Chemical Constituents of Murraya tetramera Huang and Their Repellent Activity against Tribolium castaneum
Abstract
:1. Introduction
2. Results
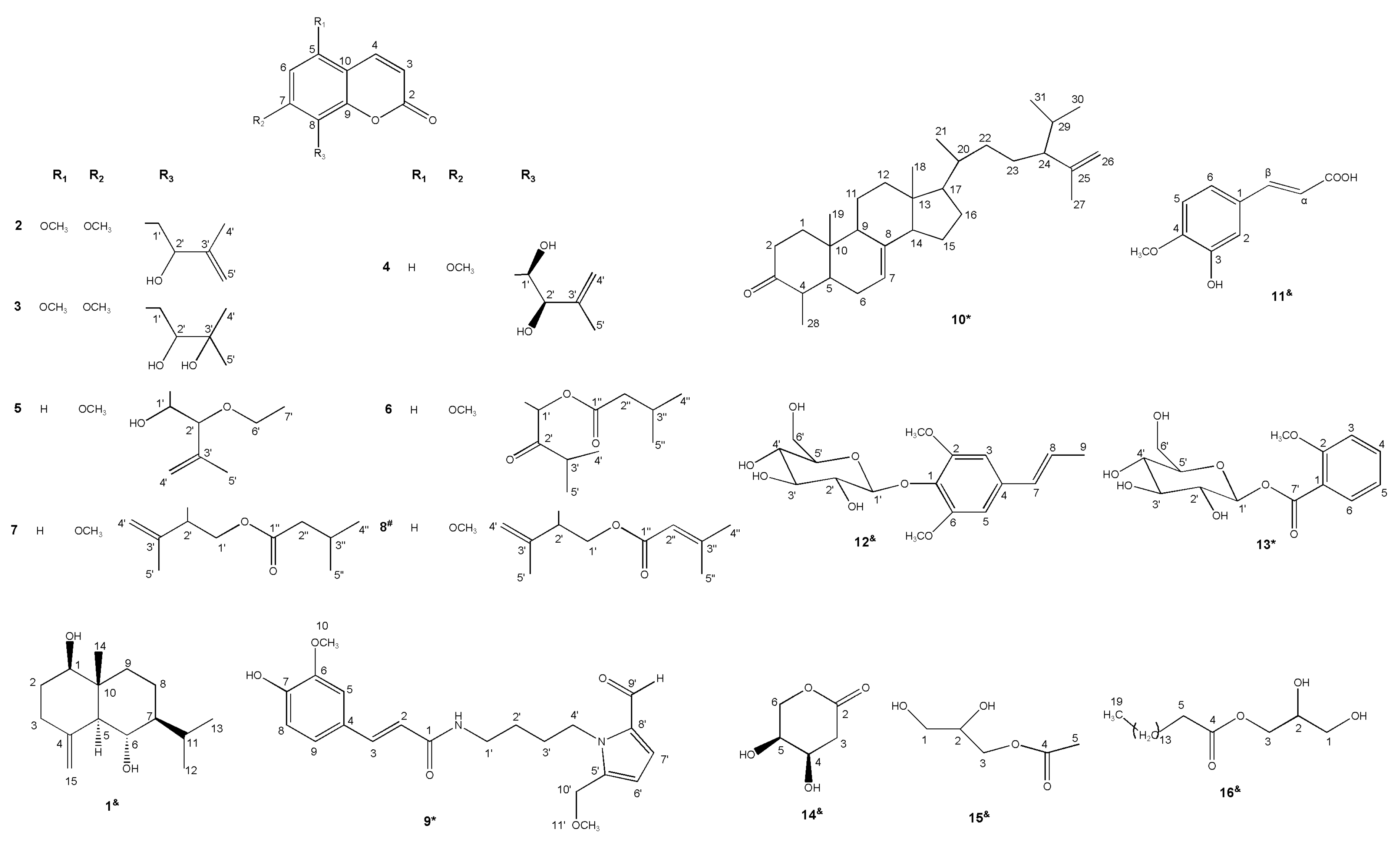
2.1. Compounds Isolated from M. tetramera
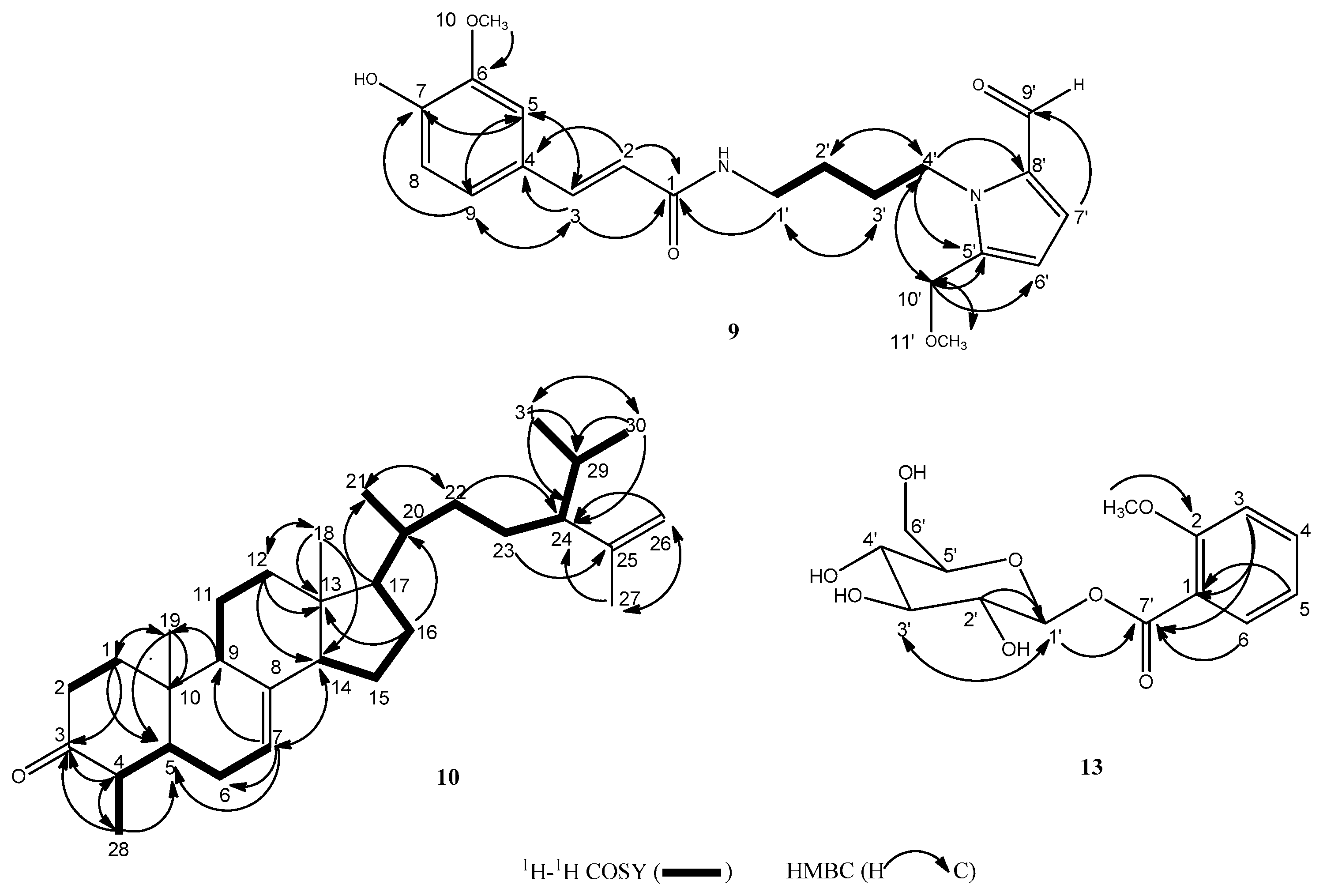
2.2. Molecular Structural Elucidation of the New Compounds
2.3. Characterization of Isolated Compounds
2.4. Repellent Activity
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Plant Material
4.3. Insects
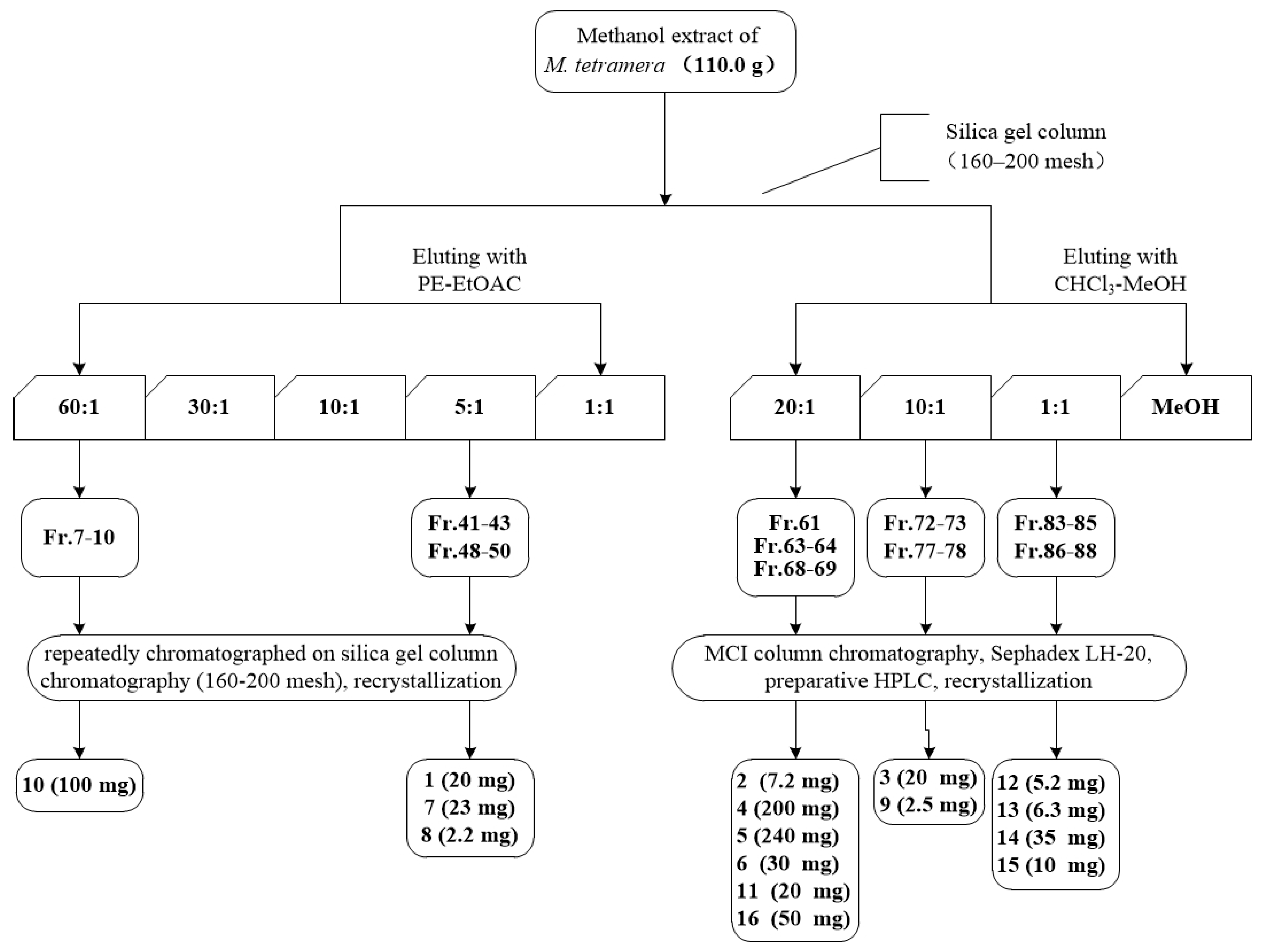
4.4. Extraction and Isolation
4.5. Repellency Tests
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, Z.L.; Ho, S.H. Bioactivity of the essential oil extracted from Evodia rutaecarpa Hook f. et Thomas against the grain storage insects, Sitophilus zeamais Motsch and Tribolium castaneum (Herbst). J. Stored Prod. Res. 1999, 35, 317–328. [Google Scholar] [CrossRef]
- Saroukolai, A.T.; Moharramipour, S.; Meshkatalsadat, M.H. Insecticidal properties of Thymus persicus essential oil against Tribolium castaneum and Sitophilus oryzae. J. Pest Sci. 2010, 83, 3–8. [Google Scholar] [CrossRef]
- Magan, N.; Hope, R.; Cairns, V.; Aldred, D. Post-harvest fungal ecology: Impact of fungal growth and mycotoxin accumulation in stored grain. Eur. J. Plant Pathol. 2003, 109, 723–730. [Google Scholar] [CrossRef]
- Zettler, J.L.; Arthur, F.H. Chemical control of stored product insects with fumigants and residual treatments. Crop Prot. 2000, 19, 577–582. [Google Scholar] [CrossRef]
- Phillips, T.W.; Throne, J.E. Biorational approaches to managing stored-product pests. Annu. Rev. Entomol. 2010, 55, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Editorial Committee of Flora of China. Flora of China; Science Press: Beijing, China, 1997; p. 145. [Google Scholar]
- Wang, Y.L.; Lu, Y.Q.; Luo, Y.P. Antifeeding activities of 45 South herbs extracts against Spodpteralitura Fabriciu. Hubei Agric. Sci. 2009, 48, 628–630. [Google Scholar]
- Li, W.Q.; Jiang, C.H.; Chu, S.S.; Zuo, M.X.; Liu, Z.L. Chemical composition and toxicity against Sitophilus zeamais and Tribolium castaneum of the essential oil of Murraya exotica aerial parts. Molecules 2010, 15, 5831–5839. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.Z.; Wu, Q.Z.; Xu, H.H.; Xie, C.L.; Wang, R. Insecticidal activity of the extracts from 40 species of plants in Hainan island against Musca domestica Linaeus. Acta Agric. Univ. Jiangxiensis 2011, 3, 476–481. [Google Scholar]
- Sun, Z.H.; Chen, B.; Zhang, S.; Hu, C. Four new eudesmanes from Caragana intermedia and their biological activities. J. Nat. Prod. 2004, 67, 1975–1979. [Google Scholar] [CrossRef] [PubMed]
- Macias, F.A.; Massanet, G.M.; Rodriguezluis, F.; Salva, J. 13C NMR of coumarins. III*-Simple coumarins. Magn. Reson. Chem. 1989, 27, 892–894. [Google Scholar] [CrossRef]
- Ito, C.; Furukawa, H.; Ishii, H.; Ishikawa, T.; Haginiwa, J. The chemical composition of Murraya paniculata. The structure of five new coumarins and one new alkaloid and the stereochemistry of murrangatin and related coumarins. J. Chem. Soc. Perkin Trans. 1 1990, 2047–2055. [Google Scholar] [CrossRef]
- Dondon, R.; Bourgeois, P.; Fery-Forgues, S. A new bicoumarin from the leaves and stems of Triphasia trifolia. Fitoterapia 2006, 77, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.M.; Qi, S.H.; Yin, H.; Xiao, Z.H.; Zhang, S. Micromelosides A-D, four new coumarins from the stem bark of Micromelum falcatum. Magn. Reson. Chem. 2009, 47, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Imai, F.; Kinoshita, T.; Sankawa, U. Constituents of the leaves of Murraya paniculata collected in Taiwan. Chem. Pharm. Bull. 1989, 37, 358–362. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Azizuddin; Khalid, A.; Sultani, S.Z.; Atta-ur-Rahman. A new coumarin from Murraya paniculata. Planta Med. 2002, 68, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Wu, J.B.; Ho, F.C. Prenylcoumarins from Murraya paniculata var. omphalocarpa (Rutaceae): The absolute configuration of sibiricin, mexoticin and omphamurin. Chem. Pharm. Bull. 1996, 44, 1208–1211. [Google Scholar]
- Gupta, R.K.; Krishnamurti, M.; Parthasarathi, J. Synthesis of some recently isolated chalcones, their analogues and corresponding flavanones. Agric. Biol. Chem. 1979, 43, 2603–2605. [Google Scholar]
- Prachayasittikul, S.; Suphapong, S.; Worachartcheewan, A.; Lawung, R.; Ruchirawat, S.; Prachayasittikul, V. Bioactive metabolites from Spilanthes acmella Murr. Molecules 2009, 14, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Kiem, P.V.; Minh, C.V.; Dat, N.T.; Cai, X.F.; Lee, J.J.; Kim, Y.H. Two new phenylpropanoid glycosides from the stem bark of Acanthopanax trifoliatus. Arch. Pharm. Res. 2003, 26, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Andreana, P.R.; McLellan, J.S.; Chen, Y.; Wang, P.G. Synthesis of 2,6-dideoxysugars via ring-closing olefinic. Org. Lett. 2002, 4, 3875–3878. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Nilaya, S.; Muraleedharan, K.M. Highly Chemoselective Esterification Reactions and Boc/THP/TBDMS Discriminating Deprotections under Samarium(III) Catalysis. Org. Lett. 2011, 13, 1932–1935. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.H.; Yang, K.; Guo, S.H.; Liu, Y.P. Studies on chemical constituents from Acropora pulchra. Nat. Prod. Res. Dev. 2003, 15, 109–112. [Google Scholar]
- Yu, H.J.; Chen, C.C.; Shieh, B.J. Two new constituents from the leaves of Magnolia coco. J. Nat. Prod. 1998, 61, 1017–1019. [Google Scholar] [CrossRef] [PubMed]
- Zulema, C.; Mena-Rejón, G.J.; Quintero-Mármol, E.; Jiménez-Díaz, A.; Quijano, L. Two new 24-isopropenyl-lanostanoids from Tillandsia brachycaulos. Z. Naturforsch. C 2003, 58, 649–654. [Google Scholar]
- Fujimatu, E.; Ishikawa, T.; Kitajima, J. Aromatic compound glucosides, alkyl glucoside and glucide from the fruit of anise. Phytochemistry 2003, 63, 609–616. [Google Scholar] [CrossRef]
- Yang, K.; You, C.X.; Wang, C.F.; Guo, S.S.; Li, Y.P.; Wu, Y.; Geng, Z.F.; Deng, Z.W.; Du, S.S. Composition and repellency of the essential oils of Evodia calcicola Chun ex Huang and Evodia trichotoma (Lour.) Pierre against three stored product insects. J. Oleo Sci. 2014, 63, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wen, R.; Zhou, Y.; Zeng, K.; Li, J.; Guo, X.; Tu, P.; Jiang, Y. Nitrogen oxide inhibitory trimeric and dimeric carbazole alkaloids from Murraya tetramera. J. Nat. Prod. 2015, 78, 2432–2439. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.N.; Zhou, Y.; Wen, R.; Shi, M.L.; Zeng, K.W.; Xia, F.; Tu, P.F.; Jiang, Y. Murradiate and murradiol, two structurally unique heterodimers of carbazole-monoterpene and carbazole-phenylethanol from Murraya tetramera. Phytochem. Lett. 2016, 15, 113–115. [Google Scholar] [CrossRef]
- You, C.X.; Yang, K.; Wang, C.F.; Zhang, W.J.; Wang, Y.; Han, J.; Fan, L.; Du, S.S.; Geng, Z.F.; Deng, Z.W. Cytotoxic compounds isolated from Murraya tetramera Huang. Molecules 2014, 19, 13225–13234. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Y.; You, C.X.; Guo, S.S.; Zhang, W.J.; Li, Y.; Geng, Z.F.; Wang, C.F.; Du, S.S.; Deng, Z.W.; Zhang, J. Chemical constituents of the essential oil extracted from Rhododendron thymifolium and their insecticidal activities against Liposcelis bostrychophila or Tribolium castaneum. Ind. Crop. Prod. 2016, 79, 267–273. [Google Scholar] [CrossRef]
- You, C.X.; Zhang, W.J.; Guo, S.S.; Wang, C.F.; Yang, K.; Liang, J.Y.; Wang, Y.; Geng, Z.F.; Du, S.S.; Deng, Z.W. Chemical composition of essential oils extracted from six Murraya species and their repellent activity against Tribolium castaneum. Ind. Crop. Prod. 2015, 76, 681–687. [Google Scholar] [CrossRef]
- Zhang, W.J.; You, C.X.; Yang, K.; Wang, Y.; Su, Y.; Geng, Z.F.; Du, S.S.; Wang, C.F.; Deng, Z.W.; Wang, Y.Y. Bioactivity and chemical constituents of the essential oil from Dendranthema indicum (L.) Des Moul. against two stored insects. J. Oleo Sci. 2015, 64, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, J.L.; Xu, S.; Zhao, N.N.; Zhou, L.; Cheng, J.; Liu, Z.L. Evaluation of repellency of some Chinese medicinal herbs essential oils against Liposcelis bostrychophila (Psocoptera: Liposcelidae) and Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2013, 106, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Zhao, N.N.; Liu, Q.Z.; Liu, Z.L.; Du, S.S.; Zhou, L.G.; Deng, Z.W. Repellent constituents of essential oil of Cymbopogon distans aerial parts against two stored-product insects. J. Agric. Food Chem. 2011, 59, 9910–9915. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool. 1998, 33, 339–347. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Treatment | PR% (Mean ± SE) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (µg/cm2) | ||||||||||
| 2 h | 4 h | |||||||||
| 78.63 | 15.73 | 3.15 | 0.63 | 0.13 | 78.63 | 15.73 | 3.15 | 0.63 | 0.13 | |
| Compound 1 | 96 ± 4 ab ** | 82 ± 7 bc | 76 ± 9 ab | 80 ± 6 a | 70 ± 8 a | 94 ± 7 a | 76 ± 9 abc | 74 ± 3 ab | 70 ± 8 a | 60 ± 8 a |
| Compound 3 | 88 ± 7 b | 64 ± 9 cd | −92 ± 9 f | −84 ± 12 e | −72 ± 12 f | 80 ± 10 ab | 56 ± 7 cd | −74 ± 9 e | −52 ± 4 d | −66 ± 7 f |
| Compound 4 | 56 ± 7 c | 50 ± 6 de | 52 ± 7 bcd | 46 ± 9 b | 40 ± 8 bc | 48 ± 9 c | 58 ± 7 bcd | 56 ± 9 ab | 52 ± 7 ab | 48 ± 9 ab |
| Compound 5 | 54 ± 4 c | 50 ± 6 de | 64 ± 9 abc | 58 ± 7 ab | 30 ± 6 c | 56 ± 9 bc | 46 ± 4 d | 62 ± 7 ab | 64 ± 9 a | 40 ± 6 abcd |
| Compound 6 | −30 ± 8 d | −34 ± 9 f | −20 ± 10 e | −26 ± 9 d | 22 ± 7 cd | −42 ± 7 c | −58 ± 9 bcd | −16 ± 7 d | −20 ± 8 c | 20 ± 6 d |
| Compound 10 | 46 ± 9 c | 40 ± 8 de | 34 ± 7 cd | 58 ± 7 ab | 64 ± 7 ab | 42 ± 7 c | 50 ± 8 d | 26 ± 4 c | 32 ± 7 b | 30 ± 8 bcd |
| Compound 11 | 88 ± 7 b | 82 ± 9 bc | 66 ± 7 abc | 62 ± 7 ab | 38 ± 9 bc | 92 ± 7 a | 74 ± 9 abc | 64 ± 9 ab | 58 ± 7 a | 46 ± 7 abc |
| Compound 14 | 32 ± 7 c | 30 ± 6 e | 22 ± 4 d | 16 ± 9 c | 18 ± 7 cd | 46 ± 9 c | 16 ± 4 e | 14 ± 7 c | −18 ± 7 c | −10 ± 8 e |
| Compound 15 | 50 ± 6 c | 46 ± 9 de | 42 ± 9 cd | −26 ± 9 d | −16 ± 9 e | 54 ± 7 bc | 48 ± 7 d | 52 ± 7 b | −30 ± 8 c | −10 ± 6 e |
| Compound 16 | 86 ± 7 b | 90 ± 6 ab | 62 ± 7 abc | 58 ± 7 ab | 24 ± 7 cd | 86 ± 9 a | 80 ± 6 ab | 76 ± 7 a | 70 ± 6 a | 22 ± 7 cd |
| DEET * | 100 ± 0 a | 98 ± 3 a | 78 ± 14 a | 66 ± 10 ab | 8 ± 5 d | 96 ± 3 a | 82 ± 8 a | 68 ± 5 ab | 54 ± 8 a | 22 ± 8 cd |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, C.-X.; Guo, S.-S.; Zhang, W.-J.; Geng, Z.-F.; Liang, J.-Y.; Lei, N.; Du, S.-S.; Deng, Z.-W. Chemical Constituents of Murraya tetramera Huang and Their Repellent Activity against Tribolium castaneum. Molecules 2017, 22, 1379. https://doi.org/10.3390/molecules22081379
You C-X, Guo S-S, Zhang W-J, Geng Z-F, Liang J-Y, Lei N, Du S-S, Deng Z-W. Chemical Constituents of Murraya tetramera Huang and Their Repellent Activity against Tribolium castaneum. Molecules. 2017; 22(8):1379. https://doi.org/10.3390/molecules22081379
Chicago/Turabian StyleYou, Chun-Xue, Shan-Shan Guo, Wen-Juan Zhang, Zhu-Feng Geng, Jun-Yu Liang, Ning Lei, Shu-Shan Du, and Zhi-Wei Deng. 2017. "Chemical Constituents of Murraya tetramera Huang and Their Repellent Activity against Tribolium castaneum" Molecules 22, no. 8: 1379. https://doi.org/10.3390/molecules22081379
APA StyleYou, C.-X., Guo, S.-S., Zhang, W.-J., Geng, Z.-F., Liang, J.-Y., Lei, N., Du, S.-S., & Deng, Z.-W. (2017). Chemical Constituents of Murraya tetramera Huang and Their Repellent Activity against Tribolium castaneum. Molecules, 22(8), 1379. https://doi.org/10.3390/molecules22081379





