Characterization of New Bioactive Enzyme Inhibitors from Endophytic Bacillus amyloliquefaciens RWL-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Growth Conditions
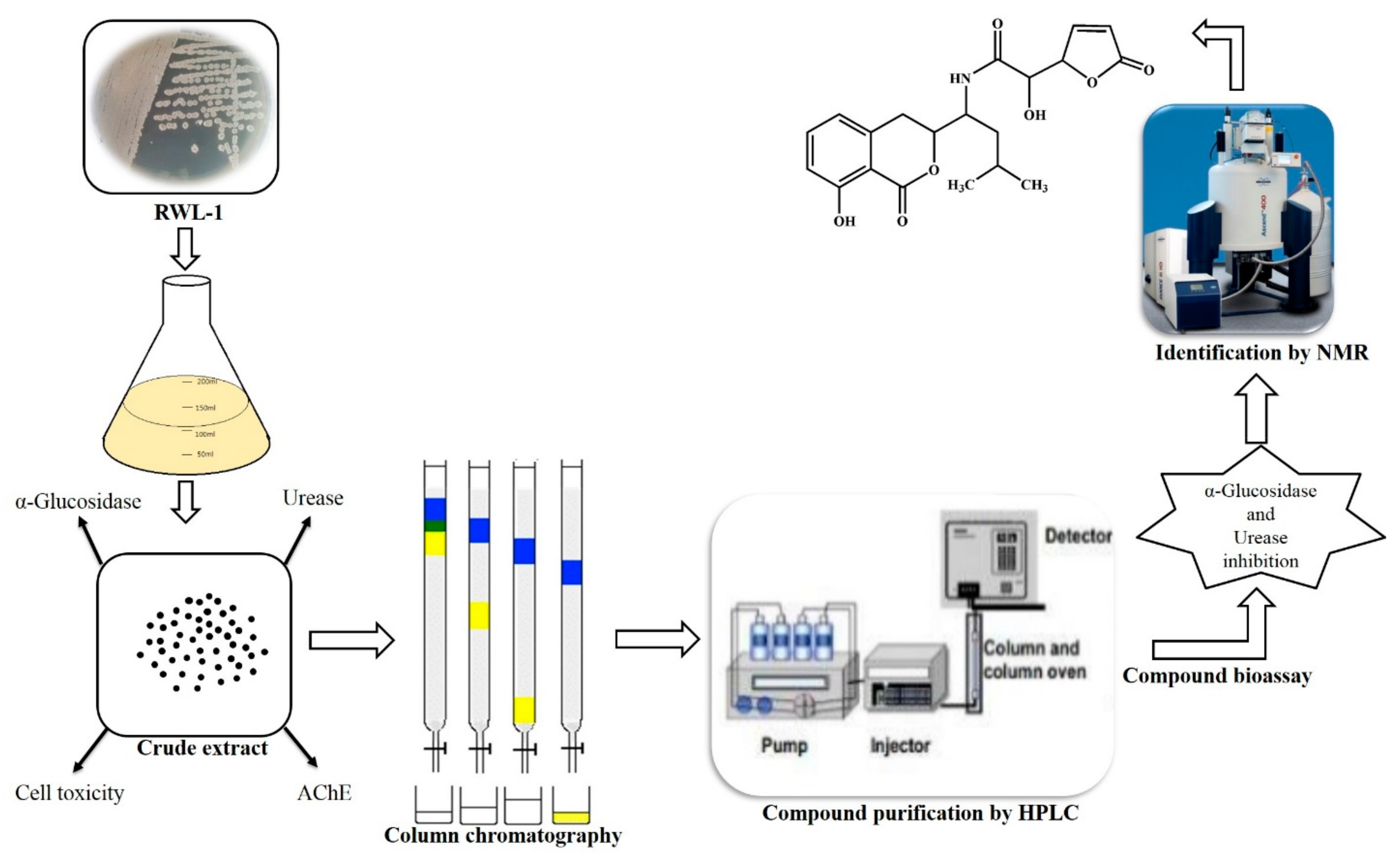
2.2. Extraction for Secondary Metabolite Identification
2.3. Secondary Metabolite Isolation
2.4. Reverse-Phase HPLC Analysis
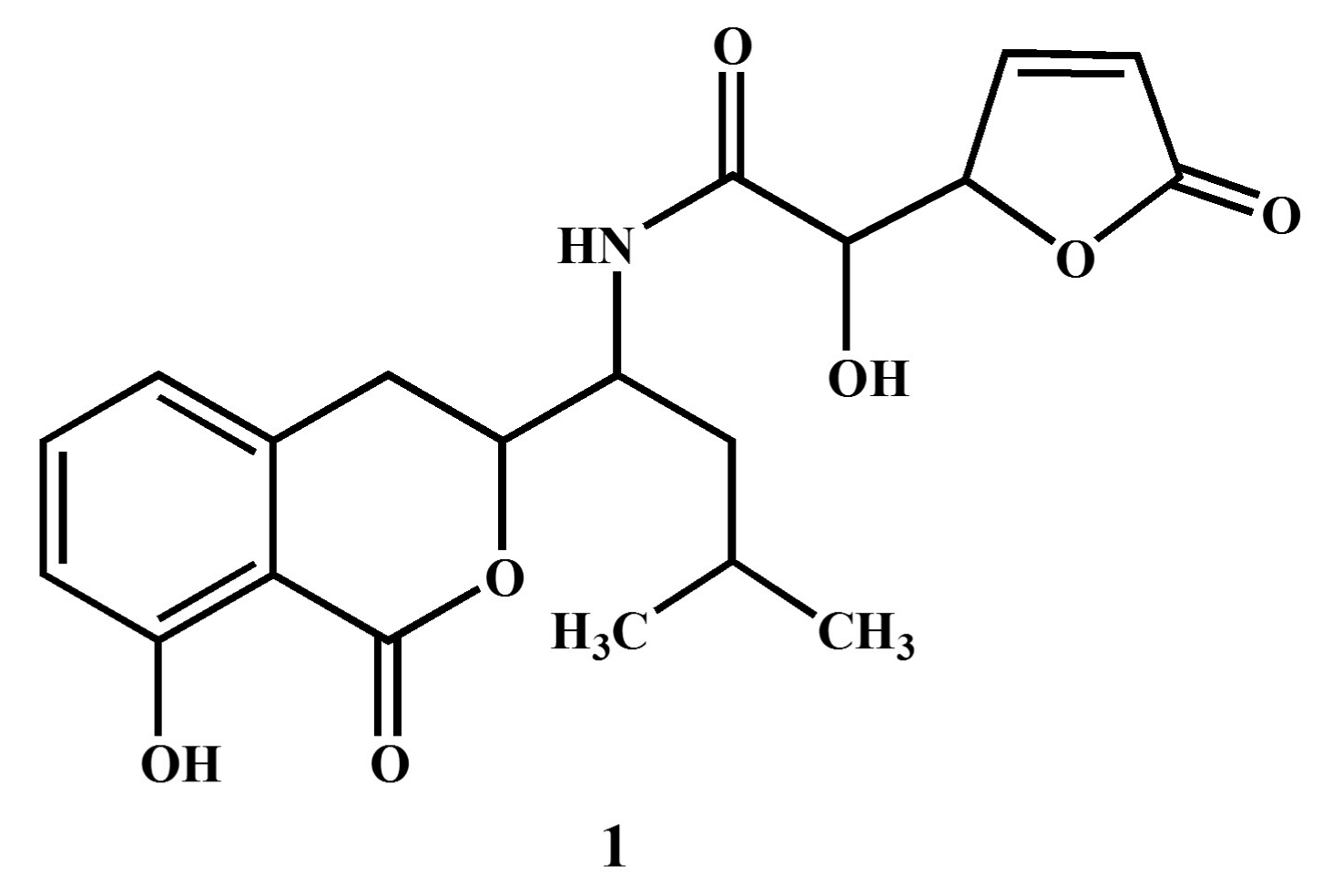
2.5. Structure Elucidation of Compound 1
2.6. In Vitro Biological Activities of RWL-1 Crude Extract
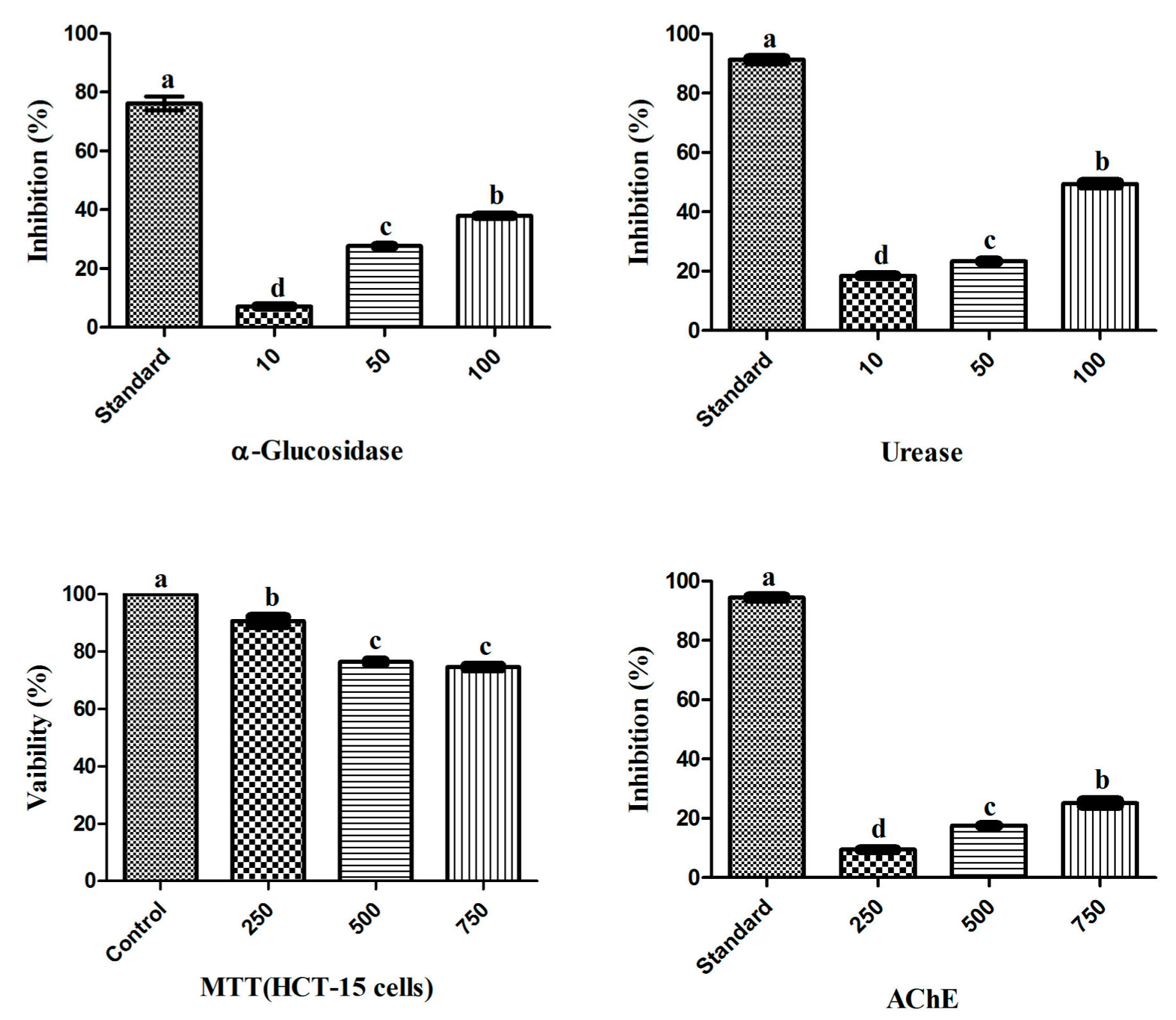
2.6.1. Urease Inhibition
2.6.2. α-Glucosidase Inhibition
2.6.3. Anticancer Assay (MTT)
2.6.4. AChE Inhibition
2.7. Statistical Analysis
3. Results and Discussion
3.1. Biological Potential of RWL-1 Crude Extract
3.2. Structural Elucidation of Compound 1
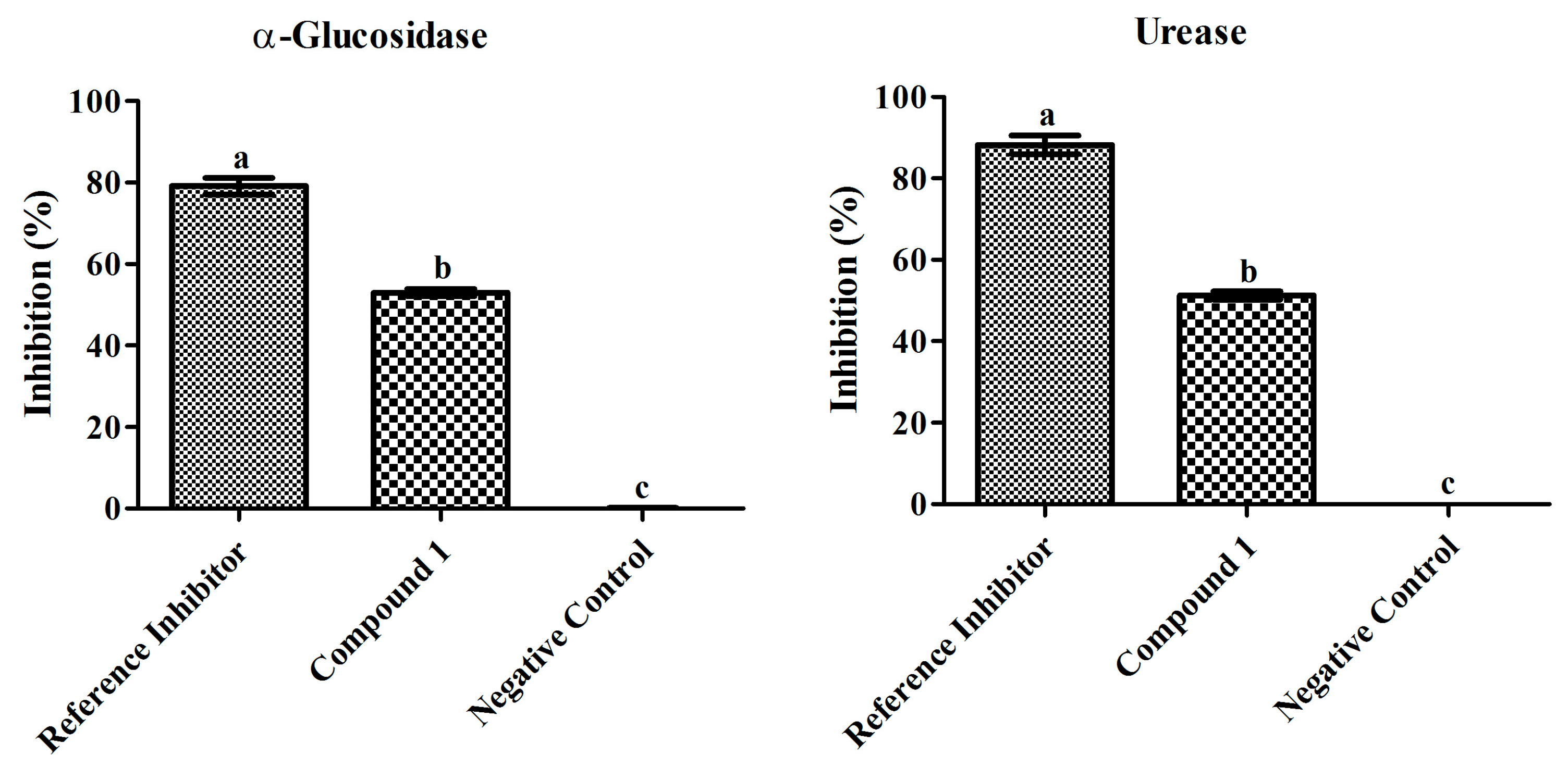
3.3. Biological Evaluation of Compound 1
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016, 106, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 2015, 35, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.N.; Padmavathy, S. Impact of endophytic microorganisms on plants, environment and humans. Sci. World J. 2014, 2014, 250693. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109. [Google Scholar] [CrossRef]
- Strobel, G.A. Endophytes as sources of bioactive products. Microbes Infect. 2003, 5. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.-K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites* *Paper presented at the British Mycological Society symposium on Fungal Bioactive Compounds, held at the University of Wales Swansea on 22–27 April 2001. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Saunders, M.; Glenn, A.E.; Kohn, L.M. Exploring the evolutionary ecology of fungal endophytes in agricultural systems: Using functional traits to reveal mechanisms in community processes. Evol. Appl. 2010, 3, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Cheplick, G.P.; Faeth, S.H. Ecology and Evolution of the Grass-Endophyte Symbiosis; OUP: New York, NY, USA, 2009; ISBN 0195308085. [Google Scholar]
- Schlaeppi, K.; Bulgarelli, D. The Plant Microbiome at Work. Mol. Plant-Microbe Interact. 2014, 28, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Aman, M.; Rai, V.R. Antifungal activity of novel indole derivative from endophytic bacteria Pantoea ananatis 4G-9 against Mycosphaerella musicola. Biocontrol Sci. Technol. 2016, 26, 476–491. [Google Scholar] [CrossRef]
- Chen, H.; Yang, C.; Ke, T.; Zhou, M.; Li, Z.; Zhang, M.; Gong, G.; Hou, T. Antimicrobial activity of secondary metabolites from Streptomyces sp. K15, an endophyte in Houttuynia cordata Thunb. Nat. Prod. Res. 2015, 29, 2223–2225. [Google Scholar] [CrossRef] [PubMed]
- Shweta, S.; Bindu, J.H.; Raghu, J.; Suma, H.K.; Manjunatha, B.L.; Kumara, P.M.; Ravikanth, G.; Nataraja, K.N.; Ganeshaiah, K.N.; Shaanker, R.U. Isolation of endophytic bacteria producing the anti-cancer alkaloid camptothecine from Miquelia dentata Bedd.(Icacinaceae). Phytomedicine 2013, 20, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; Matasyoh, J.C. Endophytes as producers of peptides: An overview about the recently discovered peptides from endophytic microbes. Nat. Prod. Bioprospect. 2014, 4, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Yuan, Q.; Shan, L.-T.; Lin, M.-A.; Cheng, D.-Q.; Li, C.-Y. Antitumor activity of bacterial exopolysaccharides from the endophyte Bacillus amyloliquefaciens sp. isolated from Ophiopogon japonicus. Oncol. Lett. 2013, 5, 1787–1792. [Google Scholar] [PubMed]
- Roy, S.; Yasmin, S.; Ghosh, S.; Bhattacharya, S.; Banerjee, D. Anti-Infective Metabolites of a Newly Isolated Bacillus thuringiensis KL1 Associated with Kalmegh (Andrographis paniculata Nees.), a Traditional Medicinal Herb. Microbiol. Insights 2016, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.T.; Mudarris, M.S.A. Separation of YbdN bioactive protein from Bacillus subtilis isolated from the Red Sea algae Sargassum sp. with bioactivity against antibiotic resistant bacterial pathogens. Mar. Sci. 2010, 21, 53–64. [Google Scholar] [CrossRef]
- Haas, D.; Défago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Eisenreich, A.; Schneider, K.; Heinemeyer, I.; Morgenstern, B.; Voss, B.; Hess, W.R.; Reva, O. Comparative analysis of the complete genome sequence of the plant growth–promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2007, 25, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.-W.; Chen, Y.-J.; Yen, Y.-H.; Wang, S.-L. The antitumor activity of the hydrolysates of chitinous materials hydrolyzed by crude enzyme from Bacillus amyloliquefaciens V656. Process Biochem. 2007, 42, 527–534. [Google Scholar] [CrossRef]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Schneider, K.; Vater, J.; Süssmuth, R.; Piel, J.; Borriss, R. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J. Biotechnol. 2009, 140, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.-J. Plant growth-promoting endophytic bacteria versus pathogenic infections: An example of Bacillus amyloliquefaciens RWL-1 and Fusarium oxysporum f. sp. lycopersici in tomato. PeerJ 2017, 5, e3107. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.-M.; Lee, I.-J. Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ. Exp. Bot. 2017, 136, 68–77. [Google Scholar] [CrossRef]
- Liu, J.J.; Huang, B.; Hooi, S.C. Acetyl-keto-β-boswellic acid inhibits cellular proliferation through a p21-dependent pathway in colon cancer cells. Br. J. Pharmacol. 2006, 148. [Google Scholar] [CrossRef] [PubMed]
- Golbabaei, S.; Bazl, R.; Golestanian, S.; Nabati, F.; Omrany, Z.B.; Yousefi, B.; Hajiaghaee, R.; Rezazadeh, S.; Amanlou, M. Urease inhibitory activities of β-boswellic acid derivatives. DARU J. Pharm. Sci. 2013, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Khan, A.L.; Al-Kharusi, L.; Hussain, J.; Al-Harrasi, A. New α-glucosidase inhibitory triterpenic acid from marine macro green alga Codium dwarkense Boergs. Mar. Drugs 2015, 13, 4344–4356. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, G.; Chen, H.; Wang, H.; Qin, S.; Zhu, W.; Xu, L.; Jiang, C.; Li, W. Antitumour and antimicrobial activities of endophytic streptomycetes from pharmaceutical plants in rainforest. Lett. Appl. Microbiol. 2008, 47, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Moyo, M.; Ndhlala, A.R.; Finnie, J.F.; Van Staden, J. Phenolic composition, antioxidant and acetylcholinesterase inhibitory activities of Sclerocarya birrea and Harpephyllum caffrum (Anacardiaceae) extracts. Food Chem. 2010, 123, 69–76. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Ouchemoukh, S.; Meziant, N.; Idiri, Y.; Hernanz, D.; Stinco, C.M.; Rodríguez-Pulido, F.J.; Heredia, F.J.; Madani, K.; Luis, J. Bioactive metabolites involved in the antioxidant, anticancer and anticalpain activities of Ficus carica L., Ceratonia siliqua L. and Quercus ilex L. extracts. Ind. Crops Prod. 2017, 95, 6–17. [Google Scholar] [CrossRef]
- Chu, C.-C.; Chen, S.-Y.; Chyau, C.-C.; Fu, Z.-H.; Liu, C.-C.; Duh, P.-D. Protective effect of Djulis (Chenopodium formosanum) and its bioactive compounds against carbon tetrachloride-induced liver injury, in vivo. J. Funct. Foods 2016, 26, 585–597. [Google Scholar] [CrossRef]
- Spanogiannopoulos, P.; Bess, E.N.; Carmody, R.N.; Turnbaugh, P.J. The microbial pharmacists within us: A metagenomic view of xenobiotic metabolism. Nat. Rev. Microbiol. 2016, 14, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Ali, L.; Hussain, J.; Rizvi, T.S.; Al-Harrasi, A.; Lee, I.-J. Enzyme inhibitory radicinol derivative from endophytic fungus Bipolaris sorokiniana LK12, associated with Rhazya stricta. Molecules 2015, 20, 12198–12208. [Google Scholar] [CrossRef] [PubMed]
- Nonejuie, P.; Trial, R.M.; Newton, G.L.; Lamsa, A.; Perera, V.R.; Aguilar, J.; Liu, W.-T.; Dorrestein, P.C.; Pogliano, J.; Pogliano, K. Application of bacterial cytological profiling to crude natural product extracts reveals the antibacterial arsenal of Bacillus subtilis. J. Antibiot. 2016, 69, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Afiyatullov, S.S.; Kalinovskaya, N.I.; Kuznetsova, T.A.; Ivanova, E.P.; Mikhailov, V.V. Antibiotics from strains of Bacillus pumilus isolated from a marine sponge Dendrilla sp. Chem. Nat. Compd. 1991, 27, 765–766. [Google Scholar] [CrossRef]
- Shimojima, Y.; Hayashi, H.; Ooka, T.; Shibukawa, M. Production, isolation and pharmacological studies of AI-77s. Agric. Biol. Chem. 1982, 46, 1823–1829. [Google Scholar] [CrossRef]
- Wang, J.; Guleria, S.; Koffas, M.A.G.; Yan, Y. Microbial production of value-added nutraceuticals. Curr. Opin. Biotechnol. 2016, 37, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, Y.; Hayashi, H. 1H-2-benzopyran-1-one derivatives, microbial products with pharmacological activity. Relationship between structure and activity in 6-[[1(S)-(3(S),4-dihydro-8-hydroxy-1-oxo-1H-2-benzopyran-3-yl)-3-methylbutyl]-amino]-4(S),5(S)-dihydroxy-6-oxo-3(S)-ammon. J. Med. Chem. 1983, 26, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Li, L.-H.; Tian, L.; Qiao, L.; Hua, H.-M.; Pei, Y.-H. Sg17-1-4, a novel isocoumarin from a marine fungus Alternaria tenuis Sg17-1. J. Antibiot. 2006, 59, 355–357. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahzad, R.; Latif Khan, A.; Ali, L.; Bilal, S.; Imran, M.; Choi, K.-S.; Al-Harrasi, A.; Lee, I.-J. Characterization of New Bioactive Enzyme Inhibitors from Endophytic Bacillus amyloliquefaciens RWL-1. Molecules 2018, 23, 114. https://doi.org/10.3390/molecules23010114
Shahzad R, Latif Khan A, Ali L, Bilal S, Imran M, Choi K-S, Al-Harrasi A, Lee I-J. Characterization of New Bioactive Enzyme Inhibitors from Endophytic Bacillus amyloliquefaciens RWL-1. Molecules. 2018; 23(1):114. https://doi.org/10.3390/molecules23010114
Chicago/Turabian StyleShahzad, Raheem, Abdul Latif Khan, Liaqat Ali, Saqib Bilal, Muhammad Imran, Kyung-Sook Choi, Ahmed Al-Harrasi, and In-Jung Lee. 2018. "Characterization of New Bioactive Enzyme Inhibitors from Endophytic Bacillus amyloliquefaciens RWL-1" Molecules 23, no. 1: 114. https://doi.org/10.3390/molecules23010114
APA StyleShahzad, R., Latif Khan, A., Ali, L., Bilal, S., Imran, M., Choi, K.-S., Al-Harrasi, A., & Lee, I.-J. (2018). Characterization of New Bioactive Enzyme Inhibitors from Endophytic Bacillus amyloliquefaciens RWL-1. Molecules, 23(1), 114. https://doi.org/10.3390/molecules23010114








