Separation and Characterization of Antioxidative and Angiotensin Converting Enzyme Inhibitory Peptide from Jellyfish Gonad Hydrolysate
Abstract
1. Introduction
2. Results and Discussion
2.1. Amino Acid Composition, ACE Inhibitory Activity and Antioxidative Activity of JGPH
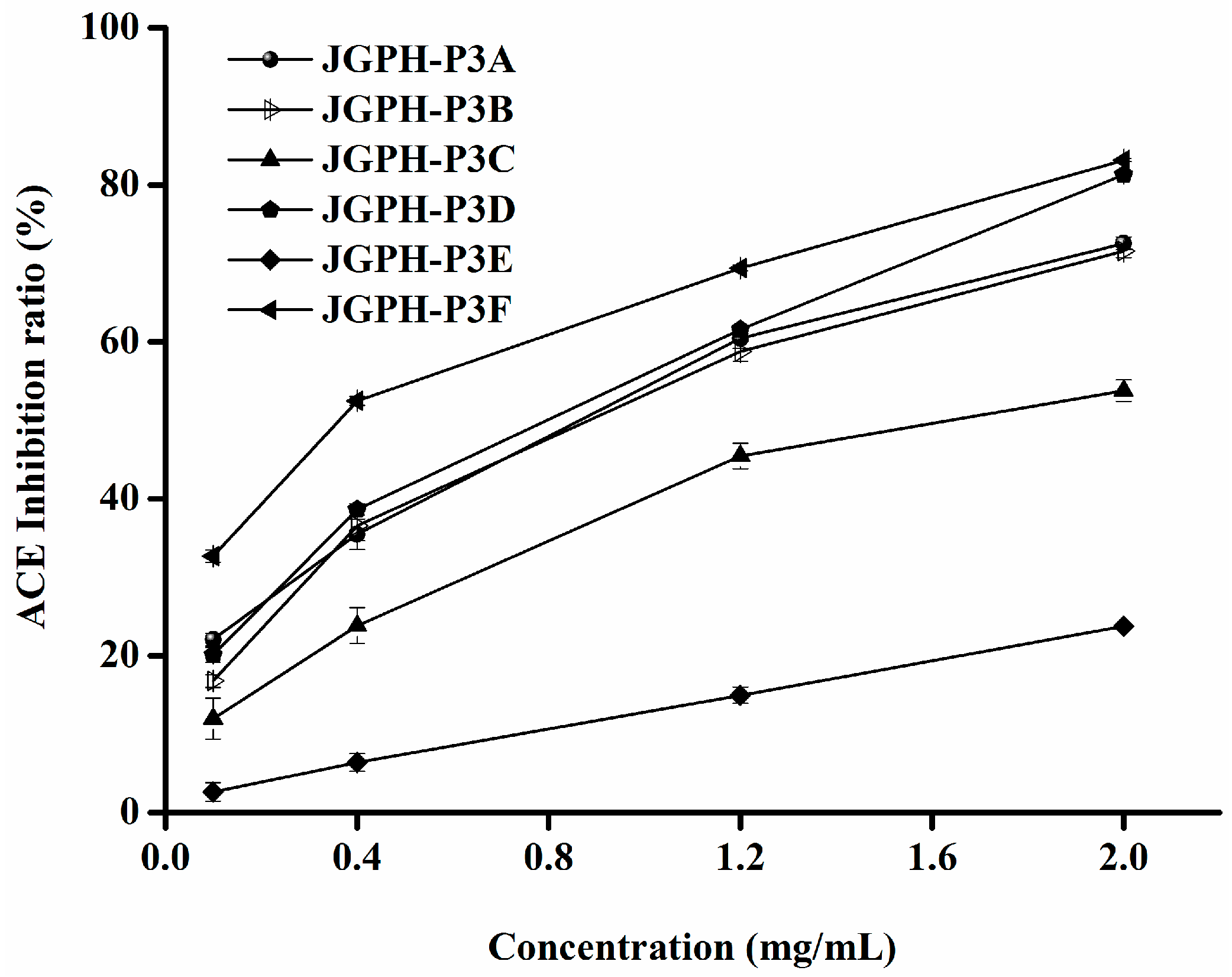
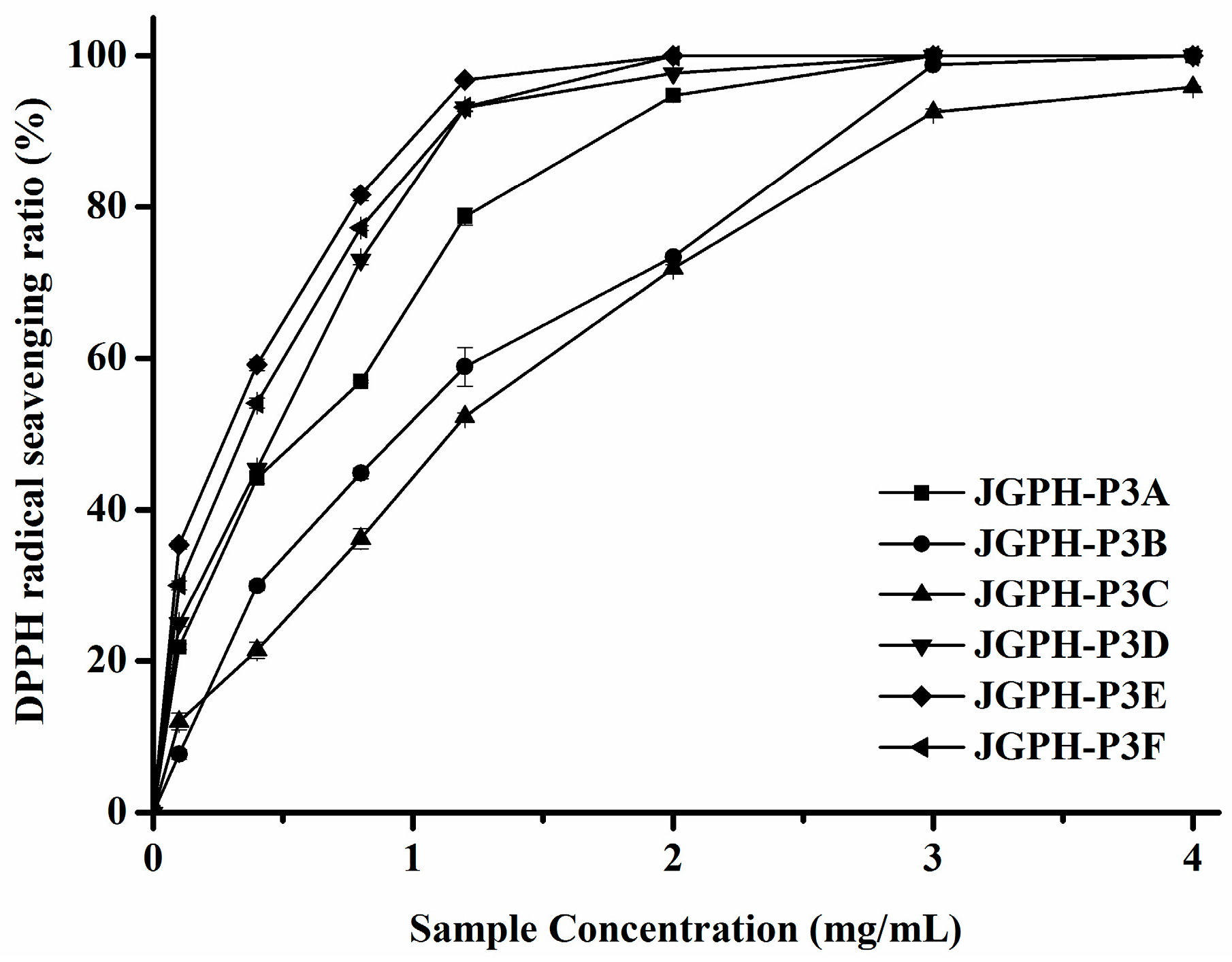
2.2. Purification of ACE Inhibitory and Antioxidative Peptide
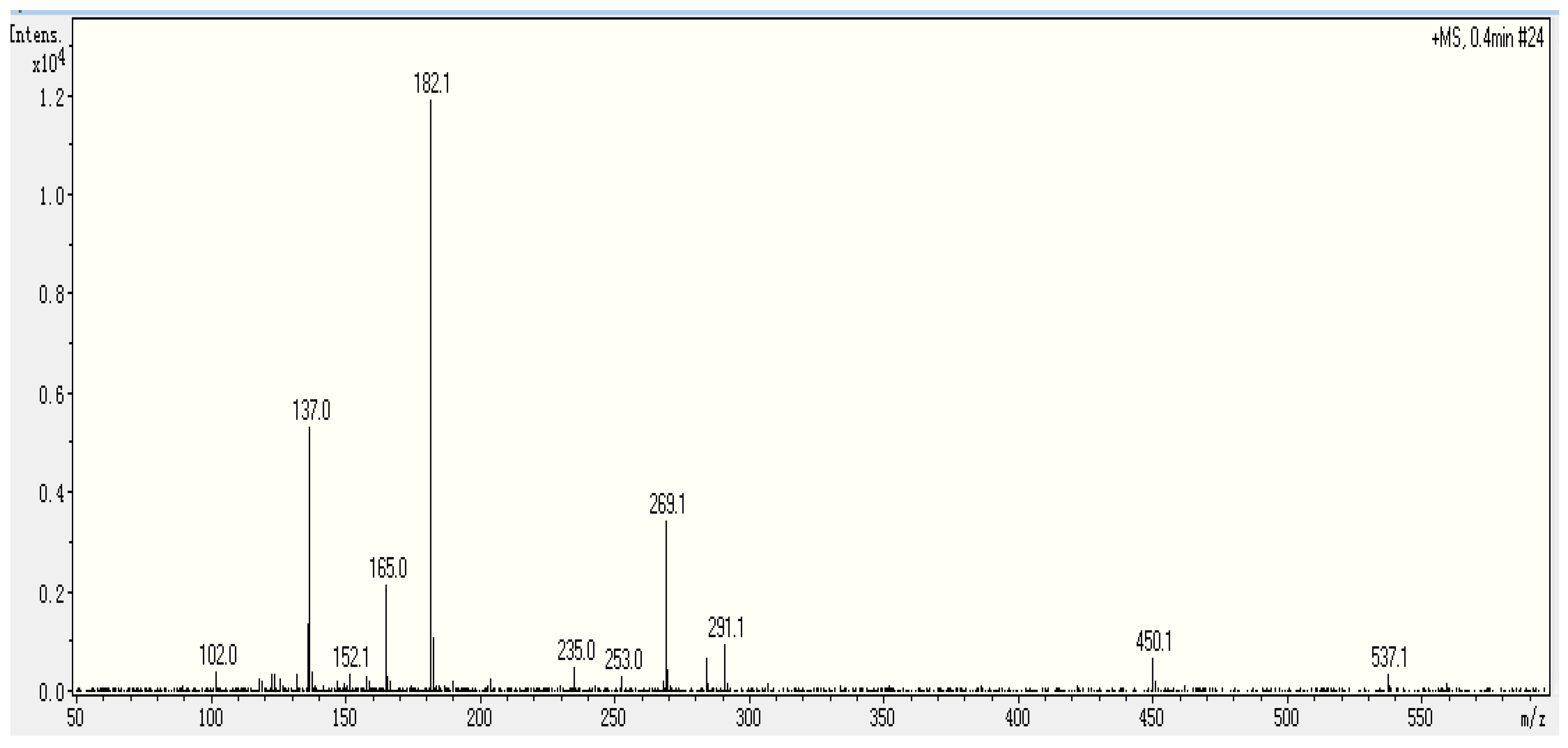
2.3. Identification Sequence of the Purified Peptide
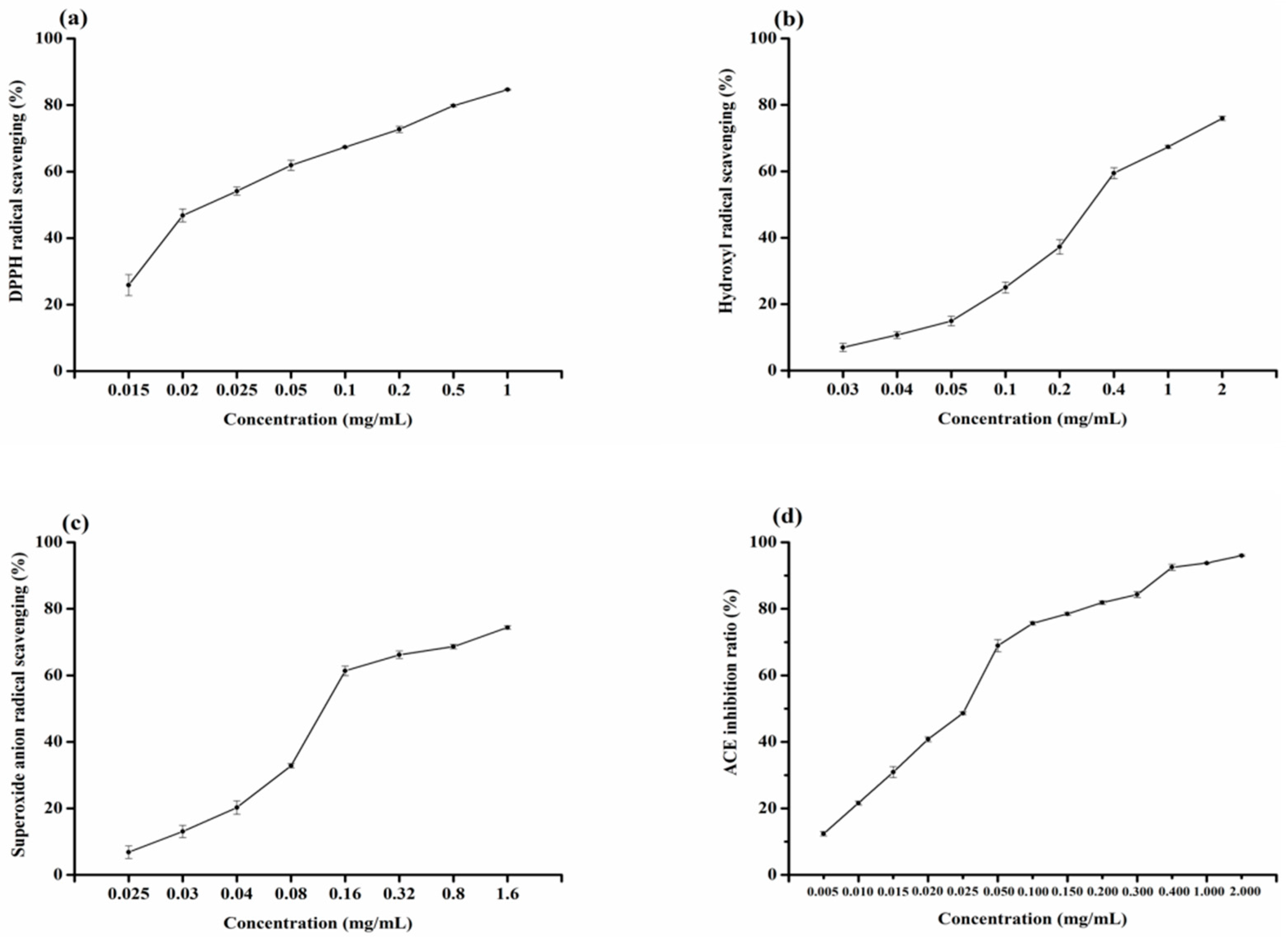
2.4. Analysis of Radical Scavenging Activity of Ser-Tyr
2.5. Analysis of ACE Inhibitory Activity of Ser-Tyr
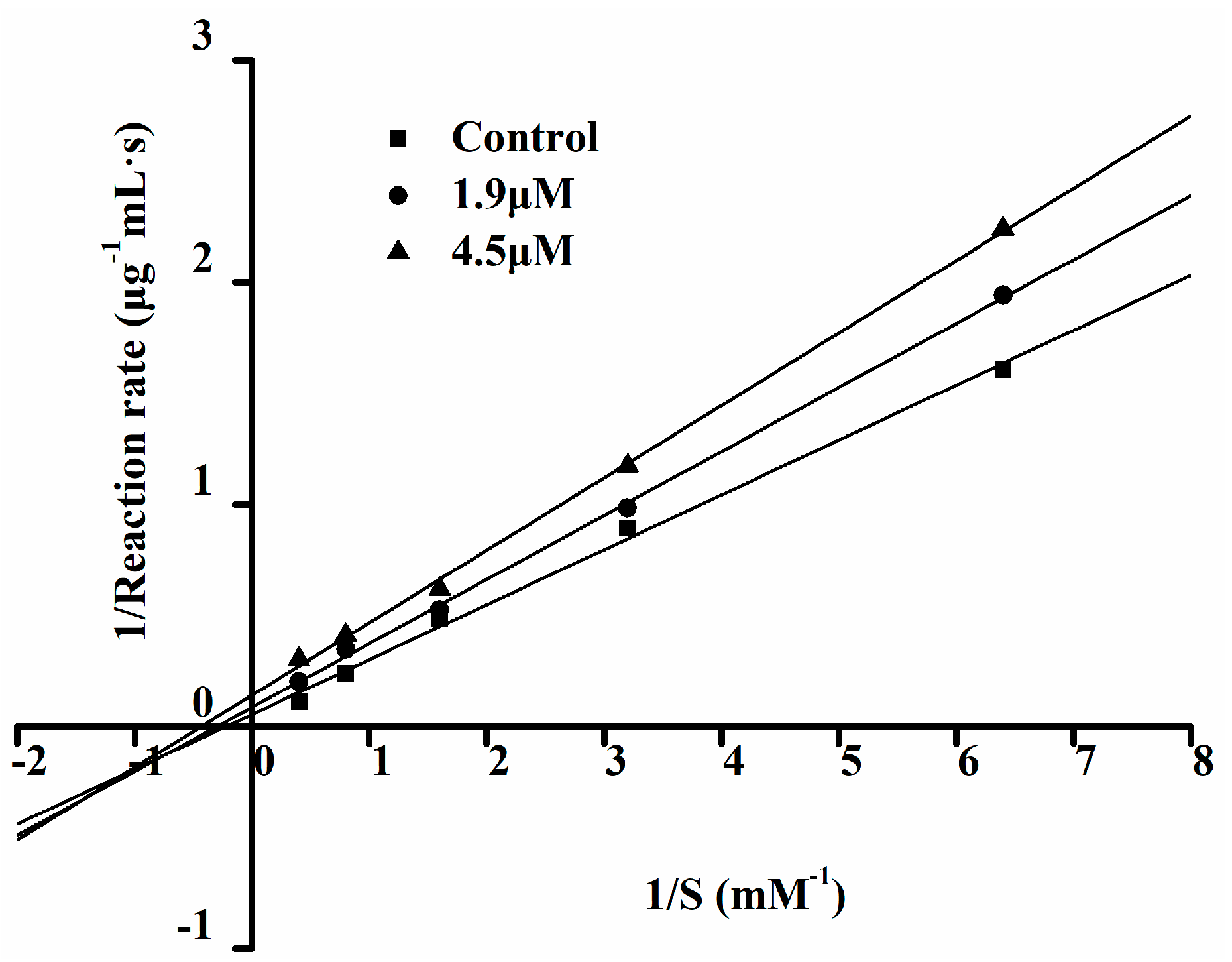
2.6. The ACE Inhibition Pattern of Ser-Tyr
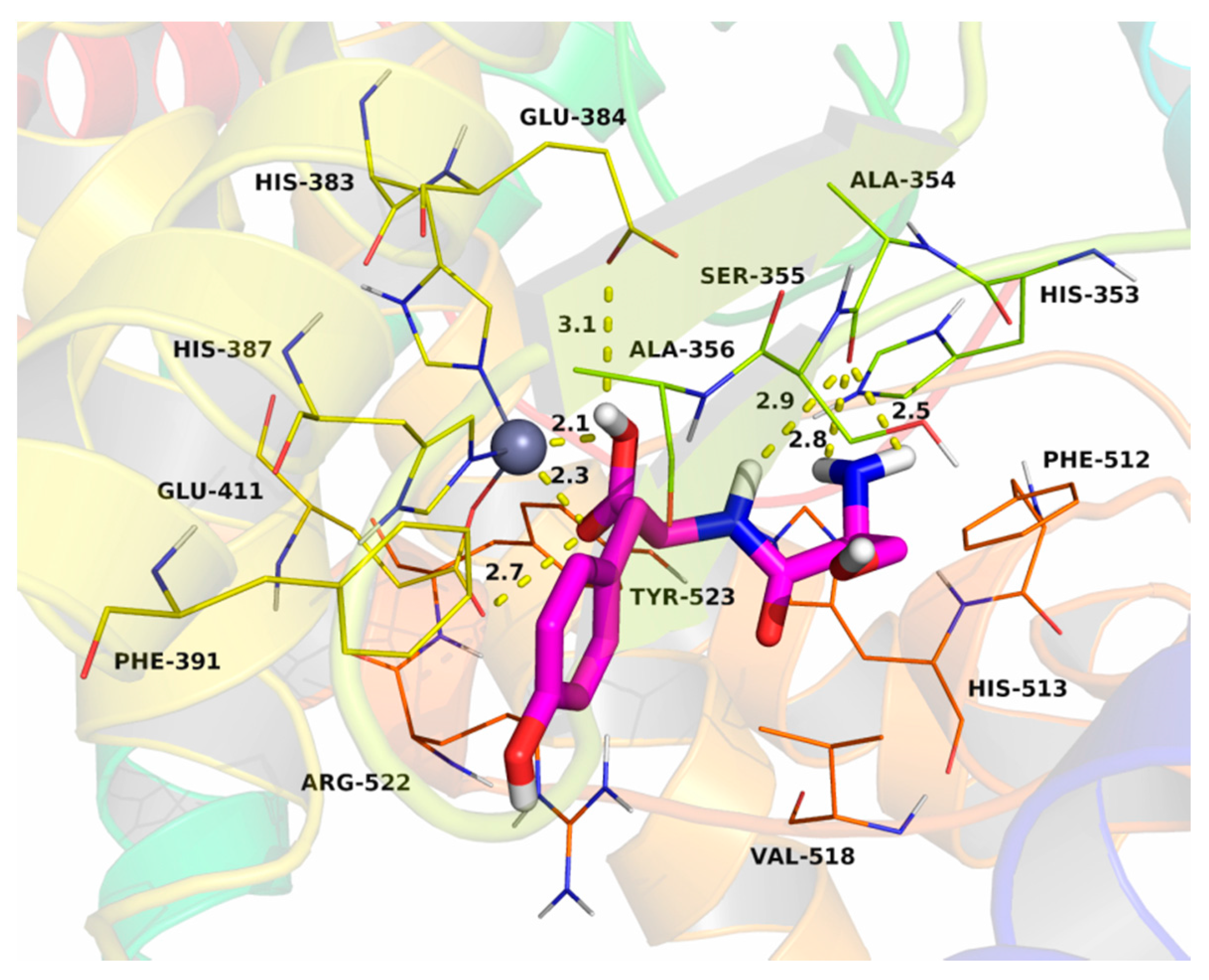
2.7. Molecular Docking
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Enzymatic Hydrolysates
3.3. Determination of Amino Acid Composition
3.4. Purification of Jellyfish Gonad Peptides
3.5. DPPH Radical Scavenging Activity
3.6. Hydroxyl Radical Scavenging Activity
3.7. Superoxide Radical Scavenging Activity
3.8. Measurement of ACE Inhibition Activity
3.9. Characterization of Peptides
3.10. Synthesis and Purity Identification of SY
3.11. Determination of the Inhibition Mode on ACE
3.12. Molecular Docking
3.13. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Soffer, R.L. Angiotensin-converting enzyme and the regulation of vasoactive peptides. Annu. Rev. Biochem. 1976, 45, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, K.E.; Ong, F.S.; Blackwell, W.L.; Shah, K.H.; Giani, J.F.; Gonzalez-Villalobos, R.A.; Shen, X.Z.; Fuchs, S.; Touyz, R.M. A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme. Pharmacol. Rev. 2012, 65, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Dicpinigaitis, P.V. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest 2006, 129, 169S–173S. [Google Scholar] [CrossRef] [PubMed]
- Cotton, J.; Hayashi, M.A.; Cuniasse, P.; Vazeux, G.; Ianzer, D.; De Camargo, A.C.; Dive, V. Selective inhibition of the C-domain of angiotensin I converting enzyme by bradykinin potentiating peptides. Biochemistry 2002, 41, 6065–6071. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.I.; Griendling, K.K. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res. 2015, 116, 531–549. [Google Scholar] [CrossRef] [PubMed]
- Kizhakekuttu, T.J.; Widlansky, M.E. Natural antioxidants and hypertension: Promise and challenges. Cardiovasc. Ther. 2010, 28, e20–e32. [Google Scholar] [CrossRef] [PubMed]
- Alemán, A.; Giménez, B.; Pérezsantin, E.; Gómezguillén, M.C.; Montero, P. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011, 125, 334–341. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, Y.; Jiang, Y.; Wang, L.; Liu, B.; Liu, J. Transport of egg white ACE-inhibitory peptide, Gln-Ile-Gly-Leu-Phe, in human intestinal Caco-2 cell monolayers with cytoprotective effect. J. Agric. Food Chem. 2014, 62, 3177–3182. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Mi, A.Y.; Koo, S.H.; Baek, H.H.; Lee, H.G. Antioxidant and ACE inhibitory activities of soybean hydrolysates: Effect of enzyme and degree of hydrolysis. Food Sci. Biotechnol. 2008, 17, 873–877. [Google Scholar]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Amoldi, A. Optimization of the enzymatic hydrolysis of Lupin (Lupinus) proteins for producing ACE-Inhibitory peptides. J. Agric. Food. 2014, 62, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, M.; Zhang, Y.; Xu, W.; Wang, C.; Wang, K.; Zhang, L. Purification and identification of an ACE inhibitory peptide from walnut protein. J. Agric. Food 2013, 61, 4097–4100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, B.; Dong, S.; Liu, Z.; Zhao, X.; Wang, J.; Zeng, M. A novel ACE inhibitory peptide isolated from Acaudina molpadioidea hydrolysate. Peptides 2009, 30, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Shen, H.X.; Xiao, Y.; Luo, Y.K. Preparation of angiotensin I converting enzyme inhibitory peptides from pearl mussel meat. Food Sci. Technol. 2007, 10, 133–136. [Google Scholar]
- Gu, R.Z.; Li, C.Y.; Liu, W.Y.; Yi, W.X.; Cai, M.Y. Angiotensin I-converting enzyme inhibitory activity of low-molecular-weight peptides from atlantic Salmon (Salmo salar L.) skin. Food Res. Int. 2011, 44, 1536–1540. [Google Scholar] [CrossRef]
- Lin, L.; Lv, S.; Li, B. Angiotensin-I-converting enzyme (ACE)-inhibitory and antihypertensive properties of squid skin gelatin hydrolysates. Food Chem. 2012, 131, 225–230. [Google Scholar] [CrossRef]
- He, H.L.; Chen, X.L.; Sun, C.Y.; Zhang, Y.Z.; Zhou, B.C. Analysis of novel angiotensin-I-converting enzyme inhibitory peptides from protease-hydrolyzed marine shrimp Acetes chinensis. J. Pept. Sci. 2006, 12, 726–733. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Jia, A.; Zhang, Y.; Zhu, H.; Zhang, C.; Sun, Z.; Liu, C. Purification and characterization of angiotensin I converting enzyme inhibitory peptides from jellyfish Rhopilema esculentum. Food Res. Int. 2013, 50, 339–343. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. Food-originating ACE inhibitors, including antihypertensive peptides, as preventive food components in blood pressure reduction. Compr. Rev. Food Sci. Food Saf. 2014, 13, 114–134. [Google Scholar] [CrossRef]
- Santos, S.D.A.D.; Martins, V.G.; Salas-Mellado, M.; Prentice, C. Evaluation of functional properties in protein hydrolysates from bluewing searobin (Prionotus punctatus) obtained with different microbial enzymes. Food Bioprocess Technol. 2011, 4, 1399–1406. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X.; Zhuang, Y. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 2012, 38, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Zhu, F.; Han, F.S. Fine salmon fish hydrolysates antioxidant study on separation and purification of bioactive peptides. Food Res. Dev. 2009, 30, 70–72. [Google Scholar] [CrossRef]
- Suetsuna, K. Isolation and characterization of angiotensin I-converting enzyme inhibitor dipeptides derived from Allium sativum L (Garlic). J. Nutr. Biochem. 1998, 9, 415–419. [Google Scholar] [CrossRef]
- Nakahara, T.; Sano, A.; Yamaguchi, H.; Sugimoto, K.; Chikata, H.; Kinoshita, E.; Uchida, R. Correction to antihypertensive effect of peptide-enriched soy sauce-like seasoning and identification of its angiotensin I-converting enzyme inhibitory substances. J. Agric. Food 2010, 58, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Castellano, P.; Aristoy, M.C.; Sentandreu, M.A.; Vignolo, G.; Toldra, F. Peptides with angiotensin I converting enzyme (ACE) inhibitory activity generated from porcine skeletal muscle proteins by the action of meat-borne Lactobacillus. J. Proteome 2013, 89, 183–190. [Google Scholar] [CrossRef] [PubMed]
- García, M.C.; Puchalska, P.; Esteve, C.; Marina, M.L. Vegetable foods: A cheap source of proteins and peptides with antihypertensive, antioxidant, and other less occurrence bioactivities. Talanta 2013, 106, 328–349. [Google Scholar] [CrossRef] [PubMed]
- Glembotski, C.C. Characterization of the peptide acetyltransferase activity in bovine and rat intermediate pituitaries responsible for the acetylation of β-endorphin and α-melanotropin. J. Biol. Chem. 1982, 257, 10501–10509. [Google Scholar] [PubMed]
- Lan, V.T.; Ito, K.; Ohno, M.; Motoyama, T.; Ito, S.; Kawarasaki, Y. Analyzing a dipeptide library to identify human dipeptidyl peptidase IV inhibitor. Food Chem. 2015, 175, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Je, J.Y.; Qian, Z.J.; Byun, H.G.; Kim, S.K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Byun, H.G.; Lee, J.K.; Park, H.G.; Jeon, J.K.; Kim, S.K. Antioxidant peptides isolated from the marine rotifer, Brachionus rotundiformis. Process Biochem. 2009, 44, 842–846. [Google Scholar] [CrossRef]
- Suetsuna, K. Antioxidant peptides from the protease digest of prawn (Penaeus japonicus) muscle. Mar. Biotechnol. (N. Y.) 2000, 2, 5–10. [Google Scholar] [CrossRef]
- Kim, S.Y.; Je, J.Y.; Kim, S.K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Kang, K.H.; Ryu, B.M.; Vo, T.S.; Jung, W.K.; Byun, H.G.; Kim, S.K. Angiotensin-I converting enzyme inhibitory peptides from antihypertensive skate (Okamejei kenojei) skin gelatin hydrolysate in spontaneously hypertensive rats. Food Chem. 2015, 174, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.G.; Kim, S.K. Structure and activity of angiotensin I converting enzyme inhibitory peptides derived from Alaskan pollack skin. J. Biochem. Mol. Biol. 2002, 35, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Lantz, I.; Glgmsta, E.-L.; Talbgck, L.; Nyberg, F. Hemorphins derived from hemoglobin have an inhibitory action on angiotensin converting enzyme activity. FEBS Lett. 1991, 287, 39–41. [Google Scholar] [CrossRef]
- Sun, M.L.; Zhang, Q.; Ma, Q.; Fu, Y.H.; Jin, W.G.; Zhu, B.W. Affinity purification of angiotensin-converting enzyme inhibitory peptides from Volutharpa ampullacea perryi protein hydrolysate using Zn-SBA-15 immobilized ACE. Eur. Food Res. Technol. 2017. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, Y.S.; Kim, S.E.; Kim, E.K.; Hwang, J.W.; Park, T.K.; Kim, B.K.; Moon, S.H.; Jeon, B.T.; Jeon, Y.J.; et al. Purification and characterization of a novel angiotensin I-converting enzyme inhibitory peptide derived from an enzymatic hydrolysate of duck skin byproducts. J. Agric. Food. Chem. 2012, 60, 10035–10040. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Li, L.; Liu, G.; Hu, S.Q. Inhibition mechanism and model of an angiotensin I-converting enzyme (ACE)-inhibitory hexapeptide from yeast (Saccharomyces cerevisiae). PLoS ONE 2012, 7, e37077. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.A.; Suman, S.P.; Konda, M.K.R.; Ramanathan, R. Effect of carbon monoxide packaging and lactate enhancement on the color stability of beef steaks stored at 1 °C for 9 days. Meat Sci. 2009, 81, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, M.; Rosen, R.T.; Ho, C.T. 2,2-diphenyl-1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum thunb. J. Agric. Food Chem. 1999, 47, 2226–2228. [Google Scholar] [CrossRef] [PubMed]
- Rosen, G.M.; Rauckman, E.J. Spin trapping of superoxide and hydroxyl radicals. Methods Enzymol. 1984, 105, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Liu, J.B.; Yu, Z.P.; Zhao, W.Z.; Lin, S.Y.; Wang, E.L.; Zhang, Y.; Hao, H.; Wang, Z.Z.; Chen, F. Isolation and identification of angiotensin-converting enzyme inhibitory peptides from egg white protein hydrolysates. Food Chem. 2010, 122, 1159–1163. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S. The protein data bank. Acta Crystallogr. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and autodocktools4: automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Model. 1999, 17, 57–61. [Google Scholar]
- Pan, D.Q.; Jiang, M.; Liu, T.T.; Wang, Q.; Shi, J.H. Combined spectroscopies and molecular docking approach to characterizing the binding interaction of enalapril with bovine serum albumin. Luminescence 2016, 31, 468–477. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Types of Amino Acids | JGPH-P1(g/100 g) | JGPH-P2(g/100 g) | JGPH-P3(g/100 g) |
|---|---|---|---|
| Asp | 4.43 ± 0.02 b | 4.77 ± 0.33 b | 3.68 ± 0.06 a |
| Glu | 5.96 ± 0.06 b | 6.20 ± 0.20 b | 4.51 ± 0.18 a |
| Ser | 2.20 ± 0.07 a | 2.18 ± 0.19 a | 1.91 ± 0.05 a |
| Arg | 2.79 ± 0.03 ab | 3.20 ± 0.26 b | 2.66 ± 0.03 a |
| Gly | 4.83 ± 0.23 a | 4.50 ± 0.22 a | 4.34 ± 0.16 a |
| Thr A | 2.60 ± 0.26 a | 2.26 ± 0.26 a | 1.91 ± 0.20 a |
| Pro C | 2.06 ± 0.03 b | 1.72 ± 0.12 a | 1.52 ± 0.03 a |
| Ala C | 2.67 ± 0.09 a | 2.95 ± 0.04 a | 2.74 ± 0.17 a |
| Val AC | 2.63 ± 0.01 b | 2.76 ± 0.07 c | 2.44 ± 0.02 a |
| Met AC | 0.85 ± 0.00 a | 0.99 ± 0.02 b | 1.01 ± 0.03 b |
| Cys | 0.22 ± 0.01 | 0.03 ± 0.01 | — |
| Ile AC | 2.01 ± 0.01 a | 2.12 ± 0.27 a | 1.99 ± 0.07 a |
| Leu AC | 3.00 ± 0.26 a | 3.42 ± 0.06 a | 3.45 ± 0.02 a |
| Trp ABC | 3.22 ± 0.16 a | 3.48 ± 0.13 a | 4.01 ± 0.03 b |
| Phe ABC | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 0.06 ± 0.01 a |
| His | 4.95 ± 0.58 a | 4.39 ± 0.20 a | 4.23 ± 0.51 a |
| Lys A | 5.91 ± 0.11 b | 6.25 ± 0.04 c | 4.92 ± 0.12 a |
| Tyr B | 1.55 ± 0.07 a | 1.69 ± 0.29 a | 4.01 ± 0.03 a |
| total | 51.93 ± 2.02 b | 52.97 ± 2.72 b | 47.40 ± 1.75 a |
| Components | DPPH Scavenging Activity (%) 1 | ACE Inhibition Activity (%) 2 |
|---|---|---|
| JGPH-P1 | 39.87 A | 79.91 a |
| JGPH-P2 | 65.68 B | 79.98 b |
| JGPH-P3 | 87.35 C | 87.35 c |
| Fractions | IC50 of DPPH Scavenging Activity (mg/mL) | IC50 of ACE Inhibitory Activity (mg/mL) |
|---|---|---|
| JGPH-P3A | 0.62 ± 0.0071 D | 1.01 ± 0.0379 b |
| JGPH-P3B | 0.95 ± 0.0314 E | 1.07 ± 0.0376 b |
| JGPH-P3C | 1.56 ± 0.0206 F | 1.67 ± 0.0412 c |
| JGPH-P3D | 0.48 ± 0.0005 C | 0.96 ± 0.0197 b |
| JGPH-P3E | 0.29 ± 0.0084 A | 4.38 ± 0.0154 d |
| JGPH-P3F | 0.38 ± 0.0095 B | 0.54 ± 0.0241 a |
| Bioactivities | IC50 (μM) |
|---|---|
| ACE inhibitory | 1164.179 ± 0.37 |
| DPPH radical | 84.623 ± 0.75 |
| GSH (DPPH radical) | 162.695 ± 0.32 |
| Hydroxyl radical | 1177.632 ± 1.86 |
| GSH (hydroxyl radical) | 7306.616 ± 0.32 |
| Superoxide radical | 456.663 ± 2.23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Song, C.; Zhao, J.; Shi, X.; Sun, M.; Liu, J.; Fu, Y.; Jin, W.; Zhu, B. Separation and Characterization of Antioxidative and Angiotensin Converting Enzyme Inhibitory Peptide from Jellyfish Gonad Hydrolysate. Molecules 2018, 23, 94. https://doi.org/10.3390/molecules23010094
Zhang Q, Song C, Zhao J, Shi X, Sun M, Liu J, Fu Y, Jin W, Zhu B. Separation and Characterization of Antioxidative and Angiotensin Converting Enzyme Inhibitory Peptide from Jellyfish Gonad Hydrolysate. Molecules. 2018; 23(1):94. https://doi.org/10.3390/molecules23010094
Chicago/Turabian StyleZhang, Qin, Chengcheng Song, Jun Zhao, Xiaomei Shi, Meiling Sun, Jing Liu, Yinghuan Fu, Wengang Jin, and Beiwei Zhu. 2018. "Separation and Characterization of Antioxidative and Angiotensin Converting Enzyme Inhibitory Peptide from Jellyfish Gonad Hydrolysate" Molecules 23, no. 1: 94. https://doi.org/10.3390/molecules23010094
APA StyleZhang, Q., Song, C., Zhao, J., Shi, X., Sun, M., Liu, J., Fu, Y., Jin, W., & Zhu, B. (2018). Separation and Characterization of Antioxidative and Angiotensin Converting Enzyme Inhibitory Peptide from Jellyfish Gonad Hydrolysate. Molecules, 23(1), 94. https://doi.org/10.3390/molecules23010094





