Synergistic Antifungal, Allelopatic and Anti-Proliferative Potential of Salvia officinalis L., and Thymus vulgaris L. Essential Oils
Abstract
:1. Introduction
2. Results and Discussions
2.1. Chemical Composition of SEO and TEO
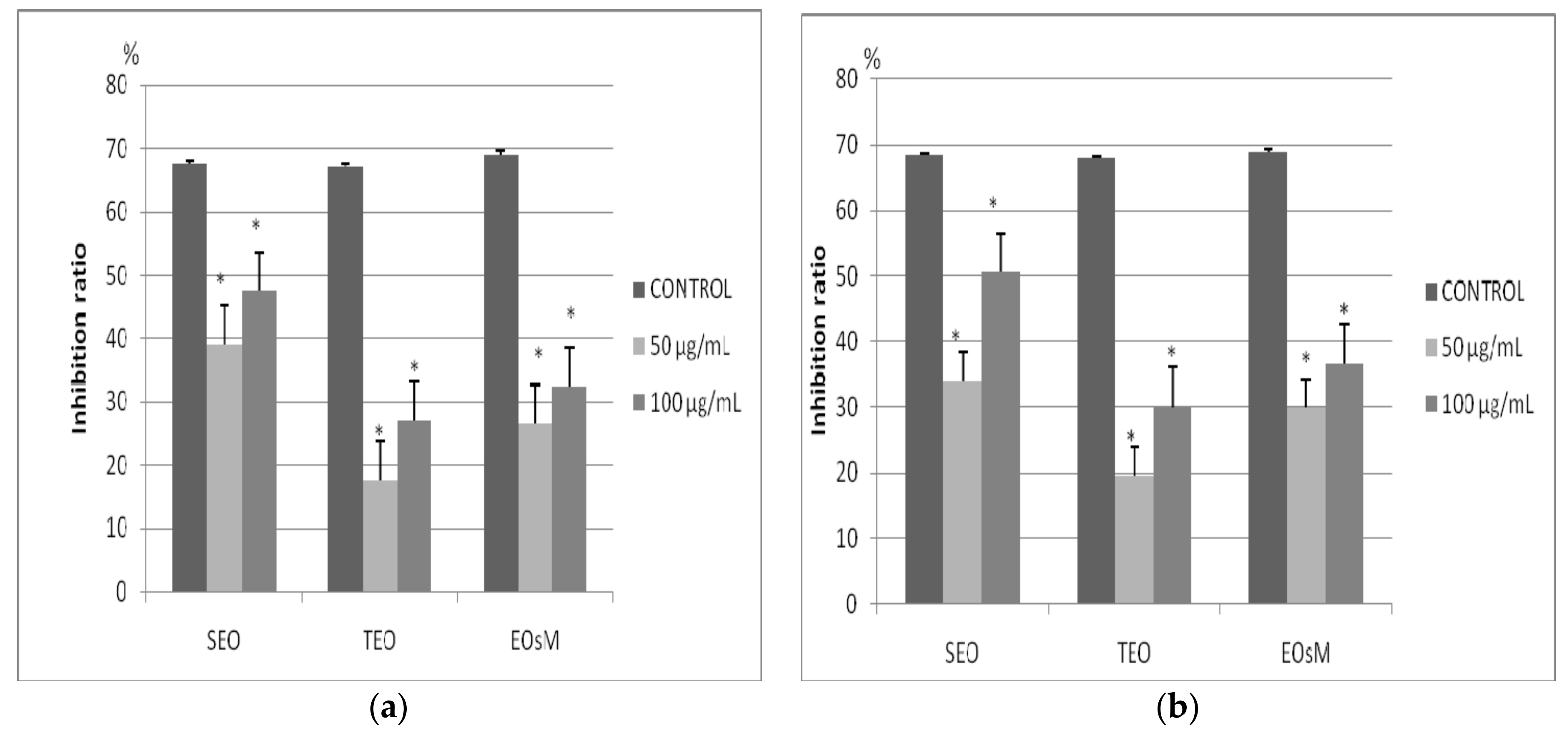
2.2. Cytotoxic Effect
2.3. Antifungal Activity
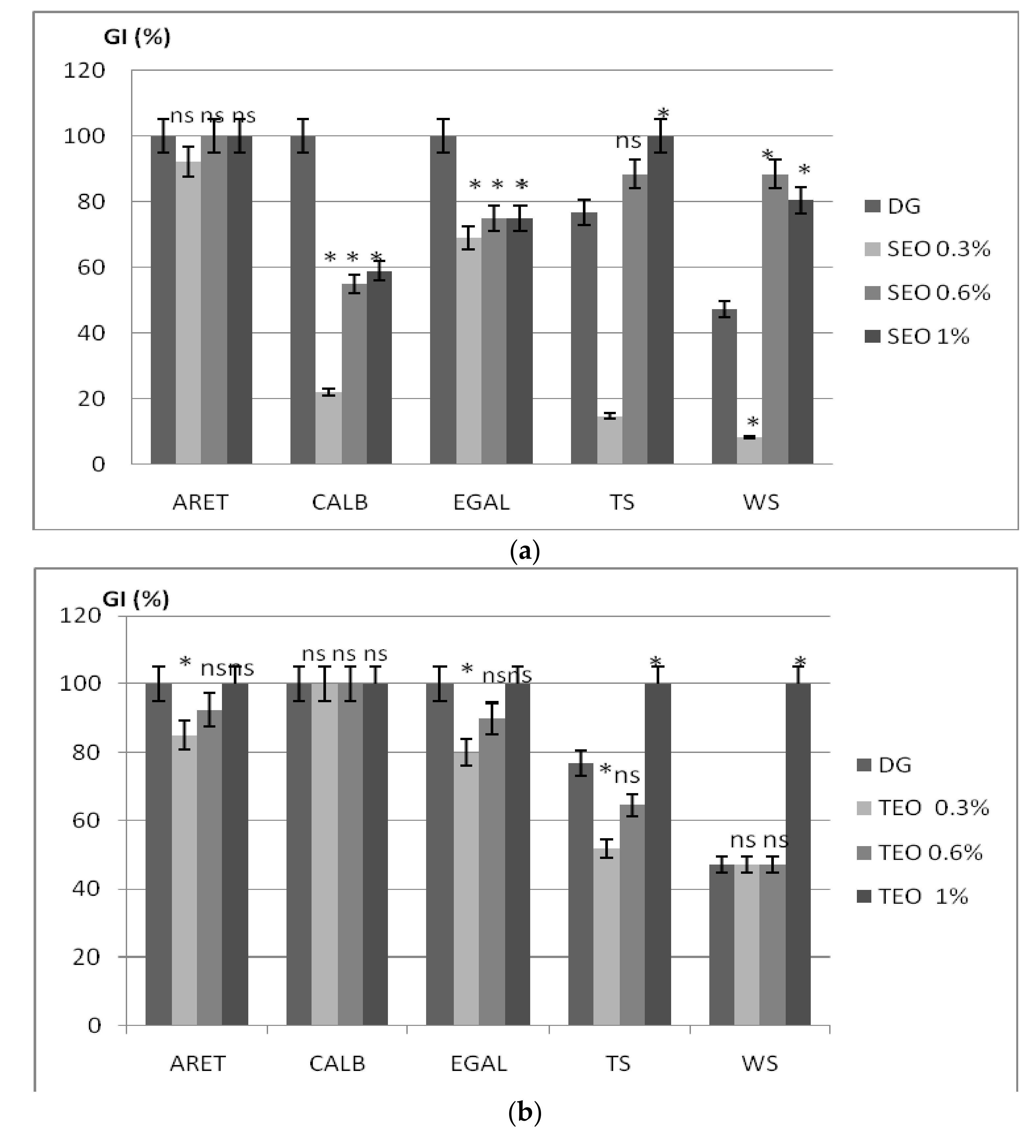
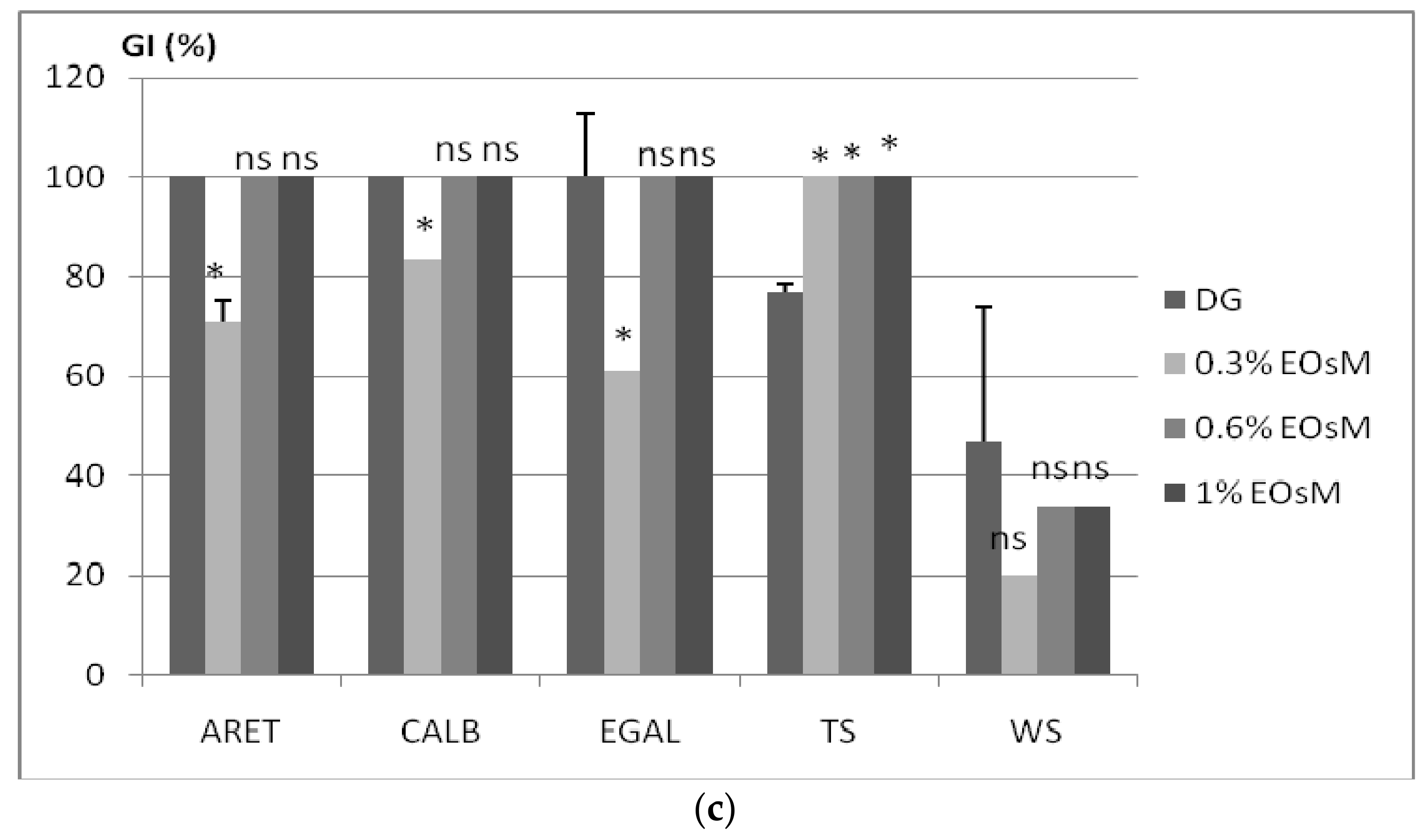
2.4. Allelopatic Potential
3. Materials and methods
3.1. Plant Material
3.2. Extraction of Essential Oils
3.3. GC-MS Analysis
3.4. MTT Proliferation Assay
3.5. Antifungal Activity
3.6. Allelopatic Potential
3.7. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bouaziz, M.; Yangui, T.; Sayadi, S.; Dhouib, A. Disinfectant properties of essential oils from Salvia officinalis L. cultivated in Tunisia. Food Chem. Toxicol. 2009, 47, 2755–2760. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.E.; Benincasa, B.I.; Fachin, A.L.; França, S.C.; Contini, S.S.H.T.; Chagas, A.C.S.; Beleboni, R.O. Thymus vulgaris L. essential oil and its main component thymol: Anthelmintic effects against Haemonchus contortus from sheep. Vet. Parasitol. 2016, 228, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Nezhadali, A.; Nabavi, M.; Rajabian, M.; Akbarpour, M.; Pourali, P.; Amini, F. Chemical variation of leaf essential oil at different stages of plant growth and in vitro antibacterial activity of Thymus vulgaris Lamiaceae, from Iran. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 87–92. [Google Scholar] [CrossRef]
- Eguchi, Y.; Widiastuti, A.; Odani, H.; Chinta, Y.D.; Shinohara, M.; Misu, H.; Kamoda, H.; Watanabe, T.; Hasegawa, M.; Sato, T. Identification of terpenoids volatilized from Thymus vulgaris L. by heat treatment and their in vitro antimicrobial activity. Physiol. Mol. Plant Pathol. 2016, 94, 83–89. [Google Scholar] [CrossRef]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L.; Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- De Carvalho, R.J.; de Souza, G.T.; Honório, V.G.; de Sousa, J.P.; da Conceição, M.L.; Maganani, M.; de Souza, E.L. Comparative inhibitory effects of Thymus vulgaris L. essential oil against Staphylococcus aureus, Listeria monocytogenes and mesophilic starter co-culture in cheese-mimicking models. Food Microbiol. 2015, 52, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Al-Bayati, F.A. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J. Ethnopharmacol. 2008, 116, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, G.; Calonico, C.; Bilia, A.R.; Barnabei, M.; Calesini, F.; Addona, R.; Mencarelli, L.; Carmagnini, L.; Di Martino, M.C.; Lo Nostro, A. Antibacterial activity of Oregano, Rosmarinus and Thymus essential oils against Staphylococcus aureus and Listeria monocytogenes in beef meatballs. Food Control 2015, 54, 188–199. [Google Scholar] [CrossRef]
- Cardile, V.; Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Arnold, N.A.; Piozzi, F. Essential oils of Salvia bracteata and Salvia rubifolia from Lebanon: Chemical composition, antimicrobial activity and inhibitory effect on human melanoma cells. J. Ethnopharmacol. 2009, 126, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Formisano, C.; Rigano, D.; Cardile, V.; Arnold, N.A.; Senatore, F. Comparative phytochemical profile and antiproliferative activity on human melanoma cells of essential oils of three Lebanese Salvia species. Ind. Crops Prod. 2016, 83, 492–499. [Google Scholar] [CrossRef]
- Al-Kalaldeh, J.Z.; Abu-Dahab, R.; Afifi, F.U. Volatile oil composition and antiproliferative activity of Laurus nobilis, Origanum syriacum, Origanum vulgare, and Salvia triloba against human breast adenocarcinoma cells. Nutr. Res. 2010, 30, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Nicolella, H.D.; de Oliveira, P.F.; Munari, C.C.; Costa, G.F.D.; Moreira, M.R.; Veneziani, R.C.S.; Tavares, D.C. Differential effect of manool—A diterpene from Salvia officinalis, on genotoxicity induced by methyl methanesulfonate in V79 and HepG2 cells. Food Chem. Toxicol. 2014, 72, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Vuković-Gačić, B.; Nikčević, S.; Berić-Bjedov, T.; Knežević-Vukčević, J.; Simić, D. Antimutagenic effect of essential oil of sage (Salvia officinalis L.) and its monoterpenes against UV-induced mutations in Escherichia coli and Saccharomyces cerevisiae. Food Chem. Toxicol. 2006, 44, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Calò, R.; Visone, C.M.; Marabini, L. Thymol and Thymus vulgaris L. activity against UVA- and UVB-induced damage in NCTC 2544 cell line. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 791, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Do Ó Pessoa, C.; Rodrigues, F.A.R.; Marques, L.G.A.; Sbardelotto, A.B. Therapeutic potential of essential oils for cancer treatment. In Medicinal Essential Oils: Chemical, Pharmacological and Therapeutic Aspects; Nova Science: New York, NY, USA, 2012; pp. 159–172. [Google Scholar]
- Pekmezovic, M.; Rajkovic, K.; Barac, A.; Senerović, L.; Arsic Arsenijevic, V. Development of kinetic model for testing antifungal effect of Thymus vulgaris L. and Cinnamomum cassia L. essential oils on Aspergillus flavus spores and application for optimization of synergistic effect. Biochem. Eng. J. 2015, 99, 131–137. [Google Scholar] [CrossRef]
- Razzaghi-Abyaneh, M.; Shams-Ghahfarokhi, M.; Rezaee, M.B.; Jaimand, K.; Alinezhad, S.; Saberi, R.; Yoshinari, T. Chemical composition and antiaflatoxigenic activity of Carum carvi L.; Thymus vulgaris and Citrus aurantifolia essential oils. Food Control 2009, 20, 1018–1024. [Google Scholar] [CrossRef]
- Nguefack, J.; Dongmo, J.B.L.; Dakole, C.D.; Leth, V.; Vismer, H.F.; Torp, J.; Guemdjom, E.F.N.; Mbeffo, M.; Tamgue, O.; Fotio, D.; et al. Food preservative potential of essential oils and fractions from Cymbopogon citratus, Ocimum gratissimum and Thymus vulgaris against mycotoxigenic fungi. Int. J. Food Microbiol. 2009, 131, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Nguefack, J.; Tamgue, O.; Dongmo, J.B.L.; Dakole, C.D.; Leth, V.; Vismer, H.F.; Amvam Zollo, P.H.; Nkengfack, A.E. Synergistic action between fractions of essential oils from Cymbopogon citratus, Ocimum gratissimum and Thymus vulgaris against Penicillium expansum. Food Control 2012, 23, 377–383. [Google Scholar] [CrossRef]
- Kohiyama, C.Y.; Yamamoto Ribeiro, M.M.; Mossini, S.A.G.; Bando, E.; Bomfim, N.D.S.; Nerilo, S.B.; Rocha, G.H.O.; Grespan, R.; Mikcha, J.M.G.; Machinski, M. Antifungal properties and inhibitory effects upon aflatoxin production of Thymus vulgaris L. by Aspergillus flavus Link. Food Chem. 2015, 173, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Gonçalves, M.J.; Oliveira, P.; Coelho, J.; Cavaleiro, C.; Salgueiro, L. Activity of Thymus caespititius essential oil and α-terpineol against yeasts and filamentous fungi. Ind. Crops Prod. 2014, 62, 107–112. [Google Scholar] [CrossRef]
- Rajkovic, K.; Pekmezovic, M.; Barac, A.; Nikodinovic-Runic, J.; Arsic Arsenijevic, V. Inhibitory effect of thyme and cinnamon essential oils on Aspergillus flavus: Optimization and activity prediction model development. Ind. Crops Prod. 2015, 65, 7–13. [Google Scholar] [CrossRef]
- Rus, C.F.; Alexa, E.; Pop, G.; Sumalan, R.M.; Copolovici, M. Antifungal activity and chemical composition of Salvia Officinalis L. essential oil. Res. J. Agric. Sci. 2015, 47, 186–194. [Google Scholar]
- Tofana, M.; Socaci, S.A.; Mudura, E. Old Principles—New Methods in the Volatile Oils Approach. Bull. UASVM Agric. 2010, 67, 439–446. [Google Scholar]
- Chon, S.U.; Nelson, C.J. Allelopathic dynamics in resource plants. In Allelopathy: Current Trends and Future Applications; Springer: Berlin/Heidelberg, Germany, 2013; pp. 81–110. [Google Scholar]
- Ben El Hadj Ali, I.; Chaouachi, M.; Bahri, R.; Chaieb, I.; Boussaïd, M.; Harzallah-Skhiri, F. Chemical composition and antioxidant, antibacterial, allelopathic and insecticidal activities of essential oil of Thymus algeriensis Boiss. et Reut. Ind. Crops Prod. 2015, 77, 631–639. [Google Scholar] [CrossRef]
- Ramezani, S.; Saharkhiz, M.J.; Ramezani, F.; Fotokian, M.H. Use of Essential Oils as Bioherbicides. J. Essent. Oil Bear. Plants 2008, 11, 319–327. [Google Scholar] [CrossRef]
- Adrar, N.; Oukil, N.; Bedjou, F. Antioxidant and antibacterial activities of Thymus numidicus and Salvia officinalis essential oils alone or in combination. Ind. Crops Prod. 2016, 88, 112–119. [Google Scholar] [CrossRef]
- Schmidt, E.; Wanner, J.; Hiiferl, M.; Jirovetz, L.; Buchbauer, G.; Gochev, V.; Girova, T.; Stoyanova, A.; Geissler, M. Chemical composition, olfactory analysis and antibacterial activity of Thymus vulgaris chemotypes geraniol, 4-thujanol/terpinen-4-ol, thymol and linalool cultivated in southern France. Nat. Prod. Commun. 2012, 7, 1095–1098. [Google Scholar] [PubMed]
- Hudaib, M.; Speroni, E.; Di Pietra, A.M.; Cavrini, V. GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J. Pharm. Biomed. Anal. 2002, 29, 691–700. [Google Scholar] [CrossRef]
- Baranauskiene, R.; Venskutonis, P.R.; Viškelis, P.; Dambrauskiene, E. Influence of nitrogen fertilizers on the yield and composition of Thyme (Thymus vulgaris). J. Agric. Food Chem. 2003, 51, 7751–7758. [Google Scholar] [CrossRef] [PubMed]
- Rus, C.; Sumalan, R.M.; Alexa, E.; Copolovici, D.M.; Pop, G.; Botau, D. Study on chemical composition and antifungal activity of essential oils obtained from representative species belonging to the Lamiaceae family. Plant Soil Environ. 2015, 61, 297–302. [Google Scholar] [CrossRef]
- Imelouane, B.; Elbachiri, A.; Wathelet, J.-P.; Dubois, J.; Amhamdi, H. Chemical composition, cytotoxic and antioxidant activity of the essential oil of Lavandula dentata. World J. Chem. 2010, 5, 103–110. [Google Scholar]
- Gali-Muhtasib, H.U.; Affara, N.I. Chemopreventive effects of sage oil on skin papillomas in mice. Phytomedicine 2000, 7, 129–136. [Google Scholar] [CrossRef]
- Privitera, G.; Napoli, E.; Luca, T.; Ruberto, G.; Castorina, S. In vitro anti-proliferative effect of Salvia officinalis essential oil and its three main components on human lung cancer cells. Am. J. Phytomed. Clin. Ther. 2014, 2, 1159–1168. [Google Scholar]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anticancer Res. 2007, 27, 3293–3299. [Google Scholar] [PubMed]
- Sertel, S.; Eichhorn, T.; Plinkert, P.K.; Efferth, T. Cytotoxicity of Thymus vulgaris essential oil towards human oral cavity squamous cell carcinoma. Anticancer Res. 2011, 31, 81–87. [Google Scholar] [PubMed]
- Itani, W.S.; El-Banna, S.H.; Hassan, S.B.; Larsson, R.L.; Bazarbachi, A.; Gali-Muhtasib, H.U. Anti colon cancer components from lebanese sage (Salvia libanotica) essential oil: Mechanistic basis. Cancer Biol. Ther. 2008, 7, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Bendif, H.; Boudjeniba, M.; Miara, M.D.; Biqiku, L.; Bramucci, M.; Lupidi, G.; Quassinti, L.; Vitali, L.A.; Maggi, F. Essential oil of Thymus munbyanus subsp. coloratus from Algeria: Chemotypification and in vitro biological activities. Chem. Biodivers. 2017, 14, e1600299. [Google Scholar]
- Miguel, M.G.; Gago, C.; Antunes, M.D.; Megías, C.; Cortés-Giraldo, I.; Vioque, J.; Lima, A.S.; Figueiredo, A.C. Antioxidant and Antiproliferative Activities of the Essential Oils from Thymbra capitata and Thymus Species Grown in Portugal. Evid. Based Complement. Altern. Med. 2015, 2015, 851721. [Google Scholar] [CrossRef] [PubMed]
- Tefiani, C.; Riazi, A.; Youcefi, F.; Aazza, S.; Gago, C.; Faleiro, M.L.; Pedro, L.G.; Barroso, J.G.; Figueiredo, A.C.; Megías, C.; et al. Ammoides pusilla (Apiaceae) and Thymus munbyanus (Lamiaceae) from Algeria essential oils: Chemical composition, antimicrobial, antioxidant and antiproliferative activities. J. Essent. Oil Res. 2015, 27, 131–139. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Chatha, S.A.S.; Latif, S.; Sherazi, S.T.H.; Ahmad, A.; Worthington, J.; Sarker, S.D. Chemical composition and bioactivity studies of the essential oils from two Thymus species from the Pakistani flora. LWT Food Sci. Technol. 2013, 50, 185–192. [Google Scholar] [CrossRef]
- Fekrazad, R.; Afzali, M.; Pasban-Aliabadi, H.; Esmaeili-Mahani, S.; Aminizadeh, M.; Mostafavi, A. Cytotoxic effect of Thymus caramanicus Jalas on human oral epidermoid carcinoma KB cells. Braz. Dent. J. 2017, 28, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, T.; Lupo, A.T.; Chinn, J.W.; Kang, R.K.L. Biological activity of essential oils and their constituents. Stud. Nat. Prod. Chem. 2000, 21, 571–631. [Google Scholar]
- Kivrak, İ.; Duru, M.E.; Öztürk, M.; Mercan, N.; Harmandar, M.; Topçu, G. Antioxidant, anticholinesterase and antimicrobial constituents from the essential oil and ethanol extract of Salvia potentillifolia. Food Chem. 2009, 116, 470–479. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ahmadi, A. Evaluation of Salvia officinalis antifungal properties on the growth and morphogenesis of Alternaria alternata under in vitro conditions. Tech. J. Eng. Appl. Sci. 2013, 3, 2062–2069. [Google Scholar]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop Prot. 2003, 22, 39–44. [Google Scholar] [CrossRef]
- Sumalan, R.M.; Alexa, E.; Poiana, M.A. Assessment of inhibitory potential of essential oils on natural mycoflora and Fusarium mycotoxins production in wheat. Chem. Cent. J. 2013, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Abu-Darwish, M.S.; Cabral, C.; Ferreira, I.V.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Al-Bdour, T.H.; Salgueiro, L. Essential oil of common sage (Salvia officinalis L.) from Jordan: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. Biomed Res. Int. 2013, 2013, 538940. [Google Scholar] [CrossRef] [PubMed]
- Tantaoui-Elaraki, A.; Beraoud, L. Inhibition of growth and aflatoxin production in Aspergillus parasiticus by essential oils of selected plant materials. J. Environ. Pathol. Toxicol. Oncol. 1994, 13, 67–72. [Google Scholar] [PubMed]
- Arslan, M.; Dervis, S. Antifungal activity of essential oils against three vegetative-compatibility groups of Verticillium dahliae. World J. Microbiol. Biotechnol. 2010, 26, 1813–1821. [Google Scholar] [CrossRef]
- Kocić-Tanackov, S.D.; Dimić, G.R. Antifungal activity of essential oils in the control of food-borne fungi growth and mycotoxin biosynthesis in food. Formatex 2013, 4, 838–849. [Google Scholar]
- Paudel, V.R.; Gupta, V.N.P. Effect of some essential oils on seed germination and seedling length of Parthenium hysterophorous L. Ecoprint Int. J. Ecol. 2009, 15, 69–73. [Google Scholar] [CrossRef]
- Cavalieri, A.; Caporali, F. Effects of essential oils of cinnamon, lavender and peppermint on germination of Mediterranean weeds. Allelopathy J. 2010, 25, 441–452. [Google Scholar]
- Angelini, L.G.; Carpanese, G.; Cioni, P.L.; Morelli, I.; Macchia, M.; Flamini, G. Essential oils from Mediterranean Lamiaceae as weed germination inhibitors. J. Agric. Food Chem. 2003, 51, 6158–6164. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dool, H.; Dec. Kratz, P. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Sangeeta. Efficacy of some medicinal plant extract against Fusarium oxysporum ssp. ciceri causing chickpea wilt. Asian J. Crop Sci. 2015, 7, 138–146. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2007. [Google Scholar]
- Atak, M.; Mavi, K.; Uremis, I. Bio-herbicidal effects of oregano and rosemary essential oils on germination and seedling growth of bread wheat cultivars and weeds. Rom. Biotechnol. Lett. 2016, 21, 11149–11159. [Google Scholar]
Sample Availability: All samples of sage and thyme essential oils are available from the authors. |
| No. Crt. | Compounds | Type of Compound | Linear Retention Indices (LRI) | % of Total |
|---|---|---|---|---|
| 1 | α-pinene | MH 1 | 1013 | 4.816 |
| 2 | β-pinene | MH | 1092 | 11.230 |
| 3 | eucalyptol | MO 2 | 1198 | 13.902 |
| 4 | o-cymene | MH | 1287 | 0.515 |
| 5 | α-thujone | MO | 1416 | 8.871 |
| 6 | camphor | MO | 1493 | 4.028 |
| 7 | camphene | MH | 1498 | 14.139 |
| 8 | d-carvone | MO | 1518 | 0.205 |
| 9 | m-menthane | MH | 1573 | 0.853 |
| 10 | p-thymol | MO | 1578 | 8.073 |
| 11 | caryophyllene | SH 3 | 1581 | 25.364 |
| 12 | trans-calamenene | MH | 1644 | 1.676 |
| 13 | valencene | MH | 1713 | 5.525 |
| 14 | terpinolene | MH | 1774 | 0.360 |
| Total | 97.624 | |||
| MH | 37.387 | |||
| SH | 25.364 | |||
| MO | 34.874 | |||
| % of SEO extraction | 3.560 | |||
| No. crt. | Compounds | Type of Compound | Linear Retention Indices (LRI) | % of Total |
|---|---|---|---|---|
| 1 | γ-terpinene | MH 1 | 1250 | 68.415 |
| 2 | p-thymol | MO 2 | 1578 | 24.721 |
| 3 | Caryophyllene | SH 3 | 1581 | 5.508 |
| 4 | Terpine-4-ol | MO | 1635 | 0.099 |
| 5 | o-thymol | MO | 1745 | 0.237 |
| 6 | p-cymene-7-ol | MO | 1824 | 0.242 |
| 7 | Caryophyllene oxide | SO 4 | 2013 | 0.222 |
| 8 | α-murolene | SH | 2120 | 0.555 |
| Total | 98.644 | |||
| MH | 68.415 | |||
| SH | 6.063 | |||
| MO | 25.299 | |||
| SO | 0.222 | |||
| % of TEO extraction | 3.040 | |||
| Variants | Fusarium graminearum | |
|---|---|---|
| 1 RG (mm) | 2 MGA (cm2) | |
| Control | 46.8 a ± 2.4 | 16.7 a ± 1.8 |
| 0.06% SEO | 45.8 b ± 2.5 | 15.9 b ± 1.7 |
| 0.12% SEO | 32.8 c ± 2.0 | 7.9 c ± 1.0 |
| 0.20% SEO | 20.7 d ± 1.8 | 2.8 d ± 0.6 |
| 0.06% TEO | 8.0 d ± 0 | 0.0 e |
| 0.12% TEO | 8.0 d ± 0 | 0.0 e |
| 0.2% TEO | 8.0 d ± 0 | 0.0 e |
| 0.06% EOsM | 9.0 d ± 0 | 0.1 e ± 0 |
| 0.12% EOsM | 8.0 d ± 0 | 0.0 e |
| 0.2% EOsM | 8.0 d ± 0 | 0.0 e |
| 0.1% thiophanate methyl | 8.0 d ± 0 | 0.0 e |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexa, E.; Sumalan, R.M.; Danciu, C.; Obistioiu, D.; Negrea, M.; Poiana, M.-A.; Rus, C.; Radulov, I.; Pop, G.; Dehelean, C. Synergistic Antifungal, Allelopatic and Anti-Proliferative Potential of Salvia officinalis L., and Thymus vulgaris L. Essential Oils. Molecules 2018, 23, 185. https://doi.org/10.3390/molecules23010185
Alexa E, Sumalan RM, Danciu C, Obistioiu D, Negrea M, Poiana M-A, Rus C, Radulov I, Pop G, Dehelean C. Synergistic Antifungal, Allelopatic and Anti-Proliferative Potential of Salvia officinalis L., and Thymus vulgaris L. Essential Oils. Molecules. 2018; 23(1):185. https://doi.org/10.3390/molecules23010185
Chicago/Turabian StyleAlexa, Ersilia, Renata Maria Sumalan, Corina Danciu, Diana Obistioiu, Monica Negrea, Mariana-Atena Poiana, Cristian Rus, Isidora Radulov, Georgeta Pop, and Cristina Dehelean. 2018. "Synergistic Antifungal, Allelopatic and Anti-Proliferative Potential of Salvia officinalis L., and Thymus vulgaris L. Essential Oils" Molecules 23, no. 1: 185. https://doi.org/10.3390/molecules23010185
APA StyleAlexa, E., Sumalan, R. M., Danciu, C., Obistioiu, D., Negrea, M., Poiana, M.-A., Rus, C., Radulov, I., Pop, G., & Dehelean, C. (2018). Synergistic Antifungal, Allelopatic and Anti-Proliferative Potential of Salvia officinalis L., and Thymus vulgaris L. Essential Oils. Molecules, 23(1), 185. https://doi.org/10.3390/molecules23010185










