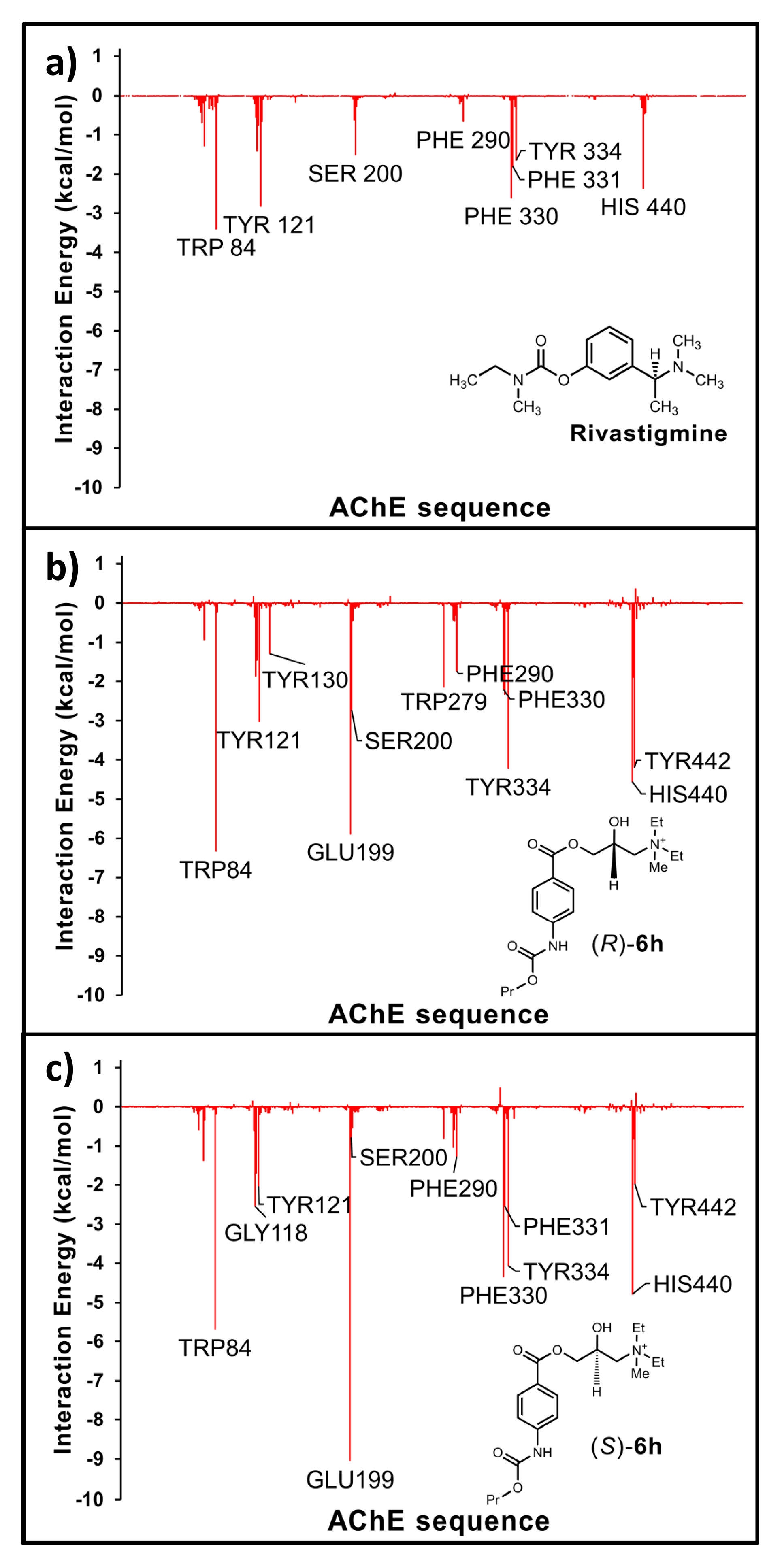
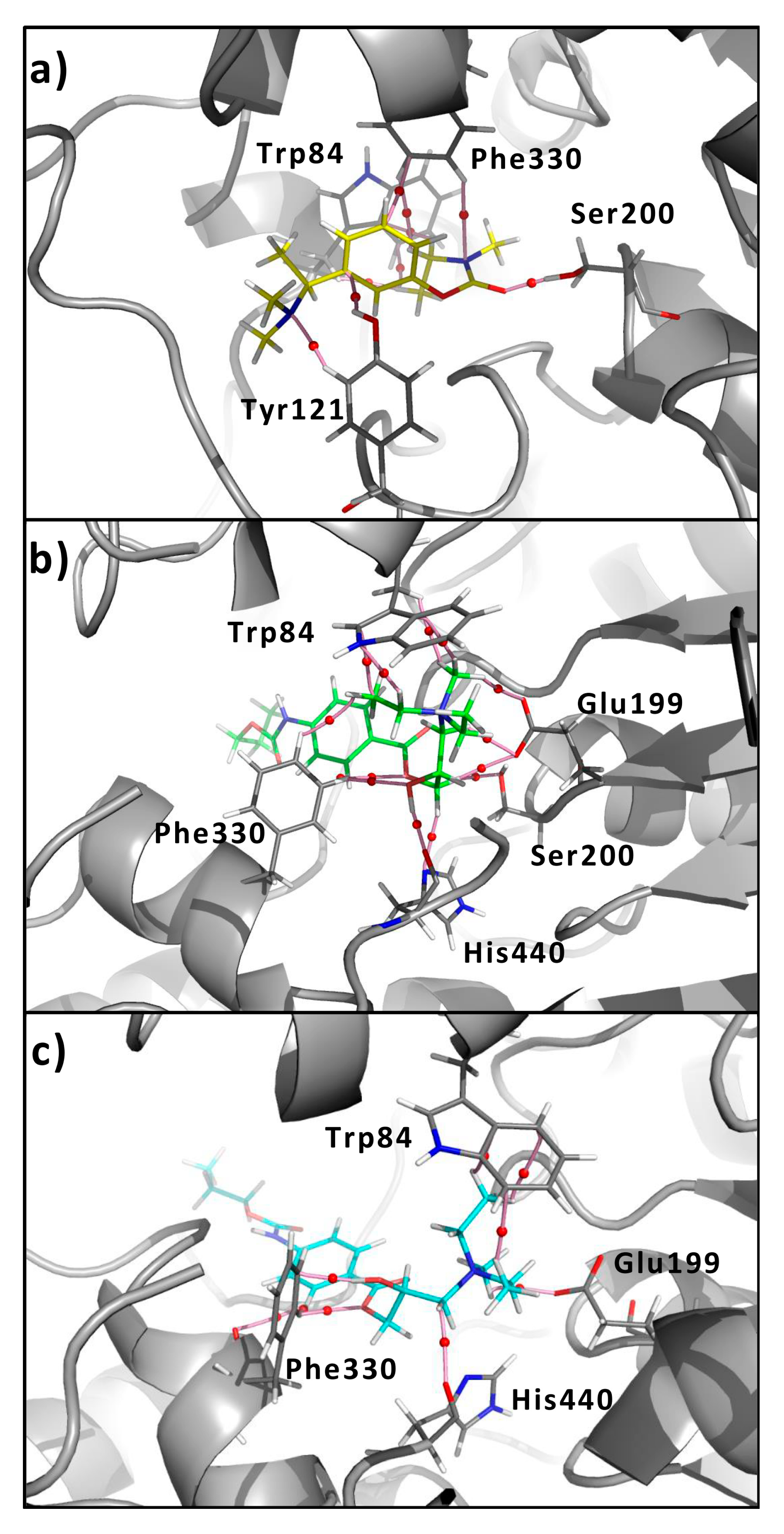
3.1. General Information
All reagents were procured from Sigma-Aldrich (St. Louis, MO, USA) and Acros Organics (Geel, Belgium) in sufficient purity, solvents from Lach-Ner (Neratovice, Czech Republic) were dried or freshly distilled if necessary. TLC Kieselgel 60 F254 plates (Merck, Darmstadt, Germany) visualized by UV irradiation (254 nm) were utilized to monitor reactions and purity of prepared substances and reverse-phase TLC RP-18 F254 plates (Merck) for the final compounds. Melting points were determined on Kofler hot-plate apparatus HMK (Franz Kustner Nacht KG, Dresden, Germany) and remain uncorrected.
Monitoring of purity of the resulting compounds ran on Dionex Ultimate 3000 Series HPLC instrument (Thermo Fisher Scientific, Waltham, MA, USA). The Chromeleon® software (version 7.2, Thermo Fisher Scientific, Waltham, MA, USA) was employed to collect the data. The purity of compound was formulated as relative proportion of the peak area of the analyte to the total area of all peaks in the chromatogram after subtracting the peak areas in the solvent chromatogram (blank). The mobile phase consisted of acetonitrile: 25 mM formate buffer (pH = 5.0) in the ratio of 3:7 (v/v). For the distribution of substances, the YMC-Triart C18 column (150 × 2 mm; 3 μm) maintained at 30 °C was used. The flow rate was set up to 0.2 mL min−1, and detection of compounds at 273 nm.
The log
kw9.0 values of synthetized compounds were measured by a Dionex Ultimate 3000 Series HPLC instrument (Thermo Fisher Scientific) controlled through the Chromeleon
® software (version 7.2). The separation was performed on ZORBAX Extend-C
18 (3 × 150 mm, 3.5 μm) column (Agilent Technologies, Waldbronn, Germany). Mobile phase consists of 0.02 M
N-[tris(hydroxymethyl)-methyl]-3-aminopropanesulfonic acid with addition of 0.15% of
n-decylamine (pH = 9.0; constituent A) and methanol to which 0.25% of
n-octanol was added (constituent B). Total flow rate reached 0.4 mL min
−1, the injection volume was 1 μL, and the column temperature was maintained at 25 °C. The detection wavelength of 254 nm was set up. One milligram of sample was dissolved in 1 mL of methanol and diluted to concentration 0.1 mg mL
−1 with 50% methanol. Retention factors of the compound were measured under isocratic conditions in the range 50:50–70:30 (A:B;
v/
v), 5 values for each factor in duplicate. Linear regression analysis was performed alongside with log
kw9.0 values calculation based on Equation (4):
where log
k represents a logarithm of an individual isocratic factor, Φ is the organic phase concentration, and
S is a constant derived from linear regression analysis. Retention factor corresponding with the neutral form (log
kw) was estimated from the apparent log
kw9.0 by using Equation (5):
CE experiments were carried out on Agilent 3D CE (Agilent Technologies, Santa Clara, CA, USA) capillary electrophoresis system equipped with an autosampler, automatic injector, photodiode array detector, and an air cooling unit for the capillary. Instrument control and analysis were performed with 3D-CE ChemStation software (Agilent Technologies). Uncoated fused-silica capillary (Agilent Technologies) of 50 μm internal diameter, the total length of 33.0 cm and effective length (to the detector) of 24.5 cm was used. Capillary cassette temperature was maintained at 25 °C with air cooling. Three milligrams of the sample were dissolved in 1 mL of 50% methanol and 10 times diluted with appropriate background electrolyte (BGE) before analysed. Samples were injected hydrodynamically at 40 mbar pressure for 4.0 s. Operating voltage was 15 kV of positive polarity. UV detection was performed at 254 nm. Mesityl oxide was used as a marker of electroosmotic flow. Effective mobilities were recorded for BGE pH values in the range from 3.5 to 11.1 (ionic strength equal to 10 mM), 4 runs at 14 different buffer pH values. The effective mobilities μ
eff were calculated according to Equation (6):
where
tmig is migration time of the analyte and
tEOF is migration time of a neutral marker compound that is of the same velocity as the electroosmotic flow (EOF).
Leff is distance from the injection end of the capillary to the detector and
Ltot is the total length of the capillary over which the voltage
U was applied. SigmaPlot for Windows version 11.0 (Systat Software GmbH, Erkrath, Germany) was employed for the nonlinear regression analysis of μ
eff/pH relationship having characteristic sigmoidal shape with inflexion point indicating p
Ka value. The pH measurements were taken with a combination glass electrode (HI 1332B, HANNA Instruments, Woonsocket, RI, USA), using a Thermo Orion 370 PerpHect
® pH meter (Thermo Fisher Scientific, Waltham, MA, USA).
High-resolution mass spectra of the final compounds were measured using a Dionex Ultimate 3000 Series high-performance liquid chromatograph (Thermo Fisher Scientific) coupled with a LTQ Orbitrap XL™ Hybrid Ion Trap-Orbitrap Fourier Transform Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with injection into HESI II.
The NMR spectra of compounds 5c,d and 6a–i in their liquid state were measured in DMSO-d6 solution on a Bruker Avance III HD NMR spectrometer 700 MHz (700.25 MHz for 1H and 176.08 MHz for a 13C nucleus; Bruker BioSpin GmbH, Rheinstetten, Germany) equipped with 5 mm sensitive triple-resonance (1H-13C-15N) cryoprobe optimized for 13C detection. The NMR spectra of all intermediates and final compounds 5a, b, e and f in the liquid state were measured in DMSO-d6 on a JNM-ECZ4OOR FT-NMR spectrometer 9.39 T (399.78 MHz for 1H and 100.53 MHz for 13C nucleus; Jeol Resonance, Tokyo, Japan) equipped with a 5 mm High Sensitivity PulseField Gradient Autotune™ probe. Chemical shifts are reported in ppm, referenced to the chemical shifts of residual solvent resonance (DMSO, 2.5 ppm for 1H and 39.5 ppm for 13C). The coupling constants (J) are reported in Hz. Spectra were recorded at the temperature 30 °C. All major signals were assigned on the basis 1H, 13C{H}, 13C-APT, 1H-1H COSY, 1H-13C HSQC, 1H-13C HMBC and 1H-15N HMBC experiments.
Infrared (IR) spectra were measured on a Nicolet Nexus FT-IR spectrometer (Thermo Scientific) using ATR (ZnSe) instrumentation. The spectra were obtained by accumulation of 12 scans with 4 cm−1 resolution in the region 4000–600 cm−1. The instrument was controlled by software Omnic v. 8.3 (Thermo Scientific).
The last reaction (
Section 3.2.5) was carried out in monomodal StartSYNTH, Microwave reactor Synthesis Labstation at 2.45 GHz (M/s, Milestone S.r.l., Milan, Italy). The device is equipped with an industrial magnetron and a microwave diffuser is located above microwave chamber with a continuous output of 0–1400 W. Temperatures were measured by infrared sensor.
3.2. Synthesis
3.2.1. Synthesis of Carbamate Intermediates
An equivalent of pyridine (0.15 mol) was added to a solution of 4-aminobenzoic acid (0.15 mol) in acetone (200 mL). Subsequently, the appropriate methyl, ethyl, propyl or butyl chloroformate (0.15 mol) was added dropwise and the reaction mixture was heated under reflux to 70 °C for 2.5 h. After evaporation of the solvent, the resulting crystals were washed with distilled water on a glass frit and were recrystallized from 50% ethanol.
4-[(Methoxycarbonyl)amino]benzoic acid (1a). Yield: 89%; Rf: 0.54 (ethyl acetate/petroleum ether/MeOH 10:1:1); m.p.: 204–207 °C; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 12.67 (s, 1H, –COOH); 10.04 (s, 1H, –NH); 7.86 (d, J = 8.6 Hz, 2H, –CHAr(–COOH)); 7.56 (d, J = 8.6 Hz, 2H, –CHAr(–NHCOO–)); 3.69 (s, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 166.9 (–COOH); 153.8 (–NHCOO–); 143.4 (–CAr(–NHCOO–)); 130.5 (–CHAr(–COOH)); 124.4 (–CAr(–COOH)); 117.3 (–CHAr(–NHCOO–)); 51.8 (–CH3).
4-[(Ethoxycarbonyl)amino]benzoic acid (1b). Yield: 96%; Rf: 0.69 (ethyl acetate/petroleum ether/MeOH 10:1:1); m.p.: 197–200 °C; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 12.66 (s, 1H, –COOH); 10.02 (s, 1H, –NH); 7.86 (d, J = 8.6 Hz, 2H, –CHAr(–COOH)); 7.57 (d, J = 8.6 Hz, 2H, –CHAr(–NHCOO–)); 4.14 (q, J = 7.3 Hz, 2H, –CH2–); 1.25 (t, J = 7.3, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 166.8 (–COOH); 153.3 (–NHCOO–); 143.3 (–CAr(–NHCOO–)); 130.3 (–CHAr(–COOH)); 124.2 (–CAr(–COOH)); 117.2 (–CHAr(–NHCOO–)); 60.4 (–CH2-); 14.3 (–CH3).
4-[(Propoxycarbonyl)amino]benzoic acid (1c). Yield: 91%; Rf: 0.80 (ethyl acetate/petroleum ether/MeOH 10:1:1); m.p.: 190–194 °C; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 12.66 (s, 1H, –COOH); 10.01 (s, 1H, –NH); 7.86 (d, J = 8.7 Hz, 2H, –CHAr(–COOH)); 7.57 (d, J = 8.7 Hz, 2H, –CHAr(–NHCOO–)); 4.05 (t, J = 6.6 Hz, 2H, –COOCH2–); 1.73–1.55 (m, 2H, –CH2CH2CH3); 0.93 (t, J = 7.5 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 166.8 (–COOH); 153.4 (–NHCOO–); 143.3 (–CAr(–NHCOO–)); 130.3 (–CHAr(–COOH)); 124.2 (–CAr(–COOH)); 117.2 (–CHAr(–NHCOO–)); 65.9 (–COOCH2–); 21.7 (–CH2CH3); 10.1(–CH3).
4-[(Butoxycarbonyl)amino]benzoic acid (1d). Yield: 70%; Rf: 0.83 (ethyl acetate/petroleum ether/MeOH 10:1:1); m.p.: 179–182 °C; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 12.66 (s, 1H, –COOH); 10.00 (s, 1H, –NH); 7.86 (d, J = 8.6 Hz, 2H, –CHAr(–COOH)); 7.57 (d, J = 8.6 Hz, 2H, –CHAr(–NHCOO–)); 4.10 (t, J = 6.8 Hz, 2H, –COOCH2–); 1.67–1.54 (m, 2H, –CH2CH2CH2CH3); 1.46–1.28 (m, 2H, –CH2CH2CH2CH3); 0.91 (t, J = 7.3 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 166.8 (–COOH); 153.4 (–NHCOO–); 143.3 (–CAr(–NHCOO–)); 130.3 (–CHAr(–COOH)); 124.2 (–CAr(–COOH)); 117.2 (–CHAr(–NHCOO–)); 64.1 (–COOCH2–); 30.4 (–CH2CH2CH3); 18.4(–CH2CH2CH3); 13.4 (–CH3).
3.2.2. Synthesis of Acyl Chloride Intermediates
Alkyl [4-(chlorocarbonyl)phenyl]carbamate 2a–d preparation: thionyl chloride (0.12 mol) was added to suspension of compound 1a–d (0.06 mol) in 150 mL of dry toluene (over P2O5). The reaction mixture was then being heated under reflux to 120 °C for 2 h. Hot solution was filtered through a frit, maternal liquor was cooled down and the precipitated crystals were filtered on sintered glass, washed with petroleum ether and dried in vacuum. Acyl chloride was used immediately to proceed to the next reaction.
3.2.3. Synthesis of Oxirane Intermediates
(Oxiran-2-yl)methanol (0.03 mol) and THF (100 mL) were added to a three-necked flask equipped with a drying calcium chloride tube, a thermometer and a dropping funnel. The mixture was cooled down to −5 °C and triethylamine (0.03 mol) was added to the mixture. Subsequently, appropriate acyl chloride (2a–d) (0.03 mol) dissolved in THF (50 mL) was dropwise added to the suspension at a rate allowing to maintain the temperature between −5 °C and 5 °C, after which the mixture was being stirred for 3 h at room temperature. The precipitated triethylammonium chloride was then filtered out of the reaction mixture. The solvent from maternal liquor was evaporated under vacuum, producing the crystals of epoxide.
Oxiran-2-ylmethyl 4-[(methoxycarbonyl)amino]benzoate (3a). Yield: 82%; Rf: 0.79 (acetone/petroleum ether 2:3); m.p.: 125–129 °C; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 10.06 (s, 1H, –NH); 7.91 (d, J = 8.8 Hz, 2H, –CHAr(–COO–)); 7.60 (d, J = 8.8 Hz, 2H, –CHAr(–NHCOO–)); 4.61 (dd, 2J = 12.4, 3J = 2.6 Hz, 1H, ArCOOCH2–); 4.09 (dd, 2J = 12.4 Hz, 3J = 6.4 Hz, 1H, ArCOOCH2–); 3.69 (s, 3H, –CH3); 3.31 (m, 1H, CH-oxiran); 2.83 (m, 1H, CH2-oxiran); 2.74 (dd, 2J = 5.0 Hz, 3J = 2.6, 1H, CH2-oxiran); 13C-NMR (100 MHz, DMSO-d6), δ (ppm): 165.1 (–COO–); 153.5 (–NHCOO–); 143.9 (–CAr(–NHCOO–)); 130.4 (–CHAr(–COO–)); 122.8 (–CAr(–COO–)); 117.4 (–CHAr(–NHCOO–)); 65.0 (ArCOOCH2–); 51.8 (–CH3); 49.0 (CH-oxiran); 43.8 (CH2–oxiran).
Oxiran-2-ylmethyl 4-[(ethoxycarbonyl)amino]benzoate (3b). Yield: 93%; Rf: 0.86 (acetone/petroleum ether 2:3); m.p.: 129–132 °C; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 10.05 (s, 1H, –NH); 7.91 (d, J = 8.8 Hz, 2H, –CHAr(–COO–)); 7.61 (d, J = 8.8 Hz, 2H, –CHAr(–NHCOO–)); 4.59 (dd, 2J = 12.4 Hz, 3J = 2.6 Hz, 1H, ArCOOCH2–); 4.16 (q, J = 7.1 Hz, 2H, –NHCOOCH2–); 4.05 (dd, 2J = 12.4 Hz, 3J = 6.4 Hz, 1H, ArCOOCH2–); 3.31 (m, 1H, CH-oxiran); 2.83 (m, 1H, CH2–oxiran); 2.72 (dd, 2J = 5.0 Hz, 3J = 2.6 Hz, 1H, CH2-oxiran); 1.26 (t, J = 7.1 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 165.1 (–COO–); 153.8 (–NHCOO–); 144.0 (–CAr(–NHCOO–)); 130.4 (–CHAr(–COO–)); 122.7 (–CAr(–COO–)); 117.4 (–CHAr(–NHCOO–)); 65.0 (ArCOOCH2–); 60.5 (–CH2CH3); 49.0 (CH-oxiran); 43.8 (CH2-oxiran); 14.4 (–CH3).
Oxiran-2-ylmethyl 4-[(propoxycarbonyl)amino]benzoate (3c). Yield: 80%; Rf: 0.87 (acetone/petroleum ether 2:3); m.p.: 110–113 °C; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 10.05 (s, 1H, –NH); 7.91 (d, J = 8.8 Hz, 2H, –CHAr(–COO–)); 7.62 (d, J = 8.8 Hz, 2H, –CHAr(–NHCOO–)); 4.60 (dd, 2J = 12.4 Hz, 3J = 2.7 Hz, 1H, ArCOOCH2–); 4.05 (m, 3H, –NHCOOCH2– and ArCOOCH2–); 3.31 (m, 1H, CH-oxiran); 2.83 (m, 1H, CH2-oxiran); 2.72 (dd, 2J = 5.0 Hz, 3J = 2.6 Hz, 1H, CH2-oxiran); 1,65 (m, 2H, –CH2CH2CH3); 0.94 (t, J = 7.4 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 165.1 (–COO–); 153.4 (–NHCOO–); 144.0 (–CAr(–NHCOO–)); 130.4 (–CHAr(–COO–)); 122.7 (–CAr(–COO–)); 117.4 (–CHAr(–NHCOO–)); 66.0 (–CH2CH2CH3); 65.0 (ArCOOCH2–); 49.0 (CH-oxiran); 43.8 (CH2-oxiran); 21.7 (–CH2CH2CH3); 10.2 (–CH3).
Oxiran-2-ylmethyl 4-[(butoxycarbonyl)amino]benzoate (3d). Yield: 72%; Rf: 0.90 (acetone/petroleum ether 2:3); m.p.: 98–101 °C; 1H-NMR (400 MHz, DMSO-d6) δ (ppm): 10.04 (s, 1H, –NH); 7.90 (d, J = 8.9 Hz, 2H, –CHAr(–COO–)); 7.61 (d, J = 8.9 Hz, 2H, –CHAr(–NHCOO–)); 4.59 (dd, 2J = 12.4 Hz, 3J = 2.7 Hz, 1H, ArCOOCH2–); 4.11 (t, J = 6.5 Hz, 2H, –NHCOOCH2–); 4.05 (dd, 2J = 12.4 Hz, 3J = 6.4 Hz, 1H, ArCOOCH2–); 3.31 (m, 1H, CH–oxiran); 2.83 (m, 1H, CH2–oxiran); 2.72 (dd, 2J = 5.0 Hz, 3J = 2.6 Hz, 1H, CH2-oxiran); 1.62 (m, 2H, –CH2CH2CH2CH3); 1.38 (m, 2H, –CH2CH2CH2CH3); 0.92 (t, J = 7.2 Hz, 3H, –CH3); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 165.1 (–COO–); 153.4 (–NHCOO–); 144.0 (–CAr(–NHCOO–)); 130.4 (–CHAr(–COO–)); 122.8 (–CAr(–COO–)); 117.3 (–CHAr(–NHCOO–)); 65.0 (ArCOOCH2–); 64.2 (–CH2CH2CH2CH3); 49.0 (CH-oxiran); 43.8 (CH2–oxiran); 30.4 (–CH2CH2CH2CH3); 18.5 (–CH2CH2CH2CH3); 13.5 (–CH3).
3.2.4. Synthesis of Tertiary Amines as Final Compounds
Compound
3a–
d (0.02 mol) in 100 mL of ethanol were added to a round bottom flask. The mixture was carefully heated to 70 °C until the oxirane-compound dissolved. Then, the mixture was cooled down to room temperature and secondary amine (
N,
N-dimethyl- or
N,
N-diethylamine respectively, 0.02 mol) was added. The reaction mixture was heated to 60 °C and allowed to stir for 5 h. Then, the solvent was evaporated under reduced pressure producing a residue which was suspended in diethyl ether (30 mL). The resulting crystalline impurities were filtered off and ethereal HCl (30 mL) was added to the filtrate producing crystals that were filtered out and recrystallized from acetone: methanol 3:1. The final products were obtained in the form of hydrochloride salt. See the
1H- and
13C-NMR spectra of compounds
5a–
f as
Supplementary Materials (
Figures S2–S13).
(2-Hydroxy-3-{4-[(methoxycarbonyl)amino]benzoyloxy}propyl)dimethylammonium chloride (5a). Yield: 75%; Rf: 0.46 (ethyl acetate/diethylamine 10:1); Rf (rev.): 0.75 (0.1 M HCl/acetone 3:2); m.p.: 199–202 °C; HPLC pur. 95.22% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3330 w; 3257 br; 2949 w; 2668 w; 1740 s; 1689 s; 1600 s; 1541 s; 1222 s; 1185 s; 1H-NMR (400 MHz, DMSO-d6): δ (ppm) = 10.15 (s, 1H, –NHCOO–); 9.97 (bs, 1H, –NH+); 7.97 (d, J = 8.7 Hz, 2H, –CHAr(–COO–)); 7.62 (d, J = 8.7 Hz, 2H, –CHAr(–NHCOO–)); 6.04 (d, J = 5.0 Hz, 1H, –OH); 4.30 (m, 1H, –COOCH2CH–); 4.20 (m, 2H, –COOCH2CH–); 3.69 (s, 3H, –CH3); 3.23 (m, 2H, –CH2NH+–); 2.82 (s, 6H, –NH+(CH3)2); 13C-NMR (100 MHz, DMSO-d6): δ (ppm) = 165.1 (–COO–); 153.8 (–NHCOO–); 144.0 (–CAr(–NHCOO–)); 130.7 (–CHAr(–COO–)); 122.9 (–CAr(–COO–)); 117.3 (–CHAr(–NHCOO–)); 66.0 (–COOCH2CH–); 63.4 (–COOCH2CH–); 58.8 (–CH2NH+–); 52.0 (–CH3); 43.7 and 41.9 (–NH+(CH3)2); HR-MS: C14H19N2O5 [M − H]− calculated 295.1299 m/z; found 295.1304 m/z.
(3-{4-[(ethoxycarbonyl)amino]benzoyloxy}-2-hydroxypropyl)dimethylammonium chloride (5b). Yield: 71%; Rf: 0.54 (ethyl acetate/diethylamine 10:1); Rf (rev.): 0.68 (0.1 M HCl/acetone 3:2); m.p.: 168–171 °C; HPLC pur. 96.42% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3346 w; 3261 br; 2987 w; 2671 s; 1737 s; 1693 s; 1597 s; 1539 s; 1216 s; 1182 s; 1H-NMR (400 MHz, DMSO-d6): δ (ppm) = 10.08–10.06 (overlap m, 2H, –NHCOO– + –NH+); 7.96 (d, J = 8.7 Hz, 2H, –CHAr(–COO–)); 7.62 (d, 2H, J = 8.7 Hz, –CHAr(–NHCOO–)); 6.03 (d, J = 5.0 Hz, 1H, –OH); 4.31 (m, 1H, –COOCH2CH–); 4.20 (m, 2H, –COOCH2CH–); 4.15 (q, J = 7.1 Hz, 2H, –CH2CH3); 3.23 (m, 2H, –CH2NH+–); 2.83 (s, 6H, –NH+(CH3)2); 1.25 (t, J = 7.1 Hz, 3H, –CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ (ppm) = 165.1 (–COO–); 153.3 (–NHCOO–); 144.0 (–CAr(–NHCOO–)); 130.6 (–CHAr(–COO–)); 122.8 (–CAr(–COO–)); 117.3 (–CHAr(–NHCOO–)); 66.0 (–COOCH2CH–); 63.4 (–COOCH2CH–); 60.5 (–CH2CH3); 58.8 (–CH2NH+–); 43.8 and 41.9 (–NH+(CH3)2); 14.4 (–CH3); HR-MS: C15H21N2O5 [M − H]− calculated 309.1456 m/z; found 309.1459 m/z.
(2-Hydroxy-3-{4-[(propoxycarbonyl)amino]benzoyloxy}propyl)dimethylammonium chloride (5c). Yield: 60%; Rf: 0.59 (ethyl acetate/diethylamine 10:1); Rf (rev.): 0.61 (0.1 M HCl/acetone 3:2); m.p.: 151–154 °C; HPLC pur. 95.15% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3356 w; 2972 w; 2672 w; 1745 s; 1699 s; 1592 s; 1537 s; 1213 s; 1177 s; 1H-NMR (700 MHz, DMSO-d6): δ (ppm) = 10.17 (bs, 1H, NH+); 10.09 (bs, 1H, –NHCOO–); 7.96 (d, J = 8.9 Hz, 2H, –CHAr(–COO–)); 7.62 (d, J = 8.9 Hz, 2H, –CHAr(–NHCOO–)); 6.04 (d, J = 5.3 Hz, 1H, –OH); 4.32 (m, 1H, –COOCH2CH–); 4.20 (m, 2H, –COOCH2CH–); 4.06 (t, J = 6.7 Hz, 2H, –CH2CH2CH3); 3.24 (m, 2H, –CH2NH+–); 2.83 (s, 6H, –NH+(CH3)2); 1.64 (m, 2H, –CH2CH2CH3); 0.93 (t, J = 7.4 Hz, 3H, –CH2CH2CH3); 13C-NMR (176 MHz, DMSO-d6): δ (ppm) = 165.10 (–COO–); 153.42 (–NHCOO–); 144.06 (–CAr(–NHCOO–)); 130.67 (–CHAr(–COO–)); 122.82 (–CAr(–COO–)); 117.30 (–CHAr(–NHCOO–)); 66.08 (–COOCH2CH–) and 66.04 (–CH2CH2CH3); 63.43 (–COOCH2CH–); 58.83 (–CH2N+–); 43.76 and 42.01 (–NH+(CH3)2); 21.82 (–CH2CH2CH3); 10.24 (–CH3); 15N (DMSO-d6): δ (ppm) = 33.5 (–NH+(CH3)2); 103.9 (–NHCOO–); HR-MS: C16H23N2O5 [M − H]− calculated 323.1612 m/z; found 323.1614 m/z.
(3-{4-[(Butoxycarbonyl)amino]benzoyloxy}-2-hydroxypropyl)dimethylammonium chloride (5d). Yield: 58%; Rf: 0.64 (ethyl acetate/diethylamine 10:1); Rf (rev.): 0.51 (0.1 M HCl/acetone 3:2); m.p.: 119–122 °C; HPLC pur. 94.73% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3350 w; 2966 w; 2669 w; 1748 s; 1696 s; 1590 s; 1538 s; 1222 s; 1178 s; 1H-NMR (700 MHz, DMSO-d6): δ (ppm) = 10.25 (bs, 1H, NH+); 10.11 (s, 1H, –NHCOO–); 7.96 (d, J = 8.9 Hz, 2H, –CHAr(–COO–)); 7.62 (m, J = 8.9 Hz, 2H, –CHAr(–NHCOO–)); 6.08 (d, J = 5.0 Hz, 1H, –OH), 4.32 (m, 1H, –COOCH2CH–); 4.20 (m, 2H, –COOCH2CH–); 4.10 (t, J = 6.6 Hz, 2H, –CH2CH2CH2CH3); 3.24 (m, 2H, –CH2N+–); 2.82 (s, 6H, –NH+(CH3)2); 1.60 (m, 2H, –CH2CH2CH2CH3), 1.37 (m, 2H, –CH2CH2CH2CH3), 0.91 (t, J = 7.3 Hz, 3H, –CH2CH2CH2CH3); 13C-NMR (176 MHz, DMSO-d6): δ (ppm) = 165.11 (–COO–); 153.40 (–NHCOO–); 144.00 (–CAr(–NHCOO–)); 130.66 (–CHAr(–COO–)); 122.82 (–CAr(–COO–)); 117.29 (–CHAr(–NHCOO–)); 66.08 (–COOCH2CH–); 64.23 (–CH2CH2CH2CH3); 63.44 (–COOCH2CH–); 58.84 (–CH2N+–); 43.72 and 42.09 (–NH+(CH3)2); 30.48 (–CH2CH2CH2CH3); 18.58 (–CH2CH2CH2CH3); 13.59 (–CH3); HR-MS: C17H25N2O5 [M − H]− calculated 337.1769 m/z; found 337.1770 m/z.
Diethyl-(2-hydroxy-3-{4-[(methoxycarbonyl)amino]benzoyloxy}propyl)ammonium chloride (5e). Yield: 78%; Rf: 0.71 (ethyl acetate/diethylamine 10:1); Rf (rev.): 0.67 (0.1 M HCl/acetone 3:2); m.p.: 165–168 °C; HPLC pur. 88.42% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3310 w; 2957 w; 2603 w; 1728 s; 1686 s; 1598 s; 1539 s; 1229 s; 1182 s; 1H-NMR (400 MHz, DMSO-d6): δ (ppm) = 10.11 (s, 1H, –NHCOO–); 9.80 (bs, 1H, NH+); 7.96 (d, J = 8.8 Hz, 2H, –CHAr(–COO–)); 7.62 (d, J = 8.8 Hz, 2H, –CHAr(–NHCOO–)); 5.99 (d, J = 5.2 Hz, 1H, –OH), 4.33 (m, 1H, –COOCH2CH–); 4.22 (m, 2H, –COOCH2CH–); 3.69 (s, 1H, –CH3); 3.25–3.09 (overlap m, 6H, –(CH2)NH+(CH2CH3)2); 1.24 (t, J = 7.2 Hz, 6H, –NH+(CH2CH3)2); 13C-NMR (100 MHz, DMSO-d6): δ (ppm) = 165.1 (–COO–); 153.8 (–NHCOO–); 143.9 (–CAr(–NHCOO–)); 130.5 (–CHAr(–COO–)); 122.9 (–CAr(–COO–)); 117.3 (–CHAr(–NHCOO–)); 66.0 (–COOCH2CH–); 63.5 (–COOCH2CH–); 53.7 (–CH2N+–); 51.8 (–CH3); 47.6 and 46.7 (–NH+(CH2CH3)2); 8.5 and 8.3 (–NH+(CH2CH3)2); HR-MS: C16H23N2O5 [M − H]− calculated 323.1612 m/z; found 323.1612 m/z.
(3-{4-[(Ethoxycarbonyl)amino]benzoyloxy}-2-hydroxypropyl)diethylammonium chloride (5f). Yield: 71%; Rf: 0.83 (ethyl acetate/diethylamine 10:1); Rf (rev.): 0.61 (0.1 M HCl/acetone 3:2); m.p.: 153–156 °C; HPLC pur. 98.17% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3326 w; 2985 w; 2608 w; 1740 s; 1685 s; 1595 s; 1536 s; 1216 s; 1177 s; 1H-NMR (700 MHz, DMSO-d6): δ (ppm) = 10.08 (s, 1H, –NHCOO–); 10.02 (bs, 1H, NH+); 7.95 (d, J = 8.8 Hz, 2H, –CHAr(–COO–); 7.62 (d, J = 8.8 Hz, 2H, –CHAr(–NHCOO–)); 6.01 (d, J = 5.1 Hz, 1H, –OH), 4.34 (m, 1H, –COOCH2CH–); 4.23 (m, 2H, –COOCH2CH–); 4.15 (q, J = 7.1 Hz, 2H, –CH2CH3); 3.27–3.14 (overlap m, 6H, –(CH2)NH+(CH2CH3)2); 1.27–1.22 (overlap m, 9H, –CH2CH3 + –NH+(CH2CH3)2); 13C-NMR (176 MHz, DMSO-d6): δ (ppm) = 165.10 (–COO–); 153.29 (–NHCOO–); 143.97 (–CAr(–NHCOO–)); 130.51 (–CHAr(–COO–)); 122.82 (–CAr(–COO–)); 117.28 (–CHAr(–NHCOO–)); 66.03 (–COOCH2CH–); 63.49 (–COOCH2CH–); 60.48 (–CH2CH3); 53.68 (–CH2N+–); 47.59 and 46.70 (–NH+(CH2CH3)2); 14.36 (–CH3); 8.44 and 8.24 (–NH+(CH2CH3)2); HR-MS: C17H25N2O5 [M − H]− calculated 337.1769 m/z; found 337.1771 m/z.
3.2.5. Synthesis of Quaternary Ammonium Salts as Final Compounds
Firstly, the salt was converted to a free base. Hydrochlorides
5a–
f were dissolved in water, NaHCO
3 (5 M) was added and the mixture was extracted with chloroform. The organic layer was dried over anhydrous Na
2CO
3, filtered and chloroform was evaporated under vacuum which provided the residue in a form of yellow oily liquid. The obtained base
4a–
d (0.01 mol), pre-dried chlorobenzene (50 mL) and the appropriate alkylating agent (0.013 mol) were added to the boiling flask and reaction was then carried out in a microwave reactor. For the specific reaction conditions see
Table 1. When the microwave reaction ended, the solution was allowed cooled down to −20 °C from 14 to 48 h and the solid compound precipitated. The crystals of the quaternary ammonium salts were filtered, washed with diethyl ether and recrystallized with ethanol. See the
1H and
13C-NMR spectra of compounds
6a–
i as
Supplementary Material (
Figures S14–S31).
(2-Hydroxy-3-{4-[(methoxycarbonyl)amino]benzoyloxy}propyl)trimethylammonium iodide (6a). Yield: 76%; Rf: 0.38 (n-PrOH/EtOH/HOAc/H2O 8:4:4:3); Rf (rev.): 0.68 (0.1 M HCl/acetone 3:2); m.p.: 190–193 °C; HPLC pur. 96.40% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3330 w, 1717 s, 1702 s, 1600 m, 1538 s, 1415 m, 1322 m, 1211 s, 1179 m, 1130 w; 1H-NMR (700 MHz, DMSO-d6): δ (ppm) = 10.04 (s, 1H, –NHCOO–); 7.97 (d, J = 8.8 Hz, 2H, –CHAr(–COO–)); 7.61 (d, J = 8.8 Hz, 2H, –CHAr(–NHCOO–)); 5.77 (d, J = 5.1 Hz, 1H, –OH); 4.44 (m, 1H, –COOCH2CH–); 4.21 (m, 2H, –COOCH2CH–); 3.70 (s, 3H, –CH3); 3.54 (m, 2H, –CH2N+–); 3.19 (s, 9H, –N+(CH3)3); 13C-NMR (176 MHz, DMSO-d6): δ (ppm) = 165.00 (–COO–); 153.68 (–NHCOO–); 143.85 (–CAr(–NHCOO–)); 130.56 (–CHAr(–COOH)); 122.77 (–CAr(–COOH)); 117.25 (–CHAr(–NHCOO–)); 67.55 (–CH2N+–); 66.20 (–COOCH2CH–); 63.52 (–COOCH2CH–); 53.47 (–N+(CH3)3); 51.83 (–CH3); HR-MS: C15H23N2O5 [M+] calculated 311.1644 m/z; found 311.1623 m/z.
Ethyl(2-hydroxy-3-{4-[(methoxycarbonyl)amino]benzoyloxy}propyl)dimethylammonium iodide (6b). Yield: 75%; Rf: 0.35 (n-PrOH/EtOH/HOAc/H2O 8:4:4:3); Rf (rev.): 0.63 (0.1 M HCl/acetone 3:2); m.p.: 198–201 °C; HPLC pur. 96.11% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3310 w, 1728 s, 1720 s, 1593 m, 1531 s, 1263 m, 1220 s, 1175 s, 1123 m, 1059 m; 1H-NMR (700 MHz, DMSO-d6): δ (ppm) = 10.08 (s, 1H, –NHCOO–); 7.97 (d, J = 8.8 Hz, 2H, –CHAr(–COOH)); 7.61 (d, J = 8.8 Hz, 2H, –CHAr(–NHCOO–)); 5.79 (bs, 1H, –OH); 4.43 (m, 1H, –COOCH2CH–); 4.21 (m, 2H, –COOCH2CH–); 3.70 (s, 3H, –CH3); 3.49–3.46 (overlap m, 4H, –CH2N+CH2CH3); 3.11 (s, 3H, –N+CH3); 3.10 (s, 3H, –N+CH3); 1.28 (t, J = 7.1 Hz, 3H, –N+CH2CH3); 13C-NMR (176 MHz, DMSO-d6): δ (ppm) = 165.05 (–COO–); 153.72 (–NHCOO–); 143.90 (–CAr(–NHCOO–)); 130.62 (–CHAr(–COO–)); 122.78 (–CAr(–COO–)); 117.25 (–CHAr(–NHCOO–)); 66.28 (–COOCH2CH–); 64.78 (–CH2N+–); 63.29 (–COOCH2CH–); 59.99 (–N+CH2CH3); 51.88 (–CH3); 50.67 and 50.46 (–N+(CH3)2); 7.92 (–N+CH2CH3); HR-MS: C16H25N2O5 [M+] calculated 325.1753 m/z; found 325.1318 m/z.
(2-Hydroxy-3-{4-[(methoxycarbonyl)amino]benzoyloxy}propyl)dimethyl(propan-2-yl)ammonium iodide (6c). Yield: 70%; Rf: 0.39 (n-PrOH/EtOH/HOAc/H2O 8:4:4:3); Rf (rev.): 0.60 (0.1 M HCl/acetone 3:2); m.p.: 164–167 °C; HPLC pur. 98.49% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3311 w, 1702 s, 1698 s, 1593 m, 1528 s, 1220 s, 1174 s, 1698 s, 1528 s, 1220 m; 1H-NMR (700 MHz, DMSO-d6): δ (ppm) = 10.07 (s, 2H, –NHCOO–); 7.97 (d, J = 8.8 Hz, 2H, –CHAr(–COO–)); 7.61 (d, J = 8.8 Hz, 2H, –CHAr(–NHCOO–)); 5.81 (d, J = 5.5 Hz, 2H, –OH); 4.46 (m, 2H, –COOCH2CH–); 4.22 (m, 2H, –COOCH2CH–); 3.86 (m, 1H, –CH(CH3)2); 3.70 (s, 3H, –CH3); 3.46 (m, 2H, –CH2N+–); 3.07 (s, 3H, –N+CH3); 3.05 (s, 3H, –N+CH3); 1.33 (m, 6H, –CH(CH3)2); 13C-NMR (176 MHz, DMSO-d6): δ (ppm) = 165.06 (–COO–); 153.72 (–NHCOO–); 143.88 (–CAr(–NHCOO–)); 130.60 (–CHAr(–COO–)); 122.82 (–CAr(–COO–)); 117.27 (–CHAr(–NHCOO–)); 67.24 (–CH2N+–); 66.39 (–COOCH2CH–); 63.31 (–COOCH2CH–); 51.87 (–CH3); 48.44 and 47.75 (–N+(CH3)2); 45.65 (–CH(CH3)2); 16.11 and 15.97 (–CH(CH3)2); HR-MS: C17H27N2O5 [M+] calculated 339.1909 m/z; found 339.2194 m/z.
(3-{4-[(Ethoxycarbonyl)amino]benzoyloxy}-2-hydroxypropyl)trimethylammonium iodide (6d). Yield: 82%; Rf: 0.41 (n-PrOH/EtOH/HOAc/H2O 8:4:4:3); Rf (rev.): 0.59 (0.1 M HCl/acetone 3:2); m.p.: 204–206 °C; HPLC pur. 97.71% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3321 w, 1698 s, 1597 m, 1538 s, 1417 m, 1318 m, 1223 s, 1178 m, 1063 m; 1H-NMR (700 MHz, DMSO-d6): δ (ppm) = 10.01 (s, 1H, –NHCOO–); 7.97 (d, J = 8.8 Hz, 2H, –CHAr(–COO–)); 7.62 (d, J = 8.8 Hz, 2H, –CHAr(–NHCOO–)); 5.77 (d, J = 5.3 Hz, 1H, –OH); 4.44 (m, 1H, –COOCH2CH–); 4.21 (m, 2H, –COOCH2CH–); 4.15 (q, J = 7.1 Hz, 2H, –CH2CH3); 3.52 (m, 2H, –CH2N+–); 3.19 (s, 9H, –N+(CH3)3); 1.26 (t, J = 7.2 Hz, 3H, –CH2CH3); 13C-NMR (176 MHz, DMSO-d6): δ (ppm) = 165.00 (–COO–); 153.22 (–NHCOO–); 143.93 (–CAr(–NHCOO–)); 130.54 (–CHAr(–COO–)); 122.68 (–CAr(–COO–)); 117.23 (–CHAr(–NHCOO–)); 67.55 (–CH2N+–); 66.18 (–COOCH2CH–); 63.51 (–COOCH2CH–); 60.45 (–CH2CH3); 53.46 (–N+(CH3)3); 14.30 (–CH3); HR–MS: C16H25N2O5 [M+] calculated 325.1753 m/z; found 325.1814 m/z.
(3-{4-[(Ethoxycarbonyl)amino]benzoyloxy}-2-hydroxypropyl)(ethyl)dimethylammonium iodide (6e) Yield: 71%; Rf: 0.37 (n-PrOH/EtOH/HOAc/H2O 8:4:4:3); Rf (rev.): 0.54 (0.1 M HCl/acetone 3:2); m.p.: 193–196 °C; HPLC pur. 97.26% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3325 w, 1728 s, 1681 s, 1598 m, 1538 m, 1225 s, 1179 m, 1065 m; 1H-NMR (700 MHz, DMSO-d6): δ (ppm) = 10.05 (s, 1H, –NHCOO–); 7.96 (d, J = 8.8 Hz, 2H, –CHAr(–COO–)); 7.62 (d, J = 8.8 Hz, 2H, –CHAr(–NHCOO–)); 5.80 (bs, 1H, –OH); 4.43 (m, 1H, –COOCH2CH–); 4.21 (m, 2H, –COOCH2CH–); 4.16 (q, J = 7.1 Hz, 2H, –CH2CH3); 3.49–3.46 (overlap m, 4H, –CH2N+CH2CH3); 3.12 (s, 3H, –N+CH3); 3.11 (s, 3H, –N+CH3); 1.29–1.25 (overlap m, 6H, –CH2CH3 + –N+CH2CH3); 13C NMR (176 MHz, DMSO-d6): δ (ppm) = 165.05 (–COO–); 153.25 (–NHCOO–); 143.99 (–CAr(–NHCOO–)); 130.60 (–CHAr(–COO–)); 122.70 (–CAr(–COO–)); 117.23 (–CHAr(–NHCOO–)); 66.28 (–COOCH2CH–); 64.77 (–CH2N+–); 63.28 (–COOCH2CH–); 60.49 (–CH2CH3); 59.98 (–N+(CH2CH3); 50.67 and 50.46 (–N+(CH3)2); 14.36 (–CH3); 7.92 (–N+CH2CH3); HR-MS: C17H27N2O5 [M+] calculated 339.1909 m/z; found 339.1906 m/z.
(3-{4-[(Ethoxycarbonyl)amino]benzoyloxy}-2-hydroxypropyl)diethylmethylammonium iodide (6f). Yield: 55%; Rf: 0.44 (n-PrOH/EtOH/HOAc/H2O 8:4:4:3); Rf (rev.): 0.58 (0.1 M HCl/acetone 3:2); m.p.: 173–176 °C; HPLC pur. 99.58% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3320 w, 1703 s, 1690 s, 1591 s, 1537 s, 1416 m, 1224 s, 1178 m, 1063 m; 1H-NMR (700 MHz, DMSO-d6): δ (ppm) = 10.05 (s, 1H, –NHCOO–); 7.96 (d, J = 8.8 Hz, 2H, –CHAr(–COO–)); 7.61 (d, J = 8.8 Hz, 2H, –CHAr(–NHCOO–)); 5.77 (d, J = 5.4 Hz, 1H, –OH), 4.42 (m, 1H, –COOCH2CH–); 4.21 (m, 2H, –COOCH2CH–); 4.15 (q, J = 7.1 Hz, 2H, –CH2CH3); 3.48–3.40 (overlap m, 6H, –(CH2)N+(CH2CH3)2); 3.04 (s, 3H, –N+CH3); 1.26–1.24 (overlap m, 9H, –CH2CH3 + –N+(CH2CH3)2); 13C-NMR (176 MHz, DMSO-d6): δ (ppm) = 165.06 (–COO–); 153.27 (–NHCOO–); 143.99 (–CAr(–NHCOO–)); 130.60 (–CHAr(–COO–)); 122.70 (–CAr(–COO–)); 117.25 (–CHAr(–NHCOO–)); 66.28 (–COOCH2CH–); 63.09 (–COOCH2CH–); 61.94 (–CH2N+–); 60.53 (–CH2CH3); 56.80 and 56.68 (–N+(CH2CH3)2); 47.59 (–N+CH3); 14.38 (–CH3); 7.64 (–N+(CH2CH3)2); HR-MS: C18H29N2O5 [M+] calculated 353.2065 m/z; found 353.2082 m/z.
(2-Hydroxy-3-{4-[(propoxycarbonyl)amino]benzoyloxy}propyl)trimethylammonium iodide (6g). Yield: 58%; Rf: 0.44 (n-PrOH/EtOH/HOAc/H2O 8:4:4:3); Rf (rev.): 0.53 (0.1 M HCl/acetone 3:2); m.p.: 149–151 °C; HPLC pur. 98.33% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3311 w, 1697 s, 1594 s, 1536 s, 1415 m, 1274 m, 1217 s, 1178 m, 1069 m; 1H-NMR (700 MHz, DMSO-d6): δ (ppm) = 10.05 (s, 1H, –NHCOO–); 7.97 (d, J = 8.9 Hz, 2H, –CHAr(–COO–)); 7.62 (d, J = 8.9 Hz, 2H, –CHAr(–NHCOO–)); 5.79 (d, J = 5.5 Hz, 1H, –OH); 4.44 (m, 1H, –COOCH2CH–); 4.20 (m, 2H, –COOCH2CH–); 4.06 (t, J = 6.7 Hz, 2H, –CH2CH2CH3); 3.52 (m, 2H, –CH2N+–); 3.19 (s, 9H, –N+(CH3)3); 1.65 (m, 2H, –CH2CH2CH3); 0.93 (t, J = 7,4 Hz, 3H, –CH2CH2CH3); 13C-NMR (176 MHz, DMSO-d6): δ (ppm) = 165.07 (–COO–); 153.37 (–NHCOO–); 144.01 (–CAr(–NHCOO–)); 130.64 (–CHAr(–COO–)); 122.70 (–CAr(–COO–)); 117.25 (–CHAr(–NHCOO–)); 67.54 (–CH2N+–); 66.26 (–COOCH2CH–); 66.02 (–CH2CH2CH3); 63.53 (–COOCH2CH–); 53.47 (–N+(CH3)3); 21.75 (–CH2CH2CH3); 10.18 (–CH3); 15N-NMR (DMSO-d6): δ (ppm) = 48.2 (–N+(CH3)2); 109.1 (–NHCOO–); HR-MS: C17H27N2O5 [M+] calculated 339.1909 m/z; found 339.1870 m/z.
Diethyl (2-hydroxy-3-{4-[(propoxycarbonyl)amino]benzoyloxy}propyl)methylammonium iodide (6h). Yield: 49%; Rf: 0.46 (n-PrOH/EtOH/HOAc/H2O 8:4:4:3); Rf (rev.): 0.53 (0.1 M HCl/acetone 3:2); m.p.: 70–73 °C; HPLC pur. 98.80% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3311 w, 2946 s, 1713 s, 1589 s, 1533 s, 1501 s, 1399 m, 1224 s, 1205 s, 1191 m, 1031 m; 1H-NMR (700 MHz, DMSO-d6): δ (ppm) = 10.05 (s, 1H, –NHCOO–); 7.96 (d, J = 8.9 Hz, 2H, –CHAr(–COO–)); 7.62 (d, J = 8.9 Hz, 2H, –CHAr(–NHCOO–)); 5.78 (d, J = 5.5 Hz, 1H, –OH), 4.42 (m, 1H, –COOCH2CH–); 4.22 (m, 2H, –COOCH2CH–); 4.06 (t, J = 6.7 Hz, 2H, –CH2CH2CH3); 3.49–3.39 (overlap m, 6H, –CH2N+(CH2CH3)2); 3.04 (s, 3H, –N+CH3); 1.65 (m, 2H, –CH2CH2CH3), 1.25 (m, 6H, –N+(CH2CH3)2), 0.93 (t, J = 7.4 Hz, 3H, –CH2CH2CH3); 13C-NMR (176 MHz, DMSO-d6): δ (ppm) = 165.08 (–COO–); 153.38 (–NHCOO–); 144.00 (–CAr(–NHCOO–)); 130.60 (–CHAr(–COO–)); 122.73 (–CAr(–COO–)); 117.29 (–CHAr(–NHCOO–)); 66.30 (–COOCH2CH–); 66.03 (–CH2CH2CH3); 63.09 (–COOCH2CH–); 61.96 (–CH2N+–); 56.82 and 56.69 (–N+(CH2CH3)2); 47.61 (–N+CH3); 21.76 (–CH2CH2CH3); 10.18 (–CH3); 7.64 (–N+(CH2CH3)2); HR-MS: C19H31N2O5 [M+] calculated 367.2222 m/z; found 367.2933 m/z.
(3-{4-[(Butoxycarbonyl)amino]benzoyloxy}-2-hydroxypropyl)trimethylammonium iodide (6i). Yield: 45%; Rf: 0.45 (n-PrOH/EtOH/HOAc/H2O 8:4:4:3); Rf (rev.): 0.47 (0.1 M HCl/acetone 3:2); m.p.: 93–96 °C; HPLC pur. 88.42% (254 nm); IR (Zn/Se ATR): ν (cm−1) = 3317 w, 2958 w, 1710 s, 1699 m, 1597 s, 1544 s, 1410 m, 1236 s, 1172 m, 1068 m; 1H-NMR (700 MHz, DMSO-d6): δ (ppm) = 10.04 (s, 1H, –NHCOO–); 7.97 (d, J = 8.9 Hz, 2H, –CHAr(–COO–)); 7.62 (m, J = 8.8 Hz, 2H, –CHAr(–NHCOO–)); 5.80 (d, J = 5.1 Hz, 1H, –OH), 4.44 (m, 1H, –COOCH2CH–); 4.20 (m, 2H, –COOCH2CH–); 4.11 (t, J = 6.6 Hz, 2H, –CH2CH2CH2CH3); 3.52 (m, 2H, –CH2N+–); 3.19 (s, 9H, –N+(CH3)3); 1.61 (m, 2H, –CH2CH2CH2CH3), 1.38 (m, 2H, –CH2CH2CH2CH3), 0.92 (t, J = 7.4 Hz, 3H, –CH2CH2CH2CH3); 13C-NMR (176 MHz, DMSO-d6): δ (ppm) = 165.07 (–COO–); 153.37 (–NHCOO–); 144.01 (–CAr(–NHCOO–)); 130.64 (–CHAr(–COO–)); 122.70 (–CAr(–COO–)); 117.25 (–CHAr(–NHCOO–)); 67.55 (–CH2N+–); 66.26 (–COOCH2CH–); 64.22 (–CH2CH2CH2CH3); 63.53 (–COOCH2CH–); 53.47 (–N+(CH3)3); 30.43 (–CH2CH2CH2CH3); 18.52 (–CH2CH2CH2CH3); 13.52 (–CH3); HR-MS: C18H29IN2O5 [M+] calculated 353.2066 m/z; found 353.2082 m/z.