Rational Design of a New Class of Toll-Like Receptor 4 (TLR4) Tryptamine Related Agonists by Means of the Structure- and Ligand-Based Virtual Screening for Vaccine Adjuvant Discovery
Abstract
:1. Introduction
2. Results and Discussion
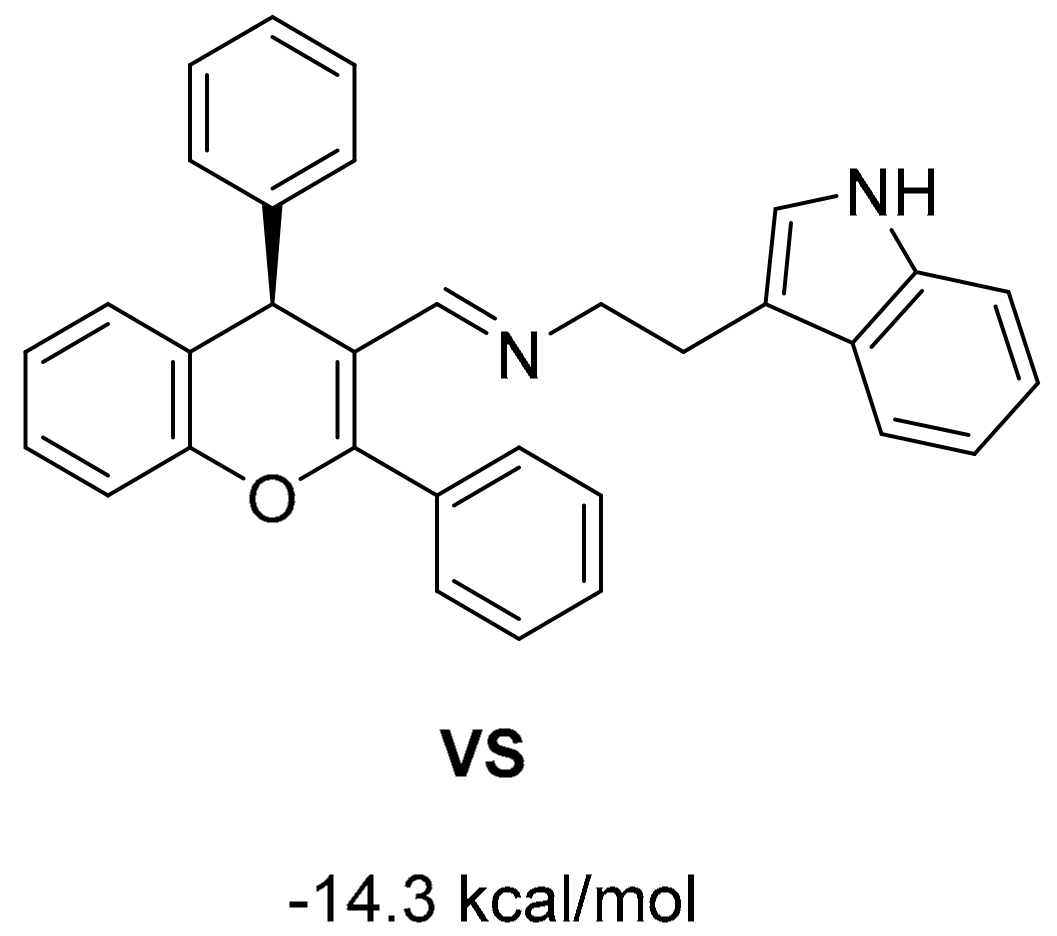
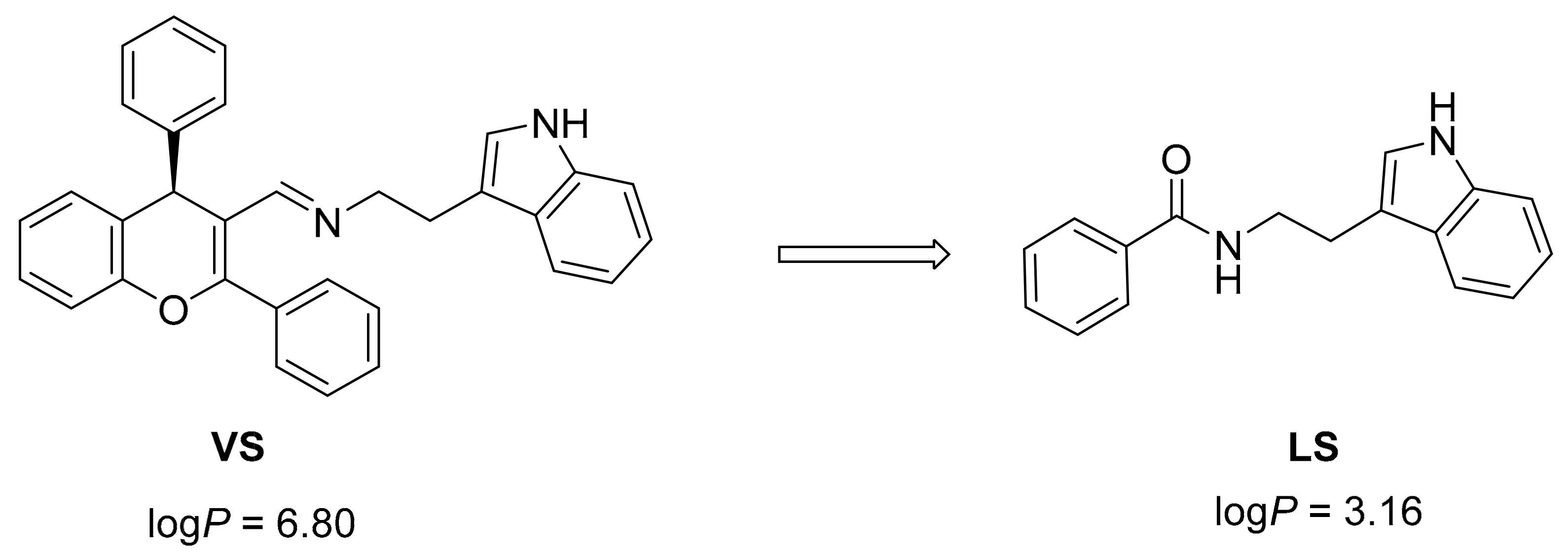
2.1. Virtual Screening and Rational Design
2.2. Chemistry
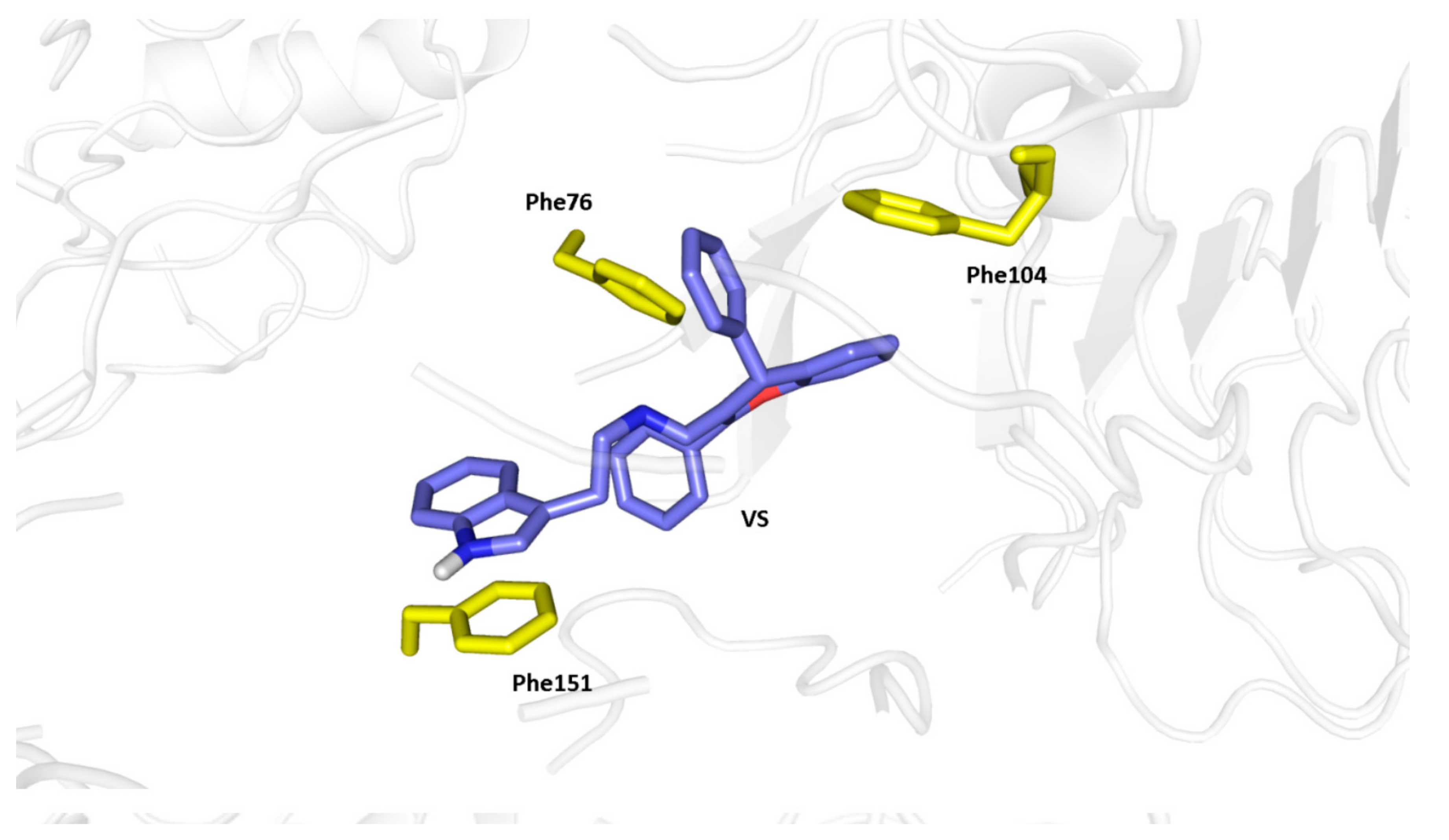
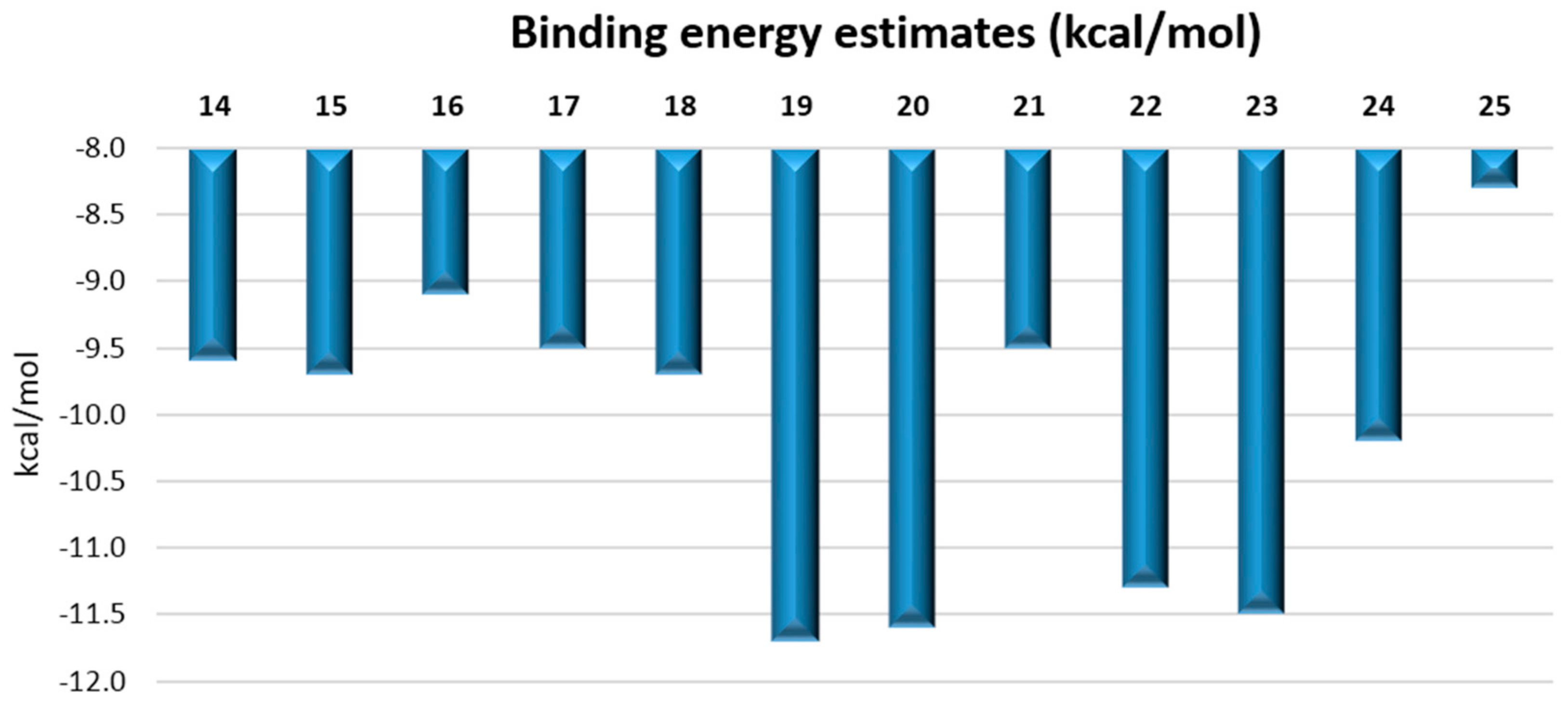
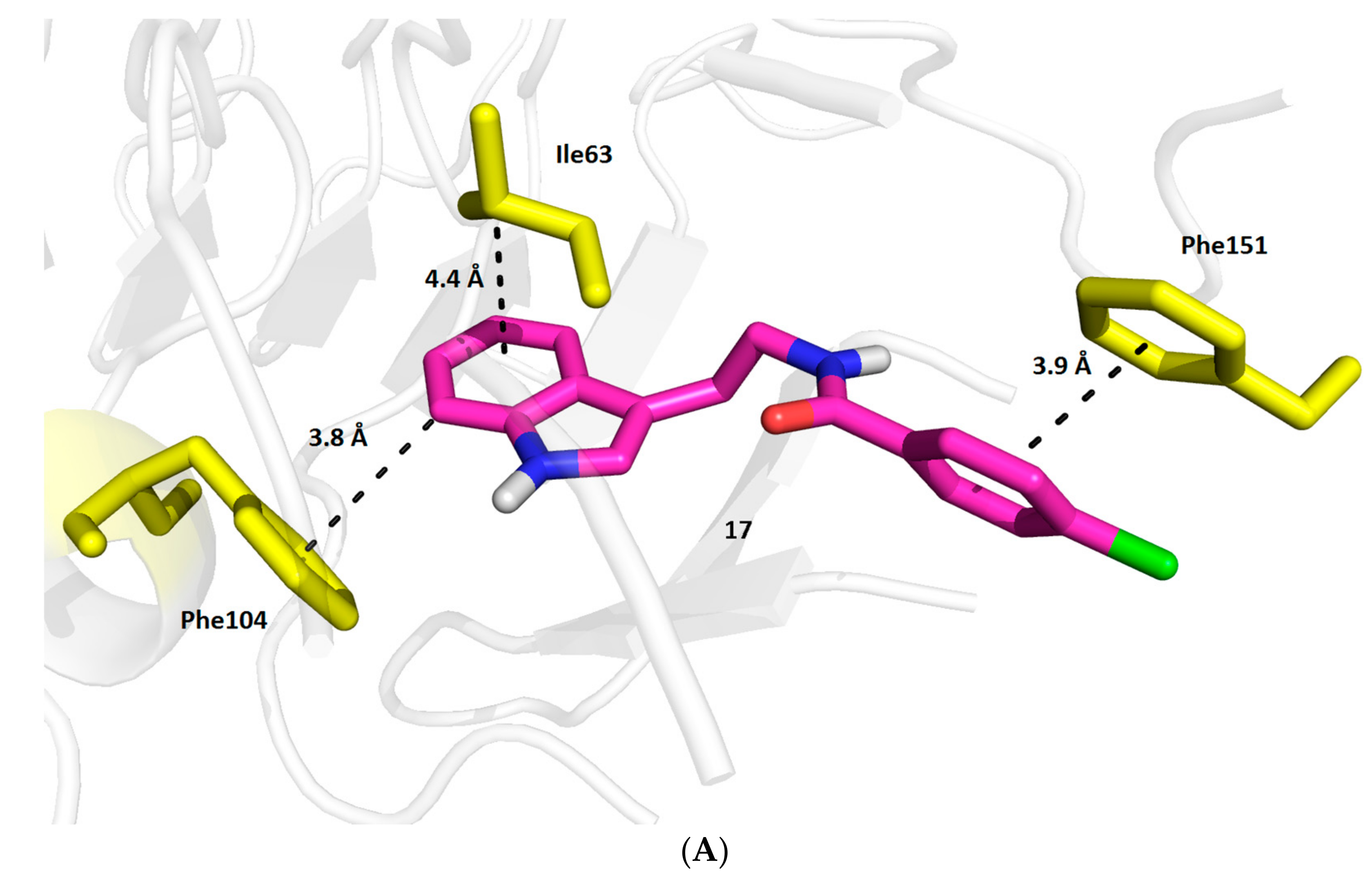
2.3. Molecular Docking Study
2.4. Molecular Dynamic Study
2.5. Cytotoxicity, In Vitro and Ex Vivo Effects
2.6. In Vitro Activity for hTLR4/MD2
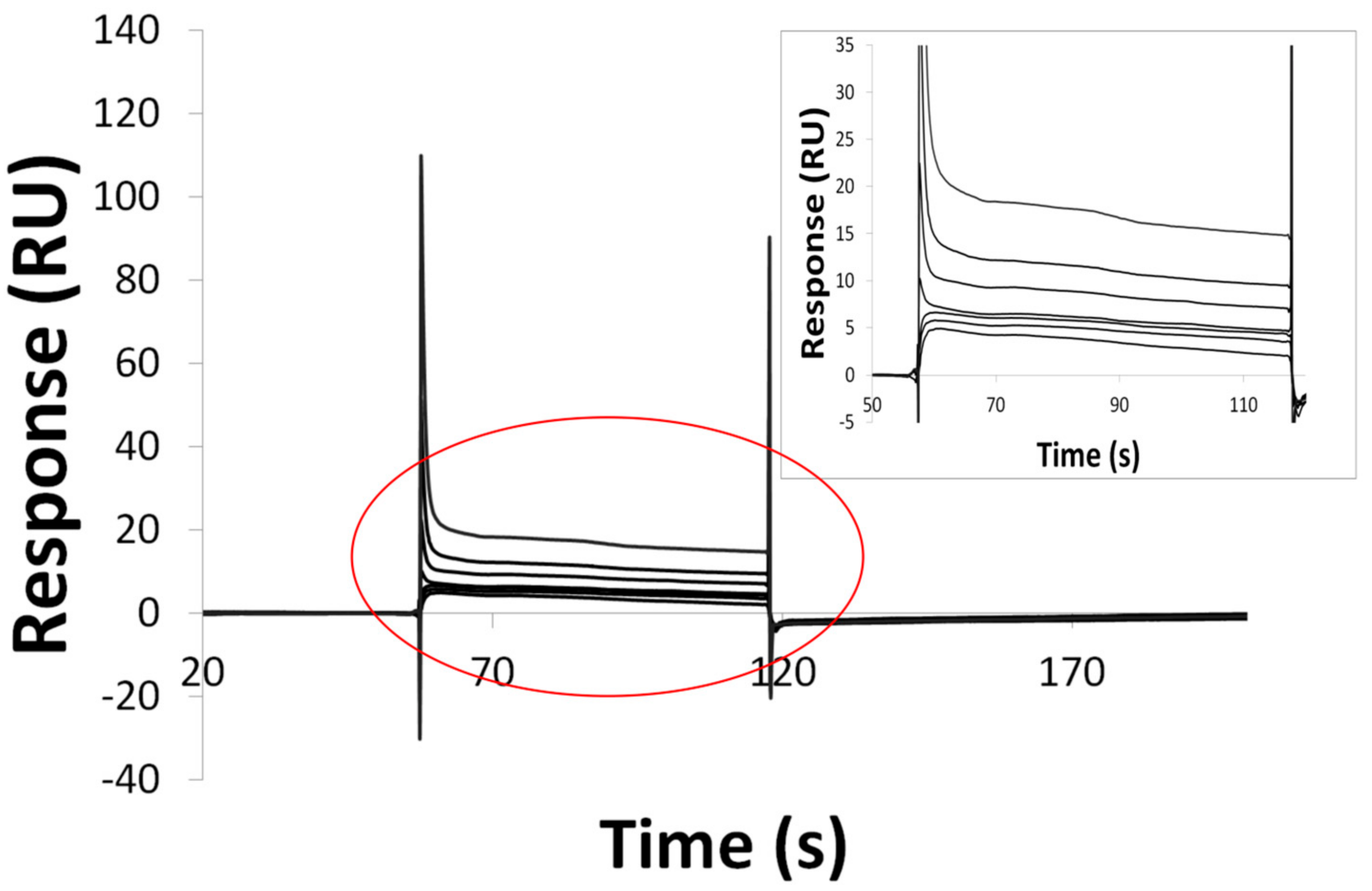
2.7. Surface Plasmon Resonance (SPR)
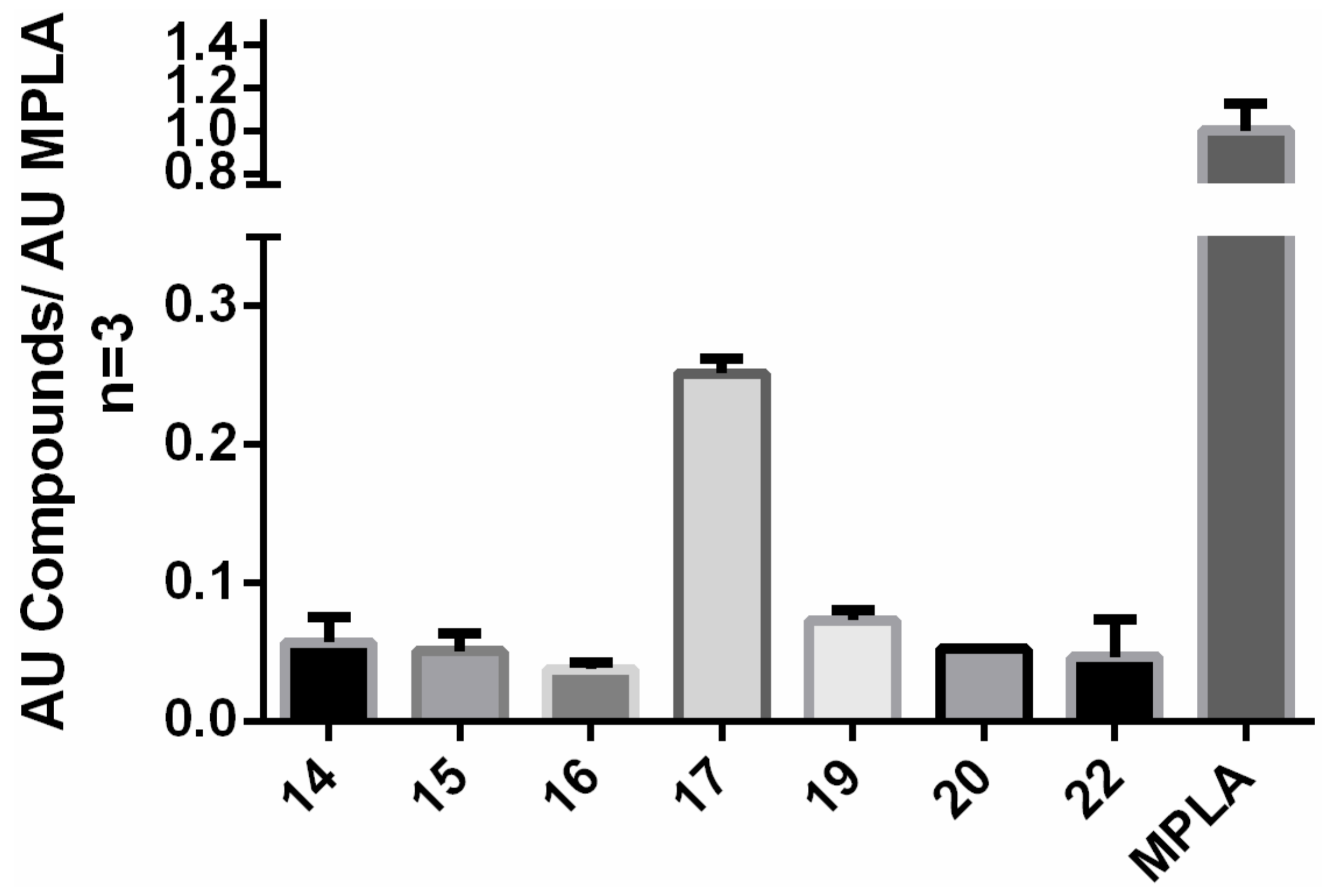
2.8. Ex Vivo Stimulation of IL-6 Production
3. Experimental Section
3.1. Virtual Screening
3.2. Molecular Docking Study
3.3. Molecular Dynamics
3.4. Chemistry General Methods
3.4.1. General Procedure for Synthesis of Compounds 14–21 by Reaction of Commercially Available Acyl Chlorides with Tryptamine (Procedure A)
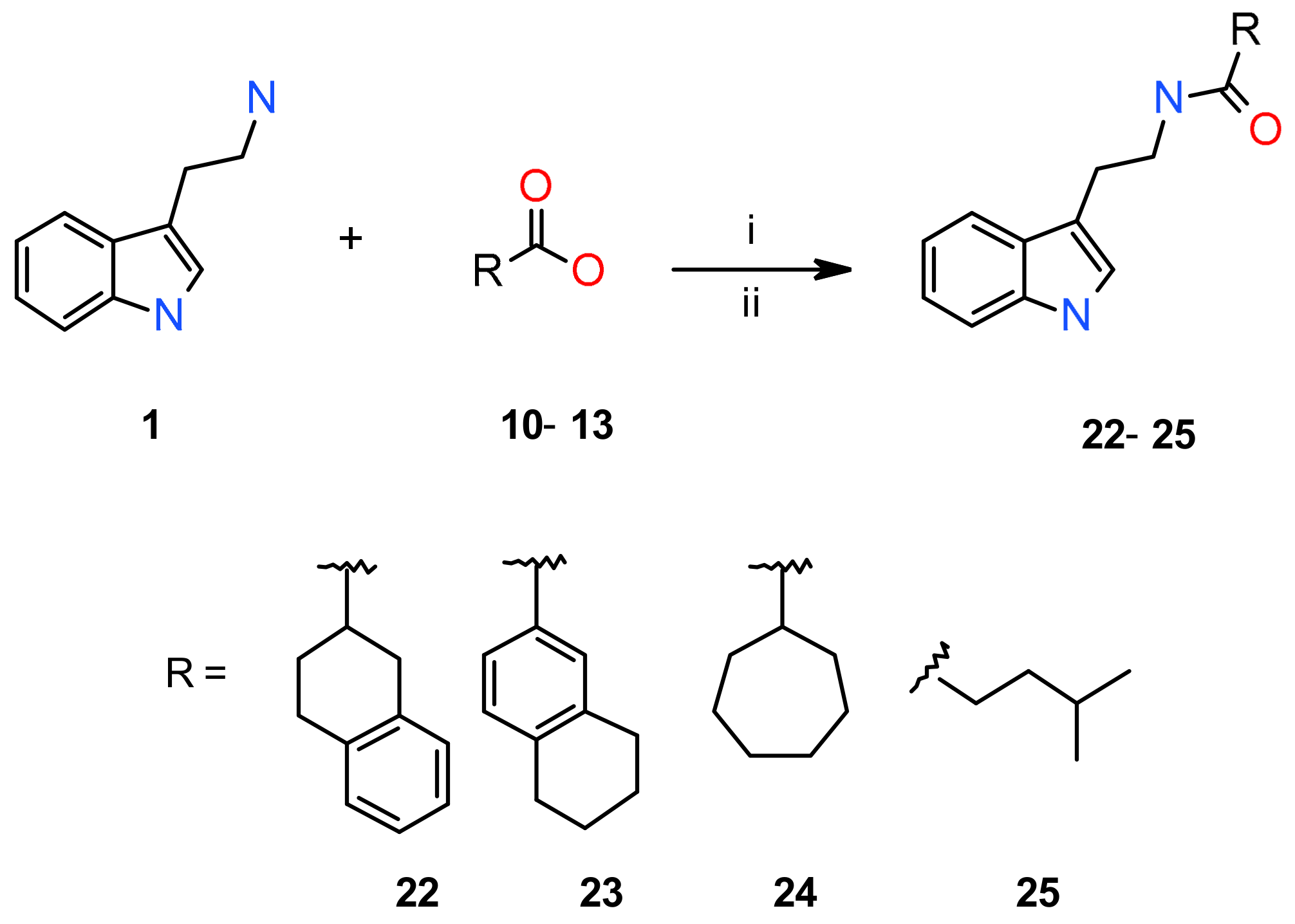
3.4.2. General Procedure for Synthesis of Compounds 22–25 by Reaction of Commercially Available Carboxylic Acids with Tryptamine (Procedure B)
3.5. In Vitro hTLR4/MD2 Activity Assessment
3.6. Surface Plasmon Resonance Spectroscopy
3.7. Cytotoxicity
3.8. Ex Vivo—IL-6 Production
4. Conclusions
Supplementary Materials
Acknowledgement
Author Contributions
Conflicts of Interest
References
- Lo, N.C.; Hotez, P.J. Public Health and Economic Consequences of Vaccine Hesitancy for Measles in the United States. JAMA Pediatr. 2017, 171, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Calandrillo, S.P. Vanishing vaccinations: Why are so many Americans opting out of vaccinating their children? Univ. Mich. J. Law Reform 2004, 37, 353–440. [Google Scholar] [PubMed]
- Freeman, M. Toxic Vaccine Adjuvants & Ingredients: The Top 10. Available online: http://freedom-articles.toolsforfreedom.com/toxic-vaccine-adjuvants-the-top-10/ (accessed on 5 October 2017).
- Kocourkova, A.; Honegr, J.; Kuca, K.; Danova, J. Vaccine ingredients: Components that influence vaccine efficacy. Mini-Rev. Med. Chem. 2017, 17, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Doja, A.; Roberts, W. Immunizations and autism: A review of the literature. Can. J. Neurol. Sci. 2006, 33, 341–346. [Google Scholar] [CrossRef] [PubMed]
- C-621/15-N. W and Others v Sanofi Pasteur MSD and Others; Court of Justice of the European Union: Luxemburg, 21 June 2017.
- O’Hagan, D.T. Vaccine Adjuvants: Preparation Methods and Research Protocols; Humana Press: New York, NY, USA, 2000. [Google Scholar]
- Ainge, G.D.; Martin, W.J.; Compton, B.J.; Hayman, C.M.; Larsen, D.S.; Yoon, S.I.; Wilson, I.A.; Harper, J.L.; Painter, G.F. Synthesis and Toll-like Receptor 4 (TLR4) Activity of Phosphatidylinositol Dimannoside Analogues. J. Med. Chem. 2011, 54, 7268–7279. [Google Scholar] [CrossRef] [PubMed]
- Honegr, J.; Soukup, O.; Dolezal, R.; Malinak, D.; Penhaker, M.; Prymula, R.; Kuca, K. Structural Properties of Potential Synthetic Vaccine Adjuvants-TLR Agonists. Curr. Med. Chem. 2015, 22, 3306–3325. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Smith, C.; Yin, H. Targeting Toll-like receptors with small molecule agents. Chem. Soc. Rev. 2013, 42, 4859–4866. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, R.; Ramalho, T.; França, T.C.; Kuca, K. Parallel Flexible Molecular Docking in Computational Chemistry on High Performance Computing Clusters. In Computational Collective Intelligence; Núñez, M., Nguyen, N.T., Camacho, D., Trawiński, B., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 9330, pp. 418–427. [Google Scholar]
- Zhang, S.T.; Cheng, K.; Wang, X.H.; Yin, H. Selection, synthesis, and anti-inflammatory evaluation of the arylidene malonate derivatives as TLR4 signaling inhibitors. Bioorg. Med. Chem. 2012, 20, 6073–6079. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.D.; Frank, M.G.; Sobesky, J.L.; Watkins, L.R.; Maier, S.F. Blocking toll-like receptor 2 and 4 signaling during a stressor prevents stress-induced priming of neuroinflammatory responses to a subsequent immune challenge. Brain Behav. Immun. 2013, 32, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Stover, A.G.; Correia, J.D.; Evans, J.T.; Cluff, C.W.; Elliott, M.W.; Jeffery, E.W.; Johnson, D.A.; Lacy, M.J.; Baldridge, J.R.; Probst, P.; et al. Structure-activity relationship of synthetic Toll-like receptor 4 agonists. J. Biol. Chem. 2004, 279, 4440–4449. [Google Scholar] [CrossRef] [PubMed]
- Lewicky, J.D.; Ulanova, M.; Jiang, Z.H. Improving the immunostimulatory potency of diethanolamine-containing lipid A mimics. Bioorg. Med. Chem. 2013, 21, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Lewicky, J.D.; Ulanova, M.; Jiang, Z.H. Synthesis of a dimeric monosaccharide lipid A mimic and its synergistic effect on the immunostimulatory activity of lipopolysaccharide. Carbohydr. Res. 2011, 346, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Hayashi, T.; Mathewson, R.D.; Nour, A.; Hayashi, Y.; Yao, S.Y.; Tawatao, R.I.; Crain, B.; Tsigelny, I.F.; Kouznetsoya, V.L.; et al. Identification of Substituted Pyrimido 5,4-b indoles as Selective Toll-Like Receptor 4 Ligands. J. Med. Chem. 2013, 56, 4206–4223. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Lv, T.; Mo, W.; Yang, X.; Gao, Y.; Chen, H. One-pot synthesis of tricyclo-1, 4-benzoxazines via visible-light photoredox catalysis in continuous flow. Tetrahedron Lett. 2017, 58, 1395–1398. [Google Scholar] [CrossRef]
- Li, Y.; Feng, T.; Liu, P.; Liu, C.; Wang, X.; Li, D.; Li, N.; Chen, M.; Xu, Y.; Si, S. Optimization of rutaecarpine as ABCA1 up-regulator for treating atherosclerosis. ACS Med. Chem. Lett. 2014, 5, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.D.; Hubble, V.B.; Ernst, R.K.; van Hoek, M.L.; Melander, R.J.; Cavanagh, J.; Melander, C. Potentiation of Francisella resistance to conventional antibiotics through small molecule adjuvants. MedChemComm 2016, 7, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Mohri, K.; Oikawa, Y.; Yonemitsu, O. Synthesis of oxazolylindole alkaloids from tryptamine and tryptophan by oxidation with 2,3-dichloro-5,6-dicyanobenzoquinone. J. Chem. Res. 1981, 12, 194–195. [Google Scholar] [CrossRef]
- Ho, B.T.; McIsaac, W.M.; Tansey, L.W. Hydroxyindole-O-methyltransferase III: Influence of the phenyl moiety on the inhibitory activities of some N-acyltryptamines. J. Pharm. Sci. 1969, 58, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, F.; Ma, Y.; Cui, X.; Cun, L.; Zhu, J.; Deng, J.; Yu, B. Asymmetric transfer hydrogenation of imines and iminiums catalyzed by a water-soluble catalyst in water. Chem. Commun. 2006, 1766–1768. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.T.; McIssaac, W.M.; Tansey, L.W.; Kralik, P.M. Hydroxyindole-O-methyltransferase II. Inhibitory Activities of Some N-Acyltryptamines. J. Pharm. Sci. 1968, 57, 1998–2000. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.T.; An, R.; Noel, M.B.; Tansey, L.W. Central nervous system depressive activity of some amides of tryptamine. J. Med. Chem. 1971, 14, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [PubMed]
- Kumari, R.; Kumar, R.; Lynn, A. g_mmpbsa—A GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef] [PubMed]
- Ohto, U.; Yamakawa, N.; Akashi-Takamura, S.; Miyake, K.; Shimizu, T. Structural Analyses of Human Toll-like Receptor 4 Polymorphisms D299G and T399I. J. Biol. Chem. 2012, 287, 40611–40617. [Google Scholar] [CrossRef] [PubMed]
- Nocedal, J.; Wright, S.J. Numerical Optimization; Springer Series in Operations Research; Springer: New York, NY, USA, 1999. [Google Scholar]
- Liu, B.; Wang, L.; Jin, Y.H. An effective PSO-based memetic algorithm for flow shop scheduling. IEEE Trans. Syst. Man Cybern. Part B Cybern. 2007, 37, 18–27. [Google Scholar] [CrossRef]
- Svajger, U.; Brus, B.; Turk, S.; Sova, M.; Hodnik, V.; Anderluh, G.; Gobec, S. Novel toll-like receptor 4 (TLR4) antagonists identified by structure- and ligand-based virtual screening. Eur. J. Med. Chem. 2013, 70, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Sigma-Aldrich, Histopaque 1077 Datasheet. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Product_Information_Sheet/1/10771pis.pdf (accessed on 6 December 2017).
- Jiang, Z.H.; Budzynski, W.A.; Qiu, D.X.; Yalamati, D.; Koganty, R.R. Monophosphoryl lipid A analogues with varying 3-O-substitution: Synthesis and potent adjuvant activity. Carbohydr. Res. 2007, 342, 784–796. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 14, 15, 16, 17, 19, 20 and 22 are available from the authors, upon request, please take into a consideration that not all samples may be available on short notice. |


| Compound | R | Yield (%) | m.p. (°C) |
|---|---|---|---|
| 14 | Phenyl | 81 a | 141.2–142.8 |
| 15 | 4-(Methyl)phenyl | 92 a | 126.8–128.2 |
| 16 | 4-(Methoxy)phenyl | 87 a | 132.8–134.3 |
| 17 | 4-(Chloro)phenyl | 66 a | 150.6–152.0 |
| 18 | 3,4-(Dichloro)phenyl | 75 a | 112.4–113.9 |
| 19 | 2-Naphtyl | 92 a | 193.2–195.0 |
| 20 | Cyclohexyl | 59 a | 104.8–106.3 |
| 21 | 1-Naphtyl | 80 a | 164.5–166.5 |
| 22 | 1,2,3,4-Tetrahydro-2-naphtyl | 81 b | 123.2–125.2 |
| 23 | 5,6,7,8-Tetrahydro-2-naphtyl | 91 b | 137.0–139.0 |
| 24 | Cycloheptyl | 90 b | 88.4–90.4 |
| 25 | Isopentyl | 87 b | 45.1–47.1 |
| Compound | Predicted Binding Energy (kcal/mol) | Compound | Predicted Binding Energy (kcal/mol) |
|---|---|---|---|
| 14 | −23.454 ± 5.368 | 20 | −19.255 ± 4.028 |
| 15 | −20.059 ± 6.248 | 21 | −19.344 ± 6.022 |
| 16 | −21.144 ± 4.535 | 22 | −21.381 ± 4.511 |
| 17 | −22.874 ± 3.929 | 23 | −18.090 ± 3.767 |
| 18 | −21.288 ± 3.531 | 24 | −23.696 ± 2.684 |
| 19 | −20.495 ± 4.284 | 25 | −18.361 ± 2.846 |
| Compound | IC50 and SEM (μM) |
|---|---|
| 14 | 297 ± 17 |
| 15 | 197 ± 41 |
| 16 | 200 ± 7 |
| 17 | 201 ± 8 |
| 18 | 892 ± 122 |
| 19 | 155 ± 12 |
| 20 | 211 ± 43 |
| 22 | 128 ± 8 |
| 23 | 98 ± 6 |
| 24 | 170 ± 10 |
| 25 | 348 ± 35 |
| Compound | c (pg/mL) |
|---|---|
| 14 | 53.501 |
| 15 | - |
| 16 | - |
| 17 | - |
| 18 | - |
| 19 | - |
| 20 | - |
| 22 | 147.64 |
| LPS-RS | - |
| MPLA | 3078.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honegr, J.; Dolezal, R.; Malinak, D.; Benkova, M.; Soukup, O.; Almeida, J.S.F.D.d.; Franca, T.C.C.; Kuca, K.; Prymula, R. Rational Design of a New Class of Toll-Like Receptor 4 (TLR4) Tryptamine Related Agonists by Means of the Structure- and Ligand-Based Virtual Screening for Vaccine Adjuvant Discovery. Molecules 2018, 23, 102. https://doi.org/10.3390/molecules23010102
Honegr J, Dolezal R, Malinak D, Benkova M, Soukup O, Almeida JSFDd, Franca TCC, Kuca K, Prymula R. Rational Design of a New Class of Toll-Like Receptor 4 (TLR4) Tryptamine Related Agonists by Means of the Structure- and Ligand-Based Virtual Screening for Vaccine Adjuvant Discovery. Molecules. 2018; 23(1):102. https://doi.org/10.3390/molecules23010102
Chicago/Turabian StyleHonegr, Jan, Rafael Dolezal, David Malinak, Marketa Benkova, Ondrej Soukup, Joyce S. F. D. de Almeida, Tanos C. C. Franca, Kamil Kuca, and Roman Prymula. 2018. "Rational Design of a New Class of Toll-Like Receptor 4 (TLR4) Tryptamine Related Agonists by Means of the Structure- and Ligand-Based Virtual Screening for Vaccine Adjuvant Discovery" Molecules 23, no. 1: 102. https://doi.org/10.3390/molecules23010102
APA StyleHonegr, J., Dolezal, R., Malinak, D., Benkova, M., Soukup, O., Almeida, J. S. F. D. d., Franca, T. C. C., Kuca, K., & Prymula, R. (2018). Rational Design of a New Class of Toll-Like Receptor 4 (TLR4) Tryptamine Related Agonists by Means of the Structure- and Ligand-Based Virtual Screening for Vaccine Adjuvant Discovery. Molecules, 23(1), 102. https://doi.org/10.3390/molecules23010102







