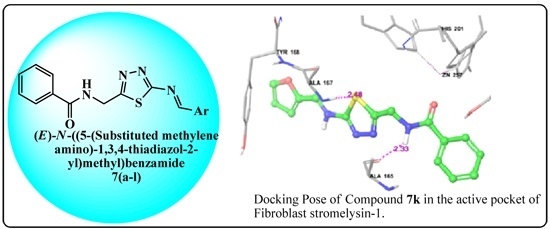
Microwave-Assisted Facile Synthesis, Anticancer Evaluation and Docking Study of N-((5-(Substituted methylene amino)-1,3,4-thiadiazol-2-yl)methyl) Benzamide Derivatives
Abstract
:1. Introduction
2. Result and Discussion
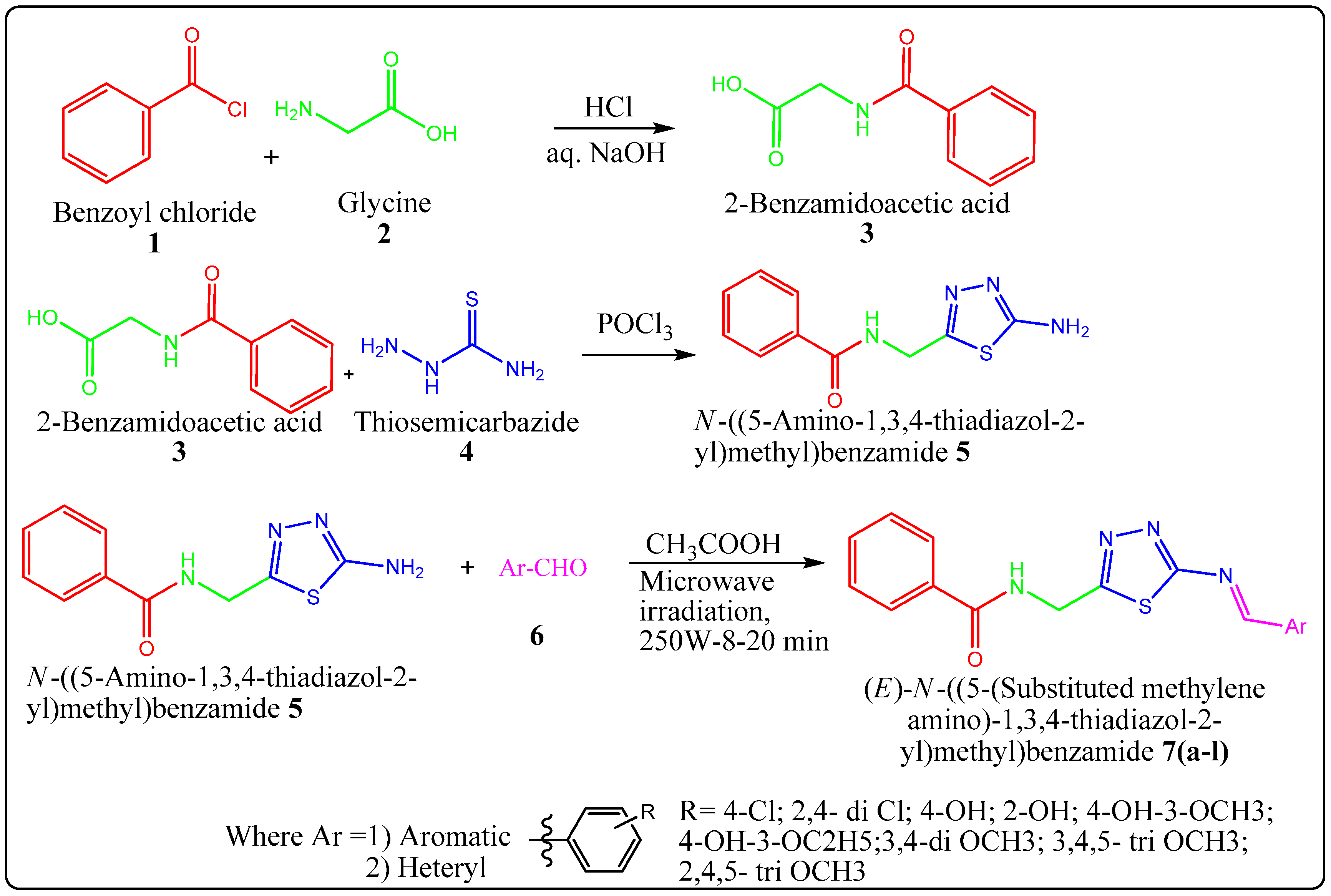
2.1. Chemistry
2.2. In Vitro Anticancer Activity
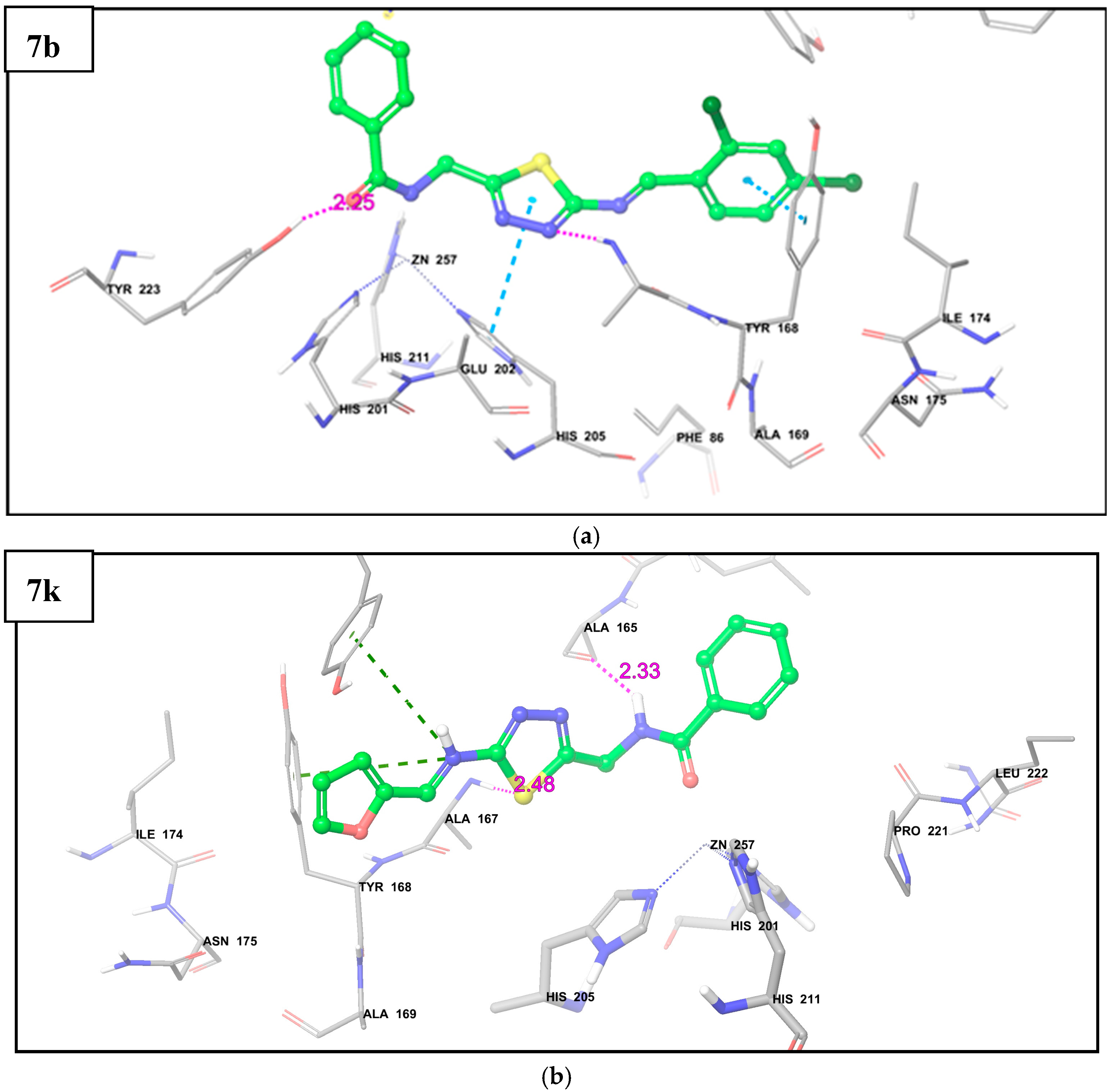
2.3. Molecular Docking
2.4. In Silico ADMET Prediction
3. Materials and Methods
3.1. General Information
3.1.1. Step I: General Process for Synthesis of 2-Benzamidoacetic Acid [34]
3.1.2. Step II: General Procedure for Synthesis of N-((5-Amino-1,3,4-thiadiazol-2-yl)methyl) Benzamide [35]
3.1.3. Step III: General procedure for synthesis of (E)-N-((5-(substituted methyleneamino)-1,3,4-thiadiazol-2-yl)methyl) benzamide 7(a–l)
3.2. In Vitro Anticancer Screening
Experimental procedure for MTT assay
3.3. Computational Study
Molecular Docking and In Silico ADMET Prediction
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patrick, G.L. An Introduction to Medicinal Chemistry, 4th ed.; Oxford University Press Inc.: New York, NY, USA, 2009; p. 519. [Google Scholar]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Gail Eckhardt, S. Development of Matrix Metalloproteinase Inhibitors in Cancer Therapy. J. Natl. Cancer Inst. 2001, 93, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.F.; Matrisian, L. Changing views of the role of matrix metalloproteinases in metastasis. J. Natl. Cancer Inst. 1997, 89, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Kahari, V.M.; Saarialho-Kere, U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann. Med. 1999, 31, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.M.; Stetler-Stevenson, W.G. Gelatinase A activity directly modulates melanoma cell adhesion and spreading. EMBO J. 1995, 14, 908–917. [Google Scholar] [PubMed]
- Kleiner, D.E.; Stetler-Stevenson, W.G. Matrix metalloproteinases and metastasis. Cancer Chemother. Pharmacol. 1999, 43, S42–S51. [Google Scholar] [CrossRef] [PubMed]
- Denis, L.J.; Verweij, J. Matrix metalloproteinase inhibitors: Present achievements and future prospects. Investig. New Drugs. 1997, 15, 175–185. [Google Scholar] [CrossRef]
- Wojtowicz-Praga, S.M.; Dickson, R.B.; Hawkins, M.J. Matrix metalloproteinase inhibitors. Investig. New Drugs. 1997, 15, 61–75. [Google Scholar] [CrossRef]
- Brown, P.D. Clinical studies with matrix metalloproteinase inhibitors. APMIS. 1999, 107, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Kaplancikli, Z.A.; Altıntop, M.D.; Atli, O.; Sever, B.; Baysal, M.; Temel, H.E.; Demirci, F.; Ozdemir, A. Synthesis and Evaluation of A New Series of Thiazole Derivatives as Potential Antitumor Agents and MMP Inhibitors. Anti-Cancer Agents Med. Chem. 2017, 17, 674–681. [Google Scholar]
- Zou, X.J.; Zhang, S.W.; Liu, Y.; Liu, Z.M.; Gao, J.W.; Song, Q.L.; Pan, Y.; Zhang, J.Z.; Li, X.J. Anti-tumor metastasis and Crystal Structure of N1-(1,3,4-thiadiazole-2-yl)-N3-m-chlorobenzoyl-urea. Chin. J. Struct. Chem. 2011, 30, 1001–1005. [Google Scholar]
- Du, H.T.; Du, H.J. Synthesis and Biological Activity of 6-(Substituted)-3-(3,4,5-trimethoxyphenyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazole. Chin. J. Org. Chem. 2010, 30, 137–141. [Google Scholar]
- Liu, F.; Luo, X.Q.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y. Synthesis and antifungal activity of novel sulfoxide derivatives containing trimethoxyphenyl substituted 1,3,4-thiadiazole and 1,3,4-oxadiazole moiety. Bioorg. Med. Chem. 2008, 16, 3632–3640. [Google Scholar] [CrossRef] [PubMed]
- Matwijczuk, A.; Kluczyk, D.; Gorecki, A.; Niewiadomy, A.; Gagos, M. Spectroscopic Studies of Fluorescence Effects in bioactive 4-(5-heptyl-1,3,4-thiadiazol-2-yl)benzene-1,3-diol and 4-(5-methyl-1,3,4-thiadiazol-2-yl)benzene-1,3-diol molecules Induced by pH Changes in Aqueous Solutions. J. Fluoresc. 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Matwijczuk, A.; Kluczyk, D.; Górecki, A.; Niewiadomy, A.; Gagos, M. Solvent Effects on Molecular Aggregation in 4-(5-Heptyl-1,3,4-thiadiazol-2-yl)benzene-1,3-diol and 4-(5-Methyl-1,3,4-thiadiazol-2-yl)benzene-1,3-diol. J. Phys. Chem. B 2016, 120, 7958–7969. [Google Scholar] [CrossRef] [PubMed]
- Kluczyk, D.; Matwijczuk, A.; Gorecki, A.; Karpinska, M.M.; Szymanek, M.; Niewiadomy, A.; Gagos, M. Molecular Organization of Dipalmitoylphosphatidylcholine Bilayers Containing Bioactive Compounds 4-(5-Heptyl-1,3,4-thiadiazol-2-yl) Benzene-1,3-diol and 4-(5-Methyl-1,3,4-thiadiazol-2-yl) Benzene-1,3-diols. J. Phys. Chem. B 2016, 120, 12047–12063. [Google Scholar] [CrossRef] [PubMed]
- Matwijczuk, A.; Kamiński, D.; Górecki, A.; Ludwiczuk, A.; Niewiadomy, A.; Maćkowski, S.; Gagos, M. Spectroscopic Studies of Dual Fluorescence in 2-((4-Fluorophenyl)amino)-5-(2,4-dihydroxybenzeno)-1,3,4-thiadiazole. J. Phys. Chem. A 2015, 119, 10791–10805. [Google Scholar] [CrossRef] [PubMed]
- Karcz, D.; Matwijczuk, A.; Boroń, B.; Creaven, B.; Fiedor, L.; Niewiadomy, A.; Gagoś, M. Isolation and spectroscopic characterization of Zn(II), Cu(II), and Pd(II) complexes of 1,3,4-thiadiazole-derived ligand. J. Mol. Struc. 2017, 1128, 44–50. [Google Scholar]
- Shrivastava, K.; Purohit, S.; Singhal, S. Studies of nitrogen and sulphur containing heterocyclic compound: 1,3,4-Thiadiazole. Asian J. Biomed. Pharm. Sci. 2013, 3, 6–23. [Google Scholar]
- Siddiqui, N.; Ahujaa, P.; Ahsana, W.; Pandey, S.N.; Alama, M.S. Thiadiazoles: Progress Report on Biological Activities. J. Chem. Pharm. Res. 2009, 1, 19–30. [Google Scholar]
- Chaudhary, D.K.; Chaudhary, R.P. Pharmacological Activities of 1,3,4 Thiadiazole Derivatives Review. Int. J. Pharm. Biol. Sci. Arch. 2013, 4, 256–264. [Google Scholar]
- Chhajed, M.; Shrivastava, A.K.; Taile, V. Synthesis of 5-arylidine amino-1,3,4-thiadiazol-2-[(N-substituted benzyol)]sulphonamides endowed with potent antioxidants and anticancer activity induces growth inhibition in HEK293, BT474 and NCI-H226 cells. Med. Chem. Res. 2014, 23, 3049–3064. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, E.J.; Mitchell, M.A.; Hendges, S.K.; Belonga, K.L.; Skaletzky, L.L.; Stelzer, L.S.; Lindberg, T.J.; Fritzen, E.L.; Schostarez, H.J.; OSullivan, T.J.; et al. Synthesis of a series of stromelysin-selective thiadiazole urea matrix metalloproteinase inhibitors. J. Med. Chem. 1999, 42, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Foda, H.D.; Zucker, S. Matrix metalloproteinases in cancer invasion, metastasis and angiogenesis. Drug Discov. Today 2001, 6, 478–482. [Google Scholar] [CrossRef]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Caddick, S. Microwave Assisted Organic Reactions. Tetrahedron 1995, 5, 10403–10432. [Google Scholar] [CrossRef]
- Finzel, B.C.; Baldwin, E.T.; Bryant, G.L.; Hess, G.F.; Wilks, J.W.; Trepod, C.M.; Mott, J.E.; Marshall, V.P.; Petzold, G.L.; Poorman, R.A.; et al. Structural characterizations of nonpeptidic thiadiazole inhibitors of matrix metalloproteinases reveal the basis for stromelysin selectivity. Protein Sci. 1998, 7, 2118–2126. [Google Scholar] [CrossRef] [PubMed]
- Hedley, P.L.; Jorgensen, P.; Schlamowitz, S.; Wangari, R.; Moolman-Smook, J.; Brink, P.A.; Kanters, J.K.; Corfield, V.A.; Christiansen, M. The genetic basis of long QT and short QT syndromes: A mutation update. Hum. Mutat. 2009, 30, 1486–1511. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, J.I.; Walker, B.D.; Campbell, T.J. HERG K+ channels: Friend and foe. Trends Pharmacol. Sci. 2001, 22, 240–246. [Google Scholar] [CrossRef]
- Chiesa, N.; Rosati, B.; Arcangeli, A.; Olivotto, M.; Wanke, E. A Novel Role for HERG K+Channels: Spike-Frequency Adaptation. J. Physiol. 1997, 501, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Aronov, A.M. Predictive in silico modeling for HERG channel blockers. Drug Discov. Today 2005, 10, 149–155. [Google Scholar] [CrossRef]
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.; Vogel, A.I. Vogel’s textbook of practical organic chemistry. Longman Sci. Tech. 1989, 5, 1056. [Google Scholar]
- Tomi, I.H.R.; Al-Daraji, A.H.R.; Al-Qaysi, R.R.T.; Hasson, M.M.; Al-Dulaimy, K.H.D. Synthesis, characterization and biological activities of some azo derivatives of aminothiadiazole derived from nicotinic and isonicotinic acids. Arab. J. Chem. 2010, 3, 687–694. [Google Scholar] [CrossRef]
- Narayanan Moorthy, N.S.H.; Vittal, U.B.; Karthikeyan, C.; Thangapandian, V.; Venkadachallam, A.P.; Trivedi, P. Synthesis, antifungal evaluation and in silico study of novel Schiff bases derived from 4-amino-5(3,5-dimethoxy-phenyl)-4H-1,2,4-triazol-3-thiol. Arab. J. Chem. 2014, 244–252. [Google Scholar] [CrossRef]
- Miglani, S.; Mishra, M.; Chawla, P. The rapid synthesis of schiff-bases without solvent under microwave irradiation and their antimicrobial activity. Der Pharm. Chem. 2012, 4, 2265–2269. [Google Scholar]
- Mosman, T.J. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of Drug Screening with Panels of Human Tumor Cell Lines Using a Microculture Tetrazolium Assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Ertl, P.; Rohde, B.; Selzer, P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment-Based Contributions and Its Application to the Prediction of Drug Transport Properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Entry | Conventional Method | Microwave Method | ||||
|---|---|---|---|---|---|---|
| Solvent | Temperature °C | Time (h) | Yield (%) | Time (min) | Yield (%) | |
| 7a | Ethanol | 80–90 | 3.30 | 78 | 8 | 95 |
| 7b | Ethanol | 80–90 | 5.10 | 77 | 12 | 92 |
| 7c | Ethanol | 80–90 | 5.00 | 78 | 12 | 94 |
| 7d | Ethanol | 80–90 | 7.20 | 66 | 15 | 94 |
| 7e | Ethanol | 80–90 | 7.25 | 52 | 15 | 92 |
| 7f | Ethanol | 80–90 | 8.00 | 48 | 18 | 92 |
| 7g | Ethanol | 80–90 | 5.25 | 76 | 12 | 95 |
| 7h | Ethanol | 80–90 | 7.30 | 66 | 15 | 88 |
| 7i | Ethanol | 80–90 | 8.00 | 68 | 20 | 86 |
| 7j | Ethanol | 80–90 | 8.00 | 48 | 20 | 88 |
| 7k | Ethanol | 80–90 | 4.15 | 44 | 10 | 85 |
| 7l | Ethanol | 80–90 | 4.25 | 56 | 10 | 84 |
| Entry | GI50 µM | ||||
|---|---|---|---|---|---|
| MCF-7 | HeLa | SKMEL-2 | HL-60 | MCF-10A | |
| 7a | 22.9 | 32.8 | 21.9 | 21.7 | >100 |
| 7b | 28.7 | 39.0 | 22.9 | 28.2 | 86.1 |
| 7c | 32.4 | 41.1 | 27.5 | 33.3 | ND |
| 7d | 36.7 | 52.4 | 34.0 | 40.2 | ND |
| 7e | 35.2 | 46.8 | 28.1 | 39.6 | ND |
| 7f | 38.4 | 49.2 | 30.0 | 37.5 | ND |
| 7g | 41.0 | 66.1 | 46.4 | 42.4 | ND |
| 7h | 46.2 | 71.7 | 49.1 | 48.2 | ND |
| 7i | 49.0 | 78.0 | 52.6 | 45.8 | ND |
| 7j | 51.4 | 78.8 | 55.7 | 49.9 | ND |
| 7k | 11.7 | 23.8 | 19.6 | 35.5 | > 100 |
| 7l | 19.0 | 28.8 | 22.0 | 29.9 | > 100 |
| ADR | < 10 | < 10 | < 10 | < 10 | ND |
| Compound | Docking Score | Compound | Docking Score |
|---|---|---|---|
| 7a | −6.61 | 7h | −6.33 |
| 7b | −6.56 | 7i | −6.61 |
| 7c | −5.93 | 7j | −5.10 |
| 7d | −5.82 | 7k | −7.56 |
| 7e | −5.84 | 7l | −7.32 |
| 7f | −5.66 | ADR | −4.33 |
| 7g | −5.26 |
| Entry | M.W a | LogP o/w b (−2.0–6.5) | n-ON c (<10) | n-OHNH d (<5) | PSA e (7–200) | log Khsa f (−1.5–1.2) | Log S g (−6–0.5) | % ABS h | #Meta i (1–8) | Log HERG j Below−5 | Lipinski Rule of 5 (≤1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7a | 356.8 | 4.82 | 5.5 | 1 | 76.2 | 0.31 | −5.8 | 98 | 2 | −6.8 | 0 |
| 7b | 391.2 | 5.34 | 5.5 | 1 | 74.4 | 0.37 | −6.1 | 99 | 2 | −6.6 | 0 |
| 7c | 338.3 | 5.18 | 6.2 | 2 | 98.4 | 0.02 | −4.7 | 89 | 3 | −6.7 | 0 |
| 7d | 338.3 | 5.17 | 6 | 2 | 98.2 | 0.03 | −4.3 | 88 | 3 | −6.5 | 0 |
| 7e | 368.4 | 5.27 | 7.2 | 1 | 133.3 | −0.04 | −4.7 | 75 | 3 | −6.6 | 0 |
| 7f | 382.4 | 5.09 | 7 | 2 | 105.7 | 0.16 | −5.5 | 91 | 4 | −6.9 | 0 |
| 7g | 352.4 | 4.91 | 6.2 | 1 | 83.9 | 0.16 | −5.1 | 100 | 3 | −6.7 | 0 |
| 7h | 382.4 | 5.20 | 7 | 1 | 89.0 | 0.19 | −5.4 | 100 | 4 | −6.7 | 0 |
| 7i | 412.2 | 4.05 | 7.7 | 1 | 95.4 | 0.17 | −5.4 | 100 | 5 | −6.5 | 0 |
| 7j | 412.2 | 4.08 | 7.7 | 1 | 97.6 | 0.19 | −5.6 | 100 | 5 | −6.6 | 0 |
| 7k | 312.3 | 3.91 | 6 | 1 | 84.7 | −0.11 | −4.0 | 94 | 3 | −6.3 | 0 |
| 7l | 328.4 | 4.11 | 5 | 1 | 85.6 | −0.21 | −4.2 | 95 | 3 | −6.2 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, S.V.; Siddiqui, S.; Seijas, J.A.; Vazquez-Tato, M.P.; Sarkate, A.P.; Lokwani, D.K.; Nikalje, A.P.G. Microwave-Assisted Facile Synthesis, Anticancer Evaluation and Docking Study of N-((5-(Substituted methylene amino)-1,3,4-thiadiazol-2-yl)methyl) Benzamide Derivatives. Molecules 2017, 22, 995. https://doi.org/10.3390/molecules22060995
Tiwari SV, Siddiqui S, Seijas JA, Vazquez-Tato MP, Sarkate AP, Lokwani DK, Nikalje APG. Microwave-Assisted Facile Synthesis, Anticancer Evaluation and Docking Study of N-((5-(Substituted methylene amino)-1,3,4-thiadiazol-2-yl)methyl) Benzamide Derivatives. Molecules. 2017; 22(6):995. https://doi.org/10.3390/molecules22060995
Chicago/Turabian StyleTiwari, Shailee V., Sumaiya Siddiqui, Julio A. Seijas, M. Pilar Vazquez-Tato, Aniket P. Sarkate, Deepak K. Lokwani, and Anna Pratima G. Nikalje. 2017. "Microwave-Assisted Facile Synthesis, Anticancer Evaluation and Docking Study of N-((5-(Substituted methylene amino)-1,3,4-thiadiazol-2-yl)methyl) Benzamide Derivatives" Molecules 22, no. 6: 995. https://doi.org/10.3390/molecules22060995
APA StyleTiwari, S. V., Siddiqui, S., Seijas, J. A., Vazquez-Tato, M. P., Sarkate, A. P., Lokwani, D. K., & Nikalje, A. P. G. (2017). Microwave-Assisted Facile Synthesis, Anticancer Evaluation and Docking Study of N-((5-(Substituted methylene amino)-1,3,4-thiadiazol-2-yl)methyl) Benzamide Derivatives. Molecules, 22(6), 995. https://doi.org/10.3390/molecules22060995