1H-NMR Profiling and Chemometric Analysis of Selected Honeys from South Africa, Zambia, and Slovakia
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
2.1.1. pH
2.1.2. Electrical Conductivity (EC)
2.1.3. Moisture Content
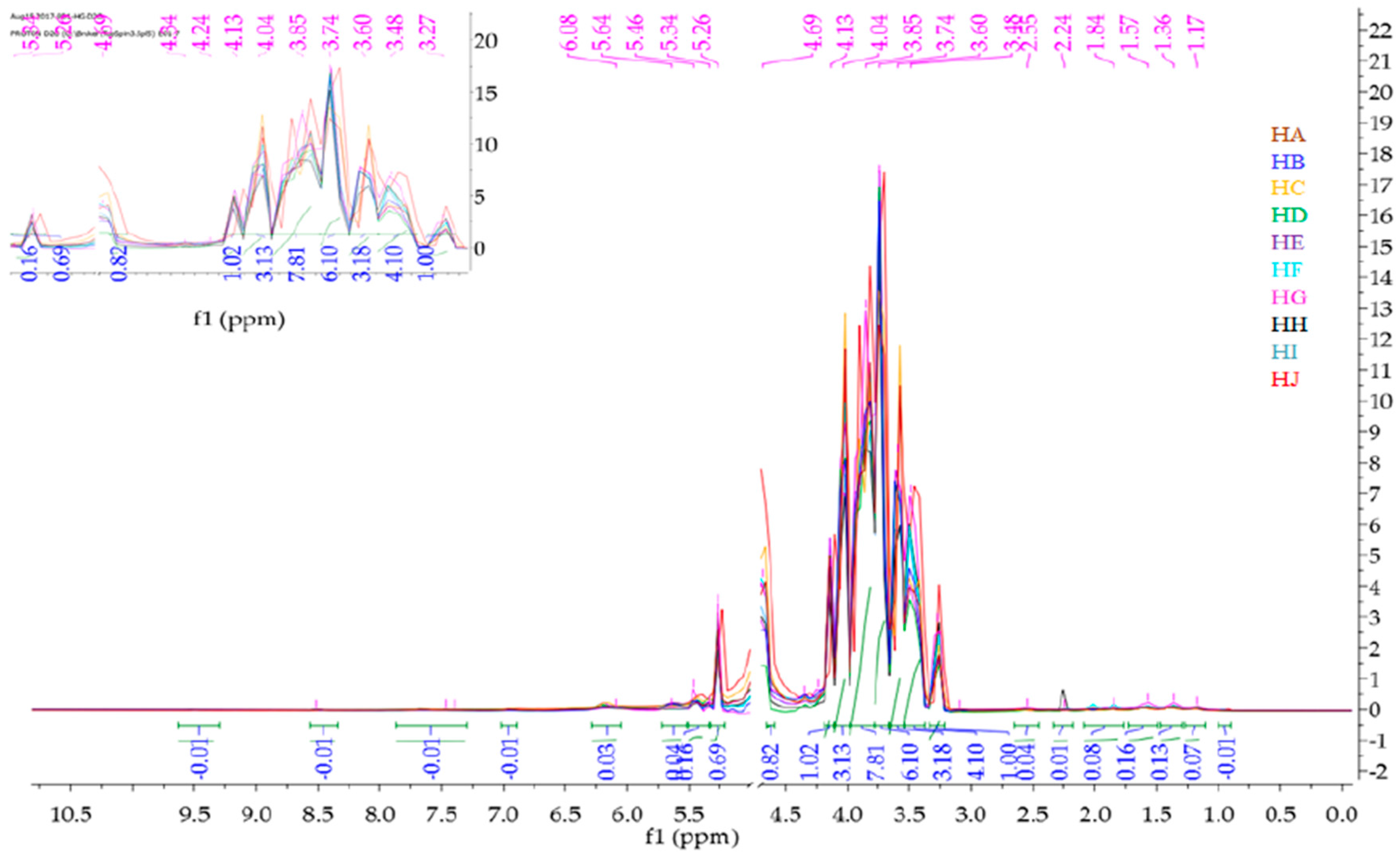
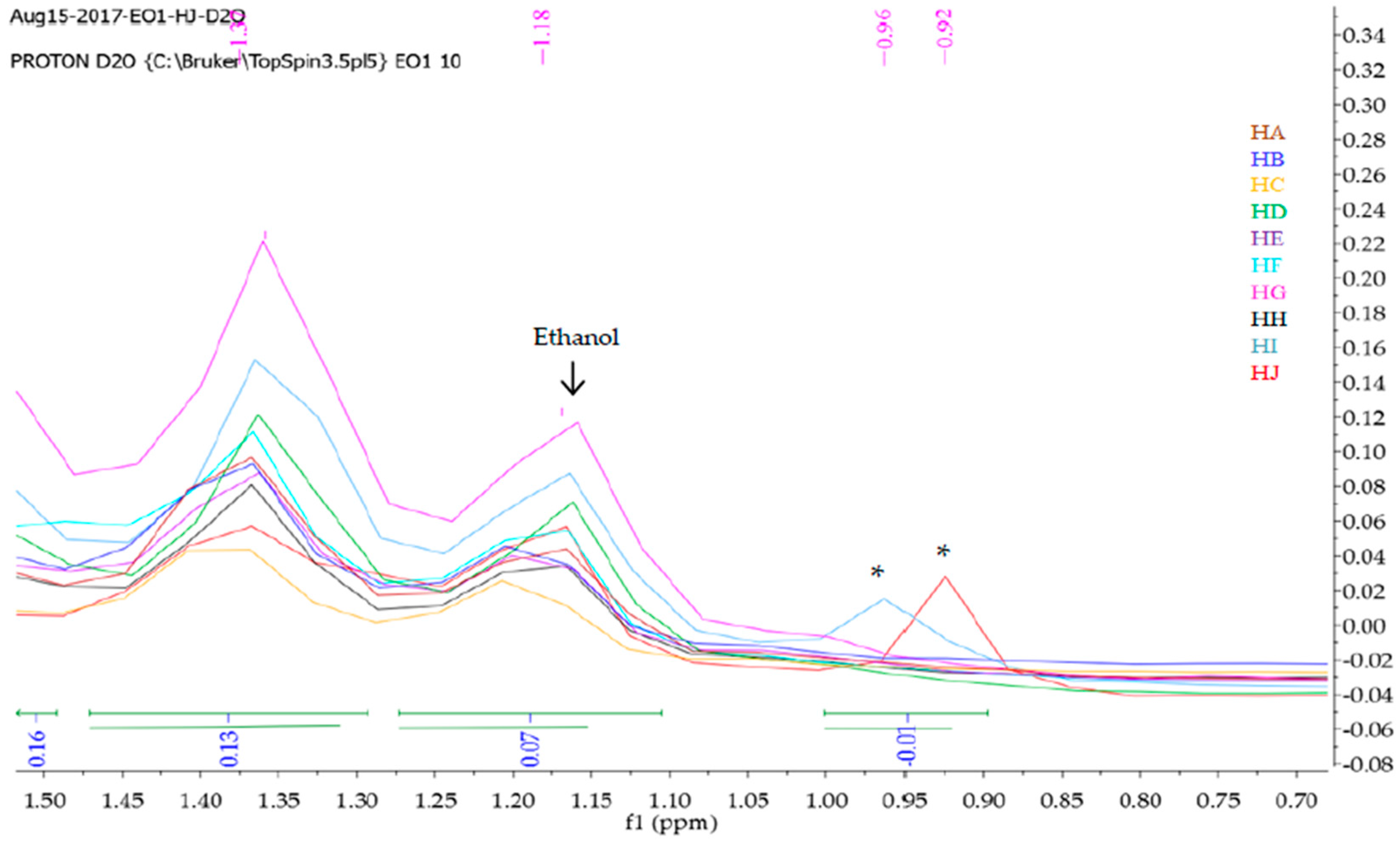
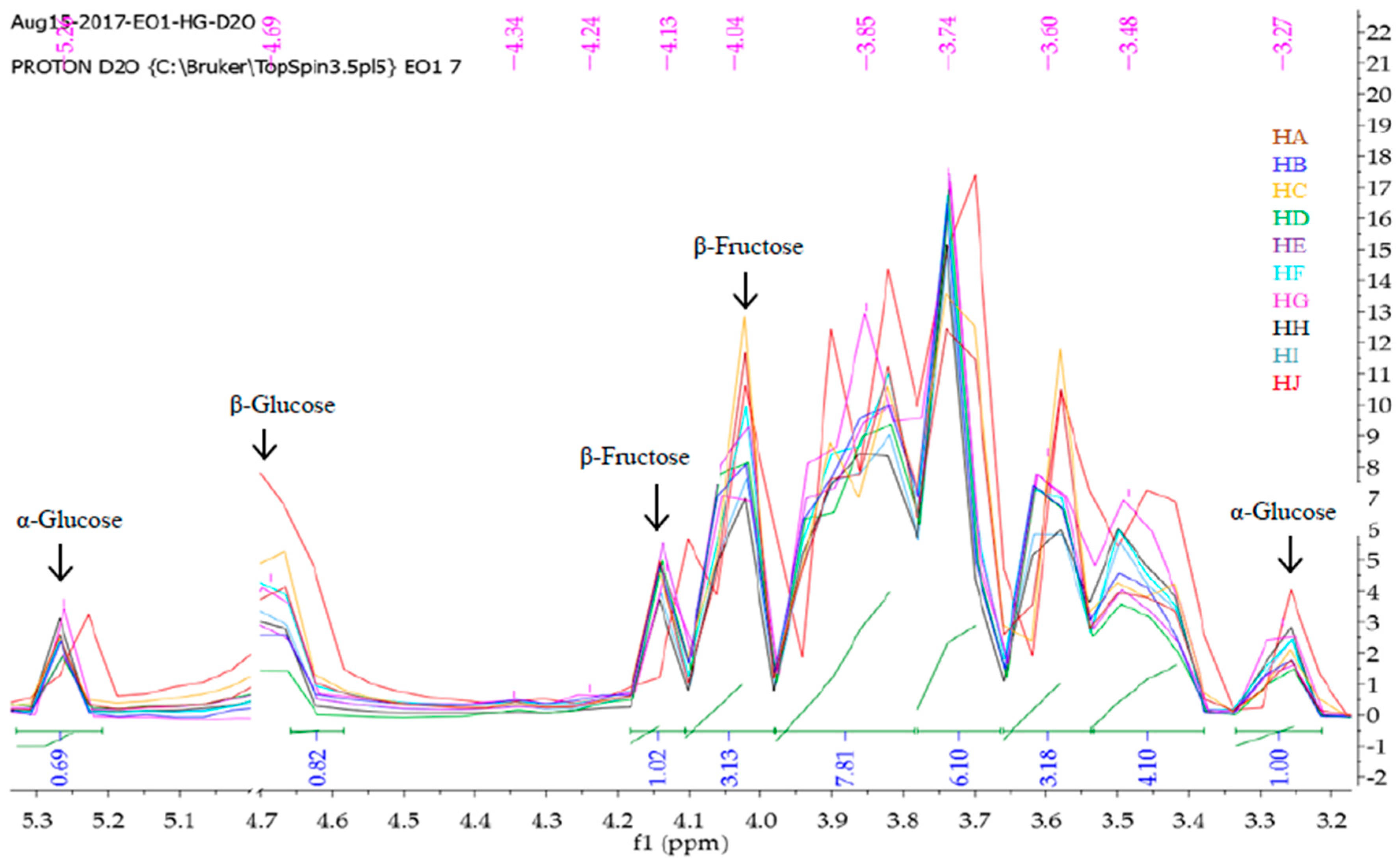
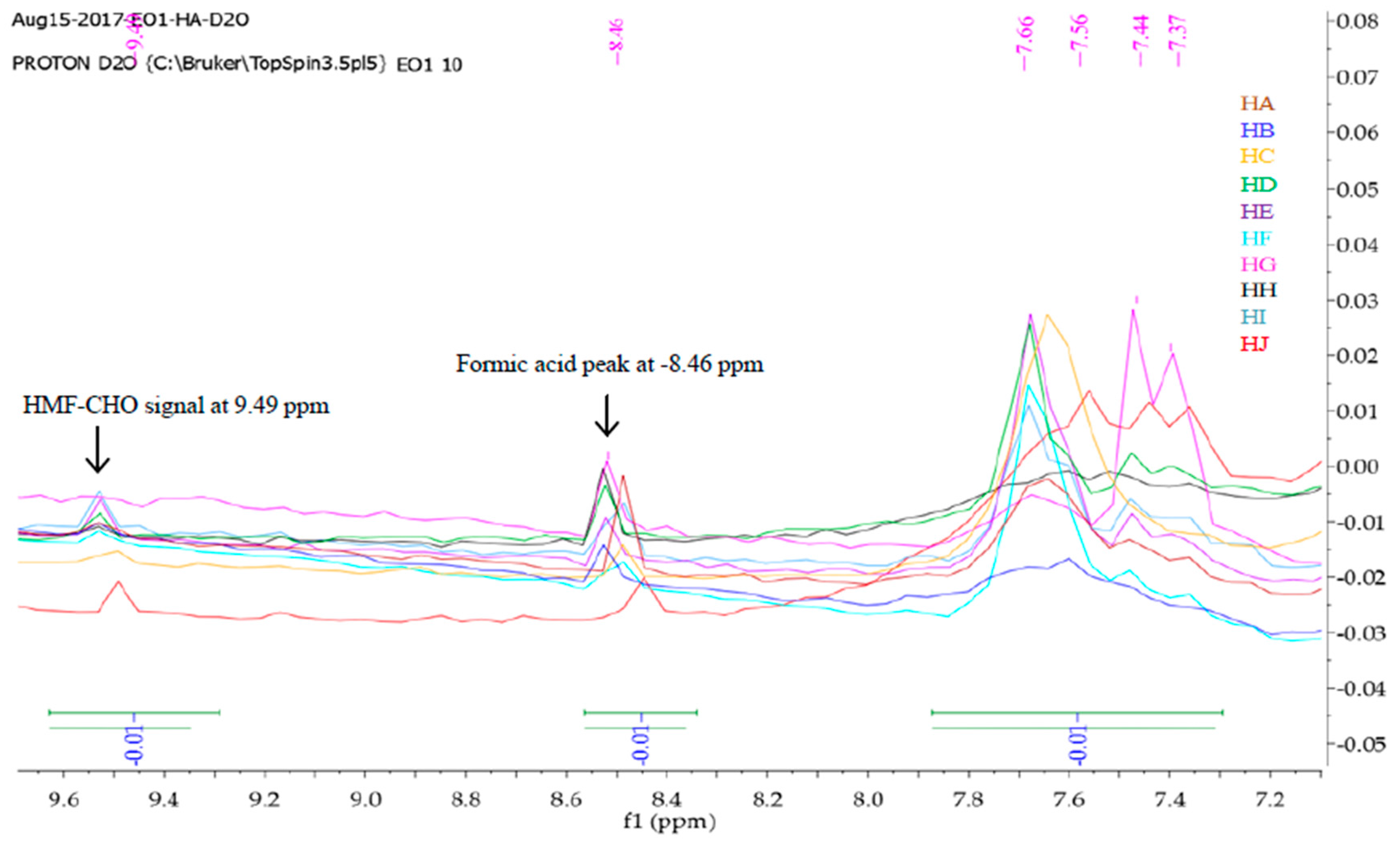
2.2. 1H-NMR Fingerprinting
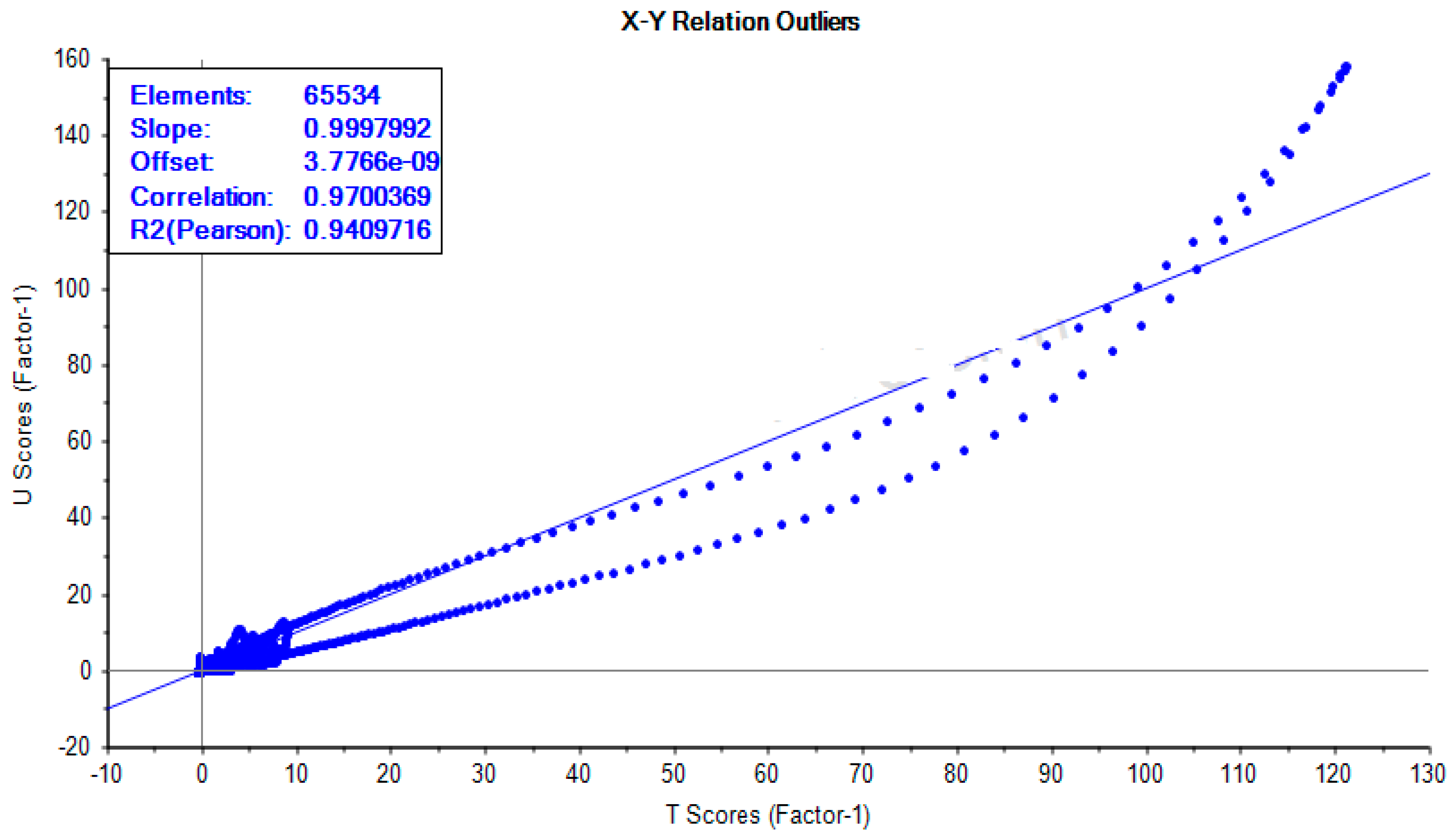
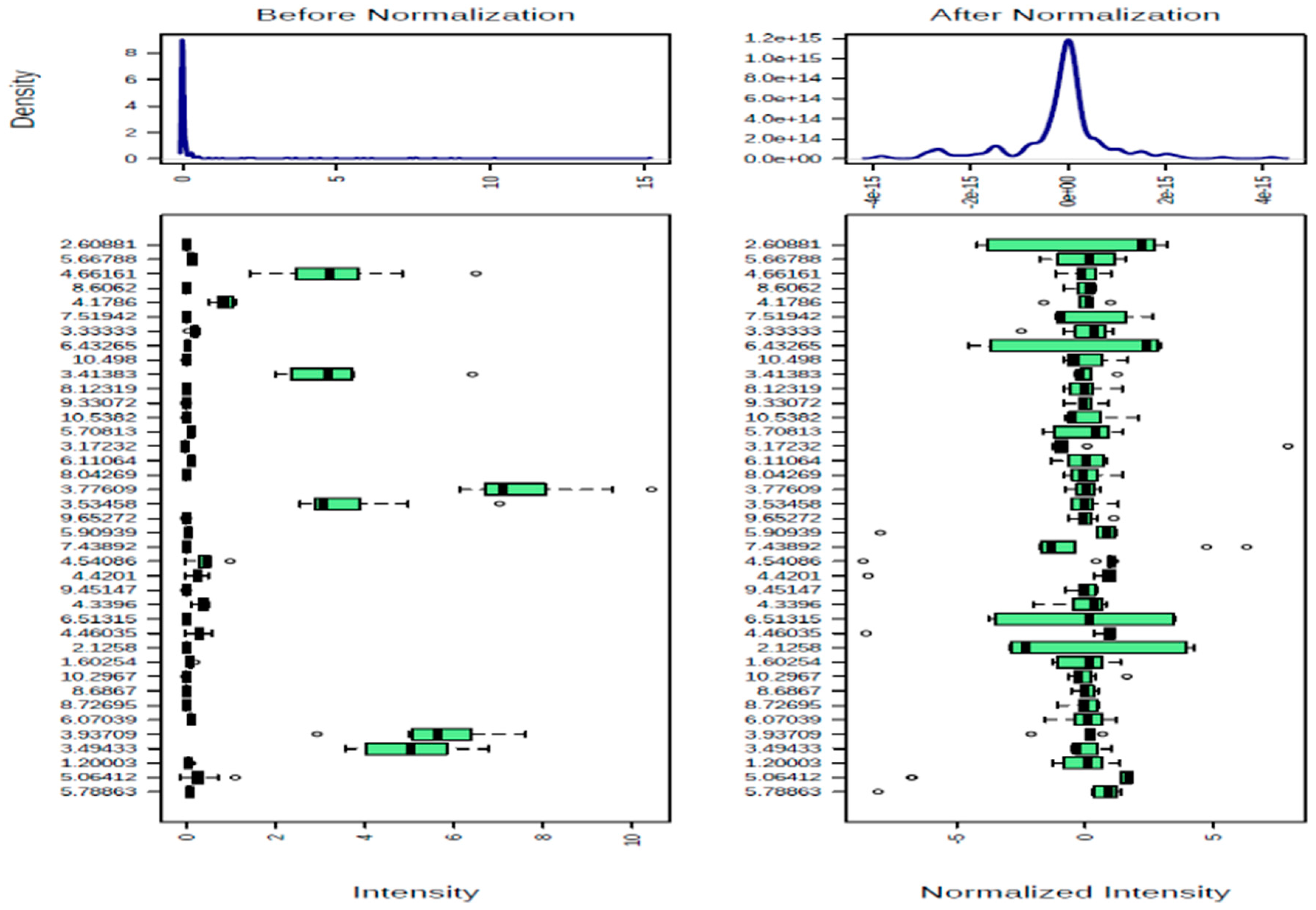
2.3. Data Validation and Transformation
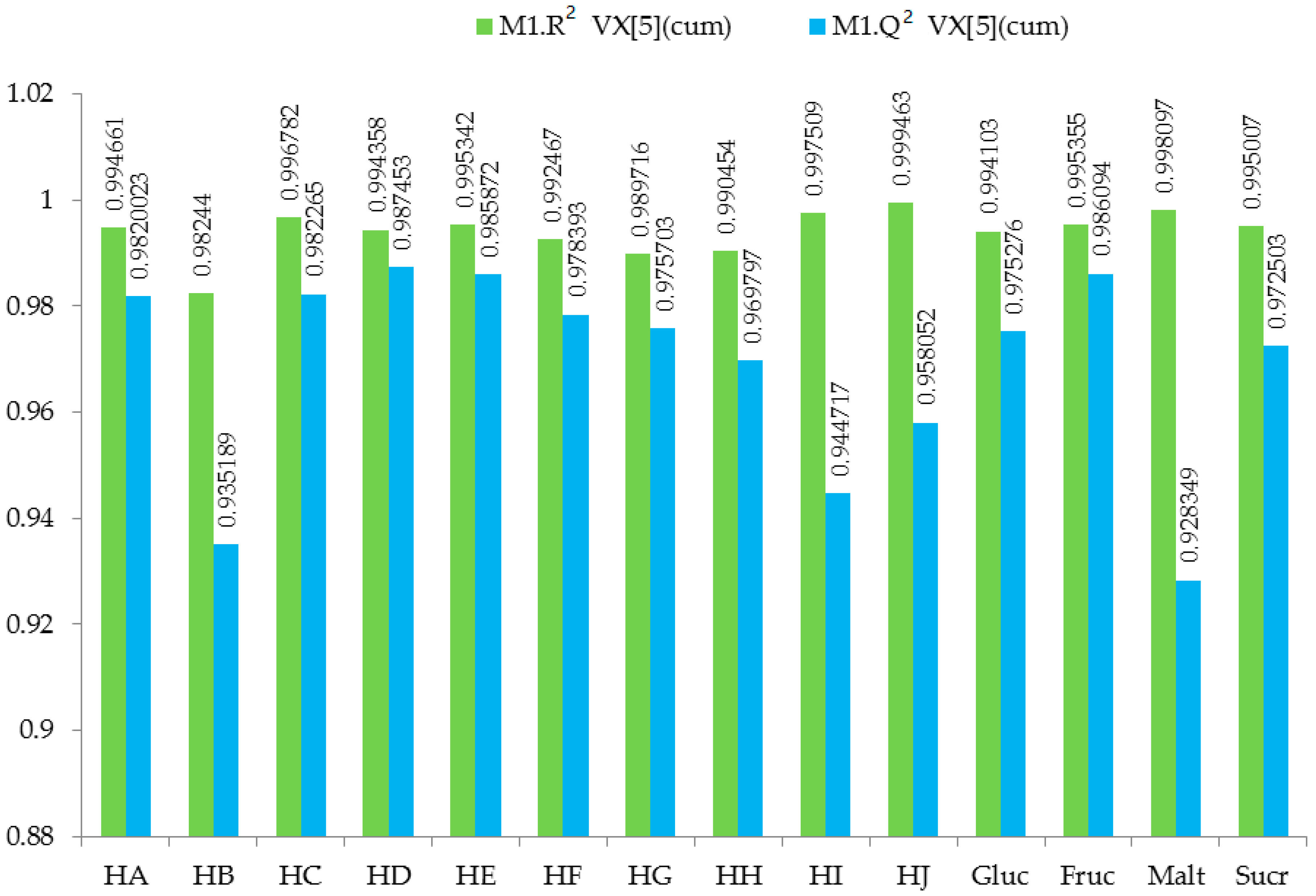
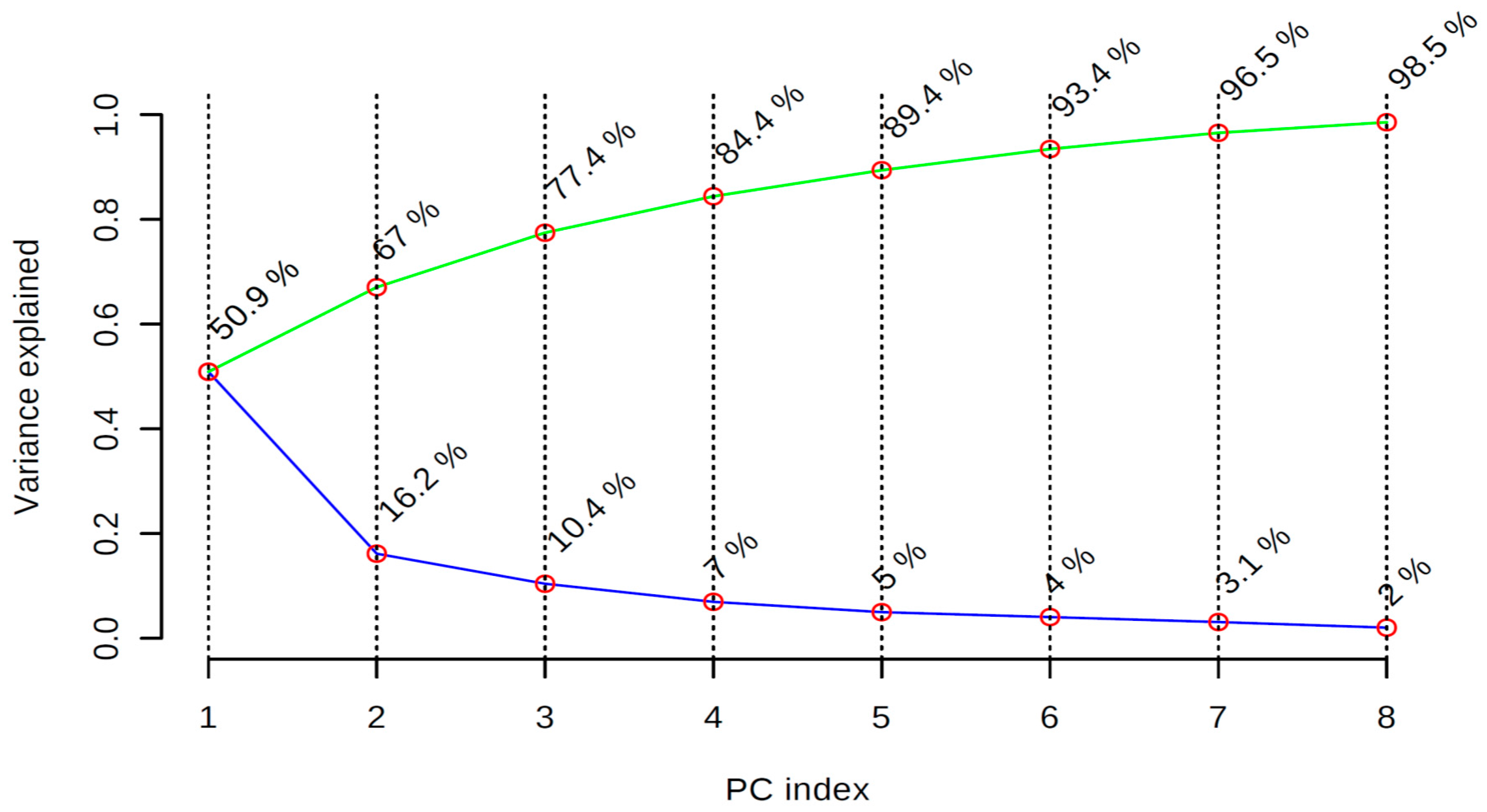
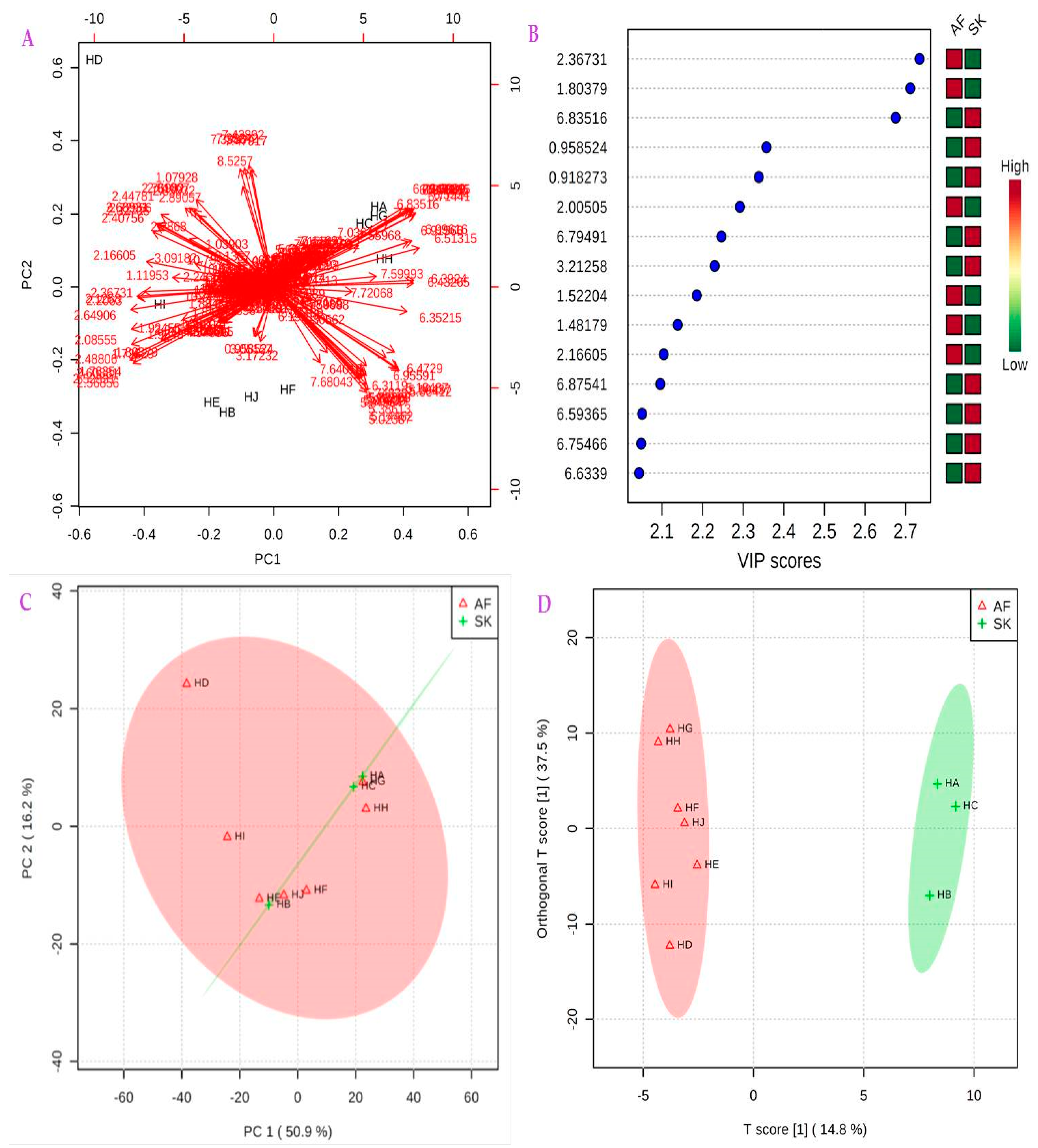
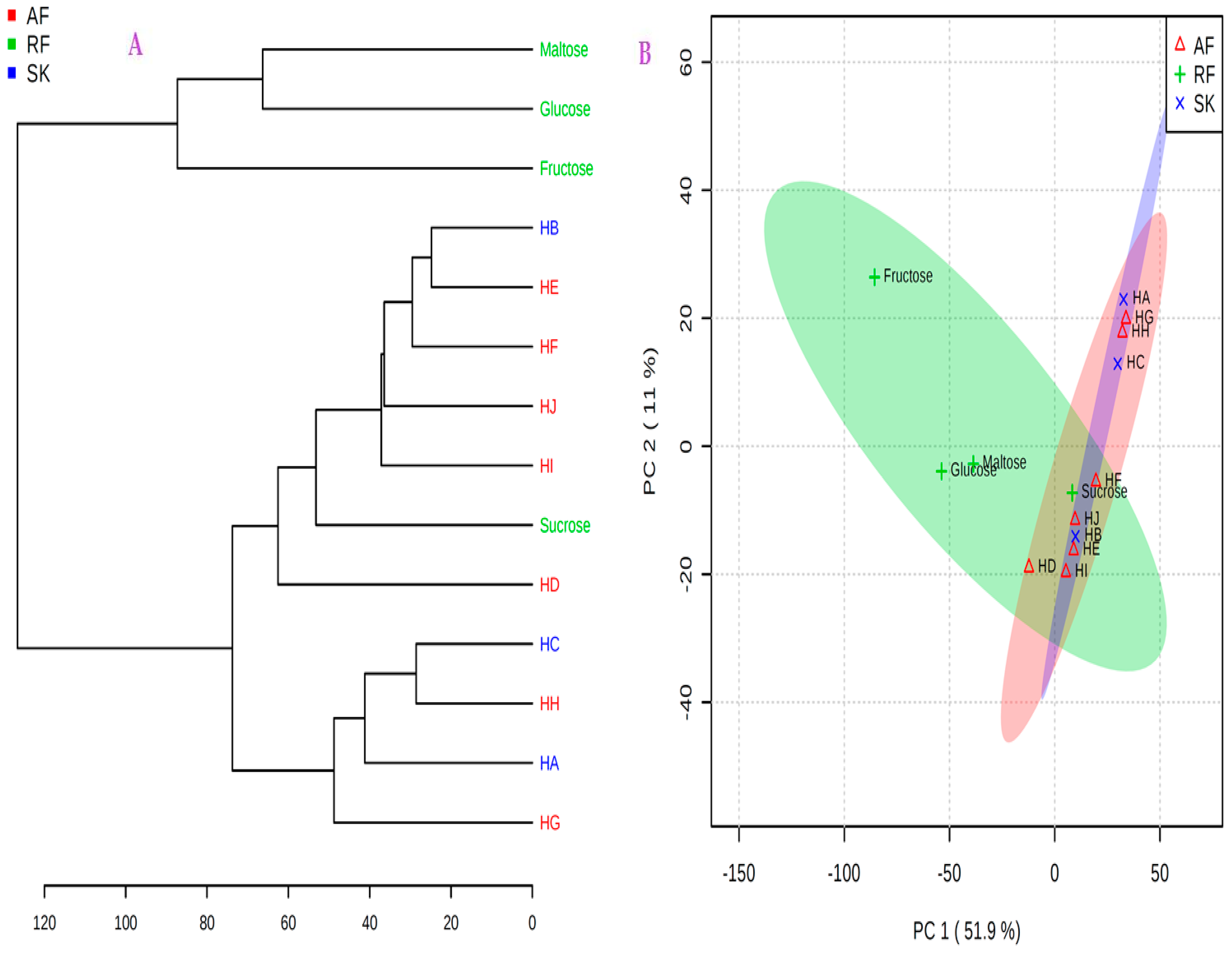
2.4. 1H-NMR Metabolomics Using Multivariate Analysis
3. Methods
3.1. Samples and Chemicals
3.2. pH
3.3. Electrical Conductivity
3.4. Moisture Content
3.5. Sample Preparation and NMR Data Pre-Treatment
3.6. Multivariate Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meekings, G.; Palomar, G.; Sarquis, M.A.; Trongé, A.F.; Nimo, M.; Ros, P.; Woodwar, P. (Eds.) Report of the Seventh Session of the Codex Committee on Sugars; ALINORM 01/25; FAO/WHO Codex Alimenatarius Commission: Geneva, Switzerland, 2001; pp. 1–33. [Google Scholar]
- European Commission. Council Directive 2001/110/EC of 20 December 2001 Relating to Honey; The Council of the European Union: Brussels, Belgium, 2002; p. 47. [Google Scholar]
- Jamróz, M.K.; Paradowska, K.; Zawada, K.; Makarova, K.; Kaźmierski, S.; Wawer, I. 1H- and 13C-NMR-based sugar profiling with chemometric analysis and antioxidant activity of herbhoneys and honeys. J. Sci. Food Agric. 2014, 94, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Nepi, M.; Pacini, E. Nectar production and presentation. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 215–264. [Google Scholar]
- Soria, A.C.; González, M.; De Lorenzo, C.; Martínez-Castro, I.; Sanz, J. Characterization of artisanal honeys from Madrid (Central Spain) on the basis of their melissopalynological, physicochemical and volatile composition data. Food Chem. 2004, 85, 121–130. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Revised Codex Standard for Honey Codex Stan 12-1981, Rev. 1 (1987), Rev. 2 (2001). Codex Stand. 1981, 12, 1–7. Available online: http://teca.fao.org/sites/default/files/resources/Annex%20A%20Codex%20Alimentarius%20Honey%20Standard.pdf (accessed on 24 January 2018).
- Felsner, M.L.; Cano, C.B.; Bruns, R.E.; Watanabe, H.M.; Almeida-Muradian, L.B.; Matos, J.R. Characterization of monofloral honeys by ash contents through a hierarchical design. J. Food Compos. Anal. 2004, 17, 737–747. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Millogo, J.; Romito, M.; Nacoulma, O.G. Physicochemical analyses of Burkina Fasan honey. Acta Vet. Brno 2005, 74, 147–152. [Google Scholar] [CrossRef]
- Picqué, C. Council Directive 2001/110/EC of 20 December 2001 Relating to Honey. Official Journal of the European Communities; The Council of the European Union: Brussels, Belgium, 2002; pp. 19–26. [Google Scholar]
- Bogdanov, S.; Ruoff, K.; Oddo, L.P. Physico-chemical methods for the characterisation of unifloral honeys: A review. Apidologie 2004, 35, 4–17. [Google Scholar] [CrossRef]
- Sereia, M.J.; Março, P.H.; Perdoncini, M.R.G.; Parpinelli, R.S.; de Lima, E.G.; Anjo, F.A. Techniques for the Evaluation of Physicochemical Quality and Bioactive Compounds in Honey; Anjo, F., Ed.; IN TECH: London, UK, 2017; pp. 194–214. [Google Scholar] [CrossRef]
- Lianda, R.L.P.; Sant’ana, L.D.O.; Echevarria, A.; Castro, R.N. Antioxidant activity and phenolic composition of Brazilian honeys and their extracts. J. Braz. Chem. Soc. 2012, 23, 618–627. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Thakur, V.; Brar, S.K. Antibacterial efficacy of raw and processed honey. Biotechnol. Res. Int. 2011, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Salehin, F.; Iqbal, A. Antibacterial efficacy of raw and commercially available honey. Afr. J. Biotechnol. 2011, 10, 11269–11272. [Google Scholar]
- Bogdanov, S. Nature and origin of the antibacterial substances in honey. LWT-Food Sci. Technol. 1997, 30, 748–753. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Clarke, A.M.; Munzhelele, T.; Green, E.; Mkwetshana, N.F.; Ndip, R.N. Selected South African honeys and their extracts possess in vitro anti-Helicobacter pylori activity. Arch. Med. Res. 2010, 41, 324–331. [Google Scholar] [CrossRef] [PubMed]
- McLoone, P.; Warnock, M.; Fyfe, L. Honey: A realistic antimicrobial for disorders of the skin. J. Microbiol. Immunol. Infect. 2016, 49, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Syazana, M.S.N.; Halim, A.; Gan, S.; Shamsuddin, S. Antiproliferative effect of methanolic extraction of tualang honey on human keloid fibroblasts. BMC Complement. Altern. Med. 2011, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Terzić, S.; Šver, L.; Bašić, I. Honey-bee products in prevention and/or therapy of murine transplantable tumours. J. Sci. Food Agric. 2005, 85, 363–370. [Google Scholar] [CrossRef]
- Al-Waili, N.S. Natural honey lowers plasma glucose, C-reactive protein, homocysteine, and blood lipids in healthy, diabetic, and hyperlipidemic subjects: Comparison with dextrose and sucrose. J. Med. Food 2004, 7, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Spiteri, M.; Rogers, K.M.; Jamin, E.; Thomas, F.; Guyader, S.; Lees, M.; Rutledge, D.N. Combination of 1H-NMR and chemometrics to discriminate manuka honey from other floral honey types from Oceania. Food Chem. 2017, 217, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Guler, A.; Bakan, A.; Nisbet, C.; Yavuz, O. Determination of important biochemical properties of honey to discriminate pure and adulterated honey with sucrose (Saccharum officinarum L.) syrup. Food Chem. 2007, 105, 1119–1125. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Cotte, J.F.; Casabianca, H.; Chardon, S.; Lheritier, J.; Grenier-Loustalot, M.F. Application of carbohydrate analysis to verify honey authenticity. J. Chromatogr. A 2003, 1021, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Padovan, G.J.; De Jong, D.; Rodrigues, L.P.; Marchini, J.S. Detection of adulteration of commercial honey samples by the 13C/12C isotopic ratio. Food Chem. 2003, 82, 633–636. [Google Scholar] [CrossRef]
- European Comission. European Commission Council Directive 2001/110/EC relating to honey. Off. J. Eur. Commun. 2002, L10, 47–52. [Google Scholar]
- Jánošík, M. (Ed.) Slovak Beekeepers Association (SZV) and Slovak Federation of Beekeepers (SVS) Program. (2004). [Annex II to Regulation (EC) No.917/2004; Statistical Office of the Slovak Republic: Bratislava, Slovakia; Available online: http://213.30.17.29/MA/apicultura/PAN_UE/Docs/SK_AGRI-2013-60986-00-03-EN-TRA-00.pdf (accessed on 24 January 2018).
- Zappalà, M.; Fallico, B.; Arena, E.; Verzera, A. Methods for the determination of HMF in honey: A comparison. Food Control. 2005, 16, 273–277. [Google Scholar] [CrossRef]
- Gok, S.; Severcan, M.; Goormaghtigh, E.; Kandemir, I.; Severcan, F. Differentiation of Anatolian honey samples from different botanical origins by ATR-FTIR spectroscopy using multivariate analysis. Food Chem. 2015, 170, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Elflein, L.; Raezke, K.P. Improved detection of honey adulteration by measuring differences between 13C/12C stable carbon isotope ratios of protein and sugar compounds with a combination of elemental analyzer-isotope ratio mass spectrometry and liquid chromatography-isotope ra. Apidologie 2008, 39, 574–587. [Google Scholar] [CrossRef]
- Cole, P.; Vasiliou, S.; Mormeneo, D.; Rosa, E. Medicinal chemistry selections from the 237th American chemical society national meeting & exposition. Drugs Future 2009, 34, 509–520. [Google Scholar]
- Schievano, E.; Stocchero, M.; Morelato, E.; Facchin, C.; Mammi, S. An NMR-based metabolomic approach to identify the botanical origin of honey. Metabolomics 2012, 8, 679–690. [Google Scholar] [CrossRef]
- Morgan, M. Experts Reveal That Three Times More Jars of Healing Manuka Are Sold around the World Than Being Produced in New Zealand. Is Your Superfood Honey FAKE? 2015. Available online: http://www.dailymail.co.uk/femail/article-3066381/Issuperfood-honey-FAKE-jars-manuka-sold-world-produced-New-Zealand.html (accessed on 24 January 2018).
- Adams, C.J.; Boult, C.H.; Deadman, B.J.; Farr, J.M.; Grainger, M.N.C.; Manley-Harris, M.; Snow, M.J. Isolation by HPLC and characterisation of the bioactive fraction of New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2008, 343, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.M.; Grainger, M.; Manley-Harris, M. The unique manuka effect: Why New Zealand manuka honey fails the AOAC 998.12 C-4 sugar method. J. Agric. Food Chem. 2014, 62, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Martin, P.; Lüllmann, C. Harmonised methods of the European Honey Commission. Apidologie 1997, 28, 1–59. [Google Scholar]
- Rattanathanalerk, M.; Chlewchan, N.; Srichum-Poung, W. Effects of thermo processing on the duality loss of pineapple juice. J. Food Eng. 2005, 66, 259–265. [Google Scholar] [CrossRef]
- Adenekan, M.O.; Amusa, N.A.; Lawal, A.O.; Okpeze, V.E. Physico-chemical and microbiological properties of honey samples obtained from Ibadan. J. Microbiol. Antimicrobial. 2010, 2, 100–104. [Google Scholar]
- Muruke, M.H. Assessment of quality of Tanzanian honey based on physicochemical properties. Food Sci. Qual. Manag. 2014, 3, 61–72. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.833.3399&rep=rep1&type=pdf (accessed on 24 January 2018).
- Singh, N.; Bath, P.K. Quality evaluation of different types of Indian honey. Food Chem. 1997, 58, 129–133. [Google Scholar] [CrossRef]
- Terrab, A.; Dıez, M.J.; Heredia, F.J. Palynological, physicochemical and colour characterization of Moroccan honeys: I. River red gum (Eucalyptus camaldulensis Dehnh) honey. Int. J. Food Sci. Technol. 2003, 38, 379–386. [Google Scholar] [CrossRef]
- Kosir, I.J.; Kidric, J. Identification of amino acids in wines by one- and two-dimensional nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 2001, 49, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Vlahov, G.; Kiprono Chepkwony, P.; Ndalut, P.K. 13C-NMR characterization of triacylglycerols of Moringa oleifera seed oil: An “oleic-vaccenic acid” oil. J. Agric. Food Chem. 2002, 50, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Lolli, M.; Bertelli, D.; Plessi, M.; Sabatini, A.G.; Restani, C. Classification of Italian honeys by 2D HR-NMR. J. Agric. Food Chem. 2008, 56, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Consonni, R.; Cagliani, L.R.; Cogliati, C. NMR characterization of saccharides in Italian honeys of different floral sources. J. Agric. Food Chem. 2012, 60, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, D.; Lolli, M.; Papotti, G.; Bortolotti, L.; Serra, G.; Plessi, M. Detection of honey adulteration by sugar syrups using one-dimensional and two-dimensional high-resolution nuclear magnetic resonance. J. Agric. Food Chem. 2010, 58, 8495–8501. [Google Scholar] [CrossRef] [PubMed]
- Kortesniemi, M.; Slupsky, C.M.; Ollikka, T.; Kauko, L.; Spevacek, A.R.; Sjövall, O.; Yang, B.; Kallio, H. NMR profiling clarifies the characterization of Finnish honeys of different botanical origins. Food Res. Int. 2016, 86, 83–92. [Google Scholar] [CrossRef]
- Boffo, E.F.; Tavares, L.A.; Tobias, A.C.T.; Ferreira, M.M.C.; Ferreira, A.G. Identification of components of Brazilian honey by 1H-NMR and classification of its botanical origin by chemometric methods. Food Sci. Tech. 2012, 49, 55–63. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H-NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Schievano, E.; Morelato, E.; Facchin, C.; Mammi, S. Characterization of markers of botanical origin and other compounds extracted from unifloral honeys. J. Agric. Food Chem. 2013, 61, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Spiteri, M.; Jamin, E.; Thomas, F.; Rebours, A.; Lees, M.; Rogers, K.M.; Rutledge, D.N. Fast and global authenticity screening of honey using 1H-NMR profiling. Food Chem. 2015, 189, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Schievano, E.; Peggion, E.; Mammi, S. 1H nuclear magnetic resonance spectra of chloroform extracts of honey for chemometric determination of its botanical origin. J. Agric. Food Chem. 2010, 58, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, S.; Bobeldijk, I.; Verheij, E.R.; Ramaker, R.; Kochhar, S.; Macdonald, I.A.; van Ommen, B.; Smilde, A.K. Large-scale human metabolomics studies: A strategy for data (pre-) processing and validation. Anal. Chem. 2006, 78, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Hankin, L. Analysis of Honey; Bulletin 847; The Connecticut Agricultural Experiment Station: New Haven, CT, USA, 1987; pp. 1–6. Available online: http://www.ct.gov/caes/lib/caes/documents/publications/bulletins/b847.pdf (accessed on 24 January 2018).
- Helrich, K. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists Inc.: Arlington, VA, USA, 1990; pp. 1028–1030. [Google Scholar]
- Bogdanov, S.; Lüllmann, C.; Martin, P.; Von Der Ohe, W.; Russmann, H.; Mossel, B.L.; D’Arcy, B.; Vorwohl, G.R.; Oddo, L.; Sabatini, A.G.; et al. Honey quality, methods of analysis and international regulatory standards: review of the work of the international honey commission. Bee World 1999, 80, 61–69. [Google Scholar] [CrossRef]
- Mairaj, G.; Akhtar, S.; Khan, A.R.; Uilah, Z.; Bibi, S.; Ali, S. Quality evaluation of different honey samples produced in Peshawar valley. Par. J. Biol. Sci. 2008, 11, 797–800. [Google Scholar]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar]
- Beretta, G.; Caneva, E.; Regazzoni, L.; Bakhtyari, N.G.; Maffei Facino, R. A solid-phase extraction procedure coupled to 1H-NMR, with chemometric analysis, to seek reliable markers of the botanical origin of honey. Anal. Chim. Acta 2008, 620, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Savorani, F.; Tomasi, G.; Engelsen, S.B. icoshift: A versatile tool for the rapid alignment of 1D-NMR spectra. J. Magn. Reson. 2010, 202, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Mazet, V.; Carteret, C.; Brie, D.; Idier, J.; Humbert, B. Background removal from spectra by designing and minimising a non-quadratic cost function. Chemom. Intell. Lab. Syst. 2005, 76, 121–133. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds HA-HJ and reference sugars are available from the authors. |

| Colour | Pfund Sacle (mm) | Colour Range |
|---|---|---|
| Water white | 1 to 8 | 0.030 or less |
| Extra white | More than 8–17 | More than 0.030–0.060 |
| White | More than 17–34 | More than 0.060–0.120 |
| Extra light amber | More than 34–50 | More than 0.120–0.188 |
| Light amber | More than 50–85 | More than 0.188–0.440 |
| Amber | More than 85–114 | More than 0.440–0.945 |
| Dark amber | More than 114 | More than 0.945 |
| Honey | Geographical | pH | Moisture Content | Electrical Conductivity |
|---|---|---|---|---|
| Code | Origin | (%) | ×102 (µS/cm) | |
| HA | Slovakia | 4.06 ± 0.006 | 17.4 ± 1.95 | 4.29 ± 0.0503 |
| HB | Slovakia | 4.01 ± 0.015 | 14.6 ± 0.116 | 1.79 ± 0.0404 |
| HC | Slovakia | 3.75 ± 0.015 | 17.7 ± 0.330 | 0.99 ± 0.0503 |
| HD | South Africa | 4.01 ± 0.015 | 15.0 ± 0.180 | 4.66 ± 0.0252 |
| HE | South Africa | 4.16 ± 0.015 | 17.4 ± 0.220 | 4.12 ± 0.111 |
| HF | South Africa | 4.27 ± 0.012 | 14.4 ± 0.19 | 4.19 ± 0.105 |
| HG | South Africa | 4.07 ± 0.01 | 14.2 ± 0.060 | 2.17 ± 0.105 |
| HH | South Africa | 4.38 ± 0.025 | 17.4 ± 0.120 | 1.78 ± 0.0436 |
| HI | Zambia | 4.29 ± 0.01 | 15.0 ± 0.350 | 6.46 ± 0.050 |
| HJ | Zambia | 4.29 ± 0.012 | 16.8 ± 1.15 | 6.59 ± 0.107 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olawode, E.O.; Tandlich, R.; Cambray, G. 1H-NMR Profiling and Chemometric Analysis of Selected Honeys from South Africa, Zambia, and Slovakia. Molecules 2018, 23, 578. https://doi.org/10.3390/molecules23030578
Olawode EO, Tandlich R, Cambray G. 1H-NMR Profiling and Chemometric Analysis of Selected Honeys from South Africa, Zambia, and Slovakia. Molecules. 2018; 23(3):578. https://doi.org/10.3390/molecules23030578
Chicago/Turabian StyleOlawode, Emmanuel O., Roman Tandlich, and Garth Cambray. 2018. "1H-NMR Profiling and Chemometric Analysis of Selected Honeys from South Africa, Zambia, and Slovakia" Molecules 23, no. 3: 578. https://doi.org/10.3390/molecules23030578
APA StyleOlawode, E. O., Tandlich, R., & Cambray, G. (2018). 1H-NMR Profiling and Chemometric Analysis of Selected Honeys from South Africa, Zambia, and Slovakia. Molecules, 23(3), 578. https://doi.org/10.3390/molecules23030578





