β-Escin Effectively Modulates HUVECs Proliferation and Tube Formation
Abstract
1. Introduction
2. Results
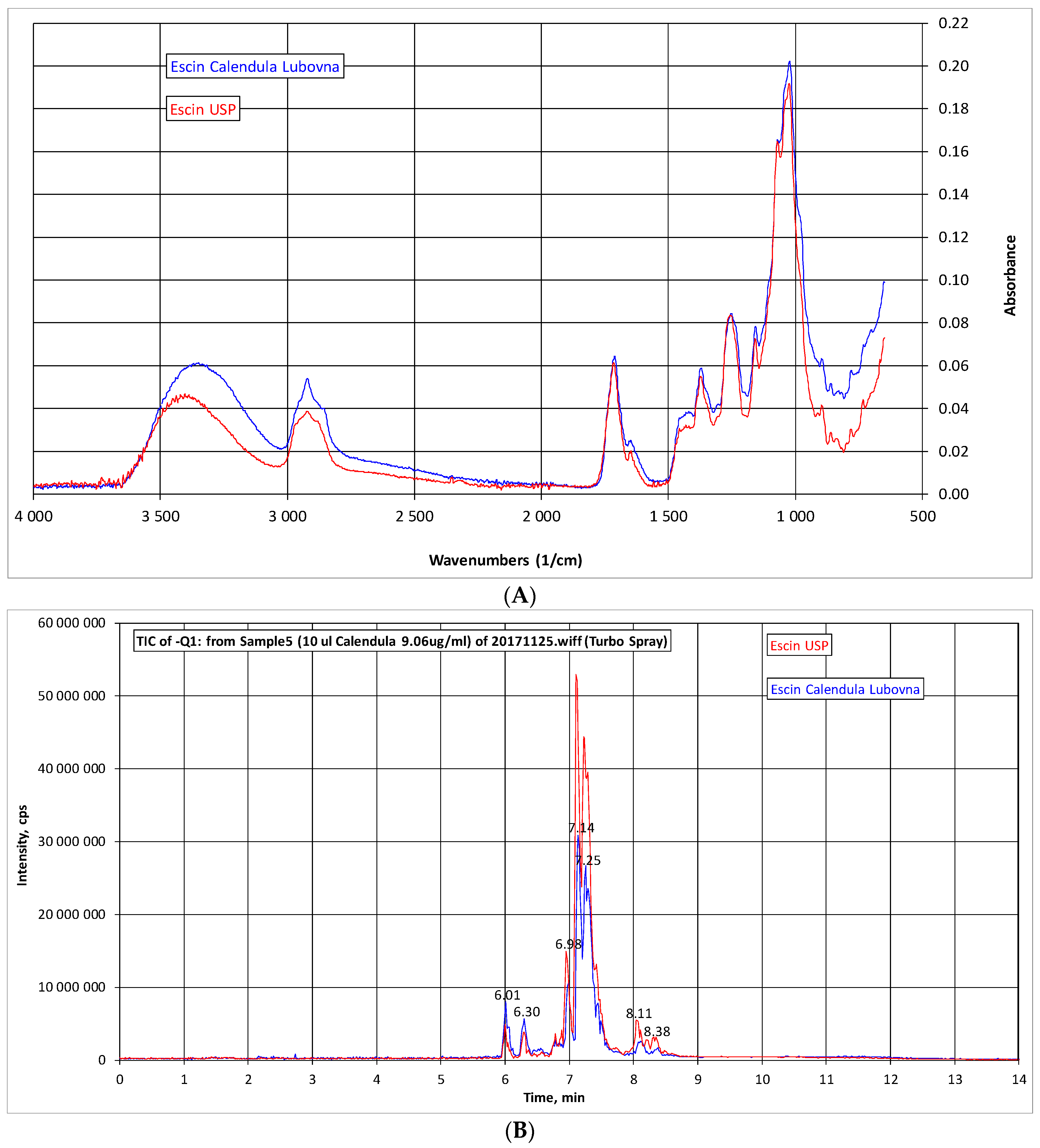
2.1. Analysis of β-Escin
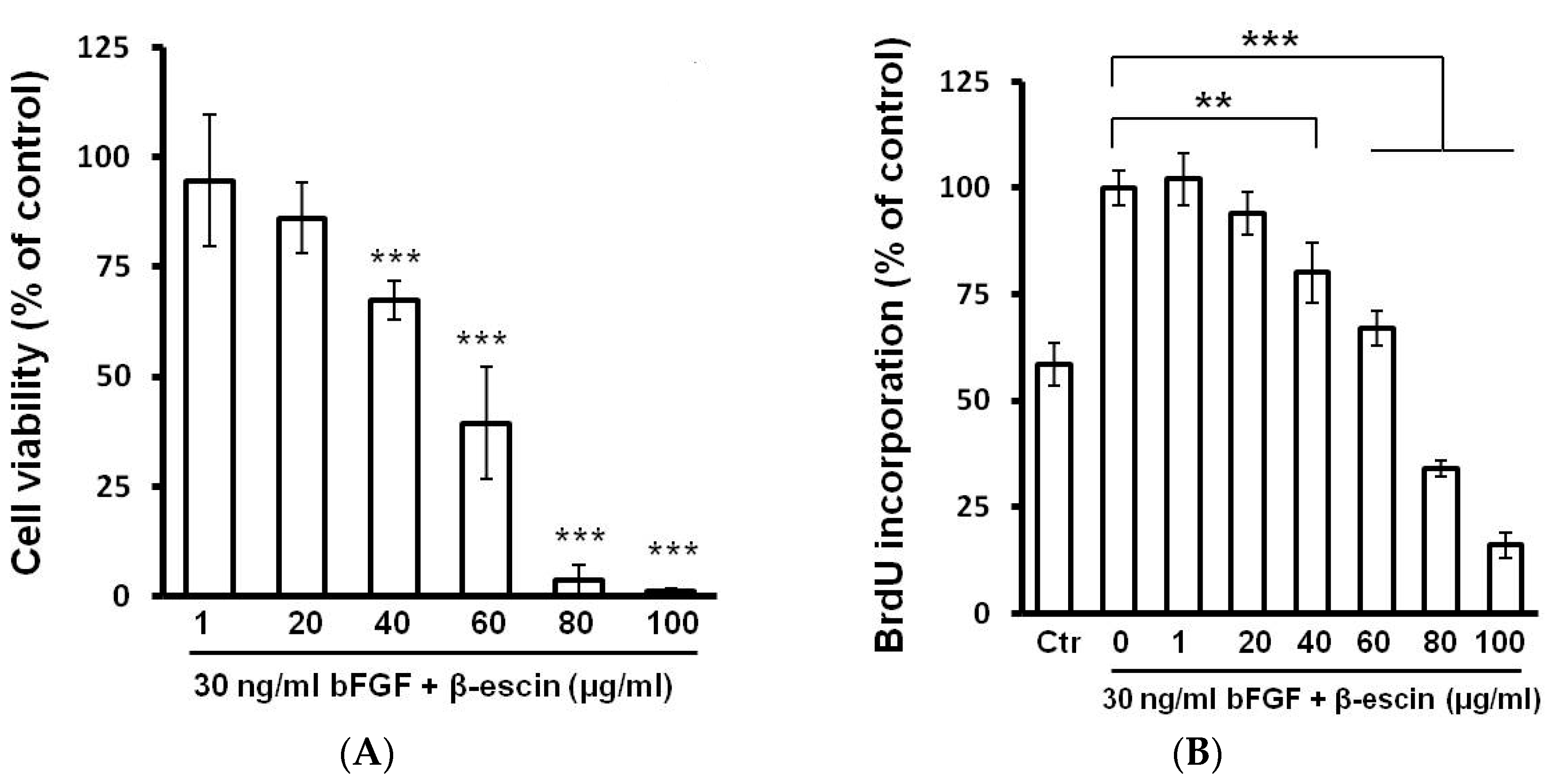
2.2. Cell Viability, BrdU Incorporation, and Cell Cycle Assay
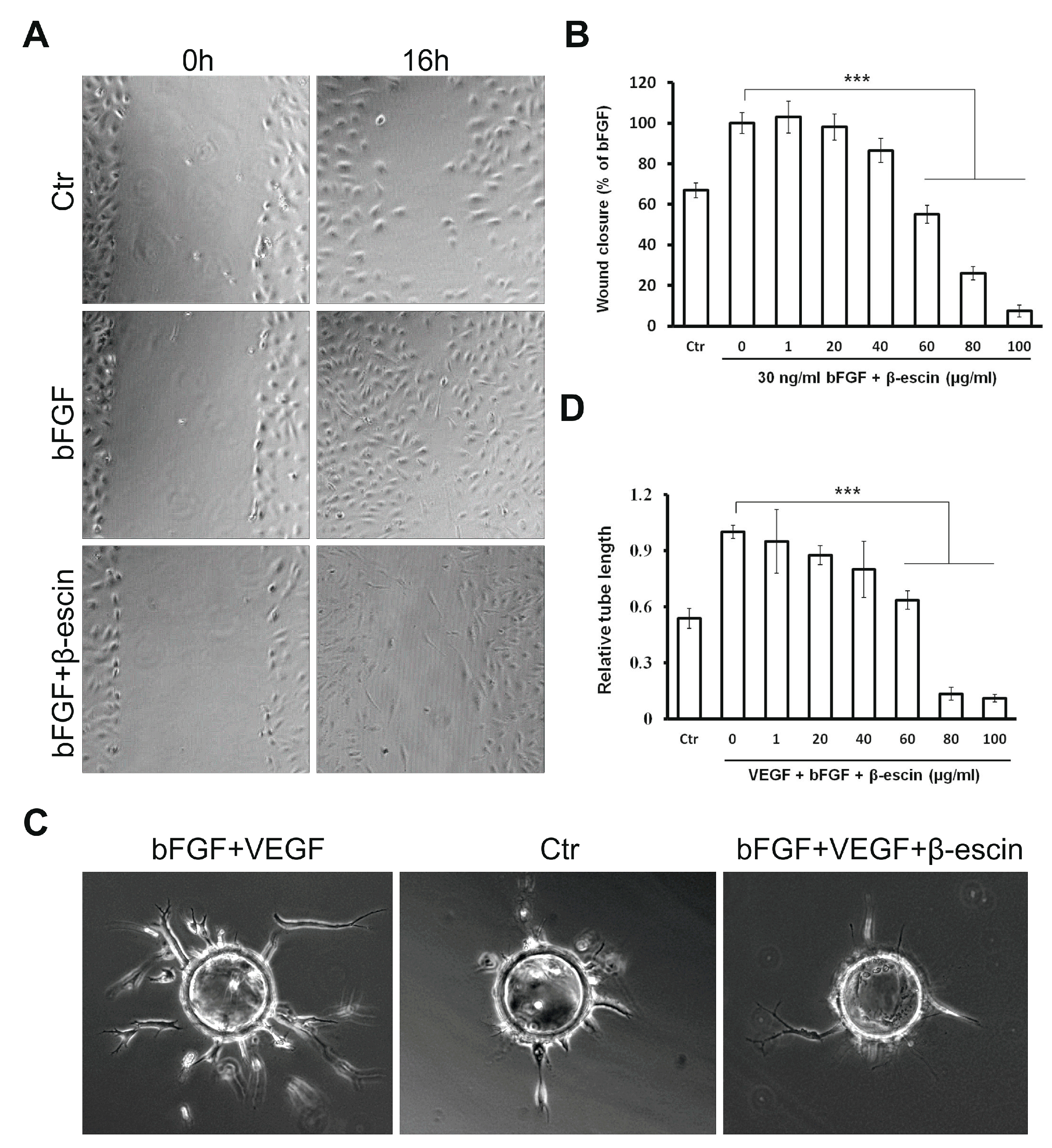
2.3. β-Escin Inhibits bFGF-Stimulated Migratory Ability Of HUVECs
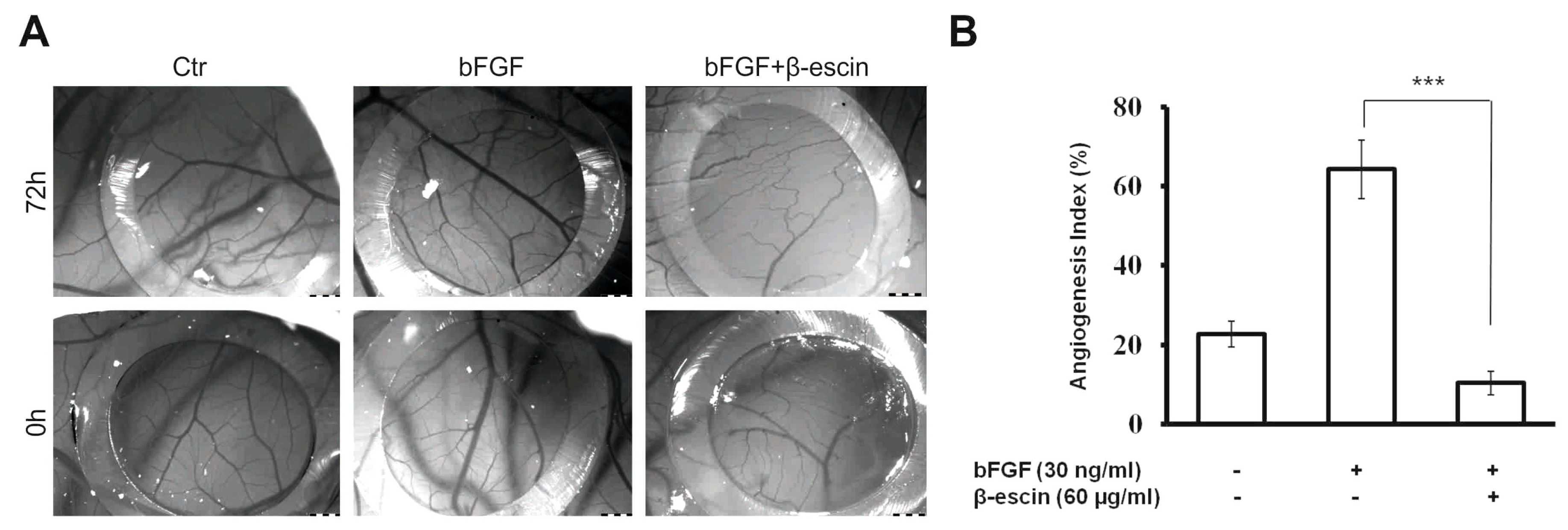
2.4. Anti-Angiogenic Effects of β-Escin on HUVECs In Vitro and in the CAM Model In Vivo
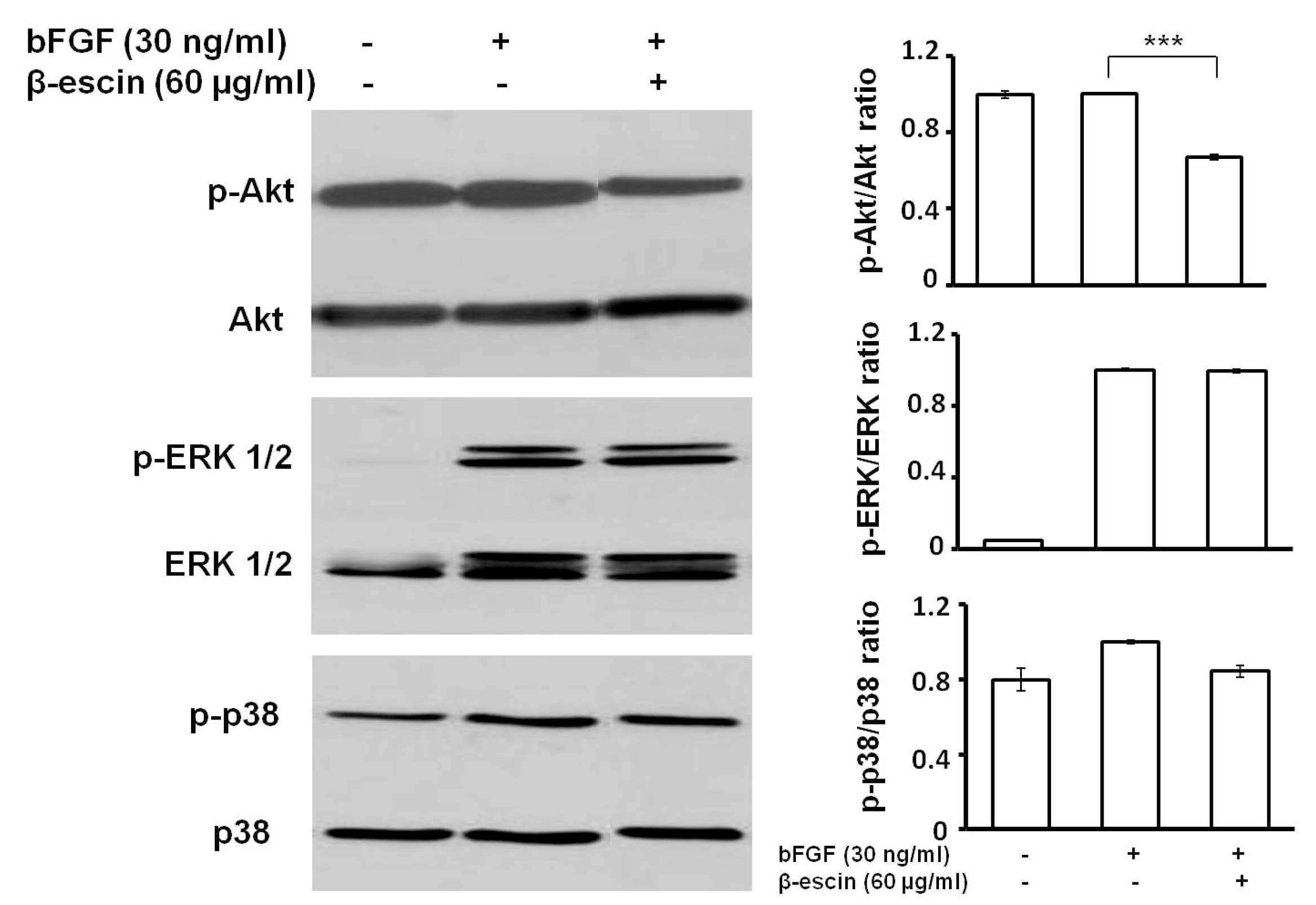
2.5. β-Escin Suppressed bFGF-Downstream Signaling Pathway
2.6. Gene Profiling of HUVECs after β-Escin Treatment
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. IR Spectra and HPLC-MS Analysis
4.4. MTS Cell Viability Assay
4.5. 5-Bromo-2′-deoxyuridine (BrdU) Cell Proliferation Assay
4.6. Analysis of Cell Cycle Distribution
4.7. Assessment of Monolayer Integrity
4.8. Two-Dimensional Migration (Wound Healing) Assay
4.9. Fibrin Gel Bead Assay
4.10. Western Blot Analysis
4.11. RNA Isolation And cDNA Synthesis
4.12. Gene Expression Profiling
4.13. PCR Data Analysis and Statistics
4.14. Chicken Embryo Chorioallantoic Membrane (CAM) Assay
4.15. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Folkman, J. Angiogenesis-dependent diseases. Semin. Oncol. 2001, 28, 536–542. [Google Scholar] [CrossRef]
- Granci, V.; Dupertuis, Y.M.; Pichard, C. Angiogenesis as a potential target of pharmaconutrients in cancer therapy. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.H.; Gootenberg, J.; Keegan, P.; Pazdur, R. FDA drug approval summary: Bevacizumab plus folfox4 as second-line treatment of colorectal cancer. Oncologist 2007, 12, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Doroshow, J.H.; Kummar, S. United states food and drug administration approved oral kinase inhibitors for the treatment of malignancies. Curr. Probl. Cancer 2013, 37, 110–144. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Mustafa, N.; Li, F.; Kannaiyan, R.; Ahn, K.S.; Kumar, A.P.; Chng, W.J.; Sethi, G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget 2014, 5, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Lee, J.H.; Jung, S.H.; Lee, S.G.; Chinnathambi, A.; Alharbi, S.A.; Yang, W.M.; Um, J.Y.; Sethi, G.; Ahn, K.S. 2,5-dihydroxyacetophenone induces apoptosis of multiple myeloma cells by regulating the MAPK activation pathway. Molecules 2017, 22, 1157. [Google Scholar] [CrossRef] [PubMed]
- Varinska, L.; Kubatka, P.; Mojzis, J.; Zulli, A.; Gazdikova, K.; Zubor, P.; Busselberg, D.; Caprnda, M.; Opatrilova, R.; Gasparova, I.; et al. Angiomodulators in cancer therapy: New perspectives. Biomed. Pharmacother. 2017, 89, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F. Potential role of natural compounds as anti-angiogenic agents in cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R. Aescin: Pharmacology, pharmacokinetics and therapeutic profile. Pharmacol. Res. 2001, 44, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Li, Y.; Murakami, T.; Ninomiya, K.; Yamahara, J.; Yoshikawa, M. Effects of escins Ia, Ib, IIa, and IIb from horse chestnut, the seeds of Aesculus hippocastanum L., on acute inflammation in animals. Biol. Pharm. Bull. 1997, 20, 1092–1095. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pittler, M.H.; Ernst, E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst. Rev. 2012, 11, CD003230. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Ogawa, S.; Jisaka, M.; Kimura, Y.; Katsube, T.; Yokota, K. Identification of novel saponins from edible seeds of japanese horse chestnut (aesculus turbinata blume) after treatment with wooden ashes and their nutraceutical activity. J. Pharm. Biomed. Anal. 2006, 41, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.N.; Zhu, X.M.; Han, L.K.; Saito, M.; Sun, Y.S.; Yoshikawa, M.; Kimura, Y.; Zheng, Y.N. Anti-obesity effects of escins extracted from the seeds of aesculus turbinata blume (hippocastanaceae). Chem. Pharm. Bull. (Tokyo) 2008, 56, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Murakami, T.; Matsuda, H.; Yamahara, J.; Murakami, N.; Kitagawa, I. Bioactive saponins and glycosides. III. Horse chestnut. (1): The structures, inhibitory effects on ethanol absorption, and hypoglycemic activity of escins Ia, Ib, IIa, IIb, and IIIa from the seeds of Aesculus hippocastanum L. Chem. Pharm. Bull. (Tokyo) 1996, 44, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Domanski, D.; Zegrocka-Stendel, O.; Perzanowska, A.; Dutkiewicz, M.; Kowalewska, M.; Grabowska, I.; Maciejko, D.; Fogtman, A.; Dadlez, M.; Koziak, K. Molecular mechanism for cellular response to beta-escin and its therapeutic implications. PLoS ONE 2016, 11, e0164365. [Google Scholar] [CrossRef] [PubMed]
- Guney, G.; Kutlu, H.M.; Iscan, A. The apoptotic effects of escin in the H-Ras transformed 5RP7 cell line. Phytother. Res. PTR 2013, 27, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Mojzisova, G.; Kello, M.; Pilatova, M.; Tomeckova, V.; Vaskova, J.; Vasko, L.; Bernatova, S.; Mirossay, L.; Mojzis, J. Antiproliferative effect of β-Escin Inhibits colonic aberrant crypt foci formation in rats and regulates the cell cycle growth by inducing p21(waf1/cip1) in colon cancer cells-escin—An in vitro study. Acta Biochim. Pol. 2016, 63, 79–87. [Google Scholar] [PubMed]
- Patlolla, J.M.; Raju, J.; Swamy, M.V.; Rao, C.V. β-Escin Inhibits colonic aberrant crypt foci formation in rats and regulates the cell cycle growth by inducing p21waf1/cip1 in colon cancer cells. Mol. Cancer Ther. 2006, 5, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; Li, F.; Rajendran, P.; Kumar, A.P.; Hui, K.M.; Sethi, G. Identification of beta-escin as a novel inhibitor of signal transducer and activator of transcription 3/janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J. Pharmacol. Exp. Ther. 2010, 334, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, G.A.; Iscan, A.; Kutlu, M. Escin reduces cell proliferation and induces apoptosis on glioma and lung adenocarcinoma cell lines. Cytotechnology 2015, 67, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, X.; Zhao, P.; Tong, B.; Wei, Z.; Dai, Y. Escin Ia suppresses the metastasis of triple-negative breast cancer by inhibiting epithelial-mesenchymal transition via down-regulating LOXL2 expression. Oncotarget 2016, 7, 23684–23699. [Google Scholar] [CrossRef] [PubMed]
- Rimmon, A.; Vexler, A.; Berkovich, L.; Earon, G.; Ron, I.; Lev-Ari, S. Escin chemosensitizes human pancreatic cancer cells and inhibits the nuclear factor-κB signaling pathway. Biochem. Res. Int. 2013, 2013, 251752. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.B.; Sung, B.; Pandey, M.K.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Escin, a pentacyclic triterpene, chemosensitizes human tumor cells through inhibition of nuclear factor-κB signaling pathway. Mol. Pharmacol. 2010, 77, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, J.M.; Qian, L.; Biddick, L.; Zhang, Y.; Desai, D.; Amin, S.; Lightfoot, S.; Rao, C.V. Beta-escin inhibits NNK-induced lung adenocarcinoma and ALDH1A1 and RhoA/ROCK expression in A/J mice and growth of H460 human lung cancer cells. Cancer Prev. Res. (Phila) 2013, 6, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yu, W.; Liu, B.; Wang, Y.; Shao, J.; Wang, J.; Xia, K.; Liang, C.; Fang, W.; Zhou, C.; et al. Escin induces caspase-dependent apoptosis and autophagy through the ROS/p38 MAPK signalling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2017, 8, e3113. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Kang, M.; Lee, Y.J.; Choi, W.S.; Chun, Y.S.; Kwak, C.; Kim, H.H. Cytotoxic effects of escin on human castration-resistant prostate cancer cells through the induction of apoptosis and G2/M cell cycle arrest. Urology 2014, 84, e981–e987. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.W.; Wang, S.J.; Zhou, Y.N.; Pan, S.H.; Sun, B. Escin augments the efficacy of gemcitabine through down-regulation of nuclear factor-κB and nuclear factor-κB-regulated gene products in pancreatic cancer both in vitro and in vivo. J. Cancer Res. Clin. Oncol. 2012, 138, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zong, X.; Ge, B.; Wang, S.; Ji, J.; Ye, Y.; Pan, L. Pilot postoperative ileus study of escin in cancer patients after colorectal surgery. World J. Surg. 2009, 33, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.Y.; Zhang, M.J.; Wang, Y.Y.; Liu, Y.H. The positive clinical therapeutically effects of escin on advanced thyroid cancer. Cancer Med. 2017, 6, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Xu, B.; Liu, J.T.; Cui, J.R. Effect of beta-escin sodium on endothelial cells proliferation, migration and apoptosis. Vasc. Pharmacol. 2008, 49, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Khalid, E.B.; Ayman, E.E.; Rahman, H.; Abdelkarim, G.; Najda, A. Natural products against cancer angiogenesis. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 14513–14536. [Google Scholar] [CrossRef] [PubMed]
- Gerwins, P.; Skoldenberg, E.; Claesson-Welsh, L. Function of fibroblast growth factors and vascular endothelial growth factors and their receptors in angiogenesis. Crit. Rev. Oncol./Hematol. 2000, 34, 185–194. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Vacca, A.; Roncali, L.; Burri, P.H.; Djonov, V. Chorioallantoic membrane capillary bed: A useful target for studying angiogenesis and anti-angiogenesis in vivo. Anat. Rec. 2001, 264, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Karar, J.; Maity, A. PI3K/Akt/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.B.; Nabha, S.M.; Atanaskova, N. Role of map kinase in tumor progression and invasion. Cancer Metast. Rev. 2003, 22, 395–403. [Google Scholar] [CrossRef]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y.; et al. Ginkgolic acid inhibits invasion and migration and TGF-β-induced EMT of lung cancer cells through PI3K/AKT/mTOR inactivation. J. Cell. Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Yap, W.N.; Arfuso, F.; Kar, S.; Wang, C.; Cai, W.; Dharmarajan, A.M.; Sethi, G.; Kumar, A.P. Targeting the PI3K/AKT signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015, 21, 12261–12273. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.C.; Wang, X.Q.; Lu, K.; Deng, X.L.; Zhang, C.W.; Luo, H.; Xu, X.D.; Chen, X.M.; Yan, L.; Wang, Y.Q.; et al. Ephrin-B2/Fc promotes proliferation and migration, and suppresses apoptosis in human umbilical vein endothelial cells. Oncotarget 2017, 8, 41348–41363. [Google Scholar] [CrossRef] [PubMed]
- Auguste, P.; Javerzat, S.; Bikfalvi, A. Regulation of vascular development by fibroblast growth factors. Cell Tissue Res. 2003, 314, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Woessner, J.F., Jr. That impish TIMP: The tissue inhibitor of metalloproteinases-3. J. Clin. Investig. 2001, 108, 799–800. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.H.; Ebrahem, Q.; Moore, N.; Murphy, G.; Claesson-Welsh, L.; Bond, M.; Baker, A.; Anand-Apte, B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): Inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat. Med. 2003, 9, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Defilippi, P.; van Hinsbergh, V.; Bertolotto, A.; Rossino, P.; Silengo, L.; Tarone, G. Differential distribution and modulation of expression of alpha 1/beta 1 integrin on human endothelial cells. J. Cell Biol. 1991, 114, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Van Hinsbergh, V.W.; Sprengers, E.D.; Kooistra, T. Effect of thrombin on the production of plasminogen activators and pa inhibitor-1 by human foreskin microvascular endothelial cells. Thromb. Haemost. 1987, 57, 148–153. [Google Scholar] [PubMed]
- Varinska, L.; van Wijhe, M.; Belleri, M.; Mitola, S.; Perjesi, P.; Presta, M.; Koolwijk, P.; Ivanova, L.; Mojzis, J. Anti-angiogenic activity of the flavonoid precursor 4-hydroxychalcone. Eur. J. Pharmacol. 2012, 691, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Poveda, B.; Quesada, A.R.; Medina, M.A. Hypericin in the dark inhibits key steps of angiogenesis in vitro. Eur. J. Pharmacol. 2005, 516, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, M.N.; Davis, J.; Hughes, C.C. Optimized fibrin gel bead assay for the study of angiogenesis. J. Vis. Exp. JoVE 2007, 3, 186. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the compound β-escin is available from the authors. |
| Treatment | Control | bFGF | bFGF + β-Escin 60 μg/mL |
|---|---|---|---|
| Sub-G1 | 2.45 ± 0.05 | 3.20 ± 0.70 | 3.80 ± 1.06 |
| G0/G1 | 72.67 ± 3.77 | 40.05 ± 1.15 ** | 42.60 ± 1.38 ** |
| S | 10.73 ± 2.07 | 18.15 ± 1.45 * | 38.65 ± 2.99 **,++ |
| G2/M | 14.15 ± 1.75 | 38.60 ± 1.90 ** | 14.95 ± 1.95 ++ |
| Gene Symbol | Gene Name | β-Escin/Control |
|---|---|---|
| EDN1 | Endothelin-1 | +2.3 |
| EFNB2 | Ephrin B2 | −2.3 |
| FGF1 | Fibroblast Growth Factor 1 | −5.3 |
| TIMP3 | Tissue Inhibitor of Metalloproteinases 3 | −4.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varinská, L.; Fáber, L.; Kello, M.; Petrovová, E.; Balážová, Ľ.; Solár, P.; Čoma, M.; Urdzík, P.; Mojžiš, J.; Švajdlenka, E.; et al. β-Escin Effectively Modulates HUVECs Proliferation and Tube Formation. Molecules 2018, 23, 197. https://doi.org/10.3390/molecules23010197
Varinská L, Fáber L, Kello M, Petrovová E, Balážová Ľ, Solár P, Čoma M, Urdzík P, Mojžiš J, Švajdlenka E, et al. β-Escin Effectively Modulates HUVECs Proliferation and Tube Formation. Molecules. 2018; 23(1):197. https://doi.org/10.3390/molecules23010197
Chicago/Turabian StyleVarinská, Lenka, Lenka Fáber, Martin Kello, Eva Petrovová, Ľudmila Balážová, Peter Solár, Matúš Čoma, Peter Urdzík, Ján Mojžiš, Emil Švajdlenka, and et al. 2018. "β-Escin Effectively Modulates HUVECs Proliferation and Tube Formation" Molecules 23, no. 1: 197. https://doi.org/10.3390/molecules23010197
APA StyleVarinská, L., Fáber, L., Kello, M., Petrovová, E., Balážová, Ľ., Solár, P., Čoma, M., Urdzík, P., Mojžiš, J., Švajdlenka, E., Mučaji, P., & Gál, P. (2018). β-Escin Effectively Modulates HUVECs Proliferation and Tube Formation. Molecules, 23(1), 197. https://doi.org/10.3390/molecules23010197







