Enantiomeric-Enriched Ferrocenes: Synthesis, Chiral Resolution, and Mathematic Evaluation of CD-chiral Selector Energies with Ferrocene-Conjugates
Abstract
1. Introduction
2. Results and Discussion
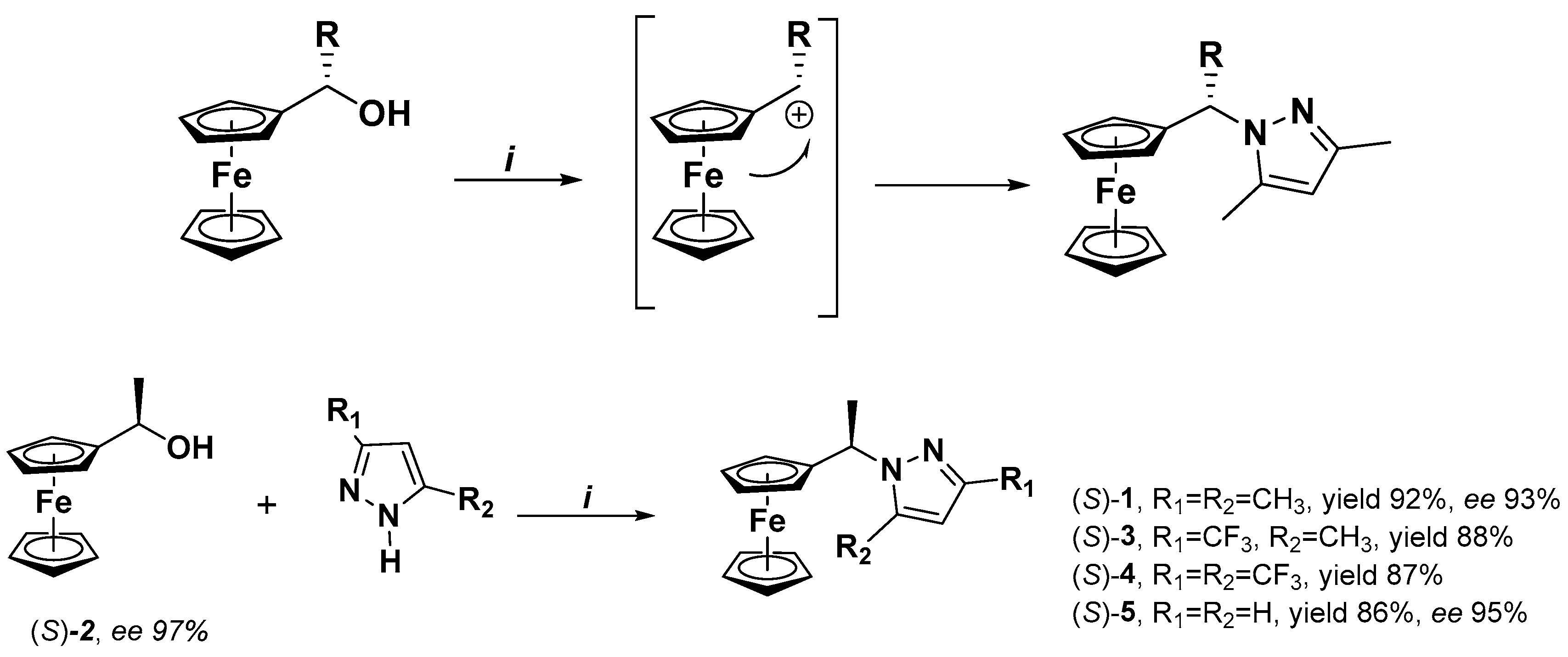
2.1. Synthesis
2.2. Enantiomeric Resolution
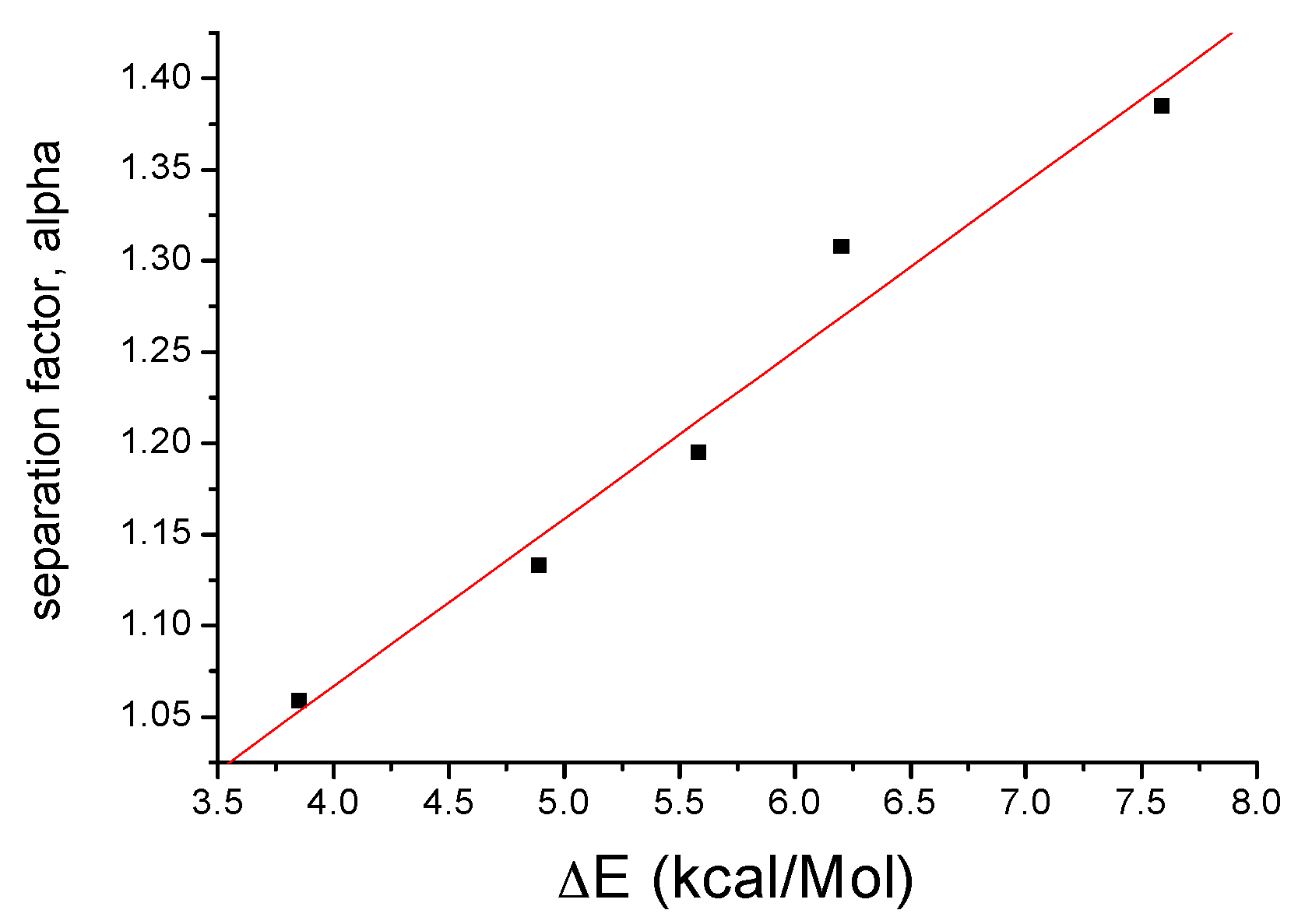
2.3. Calculations
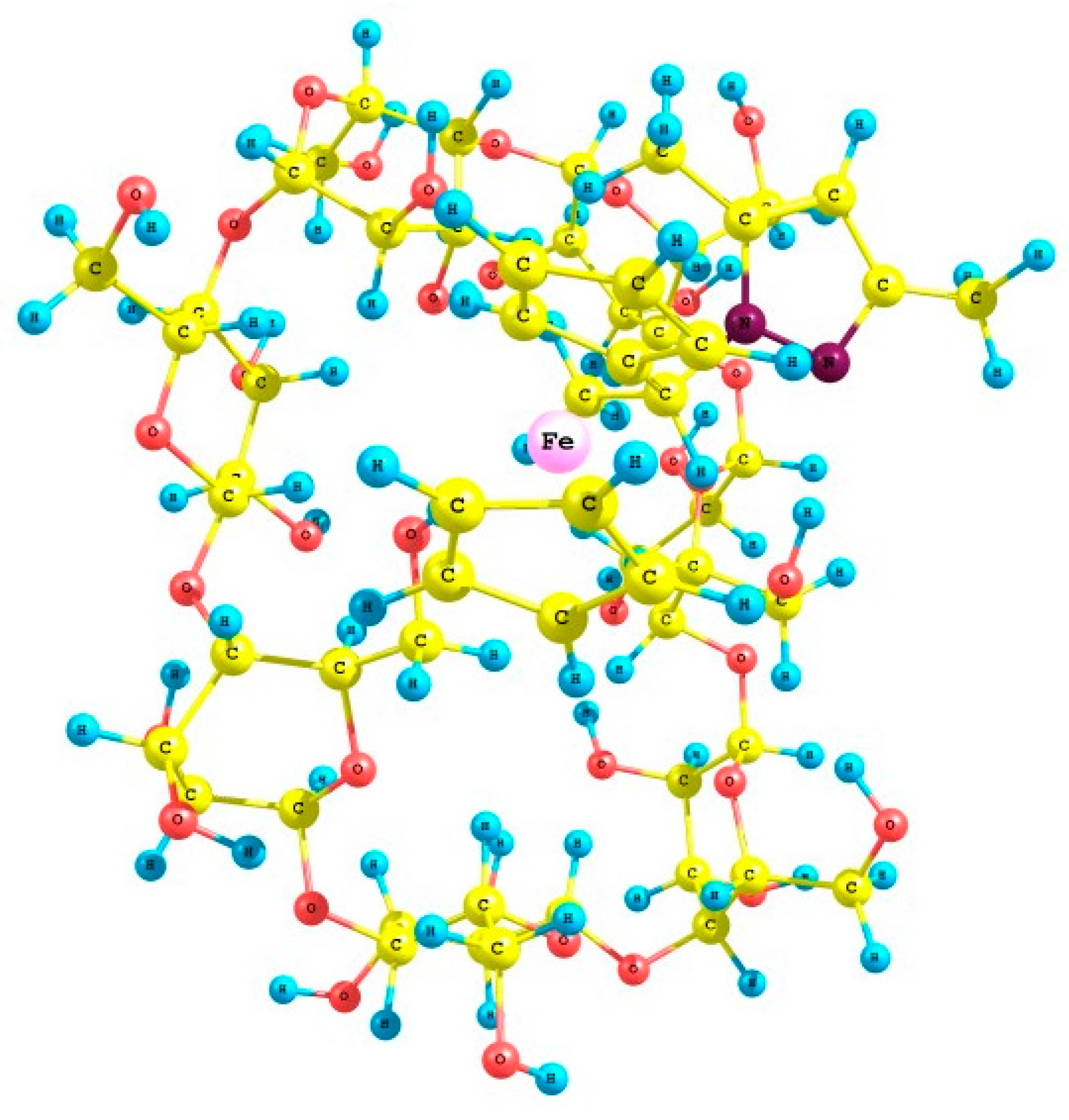
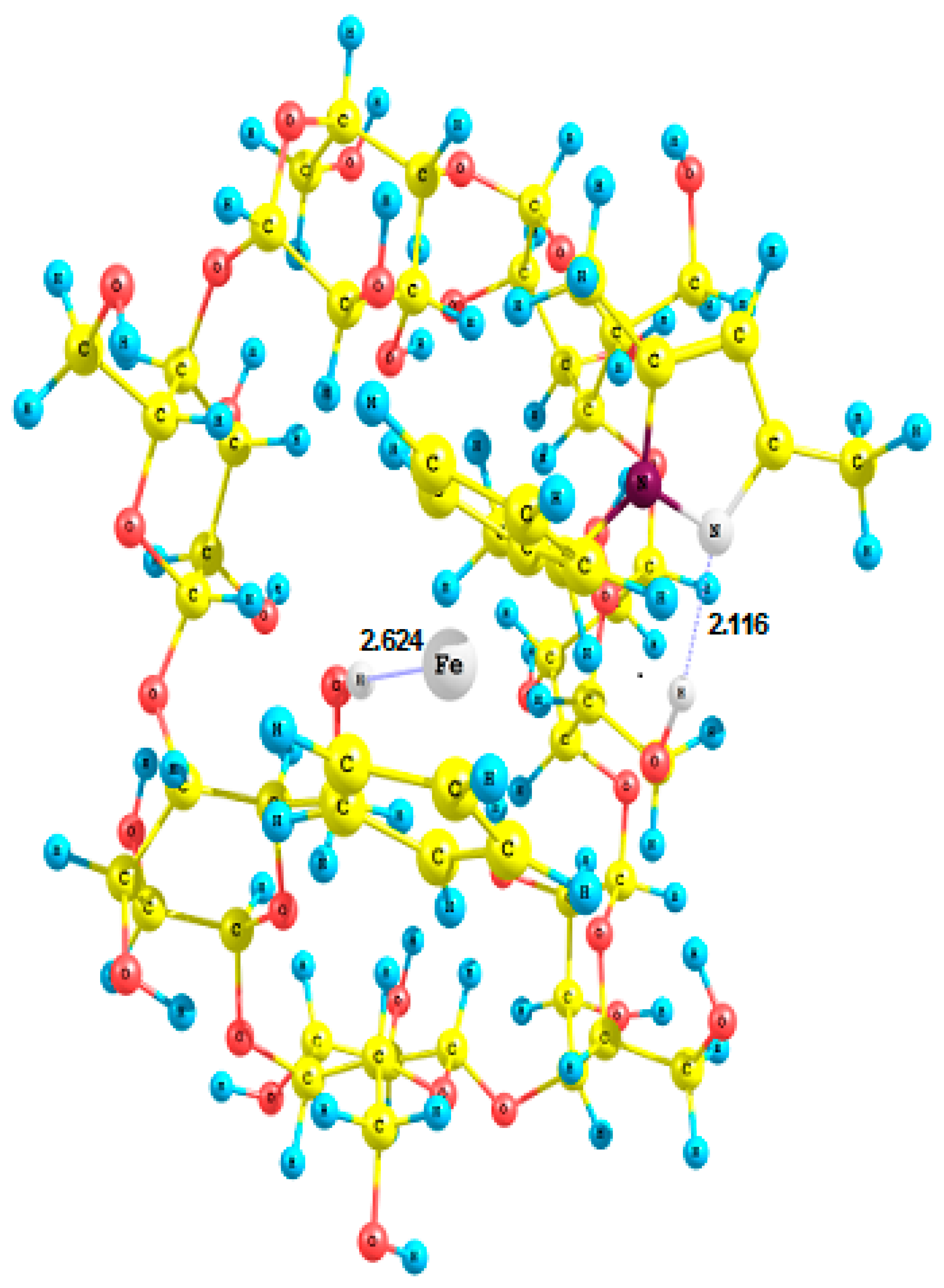
2.4. Crystal Structures
3. Experimental Section
3.1. Methods and Materials
3.2. General Procedure
3.3. Calculations
3.4. Crystallography
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The State Pharmacopoeia of the Russian Federation, XII Edition, Moscow. 2007. Available online: http://www.who.int/medicines/areas/quality_safety/quality_assurance/resources/Russian_Pharmacopoeia.pdf (accessed on 1 July 2017). (In English).
- European Pharmacopoeia. Available online: http://www.edqm.eu/en/ph-eur-reference-standards-627.html (accessed on 1 July 2017).
- Štěpnička, P. Ferrocenes: Ligands, Materials and Biomolecules; John Willey & Sons Ltd.: New York, NY, USA, 2008. [Google Scholar]
- Jaouen, G. Bioorganometallics: Biomolecules, Labeling, Medicine; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Perevalova, E.G.; Reshetova, M.D.; Grandberg, K.I. Ferrocene and Related Compounds; Nauka: Moscow, Russia, 1983. (In Russian) [Google Scholar]
- Babin, V.N.; Belousov, Y.A.; Borisov, V.I.; Gumenyuk, V.V.; Nekrasov, Y.S.; Ostrovskaya, L.A.; Sviridova, I.K.; Sergeeva, N.S.; Simenel, A.A.; Snegur, L.V. Ferrocenes as potential anticancer drugs. Facts and hypotheses. Russ. Chem. Bull. 2014, 63, 2405–2422. [Google Scholar] [CrossRef]
- Ornelas, C. Application of ferrocene and its derivatives in cancer research. New J. Chem. 2011, 35, 1973–1985. [Google Scholar] [CrossRef]
- Gasser, G.; Ott, I.; Metzler-Nolte, N. Organometallic anticancer compounds. J. Med. Chem. 2011, 54, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Nesmeyanov, A.N.; Bogomolova, L.G.; Kochetkova, N.S.; Vilchevskaya, V.D.; Palitsyn, N.P.; Andrianova, I.G.; Belozerova, O.P. Drug for Anemia and Ozena. USSR Patent 263807, 29 December 1966. [Google Scholar]
- Nesmeyanov, A.N.; Bogomolova, L.G.; Kochetkova, N.S.; Vilchevskaya, V.D.; Palitsyn, N.P.; Gorelikova, J.J.; Andrianova, I.G.; Belozerova, O.P.; Sjundjukova, V.K. Medicinal Preparation for Treating Parodontosis and Method of Treating Parodontosis. U.S. Patent 3996377, 7 December 1976. Available online: http://patentscope.wipo.int/search/en/search.jsf (accessed on 1 July 2017).
- Nesmeyanov, A.N.; Bogomolova, L.G.; Andrianova, I.G.; Vilrchevskaya, V.D.; Kochetkova, N.S. New Preparation (Ferroceron) for Treating Iron-deficient Anemia. Farm. Chem. J. 1972, 6, 269–270. [Google Scholar]
- Biot, C.; Glorian, G.; Maciejewski, L.A.; Brocard, J.S. Synthesis and antimalarial activity in vitro and in vivo of a new ferrocene-chloroquine analoge. J. Med. Chem. 1997, 40, 3715–3718. [Google Scholar] [CrossRef] [PubMed]
- Maguene, G.M.; Jakhlal, J.; Ladyman, M.; Vallin, A.; Ralambomanana, D.A.; Bousquet, T.; Maugein, J.; Lebibi, J.; Pélinski, L. Synthesis and antimycobacterial activity of a series of ferrocenyl derivatives. Eur. J. Med. Chem. 2011, 46, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Köpf-Maier, P.; Köpf, H.; Neuse, E.W. Ferricenium complexes: A new type of water-soluble antitumor agent. J. Cancer Res. Clin. Oncol. 1984, 108, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Popova, L.V.; Babin, V.N.; Belousov, Y.A.; Nekrasov, Y.S.; Snegireva, A.E.; Borodina, N.P.; Shaposhnikova, G.M.; Bychenko, O.B.; Raevskii, P.M.; Morozova, N.B.; et al. Antitumor effects of binuclear ferrocene derivatives. Appl. Organomet. Chem. 1993, 7, 85–94. [Google Scholar] [CrossRef]
- Neuse, E.W. Macromolecular ferrocene compounds as cancer drug models. J. Inorg. Organomet. Polym. Mater. 2005, 15, 3–31. [Google Scholar] [CrossRef]
- Osella, D.; Ferrali, M.; Zanello, P.; Laschi, F.; Fontani, M.; Nervi, C.; Cavigiolio, G. On the mechanism of the antitumor activity of ferrocenium derivatives. Inorg. Chim. Acta 2000, 306, 42–48. [Google Scholar] [CrossRef]
- Snegur, L.V.; Zykova, S.I.; Simenel, A.A.; Nekrasov, Y.S.; Starikova, Z.A.; Peregudova, S.M.; Ilyin, M.M.; Kachala, V.V.; Sviridova, I.K.; Sergeeva, N.S. Redox-active ferrocene-modified pyrimidines and adenine as antitumor agents: Structure, separation of enantiomers, and inhibition of DNA synthesis in tumor cells. Russ. Chem. Bull. 2013, 62, 2056–2064. [Google Scholar] [CrossRef]
- Rodionov, A.N.; Zherebker, K.Y.; Snegur, L.V.; Korlyukov, A.A.; Arhipov, D.E.; Peregudov, A.S.; Ilyin, M.M.; Ilyin, M.M., Jr.; Nikitin, O.M.; Morozova, N.B.; et al. Synthesis, structure and enantiomeric resolution ferrocenylalkyl mercaptoazoles. Antitumor activity in vivo. J. Organomet. Chem. 2015, 783, 83–91. [Google Scholar] [CrossRef]
- Jaouen, G.; Vessières, A.; Top, S. Ferrocifen type anticancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef] [PubMed]
- Rodionov, A.N.; Snegur, L.V.; Simenel, A.A.; Dobryakova, Y.V.; Markevich, V.A. Ferrocene-modification of amino acids: Synthesis and in vivo bioeffects on hippocampus. Russ. Chem. Bull. 2017, 66, 136–142. [Google Scholar] [CrossRef]
- Snegur, L.V.; Simenel, A.A.; Rodionov, A.N.; Boev, V.I. Ferrocene modification of organic compounds for medicinal applications. Russ. Chem. Bull. 2014, 63, 26–36. [Google Scholar] [CrossRef]
- Astruc, D. Why is ferrocene so exceptional? Eur. J. Inorg. Chem. 2017, 2017, 6–29. [Google Scholar] [CrossRef]
- Allenmark, S.; Kalen, K. Direct conversion (+)-N,N-dimethyl-α-ferrocenylbenzylamine into -α-ferrocenylbenzylalcohol. Adv. Synth. Catal. 1975, 16, 3175–3176. [Google Scholar] [CrossRef]
- Marquarding, D.; Klusacek, H.; Gokel, G.; Hoffmann, P.; Ugi, I. Stereoselective syntheses. VI. correlation of central and planar elements of chirality in ferrocene derivatives. J. Am. Chem. Soc. 1970, 92, 5389–5393. [Google Scholar] [CrossRef]
- Gokel, G.W.; Ugi, I.K. The retentive nucleophilic substitutions of R-alpha-ferrocenylethyl acetate. Angew. Chem. Int. Ed. 1971, 10, 191–192. [Google Scholar] [CrossRef]
- Gokel, G.W.; Marquarding, D.; Ugi, I.K. Stereoselective syntheses. VIII. retentive nucleophilic displacements of alpha-substituted alkylferrocenes. J. Org. Chem. 1972, 37, 3052–3058. [Google Scholar] [CrossRef]
- Delhaes, L.; Biot, C.; Berry, L.; Delcourt, P.; Maciejewski, L.A.; Camus, D.; Brocard, J.S.; Dive, D. Synthesis of ferroquine enantiomers: First investigation of effects of metallocenic chirality upon antimalarial activity and cytotoxicity. ChemBioChem 2002, 3, 418–423. [Google Scholar] [CrossRef]
- Ferber, B.; Top, S.; Vessières, A.; Welter, R.; Jaouen, G. Synthesis of optically pure o-formylcyclopentadienyl metal complexes of 17α-Ethynylestradiol. Recognition of the planar chirality by the estrogen receptor. Organometallics 2006, 25, 5730–5739. [Google Scholar] [CrossRef]
- Kedge, J.L.; Nguyen, H.V.; Khan, Z.; Male, L.; Ismaile, M.K.; Roberts, H.V.; Hodges, N.J.; Horswell, S.L.; Mehellou, Y.; Tucker, J.H.R. Organometallic nucleoside analogues: effect of hydroxyalkyl linker length on cancer cell line toxicity. Eur. J. Inorg. Chem. 2017, 2, 466–476. [Google Scholar] [CrossRef]
- Snegur, L.V.; Simenel, A.A.; Nekrasov, Y.S.; Morozova, E.A.; Starikova, Z.A.; Peregudova, S.M.; Kuzmenko, Y.V.; Babin, V.N.; Ostrovskaya, L.A.; Bluchterova, N.V.; et al. Synthesis, structure and redox potentials of biologically active ferrocenylalkyl azoles. J. Organomet. Chem. 2004, 689, 2473–2479. [Google Scholar] [CrossRef]
- Snegur, L.V.; Nekrasov, Y.S.; Sergeeva, N.S.; Zhilina, Z.V.; Gumenyuk, V.V.; Starikova, Z.A.; Simenel, A.A.; Morozova, N.B.; Sviridova, I.K.; Babin, V.N. Ferrocenylalkyl azoles: Bioactivity, synthesis, structure. Appl. Organomet. Chem. 2008, 22, 139–147. [Google Scholar] [CrossRef]
- Simenel, A.A.; Samarina, S.V.; Snegur, L.V.; Starikova, Z.A.; Ostrovskaya, L.A.; Bluchterova, N.V.; Fomina, M.M. O-Carboxybenzoylferrocene. Bioactivity and chemical Modifications. Appl. Organomet. Chem. 2008, 22, 276–280. [Google Scholar] [CrossRef]
- Simenel, A.A.; Morozova, E.A.; Snegur, L.V.; Zykova, S.I.; Kachala, V.V.; Ostrovskaya, L.A.; Bluchterova, N.V.; Fomina, M.M. Simple route to ferrocenylalkyl nucleobases. Antitumor activity in vivo. Appl. Organomet. Chem. 2009, 23, 219–224. [Google Scholar] [CrossRef]
- Simenel, A.A.; Dokuchaeva, G.A.; Snegur, L.V.; Rodionov, A.N.; Ilyin, M.M.; Zykova, S.I.; Ostrovskaya, L.A.; Bluchterova, N.V.; Fomina, M.M.; Rikova, V.A. Ferrocene-modified thiopyrimidines: Synthesis, enantiomeric resolution, antitumor activity. Appl. Organomet. Chem. 2011, 25, 70–75. [Google Scholar] [CrossRef]
- Boev, V.I.; Snegur, L.V.; Babin, V.N.; Nekrasov, Y.S. α-Metallocenylalkylation. Russ. Chem. Rev. 1997, 66, 613–636. [Google Scholar] [CrossRef]
- Joksovic, M.D.; Markovic, V.; Juranic, Z.D.; Stanojkovic, T.; Jovanovic, L.S.; Damljanovic, I.S.; Szécsényi, K.M.; Todorovic, N.; Trifunovic, K.M.; Vukicević, R.D. Synthesis, characterization and antitumor activity of novel N-substituted a-amino acids containing ferrocenyl pyrazole-moiety. J. Organomet. Chem. 2009, 694, 3935–3942. [Google Scholar] [CrossRef]
- Cozzi, P.G.; Zoli, L. Nucleophilic substitution of ferrocenyl alcohols “on water”. Green Chem. 2007, 9, 1292–1295. [Google Scholar] [CrossRef]
- Jiang, R.; Shen, Y.; Zhang, Y.; Xu, X.-Q.; Shao, J.; Ji, S.-J. Etherification of ferrocenyl alcohol by highly-efficient ytterbium triflate. Chin. J. Chem. 2011, 29, 1887–1893. [Google Scholar] [CrossRef]
- Jiang, R.; Chu, X.-Q.; Xu, X.; Wu, B.; Ji, S.-J. Direct C-O bond activation mediated by AcOH: A new metal-free way for α-functionalization of ferrocene alcohols. Aust. J. Chem. 2011, 64, 1530–1537. [Google Scholar] [CrossRef]
- Jiang, R.; Yuan, C.-X.; Xu, X.-P.; Ji, S.-J. Nucleophilic substitution of ferrocenyl alcohols catalyzed by bismuth (III) in aqueous medium at room temperature. Appl. Organomet. Chem. 2012, 26, 62–66. [Google Scholar] [CrossRef]
- Guillén, E.; González, A.; López, C.; Basu, P.K.; Ghosh, A.; Font-Bardía, M.; Calvis, C.; Messeguer, R. Heterodi- (Fe, Pd/Pt) and heterotrimetallic (Fe2, Pd) complexes derived from 4-(Ferrocenylmethyl)-N-(2-methoxyethyl)-3,5-diphenylpyrazole as potential antitumoral agents. Eur. J. Inorg. Chem. 2015, 22, 3781–3790. [Google Scholar] [CrossRef]
- Rodionov, A.N.; Simenel, A.A.; Korlyukov, A.A.; Kachala, V.V.; Peregudova, S.M.; Zherebker, K.Y.; Osipova, E.Y. Synthesis and properties of 5-ferrocenyl-1H-pyrazole-3-carbaldehydes. J. Organomet. Chem. 2011, 696, 2108–2115. [Google Scholar] [CrossRef]
- Topolski, M.; Rachon, J. An improved procedure for the preparation of 1-ferrocenyl-1-phenylmethylamine. Org. Prep. Proced. Int. 1991, 23, 211–213. [Google Scholar] [CrossRef]
- Fukuda, T.; Takehara, A.; Haniu, N.; Iwao, M. Synthesis of chiral primary 1-ferrocenylalkylamines via highly diastereoselective addition of organolithium compounds to ferrocenecarboxaldehyde imine derived from (S)-2-methoxy-1-phenylethylamine. Tetrahedron Asymmetry 2000, 11, 4083–4091. [Google Scholar] [CrossRef]
- Simenel, A.A. Synthesis and Properties of Ferrocenylalkylazoles. Candidate’s Dissertation, A.N. Nesmeyanov Institute of Organoelement Compounds RAS, Moscow, Russia, 2004. [Google Scholar]
- Zhou, M.-G.; Zhang, W.-Z.; Tian, S.-K. Direct enantiospecific substitution of primary α-aminoalkylferrocenes via Lewis acid-catalyzed C-N bond cleavage. Chem. Commun. 2014, 50, 14531–14534. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, U.; Hinterman, L.; Schnyder, A.; Togni, A. Synthesis and structure of pyrazole-containing ferrocenyl ligands for asymmetric catalysis. Organometallics 1995, 14, 5415–5425. [Google Scholar] [CrossRef]
- Allenmark, S. The synthetic use of α-ferrocenylcarbenium tetrafluoroborates. Tetrahedron Lett. 1974, 15, 371–374. [Google Scholar] [CrossRef]
- Ceccon, A.; Giacometti, G.; Venzo, A.; Paolucci, D.; Bonozzi, D. Complexation of α-ferrocenylmethylcarbenium tetrafluoborates by ethers; an NMR study. J. Organomet. Chem. 1980, 185, 231–239. [Google Scholar] [CrossRef]
- Kreindlin, A.Z.; Dolgushin, F.V.; Yanovsky, A.I.; Kerzina, Z.A.; Petrovskii, P.V.; Rybinskaya, M.I. Synthesis, crystal and molecular structure of [{C5Me5FeC5Me4CH2}+B{C6H3(CF3)2}4−, the first example of a structurally characterized primary ferrocenylcarbocation. J. Organomet. Chem. 2001, 616, 106–111. [Google Scholar] [CrossRef]
- Rybinskaya, M.I.; Nekrasov, Y.S.; Borisov, Y.A.; Belokon, A.I.; Kreindlin, A.Z.; Kamyshova, A.A.; Kruglova, N.V. Gaz-phase formation of mono- and dications from iron-subgroup decamethylmetallocenes and their calculation by the density functional method. J. Organomet. Chem. 2001, 631, 9–15. [Google Scholar] [CrossRef]
- Snegur, L.V.; Boev, V.I.; Nekrasov, Y.S.; Ilyin, M.M.; Davankov, V.A.; Starikova, Z.A.; Yanovsky, A.I.; Kolomiets, A.F.; Babin, V.N. Synthesis and structure of biologically active ferrocenylalkyl polyfluoro benzimidazoles. J. Organomet. Chem. 1999, 580, 26–35. [Google Scholar] [CrossRef]
- Lam, W.-S.; Kok, S.H.L.; Au-Yeung, T.T.-L.; Wu, J.; Cheung, H.-Y.; Lam, F.-L.; Yeung, C.-H.; Chan, A.S.C. An efficient approach to chiral ferrocene-based secondary alcohols via asymmetric hydrogenation of ferrocenyl ketones. Adv. Synth. Catal. 2006, 348, 370–374. [Google Scholar] [CrossRef]
- Wright, J.; Frambes, L.; Reeves, P. A simple route to chiral ferrocenyl alcohols. J. Organomet. Chem. 1994, 476, 215–217. [Google Scholar] [CrossRef]
- Armstrong, D.W.; DeMond, W.; Czech, B.P. Separation of metallocene enentiomers by liquid chromatography: Chiral recognition via cyclodextrin bonded phases. Anal. Chem. 1985, 57, 481–484. [Google Scholar] [CrossRef]
- Simenel, A.A.; Kuzmenko, Y.V.; Morozova, E.A.; Ilyin, M.M.; Gun’ko, I.F.; Snegur, L.V. Synthesis and enantiomeric resolution of ferrocenyl(alkyl)azoles. J. Organomet. Chem., 2003, 688, 138–143. [Google Scholar] [CrossRef]
- Simenel, A.A.; Kuz’menko, Y.V.; Il’in, M.M.; Gumenyuk, V.V.; Snegur, L.V.; Nekrasov, Y.S. Synthesis and properties of optically active ferrocenyl(ethyl)indazoles. Russ. Chem. Bull. 2004, 939–942. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Desiraju, G.R. A Bond by Any Other Name. Angew. Chem. Int. Ed. 2011, 50, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, F.S.; Haven, A.C., Jr. Derivatives of Dicyclopentadienyliron. J. Am. Chem. Soc. 1955, 77, 6295–6297. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–7 are available from the authors. |
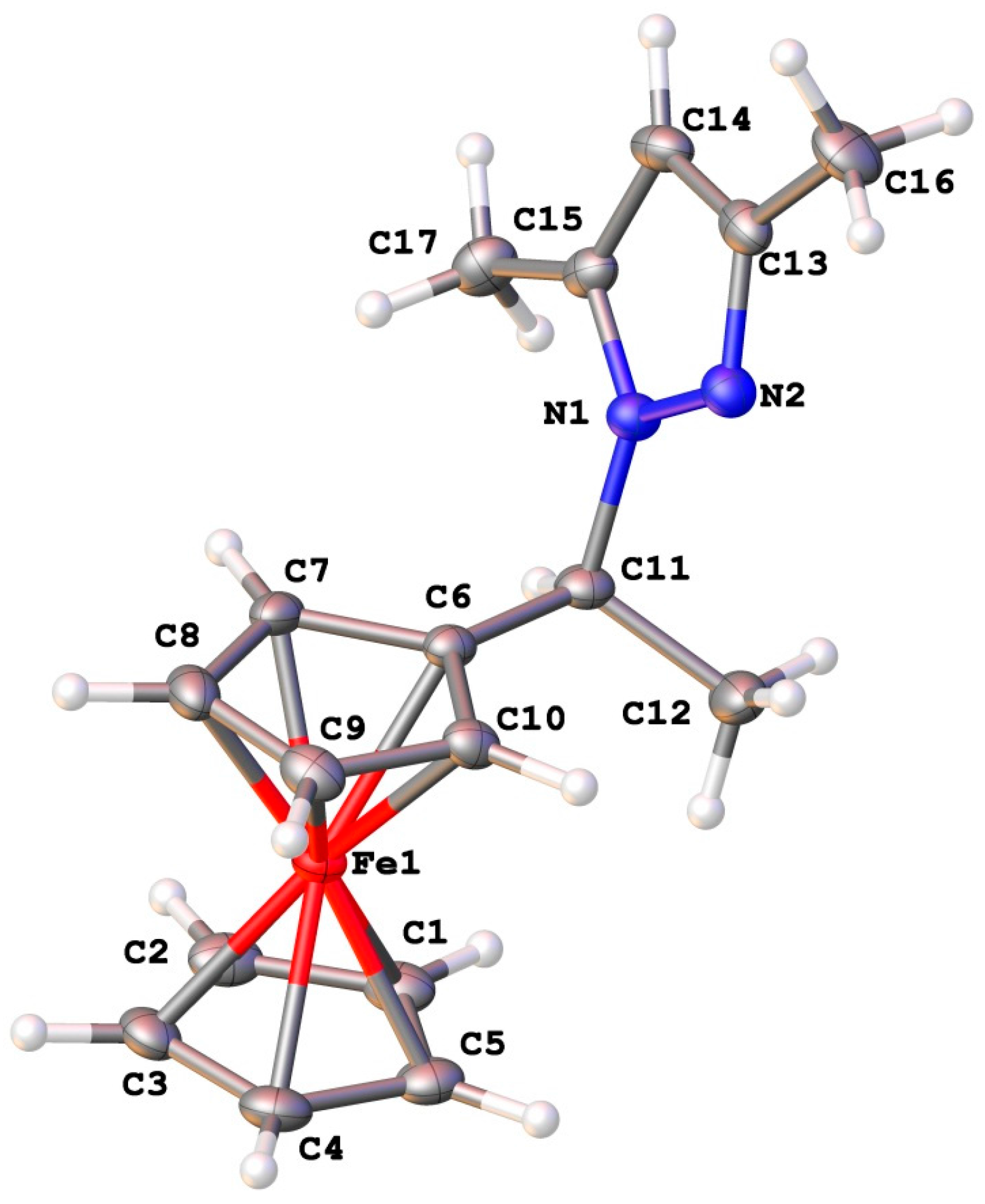
| No | Molecule | Enantiomeric Excess, ee % (a) | [α] | Concentration, mole·L−1 |
|---|---|---|---|---|
| (S)-1 | FcCH(CH3)-3,5-Me2Pz | 93 | +78.75 | 0.40 (benzene) |
| (S)-2 | FcCH(CH3)OH | 97 | +30.75 | 0.60 (methanol) |
| (S)-3 | FcCH(CH3)-3-CF3,5-CH3 Pz | not determined | +12.00 | 0.30 (benzene) |
| (S)-4 | FcCH(CH3)-3-CF3,5-CF3 Pz | not determined | +14.30 | 0.18 (benzene) |
| (S)-5 | FcCH(CH3)Pz | 95 | +16.50 | 0.70 (benzene) |
| No | Molecule (a) | Chiral Stationary Phase (b) | Separation Factor, α | ΔE, kcal/M |
|---|---|---|---|---|
| 1 | 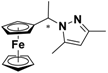 FcCH(CH3)-3,5-Me2Pz | β-CD | 1.059 | 3.85 |
| 2 | 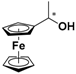 FcCH(CH3)OH | β-CD | 1.133 | 4.89 |
| 3 | 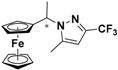 FcCH(CH3)-3-CF3,5-CH3Pz | β-CD | 1.153 | 4.94 |
| 4 | 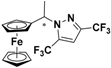 FcCH(CH3)-3-CF3,5-CF3Pz | β-CD | 1.182 | 5.25 |
| 5 | 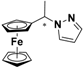 FcCH(CH3)Pz | β-CD (c) | 1.195 | 5.58 |
| 6 | 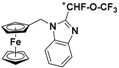 | γ-CD (d) | 1.308 | 6.20 |
| 7 | 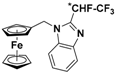 | γ-CD (d) | 1.385 | 7.59 |
| System | E, a.u. | μ, D | HOMO, a.u. | LUMO, a.u. |
|---|---|---|---|---|
| (R)-1-β-CD | −5167.4437 | 4.25 | −0.19354 | −0.02009 |
| (S)-1-β-CD | −5167.4481 | 6.25 | −0.20897 | −0.03714 |
| 1 | (S)-1 | |
|---|---|---|
| Diffractometer | Bruker Smart APEX II | CAD4 Enraf-Nonius |
| T, K | 120 | 293 |
| Empirical formula | C17H20FeN2 | C17H20FeN2 |
| Formula weight | 308.20 | 308.20 |
| Crystal system | Orthorhombic | Orthorhombic |
| Space group, Z | P212121, 4 | P212121, 4 |
| Densitycalc. (g·cm−3) | 1.396 | 1.346 |
| a (Å) | 7.8662(5) | 7.9670(16) |
| b (Å) | 8.9661(5) | 8.9670(18) |
| c (Å) | 20.7985(13) | 21.291(4) |
| V (Å3) | 1466.90(15) | 1521.0(5) |
| 2θmax (°) | 60.12 | 49.91 |
| F(000) | 648 | 648 |
| Reflections collected | 19,371 | 1649 |
| Independent reflections (R(int)) | 4301 (0.0542) | 1565 (0.0108) |
| Number of reflections with I > 2σ(I) | 3766 | 1364 |
| Parameters | 185 | 184 |
| Flack | 0.467(18) | −0.02(4) |
| Linear absorption (cm−1) | 10.19 | 9.83 |
| Goodness-of-fit (GOF) | 1.010 | 0.998 |
| R1 (I > 2σ(I)) | 0.0330 | 0.0396 |
| wR2 (all reflections) | 0.0698 | 0.1054 |
| ρmin/ρmax, e | 0.338/−0.397 | 0.366/−0.661 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snegur, L.V.; Borisov, Y.A.; Kuzmenko, Y.V.; Davankov, V.A.; Ilyin, M.M.; Ilyin, M.M., Jr.; Arhipov, D.E.; Korlyukov, A.A.; Kiselev, S.S.; Simenel, A.A. Enantiomeric-Enriched Ferrocenes: Synthesis, Chiral Resolution, and Mathematic Evaluation of CD-chiral Selector Energies with Ferrocene-Conjugates. Molecules 2017, 22, 1410. https://doi.org/10.3390/molecules22091410
Snegur LV, Borisov YA, Kuzmenko YV, Davankov VA, Ilyin MM, Ilyin MM Jr., Arhipov DE, Korlyukov AA, Kiselev SS, Simenel AA. Enantiomeric-Enriched Ferrocenes: Synthesis, Chiral Resolution, and Mathematic Evaluation of CD-chiral Selector Energies with Ferrocene-Conjugates. Molecules. 2017; 22(9):1410. https://doi.org/10.3390/molecules22091410
Chicago/Turabian StyleSnegur, Lubov V., Yurii A. Borisov, Yuliya V. Kuzmenko, Vadim A. Davankov, Mikhail M. Ilyin, Mikhail M. Ilyin, Jr., Dmitry E. Arhipov, Alexander A. Korlyukov, Sergey S. Kiselev, and Alexander A. Simenel. 2017. "Enantiomeric-Enriched Ferrocenes: Synthesis, Chiral Resolution, and Mathematic Evaluation of CD-chiral Selector Energies with Ferrocene-Conjugates" Molecules 22, no. 9: 1410. https://doi.org/10.3390/molecules22091410
APA StyleSnegur, L. V., Borisov, Y. A., Kuzmenko, Y. V., Davankov, V. A., Ilyin, M. M., Ilyin, M. M., Jr., Arhipov, D. E., Korlyukov, A. A., Kiselev, S. S., & Simenel, A. A. (2017). Enantiomeric-Enriched Ferrocenes: Synthesis, Chiral Resolution, and Mathematic Evaluation of CD-chiral Selector Energies with Ferrocene-Conjugates. Molecules, 22(9), 1410. https://doi.org/10.3390/molecules22091410







