Structural, Luminescent and Thermal Properties of Heteronuclear PdII–LnIII–PdII Complexes of Hexadentate N2O4 Schiff Base Ligand
Abstract
1. Introduction
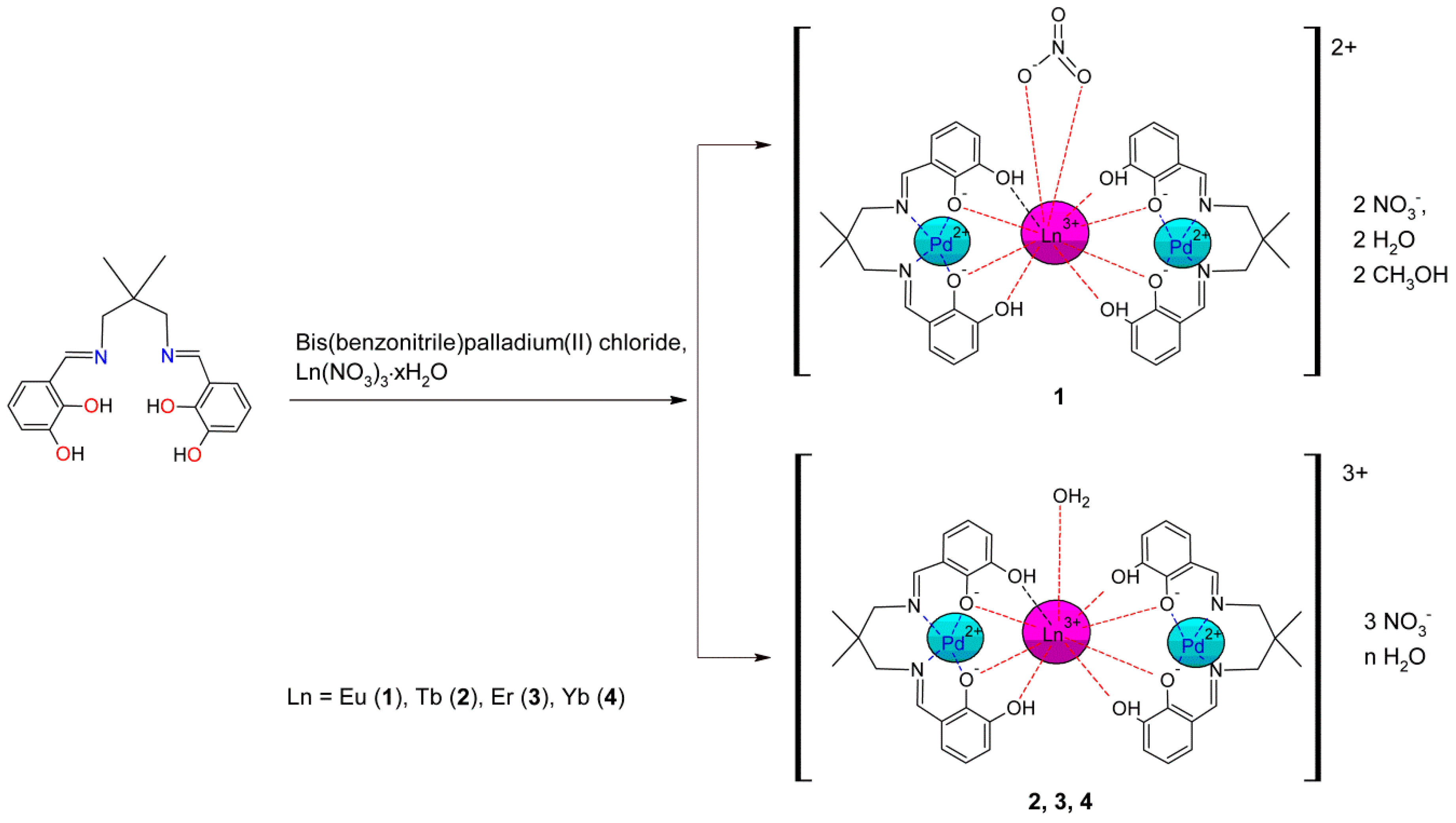
2. Results
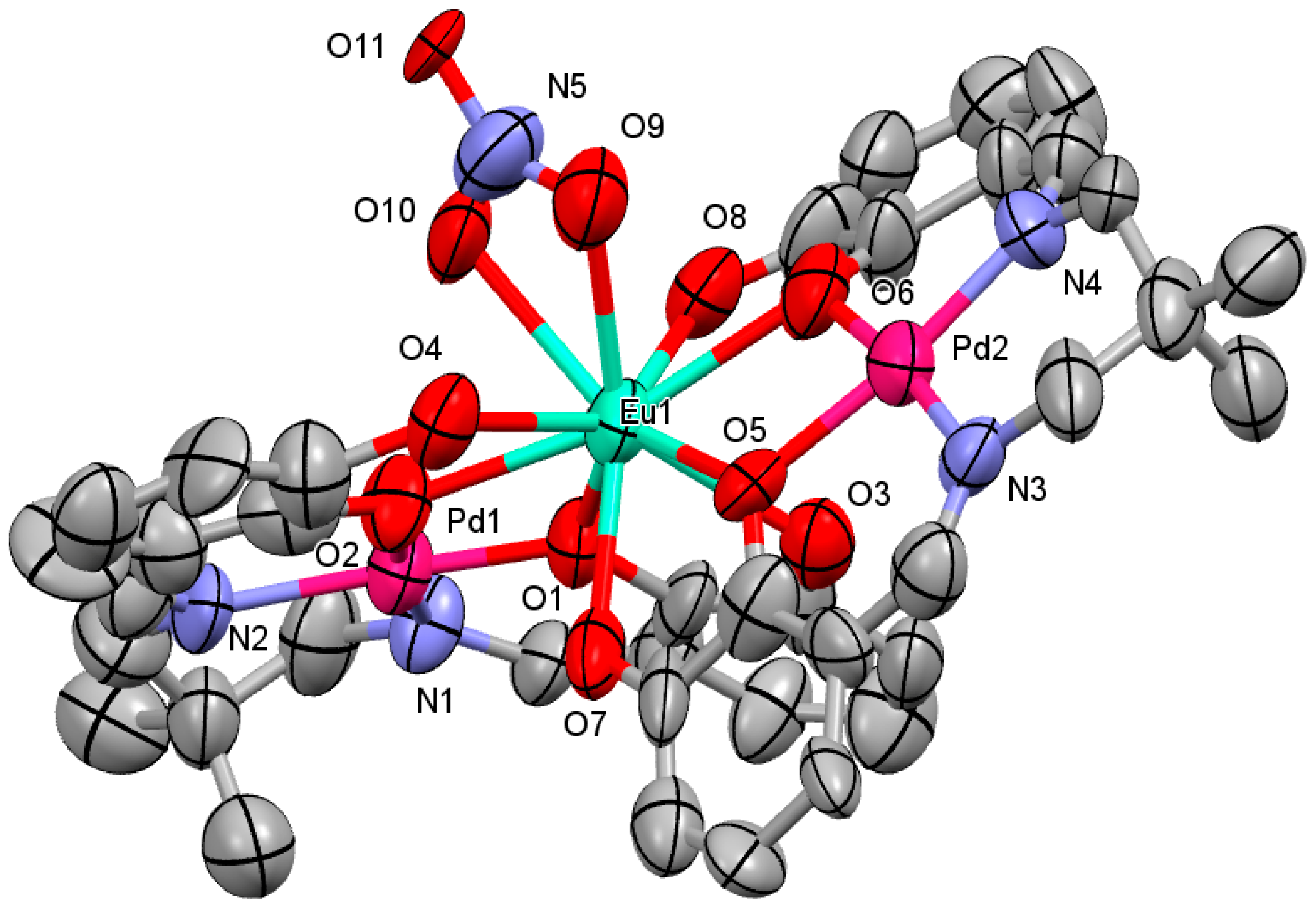
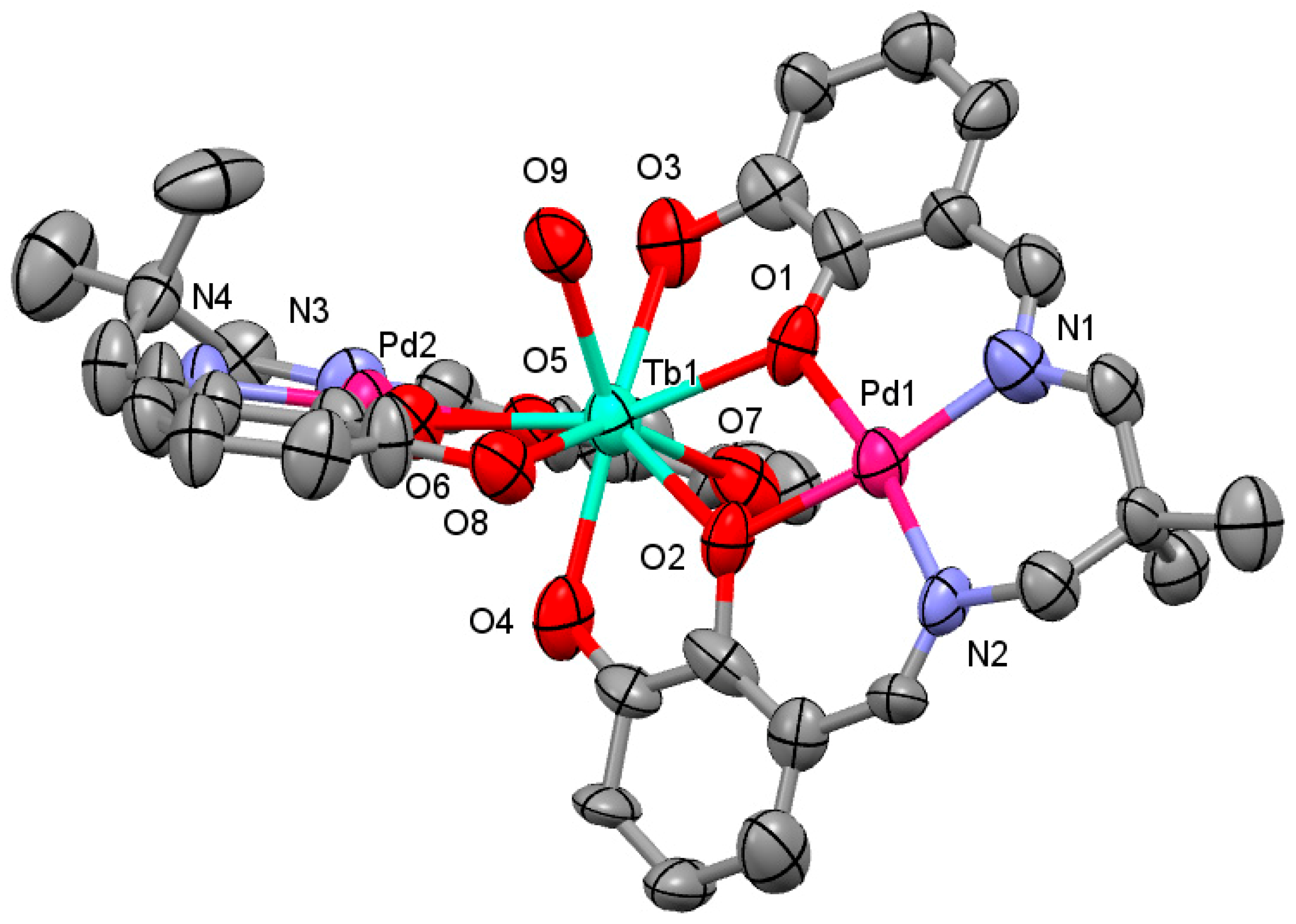
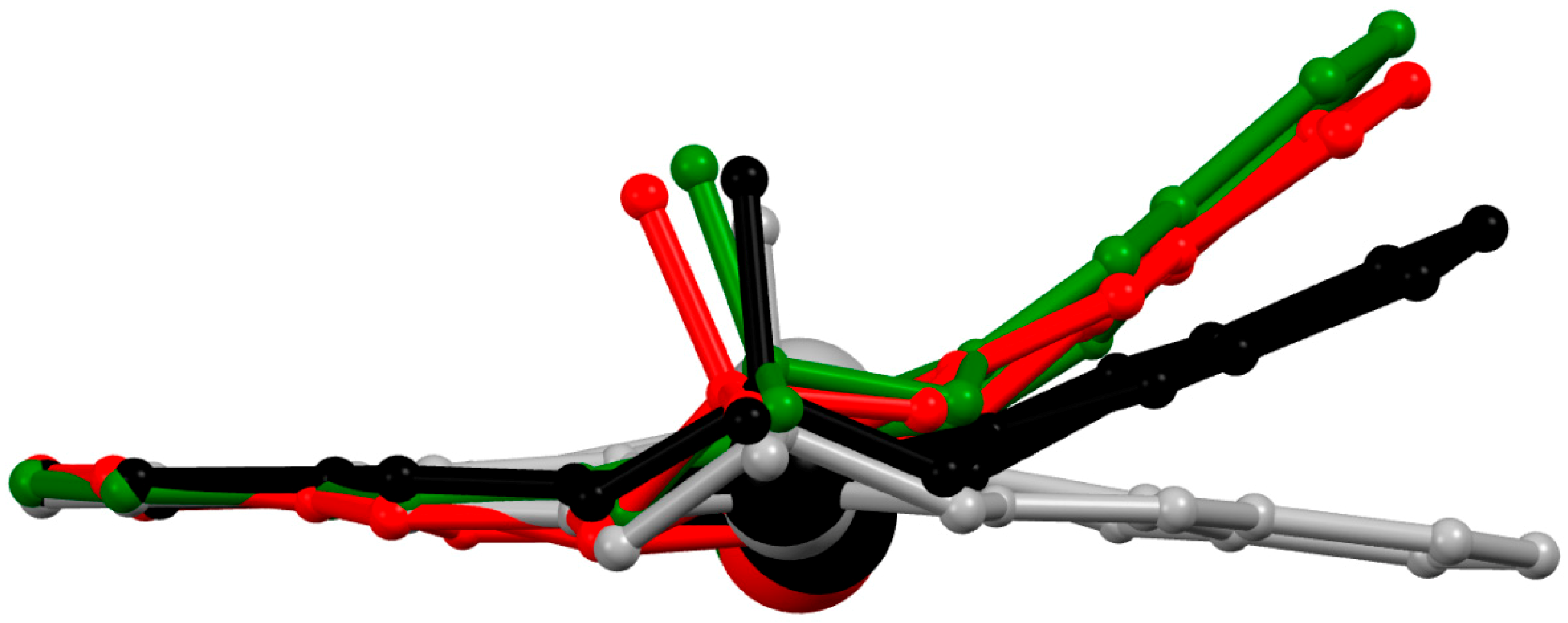
2.1. Crystal Structure
2.2. Thermal Properties
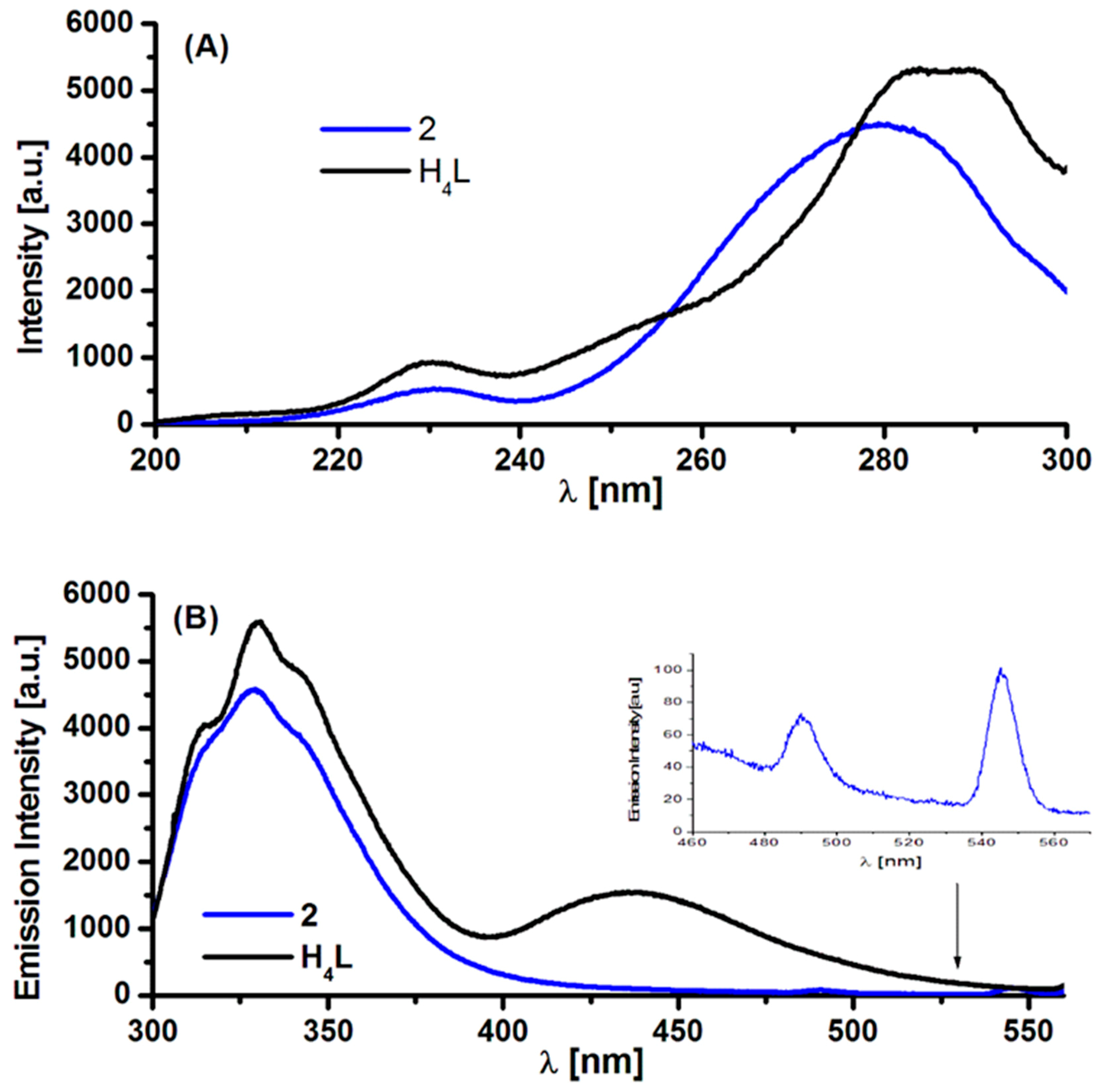
2.3. Absorption and Luminescence Spectra
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the H4L
3.3. General Procedure of Preparation of PdII–LnIII–PdII Complexes
3.3.1. [Pd2Eu(H2L)2NO3](NO3)2∙2H2O∙2CH3OH (1)
3.3.2. [Pd2Tb(H2L)2H2O](NO3)3∙3H2O (2)
3.3.3. [Pd2Er(H2L)2H2O](NO3)3∙3H2O (3)
3.3.4. [Pd2Yb(H2L)2H2O](NO3)3∙5.5H2O (4)
3.4. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Manabe, K. Palladium Catalysts for Cross-Coupling Reaction. Catalysts 2015, 5, 38–39. [Google Scholar] [CrossRef]
- Roy, D.; Uozumi, Y. Recent Advances in Palladium-Catalyzed Cross-Coupling Reactions at Ppm to Ppb Molar Catalyst Loadings. Adv. Synth. Catal. 2018, 360, 602–625. [Google Scholar] [CrossRef]
- Szwaczko, K.; Demchuk, O.M.; Mirosław, B.; Strzelecka, D.; Pietrusiewicz, K.M. Straightforward Approach to Norbornene Core Based Chiral Ligands by Tandem Cross Dehydrogenative Coupling Reactions. Tetrahedron Lett. 2016, 57, 3491–3495. [Google Scholar] [CrossRef]
- Albeniz, A.C.; Espinet, P. Palladium: Inorganic & Coordination Chemistry. In Encyclopedia of Inorganic Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar] [CrossRef]
- Balamurugan, R.; Liu, J.-H.; Liu, B.T. A Review of Recent Developments in Fluorescent Sensors for the Selective Detection of Palladium Ions. Coord. Chem. Rev. 2018, 376, 196–224. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Fujiwara, K.; Okazawa, A.; Tanaka, G.; Yoshii, S.; Nojiri, H.; Ishida, T. Chemical Trend of Ln–M Exchange Couplings in Heterometallic Complexes with Ln = Gd, Tb, Dy, Ho, Er and M = Cu, V. Chem. Commun. 2011, 47, 2110–2112. [Google Scholar] [CrossRef] [PubMed]
- Pasatoiu, T.D.; Tiseanu, C.; Madalan, A.M.; Jurca, B.; Duhayon, C.; Sutter, J.P.; Andruh, M. Study of the Luminescent and Magnetic Properties of a Series of Heterodinuclear [Zn II Ln III ] Complexes. Inorg. Chem. 2011, 50, 5879–5889. [Google Scholar] [CrossRef] [PubMed]
- Andruh, M.; Costes, J.-P.; Diaz, C.; Gao, S. 3d-4f Combined Chemistry: Synthetic Strategies and Magnetic Properties. Inorg. Chem. 2009, 48, 3342–3359. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-P.; Jones, R.A.; Wong, W.-K.; Lynch, V.; Oye, M.M.; Holmes, A.L. Design and Synthesis of a near Infra-Red Luminescent Hexanuclear Zn–Nd Prism. Chem. Commun. 2006, 0, 1836–1838. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huang, R.-W.; Zang, S.-Q.; Mak, T.C.W. Assembly of Silver(i)–organic Frameworks from Flexible Supramolecular Synthons with Pendant Ethynide Arm Attached to Biphenyl and Phenoxybenzene Skeletons. CrystEngComm 2013, 15, 4087–4093. [Google Scholar] [CrossRef]
- Amirkhanov, O.V.; Moroz, O.V.; Znovjyak, K.O.; Sliva, T.Y.; Penkova, L.V.; Yushchenko, T.; Szyrwiel, L.; Konovalova, I.S.; Dyakonenko, V.V.; Shishkin, O.V.; et al. Heterobinuclear Zn-Ln and Ni-Ln Complexes with Schiff-Base and Carbacylamidophosphate Ligands: Synthesis, Crystal Structures, and Catalytic Activity. Eur. J. Inorg. Chem. 2014, 2014, 3720–3730. [Google Scholar] [CrossRef]
- Sreejith, S.S.; Mohan, N.; Aiswarya, N.; Kurup, M.R.P. Inclusion, Pseudo-Inclusion Compounds and Coordination Polymer of Pd(II), Zn(II) and Cd(II) from Salen-Type Schiff Base Ligand with a 1,3-Diimino Spacer Group: Crystal Structures, Spectroscopic and Thermal Studies. Polyhedron 2016, 115, 180–192. [Google Scholar] [CrossRef]
- Zhang, G. A Heterotrimetallic Pd–Sm–Pd Complex for Asymmetric Friedel–Crafts Alkylations of Pyrroles with Nitroalkenes. Org. Biomol. Chem. 2012, 10, 2534–2536. [Google Scholar] [CrossRef] [PubMed]
- Cristóvão, B.; Osypiuk, D.; Miroslaw, B.; Bartyzel, A. Syntheses, Crystal Structures, Thermal and Magnetic Properties of New Heterotrinuclear CuII–LnIII–CuII complexes Incorporating N2O4-Donor Schiff Base Ligands. Polyhedron 2018, 144, 225–233. [Google Scholar] [CrossRef]
- Miroslaw, B.; Cristóvão, B.; Hnatejko, Z.; Miroslaw, B.; Cristóvão, B.; Hnatejko, Z. Heterometallic ZnII–LnIII–ZnII Schiff Base Complexes with Linear or Bent Conformation—Synthesis, Crystal Structures, Luminescent and Magnetic Characterization. Molecules 2018, 23, 1761. [Google Scholar] [CrossRef] [PubMed]
- Bartyzel, A. Synthesis, Crystal Structure and Characterization of manganese(III) Complex Containing a Tetradentate Schiff Base. J. Coord. Chem. 2013, 66, 4292–4303. [Google Scholar] [CrossRef]
- Guney, E.; Yilmaz, V.T.; Buyukgungor, O. Palladium(II) and platinum(II) Saccharinate Complexes Containing Pyridine and 3-Acetylpyridine: Synthesis, Crystal Structures, Fluorescence and Thermal Properties. Polyhedron 2011, 30, 1968–1974. [Google Scholar] [CrossRef]
- Guney, E.; Yilmaz, V.T.; Buyukgungor, O. Neutral and Cationic palladium(II) and platinum(II) Complexes of 2,2′-Dipyridylamine with Saccharinate: Syntheses, Spectroscopic, Structural, Fluorescent and Thermal Studies. Inorg. Chim. Acta 2010, 363, 2416–2424. [Google Scholar] [CrossRef]
- Guney, E.; Yilmaz, V.T.; Kazak, C. Bis(saccharinato)palladium(II) and platinum(II) Complexes with 2,2′-Bipyridine: Syntheses, Structures, Spectroscopic, Fluorescent and Thermal Properties. Polyhedron 2010, 29, 1285–1290. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, B.; Li, Y. Synthesis and Luminescence Properties of Polymer-Rare Earth Complexes Containing Salicylaldehyde-Type Bidentate Schiff Base Ligand. Luminescence 2017, 32, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; List, M.; Teasdale, I.; Redhammer, G.; Chakraborty, D.; Monkowius, U. Palladium Complexes Containing Imino Phenoxide Ligands: Synthesis, Luminescence, and Their Use as Catalysts for the Ring-Opening Polymerization of Rac-Lactide. Monatsh. Chem. 2018, 149, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Seyfi, S.; Alizadeh, R.; Ganji, M.D.; Amani, V. Palladium(II) Complexes with 1,2,4-Triazole Derivative; Ethylene Diamine as Ligands, Synthesis, Characterization, Luminesence Study; Crystal Structure Determination. Polyhedron 2017, 134, 302–315. [Google Scholar] [CrossRef]
- Micutz, M.; Iliş, M.; Staicu, T.; Dumitraşcu, F.; Pasuk, I.; Molard, Y.; Roisnel, T.; Cîrcu, V. Luminescent Liquid Crystalline Materials Based on Palladium(II) Imine Derivatives Containing the 2-Phenylpyridine Core. Dalt. Trans. 2014, 43, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.T.; Kubicki, M.; Hnatejko, Z. Two Types of Lanthanide Schiff Base Complexes: Synthesis, Structure and Spectroscopic Studies. Polyhedron 2015, 102, 224–232. [Google Scholar] [CrossRef]
- Ivanov, M.A.; Puzyk, M.V. Spectral and Luminescent Specific Features of Phenylpyridine Ethylenediamine Complexes of Pt(II), Pd(II), and Au(III). Opt. Spectrosc. 2001, 91, 869–872. [Google Scholar] [CrossRef]
- Aguiari, A.; Bullita, E.; Casellato, U.; Guerriero, P.; Tamburini, S.; Vigato, P.A. Macrocyclic and Macroacyclic Compartmental Schiff Bases: Synthesis, Characterization, X-Ray Structure and Interaction with Metal Ions. Inorg. Chim. Acta 1992, 202, 157–171. [Google Scholar] [CrossRef]
- Bermejo, M.R.; Fernández, M.I.; Gómez-Fórneas, E.; González-Noya, A.; Maneiro, M.; Pedrido, R.; Rodríguez, M.J. Self-Assembly of Dimeric MnIII–Schiff-Base Complexes Tuned by Perchlorate Anions. Eur. J. Inorg. Chem. 2007, 2007, 3789–3797. [Google Scholar] [CrossRef]
- Crysalis-Pro Software System; Rigaku Oxford Diffraction: Oxford, UK, 2016.
- Sheldrick, G.M. SHELXT—Ntegrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–4 are available from the authors. |

| Parameter | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Ln1–Pd1 | 3.612(3) | 3.5776(18) | 3.529(2) | 3.5267(7) |
| Ln1–Pd2 | 3.618(3) | 3.5615(18) | 3.546(2) | 3.5411(7) |
| Ln1–O1 | 2.283(19) | 2.348(16) | 2.348(16) | 2.362(6) |
| Ln1–O2 | 2.482(17) | 2.290(15) | 2.290(15) | 2.308(6) |
| Ln1–O3 | 2.532(19) | 2.470(18) | 2.470(18) | 2.451(6) |
| Ln1–O4 | 2.635(19) | 2.376(18) | 2.376(18) | 2.385(6) |
| Ln1–O5 | 2.396(19) | 2.337(13) | 2.337(13) | 2.334(6) |
| Ln1–O6 | 2.46(2) | 2.349(16) | 2.349(16) | 2.341(6) |
| Ln1–O7 | 2.49(2) | 2.425(17) | 2.425(17) | 2.428(6) |
| Ln1–O8 | 2.40(3) | 2.552(18) | 2.552(18) | 2.394(7) |
| Ln1–O9 | 2.57(3) | 2.43(2) | 2.43(2) | 2.328(7) |
| Ln1–O10 | 2.56(2) | – | – | – |
| Pd1–O1 | 2.070(15) | 2.019(15) | 1.979(13) | 1.980(6) |
| Pd1–O2 | 1.954(16) | 2.044(13) | 1.994(13) | 1.990(6) |
| Pd1–N1 | 1.914(18) | 1.96(2) | 1.949(14) | 1.999(7) |
| Pd1–N2 | 2.07(2) | 2.039(18) | 1.985(17) | 1.991(7) |
| Pd2–O5 | 1.989(17) | 1.956(14) | 2.008(14) | 1.989(6) |
| Pd2–O6 | 1.99(3) | 1.998(13) | 1.991(13) | 1.984(6) |
| Pd2–N3 | 2.00(2) | 2.018(19) | 1.989(16) | 1.988(8) |
| Pd2–N4 | 1.906(19) | 1.95(2) | 2.006(16) | 1.995(8) |
| Pd1–O1–Ln1 | 112.1(7) | 109.8(9) | 110.8(9) | 108.3(2) |
| Pd1–O2–Ln1 | 108.5(7) | 111.1(8) | 111.6(7) | 110.1(2) |
| Pd2–O5–Ln1 | 110.9(8) | 111.8(8) | 109.7(8) | 109.7(3) |
| Pd2–O6–Ln1 | 108.4(10) | 109.8(8) | 111.1(8) | 109.6(3) |
| Δb (Pd1; Pd2) | 0.02; 0.01 | 0.02; 0.02 | 0.03; 0 | 0.02; 0 |
| σc (Pd1; Pd2) | 10.5; 2.1 | 0.7; 9.2 | 3.0; 8.5 | 3.6; 4.4 |
| εd | 65.34 | 67.62 | 70.40 | 68.02 |
| φe | 161.70(8) | 170.26(6) | 170.92(6) | 169.45(2) |
| ψf (Pd1; Pd2) | 29.2; 39.2 | 25.2; 10.3 | 25.7; 11.6 | 27.6; 9.43 |
| Compound | λmax (abs) (a) | λex/λem (a) | λex/λem (b) |
|---|---|---|---|
| H4L | 218.0; 264.5; 298.5; 424.0 | 283/330.6; 438.2 | 380/418.0; 573.0 |
| 1 | 214.0; 286.0 | 280/329.4 | 381/465.4; 496.2 |
| 2 | 215.0; 285.5 | 280/329.2 | 380/469.6; 492.4 |
| 3 | 213.0; 284.0 | 281/328.6 | 381/469.0; 494.2 |
| 4 | 216.0; 286.0 | 279/327.8 | 382/472.2; 496.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miroslaw, B.; Cristóvão, B.; Hnatejko, Z. Structural, Luminescent and Thermal Properties of Heteronuclear PdII–LnIII–PdII Complexes of Hexadentate N2O4 Schiff Base Ligand. Molecules 2018, 23, 2423. https://doi.org/10.3390/molecules23102423
Miroslaw B, Cristóvão B, Hnatejko Z. Structural, Luminescent and Thermal Properties of Heteronuclear PdII–LnIII–PdII Complexes of Hexadentate N2O4 Schiff Base Ligand. Molecules. 2018; 23(10):2423. https://doi.org/10.3390/molecules23102423
Chicago/Turabian StyleMiroslaw, Barbara, Beata Cristóvão, and Zbigniew Hnatejko. 2018. "Structural, Luminescent and Thermal Properties of Heteronuclear PdII–LnIII–PdII Complexes of Hexadentate N2O4 Schiff Base Ligand" Molecules 23, no. 10: 2423. https://doi.org/10.3390/molecules23102423
APA StyleMiroslaw, B., Cristóvão, B., & Hnatejko, Z. (2018). Structural, Luminescent and Thermal Properties of Heteronuclear PdII–LnIII–PdII Complexes of Hexadentate N2O4 Schiff Base Ligand. Molecules, 23(10), 2423. https://doi.org/10.3390/molecules23102423








