Abstract
In our continued search for natural compounds with activity against Trypanosoma brucei, causative agent of human African trypanosomiasis (HAT, “sleeping sickness”), we have investigated extracts from the leaves and bark of the West African Holarrhena africana (syn. Holarrhena floribunda; Apocynaceae). The extracts and their alkaloid-enriched fractions displayed promising in vitro activity against bloodstream forms of T. brucei rhodesiense (Tbr; East African HAT). Bioactivity-guided chromatographic fractionation of the alkaloid-rich fractions resulted in the isolation of 17 steroid alkaloids, one nitrogen-free steroid and one alkaloid-like non-steroid. Impressive activities (IC50 in µM) against Tbr were recorded for 3β-holaphyllamine (0.40 ± 0.28), 3α-holaphyllamine (0.37 ± 0.16), 3β-dihydroholaphyllamine (0.67 ± 0.03), N-methylholaphyllamine (0.08 ± 0.01), conessimine (0.17 ± 0.08), conessine (0.42 ± 0.09), isoconessimine (0.17 ± 0.11) and holarrhesine (0.12 ± 0.08) with selectivity indices ranging from 13 to 302. Based on comparison of the structures of this congeneric series of steroid alkaloids and their activities, structure-activity relationships (SARs) could be established. It was found that a basic amino group at position C-3 of the pregnane or pregn-5-ene steroid nucleus is required for a significant anti-trypanosomal activity. The mono-methylated amino group at C-3 represents an optimum for activity. ∆5,6 unsaturation slightly increased the activity while hydrolysis of C-12β ester derivatives led to a loss of activity. An additional amino group at C-20 engaged in a pyrrolidine ring closed towards C-18 significantly increased the selectivity index of the compounds. Our findings provide useful empirical data for further development of steroid alkaloids as a novel class of anti-trypanosomal compounds which represent a promising starting point towards new drugs to combat human African trypanosomiasis.
1. Introduction
Human African trypanosomiasis (HAT) is a vector-borne parasitic disease caused by the kinetoplastid parasites, Trypanosoma brucei rhodesiense (Tbr) and Trypanosoma brucei gambiense (Tbg). The disease ranks 11th, 14th and 4th in the list of neglected tropical diseases (NTDs) for which disability-adjusted life years (DALYs), years of life lost (YLLs) due to mortality and years lived with disability (YLDs) were respectively estimated [1]. Several policy initiatives for prevention and eradication have been developed and funded by World Health Organization WHO and other international organizations [2]. These interventions have been hampered by poor access to available medications, adverse effects associated with some of the medications and incidences of resistant strains of trypanosomes. Moreover, policies geared towards control and eradication of HAT are usually impeded by incomplete and fragmented data on HAT incidence, morbidity and mortality [3] and social stigmatization of the victims in some parts of Sub-Saharan Africa [4]. In view of these obstacles, the search has been on-going to explore additional or alternative remedies from natural products with potential to eradicate HAT. Incidentally, a great number of alkaloids with anti-protozoal activity have been reported and are being explored for antitrypanosomal activity [5,6,7,8,9,10,11,12,13,14,15].
Holarrhena africana A. DC. (a synonym of H. floribunda (G. Don) T. Durand & Schinz) is a tropical tree well distributed in Nigeria and other West African countries. It belongs to the Apocynaceae family which is known to be abundant in alkaloids of various structural classes [16]. Traditionally, the leaves are used in Nigeria to treat convulsion, fever and malaria while the stem bark serves as an antimicrobial, a febrifuge and an antidote for snake venom [17]. Antibacterial and antimalarial [18,19] activities of the chemical constituents of H. africana have also been reported. A crude aqueous extract of the plant has previously been reported to show interesting preliminary in vitro anti-trypanosomal activity against Tbr. A chemically uncharacterized fraction of the aqueous extract has also shown in vivo activity against T. brucei brucei [20]. Consequently, we investigated the crude extract and its alkaloid-enriched fraction for anti-trypanosomal activity. Since the alkaloid fraction showed very strong activity, we set out to systematically isolate the alkaloids from H. africana and to test them for anti-trypanosomal activity as well as for cytotoxic activity against mammalian cells in order to assess their selectivity and structure-activity relationships (SARs).
2. Results and Discussion
2.1. Anti-Trypanosomal Activity of Crude Extracts and Alkaloid-Enriched Fractions of Holarrhena africana Leaves and Stem Bark
The crude extracts of the fresh leaves and the dried stem bark of H. africana were tested for in vitro activity against Trypanosoma brucei rhodesiense (Tbr), Trypanosoma cruzi (Tcr), Leishmania donovani (Ldon) and Plasmodium falciparum (Pf) and for cytotoxicity against L6 rat skeletal myoblasts. Since it turned out that activity was selectively observed against Tbr, the alkaloidal fraction and column chromatographic fractions were not tested against the other parasites. The results (Table 1) showed that the alkaloid-enriched fraction from the leaf extract had higher activity compared to the crude extract and the polar neutral aqueous fraction (Table 1). These findings are in line with an earlier report [20] of an uncharacterized fraction of H. africana leaves with anti-trypanosomal activity, even though the activity reported then was not attributed to the alkaloid content of the plant or to any other chemical characteristic. A similar trend was also observed in the activities of the stem bark extract and its alkaloid fraction. Consequently, both alkaloid fractions were considered potential sources of alkaloids with anti-trypanosomal activity and hence chosen for bioactivity-guided fractionation and isolation of active compounds.

Table 1.
In vitro anti-trypanosomal and cytotoxic activity of crude extracts, alkaloid fraction and CC fractions of H. africana leaves and stem bark.
2.2. Bio-Activity Guided Fractionation and Isolation of Steroid Alkaloids
The alkaloidal fraction of the leaves was fractionated into 16 fractions by column chromatography on silica gel 60. Similarly, the alkaloidal fraction of the stem bark was chromatographed on silica gel column to yield 20 major fractions. All the fractions were analyzed on TLC for the presence or absence of alkaloids using Dragendoff reagent. Positive Dragendoff reaction was obtained in four and two fractions of the leaves and stem bark respectively. The six fractions were subjected to in vitro assay against Tbr and higher activities were recorded for three fractions of the leaves and both fractions of the stem bark (Table 1). Further analysis of the fractions by UHPLC/+ESI QTOF MS/MS was carried out and the major alkaloids were partially dereplicated and marked for eventual isolation. Following repeated chromatographic separation and purification, six (1–6) and eleven (7–17) major steroid alkaloids were isolated from the leaves and stem bark respectively, in addition to one nitrogen-free steroid (18) and a non-steroid alkaloid-like compound (19) from the leaves (Figure 1). The compounds were identified as 3β-holaphyllamine (1), holaphyllamine acetamide (2), 3β-N-methylholaphyllamine (3), 3α-holaphyllamine (4), 3β-dihydroholaphyllamine (5), 3α-dihydroholaphyllamine (6), holadienine (7), holonamine (8), cona-4,6-dienin-3-one (9), cona-3,5-dienin-7-one (10), conessimine (11), isoconessimine (12), conessine (13), holarrhesine (14), holarrhetine (15), holarrheline (16), holarrhenine (17), kurchinin (18) and N3-isopentenyl adenine (19) by comparison of their 1D and 2D NMR data and their exact masses from UHPLC/+ESI QTOF MS/MS analysis, which were in agreement with previously reported data [21,22,23,24,25,26,27,28,29,30,31].
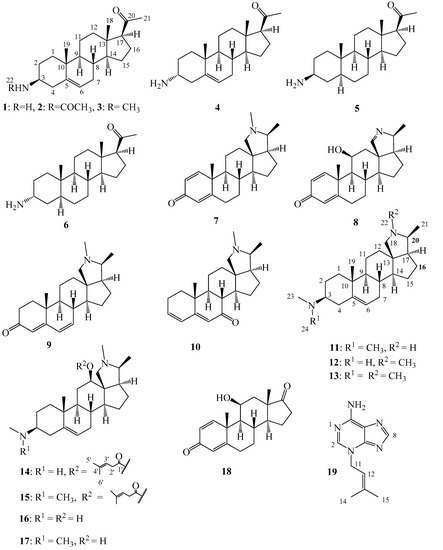
Figure 1.
Compounds isolated from the leaves (1–6, 19) and stem bark (7–18) of Holarrhena africana.
2.3. In Vitro Anti-Trypanosomal Activity of Isolated Compounds
All the isolated compounds were tested for in vitro anti-trypanosomal activity against Tbr and for cytotoxicity against L6 rat skeletal myoblasts. Impressive anti-trypanosomal activities (IC50, Tbr < 1.0 µM) were recorded for compounds 1, 3–5, 11–14. Compounds 2, 6 and 15 showed moderate activities (IC50, 1–5 µM) while compounds 7–10, 16–19 were of low activity with IC50 > 7 µM (Table 2). Interestingly, four steroid alkaloids, 11–14 showed very high activity in addition to SI > 100.

Table 2.
In vitro anti-trypanosomal activity and cytotoxicity of isolated alkaloids.
2.4. Structure-Activity and -Selectivity Relationships
A comparison of all isolated and tested steroidal compounds revealed some structure-activity relationships, as represented in Figure 2.
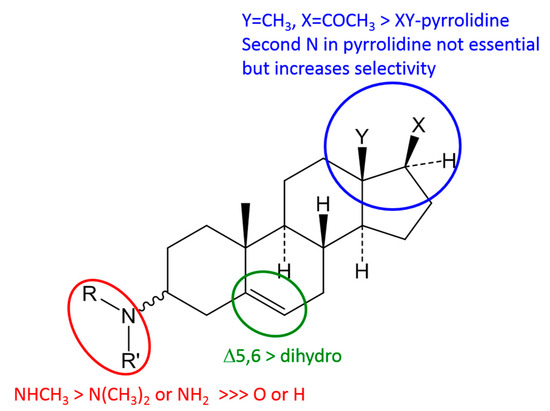
Figure 2.
Proposed basic structure-activity relationships for anti-trypanosomal activity. >sign indicates higher activity.
The major structural requirement for a strong anti-trypanosomal activity against Tbr (IC50 < 1 µM) is a basic amino group at C-3 of the steroid nucleus. Compounds without an amino group in this position such as 7–10 and 18 and also the acetamide 2 are considerably less active (IC50 between 4.8 and 14.9 µM). With respect to substitution of the 3-amino group, monomethylation appears to represent an optimum as observed when comparing monomethylated with unsubstituted (3 more active than 1), and monomethylated with dimethylated congeners (12, 14, 16 more active than 13, 15 and 17, respectively). Comparing the ∆5,6-pregnene derivatives holaphyllamine (1) and its 3α-amino analogue (4) with their two pregnane congeners 5 and 6, respectively, shows that the double bond causes a slight increase of activity. Even though 5 with a 3β-amino group is slightly more potent than the α-configured 6, the effect of stereochemistry at the 3-position appears to be small as indicated by comparison of 1 with 4 which are almost equipotent.
It is very interesting to note that an amino group as part of a pyrrolidine or pyrroline ring connecting C-20 with C-18, as present in compounds 7–17, on its own does not confer high activity (see low activity of 7–10) but rather appears to lead to a slight decrease in antitrypanosomal potency if it occurs together with the 3-amino group (compare 12 with 3). However, when combined with the C-3 amino function as in 11–17, this structural element leads to a marked decrease of cytotoxicity and increase in selectivity (e.g., SI = 33 in case of 3 and 168 for 12). This effect is strongest in compound 11 which is not methylated at the pyrrolidine amino group and shows a very favourable SI > 300.
Finally, the introduction of a lipophilic acyloxy substituent at C-12 as in 14 and 15 does not lead to dramatic effects on activity in comparison with the compounds unsubstituted at this position (12 and 13, respectively). However, deacylation of such esters leading to a free OH group at C-12 renders compounds 16 and 17 significantly less active.
3. Materials and Methods
3.1. General Experimental Procedures
Analytical and preparative TLC were performed on pre-coated, silica gel 60 F254 (Merck Chemicals GmbH, Darmstadt, Germany) with various solvent systems consisting of n-hexane, ethyl acetate and methanol saturated with aqueous ammonia as the mobile phase. The plates were visualized under UV-light at 254/360 nm and then sprayed with Dragendoff’s reagent.
3.2. Plant Material
The leaves and stem bark of Holarrhena africana were collected in March 2015 and April 2016 respectively from a forest reserve in Nsukka (6°51′28′′ N, 7°23′44′′ E), Nigeria. The identities were confirmed by comparison with a voucher specimen (No. 1342003) stored in the herbarium of the Department of Pharmacognosy and Environmental Medicines, University of Nigeria Nsukka. A voucher specimen of this present collection was deposited at the herbarium of the Institute of Pharmaceutical Biology and Phytochemistry, University of Münster (voucher No. TS-HA-01). The stem bark was air dried at room temperature and ground to 1 mm mesh size. The fresh leaves were washed with distilled water and allowed to drain completely.
3.3. Extraction and Preparation of Alkaloid-Enriched Fractions
Fresh leaves: A 2 kg fresh weight of the leaves was extracted as previously described [20]. The extract was filtered and lyophilized for 18 h to dry weight of 28.6 g. The crude extract was re-dissolved in 10% v/v MeOH/distilled water (in portions of 1.0 g to 50 mL) and basified with 1% v/v NH4OH to pH 12. The mixture was partitioned five times successively with CH2Cl2 (in portions of 1:1 ratio) in a separatory funnel. The combined lower phase was evaporated completely to yield 8.4 g (28.6% of crude extract) of alkaloid-enriched (AFL) fraction, 5.2 g of precipitate and 14.7 g polar aqueous fraction.
Stem bark: The dry powdered stem bark (500 g, in portions of 5 × 100 g) was extracted exhaustively for 12 h in a Soxhlet apparatus with a total of 4.0 L dichloromethane. The solvent was evaporated in vacuo at 40 °C. The extract yield was 58.6 g. The resulting crude extract (50 g) was re-dissolved in several portions of 10% v/v MeOH/water in a ratio of 50 mL/1 g extract, basified as described above and subsequently partitioned successively with CH2Cl2 in a separatory funnel to yield 14.5 g of alkaloid-enriched fraction (AFS), 8.1 g of precipitate, and 26.6 g polar aqueous fraction.
The crude extracts and alkaloid-enriched fractions were tested for anti-trypanosomal activity and cytotoxicity (see Table 1).
3.4. Isolation of Steroid Alkaloids
3.4.1. Isolation of Alkaloids from the Leaf Extract
The major part (8 g) of the AFL was subjected to column chromatographic (CC) separation on 560 g silica gel (E. Merck, type-60, 70–230 mesh) and eluted with gradients of (50% n-hexane to 100% EtOAc) n-hexane–EtOAc mixture v/v, each gradient saturated with NH4OH (25% v/v). The mobile phase solvent was constituted as follows: 2 L each of 1:1, 3:7 and 1:9, followed by 6 L EtOAc and finally EtOAc:MeOH (9.9:0.1). The flow rate was maintained at 1 mL/min. The eluates were analyzed at pre-determined intervals and based on their TLC profiles and positive reaction to Dragendoff’s spraying reagent, representative tubes (127–305) were collected and pooled to yield four alkaloid-containing sub-fractions, HA11 (1250 mg), HA12 (750 mg), HA13 (1680 mg) and HA14 (480 mg). These sub-fractions were tested for anti-trypanosomal activity (Table 1). Compound 19 (25.8 mg) was obtained as pure crystal by repeatedly washing sub-fraction HA11 with n-hexane. HA12 (700 mg) was re-chromatographed on silica gel 60 and eluted isocratically with NH4OH saturated-EtOAc and at flow rate of 0.5 mL/min. Compound 2 (10.0 mg) crystallized as white flaky substance while compound 1 (6.8 mg) was isolated after further purification on sephadex LH20 gel eluted with MeOH:H2O, 9:1. HA13 (1600 mg) was further separated by column chromatography on 80 g basic alumina with EtOAc:MeOH (9:1) as mobile phase to yield three sub-fractions (HA13a, HA13b, and HA13c). Compound 3 (52 mg) was isolated as a sticky white creamy solid from sub-fraction HA13b (220 mg) by repeatedly washing with n-hexane:EtOAc (9:1). HA13a (120 mg) was further chromatographed on a pre-coated preparative TLC plates (GF-254, 20 × 20 cm, E. Merck, 0.20 mm thickness, Darmstadt, Germany) developed in pre-equilibrated TLC tank containing EtOAc:MeOH (9.5:0.5) and saturated with NH4OH. The plates were scrapped according to bands identified after spraying a 2 × 20 cm cut-off segment of the plate with Dragendoff reagent, re-extracted and washed with EtOAc to furnish compounds 4 (2.5 mg; hRf 36) and 6 (3.8 mg; hRf 23). HA14 and HA13c (80 mg) were combined and further separated by CC on Sephadex LH-20 using MeOH:H2O (8:2) as eluent. The alkaloid-positive eluates were pooled and further purified on preparative TLC (silica gel 60, in NH4OH-saturated EtOAc) to furnish compound 5 (4.1 mg, hRf 22) as a creamy solid.
3.4.2. Isolation of Alkaloids from the Stem Bark Extract
Similarly, a major portion (12.0 g) of AFS was subjected to CC on 900 g silica gel 60. The column was eluted with mobile phase solvents of increasing polarity, as described above. The eluates were analyzed by TLC and, based on a positive reaction to Dragendoff reagent, it was observed that most of the alkaloidal bands were overlapping. Therefore, alkaloid positive eluates were pooled in two fractions, HF8 (eluates 86–232; 3.85 g) and HF9 (eluates 233–480; 5.33 g). These fractions were also tested for anti-trypanosomal activity (Table 1). HF8 (3.8 g) was re-chromatographed over 270 g silica gel 60. The column was eluted with 5.0 L of EtOAc (100%) saturated with NH4OH at a flow rate of 0.8 mL/min. 205 eluates were collected and representative fractions pooled into five sub-fractions (HF8a-HF8e) based on the positive reaction to the Dragendoff reagent. HF8a (80 mg) was purified on basic alumina with EtOAc:MeOH (9.5:0.5) as eluent to yield compound 10 (9.6 mg). HF8b (108 mg) and HF8c (228 mg) were combined and re-chromatographed on silica gel 60 and eluted with n-hexane:EtOAc (2:8) saturated with NH4OH to yield compounds 9 and 7 as a mixture. The mixture was separated on pre-coated preparative TLC silica plates in EtOAc (100%) saturated with NH4OH to yield 5.6 mg of 9 (hRf 65) and 4.2 mg of 7 (hRf 60) respectively. HF8d (210 mg) was chromatographed on silica gel 60 and eluted subsequently with EtOAc (100%) to yield crystals of 18 (3.8 mg) and then with EtOAc–NH4OH to yield 8 (7.9 mg) as white solid. HF8e (675 mg) was further separated on silica gel 60 (50 g) with EtOAc (100%) saturated with NH4OH, flow rate 0.5 mL/min to yield two sticky sub-fractions which were further purified on pre-coated preparative TLC with NH4OH-saturated EtOAc as mobile phase to furnish 15 (16.7 mg, hRf 55) and 14 (15.6 mg; hRf 48). HF9 (5.0 g) was re-chromatographed on silica gel 60 (300 g) and eluted with 2.5 L EtOAc (100%) followed by 3.0 L EtOAc:MeOH (9.8:0.2) both saturated with NH4OH to yield three sub-fractions, HF9a (860 mg), HF9b (510 mg) and HF9c (1050 mg). HF9a yielded further 14 and 15 in a mixture. HF9b was purified by CC on basic alumina with EtOAc:MeOH (8:2) as mobile phase to yield 13 (3.8 mg) and 12 (4.1 mg). HF9c yielded 17 (8.8 mg; hRf 58), 11 (10.8 mg; hRf 50) and 16 (5.2 mg; hRf 39) after further separation by CC on silica with EtOAc:MeOH (9:1) satutated with NH4OH as mobile phase and subsequent purification by preparative TLC (silica 60, EtOAc:MeOH (8:2) saturated with NH4OH as mobile phase).
3.4.3. Preparation of Compounds 16 and 17 by Alkaline Hydrolysis of the Esters 14 and 15
To increase the yields and further confirm 16 and 17, a simple, efficient and reliable ester hydrolysis, without racemization or other undesirable side reaction protocol [32] was adopted. 10 mg each of compound 14 and 15 was respectively hydrolyzed in CH2Cl2 using 0.5 N NaOH (dissolved in MeOH) at room temperature for 2 h; CH2Cl2:MeOH (9:1). The reaction mixtures were stirred vigorously and monitored by TLC until completion and purified by preparative TLC as previously described. The yield in each case was >70%.
3.5. Structural Characterization of Isolated Compounds
NMR spectra (1H, 13C, 1H/1H COSY, 1H/1H NOESY, 1H/13C HSQC, and 1H/13C HMBC) were recorded on Agilent DD2 400 or 600 MHz spectrometers at 25 °C in CDCl3 or CD3OD. Spectra were respectively referenced to the CDCl3 solvent signals of 1H; 7.260 ppm and 13C; 77.000 ppm or CD3OD solvent signals of 1H; 3.310 ppm and 13C; 49.000 ppm and processed with MestRENOVA v. 11 (Mestrelab Research, Chemistry Software Solutions, Santiago de Compostela, Spain) software.
3.6. Spectral Data of Isolated Compounds
3β-Holaphyllamine 1: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.5:0.5-saturated aq. NH3), 40.0; 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 5.32 (1H, dt, 5.3, 1.9, H-6), 2.60 (1H, tt, 4.1, 11.5, H-3), 2.53 (1H, t, 9.2, H-17), 2.17 (1H, m, H-16β), 2.15 (1H, m, H-4β), 2.12 (3H, s, H-21), 2.06 (1H, m, H-12α), 2.05 (1H, m, H-4α), 1.98 (1H, dq, 2.7, 5.2, H-7α), 1.85 (1H, dt, 12.7, 3.2, H-1β), 1.83 (1H, dt, 12.7, 3.2, H-1α), 1.72 (1H, m, H-2β), 1.70 (1H, m, H-2α), 1.69 (1H, m, H-15β), 1.66 (1H, m, H-16α), 1.62 (1H, m, H-11α), 1.57 (1H, m, H-7β), 1.47 (1H, m, H-8), 1.45 (1H, m, H-11β), 1.45 (1H, m, H-12β), 1.23 (1H, m, H-15α), 1.16 (1H, m, H-14), 1.0 (1H, m, H-9), 0.99 (3H, s, H-19), 0.63 (3H, s, H-18). MS (m/z): 316.2845 [M + H]+; calculated for C21H34NO+: 316.2640.
3β-Holaphyllamine acetamide 2: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.5:0.5-saturated aq. NH3), 74.0; 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 5.36 (1H, dt, 5.0, 2.2, H-6), 3.70 (1H, tdd, 14.8, 8.2, 4.5, H-3), 2.54 (1H, t, 8.9, H-17), 2.31 (2H, ddd, 13.2, 4.7, 2.2, H-4), 2.16 (1H, m, H-15α), 2.11 (3H, s, H-21), 2.05 (1H, m, H-12α), 1.98 (1H, m, H-7α), 1.95 (3H, s, H-23), 1.86 (1H, m, H-2α), 1.85 (1H, m, H-1α), 1.68 (1H, m, H-16α), 1.65 (1H, m, H-15β), 1.61 (1H, m, H-11α), 1.58 (1H, m, H-7β), 1.46 (1H, m, H-8), 1.45 (1H, m, H-12β), 1.45 (1H, m, H-11β), 1.35 (1H, m, H-2β), 1.20 (1H, m, H-16β), 1.17 (1H, m, H-1β), 1.15 (1H, m, H-14), 1.01 (1H, m, H-9), 0.98 (3H, s, H-19), 0.62 (3H, s, H-18). MS (m/z): 358.3000 [M + H]+; calculated for C23H36NO2+: 358.2746.
3β-N-Methylholaphyllamine 3: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.5:0.5-saturated aq. NH3), 54.0; 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 5.40 (1H, dt, 5.5, 2.0, H-6), 2.76 (1H, tt, 4.1, 11.7, H-3), 2.62 (3H, s, H-22), 2.54 (1H, dd, 13.3, 2.3, H-4α), 2.51 (1H, dd, 2.4, 5.0, H-17), 2.47 (1H, ddd, 13.4, 4.4, 2.3, H-4β), 2.16 (1H, m, H-15α), 2.11 (3H, s, H-21), 2.04, (1H, m, H-2α), 2.03 (1H, m, H-12α), 1.99 (1H, m, H-7α), 1.92 (1H, m, H-1α), 1.82, qd, 12.8, 3.6, H-2β), 1.66 (1H, m, H-16α), 1.64 (1H, m, H-15β), 1.57 (1H, m, H-7β), 1.57 (1H, m, H-11α), 1.46 (1H, m, H-8), 1.44 (1H, m, H-12β), 1.43 (1H, m, H-11β), 1.20 (1H, m, H-16β), 1.14 (1H, m, H-14), 1.08 (1H, m, H-1β), 1.01 (3H, s, H-19), 0.98 (1H, m, H-9), 0.60 (3H, s, H-18). MS (m/z): 330.2819 [M + H]+; calculated for C22H36NO+: 330.2797.
3α-Holaphyllamine 4: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.5:0.5-saturated aq. NH3), 36.0; 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 5.33 (1H, dt, 5.3, 2.2, H-6), 3.16 (1H, br. s, H-3), 2.56 (1H, m, H-4β), 2.51 (1H, t, 9.0, H-17), 2.15 (1H, m, H-16β), 2.10 (3H, s, H-21), 2.02 (1H, m, H-12β), 1.96 (1H, m, H-7α), 1.88 (1H, dt, 14.9, 2.7, H-4α), 1.78 (1H, dt, 14.1, 3.7, H-2β), 1.65 (1H, m, H-15β), 1.62 (1H, m, H-16α), 1.60 (1H, m, H-11β), 1.60 (1H, m, H-1α), 1.58 (1H, m, H-7β), 1.48 (1H, m, H-2α), 1.44 (1H, m, H-8), 1.42 (1H, m, H-11α), 1.42 (1H, m, H-12α), 1.36 (1H, m, H-1β), 1.18 (1H, m, H-15α), 1.13 (1H, m, H-14), 1.07 (1H, m, H-9), 0.97 (3H, s, H-19), 0.60 (3H, s, H-18). MS (m/z): 316.2827 [M + H]+; calculated for C21H34NO+: 316.2640.
3β-Dihydroholaphyllamine 5: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.5:0.5-saturated aq. NH3), 42.0; 1H-NMR (600 MHz CD3OD; δ (ppm), intensity, mult., J (Hz)): 3.05 (1H, tt, 11.9, 4.5, H-3), 2.62 (1H, t, 9.1, H-17), 2.12 (1H, m, H-16α), 2.10 (3H, s, H-21), 2.08, m, H-12α), 2.03 (1H, dt, 12.1, 3.4, H-12β), 1.86 (1H, m, H-2β), 1.84 (1H, m, H-2α), 1.82 (1H, m, H-1α), 1.74 (1H, m, H-16β), 1.60 (1H, m, H-4α), 1.42 (2H, m, H-5), 1.40 (1H, m, H-4β), 1.36 (1H, m, H-11β), 1.33 (1H, m, H-11α), 1.32 (1H, m, H-6α), 1.30 (1H, m, H-6β), 1.22 (1H, m, H-8), 1.21 (1H, m, H-14), 1.20 (2H, m, H-15), 1.08 (1H, m, H-1β), 0.97 (2H, m, H-7), 0.86 (3H, s, H-19), 0.78 (1H, ddd, 12.3, 10.7, 4.1, H-9), 0.60 (3H, s, H-18). MS (m/z): 318.2809 [M + H]+; calculated for C21H36NO+: 318.2797.
3α-Dihydroholaphyllamine 6: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.5:0.5-saturated aq. NH3), 23.0; 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 3.21 (1H, br s, H-3), 2.52 (1H, t, 9.0, H-17), 2.14 (1H, m, H-16β), 2.11 (3H, s, H-21), 2.01 (1H, dt, 12.2, 3.4, H-12β), 1.74 (1H, m, H-2α), 1.68 (1H, m, H-1α), 1.66 (1H, m, H-15α), 1.64 (1H, m, H-16α), 1.62 (1H, m, H-11α), 1.55 (1H, dd, 3.8, 13.5, H-4α), 1.48 (1H, m, H-5), 1.46 (1H, m, H-6α), 1.45 (1H, m, H-7α), 1.44 (1H, m, H-6β), 1.39 (1H, m, H-12α), 1.36 (1H, m, H-8), 1.28 (1H, m, H-7β), 1.26 (1H, m, H-11β), 1.21 (1H, m, H-4β), 1.20 (1H, m, H-2β), 1.17 (1H, m, H-15β), 1.16 (1H, m, H-14), 0.95 (1H, m, H-1β), 0.80 (1H, td, 3.2, 5.4, H-9), 0.78 (3H, s, H-19), 0.60 (3H, s, H-18). MS (m/z): 318.2788 [M + H]+; calculated for C21H36NO+: 318.2797.
Holadienine 7: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.9:0.1-saturated aq. NH3), 81.0; 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 7.04 (1H, d, 10.1, H-1), 6.22 (1H, dd, 10.1, 1.9, H-2), 6.07 (1H, t, 1.6, H-4), 3.09 (1H, br s, H-18α), 2.46 (1H, ddd, 13.4, 5.1, 1.5, H-6α), 2.45 (1H, dd, 5.1, 1.4, H-17), 2.32 (1H, ddd, 13.3, 4.2, 2.6, H-6β), 2.25 (3H, s, H-22), 2.04 (1H, m, H-7α), 1.95 (1H, br s, H-18β), 1.85 (1H, m, H-20), 1.83 (1H, m, H-11α), 1.83 (1H, m, H-12α), 1.76 (1H, dd, H-16α), 1.64 (2H, qd, H-15), 1.50 (1H, qd, 10.9, 3.8, H-8), 1.43 (1H, dd, H-16β), 1.35 (1H, m, H-12β), 1.35 (1H, m, H-11β), 1.18 (3H, s, H-19), 1.12 (1H, m, H-7β), 1.12 (1H, m, H-14), 1.08, 3H, d, H-21), 1.07 (1H, m, H-9). MS (m/z): 326.3834 [M + H]+; calculated for C22H32NO+: 326.2484.
Holonamine 8: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.9:0.1-saturated aq. NH3), 74.0; 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 7.86 (1H, d, 10.3, H-4), 7.48 (1H, d, 3.0, H-18), 6.15 (1H, dd, 10.3, 2.0, H-2), 6.10 (1H, t, 1.6, H-1), 4.05–4.10 (2H, m, H-11, H-17),), 2.51 (1H, tdd, 13.5, 5.1, 1.5, H-6α), 2.39 (1H, ddd, 13.2, 4.4, 2.5, H-6β), 2.19 (1H, dd, 12.2, 4.8, H-12α), 2.10 (1H, m, H-20), 2.06 (1H, m, H-7α), 1.91 (1H, qd, 11.4, 3.8, H-8), 1.78 (1H, ddd, 14.0, 8.2, 2.8, H-15α), 1.68 (1H, m, H-16α), 1.63 (1H, dd, 12.2, 4.8, H-12β), 1.45 (1H, ddd, 14.2, 8.5, 3.0, H-15β), 1.38 (1H, td, 11.5, 5.3, H-14), 1.36 (3H, s, H-19), 1.35 (3H, d, H-21), 1.32 (1H, m, H-9), 1.15 (1H, ddd, 12.8, 5.0, 3.8, H-7β), 0,83 (1H, m, H-16β). MS (m/z): 326.2000 [M + H]+; calculated for C21H28NO2+: 326.2120.
Cona-4,6-dienin-3-one 9: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.9:0.1-saturated aq. NH3), 84.0; 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 6.13 (1H, m, H-6), 6.02 (1H, m, H-7), 5.64 (1H, s, H-4), 2.96 (1H, d, 10.4, H-18β), 2.33 (1H, m, H-20), 2.32 (2H, m, H-2), 2.19 (1H, m, H-1α), 2.16 (3H, s, H-22), 1.86 (1H, m, H-1β), 1.85 (1H, d, H-18α), 1.78 (1H, dt, 10.7, 3.7, H-17), 1.74 (1H, dt, 12.2, 3.4, H-12α), 1.64 (2H, ddd, 17.1, 10.3, 4.2, H-15), 1.58 (1H, m, H-16α), 1.57 (1H, m, H-11α), 1.35 (1H, dt, 10.8, 3.0, H-8), 1.28 (1H, td, 12.8, 3.8, H-12β), 1.14 (1H, dq, 11.0, 2.6, H-16β), 1.08 (1H, m, H-14), 1.04 (1H, m, H-11β), 1.05 (3H, s, H-19), 0.98 (3H, d, 6.4, H-21), 0.87 (1H, ddd, 12.3, 10.5, 3.6, H-9). MS (m/z): 326.3869 [M + H]+; calculated for C22H32NO+: 326.2484.
Cona-3,5-dienin-7-one 10: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.9:0.1-saturated aq. NH3), 85.0; 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 6.14 (1H, ddd, 9.5, 5.4, 2.6, H-3), 6.06 (1H, dd, 9.7, 2.4, H-4), 5.58 (1H, s, H-6), 2.95 (1H, d, 10.3, H-18α), 2.47 (1H, ddd, 13.0, 6.5, 4.1, H-16α), 2.38 (1H, dd, 6.5, 4.6, H-20), 2.25 (2H, m, H-2), 2.22 (1H, m, H-8), 2.18 (3H, s, H-22), 1.86 (1H, m, H-1β), 1.85 (1H, m, H-18β), 1.78 (1H, m, H-12β), 1.74 (1H, m, H-17), 1.72 (1H, m, H-11β), 1.70 (1H, m, H-15β), 1.61 (1H, m, H-9), 1.42 (1H, m, H-14), 1.41 (1H, m, H-15α), 1.39 (1H, m, H-16β), 1.31 (1H, m, H-1α), 1.30 (1H, m, H-12α), 1.23 (1H, m, H-11α), 1.03 (3H, s, H-19), 1.02 (3H, d, 6.3, H-21). MS (m/z): 326.3871 [M + H]+; calculated for C22H32NO+: 326.2484.
Conessimine 11: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.9:0.1-saturated aq. NH3), 47.0; 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 5.35 (1H, dt, 5.1, 1.8, H-6), 3.19 (1H, qd, 6.5, 4.7, H-20), 2.79 (1H, d, 12.1, H-18β), 2.52 (1H, d, 12.1, H-18α), 2.27 (6H, s, H-22, H-23), 2.19 (2H, m, H-4), 2.10 (1H, m, H-3), 2.08 (1H, m, H-7α), 2.05 (1H, m, H-7β), 1.94 (1H, m, H-1α), 1.89 (1H, m, H-17), 1.88 (1H, m, H-12α), 1.78 (1H, m, H-2α), 1.69 (1H, m, H-16α), 1.69 (1H, m, H-11α), 1.64 (1H, m, H-15α), 1.51 (1H, m, H-15β), 1.46 (1H, m, H-2β), 1.41 (1H, m, H-12β), 1.32 (1H, m, H-8), 1.31 (1H, m, H-14), 1.23 (1H, m, H-11β), 1.16 (1H, m, H-16β), 1.12 (3H, d, 6.6, H-21), 1.08 (1H, m, H-1β), 0.99 (1H, m, H-9), 0.93 (3H, s, H-19). MS (m/z): 343.4082 [M + H]+; calculated for C23H39N2+: 343.3113.
Isoconessimine 12: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.9:0.1-saturated aq. NH3), 55.0; 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 5.37 (1H, td, 5.0, 2.2, H-6), 3.30 (1H, d, 11.3, H-18α), 2.73 (1H, m, H-20), 2.59 (1H, m, H-3), 2.54 (3H, s, H-23), 2.42 (3H, s, H-22), 2.35 (2H, m, H-4), 2.12 (1H, m, H-18β), 2.08 (1H, td, 5.4, 2.0, H-7α), 1.97 (1H, m, H-17), 1.92 (1H, m, H-2α), 1.89 (1H, m, H-1α), 1.82 (1H, m, H-12β), 1.82 (1H, m, H-15α), 1.68 (1H, m, H-16α), 1.67 (1H, m, H-11α), 1.61 (1H, m, H-2β), 1.60 (1H, m, H-7β), 1.51 (1H, dt, 10.7, 2.8, H-15β), 1.40 (1H, dd, 11.3, 4.1, H-12α), 1.34 (1H, m, H-8), 1.31 (1H, m, H-16β), 1.19 (3H, d, 6.5, H-21), 1.17 (1H, m, H-11β), 1.16 (1H, m, H-14), 1.08 (1H, m, H-1β), 0.97 (1H, m, H-9), 0.94 (3H, s, H-19). MS (m/z): 343.4055 [M + H]; calculated for C23H39N2+: 343.3113
Conessine 13: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.9:0.1-saturated aq. NH3), 70.0; 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 5.32 (1H, m, H-6), 2.97 (1H, d, 10.3, H-18α), 2.34 (1H, m, H-20), 2.27 (6H, s, H-23,24), 2.18 (3H, s, H-22), 2.18 (2H, m, H-4), 2.09 (1H, m, H-3), 2.06 (1H, m, H-7α), 1.87 (1H, m, H-1α), 1.85 (1H, m, H-18β), 1.82 (1H, m, H-17), 1.76 (1H, m, H-12α), 1.73 (1H, m, H-2α), 1.68 (1H, m, H-15α), 1.63 (1H, m, H-11α), 1.61 (1H, m, H-11β), 1.59 (2H, m, H-16), 1.58 (1H, m, H-7β), 1.41 (1H, m, H-2β), 1.39 (1H, m, H-15β), 1.36 (1H, m, H-8), 1.33 (1H, m, H-12β), 1.10 (1H, m, H-14), 1.04 (1H, m, H-1β), 1.01 (3H, d, 6.3, H-21), 0.93 (1H, m, H-9), 0.90 (3H, s, H-19). MS (m/z): 357.4072 [M + H]+; calculated for C24H41N2+: 357.3270
Holarrhesine 14: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.9:0.1-saturated aq. NH3), 69.0; 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 5.34 (1H, m, H-6), 5.31 (1H, ddt, 7.3, 2.9, 1.4, H-3′), 4.89 (1H, dd, 11.3, 4.2, H-12), 3.02 (2H, dp, 7.3, 1.0, H-2′), 2.84 (1H, d, 10.1, H-18β), 2.41 (3H, s, H-23), 2.34 (1H, d, 10.2, H-18α), 2.32 (1H, m, H-20), 2.27 (1H, m, H-3), 2.24 (1H, dd, 4.3, 2.1, H-4α), 2.22 (3H, s, H-22), 2.10 (1H, m, H-17), 2.08 (1H, m, H-7α), 2.00 (1H, m, H-4β), 1.78 (1H, m, H-11α), 1.78 (1H, m, H-1α), 1.74 (3H, s, H-5′), 1.71 (1H, m, H-15β), 1.65 (2H, m, H-16), 1.65 (3H, s, H-6′), 1.58 (1H, m, H-7β), 1.39 (1H, m, H-15α), 1.38 (1H, m, H-8), 1.27 (1H, m, H-2β), 1.25 (1H, m, H-2α), 1.20 (1H, m, H-11β), 1.20 (1H, m, H-14), 1.12 (1H, m, H-9), 1.08 (1H, m, H-1β), 0.98 (3H, d, 6.4, H-21), 0.93 (3H, s, H-19). MS (m/z): 455.5514 [M + H]; calculated for C29H47N2O2+: 455.3638.
Holarrhetine 15: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.9:0.1-saturated aq. NH3), 73.0; 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 5.32 (1H, dt, 4.1, 1.6, H-6), 5.30 (1H, ddt, 7.1, 2.7, 1.4, H-3′), 4.89 (1H, dd, 11.1, 4.2, H-12), 3.01 (2H, dp, 7.5, 1.0, H-2′), 2.84 (1H, d, 10.2, H-18α), 2.34 (1H, d, 10.4, H-18β), 2.32 (1H, m, H-20), 2.28 (6H, s, H-23, H-24), 2.22 (3H, s, H-22), 2.16 (2H, m, H-4), 2.10 (1H, m, H-17), 2.08 (1H, m, H-3), 2.06 (1H, m, H-7α), 1.82 (1H, m, H-1α), 1.78 (1H, dd, 10.0, 4.9, H-11α), 1.72 (1H, m, H-15β), 1.72 (1H, m, H-2α), 1.70 (3H, s, H-5′), 1.64 (3H, s, H-6′), 1.64 (1H, m, H-16α), 1.58 (1H, m, H-7β), 1.38 (1H, m, H-2β), 1.38 (1H, m, H-15α), 1.34 (1H, dd, H-8), 1.25 (1H, m, H-16β), 1.20 (1H, dd, H-14), 1.16 (1H, dd, 6.6, 3.1, H-11β), 1.12 (1H, dd, 3.6, 2.2, H-9), 1.05 (1H, m, H-1β), 0.98 (3H, d, 6.4, H-21), 0.92 (3H, s, H-19). MS (m/z): 469.5725 [M + H]+; calculated for C30H49N2O2+: 469.3794.
Holarrheline 16: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.9:0.1-saturated aq. NH3), 31.0; 1H-NMR (600 MHz CD3OD; δ (ppm), intensity, mult., J (Hz)): 5.35 (1H, dt, 5.4, 1.8, H-6), 3.58 (1H, dd, 11.0, 4.2, H-12), 2.77 (1H, d, 10.5, H-18α), 2.67 (1H, qd, 6.4, 4.9, H-20), 2.41 (1H, d, 10.5, H-18β), 2.36 (3H, s, H-23), 2.28 (1H, m, H-4α), 2.27 (1H, m, H-3), 2.25 (3H, s, H-22), 2.19 (1H, ddd, 10.8, 4.9, 3.0, H-17), 2.09 (1H, td, 5.3, 2.6, H-7α), 2.05 (1H, m, H-4β), 1.88 (1H, m, H-1α), 1.82 (1H, m, H-2β), 1.73 (1H, m, H-15β), 1.73 (1H, m, H-11α), 1.64 (1H, m, H-16β), 1.60 (1H, m, H-7β), 1.44 (1H, m, H-15α), 1.34 (1H, m, H-2α), 1.33 (1H, m, H-8), 1.32 (1H, m, H-16α), 1.15 (1H, td, 6.3, 1.5, H-14), 1.13 (1H, m, H-11β), 1.10 (1H, m, H-1β), 1.08 (1H, m, H-9), 1.04 (3H, d, 6.5, H-21), 0.95 (3H, s, H-19). MS (m/z): 359.4531 [M + H]+; calculated for C23H39N2O+: 359.3062.
Holarrhenine 17: hRf (silica gel 60 tlc, EtOAc:MeOH, 9.9:0.1-saturated aq. NH3), 55.0; 1H-NMR (600 MHz CD3OD; δ (ppm), intensity, mult., J (Hz)): 5.35 (1H, dt, 5.3, 1.9, H-6), 3.58 (1H, dd, 11.1, 4.2, H-12), 2.78 (1H, d, 10.5, H-18β), 2.68 (1H, m, H-20), 2.41 (1H, d, 10.6, H-18α), 2.27 (6H, s, H-23, H-24), 2.25 (3H, s, H-22), 2.23 (1H, m, H-4α), 2.18 (1H, m, H-4β), 2.18 (1H, m, H-14), 2.10 (1H, m, H-3), 2.07 (1H, m, H-7α), 1.90 (1H, m, H-1β), 1.78 (2H, m, H-2), 1.73 (1H, m, H-11α), 1.72 (1H, m, H-15α), 1.64 (1H, m, H-16α), 1.58 (1H, m, H-7β), 1.46 (1H, m, H-15β), 1.32 (1H, m, H-8), 1.30 (1H, m, H-16β), 1.15 (1H, m, H-17), 1.11 (1H, m, H-11β), 1.07 (1H, m, H-1α), 1.06 (1H, m, H-9), 1.04 (3H, d, 6.5, H-21), 0.95 (3H, s, H-19). MS (m/z): 373.4399 [M + H]+; calculated for C24H41N2O+: 373.3219.
Kurchinin 18: 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 7.74 (1H, d, 10.3, H-1), 6.15 (1H, dd, 2.0, 10.3, H-2), 6.10 (1H, t, 1.6, H-4), 4.10 (1H, tt, 6.1, 10.1, H-11), 2.52 (1H, dd, 5.0, 1.3, H-16α), 2.49 (1H, dt, 3.6, 1.3, H-6β), 2.47 (1H, dt, 7.7, 1.2, H-16β), 2.43 (1H, dq, 4.3, 2.5, H-6α), 2.22 (1H, dt, 5.0, 12.5, H-12α), 2.08 (1H, ddd, 6.4, 4.0, 1.5, H-7α), 1.96 (1H, dddd, 12.5, 9.0, 5.9, 1.2, H-15α), 1.83 (1H, dtd, 11.0, 3.8, 12.1, H-8), 1.57 (1H, m, H-15β), 1.35 (1H, ddd, 11.7, 5.9, 1.3, H-14), 1.32 (3H, s, H-19), 1.25 (1H, m, H-12β), 1.17 (1H, m, H-7β), 1.16 (1H, m, H-9), 0.95 (3H, s, H-18). MS (m/z): 301.3773 [M + H]+; calculated for C19H25O3: 301.1804.
N3-Isopentenyl adenine 19: 1H-NMR (600 MHz CDCl3; δ (ppm), intensity, mult., J (Hz)): 8.06 (1H, s, H-8), 8.01 (1H, s, H-2), 5.51 (1H, m, H-12), 5.00 (2H, d, 7.5, H-11), 1.85 (3H, s, H-14), 1.82 (3H, s, H-15). MS (m/z): 204.1475 [M + H]+; calculated for C10H14N5+: 204.1249.
All the spectra data were in full agreement with reported literature data [21,22,23,24,25,26,27,28,29,30,31].
3.7. In Vitro Biological Assays
In vitro tests for biological activity of the crude extracts, fractions, sub-fractions and isolated compounds against Trypanosoma brucei rhodesiense (bloodstream trypomastigotes, STIB 900 strain), Trypanosoma cruzi (amastigotes, Tulahuen C4 strain), Leishmania donovani (amastigotes, MHOM-ET-67/L82 strain), Plasmodium falciparum (intraerythrocytic form, NF54 strain) and cytotoxicity test against mammalian L6 cell line from rat skeletal myoblasts were performed according to the established standard protocol [33]. However, only the activity data against Trypanosoma brucei rhodesiense is reported due to the inactivity of the tested substances against other parasites at the concentration tested.
4. Conclusions
Steroid alkaloids from Holarrhena africana have proved to be an interesting new class of highly active and selective lead compounds against Trypanosoma brucei rhodesiense. Of the 17 steroid alkaloids isolated, eight were identified to possess significant antitrypanosomal activity with IC50 values well below 1 µM and represent potential candidates for further study. Some SARs were observed which indicates that a ∆5,6 steroid nucleus with a monomethyl amino group at C-3 and a pyrrolidine ring with the amino nitrogen placed between C-20 and C-18 represents an optimum, among the compounds isolated so far, for high antitrypanosomal activity and selectivity. This essential pharmacophoric requirement for active and selective anti-trypanosomal activity is currently under refinement in terms of 3D QSAR modelling; this modeling will provide more detailed explanations to the present findings and possibly allow activity predictions for yet untested compounds of this type, aiming at structural optimization.
Supplementary Materials
Supplementary materials are available online. The 1H and 13C NMR of all the isolated compounds are provided as supplementary Figures S1–S38.
Acknowledgments
We thank the Federal Government of Nigeria for a doctoral fellowship to Charles Nnadi at WWU, Münster through the Tertiary Education Trust Fund (TETFund) and the University of Nigeria Nsukka. This work is an activity within the Research Network Natural Products against Neglected Diseases (ResNet NPND, www.resnetnpnd.org).
Author Contributions
C.O.N. collected the plant materials, isolated and characterized the compounds, and wrote the initial draft of the manuscript. R.B. and M.K. performed the in vitro biological activity tests. T.J.S. and N.J.N. conceived and initiated the project. T.J.S. supervised the entire study and wrote the final draft of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hotez, P.J.; Alvarado, M.; Basáñez, M.; Bolliger, I.; Bourne, R.; Boussinesq, M.; Brooker, S.J.; Brown, A.S.; Buckle, G.; Budke, C.M.; et al. The Global Burden of Disease Study 2010: Interpretation and Implications for the Neglected Tropical Diseases. PLoS Negl. Trop. Dis. 2014, 8, e2865. [Google Scholar] [CrossRef] [PubMed]
- Fèvre, E.M.; Odiit, M.; Coleman, P.G.; Welburn, S.C.; Woolhouse, M.E.J. Estimating the burden of rhodesiense sleeping sickness during an outbreak in Serere, eastern Uganda. BMC Public Health 2008, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Fèvre, E.M.; Wissmann, B.V.; Welburn, S.C.; Lutumba, P. The Burden of Human African Trypanosomiasis. PLoS Negl. Trop. Dis. 2008, 2, e333. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.G. Stigma and the social burden of neglected tropical diseases. PLoS Negl. Trop. Dis. 2008, 2, e237. [Google Scholar] [CrossRef] [PubMed]
- Hopp, K.H.; Cunningham, L.V.; Bromel, M.C.; Schermeister, L.J.; Khalil, S.K. In vitro antitrypanosomal activity of certain alkaloids against Trypanosma lewisi. Lloydia 1976, 39, 375–377. [Google Scholar] [PubMed]
- Hoet, S.; Stévigny, C.; Block, S.; Opperdoes, F.; Colson, P.; Baldeyrou, B.; Lansiaux, A.; Bailly, C.; Quetin-Leclercq, J. Alkaloids from Cassytha filiformis and related aporphines: Antitrypanosomal activity, cytotoxicity, and interaction with DNA and topoisomerases. Planta Med. 2004, 70, 407–413. [Google Scholar] [PubMed]
- Nibret, E.; Sporer, F.; Asres, K.; Wink, M. Antitrypanosomal and cytotoxic activities of pyrrolizidine alkaloid-producing plants of Ethiopia. J. Pharm. Pharmacol. 2009, 61, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Scotti, M.T.; Speck-Planche, A.; Tavares, J.F.; da Silva Sobral, M.; Natália, M.; Cordeiro, D.S.; Scotti, L. Virtual Screening of Alkaloids from Apocynaceae with Potential Antitrypanosomal Activity. J. Curr. Bioinform. 2015, 10, 509–519. [Google Scholar] [CrossRef]
- Merschjohann, K.; Sporer, F.; Steverding, D.; Wink, M. In Vitro Effect of Alkaloids on Bloodstream Forms of Trypanosoma brucei and T. congolense. Planta Med. 2001, 67, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Toriizukaa, Y.; Kinoshitaa, E.; Kogurea, N.; Kitajimaa, M.; Ishiyamab, A.; Otogurob, K.; Yamadab, H.; Ōmurab, S.; Takayama, H. New lycorine-type alkaloid from Lycoris traubii and evaluation of antitrypanosomal and antimalarial activities of lycorine derivatives. Bioorg. Med. Chem. 2008, 16, 10182–10189. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.; Phillipson, J.D.; Croft, S.L.; Rock, P.; Marshall1, S.J.; Schiff, P.L., Jr. In-vitro activity of Triclisia patens and some bisbenzylisoquinoline alkaloids against Leishmania donovani and Trypanosoma brucei brucei. Phytother. Res. 2002, 16, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Hoerr, V.; Holzgrabe, U.; Stich, A. Antitrypanosomal naphthylisoquinoline alkaloids and related compounds. Die Pharm. 2003, 58, 343–346. [Google Scholar]
- Murebwayirea, S.; Frédérichb, M.; Hannaertc, V.; Jonvilleb, M.C.; Duez, P. Antiplasmodial and antitrypanosomal activity of Triclisia sacleuxii (Pierre) Diels. Phytomedicine 2008, 15, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Davis, R.A.; Sykes, M.L.; Avery, V.M.; Carroll, A.R.; Camp, D.; Quinn, R.J. Anti-trypanosomal pyridoacridine alkaloids from the Australian ascidian Polysyncraton echinatum. Tetrahedron Lett. 2010, 51, 2477–2479. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.; Biayatti, M.W.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V.; et al. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases-Part II. Curr. Med. Chem. 2012, 19, 2176–2228. [Google Scholar] [PubMed]
- Raffauf, R.F.; Flagler, M.B. Alkaloids of the Apocynaceae. Econ. Bot. 1960, 14, 37–55. [Google Scholar] [CrossRef]
- Bever, B.O. Medicinal plants in tropical West Africa. J. Ethnopharmacol. 1982, 5, 1–71. [Google Scholar] [CrossRef]
- Bogne, K.P.; Penlap, B.V.; Lontsi-David, E.F. Antibacterial activities of the extracts and conessine from Holarrhena floribunda G. DON. (Apocynaceae). Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 352–356. [Google Scholar] [PubMed]
- Fotie, J.; Scott, B.D.; MaraLeimanis, L.; Elias, G.; Geoffrey, R.; Nkenfack, E. Lupeol long-chain fatty acid esters with antimalarial activity from Holarrhena floribunda. J. Nat. Prod. 2006, 69, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Nwodo, N.J.; Brun, R.; Osadebe, P.O. In-vitro and in-vivo evaluation of the antitrypanosomal activity of fractions of Holarrhena africana. J. Ethnopharmacol. 2007, 113, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.; Sim, K.; Koyano, K.; Toyoshima, M.; Hayashi, M.; Komiyama, K. Cytotoxic and Leishmanicidal Aminoglycosteroids and Aminosteroids from Holarrhena curtisii. J. Nat. Prod. 1998, 61, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Kasal, A.; Budesinsky, M.; Griffiths, W.J. Spectroscopic Methods of Steroid Analysis. In Steroid Analysis, 2nd ed.; Makin, H.L.J., Gower, D.B., Eds.; Springer Science + Business Media B.V.: Dordrecht, The Netherlands, 2010; Volume 90. [Google Scholar]
- Zirihi, G.N.; Grellier, P.; Guede-Guina, F.; Bodo, B.; Mambu, L. Isolation, characterization and anti-plasmodial activity of steroidal alkaloids from Funtumia elastica (Preuss) Stapf. Bioorg. Med. Chem. Lett. 2005, 15, 2637–2640. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Su, J.; Ge, X.; Dong, T.; Li, X.; Wen, H.; Sun, B. Compounds with inhibitory activity on peristalsis from the seeds of Holarrhena antidysenterica. Nat. Prod. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.D.; Duan, D.Z.; Xue, W.W.; Yao, X.J.; Li, S. Steroidal alkaloids from Holarrhena antidysenterica as acetylcholinesterase inhibitors and the investigation for structure-activity relationships. Life Sci. 2012, 90, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, B.S.; Usmani, S.B.; Begum, S.; Siddiqui, C. Steroidal alkaloids and an androstane derivative from the bark of Holarrhena pubescens. Phytochemistry 1993, 33, 925–928. [Google Scholar] [CrossRef]
- Cerny, V.; Sorm, F. Steroid Alkaloids: Alkaloids of apocynaceae and buxaceae. In The Alkaloids: Chemistry and Physiology; Academic Press: New York, NY, USA, 1967; Volume 9, pp. 305–426. [Google Scholar]
- Hitchin, J.R.; Hamilton, N.M.; Jordan, A.M.; Lyons, A.J.; Ogilvie, D.J. A novel scalable and stereospecific synthesis of 3α- and 3β-amino-5α-androstan-17-ones and 3α- and 3β-amino-5α-pregnan-20-ones. Tetrahedron Lett. 2012, 53, 2868–2872. [Google Scholar] [CrossRef]
- Einhorn, J.; Monneret, C.; Khuong-Huu, Q. Alcaloïdes des feuilles de l’Holarrhena crassifolia. Phytochemistry 1972, 11, 769–777. [Google Scholar] [CrossRef]
- Wagner, H.; Seegert, K.; Sonnenbichler, H.; Ilyas, M.; Odenthal, K.P. Steroidal alkaloids of Funtumia africana. Planta Med. 1987, 53, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Vicha, J.; Malon, M.; Vesela, P.; Humpa, O.; Strnad, M.; Marek, R. 1H-, 13C-, and 15N- NMR chemical shifts for selected glucosides and ribosides of aromatic cytokinins. Magn. Reson. Chem. 2010, 48, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, V.; Skobridis, K.; Tzakos, A.G.; Ragoussis, V. A simple method for the alkaline hydrolysis of esters. Tetrahedron Lett. 2007, 48, 8230–8233. [Google Scholar] [CrossRef]
- Nour, A.M.M.; Khalid, S.A.; Kaiser, M.; Brun, R.; Abdallah, W.E.; Schmidt, T.J. The Antiprotozoal Activity of Sixteen Asteraceae Species Native to Sudan and Bioactivity-Guided Isolation of Xanthanolides from Xanthium brasilicum. Planta Med. 2009, 75, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 3, 8, 11, 14, 15, 16, 17, 19 are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).