Purification, Preliminary Characterization and Hepatoprotective Effects of Polysaccharides from Dandelion Root
Abstract
:1. Introduction
2. Results
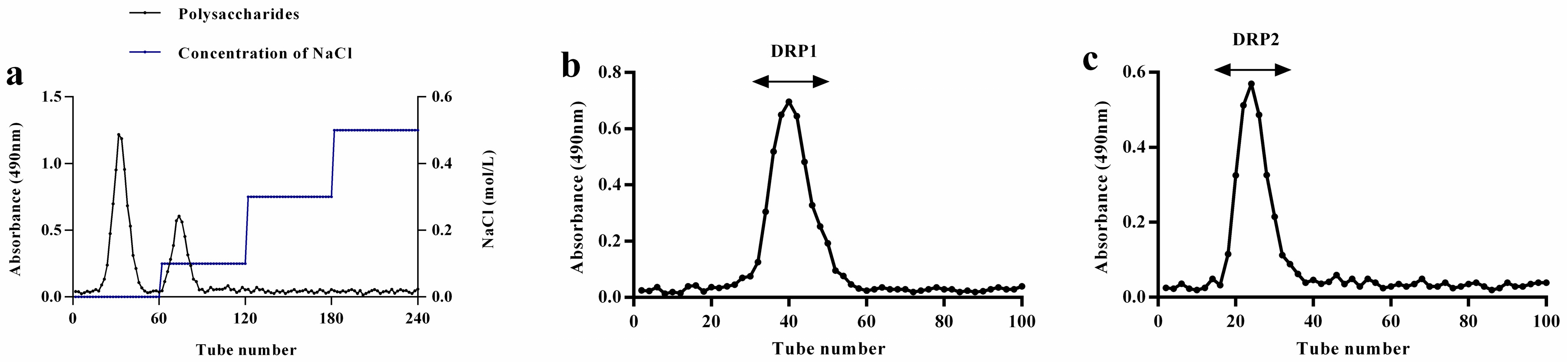
2.1. Separation and Purification of Polysaccharide
2.2. Preliminary Characterization of Polysaccharide Fractions
2.2.1. Analysis of Polysaccharide and Protein Contents
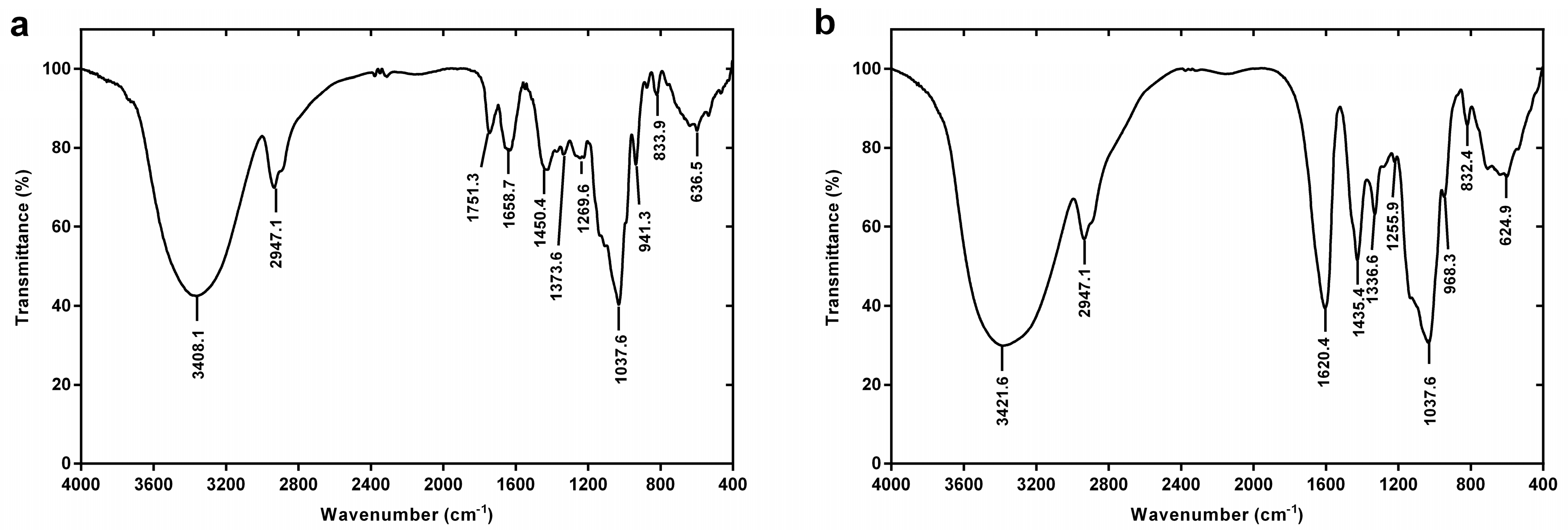
2.2.2. FT-IR Spectroscopy
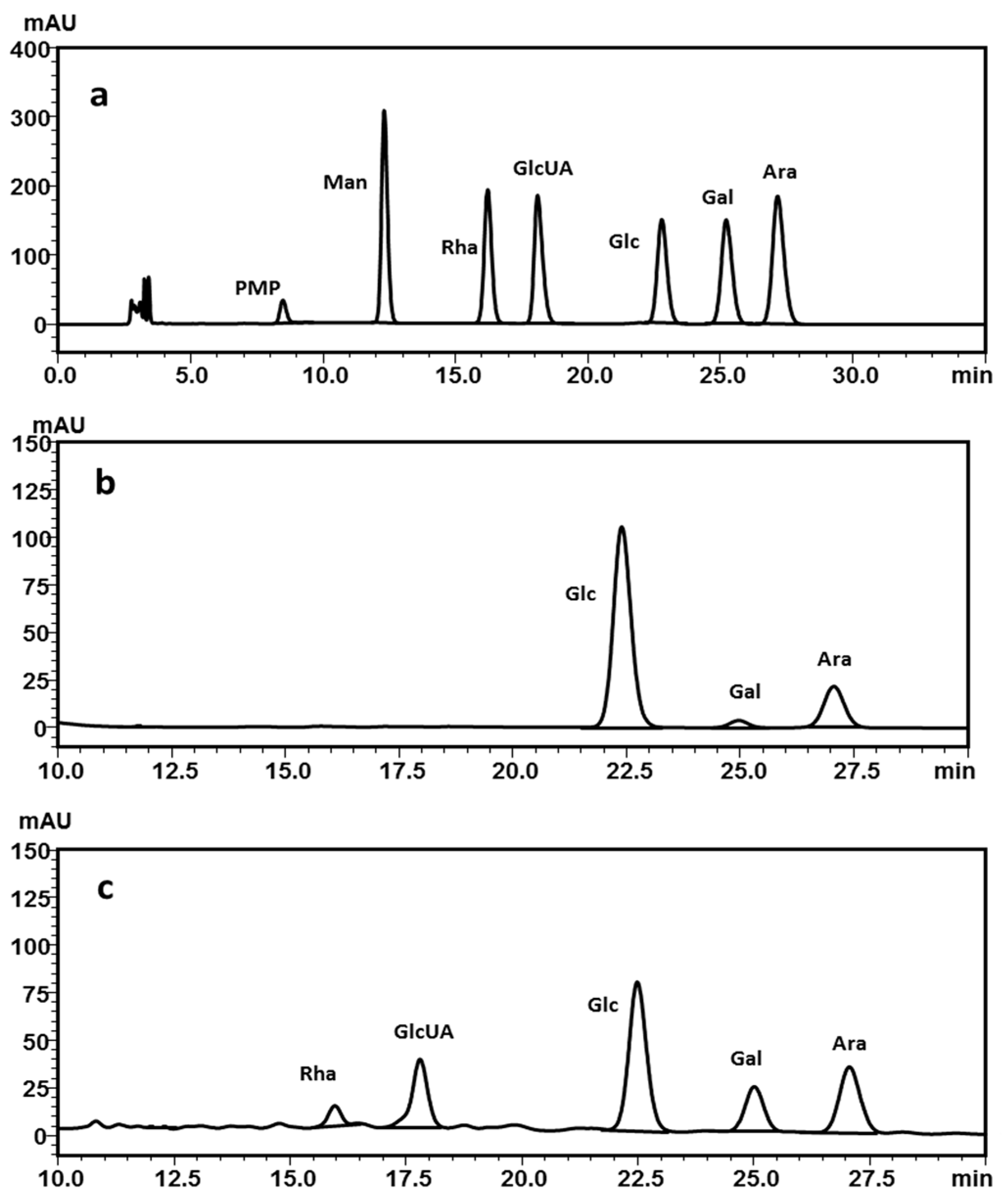
2.2.3. Monosaccharide Composition Analysis
2.2.4. Molecular Weight Analysis by Gel Permeation Chromatography
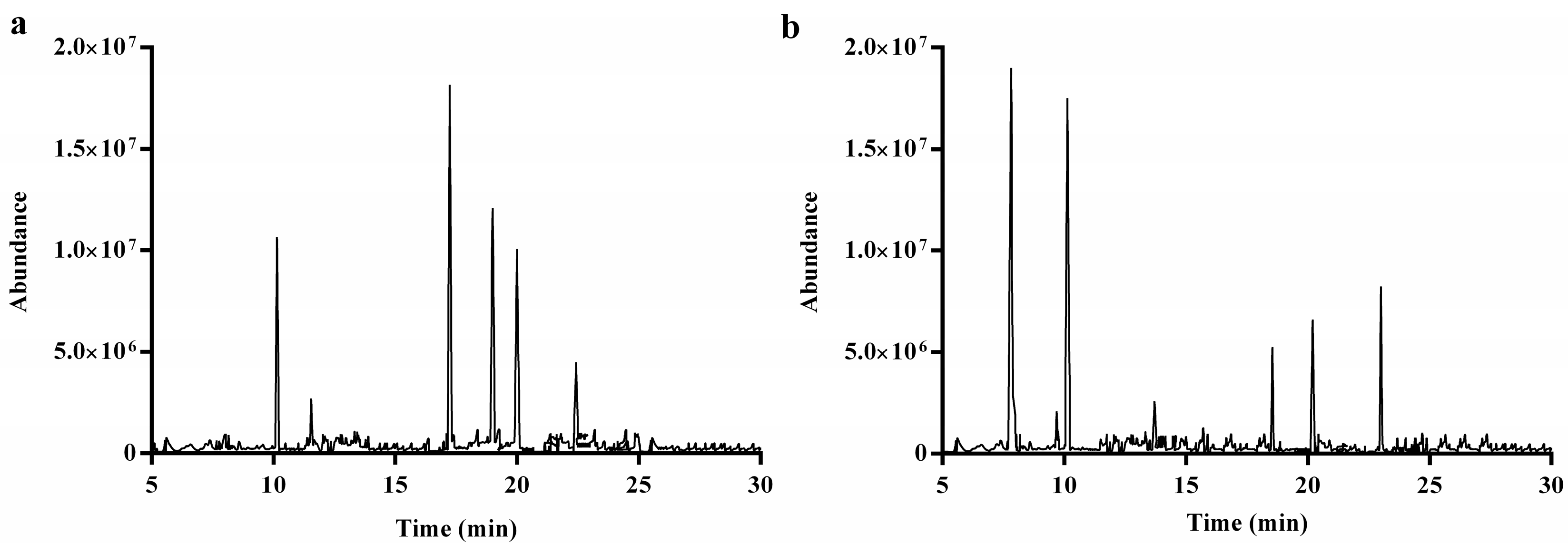
2.2.5. Methylation and GC-MS Analysis
2.3. Polysaccharide Fractions Protect against APAP-Induced Acute Liver Injury in Mice
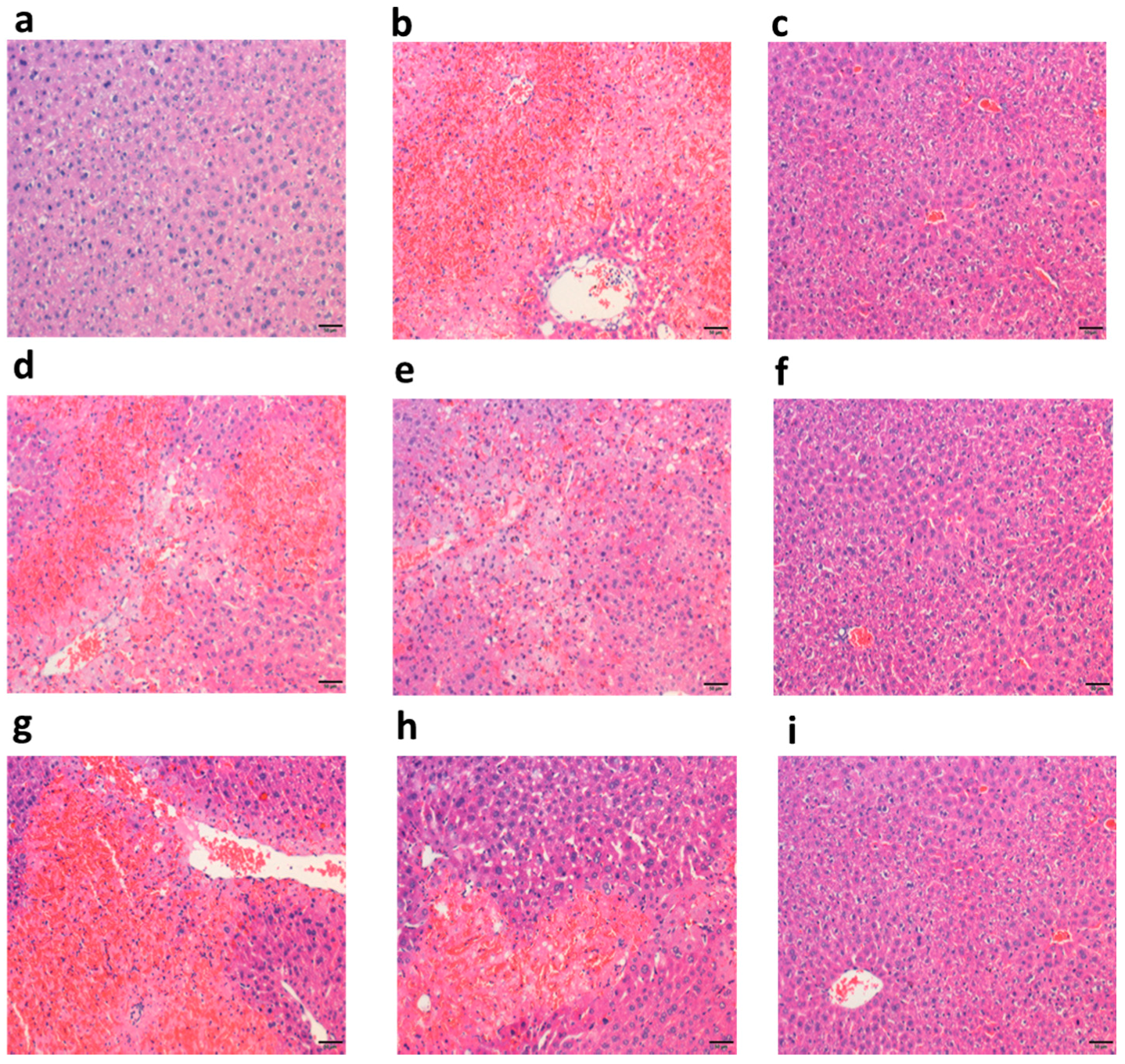
2.3.1. Polysaccharide Fractions Alleviated APAP-Induced Histology Changes in Liver
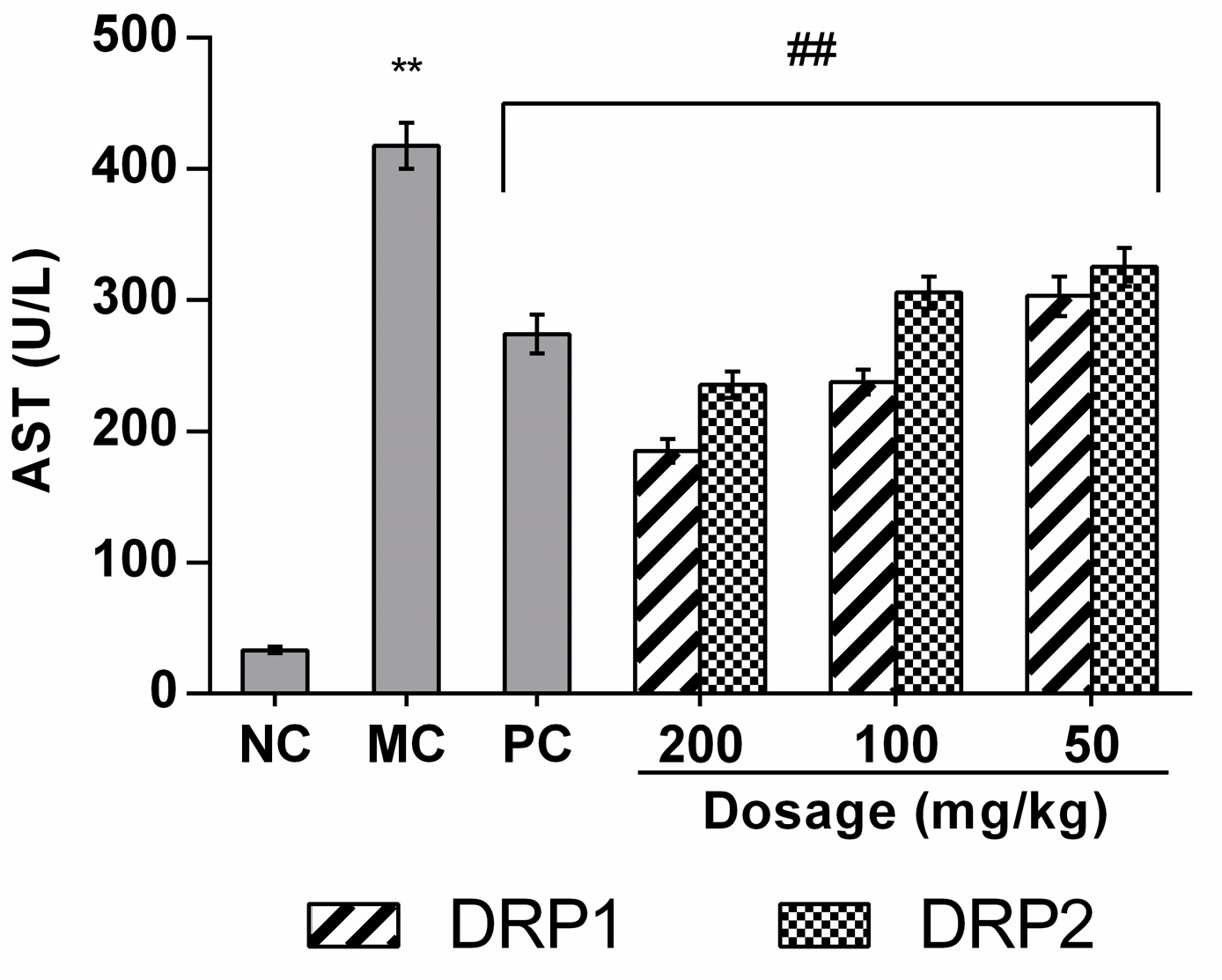
2.3.2. Effects of Polysaccharide Fractions on APAP-Induced Serum Aspartate Aminotransferases (AST) Levels
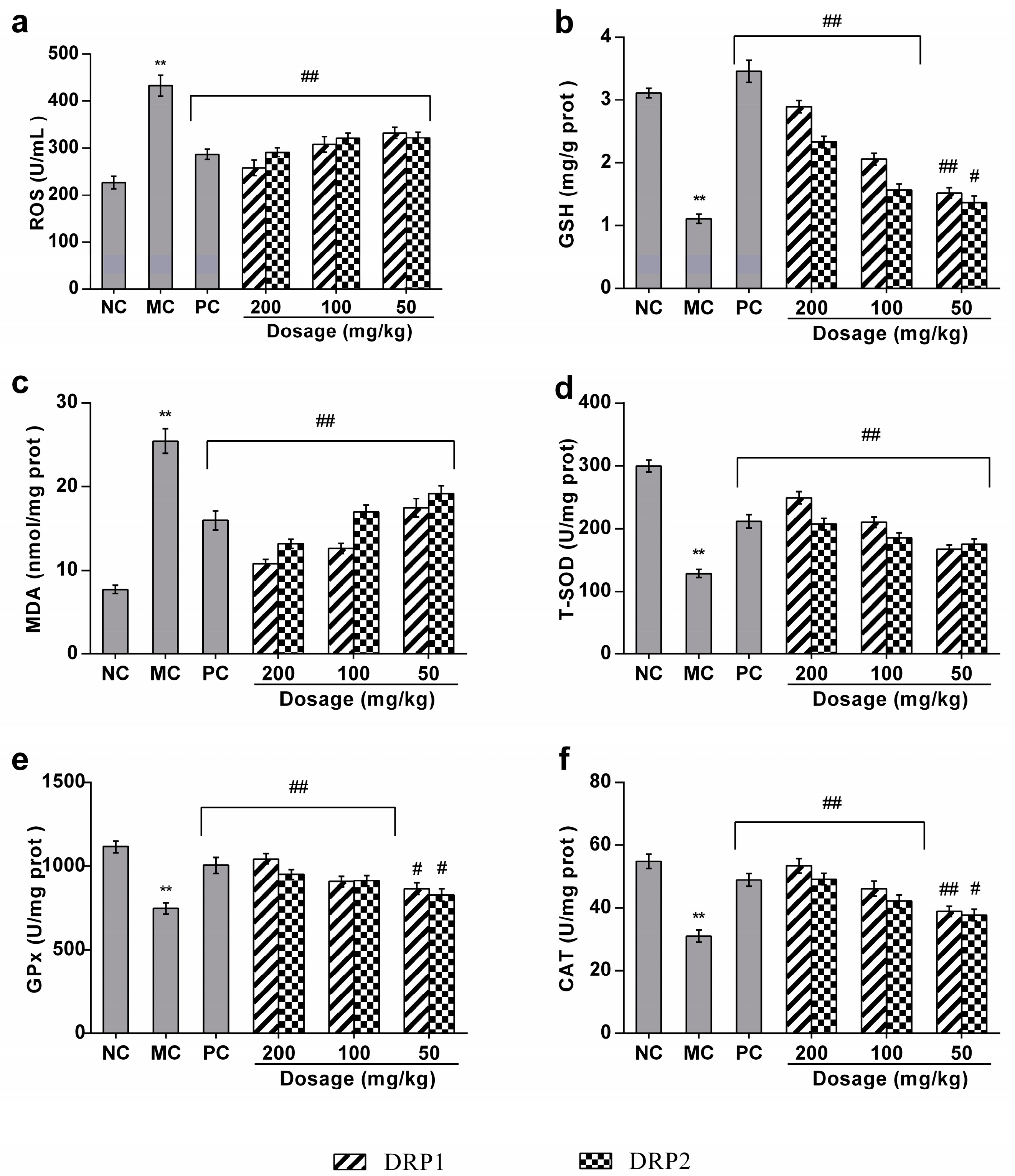
2.3.3. Polysaccharide Fractions Inhibited APAP-Induced Oxidative Stress in Liver
2.3.4. Polysaccharide Fractions Reversed the Antioxidant Enzyme Activities in Livers of APAP-Treated Mice
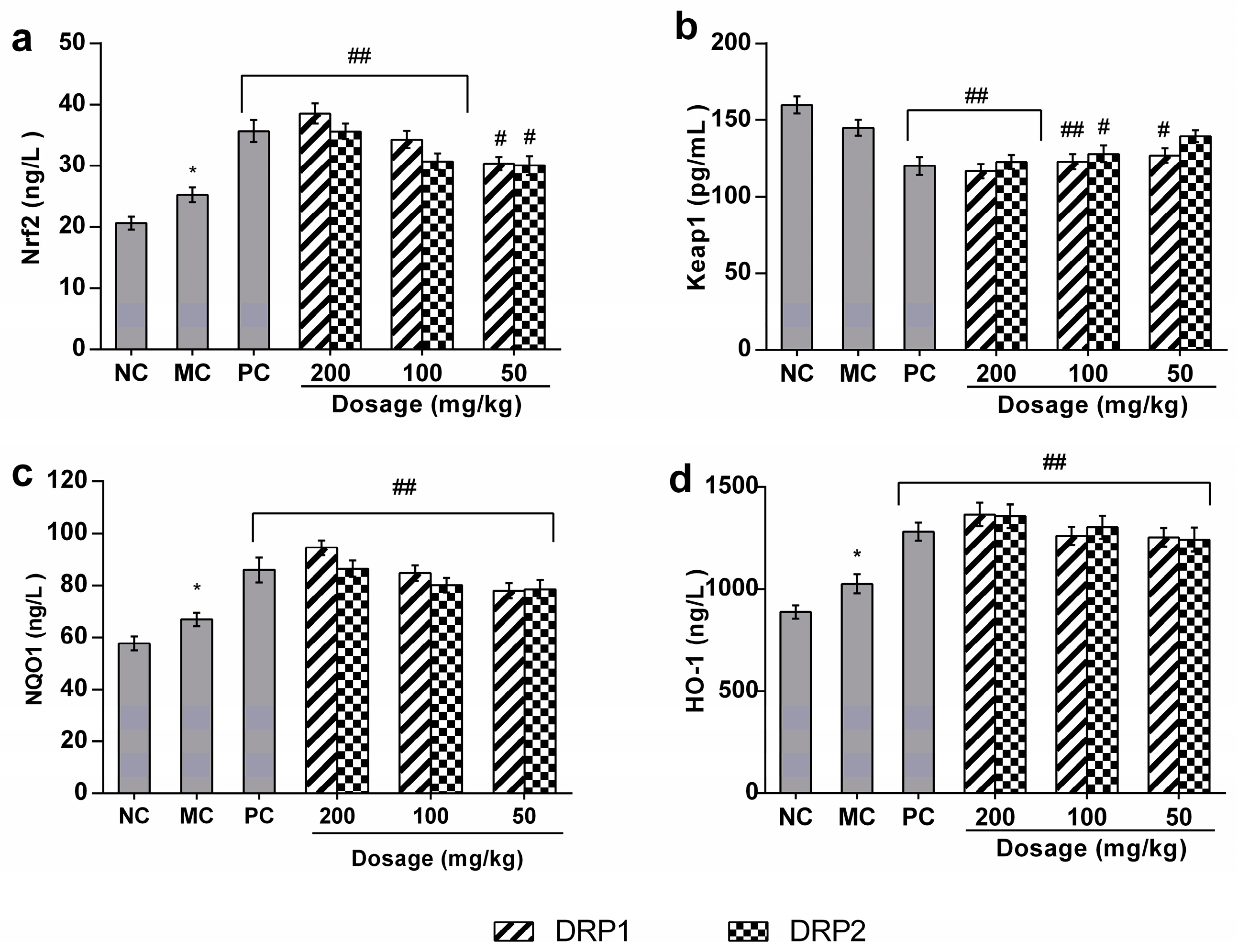
2.3.5. Polysaccharide Fractions -Mediated Protective Action Involves the Nrf2-Keap1 Pathway
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Extraction of Crude Polysaccharide
4.3. Separation and Purification of DRP
4.4. Determination of Contents of Carbohydrate and Protein
4.5. Infrared Spectroscopy Analysis
4.6. Monosaccharide Composition Analysis
4.7. Molecular Weight Determination
4.8. Methylation and GC-MS Analysis
4.9. Animals and Experimental Design
4.10. Histological Evaluations
4.11. Serum Enzyme Assay
4.12. MDA, ROS, GSH, GPx, T-SOD, and CAT Content Assay
4.13. Determination of Nrf2, Keap1, NQO1 and HO-1
4.14. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mcgill, M.R.; Sharpe, M.R.; Williams, C.D.; Taha, M.; Curry, S.C.; Jaeschke, H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Investig. 2012, 122, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Saito, C.; Lemasters, J.J.; Jaeschke, H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2010, 246, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamamoto, M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signal. 2005, 7, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kong, A.N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Reisman, S.A.; Buckley, D.B.; Tanaka, Y.; Klaassen, C.D. CDDO-Im protects from acetaminophen hepatotoxicity through induction of Nrf2-dependent genes. Toxicol. Appl. Pharmacol. 2009, 236, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Jedlicka, A.E.; Reddy, S.P.M.; Kensler, T.W.; Yamamoto, M.; Zhang, L.Y.; Kleeberger, S.R. Role of NRF2 in Protection Against Hyperoxic Lung Injury in Mice. Am. J. Respir. Cell Mol. Biol. 2002, 26, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001, 59, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Ramosgomez, M.; Kwak, M.K.; Dolan, P.M.; Itoh, K.; Yamamoto, M.; Talalay, P.; Kensler, T.W. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in Nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. USA 2001, 98, 3410–3415. [Google Scholar] [CrossRef] [PubMed]
- Okawa, H.; Motohashi, H.; Kobayashi, A.; Aburatani, H.; Kensler, T.W.; Yamamoto, M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem. Biophys. Res. Commun. 2006, 339, 79–88. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Yoo, S.; Yoon, H.G.; Park, J.; Lee, Y.H.; Kim, S.; Oh, K.T.; Lee, J.; Cho, H.Y.; Jun, W.; et al. In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem. Toxicol. 2010, 48, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B. Cellulase-assisted extraction and antibacterial activity of polysaccharides from the dandelion Taraxacum officinale. Carbohydr. Polym. 2014, 103, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Davaatseren, M.; Hur, H.J.; Yang, H.J.; Hwang, J.T.; Park, J.H.; Kim, H.J.; Kim, M.J.; Kwon, D.Y.; Sung, M.J. Taraxacum official (dandelion) leaf extract alleviates high-fat diet-induced nonalcoholic fatty liver. Food Chem. Toxicol. 2013, 58, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Kitts, D.D. Dandelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomedicine 2005, 12, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Cho, C.W.; Song, Y.S. TOP 1 and 2, polysaccharides from Taraxacum officinale, inhibit NFκB-mediated inflammation and accelerate Nrf2-induced antioxidative potential through the modulation of PI3K-Akt signaling pathway in RAW 264.7 cells. Food Chem. Toxicol. 2014, 66, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Chungmu, P.; Hyunjoo, Y.; Chang, H.K.; Song, Y.S. TOP1 and 2, polysaccharides from Taraxacum officinale, attenuate CCl4-induced hepatic damage through the modulation of NF-κB and its regulatory mediators. Food Chem. Toxicol. 2010, 48, 1255–1261. [Google Scholar]
- Shen, H.; Tang, G.; Zeng, G.; Yang, Y.; Cai, X.; Li, D.; Liu, H.; Zhou, N. Purification and characterization of an antitumor polysaccharide from Portulaca oleracea L. Carbohydr. Polym. 2013, 93, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Liu, X.; Shen, M.Y.; Nie, S.P.; Zhang, H.; Li, C.; Gong, D.M.; Xie, M.Y. Purification, physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013, 136, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yu, X.; Zhang, Y.; He, R.; Ma, H. Ultrasonic degradation, purification and analysis of structure and antioxidant activity of polysaccharide from Porphyra yezoensis Udea. Carbohydr. Polym. 2012, 87, 2046–2051. [Google Scholar] [CrossRef]
- Chen, X.M.; Jin, J.; Tang, J.; Wang, Z.F.; Wang, J.J.; Jin, L.Q.; Lu, J.X. Extraction, purification, characterization and hypoglycemic activity of a polysaccharide isolated from the root of ophiopogon japonicus. Carbohydr. Polym. 2011, 83, 749–754. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Athukorala, Y.; Jeon, Y.J. Effect of anticoagulative sulfated polysaccharide purified from enzyme-assistant extract of a brown seaweed Ecklonia cava on Wistar rats. Carbohydr. Polym. 2011, 86, 917–921. [Google Scholar] [CrossRef]
- Liu, S.P.; Dong, W.G.; Wu, D.F.; Luo, H.S.; Yu, J.P. Protective effect of angelica sinensis polysaccharide on experimental immunological colon injury in rats. World J. Gastroenterol. 2011, 9, 2786–2790. [Google Scholar] [CrossRef]
- Peng, Q.; Li, M.; Xue, F.; Liu, H. Structure and immunobiological activity of a new polysaccharide from Bletilla striata. Carbohydr. Polym. 2014, 107, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yuan, Q.; Farzana, R. Isolation, purification and immunobiological activity of a new water-soluble bee pollen polysaccharide from Crataegus pinnatifida Bge. Carbohydr. Polym. 2009, 78, 80–88. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, B.; Zhang, X.; Xu, M.; Chang, H.; Lu, X.; Ren, X. Purification of a polysaccharide from Boschniakia rossica and its synergistic antitumor effect combined with 5-Fluorouracil. Carbohydr. Polym. 2012, 89, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shah, N.P. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275. Food Chem. 2014, 165, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, M.; Qu, Z.; Xie, B. Antioxidant activities of different fractions of polysaccharide conjugates from green tea ( Camellia Sinensis ). Food Chem. 2008, 106, 559–563. [Google Scholar] [CrossRef]
- Kim, W.R.; Flamm, S.L.; Di, B.A.; Bodenheimer, H.C. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008, 47, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Nyblom, H.; Berggren, U.; Balldin, J.; Olsson, R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol. 2004, 39, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.G.; Flamm, S.L.; Gordon, F.D.; Chopra, S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am. J. Gastroenterol. 1998, 93, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Hoek, J.B.; Pastorino, J.G. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol 2002, 27, 63–68. [Google Scholar] [CrossRef]
- Cederbaum, A.I.; Lu, Y.; Wu, D. Role of oxidative stress in alcohol-induced liver injury. Arch. Toxicol. 2009, 83, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Kadiiska, M.B.; Gladen, B.C.; Baird, D.D.; Germolec, D.; Graham, L.B.; Parker, C.E.; Nyska, A.; Wachsman, J.T.; Ames, B.N.; Basu, S. Biomarkers of Oxidative Stress Study II: Are oxidation products of lipids, proteins, and DNA markers of CCl 4 poisoning? Free Radic. Biol. Med. 2005, 38, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Redmann, M.; Rajasekaran, N.S.; Darleyusmar, V.; Zhang, J. KEAP1-NRF2 signalling and autophagy in protection against oxidative and reductive proteotoxicity. Biochem. J. 2015, 469, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Gum, S.I.; Cho, M.K. Recent updates on acetaminophen hepatotoxicity: The role of Nrf2 in hepatoprotection. Toxicol. Res. 2013, 29, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.D.; Carlson, B.A.; Li, F.; Bonzo, J.A.; Yoo, M.H.; Krausz, K.W.; Conrad, M.; Chen, C.; Gonzalez, F.J.; Hatfield, D.L.; et al. Disruption of thioredoxin reductase 1 protects mice from acute acetaminophen-induced hepatotoxicity through enhanced Nrf2 activity. Chem. Res. Toxicol. 2013, 26, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Farombi, E.O.; Shrotriya, S.; Na, H.K.; Kim, S.H.; Surh, Y.J. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem. Toxicol. 2008, 46, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Yang, X.M.; Ma, H.L.; Yan, J.K.; Guo, D.Z. Purification, characterization and antitumor activity of polysaccharides extracted from Phellinus igniarius mycelia. Carbohydr. Polym. 2015, 133, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Qu, H.; Jia, J.; Kuang, C.; Wen, Y.; Yan, H.; Gui, Z. Characterization, antioxidant and antitumor activities of polysaccharides from purple sweet potato. Carbohydr. Polym. 2015, 132, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1955, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Jouraiphy, A.; Amir, S.; Winterton, P.; EI Gharous, M.; Revel, J.C.; Hafidi, M. Structural study of the fulvic fraction during composting of activated sludge-plant matter: Elemental analysis, FTIR and 13C NMR. Bioresour. Technol. 2008, 99, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Akao, E.; Suzuki, S.; Okuda, M.; Kakehi, K.; Nakamura, J. High-performance Liquid Chromatography of Reducing Carbohydrates as Strongly UV Absorbing and Electrochemically Sensitive 1-Phenyl-3-methyl-5-pyrazolone Derivatives. Anal. Biochem. 1989, 180, 351–357. [Google Scholar] [CrossRef]
- Ciucanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, X.; Wang, Y.; Tan, H.; Chen, P.; Zeng, H.; Huang, M.; Bi, H. Hepato-protective effects of six schisandra lignans on acetaminophen-induced liver injury are partially associated with the inhibition of CYP-mediated bioactivation. Chem. Biol. Interact. 2015, 231, 83–89. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Methylated Sugars | Linkages | Molar Ratios | Maior Mass Fragments (m/z) |
|---|---|---|---|
| 3,5-Me2-Ara | 1,4-linked Ara | 8.95 | 43, 88, 101, 118, 129, 145, 161, 178, 222 |
| 2,3,4,6-Me4-Gal | 1-linked Gal | 2.15 | 43, 87, 99, 102, 118, 129, 145, 172, 205 |
| 2,3,4-Me3-Glc | 1,6-linked Glc | 15.48 | 43, 87, 102, 116, 129, 144, 162, 189, 254 |
| 2,6-Me2-Glc | 1,3,4-linked Glc | 10.43 | 43, 98, 111, 127, 143, 157, 181, 217, 243 |
| 3,6-Me2-Glc | 1,2,4-linked Glc | 8.67 | 43, 88, 99, 113, 127, 130, 140, 169, 198 |
| 3,4-Me2-Glc | 1,2,6-linked Glc | 3.86 | 43, 85, 100, 118, 129, 190, 236 |
| Methylated Sugars | Linkages | Molar Ratios | Maior Mass Fragments (m/z) |
|---|---|---|---|
| 2,3,5-Me3-Ara | 1-linked Ara | 4.96 | 43, 87, 102, 116, 129, 145, 178, 220 |
| 3,4-Me2-Rha | 1,2-linked-Rha | 0.34 | 43, 89, 100, 116, 130, 143, 190 |
| 2,4-Me2-Glc | 1,3,6-linked Glc | 1.71 | 43, 87, 100, 118, 127, 139, 169, 234 |
| 2,3,4,6-Me4-Glc | 1-linked Glc | 4.58 | 43, 87, 102, 116, 127, 145, 172, 205 |
| 3,6-Me2-Glc | 1,2,4-linked Glc | 1.37 | 43, 88, 99, 113, 130, 167, 190, 218, 233 |
| 2,4,6-Me3-Gal | 1,3-linked Gal | 0.52 | 43, 87, 101, 118, 129, 143, 161, 181, 234 |
| 3,4-Me2-Gal | 1,2,6-linked Gal | 2.15 | 43, 87, 100, 116, 127, 190, 232 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Wan, D.; Yi, F.; Luan, L. Purification, Preliminary Characterization and Hepatoprotective Effects of Polysaccharides from Dandelion Root. Molecules 2017, 22, 1409. https://doi.org/10.3390/molecules22091409
Cai L, Wan D, Yi F, Luan L. Purification, Preliminary Characterization and Hepatoprotective Effects of Polysaccharides from Dandelion Root. Molecules. 2017; 22(9):1409. https://doi.org/10.3390/molecules22091409
Chicago/Turabian StyleCai, Liangliang, Dongwei Wan, Fanglian Yi, and Libiao Luan. 2017. "Purification, Preliminary Characterization and Hepatoprotective Effects of Polysaccharides from Dandelion Root" Molecules 22, no. 9: 1409. https://doi.org/10.3390/molecules22091409
APA StyleCai, L., Wan, D., Yi, F., & Luan, L. (2017). Purification, Preliminary Characterization and Hepatoprotective Effects of Polysaccharides from Dandelion Root. Molecules, 22(9), 1409. https://doi.org/10.3390/molecules22091409





