 24–33. [Google Scholar] [CrossRef]
- Masilela, N.; Nyokong, T. The interaction of silver nanoparticles with low symmetry cysteinyl metallophthalocyanines and their antimicrobial effect. J. Photochem. Photobiol. A Chem. 2013, 255, 1–9. [Google Scholar] [CrossRef]
- Lyutakov, O.; Hejna, O.; Solovyev, A.; Kalachyova, Y.; Svorcik, V. Polymethylmethacrylate doped with porphyrin and silver nanoparticles as light-activated antimicrobial material. RSC Adv. 2014, 4, 50624–50630. [Google Scholar] [CrossRef]
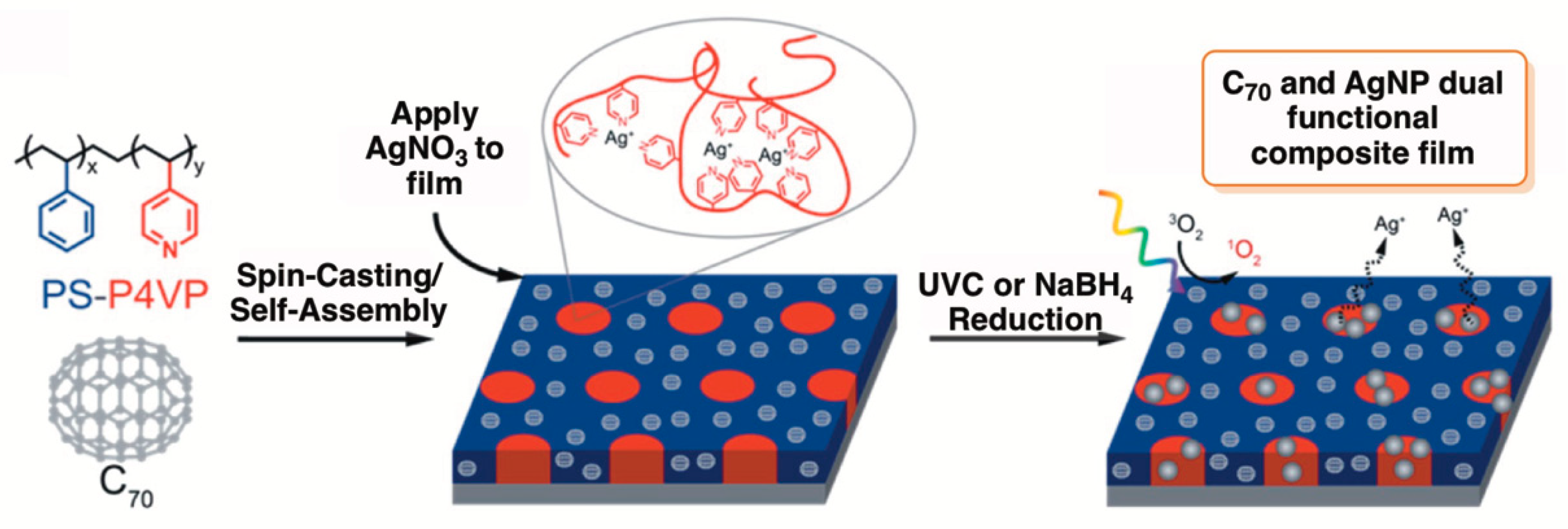
- Moor, K.J.; Osuji, C.O.; Kim, J.H. Dual-Functionality Fullerene and Silver Nanoparticle Antimicrobial Composites via Block Copolymer Templates. ACS Appl. Mater. Interfaces 2016, 8, 33583–33591. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.S.; Koohmaraie, M.; Hruska, R.L. Cell surface charge characteristics and their relationship to bacterial attachment to meat surfaces. Appl. Environ. Microbiol. 1989, 55, 832–836. [Google Scholar] [PubMed]
- Managa, M.; Nyokong, T. Photodynamic antimicrobial chemotherapy activity of gallium tetra-(4-carboxyphenyl) porphyrin when conjugated to differently shaped platinum nanoparticles. J. Mol. Struct. 2015, 1099, 432–440. [Google Scholar] [CrossRef]
- Managa, M.; Amuhaya, E.K.; Nyokong, T. Photodynamic antimicrobial chemotherapy activity of (5,10,15,20-tetrakis(4-(4-carboxyphenycarbonoimidoyl)phenyl)porphyrinato) chloro gallium(III). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Capeletti, L.B.; De Oliveira, L.F.; Gonçalves, K.D.A.; De Oliveira, F.A.; Saito, Â.; Kobarg, J.; Santos, J.H.Z.; Cardoso, M.B. Tailored silica-antibiotic nanoparticles: Overcoming bacterial resistance with low cytotoxicity tailored silica-antibiotic nanoparticles: Overcoming bacterial resistance with low cytotoxicity. Langmuir 2014. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Rogelj, S.; Zhang, P. Rose Bengal-decorated silica nanoparticles as photosensitizers for inactivation of gram-positive bacteria. Nanotechnology 2010, 21, 065102. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Brame, J.; Li, Q.; Alvarez, P.J.J. Nanotechnology for a safe and sustainable water supply: Enabling integrated water treatment and reuse. Acc. Chem. Res. 2013, 46, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Theron, J.; Walker, J.A.; Cloete, T.E. Nanotechnology and water treatment: Applications and emerging opportunities. Crit. Rev. Microbiol. 2008, 34, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Radi, S.; Abiad, C.E.; Moura, N.M.M.; Faustino, M.A.F.; Neves, M.G.P.M.S. New hybrid adsorbent based on porphyrin functionalized silica for heavy metals removal: Synthesis, characterization, isotherms, kinetics and thermodynamics studies. J. Hazard. Mater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Y.P.; Miller, K.P.; Cash, B.M.; Jones, S.; Glenn, S.; Benicewicz, B.C.; Decho, A.W. Functionalised nanoparticles complexed with antibiotic efficiently kill MRSA and other bacteria. Chem. Commun. 2014, 50, 12030–12033. [Google Scholar] [CrossRef] [PubMed]
- Sen Karaman, D.; Sarwar, S.; Desai, D.; Björk, E.; Odén, M.; Chakrabarti, P.; Rosenholm, J.M.; Chakraborti, S. Shape engineering boost antibacterial activity of chitosan coated mesoporous silica nanoparticle doped with silver: A mechanistic investigation. J. Mater. Chem. B 2016, 3292–3304. [Google Scholar] [CrossRef]
- Agnihotri, S.; Pathak, R.; Jha, D.; Roy, I.; Gautam, H.K.; Sharma, A.K.; Kumar, P. Synthesis and antimicrobial activity of aminoglycoside-conjugated silica nanoparticles against clinical and resistant bacteria. New J. Chem. 2015, 39, 6746–6755. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Menezes, J.C.J.M.D.S.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A.; Hackbarth, S.; Röder, B.; Faustino, M.A.F. Photodynamic inactivation of bioluminescent Escherichia coli by neutral and cationic pyrrolidine-fused chlorins and isobacteriochlorins. Bioorganic. Med. Chem. Lett. 2014, 24, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Villén, L.; Manjón, F.; García-Fresnadillo, D.; Orellana, G. Solar water disinfection by photocatalytic singlet oxygen production in heterogeneous medium. Appl. Catal. B Environ. 2006, 69, 1–9. [Google Scholar] [CrossRef]
- Manjón, F.; Villén, L.; García-Fresnadillo, D.; Orellana, G. On the factors influencing the performance of solar reactors for water disinfection with photosensitized singlet oxygen. Environ. Sci. Technol. 2008, 42, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Manjón, F.; Santana-Magaña, M.; García-Fresnadillo, D.; Orellana, G. Are silicone-supported [C60]-fullerenes an alternative to Ru(ii) polypyridyls for photodynamic solar water disinfection? Photochem. Photobiol. Sci. 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Manjón, F.; García-Fresnadillo, D.; Orellana, G. Water disinfection with Ru(II) photosensitisers supported on ionic porous silicones. Photochem. Photobiol. Sci. 2009, 8, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Manjón, F.; Santana-Magaña, M.; García-Fresnadillo, D.; Orellana, G. Singlet oxygen sensitizing materials based on porous silicone: Photochemical characterization, effect of dye reloading and application to water disinfection with solar reactors. Photochem. Photobiol. Sci. 2010, 9, 838–845. [Google Scholar] [CrossRef] [PubMed]
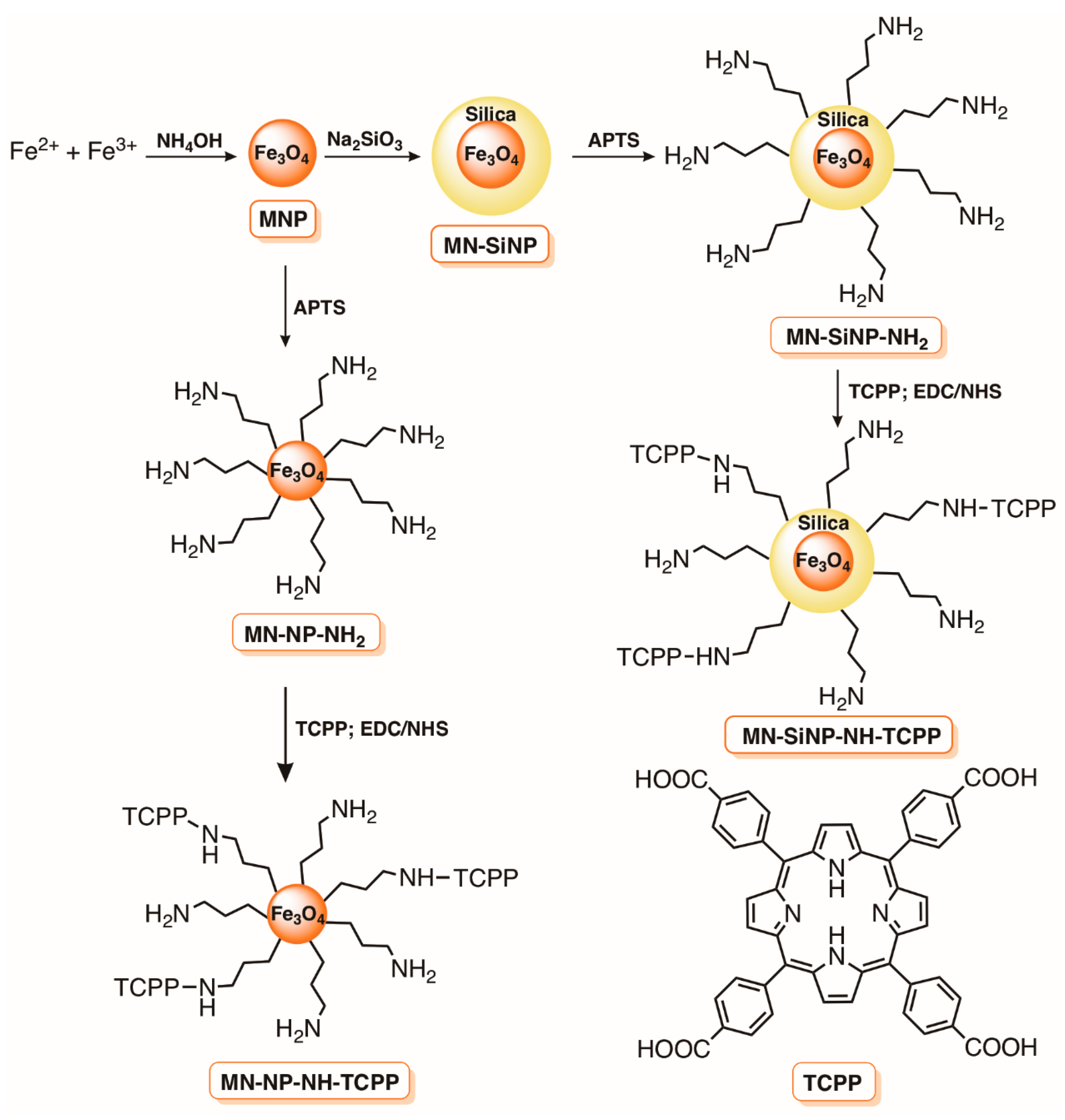
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Durantini, E.N. Photodynamic properties and photoinactivation of microorganisms mediated by 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin covalently linked to silica-coated magnetite nanoparticles. J. Photochem. Photobiol. A Chem. 2017, 346, 452–461. [Google Scholar] [CrossRef]
- Alves, E.; Rodrigues, J.M.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Lin, Z.; Cunha, Â.; Nadais, M.H.; Tomé, J.P.C.; Almeida, A. A new insight on nanomagnet-porphyrin hybrids for photodynamic inactivation of microorganisms. Dye Pigment 2014, 110, 80–88. [Google Scholar] [CrossRef]
- Alves, E.; Costa, L.; Carvalho, C.M.B.; Tomé, J.P.C.; Faustino, M.A.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Almeida, A. Charge effect on the photoinactivation of Gram-negative and Gram-positive bacteria by cationic meso-substituted porphyrins. BMC Microbiol. 2009, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.Q.; Menezes, J.C.J.M.D.S.; Pires, S.M.G.; Neves, M.G.P.M.S.; Simões, M.M.Q.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Daniel-Da-Silva, A.L.; Almeida, A.; et al. Pyrrolidine-fused chlorin photosensitizer immobilized on solid supports for the photoinactivation of Gram negative bacteria. Dye Pigment 2014, 110, 123–133. [Google Scholar] [CrossRef]
- Kuznetsova, N.A.; Yuzhakova, O.A.; Strakhovskaya, M.G.; Shumarina, A.O.; Kozlov, A.S.; Krasnovsky, A.A.; Kaliya, O.L. New heterogeneous photosensitizers with phthalocyanine molecules covalently linked to aminopropyl silica gel. J. Porphyr. Phthalocyanines 2011, 15, 718–726. [Google Scholar] [CrossRef]
- Perni, S.; Drexler, S.; Ruppel, S.; Prokopovich, P. Lethal photosensitisation of bacteria using silica-TBO nanoconjugates. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 510, 293–299. [Google Scholar] [CrossRef]
- Benabbou, A.K.; Guillard, C.; Pigeot-Rémy, S.; Cantau, C.; Pigot, T.; Lejeune, P.; Derriche, Z.; Lacombe, S. Water disinfection using photosensitizers supported on silica. J. Photochem. Photobiol. A Chem. 2011, 219, 101–108. [Google Scholar] [CrossRef]
- Alvarez, M.G.; Gómez, M.L.; Mora, S.J.; Milanesio, M.E.; Durantini, E.N. Photodynamic inactivation of Candida albicans using bridged polysilsesquioxane films doped with porphyrin. Bioorganic. Med. Chem. 2012, 20, 4032–4039. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R.; Krysteva, M.A.; Lalov, I.G.; Artarsky, S.V. Water disinfection using photosensitizers immobilized on chitosan. Water Res. 2006, 40, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Graciano, T.B.; Coutinho, T.S.; Cressoni, C.B.; de Paula Freitas, C.; Pierre, M.B.R.; de Lima Pereira, S.A.; Shimano, M.M.;; Cristina da Cunha Frange, R.; Garcia, M.T.J. Using chitosan gels as a toluidine blue O delivery system for photodynamic therapy of buccal cancer: In vitro and in vivo studies. Photodiagnosis Photodyn. Ther. 2015, 12, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.; Bourbon, A.I.; Cerqueira, M.A.; Maricato, É.; Nunes, C.; Coimbra, M.A.; Vicente, A.A. Chitosan/fucoidan multilayer nanocapsules as a vehicle for controlled release of bioactive compounds. Carbohydr. Polym. 2015, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.A.D.F.; Moura, N.M.M.; Fernandes, A.; Faustino, M.A.F.; Simões, M.M.Q.; Cavaleiro, J.A.S.; Nakagaki, S.; Almeida, A.; Cunha, Â.; Silvestre, A.J.D.; Freire, C.S.R.; et al. Control of Listeria innocua biofilms by biocompatible photodynamic antifouling chitosan based materials. Dye Pigment 2017, 137, 265–276. [Google Scholar] [CrossRef]
- Mu, H.; Zhang, A.; Zhang, L.; Niu, H.; Duan, J. Inhibitory effects of chitosan in combination with antibiotics on Listeria monocytogenes biofilm. Food Control 2014, 38, 215–220. [Google Scholar] [CrossRef]
- Portes, E.; Gardrat, C.; Castellan, A.; Coma, V. Environmentally friendly films based on chitosan and tetrahydrocurcuminoid derivatives exhibiting antibacterial and antioxidative properties. Carbohydr. Polym. 2009, 76, 578–584. [Google Scholar] [CrossRef]
- Kelly, K.A.D.; Simões, M.M.Q.; da Graça, P.M.S.; Neves, M.; Cavaleiro, J.A.S.; Ribeiro, R.R.; Wypych, F.; Nakagaki, S. Synthesis of new metalloporphyrin derivatives from [5,10,15,20-tetrakis (pentafluorophenyl)porphyrin] and 4-mercaptobenzoic acid for homogeneous and heterogeneous catalysis. Appl. Catal. A Gen. 2015, 503, 9–19. [Google Scholar] [CrossRef]
- Costa, J.I.T.; Tomé, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin: A versatile platform to novel porphyrinic materials. J. Porphyr. Phthalocyanines 2011, 15, 1116–1133. [Google Scholar] [CrossRef]
- Diogo, P.; Mota, M.; Fernandes, C.; Sequeira, D.; Palma, P.; Caramelo, F.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Gonçalves, T.; Santos, J.M. Is the chlorophyll derivative Zn(II)e6Me a good photosensitizer to be used in root canal disinfection? Photodiagnosis Photodyn. Ther. 2018, 22, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Fernandes, C.; Caramelo, F.; Mota, M.; Miranda, I.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Uliana, M.P.; de Oliveira, K.T.; Santos, J.M.; et al. Antimicrobial photodynamic therapy against endodontic Enterococcus faecalis and Candida albicans mono and mixed biofilms in the presence of photosensitizers: A comparative study with classical endodontic irrigants. Front. Microbiol. 2017, 8, 498. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Gonçalves, T.; Palma, P.; Santos, J.M. Photodynamic antimicrobial chemotherapy for root canal system asepsis: A narrative literature review. Int. J. Dent. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Kishen, A. Antibacterial efficacy of photosensitizer functionalized biopolymeric nanoparticles in the presence of tissue inhibitors in root canal. J. Endod. 2014, 40, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Carmagnola, I.; Chiono, V.; Gentile, P.; Fracchia, L.; Ceresa, C.; Georgiev, G.; Ciardelli, G. Surface modification of poly(dimethylsiloxane) by two-step plasma treatment for further grafting with chitosan-Rose Bengal photosensitizer. Surf. Coat. Technol. 2013, 223, 92–97. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Polycationic chitosan-conjugated photosensitizer for antibacterial photodynamic therapy. Photochem. Photobiol. 2012, 88, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Characterization of a conjugate between rose bengal and chitosan for targeted antibiofilm and tissue stabilization effects as a potential treatment of infected dentin. Antimicrob. Agents Chemother. 2012, 56, 4876–4884. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Darabpour, E.; Kashef, N.; Mashayekhan, S. Chitosan nanoparticles enhance the efficiency of methylene blue-mediated antimicrobial photodynamic inactivation of bacterial biofilms: An in vitro study. Photodiagnosis Photodyn. Ther. 2016, 14, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Gsponer, N.S.; Spesia, M.B.; Durantini, E.N. Effects of divalent cations, EDTA and chitosan on the uptake and photoinactivation of Escherichia coli mediated by cationic and anionic porphyrins. Photodiagnosis Photodyn. Ther. 2015, 12, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R.; Galia, A. Photobactericidal films based on regenerated cellulose. Biotechnol. Biotechnol. Equip. 1994, 8, 68–74. [Google Scholar] [CrossRef]
- Rahimi, R.; Fayyaz, F.; Rassa, M. The study of cellulosic fabrics impregnated with porphyrin compounds for use as photo-bactericidal polymers. Mater. Sci. Eng. C 2016, 59, 661–668. [Google Scholar] [CrossRef] [PubMed]
- George, L.; Müller, A.; Röder, B.; Santala, V.; Efimov, A. Photodynamic self-disinfecting surface using pyridinium phthalocyanine. Dye Pigment 2017, 147, 334–342. [Google Scholar] [CrossRef]
- Wilson, M. Light-activated antimicrobial coating for the continuous disinfection of surfaces. Infect. Control Hosp. Epidemiol. 2003, 24, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Decraene, V.; Pratten, J.; Wilson, M. Cellulose acetate containing toluidine blue and rose bengal is an effective antimicrobial coating when exposed to white light. Appl. Environ. Microbiol. 2006, 72, 4436–4439. [Google Scholar] [CrossRef] [PubMed]
- Decraene, V.; Pratten, J.; Wilson, M. Novel light-activated antimicrobial coatings are effective against surface-deposited Staphylococcus aureus. Curr. Microbiol. 2008, 57, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Ringot, C.; Sol, V.; Granet, R.; Krausz, P. Porphyrin-grafted cellulose fabric: New photobactericidal material obtained by “Click-Chemistry” reaction. Mater. Lett. 2009, 63, 1889–1891. [Google Scholar] [CrossRef]
- Ringot, C.; Saad, N.; Granet, R.; Bressollier, P.; Sol, V.; Krausz, P. Meso-functionalized aminoporphyrins as efficient agents for photo-antibacterial surfaces. J. Porphyr. Phthalocyanines 2010, 14, 925–931. [Google Scholar] [CrossRef]
- Mbakidi, J.P.; Herke, K.; Alvès, S.; Chaleix, V.; Granet, R.; Krausz, P.; Leroy-Lhez, S.; Ouk, T.S.; Sol, V. Synthesis and photobiocidal properties of cationic porphyrin-grafted paper. Carbohydr. Polym. 2013, 91, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Feese, E.; Sadeghifar, H.; Gracz, H.S.; Argyropoulos, D.S.; Ghiladi, R.A. Photobactericidal porphyrin-cellulose nanocrystals: Synthesis, characterization, and antimicrobial properties. Biomacromolecules 2011, 12, 3528–3539. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, B.L.; Feese, E.; Sadeghifar, H.; Argyropoulos, D.S.; Ghiladi, R.A. Porphyrin-cellulose nanocrystals: A photobactericidal material that exhibits broad spectrum antimicrobial activity. Photochem. Photobiol. 2012, 88, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Preuß, A.; Zeugner, L.; Hackbarth, S.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Roeder, B. Photoinactivation of Escherichia coli (SURE2) without intracellular uptake of the photosensitizer. J. Appl. Microbiol. 2013, 114, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, B.L.; Scholle, F.; Sadeghifar, H.; Francis, A.J.; Boltersdorf, J.; Weare, W.W.; Argyropoulos, D.S.; Maggard, P.A.; Ghiladi, R.A. Synthesis, characterization, and antimicrobial efficacy of photomicrobicidal cellulose paper. Biomacromolecules 2015, 16, 2482–2492. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, W.; Hu, P.; Wang, D.; Lin, F.; Xue, J.; Chen, Z. Dual antimicrobial actions on modified fabric leads to inactivation of drug-resistant bacteria. Dye Pigment 2017, 140, 236–243. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications: A. review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef] [PubMed]
- Krouit, M.; Granet, R.; Krausz, P. Photobactericidal plastic films based on cellulose esterified by chloroacetate and a cationic porphyrin. Bioorganic. Med. Chem. 2008, 16, 10091–10097. [Google Scholar] [CrossRef] [PubMed]
- Krouit, M.; Granet, R.; Krausz, P. Photobactericidal films from porphyrins grafted to alkylated cellulose-synthesis and bactericidal properties. Eur. Polym. J. 2009, 45, 1250–1259. [Google Scholar] [CrossRef]
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.O.; Ahmad, M.F.; Shadab, G.G.H.A.; Siddique, H.R. Superparamagnetic iron oxide nanoparticles based cancer theranostics: A double edge sword to fight against cancer. J. Drug Deliv. Sci. Technol. 2018, 45, 177–183. [Google Scholar] [CrossRef]
- Javanbakht, T.; Laurent, S.; Stanicki, D.; Wilkinson, K.J. Relating the surface properties of superparamagnetic iron oxide nanoparticles (spions) to their bactericidal effect towards a biofilm of streptococcus mutans. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Pelegrino, M.T.; Haddad, P.S. Chapter 24-Antimicrobial Applications of Superparamagnetic Iron Oxide Nanoparticles: Perspectives and Challenges. In Micro and Nano Technologies; Grumezescu, A.M., Ficai, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 531–550. ISBN 978-0-323-46152-8. [Google Scholar]
- Penon, O.; Marín, M.J.; Amabilino, D.B.; Russell, D.A.; Pérez-García, L. Iron oxide nanoparticles functionalized with novel hydrophobic and hydrophilic porphyrins as potential agents for photodynamic therapy. J. Colloid Interface Sci. 2016, 462, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.Y.; Oh, Y.T.; Kim, D.; Lee, E.S. Multimeric grain-marked micelles for highly efficient photodynamic therapy and magnetic resonance imaging of tumors. Int. J. Pharm. 2014, 471, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yu, X.; Wang, L.; Liu, Y.; Gao, J.; Zhang, J.; Ma, R.; Liu, R.; Zhang, Z. PEGylated fullerene/iron oxide nanocomposites for photodynamic therapy, targeted drug delivery and MR imaging. Biomaterials 2013, 34, 9666–9677. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Lim, T.G.; Kim, J.H.; Cho, Y.M.; Kim, Y.S.; Chung, U.S.; Kim, J.H.; Choi, B.W.; Koh, W.G.; Jang, W.D. Fabrication of multifunctional layer-by-layer nanocapsules toward the design of theragnostic nanoplatform. Biomacromolecules 2014, 15, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Li, H.; He, X.; Wang, K.; Hu, J.; Tan, W.; Zhang, S.; Yang, X. Preparation and antibacterial activity of Fe3O4 Ag nanoparticles. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Thandu, M.; Rapozzi, V.; Xodo, L.; Albericio, F.; Comuzzi, C.; Cavalli, S. Clicking porphyrins to magnetic nanoparticles for photodynamic therapy. Chempluschem 2014, 79, 90–98. [Google Scholar] [CrossRef]
- Thandu, M.M.; Cavalli, S.; Rossi, G.; Rizzardini, C.B.; Goi, D.; Comuzzi, C. Biological evaluation of a Porphyrin-SPION nanoconjugate as an antimicrobial magnetic photosensitizer. J. Porphyr. Phthalocyanines 2017, 21, 581–588. [Google Scholar] [CrossRef]
- Chen, C.-T.; Chen, C.-P.; Yang, J.-C.; Tsai, T. Liposome-encapsulated photosensitizers against bacteria. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Gorza, F.D.S.; da Silva, R.J.; Trescher, T.F.; Pedro, G.C.; de Sousa, M.A.O.; Souto, P.C.S.; Silva, J.R.; de Souza, N.C. Immobilization of chlorophyll by using layer-by-layer technique for controlled release systems and photodynamic inactivation. Photodiagnosis Photodyn. Ther. 2016, 15, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Nisnevitch, M.; Nakonechny, F.; Nitzan, Y. Photodynamic antimicrobial chemotherapy by liposome-encapsulated water-soluble photosensitizers. Bioorg. Khim. 2010, 36, 363–369. [Google Scholar] [CrossRef]
- Jin, C.S.; Zheng, G. Liposomal nanostructures for photosensitizer delivery. Lasers Surg. Med. 2011, 43, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Pogue, B.W.; Hasan, T. Liposomal delivery of photosensitising agents. Expert Opin. Drug Deliv. 2005, 2, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.J.; Romanowski, M. Light-activated content release from liposomes. Theranostics 2012, 2, 1020–1036. [Google Scholar] [CrossRef] [PubMed]
- Ernsting, M.J.; Worthington, A.; May, J.P.; Tagami, T.; Kolios, M.C.; Li, S.D. Ultrasound drug targeting to tumors with thermosensitive liposomes. IEEE Int. Ultrason. Symp. IUS 2011, 1–4. [Google Scholar] [CrossRef]
- Ferro, S.; Ricchelli, F.; Monti, D.; Mancini, G.; Jori, G. Efficient photoinactivation of methicillin-resistant Staphylococcus aureus by a novel porphyrin incorporated into a poly-cationic liposome. Int. J. Biochem. Cell Biol. 2007, 39, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Ferro, S.; Jori, G.; Sortino, S.; Stancanelli, R.; Nikolov, P.; Tognon, G.; Ricchelli, F.; Mazzaglia, A. Inclusion of 5-[4-(1-Dodecanoylpyridinium)]-10,15,20-triphenylporphine in Supramolecular Aggregates of Cationic Amphiphilic Cyclodextrins: Physicochemical Characterization of the Complexes and Strengthening of the Antimicrobial Photosensitizing Activity. Biomacromolecules 2009, 10, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Vimaladevi, M.; Divya, K.C.; Girigoswami, A. Liposomal nanoformulations of rhodamine for targeted photodynamic inactivation of multidrug resistant gram negative bacteria in sewage treatment plant. J. Photochem. Photobiol. B Biol. 2016, 162, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.H.; Awan, K.H.; Javed, F. Bactericidal efficacy of photodynamic therapy against Enterococcus faecalis in infected root canals: A systematic literature review. Photodiagnosis Photodyn. Ther. 2013, 10, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Longo, J.P.F.; Leal, S.C.; Simioni, A.R.; De Fátima Menezes Almeida-Santos, M.; Tedesco, A.C.; Azevedo, R.B. Photodynamic therapy disinfection of carious tissue mediated by aluminum-chloride-phthalocyanine entrapped in cationic liposomes: An in vitro and clinical study. Lasers Med. Sci. 2012, 27, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Kranz, S.; Guellmar, A.; Völpel, A.; Gitter, B.; Albrecht, V.; Sigusch, B.W. Photodynamic suppression of Enterococcus faecalis using the photosensitizer mTHPC. Lasers Surg. Med. 2011, 43, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Lüthi, M.; Besic Gyenge, E.; Engstrüm, M.; Bredell, M.; Grätz, K.; Walt, H.; Gmür, R.; Maake, C. Hypericin- and mTHPC-mediated photodynamic therapy for the treatment of cariogenic bacteria. Med. Laser Appl. 2009, 24, 227–236. [Google Scholar] [CrossRef]
- Ossmann, A.; Kranz, S.; Andre, G.; Völpel, A.; Albrecht, V.; Fahr, A.; Sigusch, B.W. Photodynamic killing of Enterococcus faecalis in dentinal tubules using mTHPC incorporated in liposomes and invasomes. Clin. Oral Investig. 2015, 19, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Plenagl, N.; Seitz, B.S.; Reddy Pinnapireddy, S.; Jedelská, J.; Brüßler, J.; Bakowsky, U. Hypericin loaded liposomes for anti-microbial photodynamic therapy of gram-positive bacteria. Phys. Status Solidi 2018, 1700837. [Google Scholar] [CrossRef]
- Lluïsa Sagristá, M.; Postigo, F.; Africa De Madariaga, M.; Pintó, R.M.; Caballero, S.; Bosch, A.; Asunción Vallés, M.; Mora, M. Photodynamic inactivation of viruses by immobilized chlorin-containing liposomes. J. Porphyr. Phthalocyanines 2009, 13, 578–588. [Google Scholar] [CrossRef]
- Tian, Y.; Shumway, B.R.; Gao, W.; Youngbull, C.; Holl, M.R.; Johnson, R.H.; Meldrum, D.R. Influence of matrices on oxygen sensing of three sensing films with chemically conjugated platinum porphyrin probes and preliminary application for monitoring of oxygen consumption of Escherichia coli (E. coli). Sensors Actuators B Chem. 2010, 150, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Emmenegger, C.; Avramenko, O.A.; Brynda, E.; Skvor, J.; Alles, A.B. Poly(HEMA) brushes emerging as a new platform for direct detection of food pathogen in milk samples. Biosens. Bioelectron. 2011, 26, 4545–4551. [Google Scholar] [CrossRef] [PubMed]
- Tsou, T.L.; Tang, S.T.; Huang, Y.C.; Wu, J.R.; Young, J.J.; Wang, H.J. Poly(2-hydroxyethyl methacrylate) wound dressing containing ciprofloxacin and its drug release studies. J. Mater. Sci. Mater. Med. 2005, 16, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Takeda, K.; Kitagawa, R.; Izutani, N.; Miki, S.; Hirose, N.; Hayashi, M.; Imazato, S. Development of sustained antimicrobial-release systems using poly(2-hydroxyethyl methacrylate)/trimethylolpropane trimethacrylate hydrogels. Acta Biomater. 2014, 10, 4285–4295. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.; Barcenilla, J.M.; Vázquez, B.; González, R.; San Román, J. Intrinsically antibacterial materials based on polymeric derivatives of eugenol for biomedical applications. Biomacromolecules 2008, 9, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, Y.; Xing, R.; Jiao, T.; Zou, Q.; Yan, X. Synergistic in vivo photodynamic and photothermal antitumor therapy based on collagen-gold hybrid hydrogels with inclusion of photosensitive drugs. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 514, 155–160. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Li, Y.; Fukushima, K.; Coady, D.J.; Engler, A.C.; Liu, S.; Huang, Y.; Cho, J.S.; Guo, Y.; Miller, L.S.; Tan, J.P.K.; et al. Broad-spectrum antimicrobial and biofilm-disrupting hydrogels: Stereocomplex-driven supramolecular assemblies. Angew. Chemie Int. Ed. 2013, 52, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yan, B.; Yang, J.; Chen, L.; Zeng, H. Novel mussel-inspired injectable self-healing hydrogel with anti-biofouling property. Adv. Mater. 2015, 27, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Shi, Y.; Pena, D.A.; Peng, L.; Yu, G. Thermally responsive hydrogel blends: A general drug carrier model for controlled drug release. Angew. Chemie. Int. Ed. 2015, 54, 7376–7380. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Rana, D.; Shirzaei Sani, E.; Portillo-Lara, R.; Gifford, J.L.; Fares, M.M.; Mithieux, S.M.; Weiss, A.S. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 2017, 139, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Belali, S.; Karimi, A.R.; Hadizadeh, M. Cell-specific and pH-sensitive nanostructure hydrogel based on chitosan as a photosensitizer carrier for selective photodynamic therapy. Int. J. Biol. Macromol. 2018, 110, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Han, D.P.; Wisniewski, S.R.; Wilson, L.A.; Barza, M.; Vine, A.K.; Doft, B.H.; Kelsey, S.F. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am. J. Ophthalmol. 1996, 122, 1–17. [Google Scholar] [CrossRef]
- Recchia, F.M.; Busbee, B.G.; Pearlman, R.B.; Carvalho-Recchia, C.A.; Ho, A.C. Changing trends in the microbiologic aspects of postcataract endophthalmitis. Arch. Ophthalmol. 2005, 123, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. Biofilms in infectious disease and on medical devices. Int. J. Antimicrob. Agents 1999, 11, 223–226. [Google Scholar] [CrossRef]
- Parsons, C.; McCoy, C.P.; Gorman, S.P.; Jones, D.S.; Bell, S.E.J.; Brady, C.; McGlinchey, S.M. Anti-infective photodynamic biomaterials for the prevention of intraocular lens-associated infectious endophthalmitis. Biomaterials 2009, 30, 597–602. [Google Scholar] [CrossRef] [PubMed]
- McCoy, C.P.; Craig, R.A.; McGlinchey, S.M.; Carson, L.; Jones, D.S.; Gorman, S.P. Surface localisation of photosensitisers on intraocular lens biomaterials for prevention of infectious endophthalmitis and retinal protection. Biomaterials 2012, 33, 7952–7958. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Cassidy, C.M.; Loughlin, R.G.; Brown, A.; Tunney, M.M.; Jenkins, M.G.; McCarron, P.A. Delivery of Methylene Blue and meso-tetra (N-methyl-4-pyridyl) porphine tetra tosylate from cross-linked poly(vinyl alcohol) hydrogels: A potential means of photodynamic therapy of infected wounds. J. Photochem. Photobiol. B Biol. 2009, 96, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, L.Y.; Chen, X.; Chen, Z.; Wu, F.G. Hydrogel-based phototherapy for fighting cancer and bacterial infection. Sci. China Mater. 2017, 60, 487–503. [Google Scholar] [CrossRef]
- Santos, C.I.M.; Gonçalves, G.; Cicuéndez, M.; Mariz, I.; Silva, V.S.; Oliveira, H.; Campos, F.; Vieira, S.I.; Marques, P.A.A.P.; Maçôas, E.M.S.; et al. Biocompatible hybrids based on nanographene oxide covalently linked to glycolporphyrins: Synthesis, characterization and biological evaluation. Carbon N. Y. 2018, 135, 202–214. [Google Scholar] [CrossRef]
- Akbari, T.; Pourhajibagher, M.; Hosseini, F.; Chiniforush, N.; Gholibegloo, E.; Khoobi, M.; Shahabi, S.; Bahador, A. The effect of indocyanine green loaded on a novel nano-graphene oxide for high performance of photodynamic therapy against Enterococcus faecalis. Photodiagnosis Photodyn. Ther. 2017, 20, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Akbari, T.; Pourhajibagher, M.; Chiniforush, N.; Shahabi, S.; Hosseini, F.; Bahador, A. Improve ICG based photodynamic properties through conjugation of icg into nano-graphene oxide against enterococcus faecalis. Avicenna J. Clin. Microbiol. Infect. 2018, 5, e64624. [Google Scholar] [CrossRef]
- Banerjee, I.; Mondal, D.; Martin, J.; Kane, R.S. Photoactivated antimicrobial activity of carbon nanotube-porphyrin conjugates. Langmuir 2010, 26, 17369–17374. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Douaisi, M.P.; Mondal, D.; Kane, R.S. Light-activated nanotube-porphyrin conjugates as effective antiviral agents. Nanotechnology 2012, 23, 105101. [Google Scholar] [CrossRef] [PubMed]
- Sah, U.; Sharma, K.; Chaudhri, N.; Sankar, M.; Gopinath, P. Antimicrobial photodynamic therapy: Single-walled carbon nanotube (SWCNT)-Porphyrin conjugate for visible light mediated inactivation of Staphylococcus aureus. Colloids Surfaces B Biointerfaces 2018, 162, 108–117. [Google Scholar] [CrossRef] [PubMed]























© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Q. Mesquita, M.; J. Dias, C.; P. M. S. Neves, M.G.; Almeida, A.; F. Faustino, M.A. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules 2018, 23, 2424. https://doi.org/10.3390/molecules23102424
Q. Mesquita M, J. Dias C, P. M. S. Neves MG, Almeida A, F. Faustino MA. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules. 2018; 23(10):2424. https://doi.org/10.3390/molecules23102424
Chicago/Turabian StyleQ. Mesquita, Mariana, Cristina J. Dias, Maria G. P. M. S. Neves, Adelaide Almeida, and M. Amparo F. Faustino. 2018. "Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy" Molecules 23, no. 10: 2424. https://doi.org/10.3390/molecules23102424
APA StyleQ. Mesquita, M., J. Dias, C., P. M. S. Neves, M. G., Almeida, A., & F. Faustino, M. A. (2018). Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules, 23(10), 2424. https://doi.org/10.3390/molecules23102424









