Design, Synthesis and Docking Studies of Novel Macrocyclic Pentapeptides as Anticancer Multi-Targeted Kinase Inhibitors
Abstract
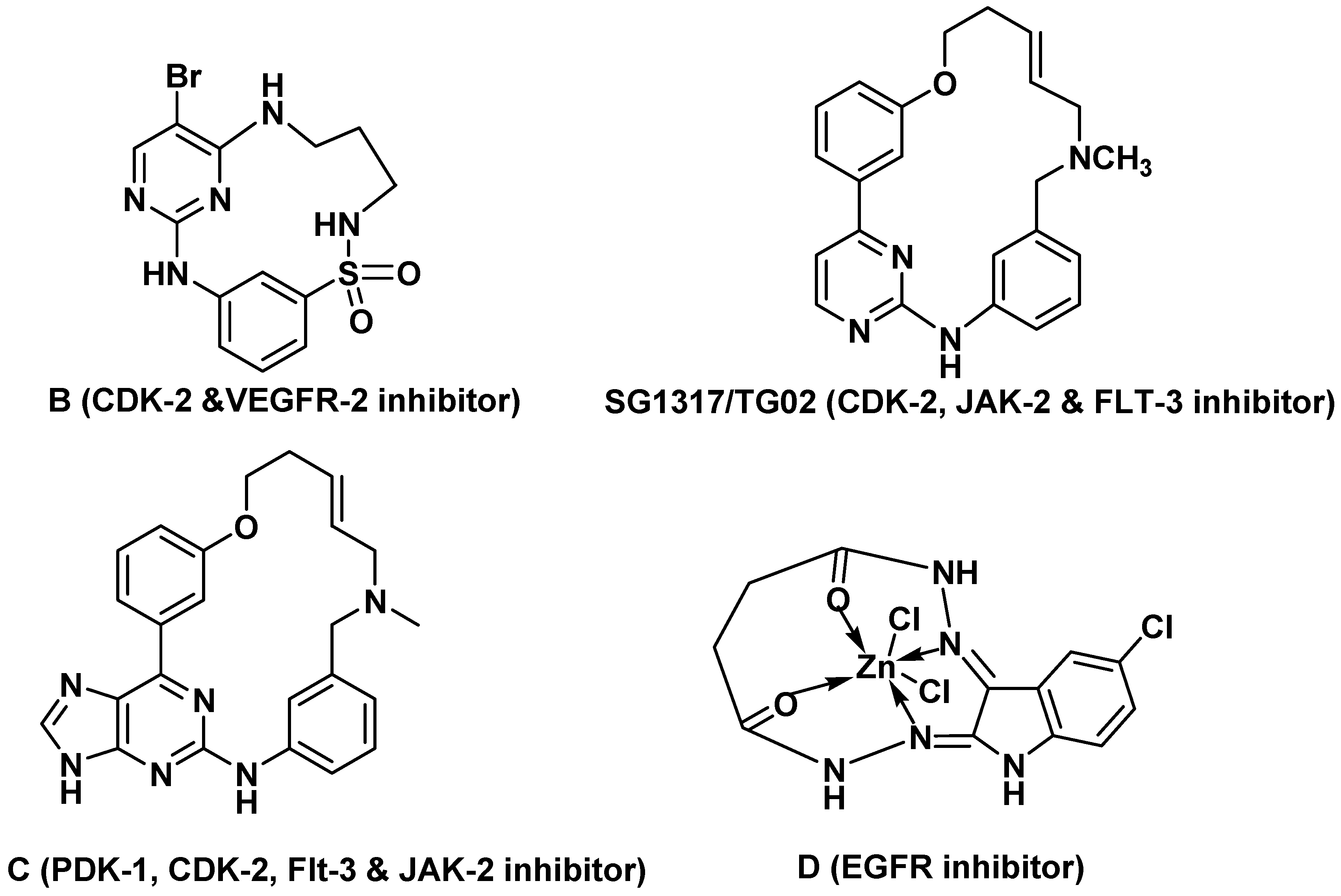
1. Introduction
2. Results and Discussion
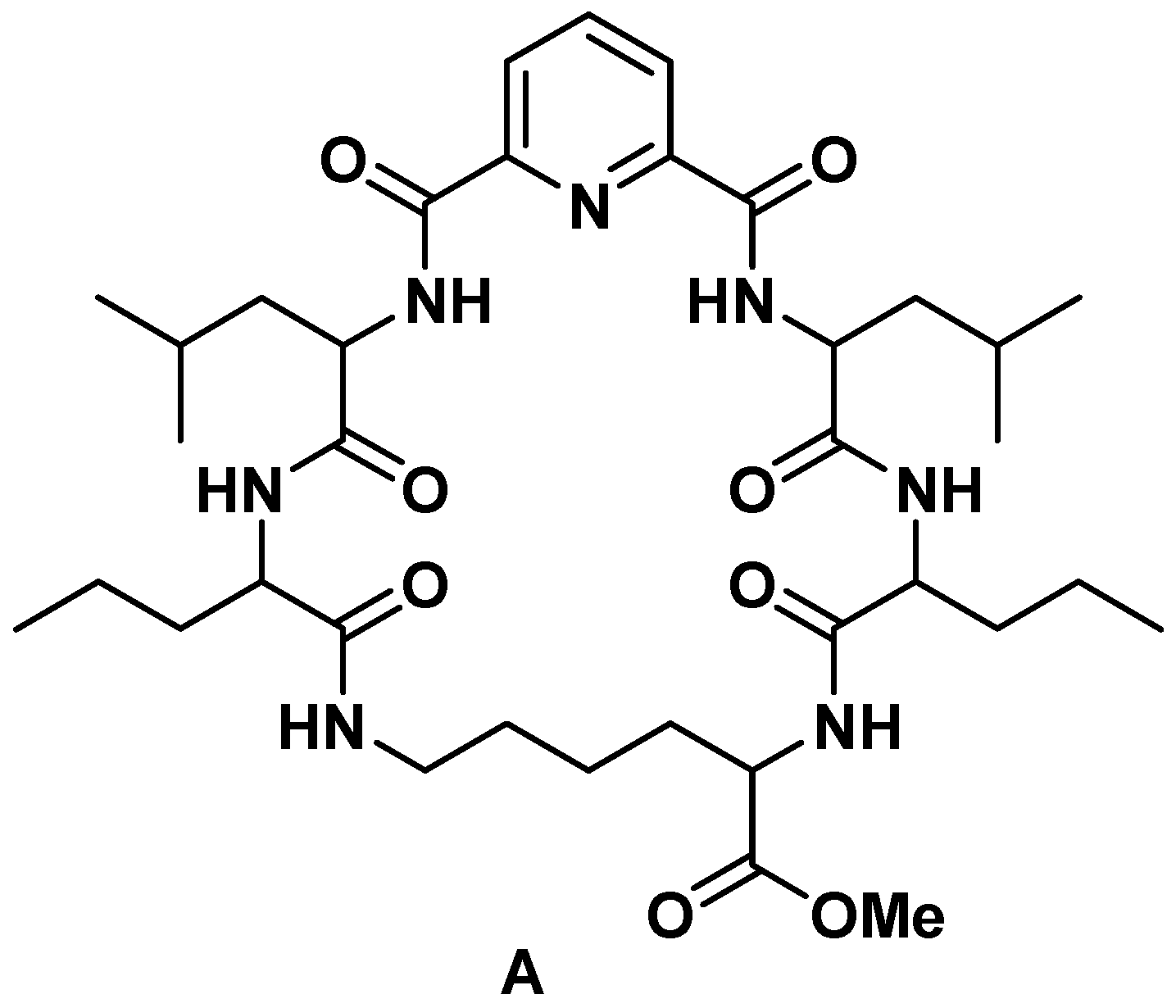
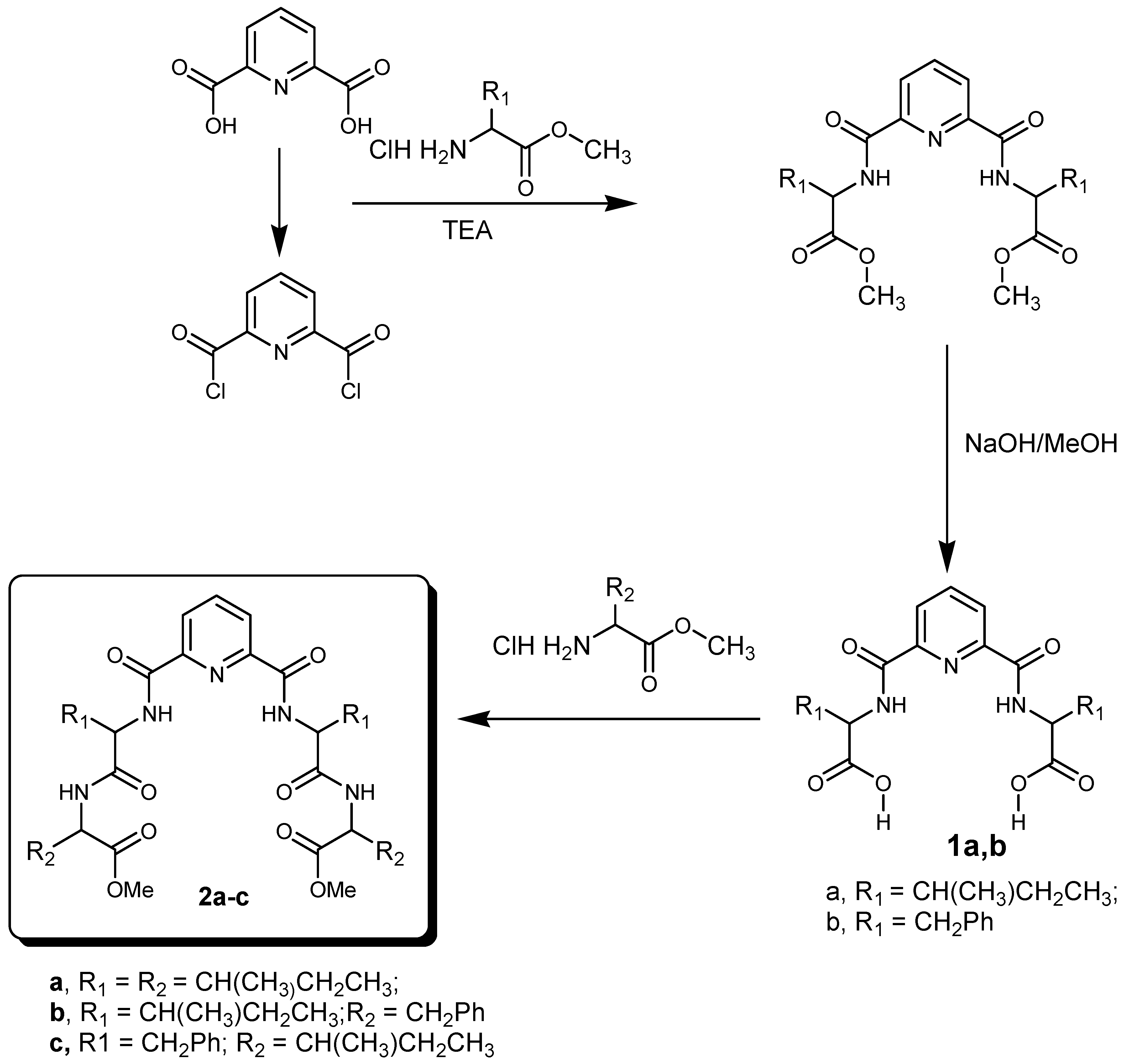
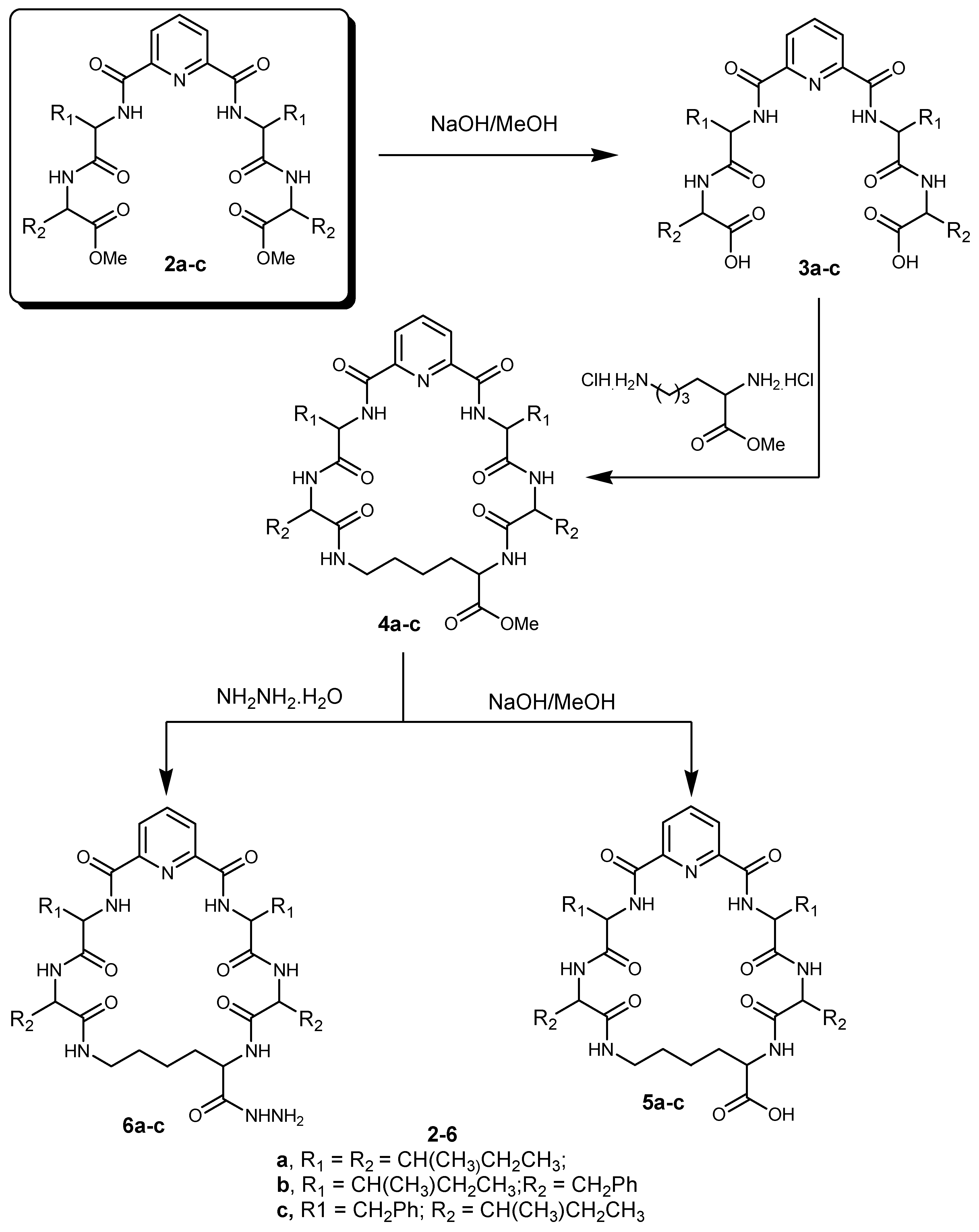
2.1. Chemistry
2.2. Anticancer Activity
2.3. In Vitro Enzymatic Assays
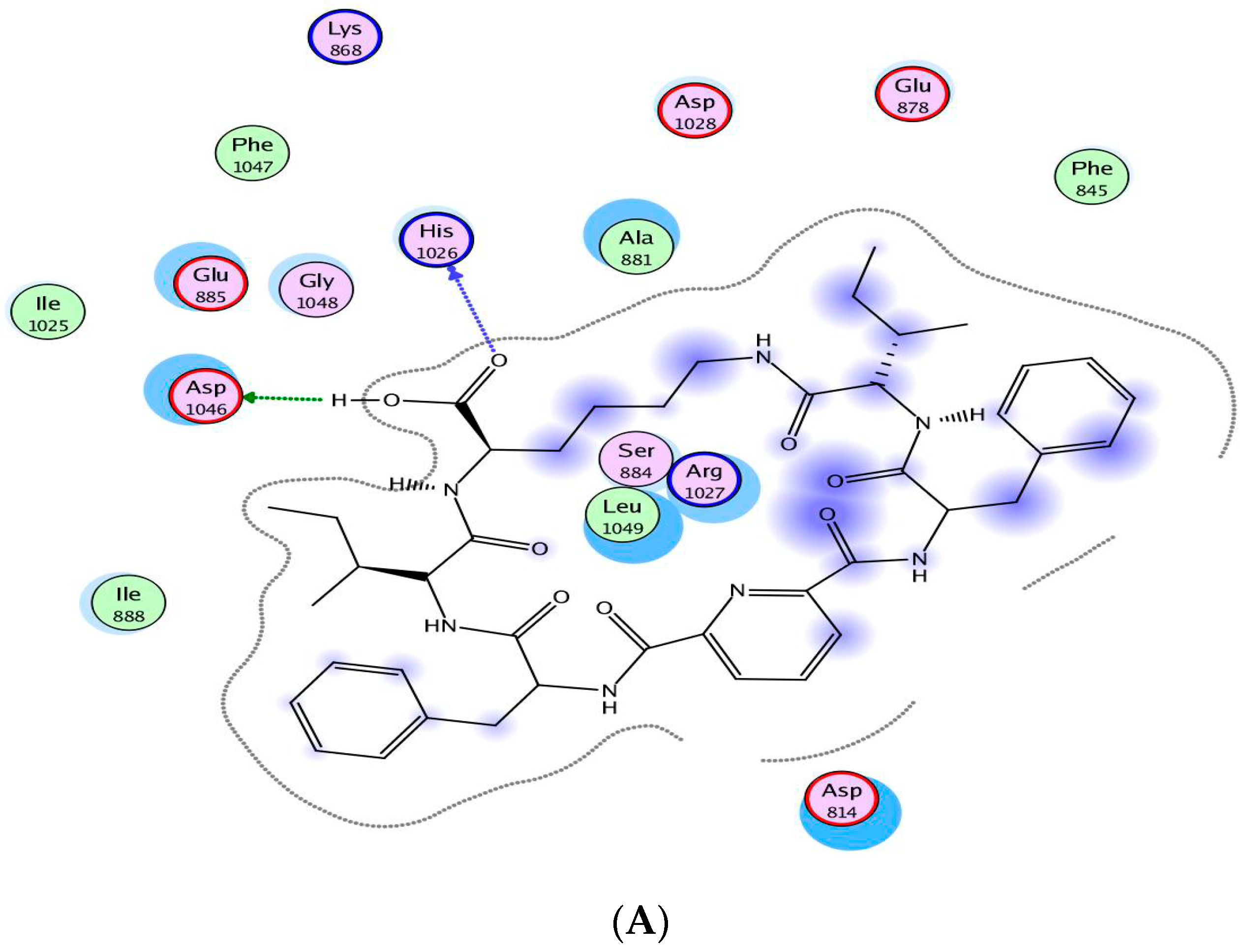
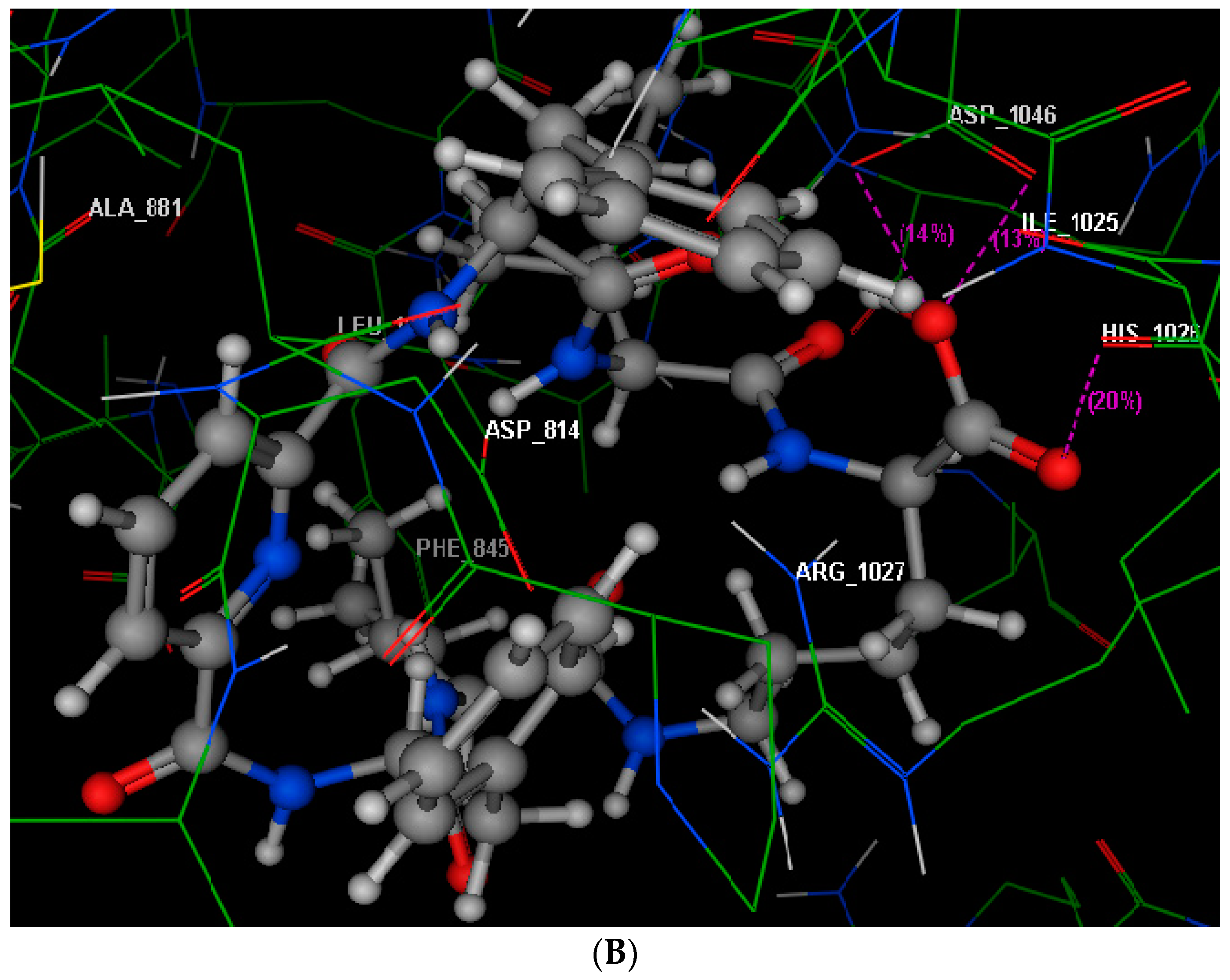
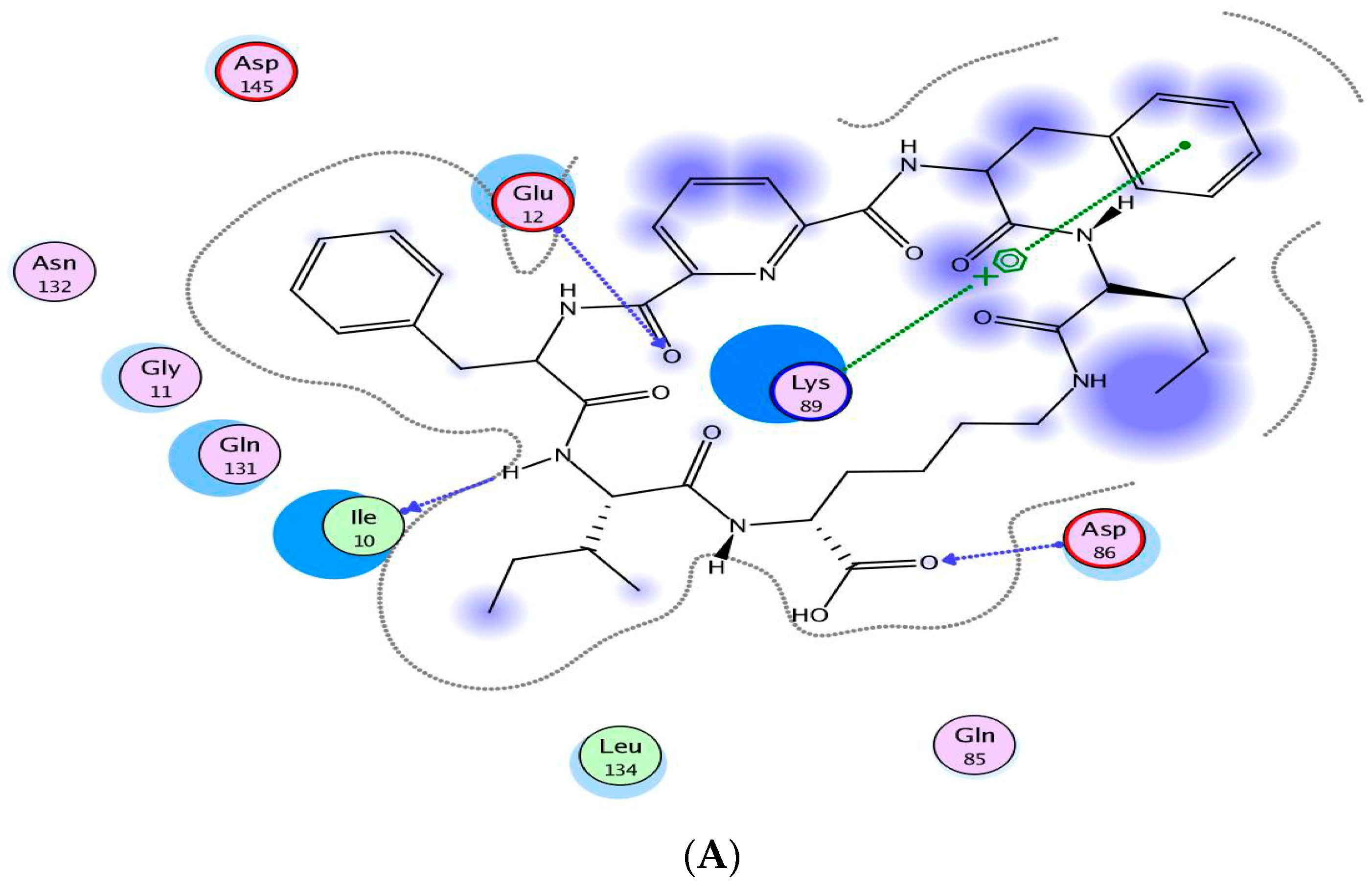
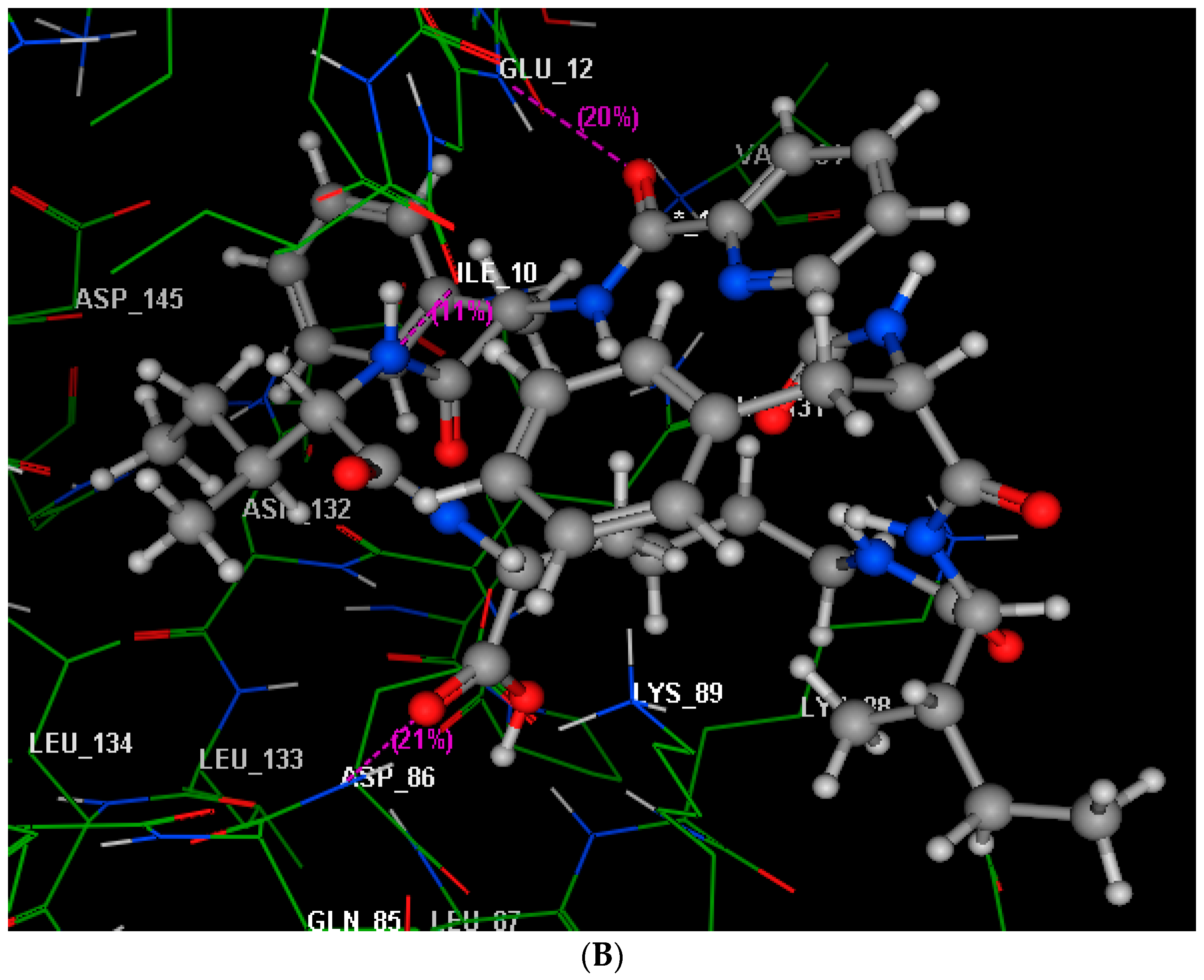
2.4. Molecular Modeling Studies
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of Nα-dipicolinoyl-bis[dipeptide methyl ester] Derivatives (2a–c)
3.1.2. Synthesis of Nα-Dipicolinoyl-bis[dipeptide]derivatives (3a–c)
3.1.3. Synthesis of Cyclo-(Nα-Dipicolinoyl)-bis-[dipeptide]-l-Lys-OMe (cyclic pentapeptide methyl esters) (4a–c)
3.1.4. Synthesis of Cyclo-(Nα-dipicolinoyl)-bis[dipeptide]-l-Lys-OH (cyclic pentapeptides) (5a–c)
3.1.5. Synthesis of Cyclo-(Nα-dipicolinoyl)-bis[dipeptide]-l-Lys-NHNH2 (cyclic pentapeptide hydrazides) (6a–c)
3.2. Anticancer Screening
3.3. In Vitro Enzymatic Assays
3.4. Molecular Modeling Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dissanayake, S.; Denny, W.A.; Gamage, S.; Sarojini, V. Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides. J. Controll. Release 2017, 250, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Driggers, E.M.; Hale, S.P.; Lee, J.; Terrett, N.K. The exploration of macrocycles for drug discovery—An underexploited structural class. Nat. Rev. Drug Discov. 2008, 7, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Krahn, D.; Ottmann, C.; Kaiser, M. Macrocyclic proteasome inhibitors. Curr. Med. Chem. 2011, 18, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Marsault, E.; Peterson, M.L. Macrocycles Are Great Cycles: Applications, Opportunities, and Challenges of Synthetic Macrocycles in Drug Discovery. J. Med. Chem. 2011, 54, 1961–2004. [Google Scholar] [CrossRef] [PubMed]
- Erb, W.; Zhu, J. From natural product to marketed drug: The tiacumicin odyssey. Nat. Prod. Rep. 2013, 30, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Mallinson, J.; Collins, I. Macrocycles in new drug discovery. Future Med. Chem. 2012, 4, 1409–1438. [Google Scholar] [CrossRef] [PubMed]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Amr, A.E.; Abo-Ghaliaa, M.H.; Abdalah, M.M. Synthesis of novel macrocyclic peptido-calix[4]arenes and peptidopyridines as precursors for potential molecular metallacages, chemosensors and biologically active candidates. Z. Naturforsch. 2006, 61b, 1335–1345. [Google Scholar] [CrossRef]
- Amr, A.E.; Abdel-Salam, O.I.; Attia, A.; Stibor, I. Synthesis of new potential bis-intercallators based on chiral pyridine-2,6-dicarbox-amides. Collect. Czech Chem. Commun. 1999, 64, 288–298. [Google Scholar] [CrossRef]
- Attia, A.; Abdel-Salam, O.I.; Amr, A.E.; Stibor, I.; Budesinsky, M. Synthesis and antimicrobial activity of some new chiral bridged macrocyclic pyridines. Egypt. J. Chem. 2000, 43, 187–201. [Google Scholar] [CrossRef]
- Naglah, A.M.; Moustafa, G.O.; Al-Omar, M.A.; Al-Salem, H.S.A.; Hozzein, W.N. Synthesis, characterization and in vitro antimicrobial investigation of novel amino acids and dipeptides based on dibenzofuran-2-sulfonyl-chloride. J. Comput. Theor. Nanosci. 2017, 14, 3183–3190. [Google Scholar] [CrossRef]
- Al-Omar, M.A.; Amr, A.E. Synthesis of some new pyridine-2,6-carboxamide-derived Schiff Bases as potential antimicrobial agents. Molecules 2010, 15, 4711–4721. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Salam, O.I.; Al-Omar, M.A.; Fayed, A.A.; Flefel, E.M.; Amr, A.E. Synthesis of new macrocyclic polyamides as antimicrobial agent candidates. Molecules 2012, 17, 14510–14521. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, H.S.A.; Naglah, A.M.; Moustafa, G.O.; Mahmoud, A.Z.; Al-Omar, M.A. Synthesis of novel tripeptides based on dibenzofuran-2-sulfonyl-[aromatic and hydroxy aromatic residues]: Towards antimicrobial and antifungal agents. J. Comput. Theor. Nanosci. 2017, 14, 3958–3966. [Google Scholar] [CrossRef]
- Moustafa, G.; Khalaf, H.; Naglah, A.; Al-Wasidi, A.; Al-Jafshar, N.; Awad, H. Synthesis, molecular docking studies, in vitro antimicrobialand antifungal activities of novel dipeptide derivatives based on n-(2-(2-hydrazinyl-2-oxoethylamino)-2-oxoethyl)-nicotinamide. Molecules 2018, 23, 761. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, S.; Amr, A.E. Synthesis and biological activities of some new (Nα-dinicotinoyl)-bis-l- leucyl lnear and macrocyclic peptides. Molecules 2014, 19, 10698–10716. [Google Scholar] [CrossRef] [PubMed]
- Amr, A.E.; Abo-Ghalia, M.H.; Abdalah, M.M. Synthesis of new (Nα-dipicolinoyl)-bis-l-valyl-l-phenyl linear and macrocyclic bridged peptides as anti-inflammatory agents. Arch. Pharm. Chem. Life Sci. 2007, 340, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Abo-Ghalia, M.H.; Amr, A.E. Synthesis and investigation of a new cyclo (Nα-pentapeptide of a breast and CNS cytotoxic activity and an ionophoric specificity. Amino Acids 2004, 26, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Masereel, B.; Dupont, L.; Laeckmann, D.; Liégeois, J.F.; Pirotte, B.; de Tullio, P.; Delarge, J. Synthesis and pharmacology of pyrid-3-ylsulfonylcyanoguanidines as diuretic. Eur. J. Med. Chem. 1995, 30, 235–240. [Google Scholar] [CrossRef]
- Abo-Ghalia, M.H.; Moustafa, G.O.; Alwasidi, A.S.; Naglah, A.M. Cytotoxic investigation of isophthaloyl cyclopentapeptides. Lat. Am. J. Pharm. 2017, 36, 1957–1962. [Google Scholar] [CrossRef]
- Moustafa, G.O.; El-Sawy, A.A.; Abo-Ghalia, M.H. Synthesis of novel cyclopeptide candidates: I-cyclo-[Nα-isophthaloyl-bis-(Glycine-amino acid)-l-lysine] derivatives with expected anticancer activity. Egypt. J. Chem. 2013, 5, 473–494. [Google Scholar] [CrossRef]
- Amr, A.E.; Mohamed, A.M.; Ibrahim, A.A. Synthesis of some new chiral tricyclic and macrocyclic pyridine derivatives as antimicrobial agents. Z. Naturforsch. 2003, 58b, 861–868. [Google Scholar] [CrossRef]
- Abo-Ghaliaa, M.H.; Amr, A.E.; Abdalah, M.M. Synthesis of some new (Nα-dipicolinoyl)-bis- l-leucyl-dl-norvalyl linear tetra and cyclic octa bridged peptides as new antiinflammatory agents. Z. Naturforsch. 2003, 58b, 903–910. [Google Scholar] [CrossRef]
- Patrick, G.L. An Introduction to Medicinal Chemistry, 3rd ed.; Oxford University Press Inc.: New York, NY, USA, 2005; pp. 489–553. ISBN 9780198749691. [Google Scholar]
- Hu, S.; Yu, H.; Zhao, L.; Liang, A.; Liu, L.; Zhang, H. Molecular docking and 3D-QSAR studies on checkpoint kinase 1 inhibitors. Med. Chem. Res. 2013, 22, 4992–5013. [Google Scholar] [CrossRef]
- Ali, S.; Singh, V.; Jain, P.; Tripathi, V. Synthesis, antibacterial, anticancer and molecular docking studies of macrocyclic metal complexes of dihydrazide and diketone. J. Saudi Chem. Soc. 2018. [Google Scholar] [CrossRef]
- Mariaule, G.; Belmont, P. Cyclin-Dependent Kinase Inhibitors as Marketed Anticancer Drugs: Where Are We Now? A Short Survey. Molecules 2014, 19, 14366–14382. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, J.; Ojha, R.; Singh, H.; Kaur, M.; Bedi, P.; Nepali, K. Design Strategies, Structure Activity Relationship and Mechanistic Insights for Purines as Kinase Inhibitors. Eur. J. Med. Chem. 2016, 112, 298–346. [Google Scholar] [CrossRef] [PubMed]
- Abo-Ghalia, M.H.; Abd El-Hamid, M.; Zweil, M.A.; Amr, A.E.; Moafi, S.A. Synthesis and reactions of new chiral linear and macrocyclic tetra- and penta-peptide candidates. Z. Naturforsch. B 2012, 67, 806–818. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE), 2008.10. Available online: https://www.chemcomp.com/MOE-Molecular_Operating_Environment.htm (accessed on 12 June 2018).
- Chemical Computing Group ULC. Available online: https://www.bloomberg.com/profiles/companies/1522230D:CN-chemical-computing-group-ulc (accessed on 23 June 2018).
- Conconi, M.T.; Marzaro, G.; Urbani, L.; Zanusso, I.; Di Liddo, R.; Castagliuolo, I.; Brun, P.; Tonus, F.; Ferrarese, A.; Guiotto, A.; et al. Quinazoline-based multi-tyrosine kinase inhibitors: Synthesis, modeling, antitumor and antiangiogenic properties. Eur. J. Med. Chem. 2013, 67, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Mctigue, M.; Murray, B.W.; Chen, J.H.; Deng, Y.; Solowiej, J.; Kania, R.S. Molecular Conformations, Interactions, and Properties Associated with Drug Efficiency and Clinical Performance Among Vegfr Tk Inhibitors. Proc. Natl. Acad. Sci. USA 2012, 109, 18281–18289. [Google Scholar] [CrossRef] [PubMed]
- Luecking, U.; Siemeister, G.; Schaefer, M.; Briem, H.; Krueger, M.; Lienau, P.; Jautelat, R. Macrocyclic Aminopyrimidines as Multitarget Cdk and Vegf-R Inhibitors with Potent Antiproliferative Activities. Chem. Med. Chem. 2007, 2, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; Elsayed, E.A.; El Dib, R.A.; Wadaan, M.; Ezzeldin, E.; Marzouk, M. Synthesis, characterization and cytotoxicity evaluation of 5-hydrazono-[1,2,4]triazolo[1,5-a]quinazolines (Part I). Lat. Am. J. Pharm. 2016, 35, 58–65. [Google Scholar]
- Al-Salahi, R.; Elsayed, E.A.; El Dib, R.A.; Wadaan, M.; Ezzeldin, E.; Marzouk, M. Cytotoxicity of new 5-hydrazono-[1,2,4]triazolo[1,5-a]quinazolines (Part II). Lat. Am. J. Pharm. 2016, 35, 66–73. [Google Scholar]
- Elsayed, E.A.; Sharaf-Eldin, M.A.; Wadaan, M. In vitro evaluation of cytotoxic activities of essential oil from Moringa oleifera seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 cell lines. Asian Pac. J. Cancer Preven. 2015, 16, 4671–4675. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, E.A.; Farooq, M.; Dailin, D.; El-Enshasy, H.A.; Othman, N.Z.; Malek, R.; Danial, E.; Wadaan, M. In vitro and in vivo biological screening of kefiran polysaccharide produced by Lactobacillus kefiranofaciens. Biomed. Res. 2017, 28, 594–600. [Google Scholar]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, W.M.; El-Sayeda, M.A.-A.; Abdel-Aziz, N.I.; El-Azab, A.S.; Ahmed, E.R.; Abdel-Aziz, A.A.-M. Synthesis, antitumour and antioxidant activities of novel a,b-unsaturated ketones and related heterocyclic analogues: EGFR inhibition and molecular modeling study. J. Enzyme Inhib. Med. Chem. 2018, 33, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Mouineer, A.A.; Zaher, A.F.; El Malah, A.A.; Sobh, E.A.E. Design, synthesis, antitumor activity, cell cycle analysis and ELISA assay for cdk-2 of a new (4-aryl-6-flouro-4h-benzo [4,5] thieno [3,2-b] pyran) derivatives. J. Chem. Pharm. Res. 2017, 9, 106–120. [Google Scholar]
- Abdullaziz, M.A.; Abdel-Mohsen, H.T.; El Kerdawy, A.M.; Ragab, F.A.F.; Ali, M.M.; Abu-bakr, S.M.; Girgis, A.S.; El Diwani, H.I. Design, synthesis, molecular docking and cytotoxic evaluation of novel 2-furybenzimidazoles as VEGFR-2 inhibitors. Eur. J. Med. Chem. 2017, 136, 315–329. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| Compound | IC50 (Mean ± SEM) (µM) | |
|---|---|---|
| MCF-7 | HepG-2 | |
| 2a | 31.64 ± 1.30 | 20.37 ± 1.36 |
| 2b | 32.58 ± 1.50 | 15.80 ± 1.66 |
| 2c | - | 35.52 ± 1.83 |
| 3a | - | 26.01 ± 2.35 |
| 3b | 25.33 ± 1.18 | 13.54 ± 1.45 |
| 3c | 29.55 ± 2.06 | 26.64 ± 1.85 |
| 4a | - | 11.59 ± 2.70 |
| 4b | 10.45 ± 1.33 | 10.25 ± 2.20 |
| 4c | 29.15 ± 1.39 | 18.84 ± 1.47 |
| 5a | 12.67 ± 2.40 | 11.19 ± 1.95 |
| 5b | 11.32 ± 1.15 | 10.09 ± 2.05 |
| 5c | 9.41 ± 1.25 | 7.53 ± 1.33 |
| 6a | 11.83 ± 1.62 | 12.44 ± 1.3 |
| 6b | 10.87 ± 1.10 | 11.53 ± 1.70 |
| 6c | - | 12.07 ± 1.68 |
| Tamoxifen | 22.40 ± 2.42 | 29.38 ± 1.15 |
| 5-Fluorouracil® | - | 43.84 ± 1.84 |
| Kinase | IC50 (Mean±SEM) (µM) | |
|---|---|---|
| 5c | Staurosporine | |
| VEGFR-2 | 0.01 ± 1.25 | 0.03 ± 1.10 |
| EGFR | 0.14 ± 1.00 | 0.02 ± 1.32 |
| PDGFRβ | 0.08 ± 1.45 | 0.07 ± 1.65 |
| CDK-2 | 0.06 ± 1.27 | 0.11 ± 1.13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amr, A.E.-G.E.; Abo-Ghalia, M.H.; Moustafa, G.O.; Al-Omar, M.A.; Nossier, E.S.; Elsayed, E.A. Design, Synthesis and Docking Studies of Novel Macrocyclic Pentapeptides as Anticancer Multi-Targeted Kinase Inhibitors. Molecules 2018, 23, 2416. https://doi.org/10.3390/molecules23102416
Amr AE-GE, Abo-Ghalia MH, Moustafa GO, Al-Omar MA, Nossier ES, Elsayed EA. Design, Synthesis and Docking Studies of Novel Macrocyclic Pentapeptides as Anticancer Multi-Targeted Kinase Inhibitors. Molecules. 2018; 23(10):2416. https://doi.org/10.3390/molecules23102416
Chicago/Turabian StyleAmr, Abd El-Galil E., Mohamed H. Abo-Ghalia, Gaber O. Moustafa, Mohamed A. Al-Omar, Eman S. Nossier, and Elsayed A. Elsayed. 2018. "Design, Synthesis and Docking Studies of Novel Macrocyclic Pentapeptides as Anticancer Multi-Targeted Kinase Inhibitors" Molecules 23, no. 10: 2416. https://doi.org/10.3390/molecules23102416
APA StyleAmr, A. E.-G. E., Abo-Ghalia, M. H., Moustafa, G. O., Al-Omar, M. A., Nossier, E. S., & Elsayed, E. A. (2018). Design, Synthesis and Docking Studies of Novel Macrocyclic Pentapeptides as Anticancer Multi-Targeted Kinase Inhibitors. Molecules, 23(10), 2416. https://doi.org/10.3390/molecules23102416







