Recent Advances in Synthesis of 4-Arylcoumarins
Abstract
1. Introduction
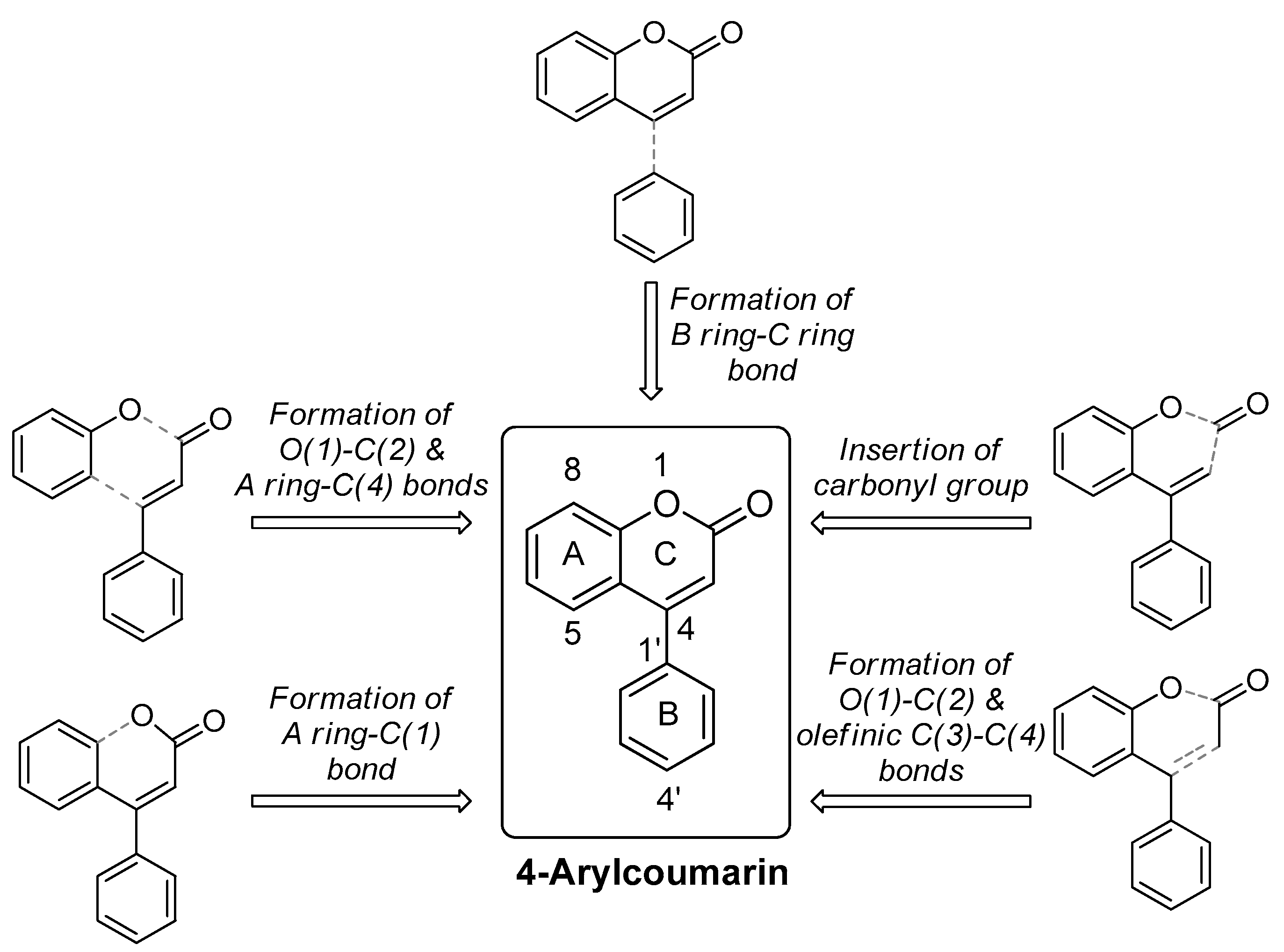
2. Synthetic Routes toward Central 2-pyrone of 4-arylcoumarin via O(1)–C(2) and A Ring–C(4) Bond Formation
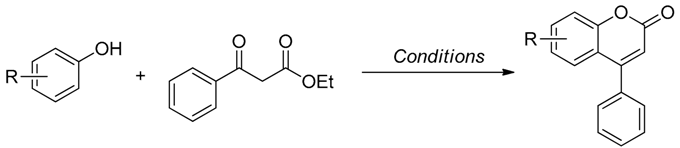
2.1. Pechmann Condensation
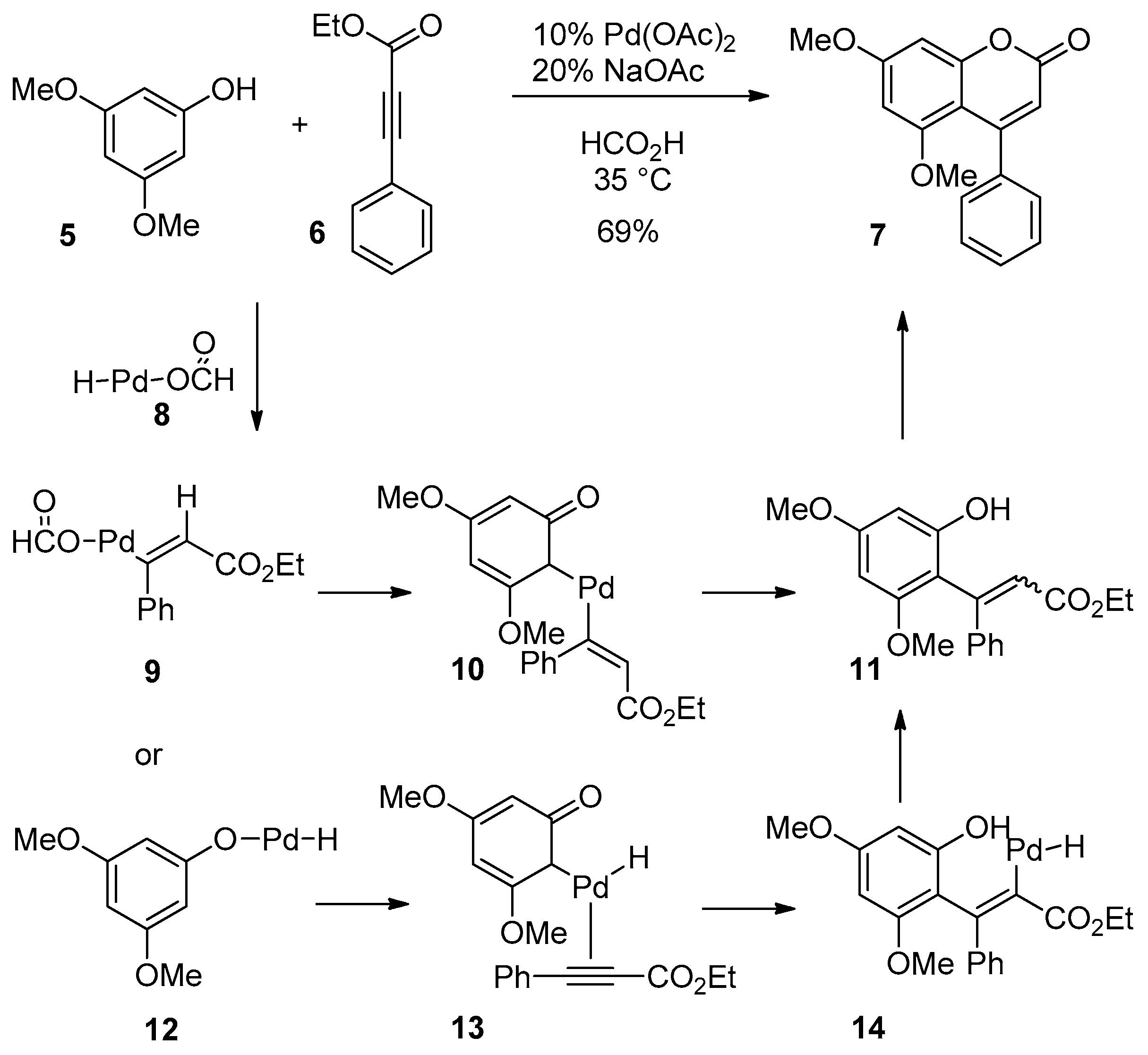
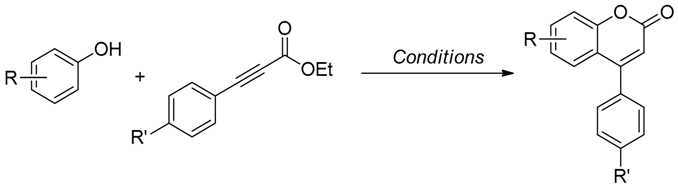
2.2. Hydroarylation of Phenylpropiolate
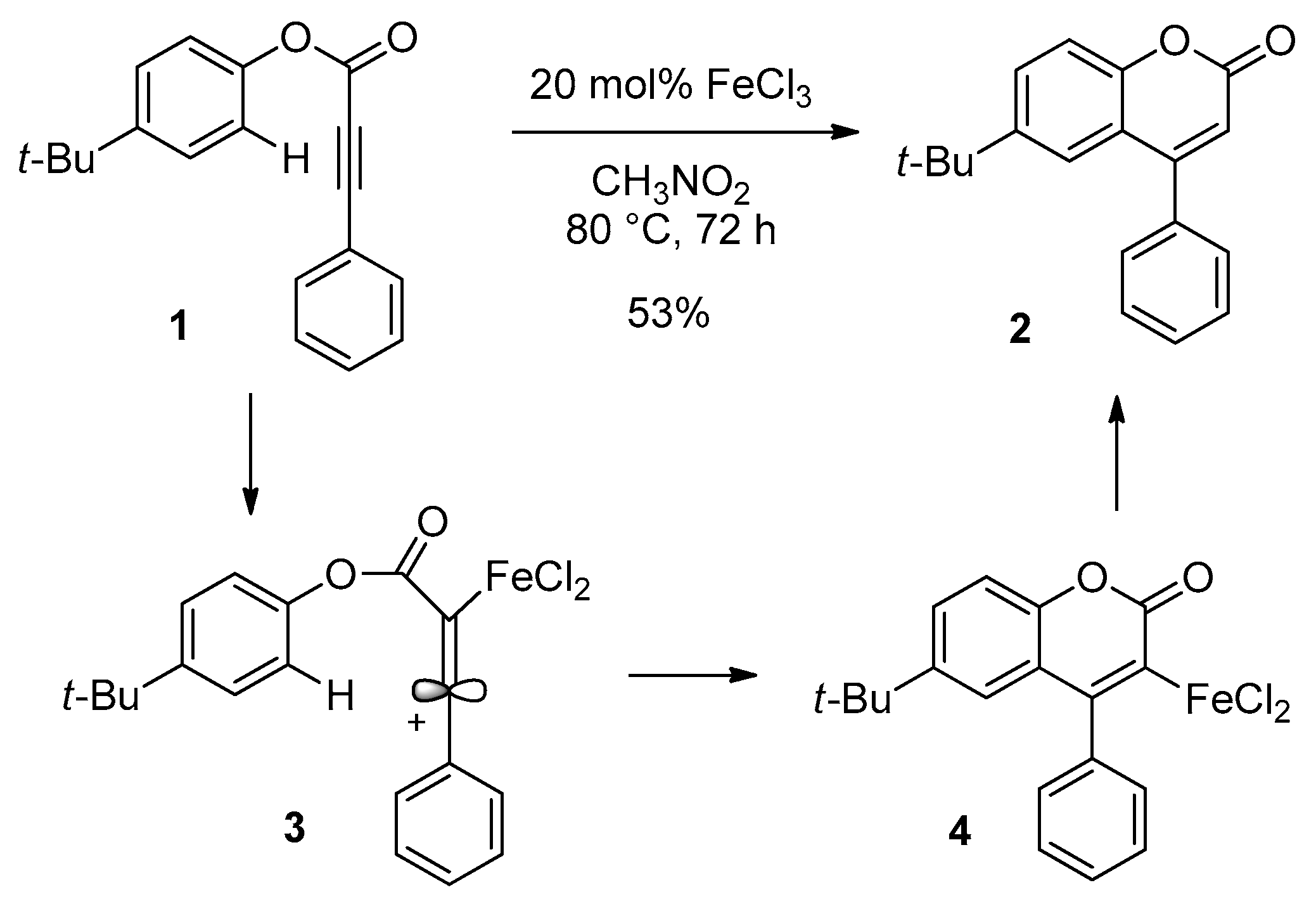
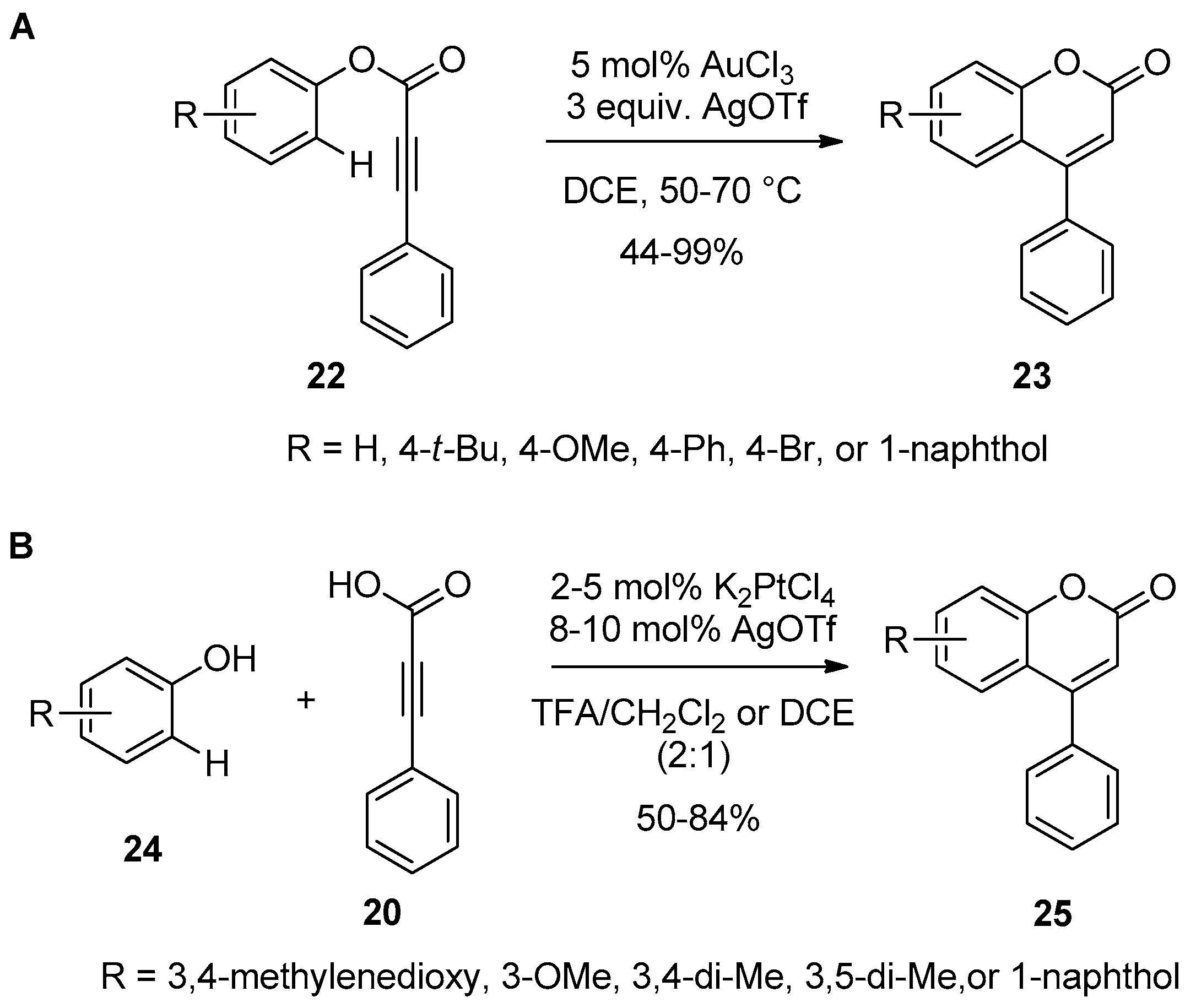
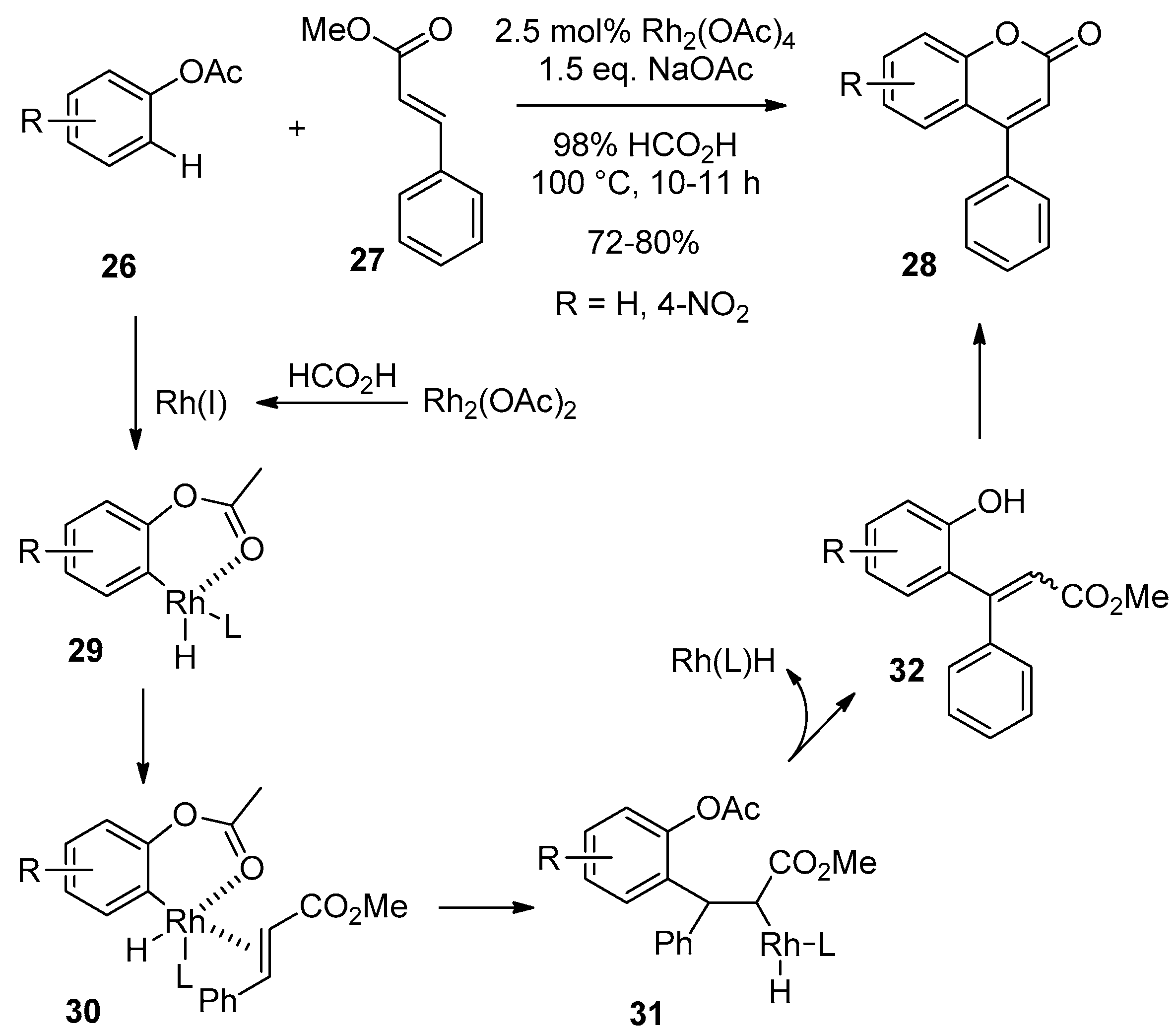
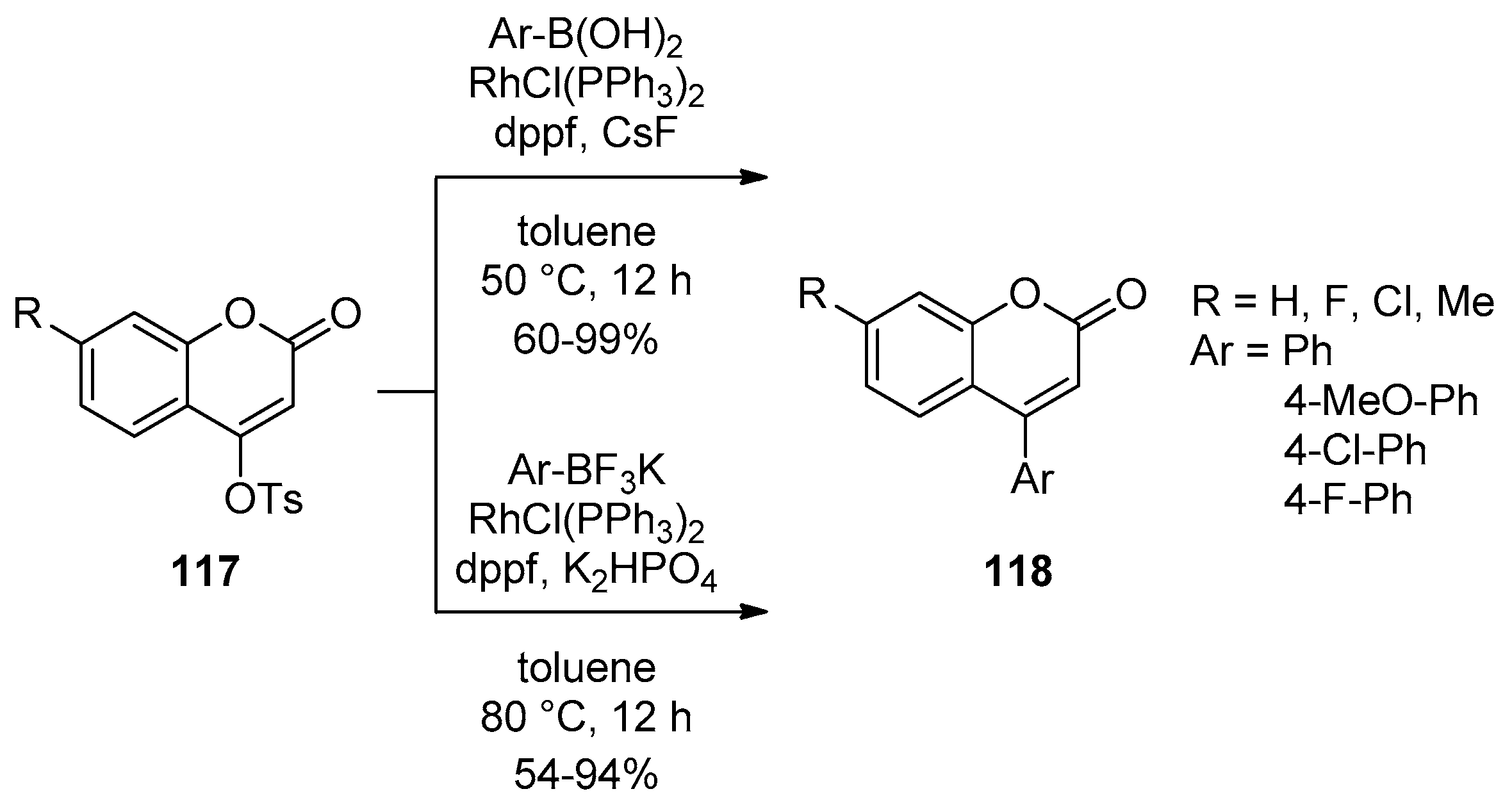
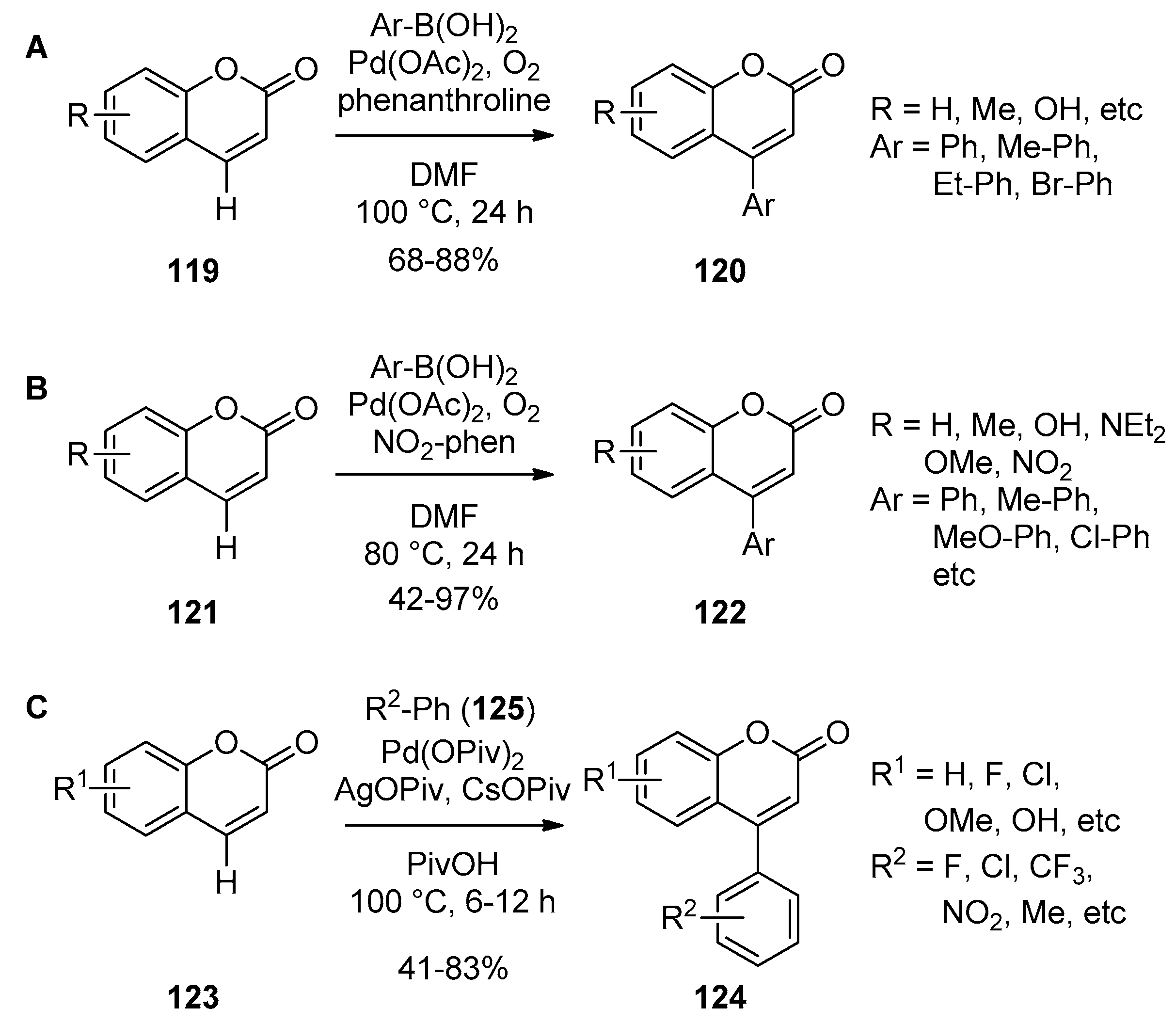
2.3. Transition-Metal-Catalyzed C–H Bond Functionalization
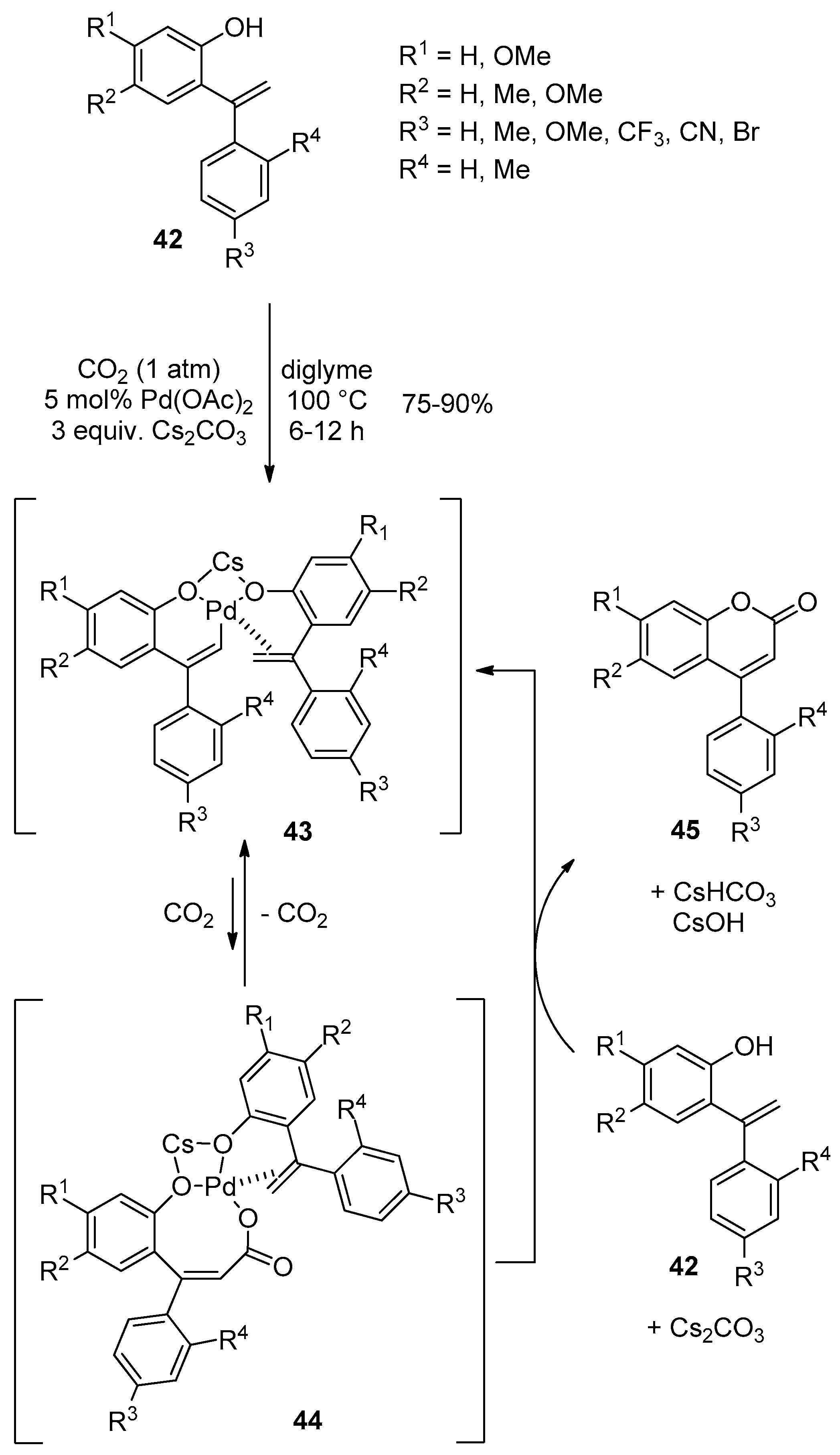
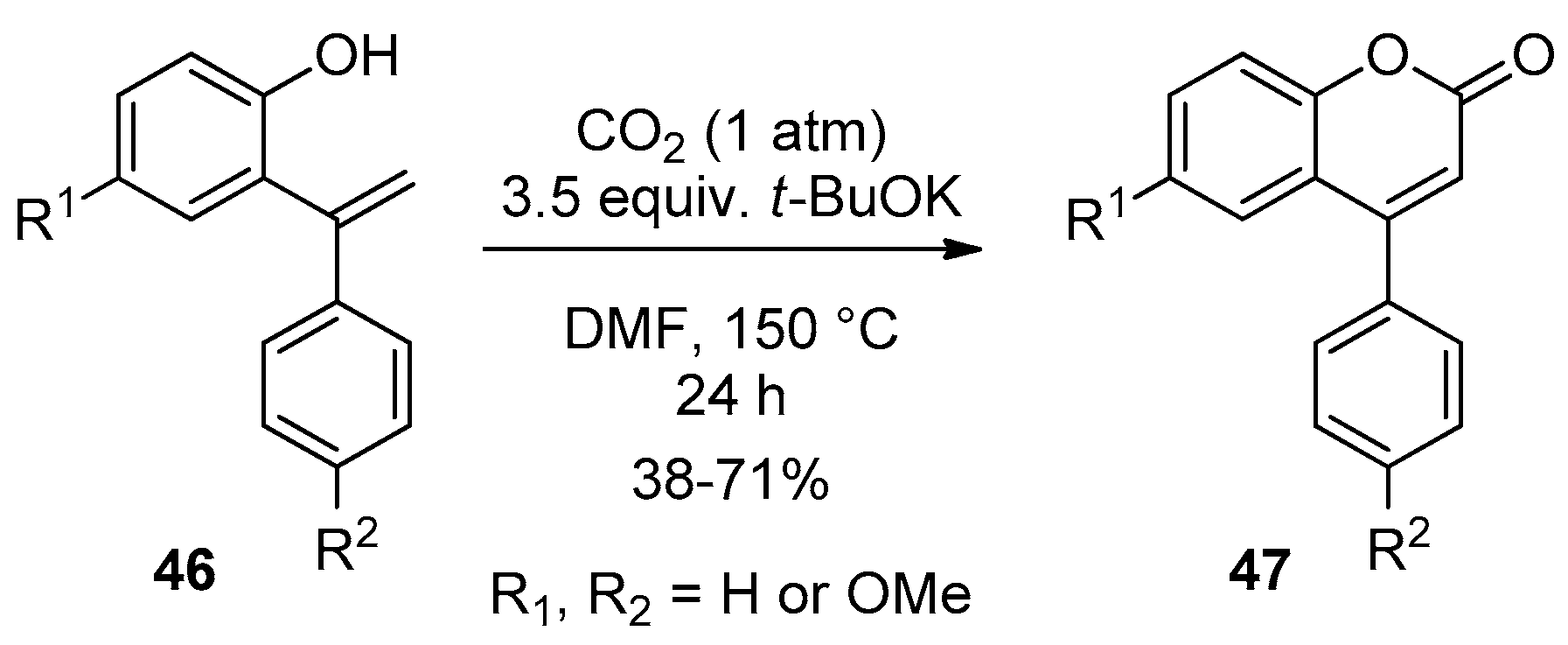
3. Cyclocarbonylation and Cyclocarboxylation toward Central 2-Pyrone of the 4-Arylcoumarin
4. Synthetic Routes toward Central 2-Pyrone of 4-Arylcoumarin via C(3)−C(4) Double Bond and O(1)−C(2) Ester Bond Formation
4.1. Wittig-Type Olefinations
4.2. Aldol-Type Olefinations
4.3. Cycloisomerization
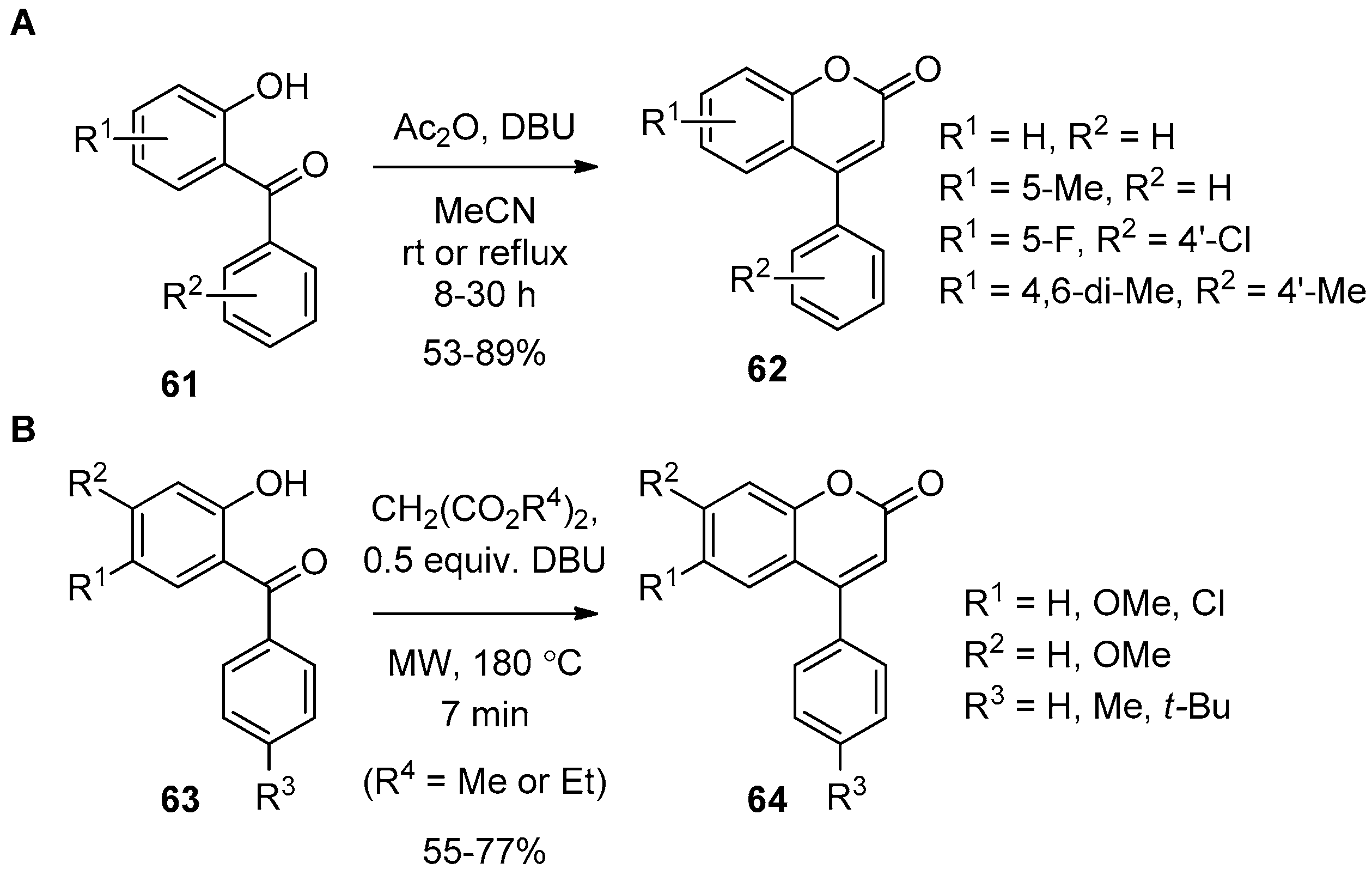
5. Oxidative Cyclization toward Central 2-Pyrone of the 4-Arylcoumarin by A Ring−O(1) Bond Formation
6. Synthesis of 4-Arylcoumarins by Introduction of An Aryl group at the 4-Position
6.1. Transition-Metal-Catalyzed Introduction of 4-Aryl Group
6.2. Domino Reactions Assisted by Transition Metal Catalysis
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ac | Acetyl |
| ASA | Alumina sulfuric acid |
| BTSA | Boric trisulfuric anhydride |
| bmim | 1-Butyl-3-methylimidazolium |
| Bu | Butyl |
| CAN | Ceric ammonium nitrate |
| COD | 1,5-Cyclooctadiene |
| Cp | Cyclopentadienyl |
| CSA | Cellulose sulfuric acid |
| Cy | Cyclohexyl |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| DCE | 1,2-Dichloroethane |
| DMA | Dimethylacetamide |
| DMF | N,N-Dimethylformamide |
| DMAP | 4-(N,N-dimethylamino)pyridine |
| dppe | 1,2-Bis(diphenylphosphino)ethane |
| dppb | 1,4-Bis(diphenylphosphino)butane |
| dppf | 1,1′-Bis(diphenylphosphino)ferrocene |
| Et | Ethyl |
| Me | Methyl |
| MSA | Molybdate sulfuric acid |
| MW | Microwave |
| Ph | Phenyl |
| Piv | Pivaloyl |
| PVSA | Polyvinyl sulfonic acid |
| Tf | Trifluoromethanesulfonyl |
| TFA | Trifluoroacetic acid |
| THF | Tetrahydrofuran |
| TMS | Trimethylsilyl |
| Ts | p-Toluenesulfonyl |
| US | Ultrasound |
| XSA | Xanthan sulfuric acid |
References
- Keri, R.S.; Budagumpi, S.; Pai, R.K.; Balakrishna, R.G. Chromones as a privileged scaffold in drug discovery: A review. Eur. J. Med. Chem. 2014, 78, 340–374. [Google Scholar] [CrossRef] [PubMed]
- Garazd, M.M.; Garazd, Y.L.; Khilya, V.P. Neoflavones. 2. Methods for synthesizing and modifying 4-arylcoumarins. Chem. Nat. Comp. 2005, 41, 245–271. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Fathi, R.; Yang, Z. Palladium-catalyzed cross-coupling reactions of 4-tosylcoumarin and arylboronic acids: Synthesis of 4-arylcoumarin compounds. Tetrahedron Lett. 2002, 43, 4395–4397. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.G.; Marrero, J.G.; Macías-Alonso, M.; González, M.C.; Córdova-Guerrero, I.; García, S.; Osegueda-Robles, A.G.T. Coumarin heterocyclic derivatives: Chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, E.C.; Tagliapietra, S.; Martina, K.; Palmisano, G.; Cravotto, G. Recent advances and perspectives in the synthesis of bioactive coumarins. RSC Adv. 2016, 6, 46394–46405. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, H.V. Neue Bildungsweise der Cumarine. Synthese des Daphnetins. Ber. Dtsch. Chem. Ges. 1884, 17, 929–936. [Google Scholar] [CrossRef]
- Tyndall, S.; Wong, K.F.; VanAlstine-Parris, M.A. Insight into the mechanism of the Pechmann condensation reaction using NMR. J. Org. Chem. 2015, 80, 8951–8953. [Google Scholar] [CrossRef] [PubMed]
- Daru, J.; Stirling, A. Mechanism of the Pechmann reaction: A theoretical study. J. Org. Chem. 2011, 76, 8749–8755. [Google Scholar] [CrossRef] [PubMed]
- Pornsatitworakul, S.; Boekfa, B.; Maihom, T.; Treesukol, P.; Namuangruk, S.; Jarussophon, S.; Jarussophon, N.; Limtrakul, J. The coumarin synthesis: A combined experimental and theoretical study. Monatsh. Chem. 2017, 148, 1245–1250. [Google Scholar] [CrossRef]
- Zambare, A.S.; Kalam Khan, F.A.; Zambare, S.P.; Shinde, S.D.; Sangshetti, J.N. Recent Advances in the Synthesis of Coumarin Derivatives via Pechmann Condensation. Curr. Org. Chem. 2016, 20, 798–828. [Google Scholar] [CrossRef]
- Sugino, T.; Tanaka, K. Solvent-free coumarin synthesis. Chem. Lett. 2001, 30, 110–111. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, S.; Makrandi, J. Modified Pechmann condensation using grinding technique under solvent-free condition at room temperature. Green Chem. Lett. Rev. 2011, 4, 127–129. [Google Scholar] [CrossRef]
- Manhas, M.S.; Ganguly, S.N.; Mukherjee, S.; Jain, A.K.; Bose, A.K. Microwave initiated reactions: Pechmann coumarin synthesis, Biginelli reaction, and acylation. Tetrahedron Lett. 2006, 47, 2423–2425. [Google Scholar] [CrossRef]
- Singh, P.R.; Singh, D.U.; Samant, S.D. Sulphamic acid-an efficient and cost-effective solid acid catalyst for the Pechmann reaction. Synlett 2004, 2004, 1909–1912. [Google Scholar]
- Moradi, L.; Rabiei, K.; Belali, F. Meglumine sulfate catalyzed solvent-free one-pot synthesis of coumarins under microwave and thermal conditions. Synth. Commun. 2016, 46, 1283–1291. [Google Scholar] [CrossRef]
- Karimi-Jaberi, Z.; Zarei, L. Synthesis of coumarins and 2, 3-dihydroquinazolin-4 (1H)-ones. Acta Chim. Slov. 2013, 60, 178–183. [Google Scholar] [PubMed]
- Sunil Kumar, B.; Thirupathi Reddy, Y.; Narsimha Reddy, P.; Kumar, P.; Rajitha, B. Selectfluor™: A simple and efficient catalyst for the synthesis of substituted coumarins under solvent-free conditions. J. Heterocycl. Chem. 2006, 43, 477–479. [Google Scholar] [CrossRef]
- Sharma, G.; Reddy, J.J.; Lakshmi, P.S.; Krishna, P.R. An efficient ZrCl4 catalyzed one-pot solvent free protocol for the synthesis of 4-substituted coumarins. Tetrahedron Lett. 2005, 46, 6119–6121. [Google Scholar] [CrossRef]
- Kumar, B.S.; Kumar, P.; Srinivasulu, N.; Rajitha, B.; Reddy, Y.T.; Reddy, P.N.; Udupi, R. Vanadium(III) chloride as an effective catalyst for the Pechmann reaction. Chem. Heterocycl. Compd. 2006, 42, 172–175. [Google Scholar] [CrossRef]
- Naik, M.A.; Mishra, B.G.; Dubey, A. A simple and efficient protocol for the synthesis of substituted comarins catalyzed by stannic(IV) chloride under solvent-free condition. React. Kinet. Catal. Lett. 2007, 91, 169–175. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, F.; Tian, Y.-P.; Li, H.-L.; Wang, J.-J. CuX2 (X = Cl, Br) as catalysts for Pechmann reaction: Synthesis of 4-substituted coumarins. J. Chem. Res. 2009, 2009, 339–341. [Google Scholar] [CrossRef]
- Khodabakhshi, S. Barium dichloride as a powerful and inexpensive catalyst for the Pechmann condensation without using solvent. Org. Chem. Int. 2012, 2012, 306162. [Google Scholar] [CrossRef]
- Kumar, S.; Saini, A.; Sandhu, J.S. LiBr-Mediated, solvent free von Pechmann reaction: Facile and efficient method for the synthesis of 2H-chromen-2-ones. Arkivoc 2007, 15, 18–23. [Google Scholar]
- Patil, S.B.; Bhat, R.P.; Raje, V.P.; Samant, S.D. Ultrasound-Assisted Pechmann Condensation of Phenols With β-Ketoesters to Form Coumarins, in the Presence of Bismuth(III) Chloride Catalyst. Synth. Commun. 2006, 36, 525–531. [Google Scholar] [CrossRef]
- Madhav, J.V.; Kuarm, B.S.; Someshwar, P.; Rajitha, B.; Thirupathi Reddy, Y.R.; Crooks, P.A. Dipyridine cobalt chloride: A novel catalyst for the synthesis of coumarins via Pechmann condensation. J. Chem. Res. 2008, 2008, 232–234. [Google Scholar] [CrossRef]
- Jung, K.; Park, Y.-J.; Ryu, J.-S. Scandium(III) Triflate–Catalyzed Coumarin Synthesis. Synthetic Commun. 2008, 38, 4395–4406. [Google Scholar] [CrossRef]
- Wang, H. Magnesium bis(trifluoromethane)sulfonimide: An efficient catalyst for the synthesis of coumarins under solvent-free conditions. Monatsh. Chem. 2013, 144, 411–414. [Google Scholar] [CrossRef]
- Alexander, V.M.; Bhat, R.P.; Samant, S.D. Bismuth(III) nitrate pentahydrate—A mild and inexpensive reagent for synthesis of coumarins under mild conditions. Tetrahedron Lett. 2005, 46, 6957–6959. [Google Scholar] [CrossRef]
- Reddy, Y.T.; Sonar, V.N.; Crooks, P.A.; Dasari, P.K.; Reddy, P.N.; Rajitha, B. Ceric ammonium nitrate (CAN): An efficient catalyst for the coumarin synthesis via Pechmann condensation using conventional heating and microwave irradiation. Synth. Commun. 2008, 38, 2082–2088. [Google Scholar] [CrossRef]
- Karami, B.; Kiani, M. Synthesis of the Coumarins via Pechmann Method in the Presence of Environmentally Friendly Y(NO3) 3× 6H2O. J. Chin. Chem. Soc. 2014, 61, 213–216. [Google Scholar] [CrossRef]
- Soleimani, E.; Khodaei, M.M.; Batooie, N.; Samadi, S. Tetrakis(acetonitrile)copper(I) hexafluorophosphate catalyzed coumarin synthesis via pechmann condensation under solvent-free condition. J. Heterocycl. Chem. 2012, 49, 409–412. [Google Scholar] [CrossRef]
- Jafari, F.; Khodabakhshi, S. MnSO4. H2O: A highly efficient and inexpensive catalyst for the synthesis of benzo-2-pyrones and benzopyrazines. Bulg. Chem. Commun. 2014, 46, 36–42. [Google Scholar]
- Moradi, L.; Belali, F. Solvent-free one-pot synthesis of coumarins using molybdate sulfuric acid as highly efficient catalyst. J. Iran. Chem. Soc. 2015, 12, 1927–1934. [Google Scholar] [CrossRef]
- Goswami, P. Dually activated organo-and nano-cocatalyzed synthesis of coumarin derivatives. Synthetic Commun. 2009, 39, 2271–2278. [Google Scholar] [CrossRef]
- Naik, M.A.; Mishra, B.G.; Dubey, A. Combustion synthesized WO3–ZrO2 nanocomposites as catalyst for the solvent-free synthesis of coumarins. Colloids Surf. A. 2008, 317, 234–238. [Google Scholar] [CrossRef]
- Wu, L.-Q.; Yan, F.-L.; Yang, C.-G.; Yang, L.-M. Silica Chloride Catalyzed Solvent-Free Synthesis of Coumarins. Chin. J. Org. Chem. 2010, 30, 1764–1767. [Google Scholar]
- Sharma, D.; Kumar, S. A facile synthesis of 2H-chromen-2-ones via Pechmann condensation under solvent free conditions using grinding technique. Green Process. Synth. 2013, 2, 151–155. [Google Scholar] [CrossRef]
- Rad-Moghadam, K.; Montazeri, N. Coumarin synthesis via pechmann condensation on silica-supported sulfuric acid under microwave irradiation. Asian J. Chem. 2009, 21, 499–503. [Google Scholar]
- Karami, B.; Kiani, M. ZrOCl2. 8H2O/SiO2: An efficient and recyclable catalyst for the preparation of coumarin derivatives by Pechmann condensation reaction. Catal. Commun. 2011, 14, 62–67. [Google Scholar] [CrossRef]
- Parhami, A.; Khalafi-Nezhad, A.; Haghighi, S.M.; Bargebid, R.; Zare, A.; Moosavi-Zare, A.R.; Nikrooz, M. Silica supported boric tri-sulfuric anhydride as a novel and efficient catalyst for solvent-free synthesis of coumarins via Pechmann condensation. Arkivoc 2012, 9, 111–121. [Google Scholar]
- Sun, R.; Gao, Y.; Ma, Y.; Yang, G.; Li, Y. SnCl4 grafted on silica gel: An efficient catalyst for solvent-free synthesis of coumarins via the Pechmann condensation. J. Iran. Chem. Soc. 2017, 14, 737–742. [Google Scholar] [CrossRef]
- Amoozadeh, A.; Ahmadzadeh, M.; Kolvari, E. Easy access to coumarin derivatives using alumina sulfuric acid as an efficient and reusable catalyst under solvent-free conditions. J. Chem. 2012, 2013. [Google Scholar] [CrossRef]
- Kuarm, B.S.; Crooks, P.A.; Rajitha, B. Polyvinylsulfonic acid: An Efficient and Recyclable Bronsted Acid Catalyst for Pechmann Condensation. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 50–53. [Google Scholar]
- Kuarm, B.S.; Madhav, J.V.; Rajitha, B. Xanthan sulfuric acid: An efficient and recyclable solid acid catalyst for Pechmann condensation. Synthetic Commun. 2012, 42, 1770–1777. [Google Scholar] [CrossRef]
- Kuarm, B.S.; Madhav, J.V.; Laxmi, S.V.; Rajitha, B.; Reddy, Y.T.; Reddy, P.N.; Crooks, P.A. Expeditious pechmann condensation by using biodegradable cellulose sulfuric acid as a solid acid catalyst. Synth. Commun. 2010, 40, 3358–3364. [Google Scholar] [CrossRef]
- Mobaraki, A.; Yasham, S.; Movassagh, B. Environmentally Sustainable Magnetic Solid Sulfonic Acid: An Efficient and Reusable Catalyst for the Pechmann Reaction. Synlett 2015, 26, 1263–1268. [Google Scholar] [CrossRef]
- Abbasi, Z.; Rezayati, S.; Bagheri, M.; Hajinasiri, R. Preparation of a novel, efficient, and recyclable magnetic catalyst, γ-Fe2O3@HAp-Ag nanoparticles, and a solvent-and halogen-free protocol for the synthesis of coumarin derivatives. Chin. Chem. Lett. 2017, 28, 75–82. [Google Scholar] [CrossRef]
- Dabiri, M.; Baghbanzadeh, M.; Kiani, S.; Vakilzadeh, Y. Alum (KAl(SO4)2·12H2O) Catalyzed One-Pot Synthesis of Coumarins under Solvent-Free Conditions. Monatsh. Chem. 2007, 138, 997–999. [Google Scholar] [CrossRef]
- Azizian, J.; Mohammadi, A.A.; Bidar, I.; Mirzaei, P. KAl(SO4)2·12H2O (alum) a reusable catalyst for the synthesis of some 4-substituted coumarins via Pechmann reaction under solvent-free conditions. Monatsh. Chem. 2008, 139, 805–808. [Google Scholar] [CrossRef]
- Kalyanam, N.; Nagarajan, A.; Majeed, M. A Single-Step Assembly of Coumarin Ring Skeleton from Oxygenated Phenols and Acetylenic Esters by Catalytic Indium Chloride in the Absence of Solvent. Synth. Commun. 2004, 34, 1909–1914. [Google Scholar] [CrossRef]
- Leao, R.A.; de Moraes, P.D.F.; Pedro, M.C.; Costa, P.R. Synthesis of coumarins and neoflavones through zinc chloride catalyzed hydroarylation of acetylenic esters with phenols. Synthesis 2011, 2011, 3692–3696. [Google Scholar] [CrossRef]
- Escobar, A.M.; Ruiz, D.M.; Autino, J.C.; Romanelli, G.P. Single-step synthesis of 4-phenyl and 3,4-dihydro-4-phenyl coumarins using a recyclable Preyssler heteropolyacid catalyst under solvent-free reaction conditions. Res. Chem. Intermediat. 2015, 41, 10109–10123. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.R.; Lu, W. FeCl3-catalyzed alkenylation of simple arenes with aryl-substituted alkynes. Org. Lett. 2007, 9, 2219–2222. [Google Scholar] [CrossRef] [PubMed]
- Kutubi, M.S.; Hashimoto, T.; Kitamura, T. Improved synthesis of coumarins by iron(III)-catalyzed cascade reaction of propiolic acids and phenols. Synthesis 2011, 2011, 1283–1289. [Google Scholar] [CrossRef]
- Fiorito, S.; Epifano, F.; Taddeo, V.A.; Genovese, S. Ytterbium triflate promoted coupling of phenols and propiolic acids: Synthesis of coumarins. Tetrahedron Lett. 2016, 57, 2939–2942. [Google Scholar] [CrossRef]
- Bennardi, D.O.; Ruiz, D.M.; Romanelli, G.P.; Baronetti, G.T.; Thomas, H.J.; Autino, J.C. Efficient microwave solvent-free synthesis of flavones, chromones, coumarins and dihydrocoumarins. Lett. Org. Chem. 2008, 5, 607–615. [Google Scholar] [CrossRef]
- Song, C.E.; Jung, D.U.; Choung, S.Y.; Roh, E.J.; Lee, S.G. Dramatic enhancement of catalytic activity in an ionic liquid: Highly practical friedel–crafts alkenylation of arenes with alkynes catalyzed by metal triflates. Angew. Chem. Int. Ed. 2004, 43, 6183–6185. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.Y.; Kim, J.H.; Choi, D.S.; Shin, U.S.; Lee, J.Y.; Song, C.E. Metal Triflate-Catalyzed Regio-and Stereoselective Friedel–Crafts Alkenylation of Arenes with Alkynes in an Ionic Liquid: Scope and Mechanism. Adv. Synth. Catal. 2007, 349, 1725–1737. [Google Scholar] [CrossRef]
- Trost, B.M.; Toste, F.D. A new palladium-catalyzed addition: A mild method for the synthesis of coumarins. J. Am. Chem. Soc. 1996, 118, 6305–6306. [Google Scholar] [CrossRef]
- Trost, B.M.; Toste, F.D.; Greenman, K. Atom Economy. Palladium-Catalyzed Formation of Coumarins by Addition of Phenols and Alkynoates via a Net C−H Insertion. J. Am. Chem. Soc. 2003, 125, 4518–4526. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Piao, D.; Kitamura, T.; Fujiwara, Y. New Method for Preparation of Coumarins and Quinolinones via Pd-Catalyzed Intramolecular Hydroarylation of C−C Triple Bonds. J. Org. Chem. 2000, 65, 7516–7522. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Piao, D.; Oyamada, J.; Lu, W.; Kitamura, T.; Fujiwara, Y. Efficient activation of aromatic C–H bonds for addition to C–C multiple bonds. Science 2000, 287, 1992–1995. [Google Scholar] [CrossRef] [PubMed]
- Oyamada, J.; Jia, C.; Fujiwara, Y.; Kitamura, T. Direct synthesis of coumarins by Pd(II)-catalyzed reaction of alkoxyphenols and alkynoates. Chem. Lett. 2002, 31, 380–381. [Google Scholar] [CrossRef]
- Kotani, M.; Yamamoto, K.; Oyamada, J.; Fujiwara, Y.; Kitamura, T. A convenient synthesis of coumarins by palladium(II)-catalyzed reaction of phenols with propiolic acids. Synthesis 2004, 2004, 1466–1470. [Google Scholar] [CrossRef]
- Oyamada, J.; Kitamura, T. Synthesis of coumarins by Pt-catalyzed hydroarylation of propiolic acids with phenols. Tetrahedron 2006, 62, 6918–6925. [Google Scholar] [CrossRef]
- Shi, Z.; He, C. Efficient Functionalization of Aromatic C−H Bonds Catalyzed by Gold(III) under Mild and Solvent-Free Conditions. J. Org. Chem. 2004, 69, 3669–3671. [Google Scholar] [CrossRef] [PubMed]
- Gadakh, S.K.; Dey, S.; Sudalai, A. Rh-Catalyzed Synthesis of Coumarin Derivatives from Phenolic Acetates and Acrylates via C–H Bond Activation. J. Org. Chem. 2015, 80, 11544–11550. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Neumann, H.; Beller, M. Synthesis of heterocycles via palladium-catalyzed carbonylations. Chem. Rev. 2012, 113, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ju, T.; Miao, M.; Han, J.-L.; Zhang, Y.-H.; Zhu, X.-Y.; Ye, J.-H.; Yu, D.-G.; Zhi, Y.-G. Transition-Metal-Free Lactonization of sp2 C–H Bonds with CO2. Org. Lett. 2017, 19, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.; Zeng, F.; Alper, H. Synthesis of coumarins via Pd-catalyzed oxidative cyclocarbonylation of 2-vinylphenols. Org. Lett. 2012, 14, 5602–5605. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-G.; Zhang, S.-S.; Jiang, C.-Y.; Wu, J.-Q.; Li, Q.; Wang, H. Cp*Co(III)-Catalyzed Annulations of 2-Alkenylphenols with CO: Mild Access to Coumarin Derivatives. Org. Lett. 2015, 17, 5404–5407. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, T.; Choi, J.-C.; Yasuda, H. Transformation of carbon dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, Y.; Fujihara, T. Carbon dioxide as a carbon source in organic transformation: Carbon–carbon bond forming reactions by transition-metal catalysts. Chem. Commun. 2012, 48, 9956–9964. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. Technological use of CO2. Chem. Rev. 2013, 114, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Sasano, K.; Takaya, J.; Iwasawa, N. Palladium(II)-catalyzed direct carboxylation of alkenyl C–H bonds with CO2. J. Am. Chem. Soc. 2013, 135, 10954–10957. [Google Scholar] [CrossRef] [PubMed]
- Bissel, P.; Nazih, A.; Sablong, R.; Lepoittevin, J.-P. Stereoselective synthesis of (R)-and (S)-4-methoxydalbergione via asymmetric catalytic hydrogenation. Org. Lett. 1999, 1, 1283–1285. [Google Scholar] [CrossRef]
- Harikrishna, K.; Rakshit, A.; Aidhen, I.S. Study of the chemoselectivity of Grignard reagent addition to substrates containing both nitrile and Weinreb amide functionalities. Eur. J. Org. Chem. 2013, 2013, 4918–4932. [Google Scholar] [CrossRef]
- Gallagher, B.D.; Taft, B.R.; Lipshutz, B.H. Asymmetric conjugate reductions of coumarins. A new route to tolterodine and related coumarin derivatives. Org. Lett. 2009, 11, 5374–5377. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cui, S. Rh(III)-catalyzed aldehyde C–H bond functionalization of salicylaldehydes with arylboronic acids. Tetrahedron 2015, 71, 8511–8516. [Google Scholar] [CrossRef]
- Upadhyay, P.K.; Kumar, P. A novel synthesis of coumarins employing triphenyl (α-carboxymethylene) phosphorane imidazolide as a C-2 synthon. Tetrahedron Lett. 2009, 50, 236–238. [Google Scholar] [CrossRef]
- Taylor, R.T.; Cassell, R.A. Trimethylsilylketene: Synthesis of coumarins via cyclization-elimination. Synthesis 1982, 1982, 672–673. [Google Scholar] [CrossRef]
- Sethna, S.M.; Shah, N.M. The Chemistry of Coumarins. Chem. Rev. 1945, 36, 1–62. [Google Scholar] [CrossRef]
- Kawase, Y.; Yamaguchi, S.; Aoyama, K.; Matsuda, M. The Fries Rearrangement of bz-Benzoyloxybenzofuran Derivatives and the Synthesis of the Furo Derivatives of 4-Phenyl-2H-chromen-2-one. B. Chem. Soc. Jpn. 1978, 51, 1907–1908. [Google Scholar] [CrossRef]
- Gaspar, A.; Matos, M.J.O.; Garrido, J.; Uriarte, E.; Borges, F. Chromone: A valid scaffold in medicinal chemistry. Chem. Rev. 2014, 114, 4960–4992. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.-T.; Lee, S.-A.; Hwang, J.-S.; Lee, K.-I. A facile synthesis of highly functionalized 4-arylcoumarins via kostanecki reactions mediated by DBU. Molecules 2011, 16, 6313–6321. [Google Scholar] [CrossRef] [PubMed]
- Crecente-Campo, J.; Pilar Vázquez-Tato, M.; Seijas, J.A. Microwave-Promoted, One-Pot, Solvent-Free Synthesis of 4-Arylcoumarins from 2-Hydroxybenzophenones. Eur. J. Org. Chem. 2010, 2010, 4130–4135. [Google Scholar] [CrossRef]
- Sridhar Reddy, M.; Thirupathi, N.; Babu, M.H. Synthesis of Substituted Coumarins and 2-Quinolinones by Cycloisomerisation of (Hydroxy/aminophenyl) propargyl Alcohols. Eur. J. Org. Chem. 2012, 2012, 5803–5809. [Google Scholar] [CrossRef]
- Li, J.; Chen, H.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Synthesis of coumarins via PIDA/I2-mediated oxidative cyclization of substituted phenylacrylic acids. RSC Adv. 2013, 3, 4311–4320. [Google Scholar] [CrossRef]
- Wattanasin, S. Novel Route to 4-Aryl Coumarins. Synth. Commun. 1988, 18, 1919–1925. [Google Scholar] [CrossRef]
- Schio, L.; Chatreaux, F.; Klich, M. Tosylates in palladium-catalysed coupling reactions. Application to the synthesis of arylcoumarin inhibitors of gyrase B. Tetrahedron Lett. 2000, 41, 1543–1547. [Google Scholar] [CrossRef]
- Ciattini, P.G.; Morera, E.; Ortar, G. 4-Stannylcoumarins as Useful Reagents in a New Approach to Neoflavonoids. Synth. Commun. 1995, 25, 2883–2894. [Google Scholar] [CrossRef]
- Lee, D.-H.; Qian, Y.; Park, J.-H.; Lee, J.-S.; Shim, S.-E.; Jin, M.-J. A Highly Active and General Catalyst for the Stille Coupling Reaction of Unreactive Aryl, Heteroaryl, and Vinyl Chlorides under Mild Conditions. Adv. Synth. Catal. 2013, 355, 1729–1735. [Google Scholar] [CrossRef]
- Boland, G.M.; Donnelly, D.M.X.; Finet, J.P.; Rea, M.D. Synthesis of neoflavones by Suzuki arylation of 4-substituted coumarins. J. Chem. Soc. Perkin Trans. 1 1996, 2591–2597. [Google Scholar] [CrossRef]
- Chen, L.; Hu, T.S.; Yao, Z.J. Development of New Pyrrolocoumarin Derivatives with Satisfactory Fluorescent Properties and Notably Large Stokes Shifts. Eur. J. Org. Chem. 2008, 6175–6182. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Xia, H.-G. Palladium-catalyzed Suzuki-Miyaura coupling of potassium aryl trifluoroborates with 4-tosyloxycoumarins or 4-tosyloxyquinolin-2(1H)-one. Tetrahedron Lett. 2006, 47, 1525–1528. [Google Scholar] [CrossRef]
- Zhang, L.A.; Meng, T.H.; Fan, R.H.; Wu, H. General and efficient route for the synthesis of 3,4-disubstituted coumarins via Pd-catalyzed site-selective cross-coupling reactions. J. Org. Chem. 2007, 72, 7279–7286. [Google Scholar] [CrossRef] [PubMed]
- Akrawi, O.A.; Nagy, G.Z.; Patonay, T.; Villinger, A.; Langer, P. Chemoselective Suzuki–Miyaura reactions of 4-trifluoromethylsulfonyloxy-6-bromocoumarin. Tetrahedron Lett. 2012, 53, 3206–3209. [Google Scholar] [CrossRef]
- Khaddour, Z.; Akrawi, O.A.; Suleiman, A.S.; Patonay, T.; Villinger, A.; Langer, P. Regioselective Suzuki-Miyaura cross-coupling reactions of the bis(triflate) of 4,7-dihydroxycoumarin. Tetrahedron Lett. 2014, 55, 4421–4423. [Google Scholar] [CrossRef]
- Rajale, T.; Sharma, S.; Stroud, D.A.; Unruh, D.K.; Miaou, E.; Lai, K.; Birney, D.M. An efficient synthesis of 4-substituted coumarin derivatives via a palladium-catalyzed Suzuki cross-coupling reaction. Tetrahedron Lett. 2014, 55, 6627–6630. [Google Scholar] [CrossRef]
- Wu, J.; Liao, Y.; Yang, Z. Synthesis of 4-substituted coumarins via the palladium-catalyzed cross-couplings of 4-tosylcoumarins with terminal acetylenes and organozinc reagents. J. Org. Chem. 2001, 66, 3642–3645. [Google Scholar] [CrossRef] [PubMed]
- Rieke, R.D.; Kim, S.H. A novel organozinc reagent 4-coumarinylzinc bromide; preparation and application in the synthesis of 4-substituted coumarin derivatives. Tetrahedron Lett. 2011, 52, 3094–3096. [Google Scholar] [CrossRef]
- Rao, M.L.N.; Venkatesh, V.; Jadhav, D.N. Palladium-Catalyzed Synthesis of 4-Arylcoumarins Using Triarylbismuth Compounds as Atom-Efficient Multicoupling Organometallic Nucleophiles. Eur. J. Org. Chem. 2010, 3945–3955. [Google Scholar] [CrossRef]
- Rao, M.L.N.; Kumar, A. Pd-catalyzed chemo-selective mono-arylations and bis-arylations of functionalized 4-chlorocoumarins with triarylbismuths as threefold arylating reagents. Tetrahedron 2014, 70, 6995–7005. [Google Scholar] [CrossRef]
- Shah, P.; Santana, M.D.; Garcia, J.; Serrano, J.L.; Naik, M.; Pednekar, S.; Kapdi, A.R. [Pd(PPh3)2(saccharinate)2]-general catalyst for Suzuki-Miyaura, Negishi cross-coupling and C–H bond functionalization of coumaryl and pyrone substrates. Tetrahedron 2013, 69, 1446–1453. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Z. Nickel-catalyzed cross-couplings of 4-diethylphosphonooxycoumarins with organozinc reagents: An efficient new methodology for the synthesis of 4-substituted coumarins. J. Org. Chem. 2001, 66, 7875–7878. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.G.; Xu, M.H.; Lin, G.Q. Nickel-catalyzed cross-coupling reactions of 4-mesylcoumarins with aryl halides: Facile synthesis of 4-substituted coumarins. Synlett. 2004, 2364–2368. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Hu, Q.S. Room temperature nickel(0)-catalyzed Suzuki-Miyaura cross-couplings of activated alkenyl tosylates: Efficient synthesis of 4-substituted coumarins and 4-substituted 2(5H)-furanones. Adv. Synth. Catal. 2004, 346, 1635–1637. [Google Scholar] [CrossRef]
- Xing, C.H.; Lee, J.R.; Tang, Z.Y.; Zheng, J.R.; Hu, Q.S. Room Temperature Nickel(II) Complexes [(4-MeOC6H4)Ni(PCy3)2OTs and Ni(PCy3)2X2]-Catalyzed Cross-Coupling Reactions of Aryl/Alkenyl Sulfonates with Arylboronic Acids. Adv. Synth. Catal. 2011, 353, 2051–2059. [Google Scholar] [CrossRef]
- Xu, L.; Li, B.J.; Wu, Z.H.; Lu, X.Y.; Guan, B.T.; Wang, B.Q.; Zhao, K.Q.; Shi, Z.J. Nickel-Catalyzed Efficient and Practical Suzuki-Miyaura Coupling of Alkenyl and Aryl Carbamates with Aryl Boroxines. Org. Lett. 2010, 12, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, L.; Gao, K. RhCl(PPh3)3/DPPF: A useful and efficient catalyst for cross-coupling reactions of activated alkenyl tosylates with arylboronic acids. Eur. J. Org. Chem. 2006, 5260–5263. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Luo, Y. Rh(I)-catalyzed cross-coupling reactions of alkenyl tosylates with potassium aryltrifluoroborates. Tetrahedron Lett. 2006, 47, 6747–6750. [Google Scholar] [CrossRef]
- Khoobi, M.; Alipour, M.; Zarei, S.; Jafarpour, F.; Shafiee, A. A facile route to flavone and neoflavone backbones via a regioselective palladium catalyzed oxidative Heck reaction. Chem. Commun. 2012, 48, 2985–2987. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Qi, Z.S.; Wang, H.F.; Fu, X.M.; Duan, C.Y. Palladium-Catalyzed Oxidative Heck Coupling Reaction for Direct Synthesis of 4-Arylcoumarins Using Coumarins and Arylboronic Acids. J. Org. Chem. 2012, 77, 2053–2057. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Hong, S. Regioselective palladium-catalyzed direct cross-coupling of coumarins with simple arenes. Chem. Commun. 2012, 48, 9613–9615. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Ahn, K.; Hong, S. Site-Selective C−H Bond Functionalization of Chromones and Coumarins. Asian J. Org. Chem. 2018, 7, 1136–1150. [Google Scholar] [CrossRef]
- Khoobi, M.; Molaverdi, F.; Alipour, M.; Jafarpour, F.; Foroumadi, A.; Shafiee, A. Palladium-catalyzed domino protodecarboxylation/oxidative Heck reaction: Regioselective arylation of coumarin-3-carboxylic acids. Tetrahedron 2013, 69, 11164–11168. [Google Scholar] [CrossRef]
- Battistuzzi, G.; Cacchi, S.; Salve, I.D.; Fabrizi, G.; Parisi, L.M. Synthesis of Coumarins in a Molten n-Bu4NOAc/n-Bu4NBr Mixture through a Domino Heck Reaction/Cyclization Process. Adv. Synth. Catal. 2005, 347, 308–312. [Google Scholar] [CrossRef]
- Barancelli, D.A.; Salles, A.G., Jr.; Taylor, J.G.; Correia, C.R. Coumarins from free ortho-hydroxy cinnamates by Heck-Matsuda arylations: A scalable total synthesis of (R)-tolterodine. Org. Lett. 2012, 14, 6036–6039. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Han, J.; Wu, X.; Xu, S.; Wang, L. Synthesis of 4-arylcoumarins via palladium-catalyzed arylation/cyclization of ortho-hydroxylcinnamates with diaryliodonium salts. Tetrahedron Lett. 2015, 56, 3809–3812. [Google Scholar] [CrossRef]
- Perez, J.M.; Cano, R.; McGlacken, G.P.; Ramon, D.J. Palladium(II) oxide impregnated on magnetite as a catalyst for the synthesis of 4-arylcoumarins via a Heck-arylation/cyclization process. RSC Adv. 2016, 6, 36932–36941. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kirai, N. Synthesis of 4-arylcoumarins via Cu-catalyzed hydroarylation with arylboronic acids. Org. Lett. 2008, 10, 5513–5516. [Google Scholar] [CrossRef] [PubMed]




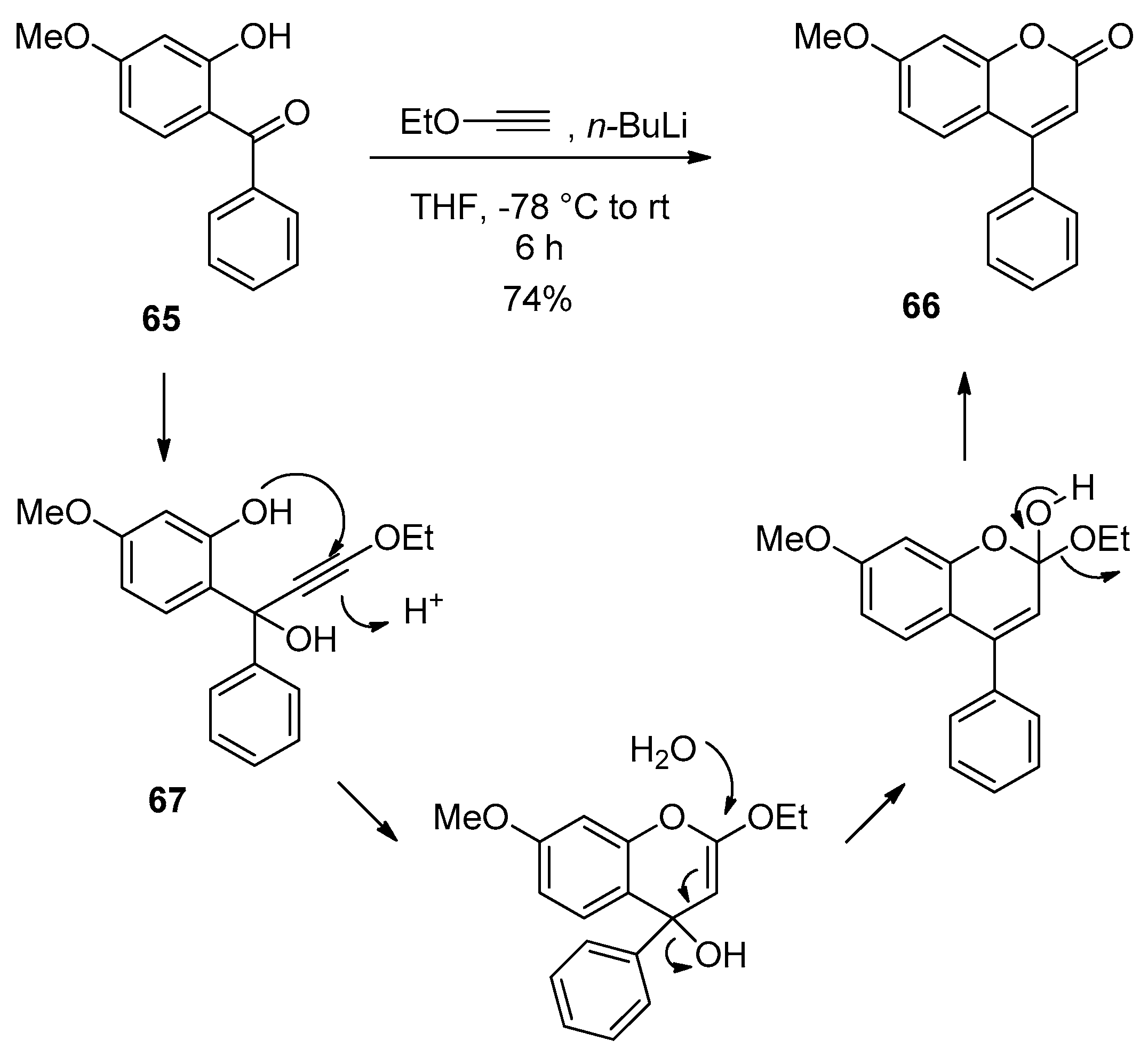
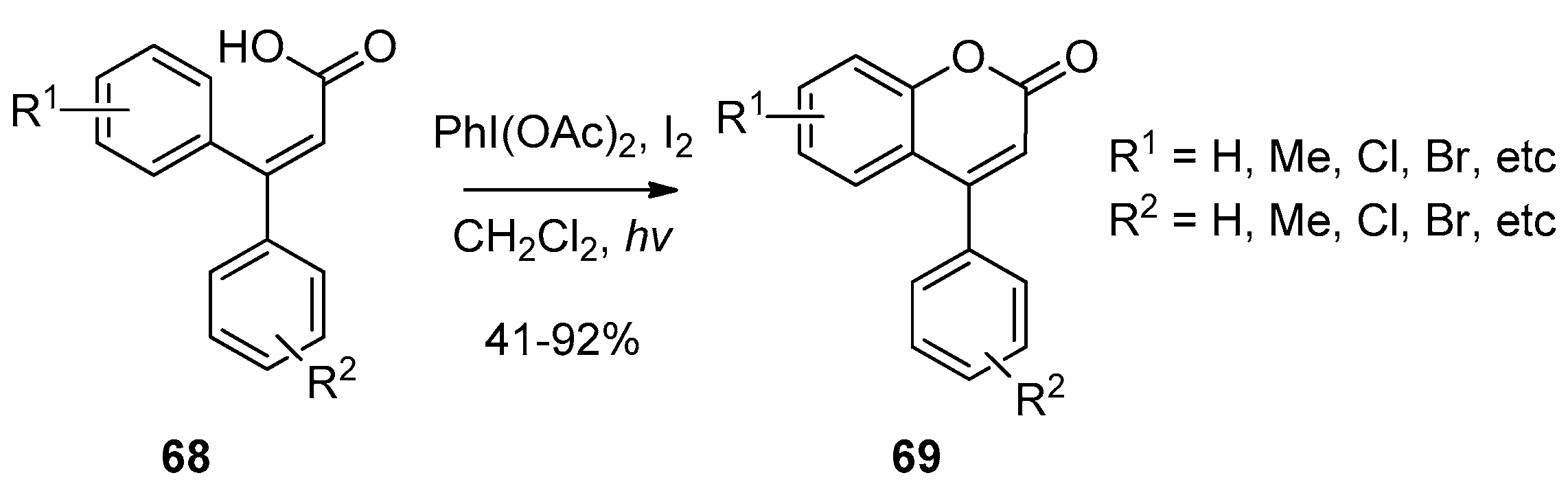
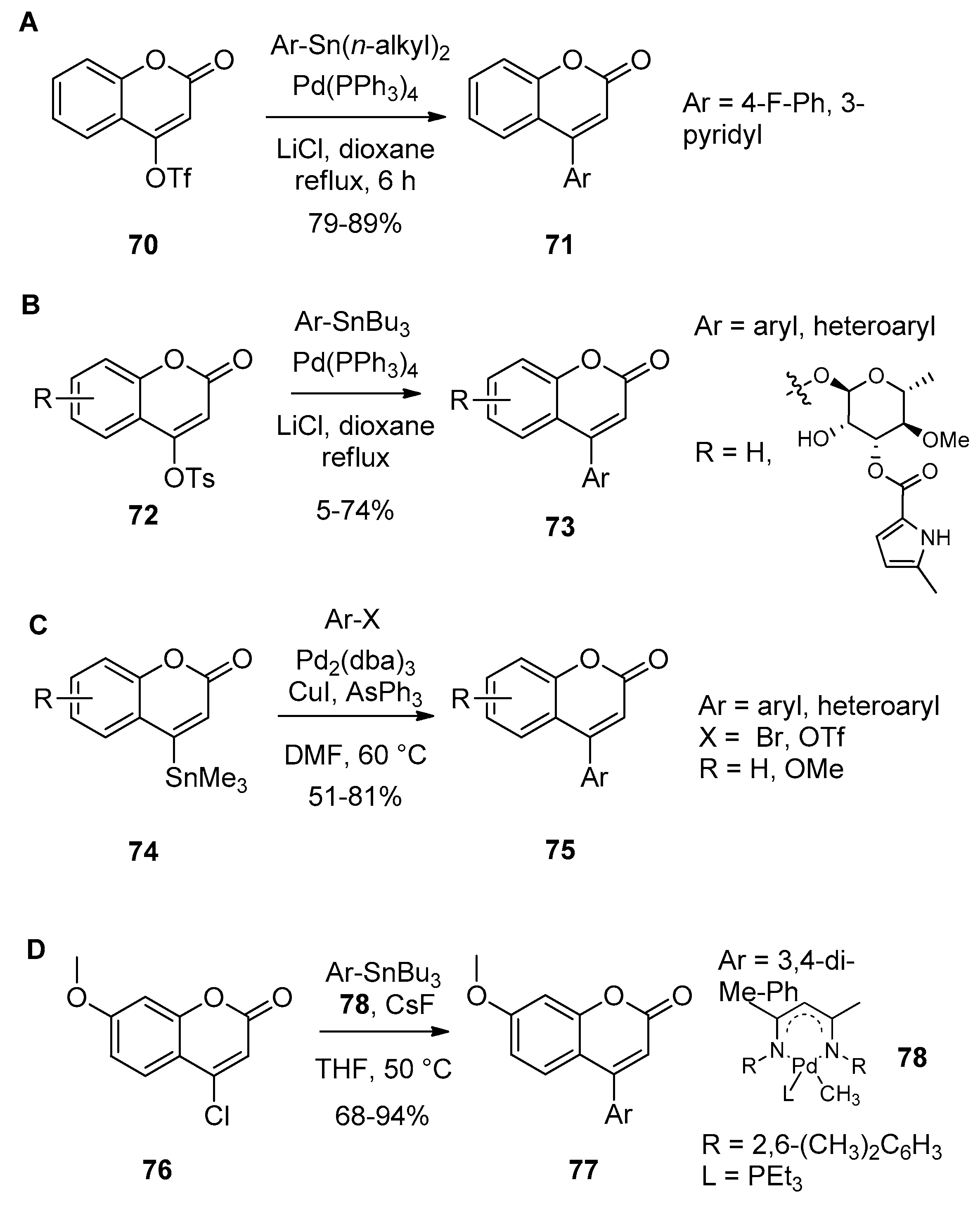
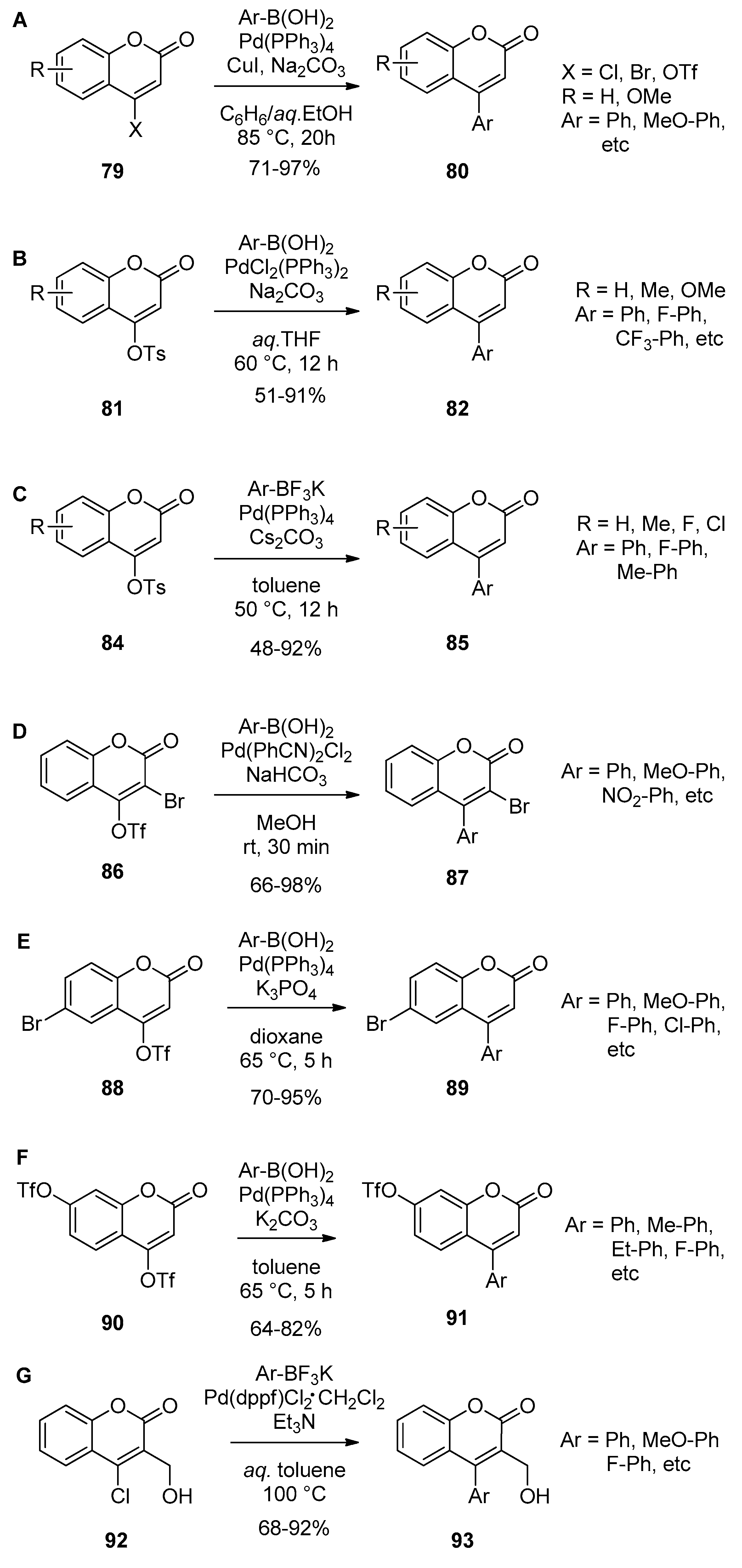





| Entry | R | Catalyst (mol%) | Temp. (°C) | Time | Yield (%) |
|---|---|---|---|---|---|
| 1 [13] | 3-OH | p-TsOH (5) | 60 | 10 min | 92 |
| 2 [14] | 3-OH, 2,3-di-OH, 3,5-di-OH | p-TsOH (200) | rt | 10 + 80 min a | 82–90 |
| 3 [15] | 3-OH, 2,3-di-OH | p-TsOH (5) | MW b | 30s | 82–92 |
| 4 [16] | 3-OH, 3,5-di-OH, 2-Me-3-OH | H2NSO3H (50) | 130 | 40–45 min | 40–45 |
| 5 [17] | 3-OH, 2,3-di-OH, 3,5-di-OH | Megluminesulfate c | 100 | 45–50 min | 88–90 |
| 6 [17] | 3-OH, 2,3-di-OH, 3,5-di-OH | Megluminesulfate c | MW d | 6–7 min | 88–92 |
| 7 [18] | 3-OH, 3,5-di-OH | Cl3CCO2H (30) | 100 | 90 min | 79 |
| 8 [19] | 3-OH, 3,5-di-OH, 2-Me-3-OH | SelectfluorTM (50) | 120 | 30–45 min | 85–93 |
| 9 [20] | 3-OH, 2,3-di-OH, 3,5-di-OH, 2-Me-3-OH | ZrCl4 (10) | rt | 10 min | 90–94 |
| 10 [21] | 3-OH, 3,5-di-OH, 2-Me-3-OH | VCl3 (10) | 50–55 | 2 h | 84–92 |
| 11 [22] | 2-OH, 3-OH, 2,3-di-OH, 3,5-di-OH, 2-Me-3-OH | SnCl4·5H2O (5) | 25 | 5–15 min | 86–94 |
| 12 [23] | 3-OH, 2,3-di-OH, 3,5-di-OH, 3-Me-5-OH | CuCl2 or CuBr2 (10) | 80 | 10–20 min | 80–97 |
| 13 [24] | 3-OH, 3-Me-5-OH | BaCl2 (10) | 100 | 50–55 min | 80–85 |
| 14 [25] | 3-OH, 2,3-di-OH, 3,5-di-OH | LiBr (10) | 75 | 15–30 min | 78–86 |
| 15 [26] | 3,5-di-OH, 3-Me-5-OH | BiCl3 (20) | US e | 35–40 min | 76–78 |
| 16 [27] | 3-OH | CoPy2Cl2 (1) | rt | 3 h | 92 |
| 17 [27] | 3-OH | CoPy2Cl2 (1) | MW f | 2.5 min | 96 |
| 18 [28] | 3,5-di-OH | Sc(OTf)3 (10) | 80 | 2 h | 89 |
| 19 [29] | H, 3-OH, 3,5-di-OH | Mg(NTf2)2 (1) | 80 | 35–60 min | 85–96 |
| 20 [30] | 3-OH, 3,5-di-OH | Bi(NO3)3·5H2O (5) | 80 | 30 min | 78–88 |
| 21 [31] | 3-OH, 2,3-di-OH, 2-Me-3-OH, 3,5-di-OH | CAN | 110 | 10–15 min | 92–96 |
| 22 [31] | 3-OH, 2,3-di-OH, 2-Me-3-OH, 3,5-di-OH | CAN | MW g | 3 min | 94–97 |
| 23 [32] | 3,5-di-OH, 3-Me-5-OH | Y(NO3)3·6H2O (10) | 90 | 45–70 min | 80 |
| 24 [33] | 3-OH | Cu(CH3CN)4PF6 (10) | rt | 20 min | 82 |
| 25 [34] | 3-OH, 3-OMe, 3-Me-5-OH | MnSO4·H2O (20) | 100 | 50–100 min | 75–90 |
| 26 [35] | 3-OH, 2,3-di-OH, 3,5-di-OH | MSA g(5) | 100 | 40–45 min | 94–95 |
| 27 [36] | 3-OH, 3-OMe, 3,5-di-OH, 3,5-di-Me, 1-naphthol | pyridine dicarboxylic acid (5) + ZnO (5) | reflux | 4–7 h | 76, 85–88 |
| 28 [37] | 2-OH, 3-OH, 2,3-OH, 1-naphthol | WO3-ZrO2 h | MW i | 90–150 s | 82–90 |
| Entry | R | Catalyst (mol%) | Temp. (°C) | Time | Yield (%) |
|---|---|---|---|---|---|
| 1 [38] | H, 3-OH, 3,5-di-OH, 3-OMe | SiO2-Cl a | 80 | 1–3 h | 67–93 |
| 2 [40] | 3-OH, 2,3-di-OH | H2SO4·SiO2 b | MW c | 5–7 min | 80–84 |
| 3 [39] | 3-OH, 2,3-di-OH, 3,5-di-OH | H2SO4·SiO2 d | rt | 5 + 10 min e | 80–90 |
| 4 [41] | 3-OH, 3,5-di-OH, 3-methyl-5-OH | ZrOCl2·8H2O/SiO2 (10) | 90 | 40–80 min | 80–94 |
| 5 [42] | 2,3-di-OH, 3,5-di-OH | BTSA·SiO2 f (40) | 85 | 1–25 min | 89–90 |
| 6 [43] | 3-OH, 2,3-di-OH, 3,5-di-OH, 2-Me-3-OH | SnClx-SiO2 g (5) | 120 | 3–5 h | 84–91 |
| 7 [44] | 3-OH | ASA h | 100 | 140 min | 91 |
| 8 [45] | 3-OH | PVSA i (10) | rt | 25 h | 91 |
| 9 [46] | 3-OH | XSA j | rt | 20 min | 96 |
| 10 [47] | 3-OH | CSA k | rt | 20 min | 96 |
| 11 [47] | 3-OH | CSA k | MW l | 2 min | 97 |
| 12 [48] | 2,3-di-OH, 3,5-di-OH, 3-methyl-5-OH | MNESA m (0.3) | 120 | 3–6 h | 66–71 |
| 13 [49] | 3-OH, 2,3-di-OH, 3,5-di-OH, 2-Me-3-OH | γ-Fe2O3@HAp-Ag NPs n | 80 | 30–42 min | 85–96 |
| 14 [50] | 3-OH, 3,5-di-OH, 2-Me-3-OH | Alum o (40) | 80 | 2–2.5 h | 90–95 |
| Entry | R | R′ | Cat. (mol%) | Solvent | Temp (°C) | Time | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 [52] | 3-OH, 3,5-di-OH, 3,4-methylenedioxy | H | InCl3(~12) | Free | 90 | 2 h | 21–55 |
| 2 [53] | 3-OH, 3,5-di-OH, 3,5-di-OMe | H | ZnCl2 (5) | Free | 100 | 5 min–12 h | 54–95 |
| 3 [54] | H, 3-OH, 3-OMe, 4-Me, 4-OMe, | H, Me, OMe | H14P5NaW30O110 (0.5) | Free | 130 | 2 h | 66–90 |
| 4 [55] | 4-t-Bu a | H | FeCl3 (20) | CH3NO2 | 80 | 72 h | 53 |
| 5 [56] | 3-OMe, 3,5-di-Me, 1-naphthol | H | FeCl3 (20) + AgOTf (60) + TFA b | DCE | 60 | 15 h | 75–80 |
| 6 [57] | H, 2-F, 3-NO2, 4-Cl | H | Yb(OTf)3 (10) | Free | MW c | 2 min | 91–98 |
| 7 [58] | H, 3-OH, 3,5-di-OH | H | WD/SiO2 d | Free | MW e | 10 min | 54–99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.-W.; Kim, N.-J.; Yun, H.; Han, Y.T. Recent Advances in Synthesis of 4-Arylcoumarins. Molecules 2018, 23, 2417. https://doi.org/10.3390/molecules23102417
Jung J-W, Kim N-J, Yun H, Han YT. Recent Advances in Synthesis of 4-Arylcoumarins. Molecules. 2018; 23(10):2417. https://doi.org/10.3390/molecules23102417
Chicago/Turabian StyleJung, Jong-Wha, Nam-Jung Kim, Hwayoung Yun, and Young Taek Han. 2018. "Recent Advances in Synthesis of 4-Arylcoumarins" Molecules 23, no. 10: 2417. https://doi.org/10.3390/molecules23102417
APA StyleJung, J.-W., Kim, N.-J., Yun, H., & Han, Y. T. (2018). Recent Advances in Synthesis of 4-Arylcoumarins. Molecules, 23(10), 2417. https://doi.org/10.3390/molecules23102417





