Development of Validated Methods and Quantification of Curcuminoids and Curcumin Metabolites and Their Pharmacokinetic Study of Oral Administration of Complete Natural Turmeric Formulation (Cureit™) in Human Plasma via UPLC/ESI-Q-TOF-MS Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. Mass Spectrometric Analysis of CUR, DMC, BDMC, COG, COS, THC, HHC and OHC
2.2. Method Validation
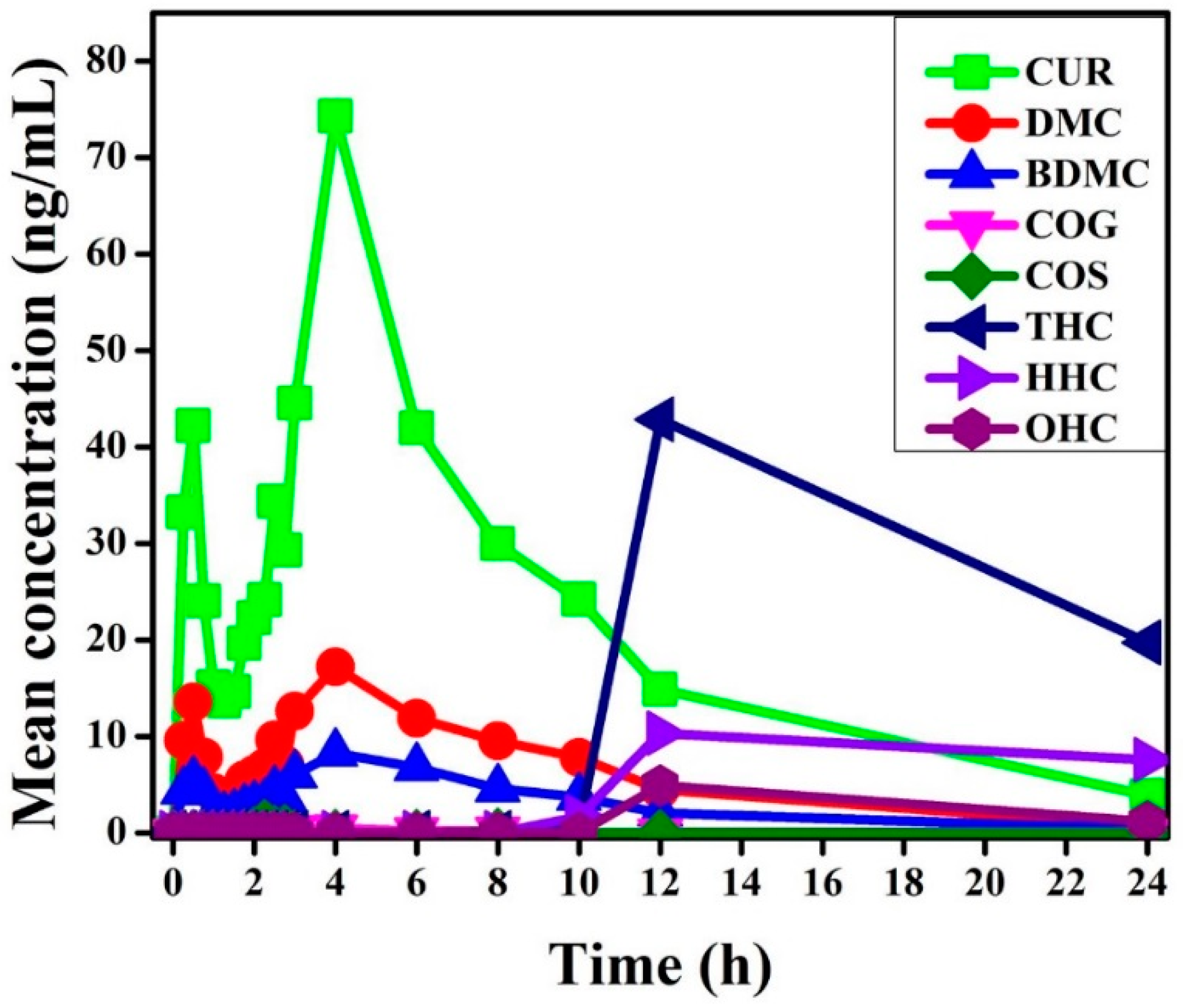
2.3. Pharmacokinetic Study of Orally Administrated Bioavailable Form of Curcumin
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instrumentation
3.3. Preparation of Standard and Quality Control Solutions
3.4. Method Validation Procedures
3.5. Study Design
3.6. Ethics and Approvals
3.7. Inclusion and Exclusion Criteria of Participants
3.8. Sample Collection and Preparation
3.9. Pharmacokinetic and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Xu, R.X.; Liu, Z. A high-throughput quantification method of curcuminoids and curcumin metabolites in human plasma via high-performance liquid chromatography/tandem mass spectrometry. J. Chromatogr. B 2014, 949, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Hsieh, T.-J.; Lu, C.-Y. Simple analytical strategy for MALDI-TOF-MS and nanoUPLC–MS/MS: Quantitating curcumin in food condiments and dietary supplements and screening of acrylamide-induced ROS protein indicators reduced by curcumin. Food Chem. 2015, 174, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Marczylo, T.H.; Steward, W.P.; Gescher, A.J. Rapid analysis of curcumin and curcumin metabolites in rat biomatrices using a novel Ultraperformance Liquid Chromatography (UPLC) method. J. Agric. Food Chem. 2009, 57, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Dempe, J.S.; Scheerle, R.K.; Pfeiffer, E.; Metzler, M. Metabolism and permeability of curcumin in cultured Caco-2 cells. Mol. Nutr. Food Res. 2013, 57, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Motomu, S.; Kiyotaka, N.; Akio, W.; Tsuyoshi, T.; Teiko, Y.; Shigefumi, K.; Fumiko, K.; Teruo, M. Comparison of the effects of curcumin and curcumin glucuronidein human hepatocellular carcinoma HepG2 cells. Food Chem. 2014, 151, 126–132. [Google Scholar]
- Lou, Y.; Zheng, J.; Hu, H.; Lee, J.; Zeng, S. Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria. J. Chromatogr. B 2015, 985, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Miyazawa, T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000, 67, 2785–2793. [Google Scholar] [CrossRef]
- Ireson, C.R.; Orr, S.; Jones, D.J.L.; Verschoyle, R.; Lim, C.K.; Luo, J.L.; Howells, L.; Plummer, S.M.; Jukes, R.M.; Williams Steward, W.P.; et al. Characterization of metabolites of the chemopreventive agent curcumin in humans and rat hepatocytes and in rat plasma and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001, 61, 1058–1064. [Google Scholar] [PubMed]
- Gopi, S.; Jacob, J.; Mathur, K.Y. Acute and subchronic oral toxicity studies of hydrogenated curcuminoid formulation ‘CuroWhite’ in rats. Toxicol. Rep. 2016, 3, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Ireson, C.R.; Jones, D.J.; Orr, S.; Coughtrie, M.W.; Boocock, D.J.; Williams, M.L.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol. Biomark. Prev. 2002, 11, 105–111. [Google Scholar]
- Amalraj, A.; Jude, S.; Varma, K.; Jacob, J.; Gopi, S.; Oluwafemi, O.S.; Thomas, S. Preparation of a novel bioavailable curcuminoid formulation (Cureit™) using Polar-Nonpolar-Sandwich (PNS) technology and its characterization and applications. Mater. Sci. Eng. C 2017, 75, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; George, R.; Jude, S.; Sriraam, V.T. Cell culture study on the cytotoxic effects of “Cureit”—A novel bio available curcumin-anti cancer effects. J. Chem. Pharm. Res. 2014, 6, 96–100. [Google Scholar]
- Gopi, S.; George, R.; Sriraam, V.T. Cell culture study on the effect of bioavailable curcumin—“Cureit” on elastase inhibition activity. Br. Biomed. Bull. 2014, 2, 545–549. [Google Scholar]
- Gopi, S.; George, R.; Sriraam, V.T. Antioxidant potential of “Cureit”—A novel bioavailable curcumin formulation. Asian J. Pharm. Tech. Innov. 2014, 2, 123–127. [Google Scholar]
- Gopi, S.; George, R.; Sriraam, V.T. Cell culture study on the effects of “Cureit” hyaluronidase inhibition—Anti aging effects. Int. J. Curr. Res. 2014, 6, 8473–8474. [Google Scholar]
- Gopi, S.; George, R.; Thomas, M.; Jude, S. A pilot cross-over study to assess the human bioavailability of “Cureit”—A bioavailable curcumin in complete natural matrix. Asian J. Pharm. Tech. Innov. 2015, 3, 92–96. [Google Scholar]
- Ahmad, N.; Warsi, M.H.; Iqbal, Z.; Samim, M.; Ahmad, F.J. Quantification of curcumin, demethoxycurcumin, and bisdemethoxycurcumin in rodent brain by UHPLC/ESI-Q-TOF-MS/MS after intra-nasal administration of curcuminoids loaded PNIPAM nanoparticles. Drug Test. Anal. 2014, 6, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.K.; Pan, M.H.; Lin-Shiau, S.Y. Recent studies on the biofunctions and biotransformations of curcumin. Biofactors 2000, 13, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Zhongfa, L.; Chiu, M.; Wang, J.; Chen, W.; Yen, W.; Fan-Havard, P.; Yee, L.D.; Chan, K.K. Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer Chemother. Pharmacol. 2012, 69, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, Z.; Jones, W.; Pavlovicz, R.E.; Liu, S.; Yu, J.; Li, P.K.; Lin, J.; Fuchs, J.R.; Marcucci, G.; et al. Curcumin is a potent DNA hypomethylation agent. Bioorg. Med. Chem. Lett. 2009, 19, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Jacob, J.; Varma, K.; Jude, S.; Amalraj, A.; Arundhathy, C.A.; George, R.; Sreeraj, T.R.; Divya, C.; Kunnumakkara, A.B.; et al. Comparative Oral Absorption of Curcumin in a Natural Turmeric Matrix with Two Other Curcumin Formulations: An Open-label Parallelarm Study. Phytother. Res. 2017, 31, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.K.; Najar, I.A.; Tikoo, M.K.; Singh, G.; Gupta, D.K.; Anand, R.; Khajuria, R.K.; Sharma, S.C.; Johri, R.K. Development of a validated UPLC-qTOF-MS Method for the determination of curcuminoids and their pharmacokinetic study in mice. Daru. J. Pharm. Sci. 2013, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.V.; Boyd, K.M.; Webb, D.J. The revision of the Declaration of Helsinki: Past, present and future. Br. J. Clin. Pharmacol. 2004, 57, 695–713. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds mentioned in the manuscript are available from the authors. |
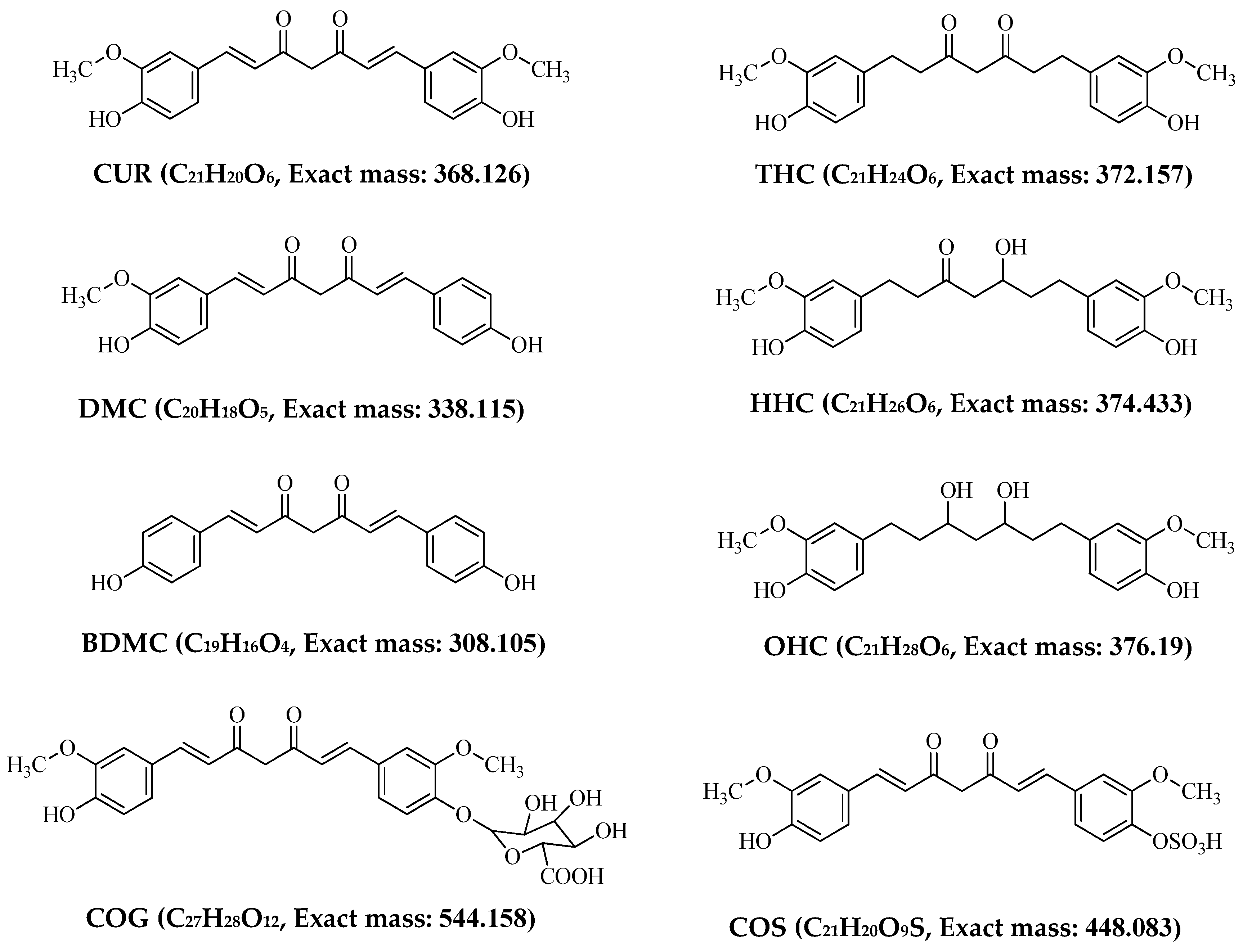
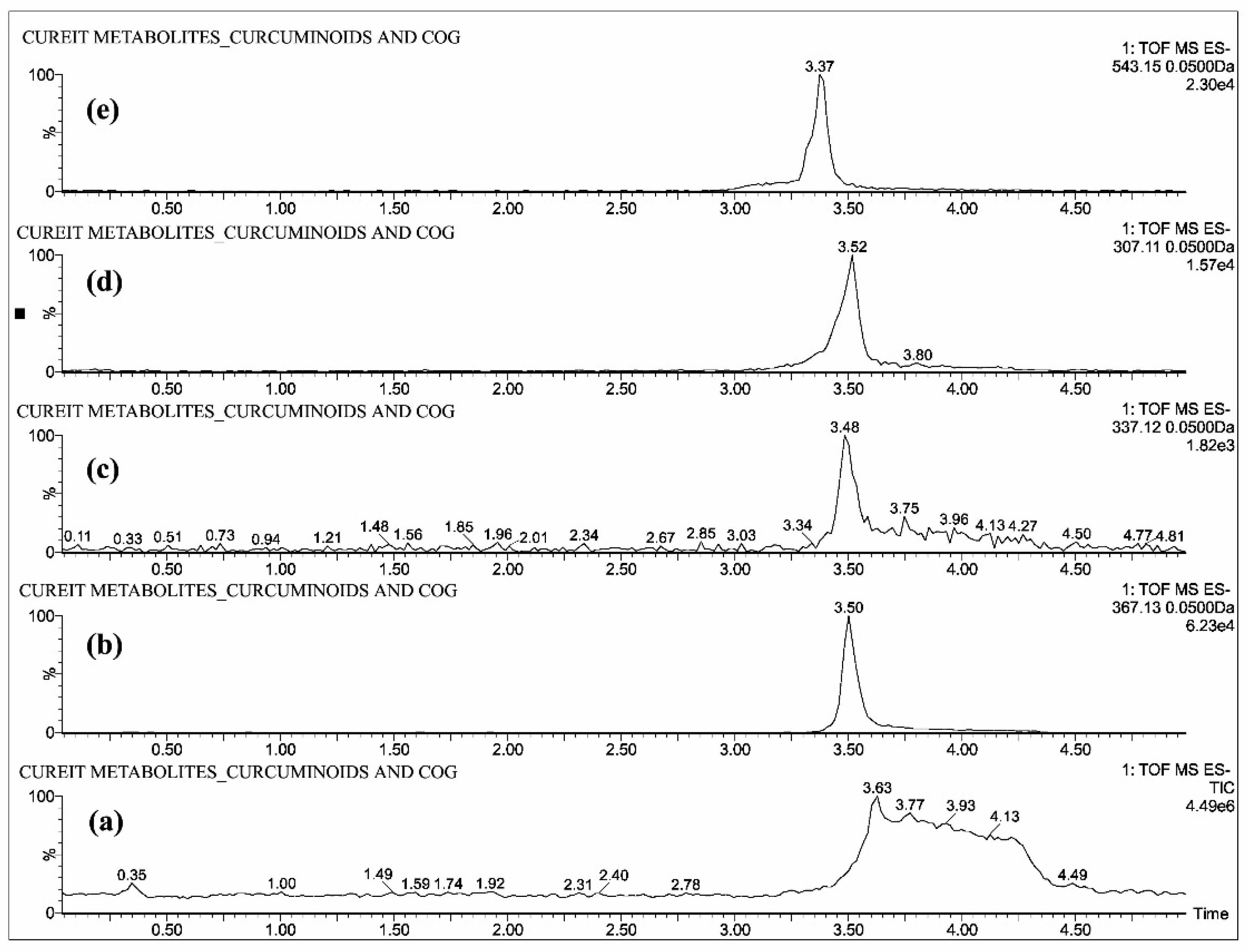
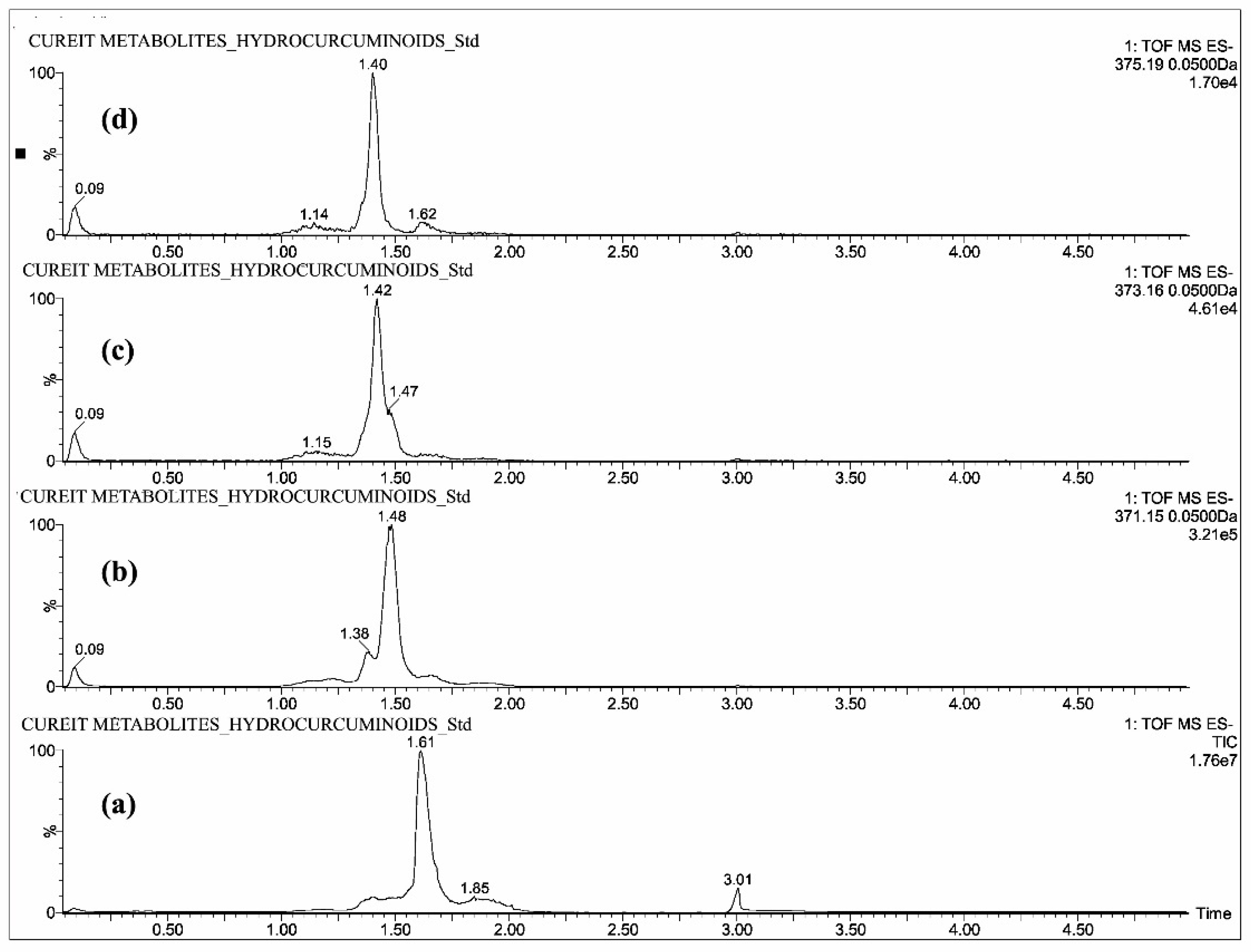
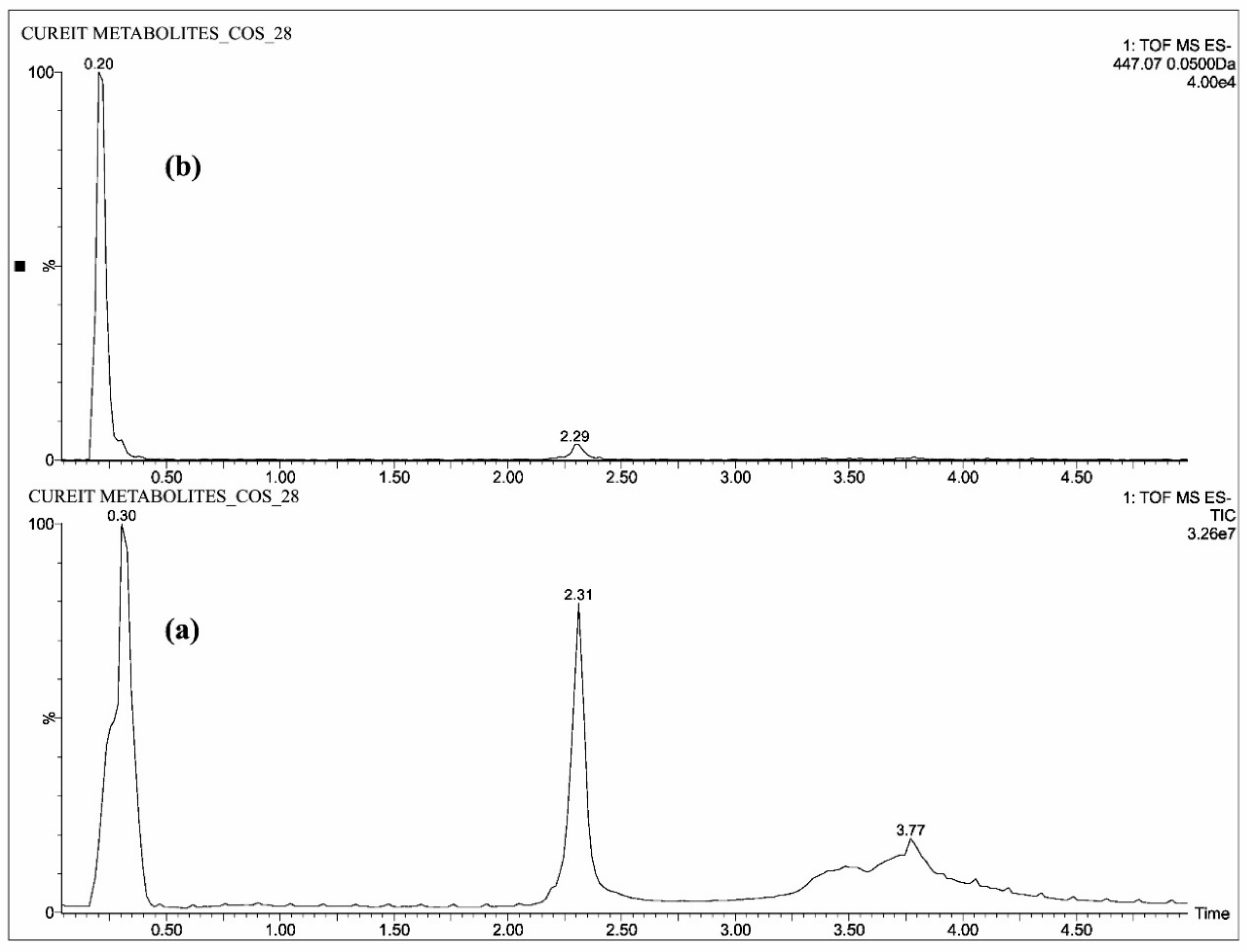
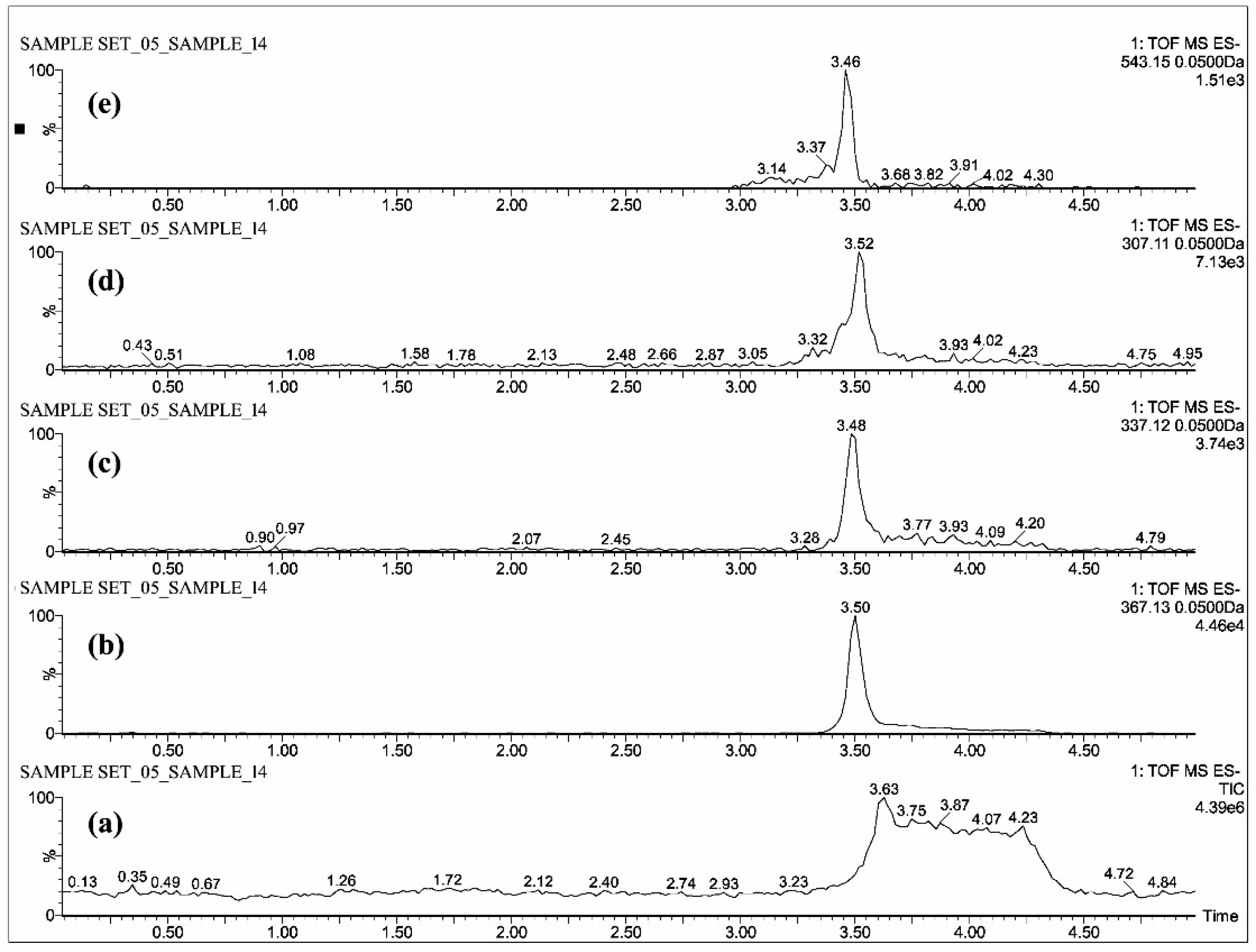
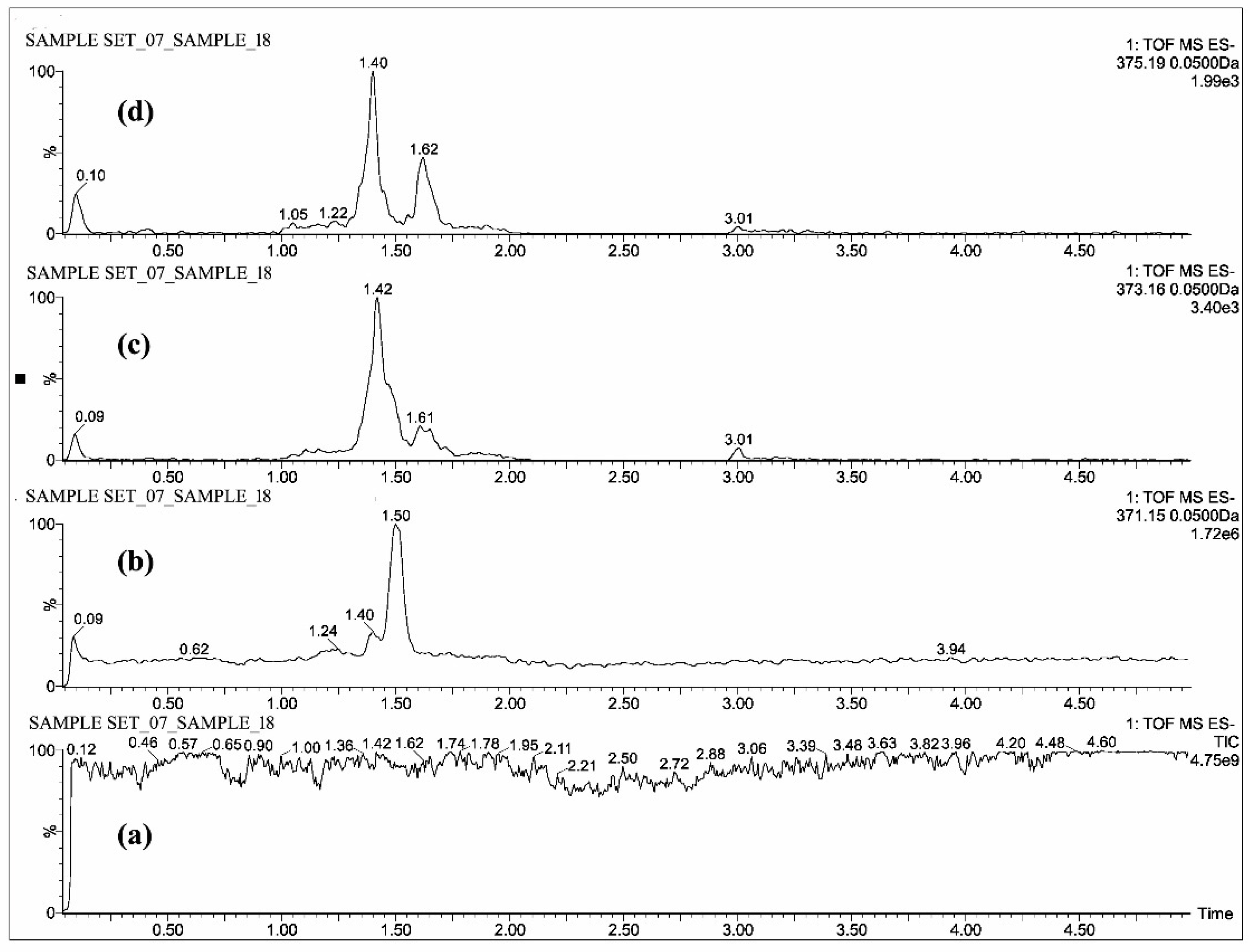
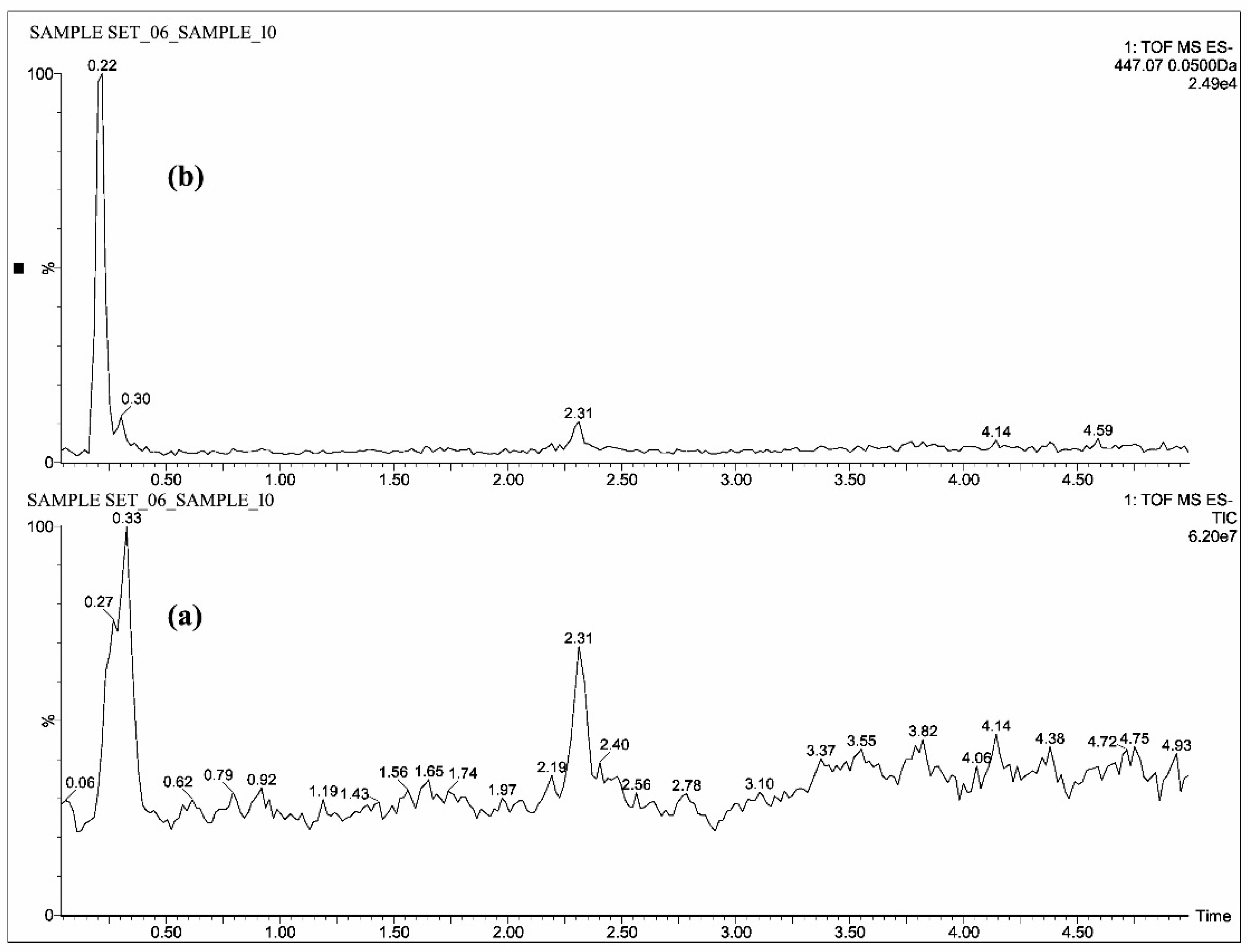
| Standards | Intensity Imax (×103 cps) | |
|---|---|---|
| Positive Mode | Negative Mode | |
| CUR | 10.8 | 42 |
| DMC | 12.3 | 57.5 |
| BDMC | 11.2 | 63.03 |
| THC | 7.9 | 43 |
| HHC | 5.1 | 22.88 |
| OHC | 0.9 | 3.52 |
| COG | 2.8 | 7.195 |
| COS | 0.18 | 1.895 |
| Compound | Nominal Conc. (ng/mL) | Intra-Assay | Inter-Assay | ||||
|---|---|---|---|---|---|---|---|
| Set 1 | Set 2 | Set 3 | Set 1 | Set 2 | Set 3 | ||
| CUR | 4 | 7.125 | 6.292 | 9.667 | 6.108 | 7.067 | 2.817 |
| 8 | 3.592 | 13.950 | −0.379 | 7.317 | 7.379 | −2.979 | |
| 16 | 1.385 | 0.577 | −2.098 | 1.288 | 2.415 | 2.354 | |
| 32 | 0.487 | 1.851 | 2.794 | 2.268 | 3.550 | 0.873 | |
| 64 | −3.866 | 1.203 | −1.182 | 1.620 | −0.235 | 1.046 | |
| 128 | 1.329 | 0.039 | −1.309 | −0.840 | 0.306 | −0.203 | |
| 256 | 0.313 | 0.384 | 0.126 | −0.780 | 0.193 | −0.212 | |
| DMC | 4 | 2.842 | 6.200 | −2.992 | 5.667 | 0.758 | 5.525 |
| 8 | 1.100 | 5.425 | −7.587 | 3.075 | 3.783 | 1.492 | |
| 16 | 2.035 | 2.271 | −0.896 | 2.023 | 1.448 | 3.040 | |
| 32 | −0.196 | 2.447 | 0.860 | 0.841 | 1.664 | 0.080 | |
| 64 | 1.882 | −0.136 | −1.713 | 0.634 | 0.556 | 0.948 | |
| 128 | −0.260 | 0.258 | −0.088 | −0.126 | 0.043 | 0.210 | |
| 256 | −0.008 | −0.086 | 0.271 | 0.143 | 0.333 | −0.760 | |
| BDMC | 4 | 0.633 | 2.392 | 3.108 | 4.192 | 0.692 | 1.767 |
| 8 | 4.317 | 2.508 | −2.871 | 7.167 | 4.854 | 0.296 | |
| 16 | 0.208 | 3.646 | 2.667 | 4.337 | 2.167 | 2.356 | |
| 32 | −0.632 | 0.948 | 0.342 | 1.740 | −0.793 | 2.111 | |
| 64 | −5.248 | 0.324 | 1.055 | 0.106 | 0.451 | −0.172 | |
| 128 | 0.779 | −0.310 | −0.105 | 0.367 | −0.182 | 0.454 | |
| 256 | 0.206 | −0.197 | 0.770 | 0.560 | −0.509 | 0.444 | |
| THC | 4 | 1.483 | 0.075 | −2.358 | −3.333 | 2.800 | −2.358 |
| 8 | 15.000 | 0.908 | 5.783 | 2.300 | −5.908 | −1.712 | |
| 16 | 4.375 | 4.583 | 5.823 | 4.104 | 1.652 | 3.748 | |
| 32 | −1.563 | −2.708 | 2.653 | 2.365 | 1.337 | 2.627 | |
| 64 | −0.573 | 0.365 | −0.009 | −0.157 | 0.038 | 0.460 | |
| 128 | −2.552 | −1.250 | −1.172 | −0.422 | −0.414 | 0.578 | |
| 256 | 0.547 | −1.094 | −0.394 | 0.165 | −0.173 | 0.642 | |
| HHC | 4 | 0.658 | 8.175 | 0.925 | 3.250 | 3.600 | 0.450 |
| 8 | −10.000 | −4.667 | 0.621 | 3.971 | −5.458 | −0.650 | |
| 16 | −5.208 | −2.083 | 4.517 | 1.106 | −0.527 | −0.215 | |
| 32 | 3.125 | 1.354 | 2.694 | 3.232 | 0.439 | −2.377 | |
| 64 | 0.990 | 0.469 | 2.398 | 1.955 | 1.144 | 0.789 | |
| 128 | 0.990 | −1.120 | 0.010 | 0.024 | −0.421 | 0.499 | |
| 256 | −0.352 | −1.328 | 0.085 | −0.304 | 0.123 | −0.096 | |
| OHC | 4 | 2.808 | 9.908 | 7.494 | 4.883 | 6.550 | −0.042 |
| 8 | 1.208 | 0.300 | 1.883 | −2.842 | 3.133 | 0.192 | |
| 16 | −3.448 | 3.789 | −0.919 | 1.071 | 2.378 | −2.581 | |
| 32 | 11.098 | −3.083 | −0.706 | −1.488 | 1.964 | 4.443 | |
| 64 | −1.296 | −0.001 | −0.800 | 1.420 | 0.310 | 0.252 | |
| 128 | −0.487 | 1.633 | 0.733 | 0.438 | −0.558 | −0.582 | |
| 256 | 2.931 | 0.624 | −0.092 | 0.774 | 0.119 | −0.122 | |
| COG | 4 | 8.833 | 5.833 | 2.833 | 1.500 | 0.083 | −1.750 |
| 8 | 11.583 | 10.458 | 9.375 | 7.333 | 5.375 | 3.708 | |
| 16 | 2.771 | 1.708 | 1.208 | 0.604 | 0.292 | −0.542 | |
| 32 | 0.354 | −0.052 | 0.177 | 0.021 | −0.125 | −0.354 | |
| 64 | 1.620 | 1.417 | 1.448 | 0.903 | 0.391 | 0.469 | |
| 128 | 0.891 | 0.646 | 0.143 | −0.339 | −0.982 | −1.070 | |
| 256 | 0.807 | 0.440 | 0.064 | 0.135 | −0.089 | −0.010 | |
| COS | 4 | 12.750 | 6.750 | 4.667 | 1.750 | 1.250 | 0.667 |
| 8 | 3.692 | 1.958 | 1.250 | 0.792 | 0.000 | −1.000 | |
| 16 | 0.479 | 0.437 | 0.081 | −0.375 | −0.648 | −0.604 | |
| 32 | 1.010 | 0.531 | 0.156 | −0.229 | −0.094 | −0.260 | |
| 64 | 0.104 | −1.615 | −0.260 | −0.260 | −0.245 | −0.391 | |
| 128 | 1.510 | 0.208 | 0.122 | 0.065 | −0.008 | −0.388 | |
| 256 | 0.833 | 0.612 | 0.208 | 0.273 | −0.508 | 0.013 | |
| Compound | Nominal Conc. (ng/mL) | Intra-Assay | Inter-Assay | ||||
|---|---|---|---|---|---|---|---|
| Set 1 | Set 2 | Set 3 | Set 1 | Set 2 | Set 3 | ||
| CUR | 4 | 7.262 | 8.894 | 9.779 | 7.997 | 7.654 | 10.411 |
| 8 | 5.277 | 6.242 | 10.188 | 9.517 | 7.312 | 3.263 | |
| 16 | 2.345 | 1.218 | 3.167 | 5.532 | 6.090 | 3.969 | |
| 32 | 1.233 | 1.895 | 3.207 | 2.714 | 2.196 | 4.052 | |
| 64 | 8.293 | 4.409 | 2.992 | 1.989 | 3.654 | 2.239 | |
| 128 | 2.096 | 1.486 | 2.736 | 3.046 | 1.734 | 3.258 | |
| 256 | 1.071 | 2.535 | 1.916 | 2.151 | 1.015 | 1.348 | |
| DMC | 4 | 5.398 | 11.478 | 3.098 | 12.374 | 3.157 | 7.751 |
| 8 | 6.095 | 11.009 | 9.751 | 4.730 | 8.282 | 2.955 | |
| 16 | 3.984 | 2.158 | 3.287 | 8.958 | 7.664 | 7.691 | |
| 32 | 0.655 | 3.251 | 5.937 | 3.342 | 1.759 | 4.120 | |
| 64 | 2.741 | 0.716 | 3.144 | 1.219 | 2.473 | 3.351 | |
| 128 | 0.972 | 1.363 | 2.816 | 1.369 | 1.503 | 1.662 | |
| 256 | 1.502 | 1.277 | 1.620 | 1.724 | 1.347 | 0.913 | |
| BDMC | 4 | 5.938 | 11.762 | 10.882 | 15.058 | 7.284 | 10.178 |
| 8 | 1.370 | 3.183 | 9.644 | 5.040 | 8.077 | 8.446 | |
| 16 | 2.632 | 7.026 | 6.027 | 3.710 | 4.717 | 2.280 | |
| 32 | 1.662 | 2.784 | 5.402 | 7.436 | 6.178 | 2.302 | |
| 64 | 9.533 | 3.070 | 4.464 | 1.186 | 2.386 | 1.088 | |
| 128 | 0.467 | 1.974 | 1.326 | 0.001 | 2.390 | 1.888 | |
| 256 | 0.554 | 1.436 | 0.913 | 0.753 | 0.548 | 0.401 | |
| THC | 4 | 5.579 | 6.357 | 13.441 | 7.371 | 3.823 | 7.225 |
| 8 | 3.919 | 5.693 | 7.311 | 9.320 | 7.633 | 8.456 | |
| 16 | 3.111 | 1.826 | 2.045 | 1.808 | 1.775 | 4.042 | |
| 32 | 4.444 | 1.617 | 1.012 | 0.678 | 1.262 | 3.167 | |
| 64 | 0.595 | 0.589 | 1.081 | 0.961 | 0.265 | 2.626 | |
| 128 | 2.567 | 2.487 | 1.856 | 1.745 | 0.813 | 1.904 | |
| 256 | 0.441 | 2.159 | 1.314 | 1.200 | 0.591 | 2.399 | |
| HHC | 4 | 8.815 | 8.591 | 12.896 | 10.771 | 9.761 | 5.472 |
| 8 | 3.675 | 4.065 | 8.366 | 4.387 | 8.154 | 7.690 | |
| 16 | 4.390 | 1.950 | 4.280 | 5.162 | 5.346 | 4.706 | |
| 32 | 2.922 | 1.780 | 2.539 | 1.988 | 3.101 | 3.041 | |
| 64 | 2.572 | 1.794 | 2.428 | 1.536 | 2.259 | 0.871 | |
| 128 | 0.473 | 1.767 | 1.153 | 0.337 | 0.732 | 1.740 | |
| 256 | 2.310 | 1.758 | 0.785 | 1.693 | 0.742 | 1.641 | |
| OHC | 4 | 5.745 | 6.905 | 5.793 | 10.637 | 7.220 | 15.026 |
| 8 | 11.047 | 4.623 | 5.516 | 4.263 | 5.420 | 9.732 | |
| 16 | 4.897 | 0.934 | 1.574 | 3.270 | 4.085 | 3.412 | |
| 32 | 6.194 | 2.930 | 2.471 | 3.221 | 2.910 | 4.476 | |
| 64 | 1.418 | 1.615 | 0.999 | 0.849 | 2.548 | 0.492 | |
| 128 | 3.845 | 2.266 | 0.866 | 2.590 | 1.290 | 0.641 | |
| 256 | 3.335 | 0.311 | 0.488 | 0.108 | 0.572 | 0.672 | |
| COG | 4 | 1.036 | 2.548 | 1.857 | 0.739 | 0.577 | 0.917 |
| 8 | 0.342 | 0.643 | 0.229 | 1.195 | 1.438 | 1.053 | |
| 16 | 0.274 | 0.284 | 0.560 | 0.404 | 0.415 | 0.837 | |
| 32 | 0.140 | 0.260 | 0.126 | 0.126 | 0.217 | 0.048 | |
| 64 | 0.988 | 0.447 | 0.170 | 0.580 | 0.322 | 0.823 | |
| 128 | 0.046 | 0.126 | 0.863 | 0.623 | 0.229 | 0.705 | |
| 256 | 0.334 | 0.089 | 0.352 | 0.096 | 0.242 | 0.022 | |
| COS | 4 | 2.614 | 1.021 | 0.365 | 1.277 | 1.132 | 1.831 |
| 8 | 0.430 | 1.691 | 1.111 | 0.559 | 0.250 | 1.456 | |
| 16 | 0.423 | 0.311 | 0.694 | 0.664 | 1.137 | 0.729 | |
| 32 | 0.449 | 0.824 | 0.437 | 0.665 | 0.325 | 0.231 | |
| 64 | 0.238 | 1.441 | 0.326 | 0.261 | 0.182 | 0.656 | |
| 128 | 0.740 | 0.238 | 0.035 | 0.016 | 0.028 | 0.327 | |
| 256 | 0.667 | 0.594 | 0.592 | 0.281 | 0.416 | 0.045 | |
| Compounds | AUC (ng·h·mL−1) | Cmax (ng·mL−1) | Tmax (h) | T½ (h) | CL (106 h−1·mL−1) | Kel (h−1) | Vd (106 mL−1) |
|---|---|---|---|---|---|---|---|
| CUR | 527.100 | 74.310 | 4.000 | 1.681 | 0.949 | 0.412 | 2.300 |
| DMC | 147.000 | 17.193 | 4.000 | 1.569 | 3.401 | 0.442 | 7.699 |
| BDMC | 72.610 | 8.280 | 4.000 | 1.480 | 6.886 | 0.468 | 14.710 |
| COG | 3.200 | 0.862 | 2.500 | 0.301 | 156.250 | 2.303 | 67.846 |
| COS | 0.900 | 1.060 | 2.250 | 0.300 | 555.560 | 2.309 | 240.580 |
| THC | 419.700 | 42.840 | 12.000 | 4.673 | 1.191 | 0.148 | 8.033 |
| HHC | 121.300 | 10.330 | 12.000 | 11.574 | 4.122 | 0.060 | 68.840 |
| OHC | 41.510 | 4.910 | 12.000 | 2.768 | 12.045 | 0.250 | 48.116 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jude, S.; Amalraj, A.; Kunnumakkara, A.B.; Divya, C.; Löffler, B.-M.; Gopi, S. Development of Validated Methods and Quantification of Curcuminoids and Curcumin Metabolites and Their Pharmacokinetic Study of Oral Administration of Complete Natural Turmeric Formulation (Cureit™) in Human Plasma via UPLC/ESI-Q-TOF-MS Spectrometry. Molecules 2018, 23, 2415. https://doi.org/10.3390/molecules23102415
Jude S, Amalraj A, Kunnumakkara AB, Divya C, Löffler B-M, Gopi S. Development of Validated Methods and Quantification of Curcuminoids and Curcumin Metabolites and Their Pharmacokinetic Study of Oral Administration of Complete Natural Turmeric Formulation (Cureit™) in Human Plasma via UPLC/ESI-Q-TOF-MS Spectrometry. Molecules. 2018; 23(10):2415. https://doi.org/10.3390/molecules23102415
Chicago/Turabian StyleJude, Shintu, Augustine Amalraj, Ajaikumar B. Kunnumakkara, Chandradhara Divya, Bernd-Michael Löffler, and Sreeraj Gopi. 2018. "Development of Validated Methods and Quantification of Curcuminoids and Curcumin Metabolites and Their Pharmacokinetic Study of Oral Administration of Complete Natural Turmeric Formulation (Cureit™) in Human Plasma via UPLC/ESI-Q-TOF-MS Spectrometry" Molecules 23, no. 10: 2415. https://doi.org/10.3390/molecules23102415
APA StyleJude, S., Amalraj, A., Kunnumakkara, A. B., Divya, C., Löffler, B.-M., & Gopi, S. (2018). Development of Validated Methods and Quantification of Curcuminoids and Curcumin Metabolites and Their Pharmacokinetic Study of Oral Administration of Complete Natural Turmeric Formulation (Cureit™) in Human Plasma via UPLC/ESI-Q-TOF-MS Spectrometry. Molecules, 23(10), 2415. https://doi.org/10.3390/molecules23102415





